Abstract
The lipid mediator sphingosine-1-phosphate (S1P), the product of sphingosine kinase (SPHK)-induced phosphorylation of sphingosine, is known to stabilize interendothelial junctions and prevent microvessel leakiness. Here, we investigated the role of SPHK1 activation in regulating the increase in pulmonary microvessel permeability induced by challenge of mice with lipopolysaccharide or thrombin ligation of protease-activating receptor (PAR)-1. Both lipopolysaccharide and thrombin increased mouse lung microvascular permeability and resulted in a delayed activation of SPHK1 that was coupled to the onset of restoration of permeability. In contrast to wild-type mice, Sphk1−/− mice showed markedly enhanced pulmonary edema formation in response to lipopolysaccharide and PAR-1 activation. Using endothelial cells challenged with thrombin concentration (50 nmol/L) that elicited a transient but reversible increase in endothelial permeability, we observed that increased SPHK1 activity and decreased intracellular S1P concentration preceded the onset of barrier recovery. Thus, we tested the hypothesis that released S1P in a paracrine manner activates its receptor S1P1 to restore the endothelial barrier. Knockdown of SPHK1 decreased basal S1P production and Rac1 activity but increased basal endothelial permeability. In SPHK1-depleted cells, PAR-1 activation failed to induce Rac1 activation but augmented RhoA activation and endothelial hyperpermeability response. Knockdown of S1P1 receptor in endothelial cells also enhanced the increase in endothelial permeability following PAR-1 activation. S1P treatment of Sphk1−/− lungs or SPHK1-deficient endothelial cells restored endothelial barrier function. Our results suggest the crucial role of activation of the SPHK1→S1P→S1P1 signaling pathway in response to inflammatory mediators in endothelial cells in regulating endothelial barrier homeostasis.
Keywords: sphingosine kinase, lung vascular permeability, thrombin, PAR-1, RhoGTPases, S1P1, S1P
The vascular endothelium forms a semipermeable barrier separating intravascular and tissue compartments. Disruption of endothelial barrier is a crucial factor in the pathogenesis of tissue inflammation, the hallmark of inflammatory diseases such as the acute respiratory distress syndrome. 1 Increased microvessel endothelial permeability leads to protein-rich alveolar edema that severely impairs oxygenation. 2 Thrombin, a serine protease, generated during sepsis and intravascular coagulation, ligates the endothelial cell surface receptor protease activating receptor 1 (PAR-1) and increases endothelial permeability.1,3–6 This increase in endothelial permeability is typically followed by a recovery period of ≈2 hours, during which barrier integrity is restored. 7,8 It has been surmised that PAR-1 signaling stimulates intrinsic repair mechanisms that restore barrier function. 7–9 Sphingosine-1-phosphate (S1P), a lipid mediator, was shown to be 1 such factor promoting endothelial barrier function.10–13 S1P binds to S1P1 receptor in endothelial cells, leading to activation of heterotrimeric G proteins of the Gi class, and signals enhancement of endothelial barrier function through Rac1-dependent adherens junction assembly and actin cytoskeletal remodeling.10–13
Sphingosine kinases (SPHKs) catalyze the formation of S1P from sphingosine (SPH).14,15 SIP in the circulation is short-lived because of degradation by S1P phosphatase and sphingosine phosphate lyase.16 Because SPHK activity is required to replenish cellular and plasma S1P concentration,17 SPHK is a crucial checkpoint regulating S1P synthesis.14,15 SPHK activity in endothelial cells largely contributes in maintaining circulating S1P concentration.16,17 SPHK is expressed as SPHK1 or SPHK2 isoforms in various cell types.15,18 In the present study, we used SPHK1 knockout mice to investigate the role of alterations in SPHK1 activity in regulating endothelial barrier function. Our results demonstrate that SPHK1-generated S1P is a crucial mechanism limiting the effects of inflammatory mediators in increasing endothelial permeability and does so by the activation of endothelial cell S1P1 signaling pathway.
Materials and Methods
Animals, cell culture and transfection, RT-PCR, SPHK activity assay, S1P measurement, confocal imaging, endothelial permeability determination, and lung vascular permeability determination are described in the expanded Material and Methods section in the online data supplement, available at http://circres.ahajournals.org.
Results
SPHK1 Limits the Increase in Lung Vascular Permeability and Edema Formation Following PAR-1 Activation
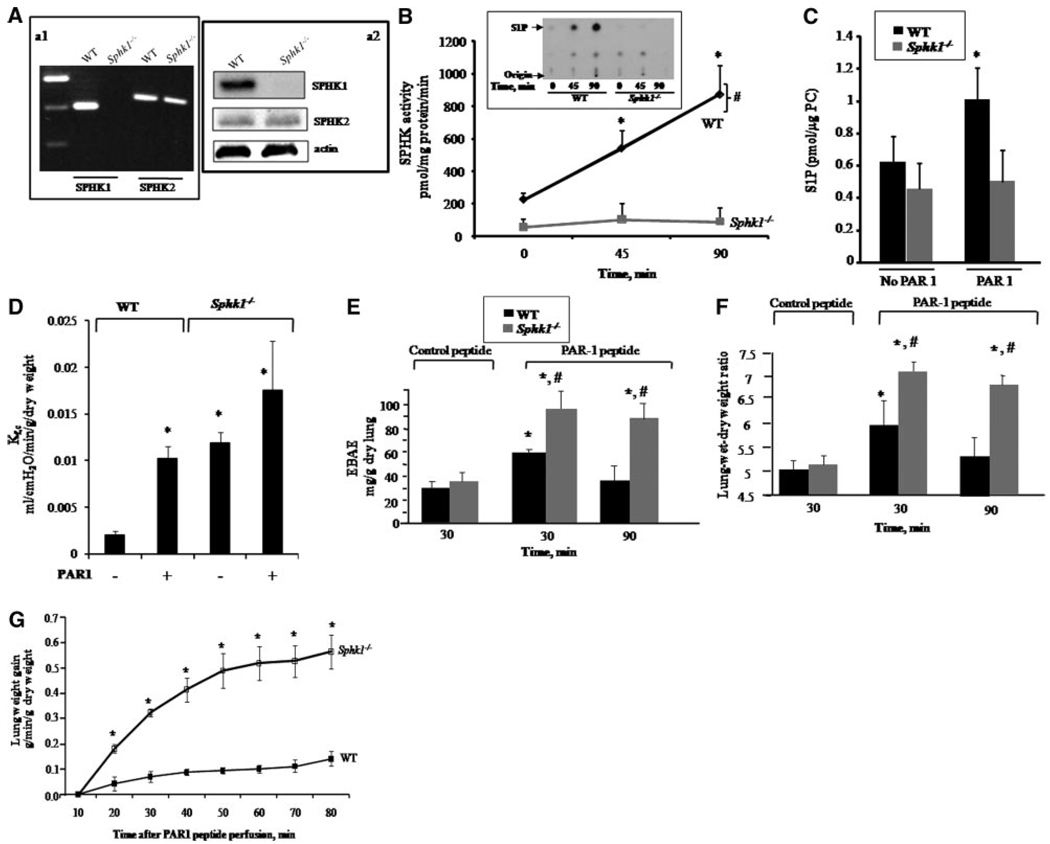
We used Sphk1−/− mice to address the role of SPHK1 in regulating S1P generation in lung microcirculation and endothelial barrier function. RT-PCR analysis of RNA isolated from lungs of wild-type (WT) and Sphk1−/− mice showed that WT lungs expressed both SPHK1 and SPHK2 isoform, whereas only SPHK1 was absent in Sphk1−/− mice (Figure 1A, a1). Similar expression of SPHK2 was seen in RNA and lysates of lungs of WT and Sphk1−/− mice (Figure 1A, a1 and a2), indicating that loss of SPHK1 did not lead to compensatory increase in SPHK2.
