Abstract
Background
The association between serum digoxin concentration (SDC) and outcomes in women with heart failure (HF) has not been well studied.
Aims
To test the hypothesis that the effect of digoxin on outcomes in women with HF is bi-directional and dependent on SDC, as in men, and is modified by ejection fraction (EF).
Methods
We studied 1,366 female participants of the Digitalis Investigation Group trial in whom data on SDC (ng/ml) were available. We calculated adjusted odds ratios (AOR) and Bonferroni-adjusted 97.5% confidence intervals (CI) for various outcomes at a median follow up of 41 months, in all women and stratified by EF 35%.
Results
Compared with placebo (26.9%), 40.3% with SDC ≥1.2 (AOR=1.80; CI=1.14- 2.86; p=0.004) and 26.6% with SDC 0.5-1.1 (AOR=1.05; CI=0.73-1.51; p=0.762) died. Respective rates for HF-hospitalizations were: placebo (32.8%), SDC ≥1.2 (38.0%) and SDC 0.5-1.1 (25.5%). For women with EF<35% (N=677), SDC 0.5-1.1 lowered odds for HF-hospitalizations (AOR=0.63; CI=0.39-1.00; p=0.026) without increasing odds for death (AOR=0.77; CI=0.47-1.26; p=0.233). In women with EF≥35% (N=689), SDC 0.5-1.1 had a borderline association with death (AOR=1.58; CI=0.92-2.72; p=0.058) but not with HF-hospitalization (AOR=0.95; CI= 0.54-1.66; p=0.826).
Conclusions
As in men, in women with HF, digoxin has a bi-directional effect based on SDC, and the beneficial effects were significant only among women with EF <35%
Keywords: serum digoxin concentration, outcomes, women, heart failure, ejection fraction
1. Introduction
A post-hoc analysis of the Digitalis Investigation Group (DIG) trial demonstrated that digoxin use was associated with a significant increased risk of death in women with heart failure (HF) and left ventricular ejection fraction (EF) <=45% [1]. These findings have prompted concerns among clinicians about the use of digoxin in women with HF [2-4]. Subsequently, another post-hoc analysis of the DIG data set demonstrated that the effects of digoxin on outcomes in men with HF and EF <=45% is bi-directional, with poor outcomes at higher serum digoxin concentration (SDC) and better outcomes at lower SDC [5].
However, critical gaps remain in our knowledge about the effects of digoxin on mortality in women.[6-9] First, the effect of various SDC on outcomes in women with HF has not been well studied [10]. This is especially important as in the DIG trial, serum creatinine, which is not a good marker of chronic kidney disease in women [11-13], was used to calculate the recommended dose of digoxin. Secondly, there are no data on the effect of digoxin on outcomes in women with relatively preserved systolic function. In the main DIG trial, the beneficial effects of digoxin was significantly more pronounced in patients with severely impaired systolic function (EF<25%) [12]. In addition, the effect of digoxin on women with relatively preserved systolic function is important as most women with HF are elderly and many have relatively preserved systolic function [14-17]. Therefore, the objective of the current analysis was to test the hypotheses that 1) as in men, in women with HF, high SDC is associated with increased risk and low SDC is associated with decreased risk of death, hospitalization, or the combined endpoint of either, and 2) that the effects of digoxin are modified by EF.
2. Methods
2.1. Study design
The DIG trial was a randomized controlled trial that enrolled 7,788 ambulatory patients with chronic HF from 302 clinical centers in the United States (186 centers) and Canada (116 centers) [11, 12]. Participants were recruited during a 31.5-month period between January 1991 and August 1993. The trial involved 6,800 patients with EF < 45% and 988 with EF ≥45%. Patients were randomized to receive digoxin versus placebo. All patients were encouraged to receive ACE inhibitors and most patients were receiving diuretics. Beta-blockers were not approved for use in HF and as such data on beta-blocker use were not collected in DIG trial. We obtained the public use version of the DIG dataset from the National Heart, Lung and Blood Institute (NHLBI) by signing a standard data use agreement protocol.
2.2. Patients
For the present study, we restricted our analysis to 1,926 women enrolled in the DIG trial in whom data on SDC were available. The study cohort was selected based on criteria similar to those used by Rathore et al. for men enrolled in the DIG trial.[5] Of the 1,926 female participants of the DIG trial, 962 women were receiving digoxin, and of these, 411 had data on SDC based on specimens collected at least 6 hours after the previous digoxin dose and 1 month after randomization. Among the 964 women receiving placebo, 955 were alive at 1 month. Merged together (411 and 955), these 1,366 women formed the study cohort for the current analysis.
2.3. Outcomes
The primary end point of the DIG study was all-cause mortality, which is also the primary outcome of interest for the current analysis. Median follow-up for the subjects in our analysis was 40.7 months (range 1.2 to 58.2 months). Vital status of all patients was collected up to December 31, 1995. Overall, vital status data were collected on 98.9% of participants [18]. Other outcomes of interest for this analysis were hospitalization due to worsening HF and the combined endpoint of all-cause mortality or HF-hospitalization.
2.4. Statistical methodology
We categorized women into those receiving placebo, those with SDC 0.5-1.1 ng/ml, and those with SDC ≥1.2 ng/ml. Because we did not observe any significant difference in outcomes between women with SDC 0.5-0.8 and SDC 0.9-1.1 ng/ml, we combined these two categories to reduce the number of comparisons and to increase the subgroup size (as the number of events among women was low). We compared baseline characteristics among the three groups and used Pearson Chi-square tests and one-way analysis of variance tests as appropriate to test for statistical significance. Values of continuous and categorical variables are respectively expressed as mean (±standard deviation) and number (percentage).
We then compared bivariate survival curves for all-cause mortality for women with higher and lower SDC versus placebo using Kaplan-Meier estimates and tested statistical significance using the log-rank test. Statistically significant overall tests were followed up by post-hoc pair-wise comparisons between placebo and SDC 0.5-1.1 ng/ml, and between placebo and SDC ≥1.2 ng/ml. Because the assumption of proportional hazard was not satisfied, we used logistic regression analyses to determine the association between digoxin use and various outcomes. In the multivariable logistic regression model, SDC 0.5-1.1 ng/ml and ≥1.2 ng/ml were entered as independent predictor variables using placebo as the reference category. Other covariates were entered in a forward stepwise fashion. Covariates used in the multivariable model included age, race, body mass index, HF duration in months, etiology of HF (ischemic, hypertensive, idiopathic, and other), comorbidities (myocardial infarction, current angina, hypertension, and diabetes), medications (ACE inhibitors, non-potassium sparing diuretics, and combined therapy with nitrates and hydralazine), NYHA functional class, current symptoms (dyspnea at rest and dyspnea on exertion) and signs (jugular venous distension, third heart sound, pulmonary râles, and lower extremity edema) of HF, estimated glomerular filtration rate, pulmonary congestion and cardiothoracic ratio > 0.5 by chest x-ray, and left ventricular EF. We estimated glomerular filtration rate (as ml /min/ 1.73 square meter) according to the Dietary Modification of Renal Disease formula [13].
