Abstract
Holocarboxylase synthetase (HCS, eukaryotic enzyme) and BirA (prokaryotic) are biotin protein ligases that catalyze the ATP-dependent attachment of biotin to apocarboxylases via the reactive intermediate, bio-5′-AMP. In this study, we examined the in vitro mechanism of biotin attachment to histone H2A in the presence of HCS and BirA. The experiment derives from our observations that HCS is found in the nucleus of cells in addition to the cytoplasm, and it has the ability to attach biotin to histones in vitro (Narang et al., Hum Mol Genet 2004; 13:15–23). Using recombinant HCS or BirA, the rate of biotin attachment was considerably slower with histone H2A than with the biotin binding domain of an apocarboxylase. However, on incubation of recombinant H2A with chemically synthesized bio-5′-AMP, H2A was observed to be rapidly labeled with biotin in the absence of enzyme. Nonenzymatic biotinylation of a truncated apocarboxylase (BCCP87) has been previously reported (Streaker and Beckett, Protein Sci 2006; 15:1928–1935), though at a much slower rate than we observe for H2A. The specific attachment sites of nonenzymatically biotinylated recombinant H2A at different time points were identified using mass spectrometry, and were found to consist of a similar pattern of biotin attachment as seen in the presence of HCS, with preference for lysines in the highly basic N-terminal region of the histone. None of the lysine sites within H2A resembles the biotin attachment consensus sequence seen in carboxylases, suggesting a novel mechanism for histone biotinylation.
Keywords: biotin, histones, nonenzymatic, holocarboxylase synthetase, mass spectrometry
Introduction
Biotin is a cofactor in carboxyl transfer reactions from microbes to animals and plants. In prokaryotes, it participates in carboxylase, transcarboxylase, and decarboxylase reactions.1 In Escherichia coli, the biotin carboxyl carrier protein (BCCP), a subunit of acetyl-CoA carboxylase, uses biotin as a cofactor.2–4 In mammals, there are five biotin-dependent carboxylases: two distinct acetyl-CoA carboxylases, β-methylcrotonyl-CoA carboxylase, propionyl-CoA carboxylase, and pyruvate carboxylase.1 In all carboxylases, biotin is covalently attached to the ɛ-amino group of a lysine residue within a highly conserved hydrophobic sequence, Ala/Val-Met-Lys-Met/Leu. Biotin attachment to carboxylases is catalyzed by a biotin protein ligase (BPL) called holocarboxylase synthetase (HCS) in mammals5, 6 and BirA in prokaryotes.7 The reaction is similar in all organisms. Biotinylation occurs in a two-step ATP-dependent process according to the following reactions:
 |
Biotin is synthesized de novo in microorganisms but, in mammals, it is a vitamin and must be obtained through the diet or recovered endogenously from pre-existing carboxylases.4 In mammals, biotin reutilization occurs through the action of biotinidase, an enzyme which hydrolyzes biotin from biotinyl-lysine (biocytin) or short peptides containing biotin which are the products of normal proteolytic digestion of holocarboxylases.8
In recent years, biotin has been described in the nucleus of cells,9 including in tumor material10–12. More recently, it has been proposed as a histone modification.13–15 Histones are highly basic proteins responsible for packaging DNA into chromatin.16–18 Roughly 25% of the amino acids in histones are arginines and lysines. The core histones (two each of histones H2A, H2B, H3, H4) form the octomeric nucleosome around which 200 bp of DNA is coiled.18 The amino-terminal domain of histones, which extends unstructured from the core domain, contains the majority of the arginines and lysines. They are subject to a variety of modifications including phosphorylation, acetylation, methylation, and ubiquitination. These modifications are associated with transcription regulation, cell cycle dynamics, and other processes involving chromatin organization. The detection of biotin as a histone modification comes from the use of antibodies made to biotinylated histone peptides.13–15 These synthetic peptides, corresponding to the N-and C-terminal tails of histones, were biotinylated through the action of biotinidase using biocytin as an exchange substrate. Antibodies made to these structures have detected biotin on histones H2A, H3, and H4. Using this method, histone H2A was found to be biotinylated at lysines 9 and 13.14
Recently, it has been shown that the biotinyl moiety of bio-5′-AMP can be transferred to a lysine residue in protein in the absence of a catalytic enzyme.19 These experiments derive from the initial observation that a mutant form of BirA could promiscuously biotinylate lysine residues in various proteins. This was ascribed to the bio-5′-AMP intermediate which is more readily dissociated by the mutant enzyme. Subsequently, it was shown that BCCP87, a truncated form of the BCCP, could be biotinylated directly using bio-5′-AMP as substrate in the absence of BirA.19 This reaction was considerably slower than enzymatic biotinylation but remained largely specific to the physiological target lysine on BCCP87, despite the presence of four other solvent accessible lysine residues in the protein. This outcome indicated that bio-5′-AMP alone, without the direction and specification of a catalytic enzyme, was capable of targeting a specific lysine. The underlying properties which drive this specificity are still unknown.
We previously showed that HCS could enzymatically add biotin to calf thymus histones using biotin and ATP as substrates.20 Furthermore, HCS was found to be a constituent of nuclei, in addition to the cytoplasm of cells, suggesting that HCS could be responsible for biotinylating histones in vivo.20 However, as histones do not contain the biotin binding motif as seen in carboxylases, the mechanism and specificity of biotin attachment to histones might be expected to be significantly different. Now, with the finding that biotin can be added specifically to the physiological lysine in carboxylases nonenzymatically, via exposure to bio-5′-AMP, we were led to explore the possibility that in vitro histone biotinylation could be the product of a nonenzymatic mechanism. We therefore sought to compare enzymatic, using BirA and HCS, and nonenzymatic biotinylation of histone lysines. We used recombinant histone H2A as the candidate for these experiments. It contains 13 lysine residues, none of which are in a domain reminiscent of the biotin binding site of carboxylases. We show that the role of BirA or HCS in histone biotinylation is restricted to the first reaction of the biotin transfer reaction, the generation of bio-5′-AMP, but the second component of the reaction, biotin transfer to a target lysine, can occur nonenzymatically. Bio-5′-AMP shows high reactivity in transferring biotin to histone H2A in the absence of enzyme. Using mass spectrometry, we show that biotin attaches to multiple sites on the histone with a bias toward the N-terminal tail of H2A in a pattern similar to that identified using biotinidase acting on synthetic peptides. We propose that the nonenzymatic component of the reaction of HCS or BirA to biotinylate histones serves as the primary mechanism for transfer of biotin to histones in vitro, which may be the origin of the difference between histone and carboxylase biotinylation.
Results
Recombinant H2A is a weak substrate for in vitro biotinylation by holocarboxylase synthetase and BirA
The in vitro ability of HCS to attach biotin to recombinant H2A was compared with its activity toward p–67, the 67–amino acid biotin carrier domain of propionyl-CoA carboxylase [see Fig. 1(A) for sequences]. Recombinant H2A, containing an N-terminal His tag, was purified using affinity chromatography in denaturing conditions. This method has been previously shown to properly refold all recombinant histone proteins by their ability to correctly associate with other recombinant histones into higher order structures, including generating a nucleosome.21 The extent of biotin attachment to recombinant H2A or p-67 was measured at 20°C using [14C]-biotin and ATP as substrates [Fig. 1(B)]. In a timed assay, biotin transfer to p-67, assessed by detection of radiolabeled protein following electrophoresis, appeared as a strong band of 10 kDa by 1 h, with a weaker band at about 20 kDa. Although the identity of this higher band is unknown, it is likely to be a dimeric form of p-67 which had not been properly denatured during SDS-PAGE sample preparation. In contrast, radiolabeled H2A was detected as a weak band by 1 h and was still increasing in intensity after 21 h. Omission of either HCS or ATP resulted in no biotin transfer to either p-67 or H2A after overnight incubation at 20°C, confirming a requirement for the bio-5′-AMP intermediate in both reactions (not shown). The quantity of radiolabeled biotin attached to each protein was estimated following an overnight incubation assay by scintillation counting of precipitated protein. A very low amount of biotin was found to be attached to H2A (0.02 biotin:1 H2A on a molar basis) when compared with the near saturation of biotin label on p-67 (0.95 biotin:1 p-67). Notably, while the maximum number of in vitro biotin attachment sites on H2A is uncertain, p-67 is expected to bind the single target lysine, as confirmed previously.22 A point mutation of the physiological target lysine in p-67 to an arginine resulted in no detectable binding with this assay, despite the presence of other lysines, thereby verifying this expectation (not shown). Substitution of HCS with BirA, the bacterial BPL, demonstrated an equal ability to biotinylate both p-67 and H2A with the same preference for biotin attachment to p-67 over H2A (not shown). From this analysis, we conclude that in vitro HCS or BirA can catalyze the attachment of biotin to recombinant H2A in the presence of biotin and ATP as substrates. However, this analysis demonstrates that H2A is a poor substrate for biotin attachment compared to the apocarboxylase biotin-attachment domain.