Figure 1.
SPHK1 counteracts PAR-1–induced increase in lung microvascular permeability. A, SPHK expression in lungs; RT-PCR of SPHK1 RNA (a1) and protein expression (a2) in WT and Sphk1 −/− lungs. a1, RNA extracted from lungs was treated with DNAase and reverse-transcribed using suitable primers as described in the expanded Materials and Methods section in the online data supplement. a2, Lung homogenates were processed for Western blotting with anti-SPHK1, anti- SPHK2, or anti–β-actin antibodies. B and C, SPHK activity and S1P concentration in lungs. Control or PAR-1 agonist peptide (1 mg/kg) were administered IV to WT and Sphk1−/− mice, and lungs were obtained at indicated times to determine SPHK enzymatic activity (B) or S1P concentrations (C). Inset in B, Representative autoradiograph showing S1P spot (arrow). Data in B and C are mean±SD of 4 to 5 experiments. *Significant increase in activity compared to 0 time in WT lungs (P<0.05). #Significant difference from SPHK1-deficient lungs (P<0.05). D, Lung microvessel permeability (measured as Kf,c) in Sphk1−/− mice. After establishing isogravimetric condition, intravascular pressure of lungs was raised by 10 cm H20 for 20 minutes. Kf,c was determined as described in the expanded Materials and Methods section. Data are expressed as mean±SEM of 3 experiments. *Significant difference from WT under basal conditions (P<0.05). E and F, PAR-1–induced increase in lung vascular permeability is augmented in Sphk1−/− mice. PAR-1 agonist (1 mg/kg) or control peptide were injected IV into mice, and, after 30 or 90 minutes, lung vascular permeability was determined by quantifying EBAE (E) or wet/dry weight ratio (F) as described in the expanded Materials and Methods section. Data are expressed as mean±SD of 3 experiments. Because the values of EBAE and wet/dry weight ratio at 30 or 90 minutes following IV administration of control peptide were similar, only 30 minutes of values are shown. *Significant difference from the corresponding control peptide group (P<0.05). #Significant difference from PAR-1 peptide-treated WT group (P<0.05). G, PAR-1 agonist peptide induces pulmonary edema in Sphk1−/− lungs. Lungs were equilibrated for 10 minutes and then perfused with PAR-1 agonist peptide, and lung wet weight was monitored for an additional 70-minute period. Data are expressed as mean±SEM of 3 experiments. *Significant difference from WT control (P<0.05).
We have shown that thrombin increases lung microvascular permeability through endothelial cell surface protease activating receptor (PAR)-1.5 Using PAR-1–specific activating peptide (TFLLRN), we determined whether PAR-1 activation alters SPHK activity in lungs. WT or Sphk1−/− mice received IV injection of either control peptide or PAR-1– activating peptide (1 mg/kg). Lungs were obtained at 45 or 90 minutes and were homogenized for determination of SPHK activity using sphingosine as substrate. SPHK was constitutively active in WT lungs (Figure 1B). PAR-1 agonist peptide significantly increased SPHK activity above basal at 45 and 90 minutes (Figure 1B). However, SPHK activity under basal conditions was decreased by 5-fold in Sphk1−/− lungs, and, importantly, it did not increase following PAR-1 activation (Figure 1B). We also determined lung S1P concentrations following PAR-1 agonist peptide administration in WT and Sphk1−/− mice. Basal S1P concentration did not differ in lungs from WT and Sphk1−/− mice (Figure 1C). PAR-1 activation significantly increased S1P concentration in WT lungs but failed to induce S1P formation above basal amount in Sphk1−/− lungs (Figure 1C). These findings demonstrate that PAR-1 agonist activates SPHK1 in vivo, which generates S1P in lungs.
We next addressed the possible role of SPHK1-induced S1P synthesis in regulating lung microvascular permeability using the isolated mouse lung preparation.5 We determined the microvessel filtration coefficient (Kf,c) in WT and Sphk1−/− lungs under basal condition and after challenge with PAR-1 agonist peptide. Basal Kf,c was significantly higher in Sphk1−/− lungs than WT lungs (Figure 1D). PAR-1 activation significantly increased Kf,c in WT lungs, and a greater increase was seen in Sphk1−/− lungs (Figure 1D). Other studies were made to determine the role of SPHK1 in regulating increased lung vascular permeability resulting from PAR-1 activation. In these studies, we quantified Evans blue albumin extravasation (EBAE) to determine transvascular albumin permeability and lung wet/dry weight ratio to quantify edema formation.19–21 Lung vascular albumin permeability was the same in WT and Sphk1−/− mice receiving a scrambled PAR-1 peptide (Figure 1E and 1F). Injection of PAR-1 peptide (IV) increased EBAE (Figure 1E) and produced edema in WT lungs (Figure 1F). Edema formation and lung vascular permeability recovered at 90 minutes in WT mice. In the absence of SPHK1, PAR-1 further augmented the increase in lung vascular permeability and edema formation (Figure 1E and 1F), indicating that SPHK1-mediated S1P generation suppresses PAR-1–induced pulmonary edema. In other studies, we determined whether loss of SPHK1 enhances the rate of edema formation following PAR-1 activation, as monitored by changes in lung wet weight in the isolated perfused lung preparation. PAR-1 agonist peptide infusion via the pulmonary artery cannula significantly increased wet weight gain in Sphk1−/− lungs compared to WT lungs (Figure 1G). Together, these results demonstrate the critical requirement of SPHK1 activity in opposing PAR-1-induced barrier dysfunction.
