Abstract
Background
Non-potassium-sparing diuretics may increase mortality and hospitalizations in heart failure patients. Most heart failure patients are older adults, yet the effect of diuretics on cause-specific mortality and hospitalizations in older adults with heart failure is unknown. The objective of this propensity-matched study was to determine the effect of diuretics on mortality and hospitalizations in heart failure patients ≥65 years.
Method
Of the 7788 Digitalis Investigation Group participants, 4036 were ≥65 years and 3271 (81%) were receiving diuretics. Propensity scores for diuretic use for each of the 4036 patients were calculated using a non-parsimonious multivariable logistic regression model incorporating all measured baseline covariates, and were used to match 651 (85%) patients not receiving diuretics with 651 patients receiving diuretics. Effects of diuretics on mortality and hospitalization at 37 months of median follow-up were assessed using matched Cox regression models.
Results
All-cause mortality occurred in 173 patients not receiving diuretics and 208 patients receiving diuretics respectively during 2056 and 1943 person-years of follow-up (hazard ratio {HR}=1.36; 95% confidence interval {CI}=1.08–1.71; p=0.009). All-cause hospitalizations occurred in 413 patients not receiving and 438 patients receiving diuretics respectively during 1255 and 1144 person-years of follow up (HR=1.18; 95% CI=0.99–1.39; p=0.063). Diuretic use was associated with significant increased risk of cardiovascular mortality (HR=1.50; 95% CI=1.15–1.96; p=0.003). and heart failure hospitalization (HR=1.48; 95% CI=1.13–1.94; p=0.005).
Conclusions
Chronic diuretic use was associated with significant increased mortality and hospitalization in ambulatory older adults with heart failure receiving angiotensin converting enzyme inhibitor and diuretics.
Keywords: Diuretics, heart failure, older adults, mortality, hospitalization
1. Introduction
In a propensity score matched cohort of ambulatory chronic heart failure, use of non-potassium-sparing diuretics was associated with increased risk of all-cause mortality and heart failure hospitalization [1]. Patients in that study had a mean age of 63 years. However, most heart failure patients are ≥65 years and heart failure is a leading cause for hospitalizations among older adults [2]. A subgroup analysis of that study suggested that diuretic associated mortality was similar in patients <65 and ≥65 years. However, patients in that subgroup analysis were not propensity score matched and the effects of diuretics on other outcomes were not reported.
Elderly HF patients are often excluded form randomized clinical trials and evidence for these patients is often extrapolated from data for younger patients. However, unlike in randomized trials, results of non-randomized studies can be more readily replicated in a cost-effective manner, obviating the need for such extrapolation. Therefore, the objective of the current analysis was to examine the effect of chronic diuretic therapy on mortality and hospitalizations (overall and cause-specific) in a propensity score matched cohort of heart failure patients ≥65 years of age.
2. Materials and methods
2.1. Study design and patients
This is a secondary analysis of the DIG trial, which was conducted in 302 centers (US=186 and Canada=116) during early 1990s [3]. Detailed of DIG trial have been previously reported [3, 4]. Of the 7888 patients, 6800 had ejection fraction ≤45%. Of the 4036 patients ≥65 years, 3271 (81%) were receiving diuretics (excluding spironolactone and other potassium-sparing diuretics). All patients were in normal sinus rhythm, and most were receiving angiotensin-converting enzyme (ACE) inhibitor. Data on beta-blocker use were not collected. The current analysis focuses on a subset of 651 pairs of patients who were matched by their propensities to receive diuretics.
2.2. Outcomes
The primary outcomes were all-cause mortality and all-cause hospitalization during 36.7 months of median follow up. Additional outcomes studied included mortality and hospitalizations due to cardiovascular causes and heart failure. Vital status was ascertained for 99% of the patients [5].
2.3. Calculation of propensity scores
We calculated propensity scores for the receipt of diuretics for each of the 4036 patients, using a non-parsimonious multivariable logistic regression model (c statistic=0.833) [1]. All baseline patient characteristics displayed in Table 1 and Figure 2 were included in the model. The propensity score for the receipt of diuretics for a patient is the conditional probability of receiving a diuretic given that patient’s measured covariates [1, 6-11].
Table 1.