In the main DIG trial, while digoxin was effective in reducing the combined outcome events in all patients, the magnitude of the effect appeared stronger for patients with severely impaired systolic function (EF <25%) [12]. To study the modification of the effects of SDC on HF outcomes by EF, we categorized women with HF into two subgroups based on the median EF of 35%. An EF cutoff of 35% has been commonly used in major HF trials to define systolic dysfunction [19-22], and is also prognostically important [23]. We then performed logistic regression analysis with EF, SDC and their interaction as terms in the model and found no significant interaction (p = 0.228 for mortality, p=0.802 for HF-hospitalization, and p=0.4041 for combined endpoint of mortality and HF-hospitalization). A possible explanation for this lack of significant interaction is the lack of sufficient power. However, because of a possible biological rationale for a heterogeneity of effect of based on EF [24], we fitted logistic models separately for each EF subgroup and for each outcome event [25-28]. All statistical tests were done using an intention to treat approach, and a two-tailed alpha of 0.05 was required to reject the null hypothesis. We used Bonferroni adjustment for the two pair-wise comparisons to correct for inflation and used a p value of 0.025 (0.05/2) to indicate significance for individual comparisons. Similarly, to achieve simultaneous 95% confidence intervals, we constructed 97.5% confidence intervals for each individual OR. Analyses were performed using SPSS software for Windows (Release 13) [29].
RESULTS
Study Population
Patients (N=1,366) had a median age of 67 years, 17.7% were non-white, and 50.4% had left ventricular EF of 35% or greater. Nine hundred and fifty five (69.9%) were receiving placebo and 411 (30.1%) patients receiving digoxin had SDC data available. Among women receiving digoxin, the median dose of digoxin received at the time when blood for SDC was drawn was 0.25 mg per day (range: 0.125 to 0.50 mg per day): 117 (28.5%) women received 0.125 mg of digoxin per day, 271 (65.9%) received 0.25 mg, 18 (4.4%) received 0.375 mg and 5 (1.2%) received 0.5 mg per day. Among women receiving digoxin, 282 (68.6%) had SDC 0.5-1.1 ng/ml and 129 (31.4%) had SDC ≥1.2 ng/ml. Compared with women receiving 0.125 mg of digoxin per day, those receiving 0.25 mg per day were more likely to have SDC ≥1.2 ng/ml (35.4% versus 21.4%; unadjusted OR =2.01; 97.5% confidence interval {CI} = 1.22 – 3.33; p=0.006). Table 1 displays the baseline characteristics of the women with higher versus lower SDC and those receiving placebo. Women with SDC ≥1.2 ng/ml were more likely to have diabetes, higher serum creatinine, chronic kidney disease, pulmonary râles, radiological evidence of pulmonary congestion, and cardiothoracic ratio >0.5.
Table 1.
Baseline Characteristics in Women with Heart Failure
| Placebo | SDC 0.5-1.1 ng/ml | SDC ≥ 1.2 ng/ml | P | |
|---|---|---|---|---|
| N = 955 | N = 282 | N=129 | ||
| Age (years), mean (±SD) | 65.4 (±11.6) | 64.4 (±11.7) | 66.0 (±11.1) | 0.311 |
| Non-white | 177 (18.5%) | 45 (16.0%) | 20 (15.5%) | 0.473 |
| Body mass index (kilogram / square meter), mean (±SD) | 27.5 (±6.7) | 26.7 (±6.7) | 28.0 (±6.9) | 0.128 |
| Primary cause of heart failure | ||||
| Ischemic | 550 (57.6%) | 165 (58.5%) | 81 (62.8%) | |
| Hypertensive | 160 (16.8%) | 47 (16.7%) | 10 (7.8%) | 0.124 |
| Others | 245 (25.7%) | 70 (24.8%) | 38 (29.5%) | |
| Comorbid conditions | ||||
| Prior myocardial infarction | 476 (49.8%) | 144 (51.1%) | 65 (50.4%) | 0.936 |
| Current angina | 280 (29.3%) | 84 (29.8%) | 28 (21.7%) | 0.180 |
| Hypertension | 530 (55.5%) | 156 (55.3%) | 77 (59.7%) | 0.653 |
| Diabetes | 330 (34.6%) | 80 (28.4%) | 50 (38.8%) | 0.066 |
| Chronic kidney disease | 554 (58.0%) | 151 (53.5%) | 87 (67.4%) | 0.030 |
| Medications | ||||
| ACE inhibitors | 886 (92.8%) | 266 (94.3%) | 120 (93.0%) | 0.652 |
| Non-potassium sparing diuretics | 799 (83.7%) | 233 (82.6%) | 116 (89.9%) | 0.146 |
| Potassium sparing diuretics | 77 (8.1%) | 30 (10.6%) | 10 (7.8%) | 0.392 |
| Potassium supplement | 289 (30.3%) | 84 (29.8%) | 39 (30.2%) | 0.988 |
| Dose of digoxin & placebo (mg / day) | 0.224 (±0.074) | 0.219 (±0.070) | 0.243 (±0.073) | 0.006 |
| Symptoms and signs of heart failure (within one month before randomization) | ||||
| Dyspnea at rest | 246 (25.8%) | 71 (25.2%) | 35 (27.1%) | 0.915 |
| Dyspnea on exertion | 758 (79.4%) | 219 (77.7%) | 97 (75.2%) | 0.502 |
| Jugular venous distension | 130 (13.6%) | 42 (14.9%) | 22 (17.1%) | 0.537 |
| Third heart sound | 212 (22.2%) | 65 (23.0%) | 30 (23.3%) | 0.932 |
| Pulmonary râles | 164 (17.2%) | 57 (20.2%) | 33 (25.6%) | 0.052 |
| Lower extremity edema | 222 (23.2%) | 66 (23.4%) | 38 (29.5%) | 0.293 |
| NYHA functional class | ||||
| I | 91 (9.5%) | 28 (9.9%) | 17 (13.2%) | |
| II | 506 (53.0%) | 154 (54.6%) | 67 (51.9%) | 0.213 |
| III | 337 (35.3%) | 95 (33.7%) | 38 (29.5%) | |
| IV | 21 (2.2%) | 5 (1.8%) | 7 (5.4%) | |
| Heart rate, beats /min, mean (±SD) | 80.6 (±12.6) | 79.7 (±13.1) | 79.4 (±11.6) | 0.411 |
| Systolic BP, mm Hg, mean (±SD) | 130.3 (±21.8) | 131.5 (±22.4) | 127.4 (±20.6) | 0.200 |
| Diastolic BP, mm Hg, mean (±SD | 75.4 (±11.7) | 76.2 (±12.1) | 73.2 (±10.7) | 0.052 |
| Chest radiograph findings | ||||
| Pulmonary congestion (now) | 147 (15.4%) | 47 (16.7%) | 35 (27.1%) | 0.004 |
| Cardiothoracic ratio > 0.5 | 697 (73.0%) | 199 (70.6%) | 106 (82.2%) | 0.042 |
| Laboratory data, mean (±SD) | ||||
| Serum creatinine (mg/dL), | 1.16 (±0.37) | 1.09 (±0.35) | 1.25 (±0.40) | <0.001 |
| Serum potassium (mEq/L) | 4.27 (±0.40) | 4.25 (±0.38) | 4.29 (±0.47) | 0.566 |
| Ejection fraction (%), mean (±SD) | 35.9 (±14.0) | 35.2 (±14.4) | 35.0 (±14.5) | 0.633 |
Abbreviation: BP= blood pressure; SDC=serum digoxin concentration
All-Cause Mortality
Overall, 384 (28.1%) patients died from all causes during the 41 months of median follow-up. Compared with the 26.9% deaths among placebo patients, 40.3% of those with SDC ≥1.2 ng/ml and 26.6% of those with SDC 0.5-1.1 ng/ml died (overall p = 0.005; individual p values for comparison of SDC ≥1.2 ng/ml and SDC 0.5-1.1 with placebo were respectively 0.002 and 0.916). Figure 1 displays the Kaplan-Meier plots for all-cause mortality in women with HF by SDC, for all women, and in those with EF of <35% and ≥35%. Among all women (overall log rank test p = 0.025), compared with placebo, a SDC ≥1.2 ng/ml was associated with higher risk of death (log rank test p =0.019), whereas SDC 0.5-1.1 ng/ml was not (log rank test p=0.401) (Figure 1 a). Among women with EF <35% (overall log rank test p=0.032), compared with placebo, those with SDC ≥1.2 ng/ml had a non-significant increased risk of death (log rank test p=0.082), while SDC 0.5-1.1 ng/ml was associated with a non-significant lower risk of death (log rank test p=0.114) (Figure 1 b). Among women with EF ≥35%, there were no overall difference in mortality risk between placebo and either SDC groups (overall log rank test p=0.315) (Figure 1 c).
