Abstract
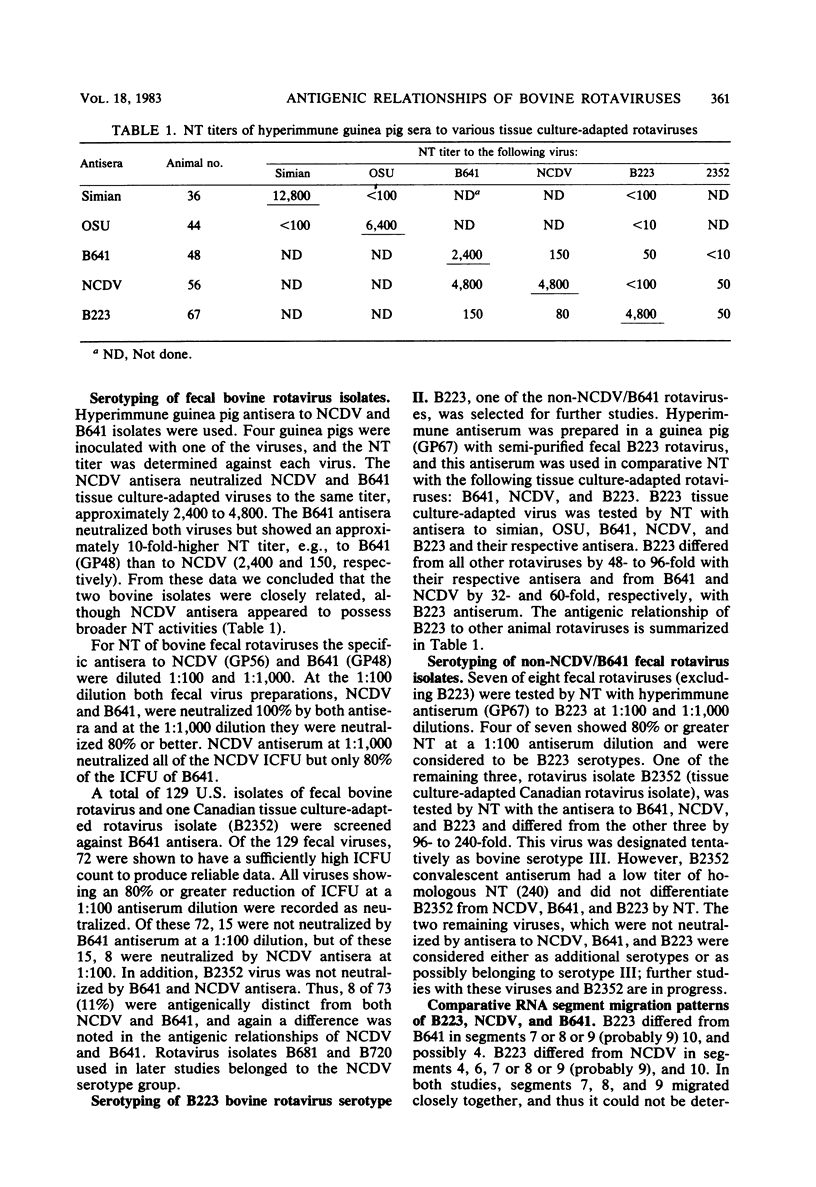
A method was further developed to screen non-tissue-culture-adapted bovine rotaviruses for serotype, using a neutralization test with infectious fecal rotavirus. One of those rotaviruses (B223) which was not blocked by antiserum to the neonatal calf diarrhea virus (NCDV) serotype was then adapted to cell culture in the presence of the antiserum for two or more passages and hyperimmune antiserum to this isolate had a 60-fold-higher homologous neutralization titer than with the NCDV serotype rotavirus. Seventy-three isolates were serotyped and eight (11%) were not of the NCDV serotype (bovine rotavirus serotype I). Of these eight, five belonged to the new bovine rotavirus serotype II and three were not typed, indicating the existence of one or more further serotypes. Cross-protection studies in gnotobiotic calves showed that cross-protection only occurred between rotaviruses of the same serotype, and even a minor serotype difference was sufficient for the calves to show a lack of cross-protection. The serotypes (I and II and the three untyped isolates) also showed differences in the rate of migration in polyacrylamide gel electrophoresis of some of their RNA segments (no. 4, 6, 7, 8, 9, 10), indicating that they were of different electropherotypes.