Baseline patient characteristics by diuretic use before and after propensity score matching
| Patient characteristics | Before propensity score match |
After propensity score match |
||||
|---|---|---|---|---|---|---|
| N (%) or mean (±SD) | No diuretic N = 765 | Diuretic N = 3271 | P value | No diuretic N = 651 | Diuretic N = 651 | P value |
| Age, years | 71.4 (±5.0) | 72.4 (±5.5) | <0.0001 | 71.6 (±5.1) | 71.5 (±5.2) | 0.794 |
| Women | 163 (21%) | 963 (29%) | <0.0001 | 144 (22%) | 155 (24%) | 0.510 |
| Non-whites | 66 (9%) | 383 (12%) | 0.015 | 61 (9%) | 66 (10%) | 0.709 |
| Body mass index, kg / m2 | 26.2 (±4.3) | 26.4 (±4.9) | 0.899 | 26.1 (±4.3) | 26.2 (±4.4) | 0.556 |
| Heart failure duration | 32 (±41) | 29 (±36) | 0.006 | 31 (±37) | 31 (±38) | 0.588 |
| Primary cause of heart failure | ||||||
| Ischemic | 600 (78%) | 2321 (71%) | 506 (78%) | 499 (77%) | ||
| Hypertensive | 71 (9%) | 391 (12%) | <0.0001 | 61 (9%) | 66 (10%) | 0.777 |
| Idiopathic | 68 (9%) | 381 (12%) | 62 (10%) | 58 (9%) | ||
| Others | 26 (3%) | 178 (5%) | 22 (2%) | 28 (2%) | ||
| Prior myocardial infarction | 558 (73%) | 2067 (63%) | <0.0001 | 468 (72%) | 463 (71%) | 0.806 |
| Current angina pectoris | 228 (30%) | 919 (28%) | 0.350 | 188 (29%) | 183 (28%) | 0.806 |
| Hypertension | 324 (42%) | 1661 (51%) | <0.0001 | 280 (43%) | 287 (44%) | 0.737 |
| Diabetes mellitus | 177 (23%) | 995 (30%) | <0.0001 | 151 (23%) | 157 (24%) | 0.744 |
| Chronic kidney disease* | 363 (48%) | 2085 (64%) | <0.0001 | 323 (50%) | 344 (53%) | 0.267 |
| Medications | ||||||
| Digoxin (pre-trial use) | 243 (32%) | 1460 (45%) | <0.0001 | 218 (34%) | 212 (33%) | 0.724 |
| Digoxin (by randomization) | 389 (51%) | 1615 (49%) | 0.470 | 321 (49%) | 341 (52%) | 0.292 |
| ACE inhibitors | 667 (87%) | 3068 (94%) | <0.0001 | 580 (89%) | 579 (89%) | 1.000 |
| Potassium-sparing diuretics | 139 (18%) | 160 (5%) | <0.0001 | 96 (15%) | 99 (15%) | 0.877 |
| Potassium supplement | 54 (7%) | 1126 (34%) | <0.0001 | 52 (8%) | 46 (7%) | 0.600 |
| Symptoms and signs of heart failure | ||||||
| Dyspnea at rest | 99 (13%) | 774 (24%) | <0.0001 | 90 (14%) | 92 (14%) | 0.936 |
| Dyspnea on exertion | 538 (70%) | 2561 (78%) | <0.0001 | 464 (71%) | 469 (72%) | 0.806 |
| Jugular venous distension | 59 (8%) | 502 (15%) | <0.0001 | 55 (8%) | 65 (10%) | 0.389 |
| Third heart sound | 124 (16%) | 802 (25%) | <0.0001 | 116 (18%) | 111 (17%) | 0.770 |
| Pulmonary râles | 85 (11%) | 727 (22%) | <0.0001 | 80 (12%) | 80 (12%) | 1.000 |
| Lower extremity edema | 103 (14%) | 790 (24%) | <0.0001 | 89 (14%) | 86 (13%) | 0.871 |
| NYHA functional class | ||||||
| I | 155 (20%) | 361 (11%) | 119 (18%) | 121 (19%) | ||
| II | 458 (60%) | 1693 (52%) | <0.0001 | 89 (59%) | 386 (59%) | 0.589 |
| III | 145 (19%) | 1131 (35%) | 136 (21%) | 131 (20%) | ||
| IV | 7 (1%) | 86 (3%) | 7 (1%) | 13 (2%) | ||
| Heart rate, rate per minute | 75 (±12) | 78 (±12) | <0.0001 | 75 (±12) | 75 (±12) | 0.749 |
| Blood pressure, mm Hg | ||||||
| Systolic | 132 (±21) | 129 (±21) | <0.0001 | 131 (±21) | 132 (±21) | 0.679 |
| Diastolic | 75 (±11) | 74 (±11) | 0.004 | 75 (±11) | 75 (±11) | 0.645 |
| Chest radiograph findings | ||||||
| Pulmonary congestion | 51 (7%) | 566 (17%) | <0.0001 | 50 (8%) | 46 (7%) | 0.751 |
| CT ratio > 0.5 | 371 (49%) | 2165 (66%) | <0.0001 | 332 (51%) | 330 (51%) | 0.956 |
| Serum concentrations | ||||||
| Creatinine, mg/dL | 1.26 (±0.33) | 1.38 (±0.41) | <0.0001 | 1.28 (±0.34) | 1.28 (±0.35) | 0.538 |
| Potassium, mEq/L | 4.41 (±0.4) | 4.35 (±0.4) | <0.0001 | 4.41 (±0.4) | 4.42 (±0.4) | 0.780 |
| Estimated glomerular filtration rate, ml/min per 1.73 m2 | 62 (±18) | 58 (±22) | <0.0001 | 61 (±17) | 61 (±18) | 0.580 |
| Ejection fraction, % | 36 (±13) | 33 (±13) | <0.0001 | 35 (±13) | 35 (±13) | 0.593 |
Chronic kidney disease was defined by estimated glomerular filtration rate <60 ml/min/1.73 square meter calculated using Modification of Diet in Renal Disease formula
Figure 2.