Figure 1.
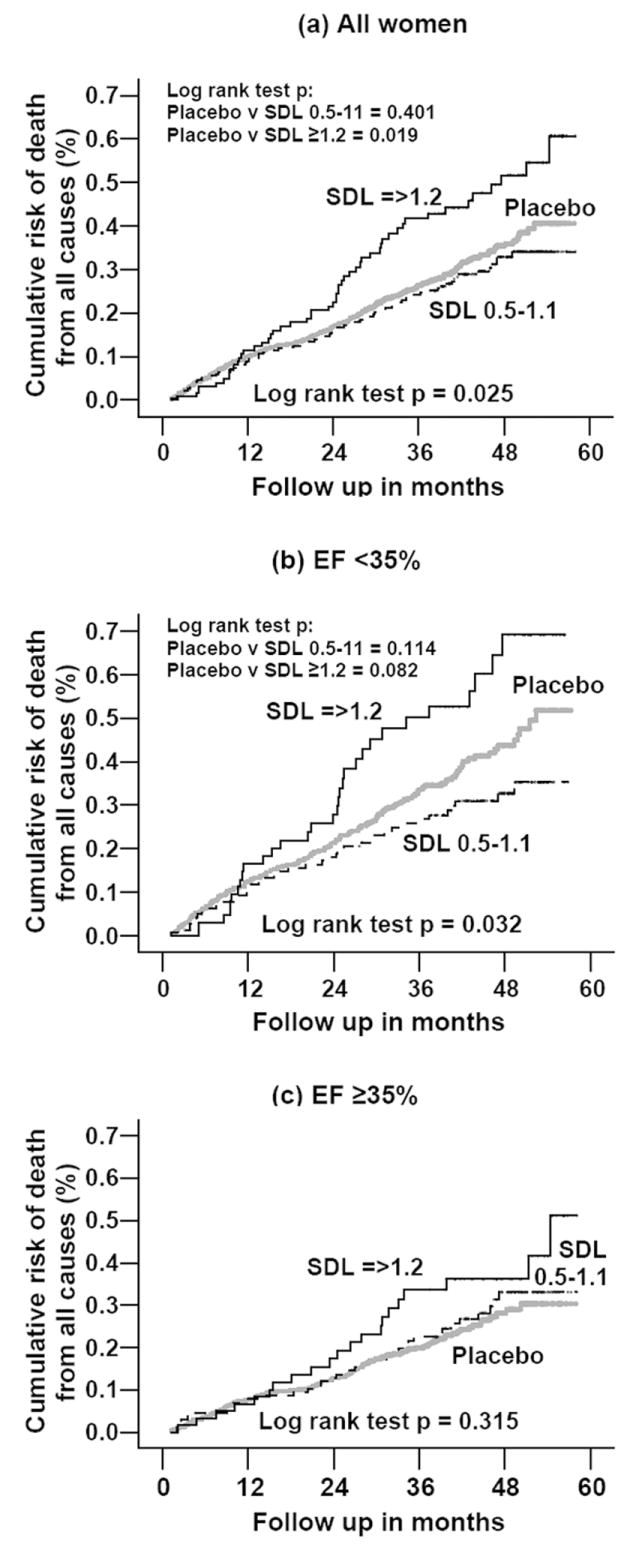
Kaplan-Meier plots of all-cause mortality by serum digoxin level, data for all women, and stratified by median ejection fraction of 35%
Table 2 displays absolute risk (AR), absolute risk reduction (ARR), unadjusted and adjusted OR, p values, and Bonferroni adjusted simultaneous CI for all-cause mortality, by SDC, for all women and in those stratified by EF 35%. Among all women, compared with those receiving placebo, those with SDC ≥1.2 ng/ml had a 13.4% increase in absolute risk and a 83% increase in relative odds of all-cause mortality (unadjusted OR=1.83; 97.5% CI = 1.19 – 2.83; p=0.002). The association remained significant after adjustments for other covariates and multiple testing (adjusted OR = 1.80; 97.5% CI = 1.14 – 2.86; p=0.004). SDC 0.5-1.1 ng/ml was not associated with any survival benefit or harm.
Table 2.