Augmented Lipopolysaccharide-Induced Increase in Lung Vascular Permeability in Sphk1−/− Mice
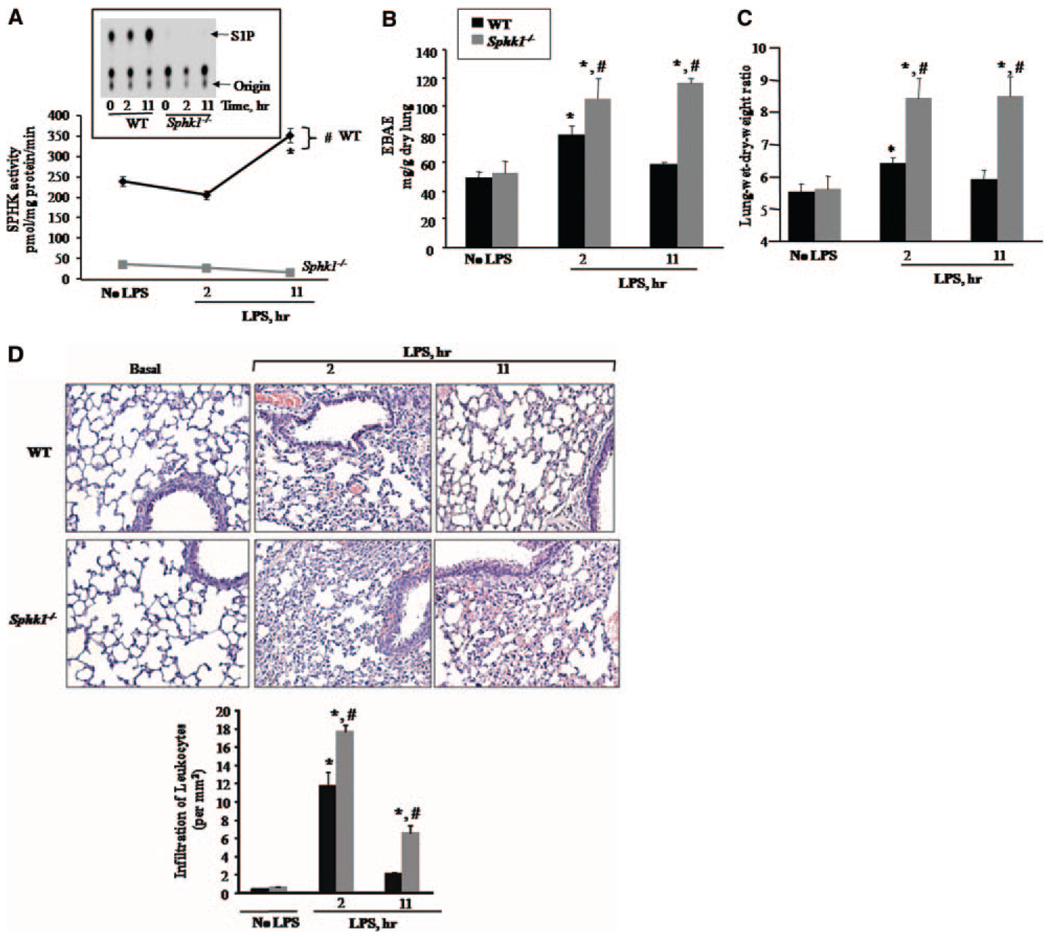
We addressed whether SPHK1 can also modulate increased lung vascular permeability provoked by lipopolysaccharide (LPS), known to cause neutrophil activation–mediated lung vascular leak.1,3–6 Sphk1−/− and WT mice received nebulized LPS by inhalation for 45 minutes as described (see expanded Materials and Methods in the online data supplement), and, after 2 or 11 hours, the mice were killed to determine SPHK activity, lung edema formation, and lung neutrophil infiltration. We observed that LPS challenge increased lung SPHK activity after 11 hours in WT mice, but not in SPHK1-deficient mice (Figure 2A). In the absence of SPHK1, LPS caused significantly greater increases in lung vascular permeability (Figure 2B) and wet/dry lung weight ratio (Figure 2C), as well as further increases in lung neutrophil sequestration (Figure 2D). The increases in lung vascular permeability and water content, as well as neutrophil sequestration, returned to normal within 11 hours in WT mice, but these responses persisted in Sphk1−/− mice.
Figure 2.
Enhanced LPS-induced pulmonary edema and neutrophil infiltration in Sphk1−/− mice. WT and Sphk1−/− mice were exposed to aerosolized LPS (1 mg/mL) dissolved in PBS or PBS alone for 45 minutes and at indicated times, lungs were isolated and perfused with PBS to remove blood. A, SPHK enzymatic activity in response to LPS. Lungs were homogenized, and lysates were used for in vitro determination of SPHK enzymatic activity. The inset displays a representative autoradiograph of S1P separation on thin-layer chromatography plates. Data are given as mean±SD of 3 experiments. *Significant difference in enzyme activity compared to “no LPS” group of WT lungs (P<0.05). #Significant difference from Sphk1−/− lungs (P<0.05). B and C, LPS-induced transvascular albumin transport and lung edema formation. Evans blue albumin transfer to lung tissue (EBAE) (B) and lung wet/dry weight ratio (C) are plotted at 2 or 11 hours after LPS challenge. Data points represent mean±SD of 3 experiments. *Significant difference from PBS group (ie, no LPS) (P<0.05). #Significant difference from LPS-challenged WT group (P<0.05). D, Quantitative analysis of increased neutrophil sequestration in Sphk1−/− mouse lungs. Lungs were fixed, sectioned, and stained with hematoxylin. The images shown at the top are representative sections, and the quantitative results for neutrophil infiltration are shown at the bottom. Note that Sphk1−/− lungs showed increased neutrophil sequestration 11 hours after LPS challenge. *Significant difference from PBS control (ie, no LPS) (P<0.05). #Significant difference in leukocyte counts compared to WT lungs after LPS challenge (P<0.05).