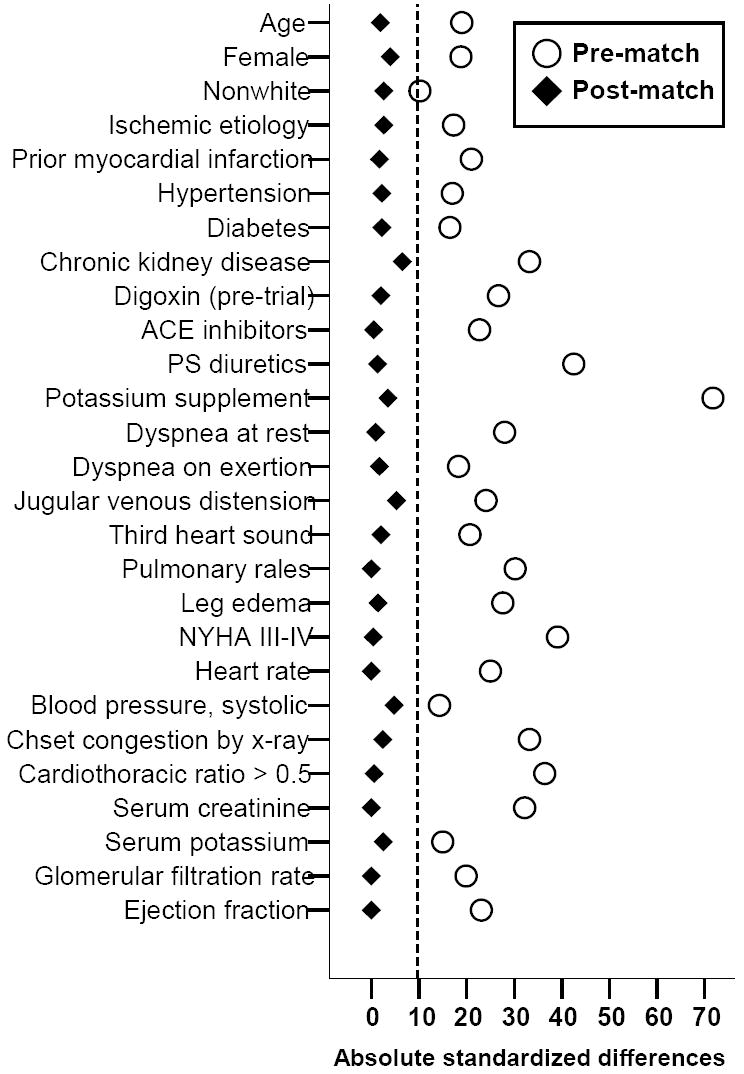
Absolute standardized differences in covariates between patients receiving and not receiving diuretic, before and after propensity score matching
2.4. Propensity scores matching
In a randomized clinical trial of diuretics, every patients receiving and not receiving diuretics would have the same 50% propensity to receive diuretics. However, in an observational study, patients are not randomly assigned to receive diuretics, and as such, the propensity to receive diuretics may vary from 0% to 100%. Generally, patients receiving and not receiving diuretics respectively would have high and low propensities (Figure 1a). As expected, patients selected for diuretic therapy were generally older and sicker with higher comorbidity burden (Table 1 and Figure 2). The goal of the propensity score matching is to find pairs of patients receiving and not receiving diuretics, who share similar propensities (Figure 1b). Using a SPSS macro, of the 765 patients not receiving diuretics, 651 (85%) were matched with 651 patients who were receiving diuretics and had similar propensity scores [1, 12].
Figure 1.

Distribution of propensity score for the receipt of diuretic (a) before, and (b) after matching, and among (c) unmatched patients
2.5. Assessment of residual bias: absolute standardized differences
After matching, we estimated covariate balance between patients receiving and not receiving diuretics using absolute standardized differences [11, 13, 14], which directly quantifies the bias in the means (or proportions) of covariates across the groups, expressed as a percentage of the pooled standard deviations. After matching, the mean propensity scores for patients receiving and not receiving diuretics wee respectively 0.66165 and 0.66201 (t-test p =0.993), which corresponded to an absolute standardized difference of 0.2%. This is in contrast to pre-match mean propensity scores of 0.85700 and 0.61145 for those receiving and not receiving diuretics (t test p <0.0001), with a corresponding absolute standardized difference of 128%.
2.6. Statistical analysis
We used Kaplan-Meier plots and matched Cox regression analysis to estimate associations of diuretic use with various outcomes [1]. To determine the effect of diuretics in the pre-match cohort, we used multivariable Cox regression analyses, adjusting for raw propensity scores. We confirmed the assumption of proportional hazards by a visual examination of the log (minus log) curves.
2.7. Sensitivity analyses
Even though our post-match cohort achieved excellent balance in all measured covariates between the two groups, we do not know if there was bias due to imbalances in unmeasured covariates. Therefore, we conducted a formal sensitivity analysis to quantify the degree of a hidden bias that would need to be present to invalidate our main conclusions [15, 16]. All statistical tests were evaluated using two-tailed 95% confidence levels, and data analyses were performed using SPSS for Windows version 14 [17].
