Abstract
Objective
Frontolimbic dysfunction is observed in borderline personality disorder (BPD), with responses to emotional stimuli that are exaggerated in the amygdala and impaired in the anterior cingulate cortex (ACC). This pattern of altered function is consistent with animal models of stress responses and depression, where hypertrophic changes in the amygdala and atrophic changes in the ACC are observed. We tested the hypothesis that BPD patients exhibit gross structural changes that parallel the respective increases in amygdala activation and impairment of rostral/subgenual ACC activation.
Methods
12 unmedicated outpatients with BPD by DSM-IV and 12 normal control (NC) subjects underwent a high-resolution T1-weighted structural MRI scan. Relative gray matter concentration (GMC) in spatially-normalized images was evaluated by standard voxel-based morphometry, with voxel-wise subject group comparisons by t test constrained to amygdala and rostral/subgenual ACC.
Results
The BPD group was significantly higher than NC in GMC in the amygdala. In contrast, the BPD group showed significantly lower GMC than the NC group in left rostral/subgenual ACC.
Conclusions
This sample of BPD patients exhibits gross structural changes in gray matter in cortical and subcortical limbic regions that parallel the regional distribution of altered functional activation to emotional stimuli among these same subjects. While the histological basis for GMC changes in adult clinical populations is poorly-known at present, the observed pattern is consistent with the direction of change, in animal models of anxiety and depression, of neuronal number and/or morphological complexity in both the amygdala (where it is increased) and ACC (where it is decreased).
Keywords: borderline personality disorder, frontolimbic, amygdala, anterior cingulate cortex, gray matter, voxel-based morphometry
INTRODUCTION
Borderline personality disorder is a serious, chronic disorder characterized by disturbances of impulse control, affect and interpersonal relationships. The neurobiological basis of the last two symptom domains remains poorly-characterized. However, emerging evidence from functional neuroimaging studies suggests that these symptoms are related to dysfunction of fronto-limbic circuits (Posner et al., 2003). Among subcortical limbic structures, the amygdala mediates the most extensive range of social and emotional processes (LeDoux, 2000; Davidson, 2002). Accordingly, BPD patients exhibit exaggerated amygdala responses to social and emotional stimuli (Donegan et al., 2002; Herpertz et al., 2001; Minzenberg et al., in press a). Among frontal cortical areas in BPD, the anterior cingulate cortex (ACC) shows impaired in vivo serotonin synthesis capacity (Leyton et al., 2001), impaired serotonergic modulation of metabolic activity (Siever et al., 1999; New et al., 2002), and greater deactivation in response to scripts eliciting memories of interpersonal abandonment (Schmahl et al., 2003a). ACC dysfunction in turn may be related to both emotion dysfunction and impaired cognitive control observed in BPD (Posner et al., 2002; Minzenberg et al., in press b). Evidence for these reciprocal changes has been found in post-traumatic stress disorder (PTSD) (Shin et al., 2004), depression (Mayberg et al., 1999) and substance abuse (London et al., 2004), which are disorders related to BPD. We have also found elevated amygdala activation, and impaired modulation of task-related ACC deactivation in response to facial emotion, measured by functional MRI (Minzenberg et al., in press a).