Absolute Risk (AR), AR Reduction (ARR), Crude and Adjusted Odds Ratios (OR) with Bonferroni adjusted confidence interval, and p values for All-Cause Mortality in Women with Heart Failure Receiving Digoxin by Serum Digoxin Concentration (SDC) Versus Placebo (Reference), Data for All Women, and Stratified by Ejection Fraction (EF) 35%
| N (%) | AR | ARR | Crude OR (97.5% CI) | P value | Adjusted* OR (97.5% CI)** | P value | ||
|---|---|---|---|---|---|---|---|---|
| All Women (N=1,366) | Placebo | 955 (69.9%) | 26.9% | Reference | 1 | Reference | 1 | Reference |
| SDC 0.5-1.1 ng/ml | 282 (20.6%) | 26.6% | 0.3% | 0.98 (0.70 – 1.39) | 0.916 | 1.05 (0.73 – 1.51) | 0.762 | |
| SDC ≥1.2 ng/ml | 129 (9.4%) | 40.3% | -13.4% | 1.83 (1.19 – 2.83) | 0.002 | 1.80 (1.14 – 2.86) | 0.004 | |
| Women with EF <35% (N=677) | Placebo | 463 (68.4%) | 32.6% | Reference | 1 | Reference | 1 | Reference |
| SDC 0.5-1.1 ng/ml | 148 (21.9%) | 27.0% | 5.6% | 0.77 (0.48 – 1.23) | 0.203 | 0.77 (0.47 – 1.26) | 0.233 | |
| SDC ≥1.2 ng/ml | 66 (9.7%) | 47.0% | -14.4% | 1.83 (1.01 – 3.32) | 0.023 | 1.77 (0.94 – 3.36) | 0.044 | |
| Women with EF ≥35% (N=689) | Placebo | 492 (71.4%) | 21.5% | Reference | 1 | Reference | 1 | Reference |
| SDC 0.5-1.1 ng/ml | 134 (19.4%) | 26.1% | -4.6 | 1.29 (0.78 – 2.13) | 0.262 | 1.58 (0.92 – 2.72) | 0.058 | |
| SDC ≥1.2 ng/ml | 63 (9.1%) | 33.3% | -11.8 | 1.82 (0.95 – 3.48) | 0.038 | 1.87 (0.93 – 3.76) | 0.045 |
Adjusted for age, sex, race, body mass index, duration of heart failure in months, etiology of heart failure, prior myocardial infarction, current angina, hypertension, diabetes, NYHA functional class, use of angiotensin-converting enzyme inhibitors, diuretics, and combination of hydralazine and nitrates, current dyspnea at rest and dyspnea on exertion, heart rate, systolic and diastolic blood pressure, current jugular venous distension, third heart sound, pulmonary râles, and lower extremity edema, estimated glomerular filtration rate, pulmonary congestion by chest x-ray, and cardiothoracic ratio >0.5. Addition adjustment for left ventricular ejection fraction less that 35% for all women.
Alpha=0.025 (0.05/2) was used to construct individual confidence intervals to achieve an overall 95% confidence intervals using Bonferroni adjustment
Among women with EF <35%, compared with placebo, SDC ≥1.2 ng/ml was associated with a 14.4% increase in absolute risk and an 83% increase in relative odds of death from all causes (unadjusted OR=1.83; 97.5% CI = 1.01 – 3.32; p=0.023). The association was unchanged after adjustment for other covariates (adjusted OR = 1.77; p=0.044), but lost its significance after Bonferroni adjustment (97.5% CI= 0.94 – 3.36). Among women with EF ≥35%, compared with placebo, SDC ≥1.2 ng/ml was associated with a 11.8% increase in absolute risk and an 82% increase in relative odds of death from all causes (unadjusted OR=1.82; 97.5% CI = 0.95 – 3.48; p=0.038). In addition, SDC 0.5-1.1 ng/ml was also associated with 4.6% increase in absolute risk and non-significant increase in relative odds of death (unadjusted OR=1.29; 97.5% CI = 0.78 –2.13; p=0.262 and adjusted OR=1.58; 97.5% CI = 0.92 – 2.72; p=0.058) compared to placebo.
Hospitalization due to worsening heart failure
Overall, 434 (31.8%) were hospitalized due to worsening HF. Compared with 32.8% of women receiving placebo, 38.0% of those with SDC ≥1.2 ng/ml (p = 0.239) and 25.5% of those with SDC 0.5-1.1 ng/ml (p = 0.021) were hospitalized (overall p = 0.020). Figure 2 displays the Kaplan-Meier plots for hospitalization due to worsening HF by SDC, for all patients, and in those with EF of <35% and ≥35%. Among all women (overall log rank test p=0.011), compared with placebo, those with SDC 0.5-1.1 ng/ml (log rank test p=0.007), but not those with SDC ≥1.2 ng/ml (log rank test p = 0.460), had lower cumulative risk of hospitalization due to worsening HF (Figure 2 a). Among women with EF <35% (overall log rank test p=0.020), compared with placebo, SDC 0.5-1.1 ng/ml (log rank test p = 0.008), but not ≥1.2 ng/ml (log rank test p=0.649) was associated with lower risk of hospitalization (Figure 2 b). Among women with EF ≥35%, however, compared with placebo, neither SDC 0.5-1.1 ng/ml nor SDC ≥1.2 ng/ml was associated with hospitalization (overall log rank test p=0.291) (Figure 2 c).
Figure 2.

Kaplan-Meier plots of heart failure hospitalization by serum digoxin level, data for all women, and stratified by median ejection fraction of 35%
Table 3 displays absolute risk (AR), absolute risk reduction (ARR), unadjusted and adjusted OR, p values, and Bonferroni adjusted simultaneous CI for HF-hospitalization, by SDC, for all women, and in those stratified by EF 35%. Among all women, compared with placebo, SDC 0.5-1.1 ng/ml was associated with a 7.3% reduction in absolute risk and a 30% reduction of relative odds of hospitalization due to HF (unadjusted OR = 0.70; 97.5% CI = 0.50 – 0.99; p=0.021). After multivariable adjustment, while the strength of the association remained essentially unchanged (adjusted OR=0.75), but it marginally lost it statistical significance (p=0.072; 97.5% CI = 0.52 – 1.07). SDC ≥1.2 ng/ml was associated with a 5.2% increase in absolute risk and a 26% increase in relative odds for HF-hospitalization (unadjusted OR = 1.26; 97.5% CI = 0.81 – 1.94; p=0.240).
Table 3.
Absolute Risk (AR), AR Reduction (ARR), Crude and Adjusted Odds Ratios (OR) with Bonferroni adjusted confidence interval, and p values for Hospitalization due to Worsening Heart Failure in Women with Heart Failure Receiving Digoxin by Serum Digoxin Concentration (SDC) Versus Placebo (Reference), Data for All Women, and Stratified by Ejection Fraction (EF) 35%
| N (%) | AR | ARR | Crude OR (97.5% CI) | P value | Adjusted* OR (97.5% CI)** | P value | ||
|---|---|---|---|---|---|---|---|---|
| All Women (N=1,366) | Placebo | 955 (69.9%) | 32.8% | Reference | 1 | Reference | 1 | Reference |
| SDC 0.5-1.1 ng/ml | 282 (20.6%) | 25.5% | 7.3% | 0.70 (0.50 – 0.99) | 0.021 | 0.75 (0.52 – 1.07) | 0.072 | |
| SDC ≥1.2 ng/ml | 129 (9.4%) | 38.0% | -5.2% | 1.26 (0.81 – 1.94) | 0.240 | 1.06 (0.67 – 1.69) | 0.772 | |
| Women with EF <35% (N=677) | Placebo | 463 (68.4%) | 40.4% | Reference | 1 | Reference | 1 | Reference |
| SDC 0.5-1.1 ng/ml | 148 (21.9%) | 29.7% | 10.7% | 0.62 (0.40 – 0.99) | 0.021 | 0.63 (0.39 – 1.00) | 0.026 | |
| SDC ≥1.2 ng/ml | 66 (9.7%) | 45.5% | -5.1% | 1.23 (0.68 – 2.23) | 0.434 | 1.09 (0.59 – 2.03) | 0.747 | |
| Women with EF ≥35% (N=689) | Placebo | 492 (71.4%) | 25.6% | Reference | 1 | Reference | 1 | Reference |
| SDC 0.5-1.1 ng/ml | 134 (19.4%) | 20.9% | 4.7 | 0.77 (0.45 – 1.30) | 0.262 | 0.95 (0.54 – 1.66) | 0.826 | |
| SDC ≥1.2 ng/ml | 63 (9.1%) | 30.2% | -4.6 | 1.25 (0.65 – 2.42) | 0.440 | 1.13 (0.58 – 2.28) | 0.706 |
Adjusted for same covariates as in Table 2.