3. Results
3.1. Patient characteristics
The post-match cohort had a mean (±SD) age of 72 (±5) years, (median 71; range 65-92), 299 (23%) were female and 127 (10%) were non-white. Balance in all measured baseline characteristics for patients receiving and not receiving diuretics, before and after propensity score matching, are displayed in Table 1 and Figure 2. Before matching, diuretic patients were older with higher symptom and comorbidity burden. After matching, all baseline covariates were balanced between the two groups and all absolute standardized differences were below 10% and most were below 5%, suggesting acceptable covariate balance and bias reduction (Table 1 and Figure 2) [1, 11, 13].
3.2. Diuretics and mortality
Mortality due to all causes occurred in 173 no-diuretic patients during 2056 person-years (rate, 841 deaths per 10000 person-years) and 208 diuretic patients during 1943 person-years (rate, 1070 deaths per 10000 person-years), with a corresponding hazard ratio (HR) of 1.38 (95% confidence interval {CI}=1.08–1.71; p=0.009; Table 2). Kaplan Meier plots for mortality for patients receiving and not receiving diuretics are displayed in Figure 3a. Mortality due to cardiovascular causes occurred in 123 (rate, 598 per 10000 person-years) no-diuretic patients and 159 (rate, 818 per 10000 person-years) diuretic patients (HR=1.50, 95% CI=1.15–1.96; p=0.003; Table 2). Rates and relative risks for other cause-specific mortality are displayed in Table 2.
Table 2.
Mortality in propensity score matched geriatric heart failure patients
| No diuretic (N=651) |
Diuretic (N=651) |
Absolute difference* (per 10000 person-year of follow up) |
Matched Hazard ratio (95% confidence interval)† |
||
|---|---|---|---|---|---|
| Cause specific mortality | Death rates per 10000 person-years of follow up (events / total years of follow up) |
P value | |||
| All-cause | 841 (173 / 2056) |
1071 (208 / 1943) |
+ 230 | 1.36 (1.08–1.71) | 0.009 |
| Cardiovascular | 598 (123 / 2056) |
818 (159 / 1943) |
+ 220 | 1.50 (1.15–1.96) | 0.003 |
| Worsening heart failure‡ | 252 (52 / 2056) |
335 (65 / 1943) |
+ 81 | 1.51 (0.99–2.32) | 0.057 |
| Other cardiovascular§¶ | 311 (71 / 2056) |
407 (94 / 1943) |
+ 95 | 1.40 (0.97–2.04) | 0.075 |
| Non-cardiovascular | 204 (42 / 2056) |
201 (39 / 1943) |
− 4 | 0.94 (0.57–1.54) | 0.800 |
| Unknown | 39 (8 / 2056) |
51 (10 / 1943) |
+ 13 | 1.60 (0.52–4.89) | 0.410 |
Absolute differences in rates of mortality per 10000 person-year of follow up were calculated by subtracting the death rates in the placebo group from the death rates in the digoxin group (before values were rounded)
Hazard ratios and confidence intervals (CI) were estimated from the matched Cox proportional-hazards models
This category includes patients who died from worsening heart failure, even if the final event was an arrhythmia
Cardiac causes under this category include deaths presumed to result from arrhythmia without evidence of worsening heart failure and deaths due to atherosclerotic coronary disease, bradyarrhythmias, low-output states, and cardiac surgery