One crucial issue that remains largely unaddressed in the study of frontolimbic dysfunction in BPD is the relationship between altered function and altered structure of these implicated regions. A variety of rodent models of stress and affective disorders have suggested a morphological basis for the functional alterations found in BPD and related disorders (see Radley & Morrison, 2005 for review). For example, chronic immobilization stress is associated with hypertrophic neuronal changes in the basolateral nucleus of the amygdala, including enhanced dendritic arborization (Vyas et al., 2002) and spine density, which parallels the emergence of anxiety-like behavior (Mitra et al., 2005). The dendritic changes may persist after a stress-free period (Vyas et al., 2004). Increased neuronal density in the lateral nucleus of the amygdala is found in adult rats subject to prenatal stress (Salm et al, 2004), and enhanced amygdala neurogenesis may be found in adult rats subject to either social stressors (Fowler et al., 2002) or bilateral olfactory bulbectomy (an established model of depression) (Keilhoff et al., 2005). In contrast, in the ACC and adjacent medial PFC, consistent atrophic changes are found in response to stressors. After restraint stress, decreased length and branching of apical dendrites are observed in layer II/III pyramidal cells in ACC and medial PFC (Radley et al., 2004, 2005; Brown et al., 2005; Cook & Wellman, 2004), which may be reversible (Radley et al., 2005). Apical dendritic reorganization of these neurons also occurs in response to chronic exogenous corticosterone administration (Wellman, 2001). Decreased spine density is also observed in layer II/II pyramidal cells of medial PFC after restraint stress (Radley et al., 2005), daily injections (Seib & Wellman, 2003) and social isolation (Silva-Gomez et al., 2003). This literature indicates that a variety of models of anxiety and depression are associated with changes in neuronal number and morphological complexity, which may be variably persistent. These changes can reasonably be expected to have effects, in predictable directions, on measures of both gross structure and function of these respective brain regions in adults who suffer from these symptoms.
Thus far, the findings of volumetric neuroimaging studies of adult populations with these disorders remains varied. Several groups have reported increased amygdala volumes among patients with bipolar affective disorder, relative to healthy control subjects (Altshuler et al., 1998, 2000; Strakowski et al., 1999; Brambilla et al., 2003). Bipolar affective disorder shares many clinical features with BPD, including affective instability, impulsivity and interpersonal disturbances, and may be overrepresented in BPD patients as a comorbid condition (Smith et al., 2004; Magill, 2004). Increases in amygdala volume have also been found in patients experiencing a first episode of major depression, in comparison to both healthy controls (Frodl et al., 2002) and patients with recurrent major depression (Frodl et al., 2003), and in temporal lobe epilepsy patients with comorbid depression (Tebartz van Elst et al., 2000) or dysthymia (Tebartz van Elst et al., 1999), relative to both epilepsy patients without comorbid mood disorders and healthy controls. However, other studies have found no change or reduced volume of the amygdala in major depression and anxiety disorders such as PTSD (reviewed in Anand & Shekhar, 2003; Sheline, 2000). In BPD, some studies have found no change in amygdala volume (Brambilla et al., 2004) while others have found reduced volume (Tebartz van Elst et al., 2003; Rusch et al., 2003; Driessen et al., 2000; Schmahl et al., 2003b). Among these latter studies, one excluded parts of the centromedian nucleus of the amygdala from the analysis (Tebartz van Elst et al., 2003), one employed a comparison group with a high incidence of mood, anxiety or substance use disorders (Schmahl et al., 2003b), and one reported stereotactic coordinates for a maximal gray matter volume difference (with voxel-based morphometry) that appears to be clearly posterior to the amygdala proper (and into the hippocampus), by either the MNI coordinate system or using the Brett-transform to Talairach space (Rusch et al., 2003). Therefore, it remains uncertain whether patients with BPD exhibit changes in amygdala volume relative to healthy control subjects. In contrast, ACC volume appears to be decreased in BPD, in both studies in which it has been examined (Tebartz van Elst et al., 2003; Hazlett et al., 2005), as it is in bipolar affective disorder (Lochhead et al., 2004; Lyoo et al., 2004; Sassi et al., 2004).
The present study
The literature addressing animal models of mood and anxiety disorders therefore suggests a morphological basis for functional changes in BPD, and there is some supportive evidence from volumetric studies of adults with mood disorders as well. Given the findings previously reported in the present study sample, of increases in amygdala activation and decreases in ACC activation to expressions of fear, we set out to test whether these functional changes are accompanied by underlying structural changes in these same brain regions. We employed voxel-based morphometry (VBM) in order to test this hypothesis. VBM offers several advantages in this regard. It is an unbiased, fully-automated procedure that involves the registration of each subject’s brain into a standard stereotactic space, to account for global variation in size and shape of individual brains, followed by the segmentation of gray matter from white matter and cerebrospinal fluid (Mechelli et al., 2005; Ashburner & Friston, 2000). The lack of investigator bias is particularly important for measuring the amygdala, which is notoriously difficult to distinguish from adjacent structures in MRI images, with great variation in the criteria that are employed to define it as a result (Brierley et al., 2002). The pre-processing of images in VBM also facilitates the pooling of subjects for between-group comparisons, and facilitates the simultaneous assessment of multiple brain regions. We utilized the “standard” VBM procedure (Mechelli et al., 2005) to obtain measures of gray matter concentration (GMC). We chose to test GMC changes as they may be expected to have a closer relationship to the neuronal morphological changes demonstrated in the animal models (see above). We hypothesized that BPD patients would exhibit GMC that is increased in the amygdala and decreased in the ACC, relative to the healthy control group.