SPHK1 Activation Is Required for Endothelial Barrier Restoration
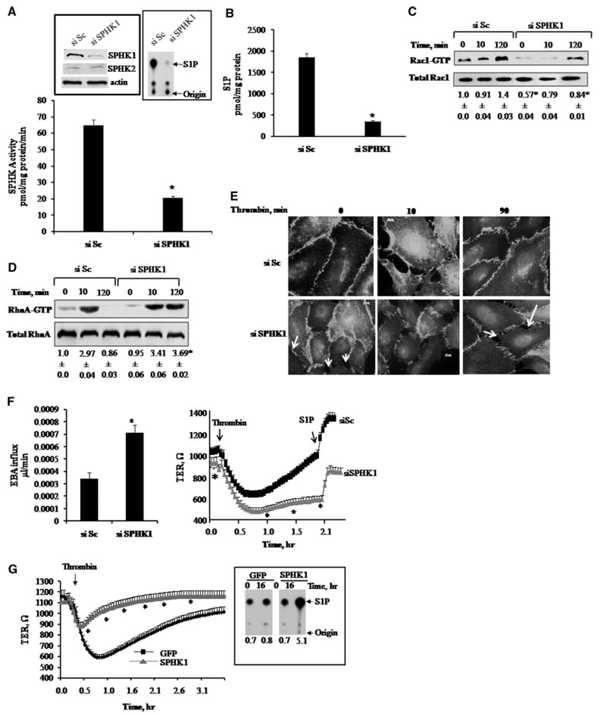
To gain insight into the mechanism of SPHK1-mediated endothelial barrier repair, we studied human pulmonary arterial endothelial (HPAE) cells in which small interfering (si)RNA was used to suppress SPHK1 expression. Cells were transfected with either scrambled siRNA (control) or SPHK1 siRNA, and after 24, 48, or 72 hours, SPHK1 Western blot analysis showed that effective knockdown of SPHK1 occurred at 72 hours posttransfection (Figure 3A, left inset). The reduction in SPHK1 expression had no effect on the expression of SPHK2 (Figure 3A, left inset). Inhibition of SPHK1 expression also markedly reduced SPHK activity compared to scrambled siRNA-transfected cells (Figure 3A, right inset). In addition, suppression of SPHK1 expression resulted in a 5-fold decrease in intracellular S1P concentration (Figure 3B).
Figure 3.
Suppression of SPHK1 in endothelial cells augments thrombin-induced increase in endothelial permeability through RhoGTPase signaling. A, SPHK activity after knockdown of SPHK1. HPAE cells were transfected with 2.4 µg of either scrambled (siSc) or SPHK1 siRNA (siSPHK1) for 72 hours. Cell lysates were used to determine SPHK activity as described in the expanded Materials and Methods section. Left Inset, Western blots showing SPHK1 knockdown. Cell lysates were immunoblotted 72 hours posttransfection using anti-SPHK1 or anti-SPHK2 antibodies to determine SPHK1 and SPHK2 expression. Immunoblot with anti-β-actin antibody was used as loading control. Note that SPHK1 knockdown had no effect on expression of SPHK2. Right Inset, Autoradiograph showing SPHK activity after SPHK1-siRNA or siSc transfection of HPAE cells. B, Effect of SPHK1 knockdown on intracellular S1P concentration. HPAE cells were pelleted and S1P was extracted as described in the expanded Materials and Methods section. The data in B are expressed as mean±SD, and experiments were repeated 3 times. *Significant difference from siSc-transfected group (P<0.05). C and D, Effect of SPHK1 knockdown on Rac-1 and RhoA activities. Cells were stimulated with thrombin for indicated times and incubated with GST-RBD or GST-PBD for 1 hour to determine between Rac1 (C) or RhoA (D) activities (bottom row in C and D) as described in the expanded Materials and Methods section. *Significant difference from siSc control at corresponding time points (P<0.05). E, Effect of SPHK1 knockdown on reannealing of endothelial junctions. HPAE cells were stimulated with thrombin for indicated times, fixed, and stained with anti–VE-cadherin antibody followed by secondary Alexa Fluor antibodies. Images were visualized using confocal microscope. The images shown are representative of 3 independent experiments. Arrows at 0 time and 90 minutes (siSPHK1) mark interendothelial gaps indicative of loss of basal barrier function. F, Effect of SPHK1 knockdown on endothelial barrier function. Left, Transendothelial albumin influx across cells transfected with siSc or siSPHK1 was determined by using Evans blue–conjugated albumin as described in the expanded Materials and Methods section. *Significant difference from siSc group (P<0.05). Right, TER in response to thrombin in endothelial cells transfected with Sc- or SPHK1-siRNA. Cells were stimulated with 50 nmol/L thrombin and changes in TER were measured. S1P (arrow) was added after the control cells recovered. *Significant difference from siSc group (P<0.05). G, Overexpression of SPHK1 counteracts thrombin-induced increase in endothelial permeability. Cells were infected with either GFP or SPHK1 adenovirus, and changes in TER and SPHK1 activity were determined 16 hours after transduction. TER was recorded following addition of 50 nmol/L thrombin (arrow). The inset shows autoradiograph of SPHK enzymatic activity in cells transducing GFP (lanes 1 and 2) or SPHK1 (lanes 3 and 4) 0 or 16 hours after adenovirus infection. Data are reported as mean±SD (n = 3). *Significant difference from GFP-transduced cells (P<0.05).