Alpha=0.025 (0.05/2) was used to construct individual confidence intervals to achieve an overall 95% confidence intervals using Bonferroni adjustment
Among women EF <35%, those with SDC 0.5-1.1 ng/ml had a 10.7 reduction in absolute risk and a 38% reduction in relative odds of HF-hospitalization (unadjusted OR = 0.62; 97.5% CI = 0.40 – 0.99; p=0.021). This association remained essentially unchanged after adjustment for other covariates (adjusted OR = 0.63; 97.5% CI = 0.39 – 1.00; p=0.026). Among women with EF ≥35%, there was no association between SDC and the risk for hospitalization due to HF.
Combined endpoint of all-cause death or heart failure hospitalization
Overall, 615 (45.0%) experienced the combined endpoint of all-cause mortality or HF-hospitalization. Compared with the 45.0% incidence of death or HF-hospitalizations among placebo women, 56.6% of those with SDC ≥1.2 ng/ml (p=0.013) and 39.7% of those with SDC 0.5-1.1 ng/ml (p=0.114) were hospitalized or died (overall p=0.006). Figure 3 displays the Kaplan-Meier plots for combined endpoint of all-cause mortality or HF-hospitalization by SDC, for all patients, and in those with EF of <35% and ≥35%. Among all women (overall log rank test p = 0.011), compared with placebo, SDC 0.5-1.1 ng/ml (log rank test p=0.020), but not SDC≥1.2 ng/ml (log rank test p = 0.145) was associated with lower cumulative risk of combined outcomes (Figure 3 a). Among women with EF <35% (overall log rank test p=0.020), similarly, compared with placebo, SDC 0.5-1.1 ng/ml (log rank test p=0.010), but not SDC≥1.2 ng/ml (log rank test p=0.354) was associated with lower risk of one of the combined outcomes (Figure 3 b). Among women with EF ≥35%, compared with placebo, neither SDC category was associated with the combined end point (overall log rank test p=0.306) (Figure 3 c).
Figure 3.
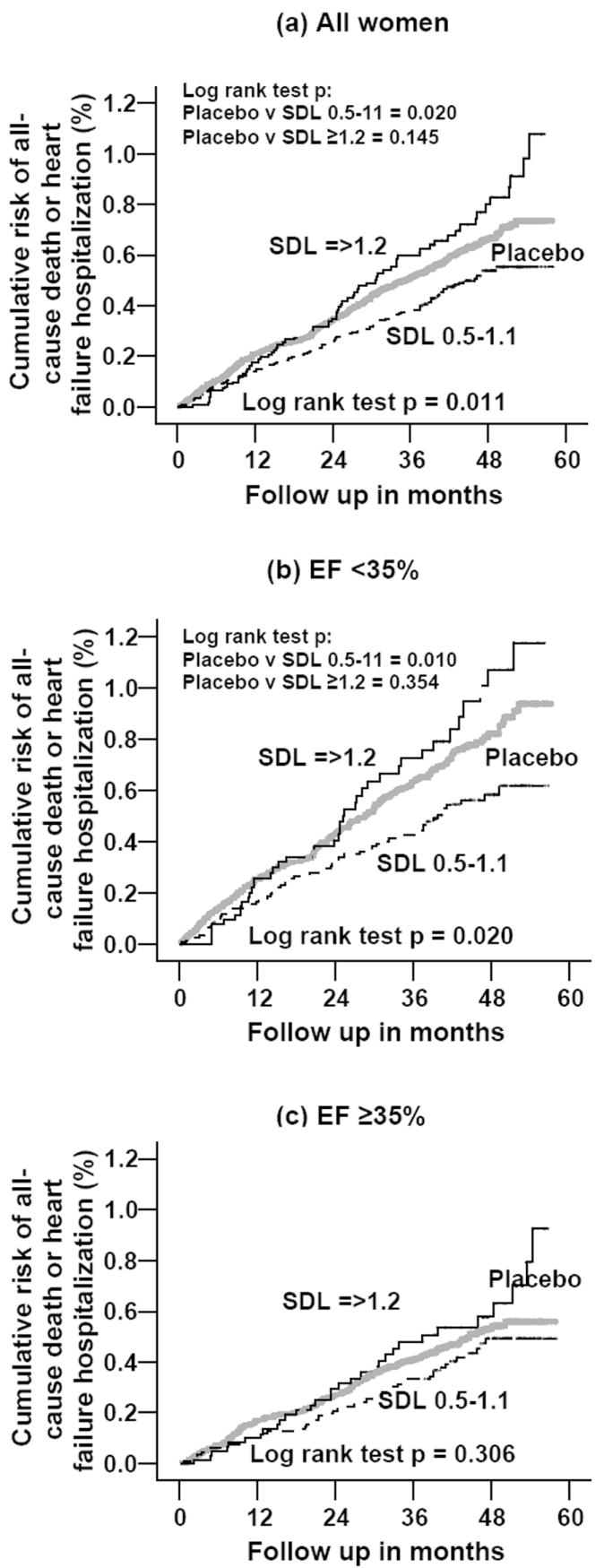
Kaplan-Meier plots of combined endpoint of all-cause death or heart failure hospitalization by serum digoxin level, data for all women, and stratified by median ejection fraction of 35%
Table 4 displays absolute risk (AR), absolute risk reduction (ARR), unadjusted and adjusted OR, p values, and Bonferroni adjusted simultaneous CI for combined endpoint, by SDC, for all women, and in those stratified by EF 35%. Among all women, compared with placebo, SDC ≥1.2 ng/ml was associated with an 11.1% increase in absolute risk and a 59% increase in relative odds (unadjusted OR = 1.59; 97.5% CI = 1.04 – 2.43; p=0.014) of the combined endpoint, which became non-significant after adjustment (adjusted OR = 1.46; 97.5% CI = 0.93 – 2.31; p=0.060). SDC 0.5-1.1 ng/ml was associated a 5.3% reduction in absolute risk and a 20% reduction in relative odds (unadjusted OR = 0.80; 97.5% CI = 0.59 – 1.10; p=0.115 and adjusted OR = 0.86; 97.5% CI = 0.62 – 1.20; p=0.322) of the combined endpoint.
Table 4.