METHODS
Subjects and Clinical Measures
This study was designed and carried out in accordance with the Declaration of Helsinki, and approved by the Mount Sinai School of Medicine Institutional Review Board, and all subjects underwent informed consent prior to participation in study procedures. The borderline personality disorder group (BPD) and Normal Control (NC) group were each comprised of 12 adults recruited from the community. The BPD group was not significantly different from the NC group in age (30.3 ± 8 versus 30.7 ± 10 years; p=.9), sex (7 male, 5 female, versus 6 male, 6 female) and race (White, Black, Latino, Asian, Mixed race: 3,1,4,3,1 versus 3,5,2,2,0; χ2 =4.53, df=4, p=.34). All subjects were free of significant medical illness, including history of head injury or other neurological illness. BPD subjects were excluded if they had lifetime co-morbid diagnoses of a primary psychotic disorder, bipolar affective disorder type I, or substance dependence. No subjects met a substance abuse diagnosis within 6 months of study and all had a negative urine drug screen prior to their scan. No BPD subjects were in active treatment with psychiatric medication at study; 7 had no lifetime history of psychiatric medication exposure and the remaining 5 had discontinued medication for a period of 4 months to 10 years. All subjects underwent diagnostic assessment with the Structured Interview for DSM-IV Personality Disorders (SIDP) (Pfohl et al., 1989), and the Structured Clinical Interview for DSM-IV Axis I (SCID-I) (First et al., 1995), with a masters or doctoral-level SCID-trained diagnostician. Consensus diagnoses were arrived at with the assistance of an expert diagnostician. Concurrent comorbid psychiatric diagnoses in the BPD group included the following: obsessive-compulsive disorder (n=1), post-traumatic stress disorder (n=1), panic disorder (n=1), intermittent explosive disorder (n=6); and among comorbid personality disorder diagnoses, paranoid (n=6), schizotypal (n=1), antisocial (n=1), narcissistic (n=5), histrionic (n=1), avoidant (n=4).
Magnetic Resonance Imaging
Subjects underwent MRI scanning in the following protocol. Structural MRI was acquired using a Siemens Allegra scanner with a T1-weighted MP-RAGE (Magnetization Prepared Rapid Gradient Echo) sequence, with contiguous axial slices of 0.82mm X 0.82 mm with 256×256 data matrix over a field of view of 210 mm, TR = 2500 msec, TE = 4.38 msec, flip angle = 8 degrees. 208 slices were acquired with a thickness of 0.82 mm each, with a zero gap to cover a full head.
Image pre-processing and inferential testing
We employed a standard VBM procedure, to obtain measures of gray matter concentration (GMC). All pre-processing and inferential testing was carried out using SPM2 (Wellcome Department of Imaging Neuroscience, University College, London). First, individual images were normalized, that is, registered to a standard MNI template brain available in the SPM2 library in a two-step process. The optimum 12-parameter affine transformation mapping individual MRI images to the template was determined, then global nonlinear shape differences were modeled by a linear combination of smooth spatial basis functions. The spatially normalized images were then segmented into gray matter, white matter and cerebrospinal fluid, with a correction for image intensity nonuniformity, and spatially smoothed with an 8 mm kernel. Voxel-wise two-sample t tests were conducted to test the hypotheses of group differences in GMC, with the threshold for statistical significance set at p<.05 and 8 contiguous voxels (resampled to a voxel size of 2×2×2 mm), and MarsBaR anatomic regions-of-interest (ROIs) used for small volume correction for multiple comparisons. These ROIs included bilateral amygdala and rostral/subgenual ACC, which are anatomic ROIs derived from the MNI single-subject template (Tzourio-Mazoyer et al., 2002) and implemented in SPM2. In this library of ROIs, the ACC is distinguished from a “Mid Cingulum” ROI, and is primarily comprised of the rostral and subgenual extent of the ACC, both anterior and inferior to the genu of the corpus callosum. Test statistics are subsequently depicted as statistical probability maps, with whole-brain maps represented by voxel-wise color-coded t statistics.