Vascular causes under this category include deaths due to stroke, embolism, peripheral vascular disease, vascular surgery, and carotid endarterectomy
Figure 3.
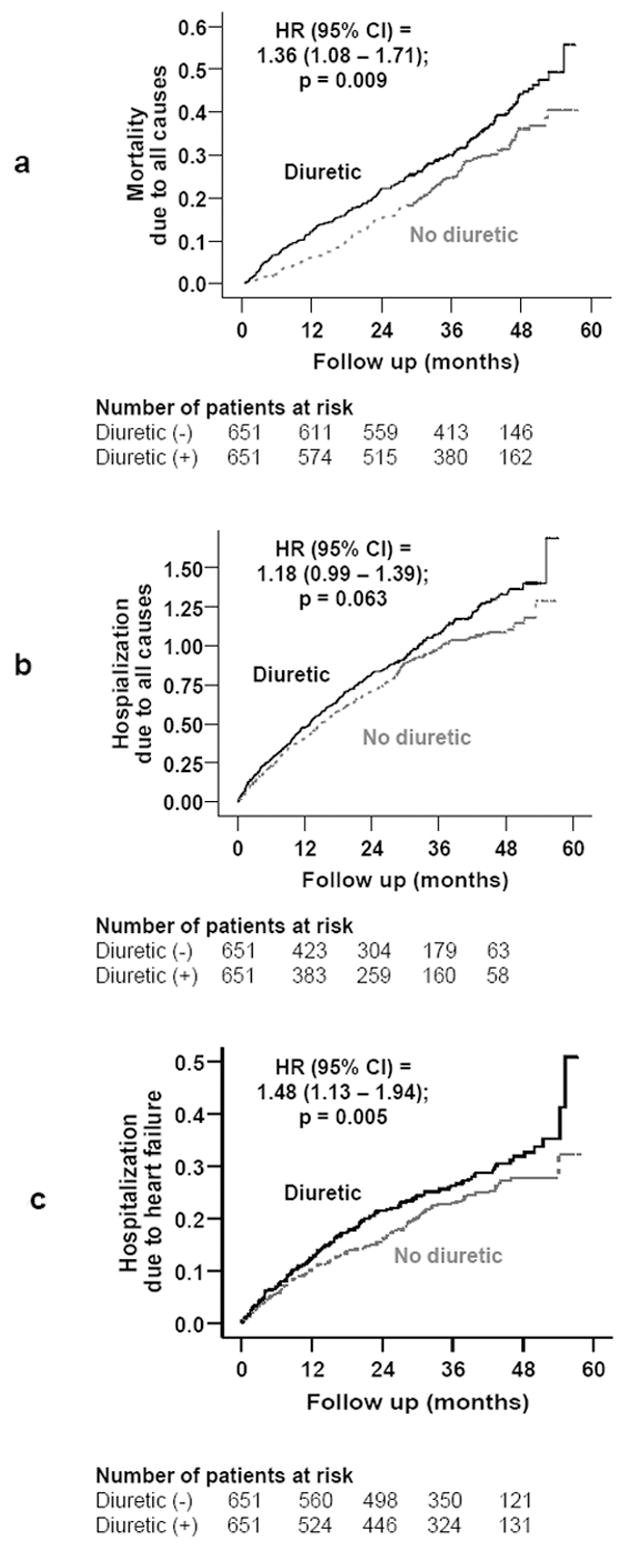
Kaplan-Meier plots for (a) all-cause mortality, (b) all-cause hospitalizations, and (c) heart failure hospitalization
3.3. Diuretics and hospitalization
Kaplan Meier plots for hospitalizations due to all causes and worsening heart failure by diuretic use are displayed in Figures 3b and 3c. Hospitalizations due to all causes occurred in 413 no-diuretic patients during 1255 person-years (rate, 3291/10000 person-years) and 438 diuretic patients during 1144 person-years (rate, 3829/10000 person-years), with a corresponding 18% non-significant increased risk (HR=1.18, 95% CI=0.99–1.39; p=0.063; Table 3). Hospitalizations due to worsening heart failure occurred in 137 patients not receiving diuretics during 1841person-years (rate, 744/10000 person-years) and 154 patients receiving diuretics during 1727 person-years (rate, 892/10000 person-years), which corresponded to a 48% significant increased risk (HR=1.48, 95% CI=1.13–1.94; p=0.005; Table 3).
Table 3.
Hospitalizations in propensity score matched geriatric heart failure patients
| Cause for hospitalization* | No diuretic (N=651) |
Diuretic (N=651) |
Absolute difference* (per 10000 person-year of follow up) |
Matched Hazard ratio (95% confidence interval)† |
P value |
|---|---|---|---|---|---|
| Hospital admission rates, per 10000 person-year of follow up (events / total years of follow up) | |||||
| All-cause | 3291 (413 / 1255) |
3829 (438 / 1144) |
+ 538 | 1.18 (0.99–1.39) | 0.063 |
| Cardiovascular | 2049 (308 / 1503) |
2155 (306 / 1420) |
+ 106 | 1.16 (0.96–1.40) | 0.124 |
| Worsening heart | 744 (137 / 1841) |
892 (154 / 1727) |
+ 147 | 1.48 (1.13–1.94) | 0.005 |
| Ventricular arrhythmia, | 118 (24 / 2027) |
110 (21 / 1912) |
− 8 | 0.82 (0.44–1.53) | 0.528 |
| SV arrhythmias§ | 175 (35 / 2000) |
136 (26 / 1914) |
− 39 | 0.88 (0.49–1.57) | 0.655 |
| AV block, bradyarrhythmia | 10 (2 / 2050) |
26 (5 / 1936) |
+ 16 | 2.00 (0.37–10.92) | 0.327 |
| Suspected digoxin toxicity | 15 (413 / 2049) |
36 (413 / 1935) |
+ 21 | 2.00 (0.50–8.00) | 0.423 |
| Myocardial infarction | 230 (46 / 2004) |
210 (40 / 1906) |
− 20 | 1.00 (0.63–1.59) | 1.00 |
| Unstable angina | 470 (89 / 1893) |
406 (73 / 1799) |
− 64 | 0.96 (0.67–1.34) | 0.798 |
| Stroke | 160 (32 / 2006) |
178 (34 / 1906) |
+ 18 | 1.17 (0.67–2.05) | 0.572 |
| Coronary revascularization¶ | 94 (19 / 2017) |
84 (16 / 1905) |
− 10 | 0.77 (0.37–1.57) | 0.467 |
| Cardiac transplantation | 0 | 0 | – | – | – |
| Other cardiovascular** | 332 (64 / 1928) |
434 (78 / 1794) |
+ 102 | 1.48 (1.03–2.13) | 0.036 |
| Respiratory infection | 244 (48 / 1970) |
246 (46 / 1871) |
+ 2 | 1.06 (0.67–1.68) | 0.814 |
| Other non-cardiovascular | 1146 (221 / 1928) |
1315 (236 / 1794) |
+ 169 | 1.21 (0.98–1.50) | 0.081 |
| Unspecified | 10 (2 / 2048) |
15 (3 / 1936) |
+ 5 | 1.50 (0.25–8.98) | 0.657 |
| Number of hospitalizations | 8837 (1109 / 1255) |
10192 (1166 / 1144) |
+ 1356 | ||
Data shown include the first hospitalization of each patient due to each cause.