Figure 1.

(A) Sequence of recombinant H2A and the biotin attachment peptide, p-67. The numbered residues are cited throughout the text. The N-terminal His-tag sequence is separated from the native protein sequence in each case with a hyphen. (B) Attachment of [14C]-biotin to recombinant H2A (16.7 kDa) or p-67 (9.9 kDa) over time. Products were subjected to PAGE under denaturing conditions. Top panel: radioblots showing incorporation of radioactivity in H2A or p-67 at the indicated times. Lower panel: Coomassie blue stain of the acrylamide gel showing equal loading. Symbols at base of lower panel: h, lane containing H2A; p, lane containing p-67.
Enzymatic versus nonenzymatic biotin attachment to recombinant H2A
We next compared enzymatic versus nonenzymatic biotinylation of H2A to determine if the second reaction, transfer of biotin from bio-5′-AMP to histone, is enzyme dependent. These reactions were conducted using HCS or BirA as the enzyme and 100 μM BCCP87 or H2A as the biotin acceptor substrate. Chemically synthesized bio-5′-AMP (100 μM or 200 μM) was used as the source of biotin. The reaction products were analyzed by MALDI-MS (see Fig. 2). As a preliminary experiment, we repeated the previously reported addition of biotin from bio-5′-AMP to BCCP87 in the presence or absence of BirA.19 A reaction time of 7 h was chosen to allow for maximal biotinylation with or without enzyme. In the presence of BirA, the reaction went to completion as evidenced by a mass shift of 226 u from the unbiotinylated peak (m/z 9335, panel i) to a biotinylated peak (m/z 9561, panel ii) [Fig. 2(A)(A)]. In contrast, incubation with bio-5′-AMP in the absence of BirA did not result in appreciable biotin attachment to BCCP87 during the 7 h of incubation [Fig. 2(B), panels i-ii]. By prolonging the incubation overnight and increasing the concentration of bio-5′-AMP to 200 μM, two-fold over BCCP87, a single biotin attachment could be observed in the absence of enzyme [Fig. 2(B), panel iii]. As previously reported and confirmed here by comparing peak heights, BCCP87 achieved ∼40% biotinylated protein using these conditions. These results are similar to those obtained in a previous study, which went on to identify the physiological target, Lys122, and the N-terminus of BCCP87 as the only sites of biotin attachment despite the presence of five accessible lysine residues in BCCP87.19
Figure 2.

Enzymatic and nonenzymatic biotinylation of BCCP87 monitored by MALDI-MS analysis. (A) Enzymatic biotinylation of BCCP87 by BirA showing mass spectra of products at (i) 0 and (ii) 420 min. (B) Nonenzymatic biotinylation of BCCP87 after (i) 0 and (ii) 420 min and (iii) overnight incubation; same substrate concentration except 200 μM bio-5′-AMP used for the overnight incubation.
The outcome was markedly different using recombinant H2A as the acceptor substrate. Incubation of recombinant H2A with 100 μM bio-5′-AMP without enzyme for 60 min resulted in a single biotin attachment, indicated by a peak with a mass shift of 226 u over the unbiotinylated protein [Fig. 3(A), panels i-ii]. Increasing the incubation time up to an overnight incubation led to an increased number of unique biotin attachments [up to five additions, Fig. 3(A), panel iii-iv, Fig. 3(B)]. This time course and pattern of biotin attachment appeared unchanged with the inclusion of either BirA or HCS (not shown).
Figure 3.

(A) Nonenzymatic biotinylation of recombinant H2A using bio-5′-AMP as substrate. Panel (i), 0 time, 100 μM bio-5′-AMP; (ii) 60 min, 100 μM bio-5′-AMP; (iii) 180 min, 100 μM bio-5′-AMP, (iv) 420 min, 100 μM bio-5′-AMP. (B) Overnight incubation with 200 μM bio-5′-AMP. (C) Plot of ratio of unbiotinylated H2A:total H2A remaining versus time in enzymatic and nonenzymatic reactions. Ratios were estimated using peak heights of relevant species.
To assess whether there was any impact of BirA in the H2A biotinylation reaction, a plot of the fractional amount of unbiotinylated H2A remaining (ratio of unbiotinylated H2A over the total amount of H2A, as measured by the peak height of detected protein by MALDI-MS analysis at both [M+H]+ and [M+2H]2+ states) versus time in the presence or absence of BirA is shown [Fig. 3(C)]. The similarity of the curves indicates that the enzyme had no influence on the rate of biotinylation of recombinant H2A and that H2A may not be recognized at all by biotin protein ligases. The accuracy of individual reaction rates will be influenced by potential nonequivalent ionization efficiencies of the biotin adducts and the presence of matrix adducts, though this technique is valid for comparative purposes.
Localization of nonenzymatic biotin attachment sites
The nonenzymatic biotinylation of BCCP87 demonstrated remarkable specificity of biotin attachment to the physiological target lysine.19 To determine if a pattern of sites is preferentially biotinylated on H2A, the sites of nonenzymatic biotin attachment at different time points, with bio-5′-AMP as substrate, were determined by MALDI-MS. Time points selected were 1 h, when H2A is predominantly unbiotinylated and singly biotinylated; 7 h, when doubly biotinylated protein is observed; and overnight, when multiple biotinylated states are observed. Two endopeptidases, trypsin and LysC, were used to generate protein fragments for MALDI-MS analysis to determine consistency of predicted modification sites and to increase coverage of the protein sequence. Two enzymes were used as it was observed that some fragments were either too large or too small to be properly analyzed using one or the other digestion. Using this approach, 65% of recombinant H2A was assigned to MALDI-ToF peaks. Missing sequences included the N-terminal His-tag [“MSYYHHHHHHLESTSLYK,” Fig. 1(A)], which is likely due to the poor ionization efficiency of a histidine rich peptide, and two interior peptides [Fragment 1: residues 18–20, fragment 2: residues 43–71, Fig. 1(A)], neither of which contains lysines and would therefore not contain a potential biotin attachment site.
The monoisotopic mass spectra from the trypsin and LysC digestions for the three treatment periods, 1 and 7 h and overnight, are presented in Figure 4 and the corresponding predicted sequences listed in Table I. Peaks labeled A–F with LysC digestion and A′–E′ with trypsin digestion match sequences observed for unmodified H2A, while peaks labeled G–P or F′–P′ are for sequences showing the addition of one, two or three biotin molecules in increments of 226 u. After the first hour of incubation with bio-5′-AMP (100 μM, equimolar with H2A), the MALDI-ToF spectra revealed the emergence of a number of biotin-containing species, most showing the addition of a single biotin and one containing two biotin molecules [Fig. 4(A), Table I]. The modified lysine(s) was deduced to be the internal lysine in each case, as biotin attachment would prevent trypsin digestion at these sites (Table I and23). Based on this interpretation, the biotin attachment sites were located predominantly in the N-terminal tail, specifically at lysines 5, 9, 13, or 15. Lysine 36, a residue within the globular domain of H2A on the lateral surface of the protein,24 was also found to be biotinylated in the first hour of incubation. Interestingly, a lysine from the linker region connecting the His-tag to the native protein (labeled as position −5 in Table I) also showed a biotin attachment. After 7 h incubation, biotin attachments were observed at additional sites, including lysines 119 and 125, which reside in the C-terminal tail of H2A, also an unstructured region of the protein [Fig. 4(B), Table I]. After overnight incubation, the biotin modifications were found to extend to lysines within the core of the protein, such as lysines 74 and 75 [Fig. 4(C), Table I]. These results suggest a higher preference of nonenzymatic biotinylation toward the N-terminal tail of H2A, though no single lysine within this region was preferentially targeted.