RESULTS
The BPD group exhibited a significant increase in GMC in the bilateral amygdala (Table 1 and Figure 1). The size, spatial location and peak magnitude of the between-group differences were remarkably similar between each amygdala bilaterally. These were largely centered in the dorso-ventral and medio-lateral dimensions of the amygdala.
Table 1.
Maxima for comparison of borderline personality disorder group versus healthy control group on gray matter concentration.
| Region | BA | MNI coordinates |
Voxels | Z | P | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| BPD > Control | |||||||
| Right Amygdala | 26 | 0 | −20 | 60 | 2.83 | .002 | |
| Left Amygdala | −24 | −2 | −20 | 54 | 2.72 | .003 | |
| Control > BPD | |||||||
| Left Subgenual Anterior | 24/32 | −4 | 22 | −8 | 41 | 2.71 | .003 |
| Cingulate Gyrus | |||||||
| 24/32 | −10 | 28 | −8 | 2.44 | .007 | ||
Figure 1. Statistical parametric map of relative increase in gray matter concentration in bilateral amygdala in BPD group compared to Control group.
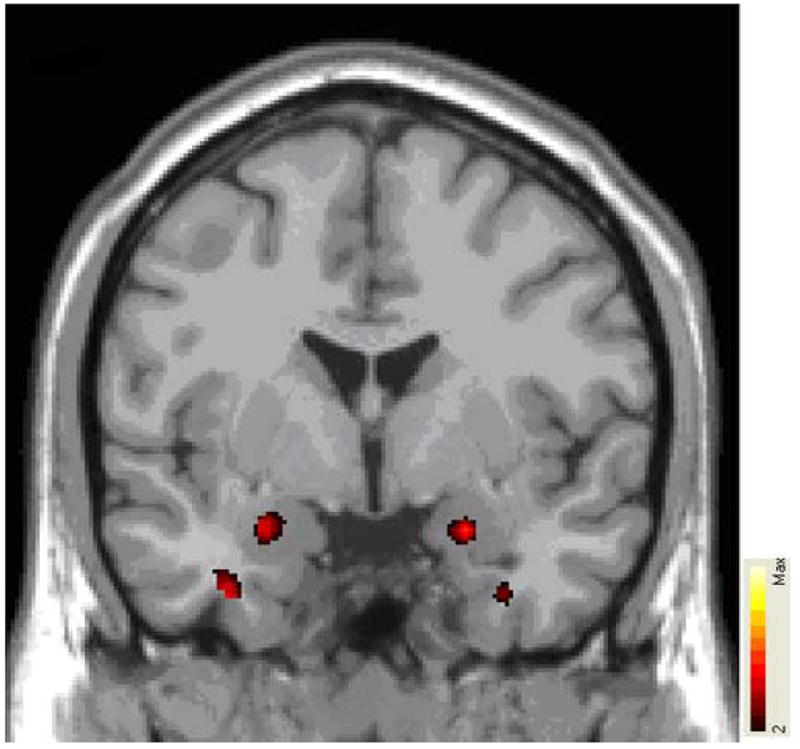
Colored areas represent significant between-group differences in signal intensity of gray matter (expressed as color-coded t statistics), overlaid on MNI single-subject template. Colored areas represent regions where BPD group has significantly increased gray matter concentration (e.g. bilateral amygdala). See text for details of image analysis. Image orientation according to neurological convention (e.g. right hemisphere to the right).