Absolute differences were calculated by subtracting the percentage of patients hospitalized in the placebo group from the percentage of patients hospitalized in the digoxin group (before values were rounded).
Hazard ratios and confidence intervals (CI) were estimated from a matched Cox proportional-hazards models that used the first hospitalization of each patient for each reason.
Supraventricular (SV) arrhythmias include Atrioventricular (AV) block and bradyarrhythmias
This category includes coronary-artery bypass grafting and percutaneous transluminal coronary angioplasty
This category includes embolism, venous thrombosis, peripheral vascular disease, hypertension, other vascular surgery, cardiac catheterization, other types of catheterization, pacemaker implantation, installation of automatic implantable cardiac defibrillator, electrophysiologic testing, transplant-related evaluation, nonspecific chest pain, atherosclerotic heart disease, hypotension, orthostatic hypotension, and valve operation
3.4. Sensitivity analysis
In the absence of hidden bias, a sign-score test for matched data with censoring provides modest evidence (p = .0351) that treatment with diuretics decreases survival time. However, results form our sensitivity analysis suggest that our results would be sensitive to a hidden bias. To attribute the lower survival time to an unobserved binary covariate rather than to the effect of the exposure to diuretics, that unobserved covariate would need to increase the odds of a patient receiving diuretics by only about 2.1%, and would also need to be unrelated to our propensity model and be an excellent predictor of mortality. Our sensitivity analysis of heart failure hospitalization yielded similar results.
4. Discussion
4.1. Key study findings
The results of the current analysis demonstrate that chronic diuretic therapy was associated with increased mortality and hospitalizations in a wide spectrum of older adults with heart failure. These findings are important as the vast majority of heart failure patients are older adults and with the increase of the mean age of the population worldwide, the number of geriatric heart failure patients is likely to increase in the coming decades.
4.2. Mechanistic insights to study findings
Activation of the renin-angiotensin-aldosterone system and the sympathetic nervous system is associated with poor outcomes in heart failure. Drugs that reduce mortality and morbidity in heart failure do so by suppressing these hormones [18]. Poor outcomes associated with diuretic therapy observed in our analysis may in part be explained by the activation of neurohormones by diuretics [19-22]. In particular, diuretic associated activation of aldosterone is associated with stimulation of pro-inflammatory cytokines, myocardial fibrosis, disease progression and poor outcomes in heart failure [23-26]. Diuretics also cause electrolyte imbalance, hypotension and worsening kidney function, which might in part explain our findings [27-30]. Even though these electrolytes, blood pressure and renal function were comparable at baseline, diuretics might have affected them during follow up.
4.3. Comparison with other studies in the literature
To the best of our knowledge, this is the first propensity-matched study of the effect of chronic diuretic therapy in an elderly cohort of heart failure patients. These data provide evidence that is stronger than Level C evidence (which is based on consensus opinions and case studies and not on study findings). However, these results need to replicated in other patient populations, particularly in contemporary heart failure patients, before this could be considered as Level B evidence (defined as data derived from a single randomized clinical trial or multiple non-randomized studies) [18].
4.4. Clinical implications
According to the American College of Cardiology and the American Heart Association 2005 chronic heart failure guideline, “diuretics are the only drugs used for the treatment of heart failure that can adequately control the fluid retention of heart failure”, yet there are “no long-term studies of diuretic therapy in heart failure, and thus, their effects on morbidity and mortality are not known” [18]. Use of diuretics for control of fluid retention is a Class I recommendation (conditions for which there is evidence and/or general agreement that a given procedure or treatment is beneficial, useful, and effective) for symptomatic heart failure [18]. Yet, this is the only Class I recommendation that is based on Level C evidence (only consensus opinion of experts, case studies, or standard-of-care) [18]. There is a growing body of evidence form laboratory animals [24, 25] and observational studies in human heart failure [19, 22, 31] that suggest potential harmful effects of diuretics. While the results of this study does not dispute the effectiveness of diuretics in controlling and treating fluid overload, they do force the question of diuretic use in heart failure patients who are asymptomatic or mildly symptomatic (New York Heart Association class I or II). About 80% of the patients in our analysis belonged to these two classes.
For symptomatic chronic heart failure patients (New York Heart Association Class III-IV) with fluid overload, diuretics are essential to achieve euvolemia. For such patients there are currently no alternative pharmacotherapeutic options but to use diuretics. With the growing evidence of the potential harmful effects of diuretics, clinicians may encourage stricter salt restriction and thus reduce the need for diuretic use. Diuretics are known to increase salt appetite [32] and diuretics may be more harmful in a salt-rich environment [33, 34]. Another alternative is to consider adding low-dose digoxin, instead of increasing the dose of diuretics, to relieve heart failure symptoms [35]. Clinicians also might consider using torsemide instead of furosemide, as the former is less likely to cause neurohormonal activation and may even reverse neurohormone-induced myocardial fibrosis and reduce mortality [24, 36-39]. However, torsemide is expensive and the long-term effects of torsemide in heart failure are largely unknown.