Figure 4.

Identification of nonenzymatic biotin attachment sites on H2A. H2A was incubated with an equimolar amount of bio-5′-AMP for the indicated times. The sites of biotin attachment were determined using LysC or trypsin digestion followed by MALDI-MS. Peaks with assigned peptide sequence are labeled, and sequence data is given in Table I.
Table I.
Masses Observed in MALDI-ToF Spectra as Shown in Figure 3
| LysC digestion | |||
|---|---|---|---|
| Peak label | Observed mass | Sequence | Residues |
| A | 738.35 | TESHHK | 119–124 |
| B | 910.46 | AGFMSGRGK | His-tag–5 |
| C | 1038.55 | KAGFMSGRGK | His-tag–5 |
| D | 1280.65 | AGFMSGRGKQGGK | His-tag–9 |
| E | 1931.17 | VTIAQGGVLPNIQAVLLPK | 99–118 |
| F | 2429.37 | TRIIPRHLQLAIRNDEELNK | 75–95 |
| G | 870.49 | ARAK13AK + Biotin | 10–15 |
| H | 1041.56 | QGGK9ARAK + Biotin | 6–13 |
| I | 1092.53 | K118TESHHK + Biotin | 118–124 |
| J | 1163.56 | TESHHK124AK + Biotin | 119–126 |
| K | 1466.77 | QGGK9ARAK13AK + 2 Biotin | 6–15 |
| L | 1264.63 | K--5AGFMSGRGK + Biotin | His-tag–5 |
| M | 1506.73 | AGFMSGRGK5QGGK + Biotin | His-tag–9 |
| N | 1634.83 | KAGFMSGRGK5QGGK + Biotin | His-tag–9 |
| O | 1860.90 | K--5AGFMSGRGK5QGGK + 2 Biotin | His-tag–9 |
| Potential LysC nonspecific cleavages (upstream residue in parentheses) | |||
| P | 1130.58 | (A)IRNDEELNK | 110–118 |
| Trypsin digestion | |||
| A′ | 709.33 | GNYAER | 37–42 |
| B′ | 725.34 | AGFMSGR | His-tag–3 |
| C′ | 944.53 | AGLQFPVGR | 21–29 |
| D′ | 1219.69 | LLRKGNYAER | 33–42 |
| E′ | 2342.45 | LLGKVTIAQGGVLPNIQAVLLPK | 96–118 |
| F′ | 842.43 | QGGK9AR + Biotin | 6–11 |
| G′ | 870.50 | ARAK13AK + Biotin | 10–15 |
| H′ | 1063.50 | K36GNYAER + Biotin | 36–42 |
| I′ | 1079.51 | K-5AGFMSGR + Biotin | His-tag–3 |
| J′ | 1126.59 | AK13AK15TR + 2 Biotin | 12–15 |
| K′ | 1163.56 | TESHHK124AK + Biotin | 119–126 |
| L′ | 1213.58 | DNK74K75TR + 2 Biotin | 72–77 |
| M′ | 1253.62 | GK5QGGK9AR + 2 Biotin | 4–11 |
| N′ | 1389.64 | TESHHK124AK + 2 Biotin | 119–126 |
| O′ | 1928.93 | K118TESHHK124AK126GK + 3 Biotin | 118–128 |
| P′ | 1941.17 | K75TRIIPRHLQLAIR + Biotin | 75–88 |
To confirm the assignment of biotin attachment sites in recombinant H2A, biotinylated peptides were sequenced by tandem mass spectrometry. Those spectra with the most significant assignments are illustrated (see Fig. 5). All observed spectra matched the results obtained by MALDI-TOF. Peptide GK5QGGK9AR [Fig. 5(A); numbered residue in bold indicates biotin attachment] was found to contain biotin at both lysines 5 and 9, confirming the sequence obtained for fragment M′ (Table I). Similarly, peptide AK13AK15TR [Fig. 5(B)] was biotinylated at both lysines, matching the sequence predicted for fragment J′. Peptide K36GNYAER [Fig. 5(C)] was biotinylated at lysine 36, matching fragment H′. Other peptides with attached biotin were assigned which located lysines 15, 125, and 127 as target sites, though the signals were typically weak (not shown). In addition, both lysines within the linker region of the His-tag, positions −5 and −6, were found to contain an attached biotin, although not at the same time [Fig. 5(D), biotin at K−5].
Figure 5.
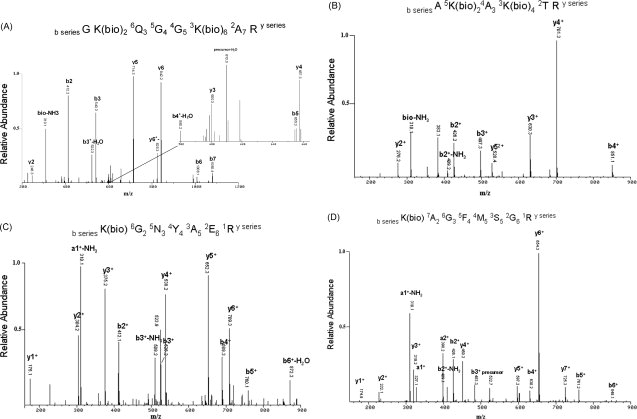
Tandem mass spectrometry analysis of biotinylation sites on recombinant H2A. Recombinant H2A incubated overnight with bio-5′-AMP was digested with trypsin and enriched on an avidin cartridge. Tandem mass spectra of the most significant assignments are shown (panels A–D), along with the matched sequence above each spectrum.
Significantly, a dominant peak at 310 u was observed in several mass spectra, including those presented here [Fig. 5(A–D)], which was not observed when analyzing unbiotinylated peptides. This mass corresponds to a novel marker of biotinylated lysine produced by loss of ammonia from the immonium ion of lysine (84 u) plus the attached biotin (226 u). Such marker ions, obtained under low-energy CID conditions, have been used diagnostically to detect specific modifications of the ɛ-amine of lysine, such as lysine acetylation.25 The 310 u peak is labeled as bio-NH3 when it is derived from internal a-type plus y-type cleavage of the peptide backbone or a1±-NH3 when the modified lysine is at the N-terminal end (a-type cleavage only).
The biotinylation of H2A was repeated in the presence of HCS, biotin, and ATP to determine if the sites of biotin attachment in recombinant H2A are the same when generated enzymatically. This assay requires the HCS-dependent synthesis of bio-5′-AMP and its dissociation from the enzyme for the biotin to be subsequently attached to H2A. The reaction, with each substrate at 50 μM, was allowed to run overnight to significantly label H2A [Fig. 1(B)]. Biotin-containing fragments from H2A were generated using in-gel trypsin digestion, and the isolated peptides were affinity purified on an avidin cartridge. The peptide sequences were detected by MALDI-MS (not shown). From this analysis, lysines 9, 13, and 15 were identified as biotin attachment sites (peptides F′, G′, and J′, Table I). The other biotin attachment sites observed above using nonenzymatic conditions were not detected with this reaction. The results appear equivalent to those obtained at the earliest stages of the nonenzymatic reaction, as would be expected for a more limiting supply of the bio-5′-AMP product of the enzymatic reaction.