We have shown that thrombin induces a rapid increase in endothelial permeability resulting from disruption of adherens junctions followed by recovery when junctions reanneal to restore the barrier.1,7 Because RhoA and Rac1 GTPases signal these time-dependent alterations in endothelial barrier function in response to PAR-1 agonist,7,8 we determined the role of SPHK1 knockdown and decreased S1P generation on RhoA and Rac1 activities and adherens junction assembly following thrombin challenge. In scrambled siRNA-transfected cells, thrombin increased RhoA activity and disrupted adherens junctions within 10 minutes, but thrombin did not alter Rac1 activity. However, at 2 hours after thrombin challenge, RhoA activity declined to near basal value, whereas Rac1 activity increased in association with reannealing of adherens junctions (Figure 3C through 3E). Knockdown of SPHK1 reduced basal Rac1 activity and disrupted adherens junctions in the control endothelium, leading to formation of interendothelial gaps. Thrombin induced prolonged activation of RhoA in SPHK1 knockdown cells, Rac1 activity remained suppressed, and the junctions did not reanneal (Figure 3C through 3E). SPHK1 knockdown also significantly increased basal endothelial permeability determined by transendothelial transfer of Evans blue-conjugated albumin across the endothelial monolayer (Figure 3F, left). Using transendothelial electric resistance (TER) to assess reannealing of junctions, we observed that basal TER values were significantly lower in SPHK1-siRNA transfected cells than scrambled siRNA-transfected cells (Figure 3F, right). Thrombin decreased TER in scrambled siRNA-transfected cells, which fully recovered within 2 hours (Figure 3F, right). However, after SPHK1 knockdown, thrombin also decreased TER in HPAE cells, but, in contrast to control cells, TER did not return to basal value during the 2-hour period (Figure 3F, right). To address whether SPHK1 knockdown perturbed S1P1 receptor function, we added 1 µmol/L S1P to control cells or SPHK1 siRNA-transfected cells. S1P enhanced endothelial barrier function in control cells (Figure 3F, right), whereas it restored barrier function in the SPHK1-deficient cells (Figure 3F, right).
We next addressed whether increasing SPHK1 activity would prevent thrombin-induced endothelial barrier dysfunction. Figure 3G shows SPHK activity and TER in HPAE cells infected with green fluorescent protein (GFP) (control) or SPHK1 adenovirus. Increasing SPHK1 activity by 5-fold attenuated the thrombin-induced decrease in TER and promoted endothelial barrier recovery, demonstrating the requirement of SPHK1 activation in not only maintaining normal endothelial barrier function but also promoting recovery of barrier function after endothelial junctional disruption induced by thrombin.
SPHK1-Generated S1P Restores Endothelial Barrier Function by Activating S1P1 Receptor
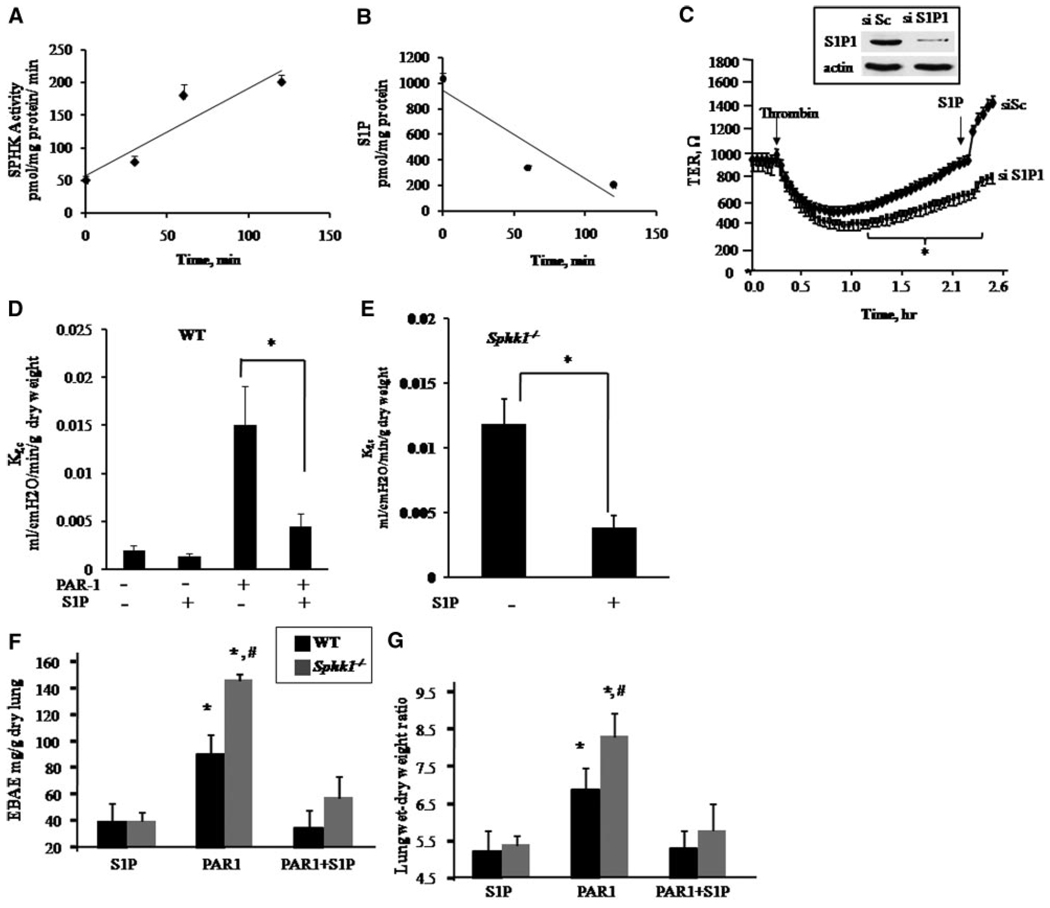
We performed RT-PCR using total RNA isolated from HPAE cells or mouse lungs to identify expression of S1P receptors. Both HPAE cells and mouse lungs express S1P1 receptor (Figure I in the online data supplement), which is known to signal S1P-mediated enhancement of barrier function.10–13 To address the role of S1P generated by SPHK1 in the mechanism of restoration of endothelial barrier function, we first measured SPHK activity and intracellular S1P concentration in HPAE cells following thrombin challenge. Thrombin increased SPHK activity in the same time frame as recovery of endothelial barrier function and concomitantly decreased intracellular S1P concentration (Figure 4A and 4B). We next suppressed S1P1 receptor expression using siRNA to determine whether the receptor could be activated in a paracrine manner during recovery from thrombin-induced endothelial permeability increase. As shown in Figure 4C, knockdown of S1P1 receptor prevented recovery from thrombin-induced permeability increase. As a positive control, we demonstrated that S1P enhanced barrier (indicated by a sharp rise in TER) in cells transfected with scrambled siRNA. We observed that S1P had no effect on TER in cells transfected with S1P1 siRNA.