Because aldosterone is one of the key neurohormones activated by diuretics, one might speculate whether aldosterone antagonists might reduce the deleterious effects of diuretics. In the RALES trial, in which all patients were receiving loop diuretics, therapy with spironolactone, an aldosterone antagonist, was associated with reduced mortality [39]. However, >99% of patients in that trial were in New York Heart Association Class III-IV and effectiveness of aldosterone in mildly symptomatic heart failure is currently being studied [40]. Data from recent reports that ultrafiltration may be safe and more effective that diuretics in controlling fluid overload raise the provocative question of whether or not patients should be allowed to temporarily become volume overloaded off of diuretics, and then be treated with intermittent ultrafiltration [41, 42].
4.5. Study limitations
Potential limitations of propensity score matching are bias due to unmeasured or hidden covariates, and incomplete and/or inexact matching. The results of our study were sensitive to a potential hidden covariate [15, 16]. However, sensitivity analysis cannot determine if such a bias existed. For a hidden variable to explain away our findings, it would need to increase the odds of a patient receiving diuretics by only about 2.1%. However, it is difficult to contemplate a binary covariate that would increase the odds of diuretic use and yet be completely unrelated to age, dyspnea, pulmonary edema, elevated jugular venous pressure or higher New York Heart Association class, and be an excellent predictor of mortality. Physician preference by specialty is unlikely to be an issue as all DIG investigators were cardiologists. Our matching was also of high quality (Figure 1b). We were able to match 85% of patients not receiving diuretics, in contrast to about 60% adequate matching in other studies [11, 43]. Patients excluded were at the extremes of the propensity score range (Figure 1c) and inclusion of those patients would have introduced bias into our study. Thus, while exclusion of patients might limit generalizability, it has improved internal validity of our findings. DIG participants were predominantly white and male, with normal sinus rhythm and from the pre-beta-blocker era.
4.6. Future directions
Randomized clinical trials are needed to determine definitively the long-term effects of chronic diuretic therapy in mild to moderate heart failure. However, randomized clinical trials may be unethical or impractical in advanced and symptomatic heart failure patients. The issue of the long-term effect of chronic diuretic therapy in these patients may need to be resolved in a well-designed prospective follow-up study using propensity score methods.
4.7. Conclusions
Chronic diuretic therapy was associated with increased mortality and hospitalization in a wide spectrum of ambulatory older adults with chronic mild to moderate systolic and diastolic heart failure. These results based on non-randomized studies should be interpreted with caution. In the interim, these data serve as a reminder of the potential harmful effects of diuretics and the need for future prospective studies.
Acknowledgments
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
Funding/Support Dr. Ahmed is supported by the National Institutes of Health through grants from the National Institute on Aging (1-K23-AG19211-04) and the National Heart, Lung, and Blood Institute (1-R01-HL085561-01 and P50-HL077100).
Footnotes
Conflict of Interest Disclosures: None
Author Contributions Dr. Ahmed conceived the study hypothesis, designed the study, and wrote the first draft of the manuscript. Dr. Ahmed conducted the statistical analyses in consultation with Dr. Love. All authors interpreted the data, participated in critical revision of the paper for important intellectual content, and approved the final version of the article. Dr. Ahmed had full access to the data.
References
- 1.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study, using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 3.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–33. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 4.The Digitalis Investigation Group. Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 5.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–30. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 7.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Asso. 1984;79:516–524. [Google Scholar]
- 8.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–63. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 9.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 10.Rubin DB. On principles for modeling propensity scores in medical research. Pharmacoepidemiol Drug Saf. 2004;13:855–7. doi: 10.1002/pds.968. [DOI] [PubMed] [Google Scholar]
- 11.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–98. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 12.Macro Levesque R. In: SPSS® Programming and Data Management, 2nd Edition. A Guide for SPSS® and SAS® Users. 2. Levesque R, editor. Chicago IL: SPSS Inc; [June 4, 2005]. 2005. Available online at: http://www.spss.com/spss/data_management_book.htm. [Google Scholar]
- 13.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Love TE. Using Propensity Scores: Stratification, Adjustment, Sensitivity and Strategies. [June 4, 2005]; Available at http://www.chrp.org/propensity/
- 15.Rosenbaum PR. Sensitivity to Hidden Bias. In: Rosenbaum PR, editor. Observational Studies. 2. New York: Springer-Verlag; 2002. pp. 110–124. [Google Scholar]
- 16.Rosenbaum PR. Sensitivity analysis for matching with multiple controls. Biometrika. 1988;75:577–81. [Google Scholar]