Specificity of targeting biotin attachment
The above experiments demonstrate a preferred pattern of biotin attachment to H2A, but the criteria for attachment are not obvious. In BCCP87 and p-67, there are five and four lysines within the sequence, respectively. Yet, the biotinylation target is the lysine within the highly conserved Ala-Met-Lys-Met motif, corresponding to the biotin attachment site in carboxylases. Recombinant histone H2A, on the other hand, contains many target lysines within strongly basic environments [Fig. 1(A)]. To assess whether the local sequence within H2A has a role in targeting biotin attachment, the reactivity of bio-5′-AMP towards synthetic peptides of H2A and p-67 encompassing the target sites were compared. For H2A, a peptide was synthesized containing the cluster of three target lysines at residues 5, 9, and 13 (H2A-peptide RGK5QGGK9ARAK13A; pI, 12.02, MW 1226). For p-67, a peptide was synthesized containing the acceptor lysine at residue 61 along with a second downstream lysine that is not biotinylated (p-67-peptide QEICVIEAMK61MQNSMTAGK70T; pI, 6.14, MW 2212). The biotinylation assay was conducted by incubating each peptide with an equimolar amount of bio-5′-AMP for 7 h, and biotin attachment was evaluated by MALDI-MS of the reaction products (see Fig. 6). The unbound mass of the H2A-peptide is 1226.7 u [Fig. 6(A)]. After 7 h incubation, three peaks were detected, representing the addition of one (1452.6 u), two (1678.7 u), and three biotin groups (1904.6 u) [Fig. 6(B)]. No peak was detected for unmodified H2A-peptide (1226.73 u), indicating that all the peptide in the reaction was modified at least once. A peak at 1502.7 u in the starting material [Fig. 6(A)] is likely a contaminant related to the H2A peptide segment. It appears to have been biotinylated once to generate the peak observed at 1728.7 u in Figure 6(B). In contrast, the unmodified p-67 peptide, with a mass of 2212.8 u [Fig. 6(C)], remained present after 7 h incubation with bio-5′-AMP [Fig. 6(D)]. A weak signal indicating a single biotin addition to p-67 (∼2440 u) was detected, indicating that the reaction did progress to some extent. As before, the ionization efficiency for both peptides with and without modification is not known. However, because the unmodified H2A peptide disappeared while the unmodified p-67 peptide was still detected after incubation with biotinyl-AMP, the increased susceptibility of the H2A-peptide towards biotinylation compared with the p-67 peptide is apparent. This dramatic difference in biotinylation suggests that the rules for nonenzymatic biotin attachment are contained within the pattern of these short sequences and that the sequence of p-67 does not share these properties.
Figure 6.

Nonenzymatic biotin attachment to synthetic peptides of H2A containing Lys 5, 9 and 13 and p-67 containing Lys 61 and 70 (complete sequences in Materials and Methods). Panels A, B. H2A peptide at 0 time (A) and after 7 hours incubation (B). Expected masses are: for unbiotinylated peptide, 1226 units; +1 biotin, 1452 units; +2 biotin, 1678 units; +3 biotin, 1904 units. Note complete disappearance of unbiotinylated peptide following incubation. Panels C, D. p-67 peptide at 0 time (C) and after 7 h incubation (D). Expected masses are: for unbiotinylated peptide, 2212 units; +1 biotin, 2438 units.
Discussion
In recent years, the proposed role for biotin as a histone modification has taken it beyond its traditional role as a carboxyl carrier on biotin-dependent carboxylases to proposed involvement in gene regulation, cell proliferation, and response to DNA damage.26 The detection of HCS in cell nuclei and the HCS-catalyzed biotinylation of histones in vitro suggested that HCS might be responsible for biotin attachment to histones in vivo.20 However, the recent finding of Streaker and Beckett19 that the bio–5′-AMP product of BirA was sufficient, in the absence of enzyme, to produce biotin transfer to BCCP87 led us to examine the behavior of HCS and BirA toward histones, that is, whether the rate and specificity of biotin addition to histones depended on the BPL or whether the role of the biotin ligase might be limited to the generation of bio-5′-AMP.
Using bio-5′-AMP as a substrate in the absence of enzyme, we showed that biotin can be covalently attached to several lysine residues of histone H2A. Biotin addition was observed at lysine positions 5, 9, 13, 15 (N-terminal tail), 119, 125, 127 (C-terminal tail) and 36, 74, and 75 (core region), with preference shown for the N-terminal tail. The absence of increased biotin attachment with the addition of HCS or BirA suggests that the role of the enzyme in histone biotinylation, at least in vitro, is limited to the synthesis of the bio-5′-AMP intermediate and that the enzyme does not participate in biotin transfer. This is unlike the mechanism of biotin attachment to BCCP87 which, while it could be accomplished nonenzymatically with bio-5′-AMP as substrate, was accelerated in the presence of BirA.19
These results have implications for reported in vivo biotinylation of histones. Biotin attachment sites have been reported for histones H2A, H3, and H4.13–15 Five sites have been identified on histone H2A at lysines 9, 13, 125, 127, and 129, with the first two confirmed by detection with antibodies to biotinylated peptides.14 None of these sites are found in sequences related to the well recognized target sequence of biotin-dependent carboxylases, Ala/Val-Met-Lys-Met/Leu.2 The antibodies were generated to biotinylated histone peptides produced by treating candidate peptides with biotinidase and biocytin. The similarity of the biotin attachment sites produced by treatment with bio-5′-AMP with those generated by biotinidase and biocytin opens the possibility that the biotin transfer component of this reaction may also be nonenzymatic in nature. A recent publication demonstrated that BirA was capable of attaching biotin to the lysines at residues 4, 9, 18, and 23 in peptides synthesized to histone H3.27 These sites are similar to those observed (4, 9, and 13) using biotinidase as a biotin-transfer catalyst.15 Though a different histone protein was examined, these results coincide with what is observed here: that HCS and BirA both attach biotin to residues also observed as biotinylation target sites by biotinidase in histone H2A.
The physiochemical involvement of the local environment in histone biotinylation remains unclear, although we note that the attachment sites in H2A, as well as the proposed attachment sites in the remaining histones, tend to be surrounded by neutral or positively charged residues in largely positively charged regions of the protein. The nature of these domains suggest that the electrostatic interaction between the negative phosphate group from the adenylate group on bio-5′-AMP and the positively charged lysine or arginine side chain on the histone are the key elements that bring these molecules together. In a study of the interaction between mononucleotides and poly-lysine or poly-arginine peptides, electrostatic attractions were shown to be the initial force governing the interaction which, at a high concentration of the nucleotide, led to positioning and adsorption of the nucleotide base along the polyamino acid chain.28,29 Underscoring the importance of charge around the target lysine, we showed that a highly basic 12-mer peptide of H2A, residues 3–14, was readily biotinylated at all three Lys residues in the presence of bio-5′-AMP, whereas the neutral p-67 peptide, residues 660–679, containing two Lys residues, one the target Lys for in vivo biotinylation, was only trace biotinylated in the absence of enzyme. These results mimic the response of full-length H2A, which was rapidly biotinylated nonenzymatically on the N-terminal tail, when compared with BCCP which was minimally labeled after an overnight incubation. These results suggest that clusters of positively charged amino acids, such as the N-terminal tail of histones, promote rapid, electrostatic interaction with bio-5′-AMP, whereas the neutral region of p-67 or BCCP requires an enzyme catalyst to drive the reaction forward. The catalytic power of this interaction may be increased if the H2A peptide and N-terminal domain examined formed a secondary structure, such as an α-helix, that would cluster each lysine on one side of the structure. One study found that incubation of equimolar amounts of polyanions with a peptide generated to the lysine-rich region of yeast aspartyl-tRNA synthetase induced the formation of an α-helix from a random coil, resulting in clustering of the cationic residues to one face of the structure, which provided a polycationic interface.30 Indeed, studies have shown that a proportion of the histone tails adopt a helical formation, even when dissociated from nucleosomal DNA.31 It is interesting to speculate that a similar conformation is produced by the H2A peptide or full-length protein when incubated with equimolar amounts of anionic bio-5′-AMP.