In contrast, the BPD group exhibited a significant decrease in GMC in the left rostral/subgenual ACC (Table 1 and Figure 2). This area has previously shown altered hemodynamic activation to facial emotion in the BPD group (Minzenberg et al., in press a).
Figure 2. Statistical parametric map of relative decrease in gray matter concentration in subgenual anterior cingulate cortex in BPD group compared to Control group.
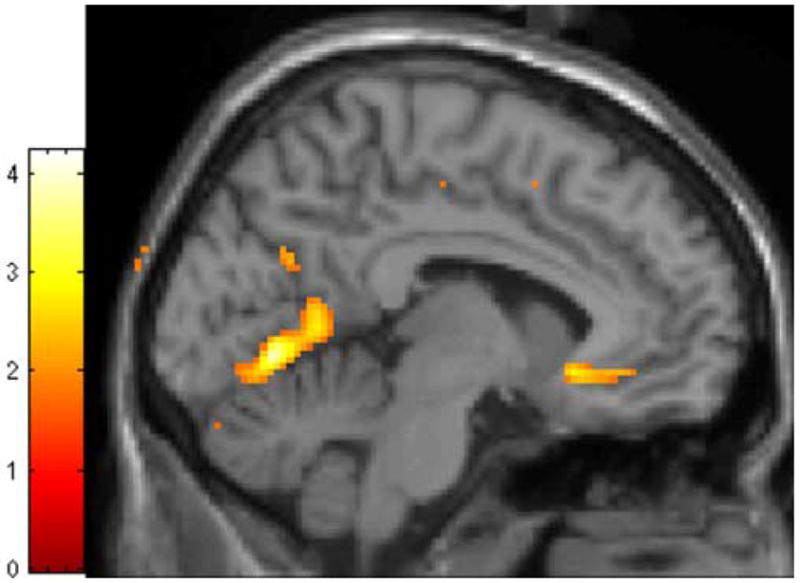
Colored area represents significant between-group differences in signal intensity of gray matter concentration (expressed as color-coded t statistic), overlaid on MNI single-subject template. Colored area in frontal cortex represents region where BPD group has significantly decreased gray matter concentration (subgenual ACC). See text for details of image analysis.
DISCUSSION
In the present study we tested group differences in gray matter concentration between a sample of unmedicated outpatients with BPD and a group of healthy control subjects. We hypothesized that the BPD group would exhibit regional GMC changes that parallel the topographic distribution of changes in functional activation to emotional stimuli in these same subjects. In particular, we predicted that the BPD group would show GMC that is increased in the amygdala and decreased in the ACC, pursuant to the literature on regional neuronal morphological changes in animal models of anxiety and depression, and current models of frontolimbic dysfunction in the processing of social and emotional stimuli among patients with BPD and related disorders. The present results support this model of BPD pathophysiology, and are consistent with a putative basis in altered number and/or morphology of neurons found in these brain regions.
This study is the first of its kind to evaluate structural brain changes in a sample of BPD patients who have also performed a functional imaging paradigm of social/emotional information processing. These structural changes are consistent with models of frontolimbic dysfunction in BPD, which postulate that exaggerated amygdala activity and impaired ACC activity occur to give rise to symptoms of affective instability and interpersonal disturbance. The present results suggest that, where altered activity is found in functional neuroimaging studies of clinical populations such as BPD patients, this may arise from changes in the underlying structure of regional masses of gray matter. While the histological basis for GMC changes in adult clinical populations is poorly-known at present, the observed pattern is consistent with the direction of change, in animal models of anxiety and depression, of neuronal number and/or morphological complexity in both the amygdala, where it is increased (Vyas et al., 2002; Fowler et al., 2002; Vyas et al., 2004; Salm et al, 2004; Mitra et al., 2005; Keilhoff et al., 2005) and ACC, where it is decreased (Radley et al., 2004, 2005; Brown et al., 2005; Cook & Wellman, 2004; Seib & Wellman, 2003; Silva-Gomez et al., 2003). The increased amygdala gray matter (compared to control subjects) is likely to be related to patterns of relatively increased functional activation in a straightforward manner, as the BOLD signal appears to be most closely related to local field potentials, which arise primarily from dendritic membrane potentials in a local region (Logothetis & Wandell, 2004). In contrast, the phenomenon of task-related deactivation, which was previously tested in our study of subgenual ACC response to facial emotion in these subjects, remains uncharacterized at the local circuit or cellular level, though it has been proposed to have a basis in local neuronal inhibition (Nair, 2005). In our earlier study, the healthy controls showed a deactivation in the subgenual ACC in response to neutral faces, which was positively modulated in response to fearful expressions, that is, the ACC deactivation was attenuated by emotional expressions. In contrast, the BPD patients in that study exhibited a tendency toward further deactivation in response to the emotional content of facial expressions (Minzenberg et al., in press a). This suggests that in the subgenual ACC, the modulation of local inhibition that supports ongoing task processing is impaired in BPD. This may be related in some unidentified manner to the impaired serotonergic function found in the ACC in studies of in vivo serotonin activity among BPD patients (Leyton et al., 2001; Siever et al., 1999; New et al., 2002). This hypothesis can be tested in the future with the use of serotonergic agents during the evaluation of task-related deactivation by fMRI.