Absolute Risk (AR), AR Reduction (ARR), Crude and Adjusted Odds Ratios (OR) with Bonferroni adjusted confidence interval, and p values for the Combined Endpoint of All-Cause Mortality or Heart Failure Hospitalization in Women with Heart Failure Receiving Digoxin by Serum Digoxin Concentration (SDC) Versus Placebo (Reference), Data for All Women, and Stratified by Ejection Fraction (EF) 35%
| N (%) | AR | ARR | Crude OR (97.5% CI) | P value | Adjusted* OR (97.5% CI)** | P value | ||
|---|---|---|---|---|---|---|---|---|
| All Women (N=1,366) | Placebo | 955 (69.9%) | 45.0% | Reference | 1 | Reference | 1 | Reference |
| SDC 0.5-1.1 ng/ml | 282 (20.6%) | 39.7% | 5.3% | 0.80 (0.59 – 1.10) | 0.115 | 0.86 (0.62 – 1.20) | 0.322 | |
| SDC ≥1.2 ng/ml | 129 (9.4%) | 56.6% | -11.1% | 1.59 (1.04 – 2.43) | 0.014 | 1.46 (0.93 – 2.31) | 0.060 | |
| Women with EF <35% (N=677) | Placebo | 463 (68.4%) | 52.9% | Reference | 1 | Reference | 1 | Reference |
| SDC 0.5-1.1 ng/ml | 148 (21.9%) | 42.6% | 10.3% | 0.66 (0.43 – 1.01) | 0.029 | 0.66 (0.42 – 1.03) | 0.037 | |
| SDC ≥1.2 ng/ml | 66 (9.7%) | 63.6% | -10.7% | 1.56(0.85 – 2.87) | 0.104 | 1.37 (0.72 – 2.61) | 0.274 | |
| Women with EF ≥35% (N=689) | Placebo | 492 (71.4%) | 37.6% | Reference | 1 | Reference | 1 | Reference |
| SDC 0.5-1.1 ng/ml | 134 (19.4%) | 36.6% | 1.0 | 0.96 (0.61 – 1.51) | 0.862 | 1.21 (0.74 – 1.98) | 0.380 | |
| SDC ≥1.2 ng/ml | 63 (9.1%) | 49.2% | -11.6 | 1.61 (0.88 – 2.94) | 0.077 | 1.50 (0.78 – 2.90) | 0.166 |
Adjusted for same covariates as in Table 2.
Alpha=0.025 (0.05/2) was used to construct individual confidence intervals to achieve an overall 95% confidence intervals using Bonferroni adjustment
Among women with EF<35%, compared with placebo, SDC 0.5-1.1 ng/ml was associated with 10.3% reduction in absolute risk and a 34% reduction in relative odds (unadjusted OR = 0.66; 97.5% CI = 0.43 – 1.01; p=0.029) of death or HF-hospitalization, which remained unchanged after multivariable adjustment OR = 0.66; 97.5% CI = 0.42 – 1.03; p=0.037). The effect of SDC ≥1.2 ng/ml was similar to that noted among all women. Among women with EF ≥35%, neither SDC category was associated with the combined outcomes.
DISCUSSION
The results of our study demonstrate that as in men [5], the effect of digoxin in women with HF varies with SDC, and that the effect appears to be modified by left ventricular systolic function. We observed that in women with HF, digoxin at SDC 1.2 ng/ml and higher were associated with higher risk of death but no effect on the risk of HF-hospitalization or combined endpoints. A SDC of 0.5-1.1 ng/ml, on the other hand, was associated with a 7% and 5% absolute reduction respectively of HF- hospitalization and the combined endpoint of death or HF-hospitalization, without any effect on mortality. Among women with EF <35%, at SDC of 0.5-1.1 ng/ml, use of digoxin was associated with unadjusted 11% and 10% absolute reduction in the risk of respectively HF-hospitalization and the combined endpoint of death or HF-hospitalization. On the other hand, neither category of SDC was associated with any mortality or hospitalization benefits in women with EF ≥35%.
These findings from our study are of clinical and public health importance. HF is primarily a disorder of the elderly, women constitute over half of these older HF patients and most have preserved systolic function. With the aging of the population, the proportion of women with HF is also likely to increase in the coming decades. However, HF patients who are elderly, women and have preserved systolic function are typically not enrolled in clinical trials [30], and management guidelines for these patients are often extrapolated from trials involving predominantly younger male patients with systolic HF.
Use of digoxin in women with HF and severely impaired systolic function
Previous post-hoc analyses of the DIG trial either did not account for SDC or did not include women [1, 5], leaving significant gaps in our knowledge about the effects of digoxin at various SDC on outcomes in women with HF [6-9]. The results of our analysis demonstrate a bi-directional effect of digoxin in women with HF similar to that observed in men. There is no biological basis for a different outcome of digoxin therapy in women with HF. The theory of interaction of digoxin with hormone replacement therapy[1] has been questioned [7]. In addition, with the recent findings of the harmful effects of hormone replacement therapy [31, 32], it is likely that most elderly women with HF would not be receiving hormone replacement therapy in the future. Our results suggest that at appropriate low SDC digoxin might still have a role in women with HF and advanced systolic dysfunction who are symptomatic on maximum neurohormonal suppression. However, the dose of digoxin must be carefully adjusted to achieve an optimal SDC [6, 10], preferably to 1.1 ng/ml or lower.
Use of digoxin in women with HF and relatively preserved systolic function
The current recommendations [33, 34] for the use of digoxin to reduce symptoms and hospitalizations due to worsening HF are based on the DIG trial [12], which demonstrated that the use of digoxin was associated with a decrease in the risk of HF-hospitalizations and combined endpoint event of death or hospitalization due to HF, without any effect on all-cause mortality. Similar results were found in the ancillary trial involving patients with EF>45% in which the use of digoxin was associated with an 18% non-significant reduction (risk ratio, 0.82; 95% CI, 0.63 – 1.07) in the risk of the combined endpoint of death or hospitalization due to worsening HF without any effect on overall mortality [12]. Based on these results, the use of digoxin in HF patients with preserved systolic function (EF>45%) is considered a Class- IIb indication (usefulness and efficacy less well established by evidence / opinion) based on Level-C (consensus opinion of experts) evidence [33, 35]. Nonetheless, digoxin continues to be frequently used in HF patients with preserved systolic function [36-41]. Results of our analysis raise question about the continued use of digoxin in women with HF and relatively normal systolic function.
