Abstract
The potential gain in life expectancy which could result from the complete elimination of mortality from cancer in the U.S. would not exceed 3 years if one were to consider cancer independently of other causes of death. In this paper, we review evidence of trade-offs between cancer and aging as well as between cancer and other diseases, which, if taken into account, may substantially increase estimates of gain in life expectancy resulting from cancer eradication. We also used the Multiple Causes of Death (MCD) data to evaluate correlations among mortalities from cancer and other major disorders including heart disease, stroke, diabetes, Alzheimer’s, Parkinson’s diseases, and asthma. Our analyses revealed significant negative correlations between cancer and other diseases suggesting stronger population effects of cancer eradication. Possible mechanisms of the observed dependencies and emerging perspectives of using dependent competing risks models for evaluating the effects of reduction of mortality from cancer on life expectancy are discussed.
Keywords: Cancer, Dependent risks, Multiple Causes of Death data, Aging, Apoptosis
1. Introduction
To compare the effects of public health policies on population’s characteristics, researchers estimate potential gain in life expectancy resulted from eradication or reduction of selected causes of death. Keyfitz (1977) estimated that eradication of cancer will result in 2.27 years of increase in male life expectancy at birth (or by 3% compared to its 1964 level). Lemaire (2005) found that potential gain in the U.S. life expectancy resulting from cancer eradication will not exceed 3 years for both genders. Conti et al. (1999) calculated that potential gain in life expectancy resulting from cancer eradication in Italy is 3.84 years for males and 2.77 years for females.
All these calculations assumed independence between causes of death. The use of such an assumption would be completely justified more than a century ago when leading causes of death were infectious diseases. However, for today’s populations in developed countries, where deaths from chronic non-communicable diseases took a lead, this assumption is no longer valid. An important feature of such diseases is that they often develop in clusters manifesting a positive correlation with each other. The common opinion was that in case of such dependence the effect of cancer eradication on life expectancy would be even smaller. As Keyfitz (1977) wrote: “since the most common kind of dependence must be a positive one people saved from cancer would be more susceptible to heart and other diseases.”
However, factors associated with increased vulnerability to one disease may, in principle, be protective against other pathology and even favor overall survival and longevity if the protective effect overweighs the detrimental one. If so, then a reasonable strategy would be targeting the risk of death from all causes combined rather than risks of separate diseases independently as it takes place in many of the today’s medical programs.
The results of recent studies suggest a number of underlying mechanisms for a possible negative correlation between cancer and several other diseases. One is associated with differential expression of genes involved in apoptotic pathways. For example, genetic variants favoring high intensity of apoptosis in tissues may better protect organism from cancer but instead increase its chances of death from heart disease (in which the amount of cells dying from ischemia is essential for prognosis). Oppositely, genes responsible for the lower levels of apoptosis may contribute to better survival from acute heart disease, but increase chances of death from cancer. Such a trade-off results in a negative correlation between mortality rates from cancer and coronary heart disease (CHD).
The purpose of this article is to show that studying mechanisms of dependence between diseases opens new opportunities for improving human health, as well as for better understanding its connection to aging and longevity. A popular concept about the trade-off between cancer and aging implies that the higher risk of cancer in modern human populations may associate with increased longevity. This is because genetic factors that enhance body’s susceptibility to cancer may also decelerate its aging (see van Heemst et al., 2005; Ukraintseva and Yashin, 2005, and references therein). At the same time, there are experimental studies of factors affecting aging/longevity of laboratory animals, in which increased life span is accompanied by the reduction in the cancer risk. Indeed, in experimental rodents, calorie restriction extends maximum life span, while decreases the risk of developing cancer (Weindruch and Walford, 1982; Hursting et al., 2003). The question arises: to what extent can the increases in longevity observed in both human and experimental animal studies be attributed to the effects of deceleration in some fundamental aging process, as compared to the effects of prevention of the occurrence of chronic diseases, or the effects of changing balance between those disease risks that are inherently negatively correlated?
The correlation between causes of death can be evaluated using the U.S. Data on Multiple Causes of Death (http://www.cdc.gov/nchs/products/elec_prods/subject/mortmcd.htm#1999–2002). The importance of such analyses was demonstrated by Stallard (2002) who evaluated associations of diseases and their secular trends by examining statistics on the joint distributions of causes of death for the years 1980, 1990, and 1998. Estimating ratios of observed to expected age-standardized joint frequencies of each pair of 15 selected conditions, he found 57 associations, which were equivalent to a positive correlation of the disease indicator variables. He also demonstrated that Alzheimer’s disease (in conditions) accompanies cancer deaths significantly less frequently (up to 5 times) than expected. Stallard argued that any analysis of cause-specific mortality that does not account for the joint occurrence of multiple diseases among elderly decedents, as well as the difficulties inherent in selecting one of these diseases as the underlying cause, will be incomplete.