Limitations of this study include the modest sample size. While we found support for our hypotheses, the reliability of these findings would inevitably be enhanced with a larger sample size. Nevertheless, our results resolve well with the predictions from various empirical literatures, including the models of the functional neuroanatomy of BPD symptomatology, volumetric and functional neuroimaging studies of related disorders, and studies of regional morphological changes in animal models of anxiety and depression. Furthermore, we undertook this analysis in order to provide a unique report on the identical sample that performed a functional MRI paradigm of social/emotional processing, in order to compare functional and structural changes in these patients.
Conclusion
BPD is characterized by disturbances in frontolimbic activity, with evidence for reciprocal changes in amygdala and rostral/subgenual ACC. The structural basis for these changes may reside in altered gray matter in these regions, with animal models of early stress and depression suggesting hypertrophic and atrophic changes in neurons in the amygdala and ACC, respectively. Future investigations may benefit from directly addressing the hypothesized histopathology underlying these neuroimaging findings, and clarifying the structural and functional neural signatures that establish overlapping versus distinct expressions of phenomenology in these and related clinical populations.
Acknowledgments
This work was supported in part by a grant from the Borderline Personality Disorder Research Foundation to Dr. Siever, 1 RO1 MH067918-01A1 from NIMH to Dr. New, the VA VISN3 MIRECC, and 5 M01 RR00071 for the Mount Sinai General Clinical Research Center from the National Center for Research Resources, National Institutes of Health. These sources of support had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The authors have no actual or potential conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Archives of General Psychiatry. 1998;55(7):663–4. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, Wilkins J, Gerner R, Mintz J. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biological Psychiatry. 2000;48(2):147–62. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- Anand A, Shekhar A. Brain imaging studies in mood and anxiety disorders: special emphasis on the amygdala. Annals of the New York Academy of Science. 2003;985:370–88. doi: 10.1111/j.1749-6632.2003.tb07095.x. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Soloff PH, Sala M, Nicoletti MA, Keshavan MS, Soares JC. Anatomical MRI study of borderline personality disorder patients. Psychiatry Research: Neuroimaging. 2004;131(2):125–133. doi: 10.1016/j.pscychresns.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Harenski K, Nicoletti M, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. MRI investigation of temporal lobe structures in bipolar patients. Journal of Psychiatric Research. 2003;37(4):287–95. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- Brierley B, Shaw P, David AS. The human amygdala: a systematic review and meta-analysis of volumetric magnetic resonance imaging. Brain Research Brain Research Reviews. 2002;39(1):84–105. doi: 10.1016/s0165-0173(02)00160-1. [DOI] [PubMed] [Google Scholar]
- Brown SM, Henning S, Wellman CL. Mild, Short-term Stress Alters Dendritic Morphology in Rat Medial Prefrontal Cortex. Cerebral Cortex. 2005;15(11):1714–22. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. Journal of Neurobiology. 2004;60(2):236–48. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biological Psychiatry. 2002;51(1):68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, Gore JC, Olson IR, McGlashan TH, Wexler BE. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biological Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, Osterheider M, Petersen D. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Archives of General Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, Benjamin LS. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I (SCID-I) Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. Journal of Neurobiology. 2002;51(2):115–28. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl E, Zetzsche T, Bottlender R, Born C, Groll C, Jager M, Leinsinger G, Hahn K, Moller HJ. Enlargement of the amygdala in patients with a first episode of major depression. Biological Psychiatry. 2002;51(9):708–14. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jager M, Groll C, Bottlender R, Leinsinger G, Moller HJ. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biological Psychiatry. 2003;53(4):338–44. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, New AS, Newmark R, Haznedar MM, Lo JN, Speiser LJ, Chen AD, Mitropoulou V, Minzenberg M, Siever LJ, Buchsbaum MS. Reduced Anterior and Posterior Cingulate Gray Matter in Borderline Personality Disorder. Biological Psychiatry. 2005;58(8):614–623. doi: 10.1016/j.biopsych.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SG, Willmes K, Thron A, Sass H. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biological Psychiatry. 2001;50:292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Becker A, Grecksch G, Bernstein HG, Wolf G. Cell Proliferation is Influenced by Bulbectomy and Normalized by Imipramine Treatment in a Region-Specific Manner. Neuropsychopharmacology. 2006;31(6):1165–1176. doi: 10.1038/sj.npp.1300924. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leyton M, Okazawa H, Diksic M, Paris J, Rosa P, Mzengeza S, Young SN, Blier P, Benkelfat C. Brain Regional alpha-[11C] methyl-L-tryptophan trapping in impulsive subjects with borderline personality disorder. American Journal of Psychiatry. 2001;58(5):775–82. doi: 10.1176/appi.ajp.158.5.775. [DOI] [PubMed] [Google Scholar]
- Lochhead RA, Parsey RV, Oquendo MA, Mann JJ. Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxel-based morphometry. Biological Psychiatry. 2004;55:1154–1162. doi: 10.1016/j.biopsych.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Kim MJ, Stoll AL, Demopulos CM, Parow AM, Dager SR, Friedman SD, Dunner DL, Renshaw PF. Frontal lobe gray matter density decreases in bipolar I disorder. Biological Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annual Review of Physiology. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Archives of General Psychiatry. 2004;61(1):73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- Magill CA. The boundary between borderline personality disorder and bipolar disorder: current concepts and challenges. Canadian Journal of Psychiatry. 2004;49(8):551–6. doi: 10.1177/070674370404900806. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: methods and applications. Current Medical Imaging Review. 2005 epub ahead of print. [Google Scholar]
- Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Frontolimbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Research: Neuroimaging. doi: 10.1016/j.pscychresns.2007.03.006. (in press a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Poole JH, Vinogradov S. A neurocognitive model of borderline personality disorder: effects of childhood sexual abuse and relationship to adult social attachment disturbance. Development and Psychopathology. doi: 10.1017/S0954579408000163. (in press b) [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Science. 2005;102(26):9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair DG. About being BOLD. Brain Research Brain Research Reviews. 2005;50(2):229–43. doi: 10.1016/j.brainresrev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Buchsbaum MS, Goodman M, Reynolds D, Mitropoulou V, Sprung L, Shaw RB, Jr, Koenigsberg H, Platholi J, Silverman J, Siever LJ. Blunted prefrontal cortical 18fluorodeoxyglucose positron emission tomography response to meta-chlorophenylpiperazine in impulsive aggression. Archives of General Psychiatry. 2002;59(7):621–629. doi: 10.1001/archpsyc.59.7.621. [DOI] [PubMed] [Google Scholar]
- Pfohl BM, Blum N, Zimmerman M, Stangl D. Structured Interview for DSM-III-R Personality: SIDP-R. Iowa City, IA: Author; 1989. [Google Scholar]