Similarities and differences in the effects of digoxin in men and women with systolic HF
A closer examination of the our results and those from Rathore et al. [5] reveals several similarities of the effects of digoxin on outcomes in both men and women. We observed similar harmful effects of SDC ≥1.2 ng/ml in women as previously observed in men. In men, SDC of ≥1.2 ng/ml was associated with a significant 34% increased risk of all-cause mortality [5]. In comparison, we noted a significant 83% increased odds of death among women with the same SDC (Table 2). The greater magnitude of the deleterious effects of digoxin observed in women is likely due to the fact that women are more likely to achieve higher serum concentrations than men.[10] This might have been due in part to using serum creatinine to determine the dose of digoxin in the DIG trial [11, 12], which is likely to have underestimated chronic kidney disease in women [13]. We noted that among the participants in the DIG trial in whom data on SDC were available, the prevalence of chronic kidney disease (based on estimated glomerular filtration rate <60 ml/min/1.73 m2) was higher among women (57.9% versus 42.7% in men; p<0.001), and the mean SDC was significantly higher in women (1.05 ng/ml versus 0.96 ng/ml in men; p<0.001) (data not shown). In addition, more women (31.4%) than men (23.6%) had SDC ≥1.2ng/ml (p=0.002) (data not shown) suggesting a higher population attributable risk of death associated with higher SDC in women [42, 43]. Compared to men, women in the DIG trial were also more likely to be older, have severe HF and be on diuretics, have higher classes of NYHA symptoms, and diabetes [1]. It is unknown if the effect of digoxin in women was affected by any of these covariates [9].
We also noted similar beneficial effects of low SDC on reduction of risk of hospitalization in women as in men. In men, SDC of 0.5-0.8 ng/ml was associated with a significant 48% reduction in the risk of HF-hospitalization [5], which is similar to a significant 30% reduction in the odds of HF-hospitalization in women with SDC 0.5-1.1 ng/ml (Table 3). This apparent lower benefit in women at low SDC might be explained by the inclusion of women with SDC 0.8-1.1 ng/ml into our low SDC group. Another possible explanation for the differential estimates of risks between the two studies is different methods used in our studies. We used logistic regression modeling to estimate the effect of digoxin on outcomes. When the incidence of an outcome is common (>10%), the OR overestimates the risk ratio when it is more than unity (as in the case for SDC ≥1.2 ng/ml) and underestimates when it is less than unity (as would be case for SDC 0.5-1.1 ng/ml) [44].
We also noted several apparent differences between our findings and those of Rathore [5]. Unlike in men, lower SDC was not associated with any reduction in death in women. The lack of effect on mortality at SDC 0.5-1.1 ng/ml might in part due to the fact that we combined SDC 0.5-0.8 ng/ml and SDC 0.9-1.1 ng/ml into one group. In men, SDC 0.9-1.1 was not associated with mortality or hospitalization [5]. At SDC 0.5-1.1 ng/ml, as in men, women experienced a significant reduction in HF hospitalization. Among women with EF<35%, on the other hand, we noted a 5.6% absolute reduction and a non-significant 23% relative reduction in unadjusted death in those with SDC 0.5-1.1 ng/ml, which is in the same direction and of similar magnitude (a significant 28% reduction) to that observed in men with SDC 0.5-0.8 ng/ml [5]. In addition, we observed a 10.3% absolute reduction and a 34% relative reduction in the combined endpoint events in women with SDC 0.5-1.1 ng/ml (Table 4). These effects are similar to the effects reported by the main DIG trial (no reduction in death, but reduction in HF hospitalization and combined endpoints). The finding that the favorable effects of digoxin at lower serum concentrations were more pronounced in women with lower (versus higher) EF values suggest a possible modification of the effect of digoxin on mortality at SDC 0.5-1.1 ng/ml by EF. Even though we did not observe any significant interaction, likely due to lack of power, our findings are consistent with the data reported in the DIG trial [12], in which patients with EF <25% had a 32% risk reduction in the combined endpoint events compared with a 20% risk reduction for those with EF 25-45% (p for interaction 0.05). In addition, it is also consistent with the data from other studies that demonstrate a greater benefit from treatment in general in patients with higher severity of disease.[24] Low EF is a marker of HF severity and is associated with poor outcomes [23, 45].
Strengths and limitations
To our knowledge, this is the first report suggesting a potential beneficial role of digoxin in women with HF and moderate to severe systolic dysfunction (EF<35%), if used with caution to achieve lower serum concentrations (SDC 0.5-1.1 ng/ml). In addition, we also noted that in women with less impaired systolic function (EF ≥35%) digoxin at lower serum concentrations (0.5-1.1 ng/ml) was neither beneficial nor harmful. However, digoxin at higher SDC (≥1.2 ng/ml) is harmful in all women with HF. These results are complementary to the data provided by Rathore et al. [1, 5] and to the existing body of knowledge about the use of digoxin in women with HF. One of the strengths of our study is that it is based on data from the largest randomized trial of digitalis in HF to date with available laboratory data on serum concentration of digoxin.
However, some limitations of our study must be recognized [24]. One of the potential limitations of our study is that we combined HF patients with EF 35-55% and those with EF>55% into one group. Patients with EF 35-55% are likely to have some degree of systolic dysfunction. As such, our analysis may not strictly apply to HF patients with normal systolic function. However, prognostically, HF patients with EF 35-55% are closer to those with EF>55% than to EF<35%. Curtis et al. demonstrated that among the participants of the DIG trial, HF patients with EF 36-45%, 46-55%, and >55% had similar unadjusted mortality rates (respectively 25.6%, 23.3% and 23.5%) [23]. In contrast, unadjusted mortality rates for those with EF 26-35%, 16-25% and ≤15% were respectively 31.4%, 41.7% and 51.7%, which remained essentially unchanged after adjustment of other covariates [23].
Another limitation of the current analysis is that patients with atrial fibrillation were excluded from the DIG trial. Atrial fibrillation is common in patients with HF and is associated with poor prognosis [46, 47]. Digoxin may exert benefit in such patients by slowing the ventricular rate. We also lacked of sufficient power to detect significant interactions. In the DIG trial, 24.7% (1926/7788) of all participants were women. Nevertheless, the direction and the magnitude of most key associations observed in women in our analysis were similar to those previously reported in men. Even though we did not observe a significant interaction between EF and SDC, there is reasonable clinical and biological basis to support a possible heterogeneity of effect of digoxin by EF [12, 24].
The level of evidence of the findings of our post-hoc analysis is not strong enough to make definitive practice recommendations. The benefit of digoxin at lower SDC in women with HF and EF<35% should be replicated by other observational studies, since another large randomized controlled trial of digoxin in HF is unlikely. Nonetheless, it is tempting to recommend that clinicians should avoid using digoxin in older women with HF and relatively preserved systolic function. On the other hand, digoxin might be used with caution on an individual basis for women with HF and EF <35% who are symptomatic despite neurohormonal suppression [6, 10].
Conclusion
In conclusion, we demonstrated that among women with HF, SDC of ≥1.2 ng/ml was associated with increased risk of deaths and that SDC 0.5-1.1 ng/ml was associated with decreased risk of HF hospitalization. These beneficial effects of lower SDC were significant among women with EF <35%, but not in those with EF ≥35%. These findings suggest that women with HF and EF<35% may benefit from digoxin if care is taken to maintain the SDC between 0.5 and 1.1 ng/dl.
Acknowledgments
The Digitalis Investigation Group (DIG) study was conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the Digitalis Investigation Group (DIG) Investigators. This manuscript has been reviewed by NHLBI for scientific content and consistency of data interpretation with previous The Digitalis Investigation Group (DIG) publications and significant comments have been incorporated prior to submission for publication.