- 17.SPSS for Windows, Rel. 14. program. SPSS Inc.; Chicago, IL: 2006. [Google Scholar]
- 18.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). Developed in Collaboration With the American College of Chest Physicians and the International Society for Heart and Lung Transplantation. Endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. Published online before print September 13, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Ann Intern Med. 1985;103:1–6. doi: 10.7326/0003-4819-103-1-1. [DOI] [PubMed] [Google Scholar]
- 20.Knight RK, Miall PA, Hawkins LA, Dacombe J, Edwards CR, Hamer J. Relation of plasma aldosterone concentration to diuretic treatment in patients with severe heart disease. Br Heart J. 1979;42:316–25. doi: 10.1136/hrt.42.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayliss J, Norell M, Canepa-Anson R, Sutton G, Poole-Wilson P. Untreated heart failure: clinical and neuroendocrine effects of introducing diuretics. Br Heart J. 1987;57:17–22. doi: 10.1136/hrt.57.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis GS, Benedict C, Johnstone DE, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD) Circulation. 1990;82:1724–9. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 23.Brilla CG, Rupp H, Funck R, Maisch B. The renin-angiotensin-aldosterone system and myocardial collagen matrix remodelling in congestive heart failure. Eur Heart J. 1995;16 Suppl O:107–9. doi: 10.1093/eurheartj/16.suppl_o.107. [DOI] [PubMed] [Google Scholar]
- 24.Lopez B, Querejeta R, Gonzalez A, Sanchez E, Larman M, Diez J. Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J Am Coll Cardiol. 2004;43:2028–35. doi: 10.1016/j.jacc.2003.12.052. [DOI] [PubMed] [Google Scholar]
- 25.McCurley JM, Hanlon SU, Wei SK, Wedam EF, Michalski M, Haigney MC. Furosemide and the progression of left ventricular dysfunction in experimental heart failure. J Am Coll Cardiol. 2004;44:1301–7. doi: 10.1016/j.jacc.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 26.Weber KT. Furosemide in the long-term management of heart failure: the good, the bad, and the uncertain. J Am Coll Cardiol. 2004;44:1308–10. doi: 10.1016/j.jacc.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed A, Rich MW, Sanders PW, et al. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–8. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed A, Zannad F, Love TE, Tallaj J, Gheorghiade M, Pitt B. A propensity matched study of the association of low serum potassium levels and mortality in chronic heart failure. European Heart Journal. 2007;28(11):1334–43. doi: 10.1093/eurheartj/ehm091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gheorghiade M, Abraham WT, Albert NM, et al. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007;28(8):980–88. doi: 10.1093/eurheartj/ehl542. [DOI] [PubMed] [Google Scholar]
- 30.Gheorghiade M, Abraham WT, Albert NM, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. Jama. 2006;296:2217–26. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 31.Domanski M, Norman J, Pitt B, Haigney M, Hanlon S, Peyster E. Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 2003;42:705–8. doi: 10.1016/s0735-1097(03)00765-4. [DOI] [PubMed] [Google Scholar]
- 32.Rowland NE, Morian KR. Roles of aldosterone and angiotensin in maturation of sodium appetite in furosemide-treated rats. Am J Physiol. 1999;276:R1453–60. doi: 10.1152/ajpregu.1999.276.5.R1453. [DOI] [PubMed] [Google Scholar]
- 33.Rocha R, Funder JW. The pathophysiology of aldosterone in the cardiovascular system. Ann N Y Acad Sci. 2002;970:89–100. doi: 10.1111/j.1749-6632.2002.tb04415.x. [DOI] [PubMed] [Google Scholar]
- 34.Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689–97. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27:178–86. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muniz P, Fortuno A, Zalba G, Fortuno MA, Diez J. Effects of loop diuretics on angiotensin II-stimulated vascular smooth muscle cell growth. Nephrol Dial Transplant. 2001;16 Suppl 1:14–7. doi: 10.1093/ndt/16.suppl_1.14. [DOI] [PubMed] [Google Scholar]
- 37.Cosin J, Diez J. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail. 2002;4:507–13. doi: 10.1016/s1388-9842(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 38.Murray MD, Deer MM, Ferguson JA, et al. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med. 2001;111:513–20. doi: 10.1016/s0002-9343(01)00903-2. [DOI] [PubMed] [Google Scholar]
- 39.Muller K, Gamba G, Jaquet F, Hess B. Torasemide vs. furosemide in primary care patients with chronic heart failure NYHA II to IV--efficacy and quality of life. Eur J Heart Fail. 2003;5:793–801. doi: 10.1016/s1388-9842(03)00150-8. [DOI] [PubMed] [Google Scholar]
- 40.ClinicalTrials.Gov. National Institutes of Health. A Comparison Of The Endpoints Of Death From A Cardiovascular Cause and Hospitalization For Worsening Heart Failure, In Patients Who Have NYHA Class II Heart Failure When They Are Treated With Eplerenone Or Placebo In Addition To Standard Heart Failure Medicines. [July 14, 2007]; http://clinicaltrials.gov/ct/show/NCT00232180?order=4.
- 41.Costanzo MR, Saltzberg M, O’Sullivan J, Sobotka P. Early ultrafiltration in patients with decompensated heart failure and diuretic resistance. J Am Coll Cardiol. 2005;46:2047–51. doi: 10.1016/j.jacc.2005.05.099. [DOI] [PubMed] [Google Scholar]
- 42.Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am CollCardiol. 2007;49:675–83. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 43.Gum PA, Thamilarasan M, Watanabe J, Blackstone EH, Lauer MS. Aspirin use and all-cause mortality among patients being evaluated for known or suspected coronary artery disease: A propensity analysis. JAMA. 2001;286:1187–94. doi: 10.1001/jama.286.10.1187. [DOI] [PubMed] [Google Scholar]