The mechanism of biotin attachment utilizes a nucleophilic lysine, which attacks the electrophilic carbonyl group in bio-5′-AMP. For the ɛ-amino group of lysine to be nucleophilic, it must be uncharged. However, the pKa of free lysine in aqueous solution is 10.5, and therefore the composition of lysine at neutral pH is strongly protonated.32 In order for the reaction to proceed, the pKa of the target lysine(s) must be reduced. In the case with BCCP and p-67, this is accomplished through the interaction with BPL. As shown for the BPL-BCCP complex from Pyrococcus horikoshii, specific residues on both BCCP and the BPL direct the positioning of the target lysine in proximity to the bound substrates in the active site of the enzyme.33 The precise positioning of BCCP allows an aspartate residue of BPL (Asp104 in P. horikoshii) to deprotonate the ɛ-amino group of the target lysine, thereby increasing its nucleophilic character.33 We have shown that BPL does not interact with histone H2A, and the biotinylation reaction proceeds rapidly without a catalytic enzyme acting on the peptide or full-length protein. It is likely that, due to the sequence-proximity of lysines in the H2A peptide and protein, the electrostatic repulsion of the neighboring protonated lysines would increase with each proton addition.34 Following this hypothesis, the pKa of the target lysine would be decreased by coulombic interaction with its neighboring protonated lysine side chains, generating the required nucleophile. It has been observed in various enzymes, such as acetoacetate decarboxylase, that the pKa of the target lysine is drastically reduced by electrostatic interactions with neighboring charged lysines.34–36 This pKa perturbation has been verified in acetoacetate decarboxylase by measuring changes in the target lysine's pKa following mutagenesis of the neighboring lysine.35 The structure of histone H2A in the nucleosome predicts that lysines 9 and 13 are 4.1 Å apart, which, at that distance would likely result in pKa perturbation.37 Given the presumed unconstrained structure of H2A in solution, different conformers may bring different Lys or Lys-Arg pairs in proximity to one another, resulting in biotin attachment at different lysines. Curiously, H2A is reported to contain biotin attachment sites on lysines 9 and 13 in vivo, suggesting that these sites may in fact be chemically favored in a similar manner when using biotinidase.14 Comparatively, the target lysine in BCCP is over 10 Å in distance from the nearest basic residue, suggesting pKa alteration to be minimal [Fig. 7(B)]. Similarly, the lysines in the H2A peptide are close together, and their spacing suggests they may be tightly clustered in a helical structure, which would increase any electrostatic influence between lysines. However, in the p-67 peptide, the lysines are far apart, and unlikely to influence each other in this manner. Therefore, the special proximity of positively charged residues in histones and carboxylases likely plays a critical role in defining the difference in biotin attachment between these two proteins.
Figure 7.
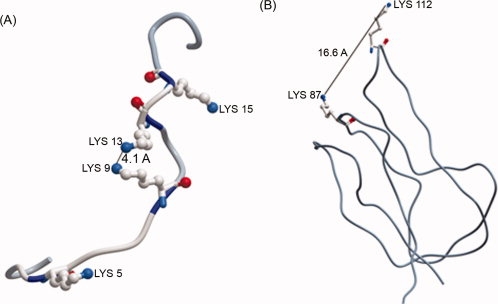
Intramolecular distance between adjacent lysines in (A) the N-terminal tail of histone H2A (1KX5) and (B) BCCP from Pyrococcus horikoshi (2EVB). Relevant lysines are labeled and shown in ball-and-stick format. The distance between the ɛ-amino groups of neighboring lysines is given in angstroms (Å). Structural manipulation was conducted using the Molsoft ICM-Browser software. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]:
With the proposed in vitro mechanism for biotin addition to histones, that is, enzymatic generation of bio-5′-AMP and nonenzymatic transfer of biotin to histone lysines, it is unclear how histones might be biotinylated in vivo. It is unlikely that a high local concentration of bio-5′-AMP could be generated and dissociated from the enzyme in close proximity to histone substrates. Furthermore, providing a necessity for direct interaction between HCS and histone H2A, such an interaction in vivo would have to involve the participation of other proteins. Histone modifying enzymes are commonly involved in large complexes, which consist of an array of proteins such as transcription factors, corepressors and scaffolding proteins. These complexes are at times involved in regulating the catalytic activity of a histone modifier, or in bringing the enzyme close to the target histone. One recent report on tension-induced/inhibited protein-6 (TIP-6) had defined the function of this novel protein in recruitment of p300; to position this histone acetyltransferase in close proximity to H3/H4 enabling acetylation, and in optimization of p300 activity, possibly by a change in p300 confirmation.38 The SANT (switching-defective protein 3 [Swi3], adaptor 2 [Ada2], nuclear receptor corepressor [N-CoR], and transcription factor IIIB [TFIIIB]) domain, which is commonly found in histone-modifying enzymes, was found to be essential for these functions. Thus, a more complex process than implicated in vitro would be required for HCS to be involved in histone biotinylation in vivo. HCS has been observed to be attached to the nuclear lamina, and association with chromatin sites remains possible.20 In Drosophila, for example, HCS has been localized to euchromatic bands in polytene chromosomes, suggesting the enticing possibility of histone biotinylation at such sites.39
Complicating the question of in vivo histone biotinylation is a report of caution expressed about the use of avidin or streptavidin to detect biotin in histones, with the suggestion that the affinity of avidins for histones is not mediated solely through biotin.40 Such concerns increase the importance of understanding the in vitro mechanism and specificity of biotin attachment and suggest great care in the use of in vitro-based tools to implicate in vivo processes. Despite this caution, the specificity and reactivity of bio-5′-AMP toward histones opens a new dimension to the capacity for histone modification, including the generation of biotinylated histone reagents using enzymes such as BirA or HCS to catalyze the first reaction.
Materials and Methods
Expression and purification of recombinant H2A, HCS and p-67
The cDNA clone for human H2A (H2A-M) in the sequencing vector pT7T3D-Pac was generously donated by S. Bell (Image No:1,687,138, The Centre for Applied Genomics, Montreal, Canada). The coding sequence of this protein was cloned by BP and LR recombination into the Gateway vectors pDONR221 and pDEST17, respectively, following the supplier's instructions. This fuses a noncleavable 6-histidine tag to the N-terminal end of the protein (Invitrogen). The primers used to generate attB recombination sites flanking the insert were as follows: 5′ primer-GGGGACAAGTTTGTACAAAA AAGCAGGCTTCATGTCTGGACGTGGCAAG, and 3′ primer-GGGGACCACTT TGTACAAGAAAGCTGGGTCCTAGGCCTGATTCAATACT. Two hundred and fifty millilitres of culture was grown in Luria-Burtani broth at 37°C with antibiotics to an optical density of 0.4–0.6. Expression was induced by adding 0.2% arabinose, and cells were allowed to grow for an additional 6 h and harvested by centrifugation (5000g/20 min/4°C). Recombinant histone H2A was purified by affinity chromatography under denaturing conditions and refolded by dialysis, as was previously reported.21 In brief, the cells were lysed with a French Press (Fisher Scientific) at 16000 psi in a solution containing 6M guanidine-hydrochloride in 50 mM Tris-HCl (pH 8.0) and the soluble fraction was collected. The protein was purified by maintaining 6M guanidine-hydrochloride in 50 mM Tris-HCl (pH 8.0) in column wash and elution buffers. The protein was bound to nickel charged resin (Ni-NTA agarose, Qiagen), washed in 20 mM imidazole, and eluted with 250 mM imidazole. The eluate was slowly dialyzed overnight into 50 mM Tris-Cl/100 mM NaCl/5% glycerol (pH 8.0). No protein precipitation was observed after dialysis, and the protein was found to be stable for several months at 4°C. This expression typically yielded 30 mg of pure protein. The protein concentration, which was maintained at 3–4 mg/mL, was determined using the BCA protein assay (Pierce) using purified calf thymus histones (Roche) as standards.
For the generation and purification of recombinant HCS, full length (residues 58–576) HCS was subcloned into pGEX 4T-1 (Amersham Biosciences) by introducing EcoRI and XhoI at the 5′ and 3′ ends, respectively (5′ primer-GGGGGAATTCATGGAGCATGT TGGCAGAG; 3′ primer-GGGGCTCGAGTTACCGCCGT TTGGGGAG) to generate an N-terminally tagged GST-HCS fusion protein. Recombinant GST-HCS was expressed in BL21 cells by growing initially at 37°C for 3–4 h, inducing with 0.1 mM IPTG and expressing at 25°C for 8 h. Cells were lysed using a French press (12,000 psi, 2 passes), and mixed for 1 h with glutathione-Sepharose (Amersham Biosciences) resin. Bound protein was washed with 30 column volumes of PBS/1% triton/0.5M NaCl (pH 8.0), 50 mM Tris-HCl/0.5M NaCl (pH 8.0), and 50 mM Tris-HCl (pH 8.0). Protein was eluted with 50 mM Tris-HCl/10 mM reduced glutathione and verified by SDS-PAGE analysis. Five milligrams of purified protein (∼80% purity) from 1 L of medium was generated using this method. The GST tag was cleaved from HCS by an overnight digestion at room temperature with thrombin (GE Lifesciences, 1 unit thrombin to 500 μg fusion protein).