Full text
PDF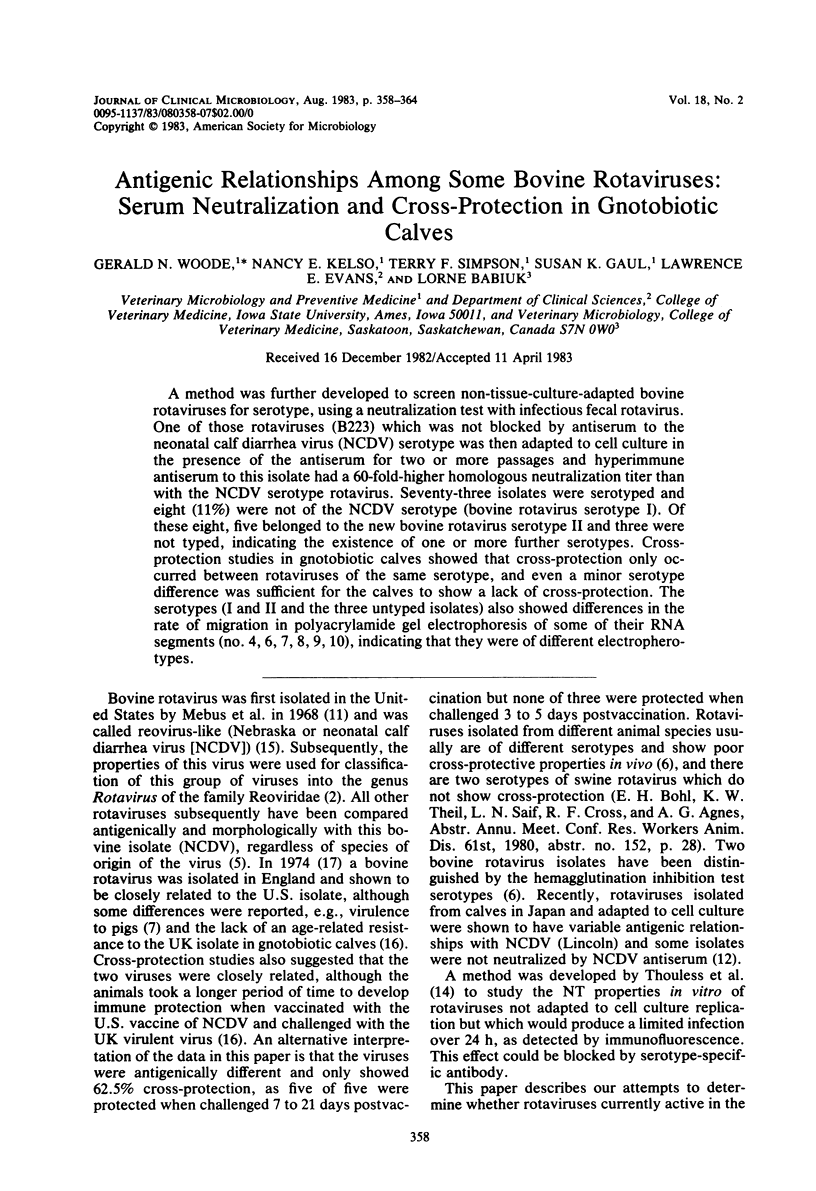




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridger J. C., Brown J. F. Development of immunity to porcine rotavirus in piglets protected from disease by bovine colostrum. Infect Immun. 1981 Mar;31(3):906–910. doi: 10.1128/iai.31.3.906-910.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire J. B., Woode G. N. Classification of rotaviruses: report from the World Health Organization/Food and Agriculture Organization Comparative Virology Program. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):519–521. [PubMed] [Google Scholar]
- Dyall-Smith M. L., Holmes I. H. Gene-coding assignments of rotavirus double-stranded RNA segments 10 and 11. J Virol. 1981 Jun;38(3):1099–1103. doi: 10.1128/jvi.38.3.1099-1103.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberts T. J., Sample R. H., Glick M. R., Ellis G. H. A simplified, colorimetric micromethod for xylose in serum or urine, with phloroglucinol. Clin Chem. 1979 Aug;25(8):1440–1443. [PubMed] [Google Scholar]
- Flewett T. H., Woode G. N. The rotaviruses. Arch Virol. 1978;57(1):1–23. doi: 10.1007/BF01315633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaul S. K., Simpson T. F., Woode G. N., Fulton R. W. Antigenic relationships among some animal rotaviruses: virus neutralization in vitro and cross-protection in piglets. J Clin Microbiol. 1982 Sep;16(3):495–503. doi: 10.1128/jcm.16.3.495-503.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. A., Bridger J. C., Chandler R. L., Woode G. N. Gnotobiotic piglets experimentally infected with neonatal calf diarrhoea reovirus-like agent (Rotavirus). Vet Pathol. 1976;13(3):197–210. doi: 10.1177/030098587601300304. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Nishioka N., Hashiguchi Y., Kuniyasu C. Neutralizing patterns of anti-bovine rotavirus (Lincoln) serum against cytopathic bovine rotaviruses isolated from calves in Japan. Microbiol Immunol. 1981;25(10):1097–1100. doi: 10.1111/j.1348-0421.1981.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Spence L., Fauvel M., Petro R., Babiuk L. A. Comparison of rotavirus strains by hemagglutination inhibition. Can J Microbiol. 1978 Apr;24(4):353–362. doi: 10.1139/m78-059. [DOI] [PubMed] [Google Scholar]
- Thouless M. E., Bryden A. S., Flewett T. H., Woode G. N., Bridger J. C., Snodgrass D. R., Herring J. A. Serological relationships between rotaviruses from different species as studied by complement fixation and neutralization. Arch Virol. 1977;53(4):287–294. doi: 10.1007/BF01315627. [DOI] [PubMed] [Google Scholar]
- Welch A. B. Purification, morphology and partial characterization of a reovirus-like agent associated with neonatal calf diarrhea. Can J Comp Med. 1971 Jul;35(3):195–202. [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Bew M. E., Dennis M. J. Studies on cross protection induced in calves by rotaviruses of calves, children and foals. Vet Rec. 1978 Jul 8;103(2):32–34. doi: 10.1136/vr.103.2.32. [DOI] [PubMed] [Google Scholar]
- Woode G. N., Bridger J. C., Hall G., Dennis M. J. The isolation of a reovirus-like agent associated with diarrhoea in colostrum-deprived calves in Great Britain. Res Vet Sci. 1974 Jan;16(1):102–105. [PubMed] [Google Scholar]
- Woode G. N., Reed D. E., Runnels P. L., Herrig M. A., Hill H. T. Studies with an unclassified virus isolated from diarrheic calves. Vet Microbiol. 1982 Jul;7(3):221–240. doi: 10.1016/0378-1135(82)90036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