In this paper, we first review experimental evidence about connection between cancer and aging/longevity in laboratory animals as well as about relationships between cancer and longevity in humans. Then we investigate possible trade-offs among major diseases (causes of death) of the elderly using the Multiple Cause of Death (MCD) data. We evaluate frequencies and associations among the specific diagnoses that appear most often in the death certificates and are overall responsible for the majority of deaths in the U.S., to explore a character of correlations among the causes of death, evaluate their temporal trends, and suggest a plausible interpretation of the findings. The results of this study suggest that understanding mechanisms of trade-offs between major elderly disorders may have important health care applications and be also essential for understanding the effects of aging versus the effects of balancing disease risks/mortalities on longevity.
2. Background
2.1. Evidence of trade-off between cancer and aging in laboratory animals
Studying the role of p53 in the connection between cancer and cellular aging, Campisi (2002), (2003) suggested that longevity may depend on a balance between tumor suppression and tissue renewal mechanisms. Tyner et al. (2002) and Donehower (2002) showed that mice carrying the p53 mutation with a phenotypic effect analogous to the up-regulation of this gene have a lower risk of cancer development but their life span is reduced and accompanied by early tissue atrophy. Long-living mutant mice, p66shc−/−, have shown an impaired p53 apoptotic response (Migliaccio et al., 1999). Introducing the null p53 allele has protected Ku80−/− and mTR−/− mice from premature aging (Vogel et al., 1999; Chin et al., 1999), indicating that the senescence phenotypes were p53-dependent (Lim et al., 2000). García-Cao et al. (2002) examined properties of “super p53 mice.” This type of mice was produced by transgenic introduction of one or two copies of p53. The mice were tumor resistant and did not exhibit any traits consistent with accelerated aging. Bauer et al. (2005) and Bauer and Helfand (2006) found that reduction of p53 activity in flies leads to lifespan extension. Although the mechanism by which p53 regulates lifespan remains to be determined, these findings highlight the possibility that careful manipulation of p53 activity during adult life may result in beneficial effects on healthy lifespan. In Ukraintseva and Yashin (2003a,b, 2004), we suggested that the trade-off between cancer and aging may result from the opposite manifestation of apoptotic and growth signaling pathways in cancer and aging cells. Golubovski et al. (2006) examined survival in populations of Drosopila with mutated lgl tumor suppressor gene. Its ortholog, Hugl-1 is found in humans where it is mutated in 75% of the gut and prostate tumors in humans. The authors showed that animals heterozygous for the loss-of-function lgl gene display a survival and longevity advantage of the lgl loss-of-function under stressful temperature conditions (high 29 °C and low 16 °C). One possible explanation of this stress-adaptive effect of reduced tumor suppressor dose could be a better resistance of Drosophila post-mitotic cells to a stress-induced apoptosis at old ages.
2.2. Trade-off between cancer and longevity in humans
Dumont et al. (2003) showed that in humans, replacement of arginine (Arg) by proline (Pro) at position 72 of human p53 decreases its ability to initiate apoptosis, suggesting that these two polymorphisms may differently affect longevity and vulnerability to cancer. Recently, van Heemst et al. (2005) demonstrated that individuals with Pro/Pro genotype of p53 corresponding to reduced apoptosis in cells have a significantly increased both overall survival (by 41%) and mortality (2.54 fold) from cancer along with a decreased risk of death from CVD at the ages 85+. It was suggested that in humans, p53 may protect against cancer but at a cost of longevity. One may suppose that individuals carrying alleles manifested in a lowered intensity of apoptosis in a tissue are more vulnerable to cancer but less vulnerable to stroke or myocardial infarction (MI) (Ukraintseva and Yashin, 2005). Ørsted et al. (2007) found that the overall 12-year survival was increased in p53 Pro/ Pro versus Arg/Arg homozygotes despite of potential cancer favoring properties of the former genotype. Overall, human studies indicate that it is not clear what kind of trade-off contributes more to longevity: the one between cancer and aging or the one between predisposition to cancer and other disease (such as CVD).
2.3. Increased longevity can be associated with decreased chances of cancer
Despite existing evidence in support of the notion about tradeoff between cancer and aging, there are plenty of experimental studies in which an increase in longevity is accompanied by the reduction in the cancer risk. Several studies demonstrated a possibility of simultaneous shifting of cancer incidence and survival curves to the right under treatment with drugs suggested as candidate “anti-aging” interventions (Anisimov, 1987,1998; Anisimov et al., 2000, 2001; Yashin et al., 2001). Treatment with l-DOPA (3,4-dihydroxy-l-phenylalanine) of female C3H/Sn mice increased their maximal life span together with a significant increase in tumor latency (Dilman and Anisimov, 1980). The monoamine oxidase inhibitor Deprenyl (anti-Parkinson’s treatment) increased the life span of experimental mice, rats, and dogs (Ivy et al., 1994; Kitani et al., 1994; Piantanelli et al., 1994) and also inhibited the development of spontaneous and induced tumors (Kitani et al., 1994; ThyagaRajan et al., 1995; ThyagaRajan and Quadri, 1999). Synthetic pineal tetrapeptide ALA-GLU-ASP-GLY (Epithalon) increased the life span of CBA mice and suppressed spontaneous neoplasm development (Anisimov et al., 2000).