Truncated apocarboxylase (p-67) was expressed in E. coli for affinity purification. P-67 is the 67 amino acid carboxyl-terminal fragment of the α-subunit of human PCC, which acts as an independent domain for biotinylation.22 The cDNA of this peptide was cloned by LR recombination into the Gateway vector pDEST17, which fuses a 6-histidine tag to the N-terminal end of the protein (Invitrogen). The primers used to generate recombination sites flanking the insert were as follows: 5′ primer-CACCCTGCGTTCCCCGA and 3′ primer-TCATTCCAGCTCCACGAG. One hundred millilitres of culture were grown in Luria-Burtani broth at 30°C with antibiotics to an optical density of 1.2–1.4. Cells were allowed to grow for 3–4 h and harvested by centrifugation (5000g/4°C/20 min). The cells were lysed using a French Press (Fisher Scientific) at 12,000 psi, and the soluble fraction was collected. The peptide was purified using nickel charged resin (Ni-NTA Agarose, Qiagen), eluting the pure protein with 250 mM imidazole. The eluate was dialyzed into 20 mM Tris-Cl/5% glycerol including protease inhibitors. This expression yielded 20 mg of pure protein.
Biotin attachment assays comparing p-67 and H2A as substrates
To determine the biotin attachment activity of HCS toward H2A or p-67, reaction mixtures were prepared containing 0.3 μM HCS (final concentration), 25 μM p-67 or recombinant H2A, 25 μM [14C]-biotin (specific activity 50 mCi/mmol, GE Lifesciences), and 25 μM ATP brought to a total volume of 20 μL with 10 mM Tris-HCl (pH 7.5 at 20°C), 200 mM KCl, 2.5 mM MgCl2. Assays were conducted at 20°C and 37°C. Samples were taken at 0, 2.5, 60, 420 min, and overnight. The reaction was stopped by adding 5 μL SDS-electrophoresis buffer with heating (95°C/5min). These samples were run on a 16.5% Tris-tricine discontinuous gel, and dried in cellophane for direct exposure to a phosphoimager screen (GE Lifesciences). The proportion of biotin attached to p67 or recombinant H2A was determined by adding nine volumes of 95% ethanol to the reaction mixture described above after 21 h incubation. The protein precipitate was collected by centrifugation (15,000g/15 min/4°C) and washed twice in acetone. The pellet was resuspended in 5% SDS and the radioactive content was determined by scintillation counting. The molar amount of incorporated biotin in the resuspended mixture was estimated through the specific activity of [14C]-biotin, detected through radioactivity from scintillation counts (Beckman Scintillation Counter LS 3801). Protein was determined using the BCA method as above. Data were converted to molar equivalents to determine the ratio of [14C]-biotin incorporated into the protein substrate on a molar basis.
Enzymatic and nonenzymatic biotin attachment to recombinant H2A
The nonenzymatic attachment of biotin to recombinant H2A or BCCP87 was assayed as described previously for BCCP87.19 BCCP87 and bio-5′-AMP were generously provided by D. Beckett, University of Maryland.41,42 The experiments on BCCP87 were the same as reported19 and were meant to act as controls for the biotinylation of H2A. The enzymatic biotinylation of H2A and BCCP87 using HCS or BirA followed methods as previously described. Enzymatic reactions were prepared consisting of 100 μM H2A or BCCP87, 100 μM bio-5′-AMP and 1 μM BirA or HCS, in a total of 50 μL in 10 mM Tris HCl (pH 7.5 at 20°C), 200 mM KCl, and 2.5 mM MgCl2. Incubations were at 20°C, and 10 μL aliquots were removed at 0, 60, 180, and 420 min. The reaction was stopped with the addition of 10 μL 1.0% trifluoroacetic acid, desalted using a C18 ZipTip (Millipore), eluted in 3 μL 50 mM sinapinic acid (Roche) in 70% acetonitrile/30% of 0.1% trifluoroacetic acid in water, and spotted directly in 1.0 μL aliquots on a MALDI analytical plate. Nonenzymatic biotinylation reactions were prepared in the same way, except that BirA or HCS was omitted from the solution. An overnight (16 h) reaction under both enzymatic and nonenzymatic conditions with 200 μM biotinyl-5′AMP was also included for both BCCP87 and H2A. Mass spectrometric measurements of full-length BCCP87 and H2A were conducted at the University of Maryland using an Axima CFR instrument (Shimadzu). The monoisotopic masses of H2A and BCCP87 were acquired in linear mode using bovine trypsinogen measured at both [M+H]± and [M+2H]2+ and bovine insulin (Sigma-Aldrich) as standards. The percentage of unbiotinylated protein remaining at each time point was calculated by dividing the intensity of the peak at the unbiotinylated mass by the sum of unbiotinylated and biotinylated peak intensities. Protein masses at both [M+H]± and [M+2H]2+ states were measured and averaged. This method, as described previously, was shown to correlate with known ratios of apoBCCP87 and biotinylated BCCP87.19
Identification of in vitro biotin attachment sites on recombinant H2A
To determine the sites of biotin attachment on H2A, nonenzymatic reactions, using the intermediate bio-5′-AMP, were conducted as above for 60 and 420 min and overnight at 20°C. Reactions were stopped by adding an equal volume of 1.0% trifluoroacetic acid and desalted using a G25 MicroSpin Column, which had been equilibrated in water (GE Lifesciences). Eluted protein was lyophilized in a speed vacuum and resuspended in 1% SDS. The protein was denatured by heating at 95°C for 10 min. The detergent was diluted to 0.1% with the addition of 50 mM ammonium bicarbonate, pH 8.0, when digesting with 1:20 (w/w) trypsin (Princeton Separations), or with 100 mM ammonium bicarbonate, pH 8.0 when digesting with 1:20 (w/w) LysC (Sigma Aldrich). Protein digested with trypsin was incubated for 16 h at 30°C, whereas protein digested with LysC was incubated for 16 h at 37°C. Unbiotinylated H2A was included in both LysC and trypsin digestions as a control. Peptides were then analyzed by MALDI-TOF. Peptide mass fingerprinting was conducted at the Southern Alberta Mass Spectrometry (SAMS) Centre at the University of Calgary, using a Voyager-DE STR in reflectron mode. Samples were desalted with C18ZipTips as described above, except peptides were eluted with 0.1% TFA/50% acetonitrile (3 μL). The peptide sample was then spotted on a MALDI plate (typically 0.5 μL), followed by an equal volume of matrix (5 mg/ml α-cyano-4-hydroxycinnamic acid in 0.1% TFA/50% acetonitrile). Angiotensin (Fluka) was included as a standard. One hundred to 300 shots were accumulated to generate an acceptable spectrum, and acquired monoisotopic masses were used to predict peptide sequences using FindMod freeware.43 All spectral data was manipulated using “MoverZ” (Genomic Solutions).
The sites of nonenzymatic biotin attachment on samples subjected to overnight incubation were further analyzed by tandem mass spectrometry. In this case, protein fragments containing biotin were isolated by affinity chromatography using ICAT Avidin Cartridges (Applied Biosystems), as per manufacturer's instructions. Elution was in 30% acetonitrile/0.1% trifluoroacetic acid. The purified peptide was lyophilized to dryness in a Speedvac vacuum concentrator and analyzed at the Proteomics Facility at the Institute of Systems Biology, Seattle, by tandem mass spectrometry. All the samples were separated by a coupled reversed-phase liquid chromatography system (Agilent HP 1100 quaternary LC pump, LC-Packings FAMOS autosampler) and analyzed on an ion-trap ESI-MS (LCQ-Deca ion trap Classic, Thermo-Electron). Specific precursor ion(s) within a narrow m/z range were automatically selected for fragmentation via collision-induced dissociation (CID), generating tandem mass spectra. The raw data was acquired with Xcalibur, version 1.0. MS peaks were selected without smoothing, and MS-MS data were centroided. The intensity cutoff for MS-MS was 50,000 counts/s. Raw data was converted to mzXML format using ReAdW (Institute of Systems Biology, http://tools. proteomecenter.org/software.php). Peak lists were searched against all known protein sequences in an in-house NCI bovine database including the recombinant H2A sequence using Sequest v.27.44 The precursor mass tolerance was set at 3.0, and the ion fragment mass tolerance was set to 0.5 Da. Biotin bound to lysine was specified as a variable modification. All peptide masses were reported as average mass units. The search tool Sequest was used to find a peptide sequence in a generated protein database that best correlated with the fragment ions in the MS/MS spectrum. Identifications were analyzed using the INTERACT program and validated using Peptide Prophet and Protein Prophet.45 Data was presented using Comet Spectrum View.