Figure 4.
SPHK1-S1P signaling restores endothelial barrier integrity through activation of S1P1 receptor. A, Thrombin increases SPHK1 activity. Lysates from HPAE cells stimulated with 50 nmol/L thrombin for indicated times were used to determine SPHK activity as described in Materials and Methods. B, Thrombin decreases intracellular S1P concentration. HPAE cells stimulated with 50 nmol/L thrombin for indicated times were centrifuged and S1P was quantified using liquid chromatography–mass spectrometry, as described in the expanded Materials and Methods section. In A and B, data are expressed as mean±SD of at least 3 experiments. Regression line of best fit has been drawn through data points. C, Downregulation of S1P1 receptor prevents endothelial barrier repair. HPAE cells plated on gold electrodes were transfected with 2.4 µg of scrambled or S1P1 siRNA, and, after 48 hours, changes in TER were recorded on addition of 50 nmol/L thrombin (arrow). When control cells recovered, they received 1 µmol/L S1P to confirm that S1P requires S1P1 to restore barrier function. Inset in C shows immunoblot of S1P1 using anti-S1P1 antibody in cells after 48 hours of transfection. Immunoblotting with anti-β-actin antibody was used as a loading control. D and E, Activation of S1P1 by S1P reverses the PAR-1–induced increase in lung microvessel permeability in WT mice and restores basal microvessel permeability in Sphk1−/− mice. Intravascular pressure was raised by 10 cm H20 for 20 minutes in isogravimetric lungs, and Kf,c was determined as described in the expanded Materials and Methods section. WT (D) or Sphk1 −/− lungs (E) were perfused with 1 µmol/L S1P for 15 minutes and/or 10 µmol/L PAR-1 peptide for 20 minutes, as indicated. Kf,c values were obtained. Individual Kf,c values are the mean of 3 to 4 consecutive determination per lung preparation. *Significant effect of S1P on Kf,c values in WT or Sphk1−/− lungs (P<0.05). F and G, S1P prevents PAR-1–induced lung microvascular permeability increase in Sphk1−/− mice. Mice were challenged with either PBS or PAR-1 peptide for 15 minutes, followed by administration of 1 µmol/L S1P. Lung vascular permeability was determined by quantifying EBAE (F) or wet/dry weight ratio (G) as described in the expanded Materials and Methods section. The data are given as mean±SD of 4 experiments. *Significantly different from PBS control group. #Significantly different from WT group after PAR-1 challenge (P<0.05).
We next determined whether agonist activation of S1P1 could block the increase in vascular permeability induced by PAR-1 activation. WT lungs were perfused with S1P for 15 minutes, followed by 20 minutes of perfusion with PAR-1 agonist peptide to activate SIP1 and PAR-1 sequentially, and lung vascular permeability was measured by determining the Kf,c. In WT lungs, pretreatment with S1P prevented the PAR-1–induced increase in lung microvascular permeability without affecting basal permeability (Figure 4D). In SPHK1-null lungs, S1P reversed the increase in basal lung microvascular permeability (Figure 4E).
We also determined whether S1P administration could reverse the increased transvascular albumin permeability and lung edema formation following PAR-1 receptor activation in WT or Sphk1−/− mice. S1P or vehicle (control) was administered IV 15 minutes following IV administration of PAR-1 agonist peptide. With PAR-1 stimulation alone, Sphk1−/− lungs had greater transvascular albumin leakage and edema formation than WT lungs, consistent with the data shown in Figure 1F and 1G. As can be seen, S1P injection fully restored albumin leakage and edema formation induced by PAR-1 activation in both WT and Sphk1−/− lungs.
Discussion
Our findings have identified the indispensable role of endothelial SPHK1 activity in mediating the reversal of increased endothelial permeability. We showed that SPHK1 activation prevented the pulmonary edema elicited by 2 diverse inflammatory stimuli, thrombin and LPS, suggesting that SPHK1 serves a critical antiinflammatory function. The primary observation was that both thrombin and LPS activated SPHK1, leading to release of S1P from the endothelium, which by activating SIP1 promoted reannealing of adherens junctions, thereby restoring endothelial permeability. The results support the concept the SIP acts locally in a paracrine manner.14–17 Thus, based on our data, we propose that S1P generated by upregulation of SPHK1 activity is a negative feedback mechanism that serves to limit the increase in endothelial permeability arising from diverse inflammatory stimuli.
We showed that endothelial cells and lung both expressed the 2 isoforms of SPHK, SPHK1 and SPHK2.14,15 Both enzymes generate S1P following activation18,22–28; however, our data suggest that endothelial barrier function regulation is primarily the function of SPHK1. Lung tissue of Sphk1−/− mice had markedly reduced SPHK enzymatic activity compared to WT mouse lungs whereas the S1P concentration did not differ. Although the basis of normal lung S1P concentration in Sphk1−/− mice is not clear, studies have reported near normal S1P concentrations in other organs of SPHK1-null mice despite the low plasma S1P concentration.29 In lungs of Sphk1−/− mice, we found that SPHK2 did not compensate for the absence of SPHK1 because SPHK2 expression was similar to WT and SPHK activity of Sphk1−/− lungs was markedly reduced. Because sphingosine phosphatase or sphingosine phosphate lyase rapidly degrade S1P,14,15 a possible explanation for the normal lung tissue SIP concentration in Sphk1−/− mice may be compensatory reductions in enzymatic activities of sphingosine phosphatase or sphingosine phosphate lyase. The fact that SPHK2 did not compensate for the absence of SPHK1 and the endothelial permeability response was greatly enhanced in Sphk1−/− mice suggests that SPHK1 is the primary isoform responsible for endothelial barrier regulation.