Funding/Support: Dr. Ahmed is supported by a National Institutes of Health / National Institute on Aging award 1-K23-AG19211-01.
References
- 1.Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med. 2002;347:1403–11. doi: 10.1056/NEJMoa021266. [DOI] [PubMed] [Google Scholar]
- 2.Rideout E. Digoxin increased risk of death in women, but not men, with heart failure. Evid Based Nurs. 2003;6:80. doi: 10.1136/ebn.6.3.80. [DOI] [PubMed] [Google Scholar]
- 3.Rathore SS, Krumholz HM. Digoxin therapy for heart failure: safe for women? Ital Heart J. 2003;4:148–51. [PubMed] [Google Scholar]
- 4.See S, Bruno P. Digoxin increases mortality among women with congestive heart failure. J Fam Pract. 2003;52:106–111. [PubMed] [Google Scholar]
- 5.Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. Jama. 2003;289:871–8. doi: 10.1001/jama.289.7.871. [DOI] [PubMed] [Google Scholar]
- 6.Eichhorn EJ, Gheorghiade M. Digoxin--new perspective on an old drug. N Engl J Med. 2002;347:1394–5. doi: 10.1056/NEJMp020118. [DOI] [PubMed] [Google Scholar]
- 7.Hallberg P, Michaelsson K, Melhus H. Digoxin for the treatment of heart failure. N Engl J Med. 2003;348:661–3. author reply 661-3. [PubMed] [Google Scholar]
- 8.Hudson M, Pilote L. Digoxin for the treatment of heart failure. N Engl J Med. 2003;348:661–3. author reply 661-3. [PubMed] [Google Scholar]
- 9.Moulias S, Tigoulet F, Meaume S. Digoxin for the treatment of heart failure. N Engl J Med. 2003;348:661–3. doi: 10.1056/NEJM200302133480718. author reply 661-3. [DOI] [PubMed] [Google Scholar]
- 10.Gheorghiade M, Adams KF, Jr, Colucci WS. Digoxin in the management of cardiovascular disorders. Circulation. 2004;109:2959–64. doi: 10.1161/01.CIR.0000132482.95686.87. [DOI] [PubMed] [Google Scholar]
- 11.The Digitalis Investigation Group. Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 12.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–33. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 14.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 15.Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–9. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- 16.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–37. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed A, Roseman JM, Duxbury AS, Allman RM, DeLong JF. Correlates and outcomes of preserved left ventricular systolic function among older adults hospitalized with heart failure. Am Heart J. 2002;144:365–72. doi: 10.1067/mhj.2002.124058. [DOI] [PubMed] [Google Scholar]
- 18.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–30. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 19.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 20.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–55. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 21.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 22.The Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–67. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 23.Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–42. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 24.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–86. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 25.Rothman KJ. The estimation of synergy or antagonism. Am J Epidemiol. 1976;103:506–11. doi: 10.1093/oxfordjournals.aje.a112252. [DOI] [PubMed] [Google Scholar]
- 26.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–6. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Matthews JN, Altman DG. Interaction 3: How to examine heterogeneity. Bmj. 1996;313:862. doi: 10.1136/bmj.313.7061.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sciacqua A, Scozzafava A, Pujia A, Maio R, Borrello F, Andreozzi F, et al. Interaction between vascular dysfunction and cardiac mass increases the risk of cardiovascular outcomes in essential hypertension. Eur Heart J. 2005 doi: 10.1093/eurheartj/ehi112. [DOI] [PubMed] [Google Scholar]
- 29.SPSS for Windows, Rel. 13 program. SPSS Inc.; 2005. [Google Scholar]
- 30.Lindenfeld J, Krause-Steinrauf H, Salerno J. Where are all the women with heart failure? J Am Coll Cardiol. 1997;30:1417–9. doi: 10.1016/s0735-1097(97)00343-4. [DOI] [PubMed] [Google Scholar]
- 31.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 32.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 33.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, et al. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation. 2001;104:2996–3007. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 34.Remme WJ, Swedberg K. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J. 2001;22:1527–60. doi: 10.1053/euhj.2001.2783. [DOI] [PubMed] [Google Scholar]
- 35.Konstam MA. “Systolic and diastolic dysfunction” in heart failure? Time for a new paradigm. J Card Fail. 2003;9:1–3. doi: 10.1054/jcaf.2003.9. [DOI] [PubMed] [Google Scholar]
- 36.Aronow WS. Prevalence of appropriate and inappropriate indications for use of digoxin in older patients at the time of admission to a nursing home. J Am Geriatr Soc. 1996;44:588–90. doi: 10.1111/j.1532-5415.1996.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed A, Allman RM, DeLong JF. Inappropriate use of digoxin in older hospitalized heart failure patients. J Gerontol A Biol Sci Med Sci. 2002;57:M138–43. doi: 10.1093/gerona/57.2.m138. [DOI] [PubMed] [Google Scholar]
- 38.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–55. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 39.Varela-Roman A, Gonzalez-Juanatey JR, Basante P, Trillo R, Garcia-Seara J, Martinez-Sande JL, et al. Clinical characteristics and prognosis of hospitalised inpatients with heart failure and preserved or reduced left ventricular ejection fraction. Heart. 2002;88:249–54. doi: 10.1136/heart.88.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Philbin EF, Rocco TA, Jr, Lindenmuth NW, Ulrich K, Jenkins PL. Systolic versus diastolic heart failure in community practice: clinical features, outcomes, and the use of angiotensin-converting enzyme inhibitors. Am J Med. 2000;109:605–13. doi: 10.1016/s0002-9343(00)00601-x. [DOI] [PubMed] [Google Scholar]
- 41.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–81. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 42.Hennekens CH, Buring JE. Measures of Disease Frequency and Association. In: Mayrent SL, editor. Epidemiology in Medicine. Boston/ Toronto: Little, Brown and Company; 1987. pp. 55–98. [Google Scholar]
- 43.Deubner DC, Tyroler HA, Cassel JC, Hames CG, Becker C. Attributable risk, population attributable risk, and population attributable fraction of death associated with hypertension in a biracial population. Circulation. 1975;52:901–8. doi: 10.1161/01.cir.52.5.901. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. Jama. 1998;280:1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 45.Kerzner R, Gage BF, Freedland KE, Rich MW. Predictors of mortality in younger and older patients with heart failure and preserved or reduced left ventricular ejection fraction. Am Heart J. 2003;146:286–90. doi: 10.1016/S0002-8703(03)00151-0. [DOI] [PubMed] [Google Scholar]
- 46.Swedberg K, Olsson LG, Charlesworth A, Cleland J, Hanrath P, Komajda M, et al. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long-term treatment with beta-blockers: results from COMET. Eur Heart J. 2005 doi: 10.1093/eurheartj/ehi166. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed A, Thornton P, Perry GJ, Allman RM, DeLong JF. Impact of atrial fibrillation on mortality and readmission in older adults hospitalized with heart failure. Eur J Heart Fail. 2004;6:421–6. doi: 10.1016/j.ejheart.2003.11.011. [DOI] [PubMed] [Google Scholar]