To determine the sites of biotin attachment in enzymatic reactions, reaction mixes were prepared as follows: 2 μM (final concentration) of bacteria-expressed HCS was mixed with 5× biotinylation buffer [250 mM reduced glutathione (Sigma), 125 mM MgCl2 and 1.5M Tris-Cl, pH 7.5] plus 100 μM ATP, 50 μM recombinant H2A and 50 μM biotin (Sigma) to a final volume of 100 μL. The reaction was incubated overnight at 37°C and was stopped by the addition of an equal volume of SDS sample buffer. Samples were heated to 95°C for 5 min and were resolved by discontinuous SDS polyacrylamide gel electrophoresis using 16.5% Tris-tricine polyacrylamide gels, run for 4 h at 10 mA. The gel was stained with Coomassie blue. Bands containing recombinant H2A were excised for in-gel digestion. Gel pieces were washed twice in 50% acetonitrile, and the protein band was destained with multiple changes of 25 mM ammonium bicarbonate (pH 8.0)/50% acetonitrile. The gel pieces were dehydrated with 100% acetonitrile and dried with a speed vacuum for 15 min. Trypsin (1:20 w/w) in 50 mM ammonium bicarbonate pH 8.0 was added to the dehydrated gel to an approximate volume of 20 μL and digested overnight at 37°C. Peptides were extracted from the gel by adding 100 μL acetonitrile and shaking for 30 min at room temperature. The peptides were lyophilized, and biotin-containing peptides were purified on an ICAT avidin column as described above. Biotinylated peptide sequences from this reaction were measured from monoisotopic mass units by MALDI-MS analysis at the SAMS Centre, as described earlier. Unbiotinylated H2A was similarly treated and purified as a control.
Peptide analysis
Peptides, containing biotin attachment region sequences from p-67, and histone H2A, were compared for their ability to be nonenzymatically biotinylated. Peptides were synthesized spanning the region in histone H2A that contains the biotinylation sites at Lys-5, 9, and 13: RGK5QGGK9ARAK13A, and in p-67 that contains the physiological target site Lys-61 along with a second lysine: QEICVIEAMK61MQNSMTAGKB70T. Peptides were synthesized using Fmoc chemistry on an automated solid-phase system at a local facility (D. McMaster, Peptide Synthesis, University of Calgary). Synthesis of 4–10 mg peptide was performed on a 12 reactor PTI Symphony synthesizer (Protein Technologies) and purified by HPLC (Waters). Peptide molecular mass was verified from their monoisotopic mass units using MALDI-TOF analysis. Lyophilized peptides were prepared as 1 mg/ml solutions dissolved in water.
Nonenzymatic biotinylation reactions using the peptide substrates were prepared and analyzed as follows: 1 nmol peptide was incubated with 1 nmol biotinyl-AMP to a total volume of 10 μL in 10 mM Tris HCl (pH 7.5), 200 mM KCl, and 2.5 mM MgCl2 for 0 and 7 h at 20°C. Reactions were stopped by pipetting 10 μL 0.1% trifluoroacetic acid into the reaction mixture and immediately desalted using a C18 ZipTip. The monoisotopic mass of each peptide sample was directly analyzed by MALDI-MS at the SAMS Centre at the University of Calgary, as described earlier.
Acknowledgments
We are grateful to Ruedi Aebersold for advice and initial access to the facilities at the Institute for Systems Biology, Seattle, to A. Leon-del-Rio, and D. Beckett for helpful comments on the manuscript and to D. Beckett for hosting S.H. in her laboratory and providing specialized reagents.
Glossary
Abbreviations
- BCCP
biotin carboxyl carrier protein
- bio-5′-AMP
biotinyl-5′-adenylate
- HCS
holocarboxylase synthetase
- m/z
mass/charge
- MALDI
matrix-assisted laser desorption/ionization
- MS
mass spectrometry
- MW
molecular weight
References
- 1.Wood HG, Barden RE. Biotin enzymes. Annu Rev Biochem. 1977;46:385–413. doi: 10.1146/annurev.bi.46.070177.002125. [DOI] [PubMed] [Google Scholar]
- 2.Chapman-Smith A, Cronan JE., Jr In vivo enzymatic protein biotinylation. Biomol Eng. 1999;16:119–125. doi: 10.1016/s1050-3862(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 3.Pacheco-Alvarez D, Solorzano-Vargas RS, Del Rio AL. Biotin in metabolism and its relationship to human disease. Arch Med Res. 2002;33:439–447. doi: 10.1016/s0188-4409(02)00399-5. [DOI] [PubMed] [Google Scholar]
- 4.Streit WR, Entcheva P. Biotin in microbes, the genes involved in its biosynthesis, its biochemical role and perspectives for biotechnological production. Appl Microbiol Biotechnol. 2003;61:21–31. doi: 10.1007/s00253-002-1186-2. [DOI] [PubMed] [Google Scholar]
- 5.Leon-Del-Rio A, Leclerc D, Akerman B, Wakamatsu N, Gravel RA. Isolation of a cDNA encoding human holocarboxylase synthetase by functional complementation of a biotin auxotroph of Escherichia coli. Proc Natl Acad Sci USA. 1995;92:4626–4630. doi: 10.1073/pnas.92.10.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki Y, Aoki Y, Ishida Y, Chiba Y, Iwamatsu A, Kishino T, Niikawa N, Matsubara Y, Narisawa K. Isolation and characterization of mutations in the human holocarboxylase synthetase cDNA. Nat Genet. 1994;8:122–128. doi: 10.1038/ng1094-122. [DOI] [PubMed] [Google Scholar]
- 7.Eisenberg MA, Prakash O, Hsiung SC. Purification and properties of the biotin repressor. A bifunctional protein. J Biol Chem. 1982;257:15167–15173. [PubMed] [Google Scholar]
- 8.Wolf B, Grier RE, Secor M, Jr, Heard GS. Biotinidase deficiency: a novel vitamin recycling defect. J Inherit Metab Dis 8 Suppl. 1985;1:53–58. doi: 10.1007/BF01800660. [DOI] [PubMed] [Google Scholar]
- 9.Dakshinamurti K, Mistry SP. Tissue and intracellular distribution of biotin-C-1400H in rats and chicks. J Biol Chem. 1963;238:294–296. [PubMed] [Google Scholar]
- 10.Hasegawa Y, Ishida Y, Kato K, Ijiri R, Miyake T, Nishimata S, Watanabe T, Namba I, Hayabuchi Y, Kigasawa H, Tanaka Y. Pancreatoblastoma. A case report with special emphasis on squamoid corpuscles with optically clear nuclei rich in biotin. Acta Cytol. 2003;47:679–684. doi: 10.1159/000326588. [DOI] [PubMed] [Google Scholar]
- 11.Nakatani Y, Kitamura H, Inayama Y, Ogawa N. Pulmonary endodermal tumor resembling fetal lung. The optically clear nucleus is rich in biotin. Am J Surg Pathol. 1994;18:637–642. doi: 10.1097/00000478-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Yokoyama S, Kashima K, Inoue S, Daa T, Nakayama I, Moriuchi A. Biotin-containing intranuclear inclusions in endometrial glands during gestation and puerperium. Am J Clin Pathol. 1993;99:13–17. doi: 10.1093/ajcp/99.1.13. [DOI] [PubMed] [Google Scholar]
- 13.Camporeale G, Shubert EE, Sarath G, Cerny R, Zempleni J. K8 and K12 are biotinylated in human histone H4. Eur J Biochem. 2004;271:2257–2263. doi: 10.1111/j.1432-1033.2004.04167.x. [DOI] [PubMed] [Google Scholar]