SPHK1 activity was markedly increased in WT mouse lungs following PAR-1 activation or LPS challenge, resulting in enhanced S1P generation and accompanied by reduced lung edema formation. SPHK1 activity was required for the restoration of lung vascular permeability after the vascular leak induced by either PAR-1 activation or LPS challenge because the permeability did not return to control values in the absence of SPHK1 expression. The lungs of these animals developed persistent edema. To address mechanisms of SPHK1-mediated endothelial barrier protection, we determined functional consequences of SPHK1 knockdown in endothelial monolayers. Knockdown of SPHK1 decreased both SPHK activity and intracellular SIP concentration by 80%. Also, SPHK1 deficiency markedly increased basal endothelial permeability to albumin and challenge of these cells with thrombin resulted in prolonged increase in permeability in contrast to full recovery seen within 2 hours in control endothelial cells. Thus, SPHK1 knockdown abrogated the recovery process and enhanced the permeability increase. Moreover, overexpression of SPHK1 counteracted PAR-1– induced barrier dysfunction. The knockdown of the SIP G protein– coupled receptor S1P1 in endothelial cells abolished the recovery process in response to thrombin challenge. We interpret these findings as suggesting that SPHK1-generated S1P from endothelial cells activates S1P1 receptor in a paracrine manner to restore barrier function. The mechanism of S1P release from endothelial cells remains an enigma. It is possible that ATP-binding cassette (ABC) transporters are involved because they are known to export S1P from cells.14,15
We also showed that the basal lung vascular permeability of lungs from Sphk1−/− mice was considerably higher than WT mice. We did not observe gross alterations in lung morphology or differences in basal S1P concentrations that could account for the higher basal permeability in knockout mouse lungs. However, there was a strong correlation between constitutive SPHK1 activity and basal barrier function. Thus, a possible explanation may be that lower enzymatic activity and reduced release of S1P and S1P1 activation in the endothelium of Sphk1−/− lungs impaired endothelial barrier function. This scenario is likely because administration of S1P restored basal barrier function in Sphk1−/− lungs to near normal values.
RhoA and Rac1 have opposing effects in regulating endothelial barrier function. RhoA signals increased endothelial permeability and Rac1 reduces the response.1 We addressed the possibility that SPHK1 generation of S1P would either prevent RhoA activation or induce activation of Rac1. Inhibition of SPHK1 expression in endothelial cells augmented RhoA activity but also suppressed Rac1 activity in response to thrombin. Both of these changes in RhoA and Rac1 activities help to explain the persistent increase in endothelial permeability seen after SPHK1 knockdown. Moreover, over-expression of SPHK1 fully counteracted the thrombin-induced increase in endothelial permeability. The basis of the different effects of SPHK1-generated S1P on RhoA and Rac1 activities is not clear, but it likely involves S1P regulation of activities of GDI-1 (GDP dissociation inhibitor-1), GEFs (guanine nucleotide exchange factors), and GAPs (GTPase-activating proteins) in an orchestrated manner such that RhoA and Rac1 activities change in the opposing manner.30 Thus, a simple model to explain the effects of SPHK1-generated S1P may be that S1P acts as a rheostat that inactivates RhoA and activates Rac1, thereby restoring endothelial barrier.
Vascular injury is associated with activation of the coagulation cascade and release of thrombin, which increases endothelial permeability by activating endothelial cell surface PAR-1 signaling.1,3–6 Moreover, sepsis is known to upregulate the expression of PAR-1 receptor, suggesting a commonality between sepsis and PAR-1 activation.31 However, recent studies suggest that PAR-1 activation under specific conditions may signal endothelial barrier protection.6,9,32 Activated protein C, which reduces mortality of septic patients,33 was shown to convert PAR-1 signaling from being barrier-disruptive to barrier-protective.34,35 Pepducin, a cell-penetrating PAR-1–activating peptide, was protective during the later phase of sepsis, but its effect was mediated via upregulation and cross-activation of PAR-2.9 In the present study, we add to mechanisms by which PAR-1 activation may be protective. We describe a novel PAR-1–activated mechanism of endothelial barrier involving SPHK1 activation. This mechanism apparently does not require the upregulation of PAR-2 gene because S1P was sufficient to repair barrier after PAR-1 activation and in a time frame that precludes gene expression.
The signaling pathway between PAR-1 and the activation of SPHK1 is not known. Evidence shows that translocation of SPHK1 to the membrane is required to catalyze S1P formation from sphingosine.14,15 Extracellular signal-regulated kinase activation downstream of protein kinase C has been shown to phosphorylate SPHK1, leading to its activation.15,36 Binding with phosphatidyl serine and calmodulin also facilitates SPHK1 translocation.37–39 Because PAR-1 activates protein kinase C,1 SPHK1 activation may involve phosphorylation by protein kinase C pathway, which may translocate it to endothelial plasma membrane, resulting in S1P production and activation of S1P1 receptor and junctional reannealing. The present results have significant in vivo implications for diseases resulting from a pathological increase in vascular leakiness, such as acute lung injury, and may help in the development of novel therapeutics for specific activation of SPHK1 in endothelial cells.
Supplementary Material
Acknowledgments
We thank Drs Richard Proia (National Institute of Diabetes and Digestive and Kidney Diseases) for providing SPHK1-null mice and Maria Trojanowska (Medical University of South Carolina) for adenoviral-SPHK1 construct and Andrew Lee (Children’s Hospital Oakland Research Institute) for his technical support.
Sources of Funding
Supported by NIH grants RO1 CA77528 (to J.S.), HL45638 (to A.B.M.), and HL71794 and HL 84153 (to D.M.).