- Posner MI, Rothbart MK, Vizueta N, Levy KN, Evans DE, Thomas KM, Clarkin JF. Attentional mechanisms of borderline personality disorder. Proceedings of the National Academy of Science. 2002;99 (25):16366–16370. doi: 10.1073/pnas.252644699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Vizueta N, Thomas KM, Levy KN, Fossella J, Silbersweig D, Stern E, Clarkin J, Kernberg O. An approach to the psychobiology of personality disorders. Development and Psychopathology. 2003;15(4):1093–1106. doi: 10.1017/s0954579403000506. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Research Reviews. 2005;4(2):271–287. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated Stress Induces Dendritic Spine Loss in the Rat Medial Prefrontal Cortex. Cerebral Cortex. 2006;16(3):313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125(1):1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rusch N, van Elst LT, Ludaescher P, Wilke M, Huppertz HJ, Thiel T, Schmahl C, Bohus M, Lieb K, Hesslinger B, Hennig J, Ebert D. A voxel-based morphometric MRI study in female patients with borderline personality disorder. Neuroimage. 2003;20(1):385–392. doi: 10.1016/s1053-8119(03)00297-0. [DOI] [PubMed] [Google Scholar]
- Salm AK, Pavelko M, Krouse EM, Webster W, Kraszpulski M, Birkle DL. Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Brain Research Developmental Brain Research. 2004;148(2):159–67. doi: 10.1016/j.devbrainres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Sassi RB, Brambilla P, Hatch JP, Nicoletti MA, Mallinger AG, Frank E, Kupfer DJ, Keshavan M, Soares JC. Reduced left anterior cingulate volumes in untreated bipolar patients. Biological Psychiatry. 2004;56:467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Elzinga BM, Vermetten E, Sanislow C, McGlashan TH, Bremner JD. Neural correlates of memories of abandonment in women with and without borderline personality disorder. Biological Psychiatry. 2003a;54(2):142–51. doi: 10.1016/s0006-3223(02)01720-1. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. Magnetic resonance imaging of HPC and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Research. 2003b;122(3):193–8. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- Seib LM, Wellman CL. Daily injections alter spine density in rat medial prefrontal cortex. Neuroscience Letters. 2003;337(1):29–32. doi: 10.1016/s0304-3940(02)01287-9. [DOI] [PubMed] [Google Scholar]
- Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biological Psychiatry. 2000;48(8):791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2004;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Buchsbaum MS, New AS, Spiegel-Cohen J, Wei T, Hazlett EA, Sevin E, Nunn M, Mitropoulou V. d, l-fenfluramine response in impulsive personality disorder assessed with [18F]fluorodeoxyglucose positron emission tomography. Neuropsychopharmacology. 1999;20(5):413–23. doi: 10.1016/S0893-133X(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Silva-Gomez AB, Rojas D, Juarez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Research. 2003;983(1–2):128–36. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Muir WJ, Blackwood DH. Is borderline personality disorder part of the bipolar spectrum? Harvard Review of Psychiatry. 2004;12(3):133–9. doi: 10.1080/10673220490472346. [DOI] [PubMed] [Google Scholar]
- Soares JC, Mann JJ. The anatomy of mood disorders--review of structural neuroimaging studies. Biological Psychiatry. 1997;41(1):86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Archives of General Psychiatry. 1999;56(3):254–60. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Tebartz van Elst L, Hesslinger B, Thiel T, Geiger E, Haegele K, Lemieux L, Lieb K, Bohus M, Hennig J, Ebert D. Frontolimbic brain abnormalities in patients with borderline personality disorder: a volumetric magnetic resonance imaging study. Biological Psychiatry. 2003;54(2):163–171. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- Tebartz van Elst L, Woermann F, Lemieux L, Trimble MR. Increased amygdala volumes in female and depressed humans. A quantitative magnetic resonance imaging study. Neuroscience Letters. 2000;281(2–3):103–6. doi: 10.1016/s0304-3940(00)00815-6. [DOI] [PubMed] [Google Scholar]
- Tebartz van Elst L, Woermann FG, Lemieux L, Trimble MR. Amygdala enlargement in dysthymia--a volumetric study of patients with temporal lobe epilepsy. Biological Psychiatry. 1999;46(12):1614–23. doi: 10.1016/s0006-3223(99)00212-7. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667–73. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. Journal of Neurobiology. 2001;49(3):245–53. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]