- 14.Chew YC, Camporeale G, Kothapalli N, Sarath G, Zempleni J. Lysine residues in N-terminal and C-terminal regions of human histone H2A are targets for biotinylation by biotinidase. J Nutr Biochem. 2006;17:225–233. doi: 10.1016/j.jnutbio.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobza K, Camporeale G, Rueckert B, Kueh A, Griffin JB, Sarath G, Zempleni J. K4, K9 and K18 in human histone H3 are targets for biotinylation by biotinidase. FEBS J. 2005;272:4249–4259. doi: 10.1111/j.1742-4658.2005.04839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davie JR. Covalent modifications of histones: expression from chromatin templates. Curr Opin Genet Dev. 1998;8:173–178. doi: 10.1016/s0959-437x(98)80138-x. [DOI] [PubMed] [Google Scholar]
- 17.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 18.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 19.Streaker ED, Beckett D. Nonenzymatic biotinylation of a biotin carboxyl carrier proteunusual reactivity of the physiological target lysine. Protein Sci. 2006;15:1928–1935. doi: 10.1110/ps.062187306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narang MA, Dumas R, Ayer LM, Gravel RA. Reduced histone biotinylation in multiple carboxylase deficiency patients: a nuclear role for holocarboxylase synthetase. Hum Mol Genet. 2004;13:15–23. doi: 10.1093/hmg/ddh006. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka Y, Tawaramoto-Sasanuma M, Kawaguchi S, Ohta T, Yoda K, Kurumizaka H, Yokoyama S. Expression and purification of recombinant human histones. Methods. 2004;33:3–11. doi: 10.1016/j.ymeth.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Leon-Del-Rio A, Gravel RA. Sequence requirements for the biotinylation of carboxyl-terminal fragments of human propionyl-CoA carboxylase alpha subunit expressed in Escherichia coli. J Biol Chem. 1994;269:22964–22968. [PubMed] [Google Scholar]
- 23.Inouye S, Nakamura M. Identification of biotinylated lysine residues in the photoprotein aequorin by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry peptide mapping after lysine-specific endopeptidase digestion. Anal Biochem. 2003;316:216–222. doi: 10.1016/s0003-2697(03)00055-1. [DOI] [PubMed] [Google Scholar]
- 24.Mersfelder EL, Parthun MR. The tale beyond the tail: histone core domain modifications and the regulation of chromatin structure. Nucleic Acids Res. 2006;34:2653–2662. doi: 10.1093/nar/gkl338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JY, Kim KW, Kwon HJ, Lee DW, Yoo JS. Probing lysine acetylation with a modification-specific marker ion using high-performance liquid chromatography/electrospray-mass spectrometry with collision-induced dissociation. Anal Chem. 2002;74:5443–5449. doi: 10.1021/ac0256080. [DOI] [PubMed] [Google Scholar]
- 26.Zempleni J. Uptake, localization, and noncarboxylase roles of biotin. Annu Rev Nutr. 2005;25:175–196. doi: 10.1146/annurev.nutr.25.121304.131724. [DOI] [PubMed] [Google Scholar]
- 27.Kobza K, Sarath G, Zempleni J. Prokaryotic BirA ligase biotinylates K4, K9, K18 and K23 in histone H3. BMB Rep. 2008;41:310–315. doi: 10.5483/bmbrep.2008.41.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lacey JC, Jr, Pruitt KM. Drug-biomolecule interactions: interactions of mononucleotides and polybasic amino acids. J Pharm Sci. 1975;64:473–477. doi: 10.1002/jps.2600640335. [DOI] [PubMed] [Google Scholar]
- 29.Schott H, Eckstein H. Studies on interactions between immobilized lysine residues and oligomers of thymidylic and deoxyadenylic acids. Eur J Biochem. 1980;104:79–84. doi: 10.1111/j.1432-1033.1980.tb04402.x. [DOI] [PubMed] [Google Scholar]
- 30.Agou F, Yang Y, Gesquiere JC, Waller JP, Guittet E. Polyanion-induced alpha-helical structure of a synthetic 23-residue peptide representing the lysine-rich segment of the N-terminal extension of yeast cytoplasmic aspartyl-tRNA synthetase. Biochemistry. 1995;34:569–576. doi: 10.1021/bi00002a023. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Moore SC, Laszckzak M, Ausio J. Acetylation increases the alpha-helical content of the histone tails of the nucleosome. J Biol Chem. 2000;275:35013–35020. doi: 10.1074/jbc.M004998200. [DOI] [PubMed] [Google Scholar]
- 32.Atkins P. The Elements of Physical Chemistry. 3rd. New York: W.H. Freeman and Company; 2001. Consequences of equilibrium; pp. 167–185. [Google Scholar]
- 33.Bagautdinov B, Matsuura Y, Bagautdinova S, Kunishima N. Protein biotinylation visualized by a complex structure of biotin protein ligase with a substrate. J Biol Chem. 2008;283:14739–14750. doi: 10.1074/jbc.M709116200. [DOI] [PubMed] [Google Scholar]
- 34.Westheimer FH. Coincidences, decarboxylation, and electrostatic effects. Tetrahedron. 1995;51:3–20. [Google Scholar]
- 35.Highbarger LA, Gerlt JA, Kenyon GL. Mechanism of the reaction catalyzed by acetoacetate decarboxylase. Importance of lysine 116 in determining the pKa of active-site lysine 115. Biochemistry. 1996;35:41–46. doi: 10.1021/bi9518306. [DOI] [PubMed] [Google Scholar]
- 36.Johnsson K, Allemann RK, Widmer H, Benner SA. Synthesis, structure and activity of artificial, rationally designed catalytic polypeptides. Nature. 1993;365:530–532. doi: 10.1038/365530a0. [DOI] [PubMed] [Google Scholar]
- 37.Davey CA, Sargent DF, Luger K, Maeder AW, Richmond TJ. Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 a resolution. J Mol Biol. 2002;319:1097–1113. doi: 10.1016/S0022-2836(02)00386-8. [DOI] [PubMed] [Google Scholar]
- 38.Badri KR, Zhou Y, Dhru U, Aramgam S, Schuger L. Effects of the SANT domain of tension-induced/inhibited proteins (TIPs), novel partners of the histone acetyltransferase p300, on p300 activity and TIP-6-induced adipogenesis. Mol Cell Biol. 2008;28:6358–6372. doi: 10.1128/MCB.00333-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camporeale G, Giordano E, Rendina R, Zempleni J, Eissenberg JC. Drosophila melanogaster holocarboxylase synthetase is a chromosomal protein required for normal histone biotinylation, gene transcription patterns, lifespan, and heat tolerance. J Nutr. 2006;136:2735–2742. doi: 10.1093/jn/136.11.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey LM, Ivanov RA, Wallace JC, Polyak SW. Artifactual detection of biotin on histones by streptavidin. Anal Biochem. 2008;373:71–77. doi: 10.1016/j.ab.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Lane MD, Rominger KL, Young DL, Lynen F. The enzymatic synthesis of holotranscarboxylase from apotranscarboxylase and (+)-biotin. II. Investigation of the reaction mechanism. J Biol Chem. 1964;239:2865–2871. [PubMed] [Google Scholar]
- 42.Nenortas E, Beckett D. Purification and characterization of intact and truncated forms of the Escherichia coli biotin carboxyl carrier subunit of acetyl-CoA carboxylase. J Biol Chem. 1996;271:7559–7567. doi: 10.1074/jbc.271.13.7559. [DOI] [PubMed] [Google Scholar]
- 43.Wilkins MR, Gasteiger E, Gooley AA, Herbert BR, Molloy MP, Binz PA, Ou K, Sanchez JC, Bairoch A, Williams KL, Hochstrasser DF. High-throughput mass spectrometric discovery of protein post-translational modifications. J Mol Biol. 1999;289:645–657. doi: 10.1006/jmbi.1999.2794. [DOI] [PubMed] [Google Scholar]
- 44.Yates JR, III, Eng JK, McCormack AL, Schieltz D. Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal Chem. 1995;67:1426–1436. doi: 10.1021/ac00104a020. [DOI] [PubMed] [Google Scholar]
- 45.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]