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 2.Mehta D, Bhattacharya J, Matthay MA, Malik AB. Integrated control of lung fluid balance. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1081–L1090. doi: 10.1152/ajplung.00268.2004. [DOI] [PubMed] [Google Scholar]
- 3.Esmon CT. Role of coagulation inhibitors in inflammation. Thromb Haemost. 2001;86:51–56. [PubMed] [Google Scholar]
- 4.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 5.Vogel SM, Gao X, Mehta D, Ye RD, John TA, Andrade-Gordon P, Tiruppathi C, Malik AB. Abrogation of thrombin-induced increase in pulmonary microvascular permeability in PAR-1 knockout mice. Physiol Genomics. 2000;4:137–145. doi: 10.1152/physiolgenomics.2000.4.2.137. [DOI] [PubMed] [Google Scholar]
- 6.Camerer E, Cornelissen I, Kataoka H, Duong DN, Zheng YW, Coughlin SR. Roles of protease-activated receptors in a mouse model of endotoxemia. Blood. 2006;107:3912–3921. doi: 10.1182/blood-2005-08-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kouklis P, Konstantoulaki M, Vogel S, Broman M, Malik AB. Cdc42 regulates the restoration of endothelial barrier function. Circ Res. 2004;94:159–166. doi: 10.1161/01.RES.0000110418.38500.31. [DOI] [PubMed] [Google Scholar]
- 8.Holinstat M, Knezevic N, Broman M, Samarel AM, Malik AB, Mehta D. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J Biol Chem. 2006;281:2296–2305. doi: 10.1074/jbc.M511248200. [DOI] [PubMed] [Google Scholar]
- 9.Kaneider NC, Leger AJ, Agarwal A, Nguyen N, Perides G, Derian C, Covic L, Kuliopulos A. ‘Role reversal’ for the receptor PAR1 in sepsis-induced vascular damage. Nat Immunol. 2007;8:1303–1312. doi: 10.1038/ni1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal. 2005;17:131–139. doi: 10.1016/j.cellsig.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Singleton PA, Dudek SM, Chiang ET, Garcia JG. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J. 2005;19:1646–1656. doi: 10.1096/fj.05-3928com. [DOI] [PubMed] [Google Scholar]
- 12.Mehta D, Konstantoulaki M, Ahmmed GU, Malik AB. Sphingosine-1-phosphate mobilizes intracellular Ca2+ to induce Rac activation and adherens junction assembly in endothelial cells. J Biol Chem. 2005;280:17320–17328. doi: 10.1074/jbc.M411674200. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki H, Hla T, Lee MJ. Sphingosine-1-phosphate signaling in endothelial activation. J Atheroscler Thromb. 2003;10:125–131. doi: 10.5551/jat.10.125. [DOI] [PubMed] [Google Scholar]
- 14.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 15.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, Wu M, Parikh NS, Khan F, Proia RL, Hla T. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397:461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Kalari SK, Usatyuk PV, Gorshkova I, He D, Watkins T, Brindley DN, Sun C, Bittman R, Garcia JG, Berdyshev EV, Natarajan V. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J Biol Chem. 2007;282:14165–14177. doi: 10.1074/jbc.M701279200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 20.Barnard JW, Biro MG, Lo SK, Ohno S, Carozza MA, Moyle M, Soule HR, Malik AB. Neutrophil inhibitory factor prevents neutrophildependent lung injury. J Immunol. 1995;155:4876–4881. [PubMed] [Google Scholar]
- 21.Schaphorst KL, Chiang E, Jacobs KN, Zaiman A, Natarajan V, Wigley F, Garcia JG. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am J Physiol Lung Cell Mol Physiol. 2003;285:L258–L267. doi: 10.1152/ajplung.00311.2002. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim FB, Pang SJ, Melendez AJ. Anaphylatoxin signaling in human neutrophils. A key role for sphingosine kinase. J Biol Chem. 2004;279:44802–44811. doi: 10.1074/jbc.M403977200. [DOI] [PubMed] [Google Scholar]
- 23.Melendez AJ, Ibrahim FB. Antisense knockdown of sphingosine kinase 1 in human macrophages inhibits C5a receptor-dependent signal transduction, Ca2+ signals, enzyme release, cytokine production, and chemotaxis. J Immunol. 2004;173:1596–1603. doi: 10.4049/jimmunol.173.3.1596. [DOI] [PubMed] [Google Scholar]
- 24.Okada T, Ding G, Sonoda H, Kajimoto T, Haga Y, Khosrowbeygi A, Gao S, Miwa N, Jahangeer S, Nakamura S. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J Biol Chem. 2005;280:36318–36325. doi: 10.1074/jbc.M504507200. [DOI] [PubMed] [Google Scholar]
- 25.Pi X, Tan SY, Hayes M, Xiao L, Shayman JA, Ling S, Holoshitz J. Sphingosine kinase 1-mediated inhibition of Fas death signaling in rheumatoid arthritis B lymphoblastoid cells. Arthritis Rheum. 2006;54:754–764. doi: 10.1002/art.21635. [DOI] [PubMed] [Google Scholar]
- 26.Vlasenko LP, Melendez AJ. A critical role for sphingosine kinase in anaphylatoxin-induced neutropenia, peritonitis, and cytokine production in vivo. J Immunol. 2005;174:6456–6461. doi: 10.4049/jimmunol.174.10.6456. [DOI] [PubMed] [Google Scholar]
- 27.Xia P, Wang L, Gamble JR, Vadas MA. Activation of sphingosine kinase by tumor necrosis factor-alpha inhibits apoptosis in human endothelial cells. J Biol Chem. 1999;274:34499–34505. doi: 10.1074/jbc.274.48.34499. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Xing XP, Holmes A, Wadham C, Gamble JR, Vadas MA, Xia P. Activation of the sphingosine kinase-signaling pathway by high glucose mediates the proinflammatory phenotype of endothelial cells. Circ Res. 2005;97:891–899. doi: 10.1161/01.RES.0000187469.82595.15. [DOI] [PubMed] [Google Scholar]
- 29.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- 30.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 31.Jesmin S, Gando S, Zaedi S, Sakuraya F. Differential expression, time course and distribution of four PARs in rats with endotoxin-induced acute lung injury. Inflammation. 2007;30:14–27. doi: 10.1007/s10753-006-9017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 33.Kerschen EJ, Fernandez JA, Cooley BC, Yang XV, Sood R, Mosnier LO, Castellino FJ, Mackman N, Griffin JH, Weiler H. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204:2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bae JS, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–3916. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 cross activation. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 36.Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, Wattenberg BW. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutherland CM, Moretti PA, Hewitt NM, Bagley CJ, Vadas MA, Pitson SM. The calmodulin-binding site of sphingosine kinase and its role in agonist-dependent translocation of sphingosine kinase 1 to the plasma membrane. J Biol Chem. 2006;281:11693–11701. doi: 10.1074/jbc.M601042200. [DOI] [PubMed] [Google Scholar]
- 38.Serrano-Sanchez M, Tanfin Z, Leiber D. Signaling pathways involved in sphingosine kinase activation and sphingosine-1-phosphate release in rat myometrium in late pregnancy: role in the induction of COX2. Endocrinology. 2008;149:4669–4679. doi: 10.1210/en.2007-1756. [DOI] [PubMed] [Google Scholar]
- 39.Stahelin RV, Hwang JH, Kim JH, Park ZY, Johnson KR, Obeid LM, Cho W. The mechanism of membrane targeting of human sphingosine kinase 1. J Biol Chem. 2005;280:43030–43038. doi: 10.1074/jbc.M507574200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.