The most proved “anti-aging” treatment, caloric restriction, typically increases the maximal life span together with reducing the tumor incidence in experimental rodents (Weindruch and Walford, 1982; Blackwell et al., 1995; Hursting et al., 2003). In Sheldon et al. (1995), an increase in lifespan of the food restricted animals was achieved primarily by a decrease in incidence and delay of onset of fatal tumors, of which lymphoma was the most prominent. Because the rate of apoptosis is significantly and consistently higher in food-restricted animals regardless of age, James et al. (1998) suggested that the caloric restriction may have the cancer-protective effect primarily due to upregulated apoptosis. Animal experiments with amplification of dose of tumor suppressor genes show that the effect on longevity depends on how such amplification has been performed. The reduction of cancer mortality in super p53 mice was not accompanied by the decline in longevity in contrast to the Donehower’s p53 mutant mice (García-Cao et al., 2002). This problem, typical of dependent competing risks, emphasizes the importance of studying various pathways of reduction of cancer mortality.
3. Data and methods
3.1. The Multiple Cause of Death (MCD)
The Multiple Cause of Death (MCD) data files contain information about underlying and secondary causes of death in the U.S. during 1968–2004. Totally, they include more than 65 million individual death certificate records. The information available in death certificates includes the date of death, geographic location (region, state, county, division) of death, place of residence (region, state, county, city, and population size), sex, race, age, marital status, state of birth, and origin of descent. In our study, we addressed the period 1979–2004. The cause of death fields were coded using ICD-9-CM for 1979–1998 and ICD-10 for 1999 and later. The data were collected from death certificates filed in the vital statistics offices of each state and the District of Columbia.
The list of diseases of interest includes acute coronary heart disease (CHD), stroke (acute cerebrovascular accident, CVA), cancer (malignant neoplasm), diabetes mellitus, asthma, Parkinson’s disease (PD) and Alzheimer’s disease (AD). These are represented by the following ICD-9-CM and ICD-10 codes: Cancer, for all sites combined: ICD-9-CM (140–208) and ICD-10 (C00-C97); acute CHD: ICD-9-CM (410, 411, 413) and ICD-10 (I20-I24); Stroke (CVA): ICD-9-CM (431, 436) and ICD-10 (I61, I64); Diabetes mellitus (excluding gestational): ICD-9-CM (250, 648.0, V77.1) and ICD10 (E10-E14); Asthma: ICD-9-CM (493) and ICD-10 (J45-J46); PD: ICD-9-CM (332) and ICD-10 (G20, F02.3); AD: ICD-9-CM (331.0 and 290.1) and ICD-10 (F00.x, G30.x).
Note that stroke (CVA) can be represented by a broader number of ICD-9-CM and ICD-10 codes. For example, in ICD-10 the whole group of I63.x codes represents cerebral infarction (WHO, 2007; Kokotailo and Hill, 2005). We, however, deliberately omitted this group to maintain better correspondence between ICD-9-CM and ICD-10 based on the codes availability in our study. That is, ICD-10 codes I63.x generally correspond to ICD-9-CM codes 433.x1 and 434.x1 (when the fifth digit is 1), both representing cerebral infarction. The fifth digits were, however, unavailable in the MCD data, so the use of the codes without fifth digit would allow us to achieve only approximate correspondence between two coding systems (e.g., if to use ICD-9-CM (431, 436, 433, 434) and ICD-10 (I61, I63, I64) for stroke), which would mean unnecessary loss of accuracy in our study.
3.2. Frequencies of diseases
The frequencies of diseases as well as joint disease frequencies are calculated as a ratio of the total numbers of respective ICD codes or their selected combinations that appeared in death certificates of selected population to the total numbers of deaths. Note that since several diseases can appear in the same death certificate as underlying or secondary causes of death, the frequencies are summed to n × 100% where n is the mean number of diseases per death certificate. Frequencies defined in this way take into account the contribution of secondary causes of death and illustrate a relative burden of a disease among others.
The correlations are calculated in terms of the above frequencies (f1 and f2) and joint frequencies (f12) which reflect a pattern of common appearance of a pair of diseases. The correlation coefficient was calculated using the formula
This coefficient changes between −1 and 1. The border value r = 1 corresponds to the situation when codes of the two conditions appear only together; then f12 = f1 =f2 and r = 1. The correlation is equal to −1 when in each death record there is a code from one (and only one) of two considered conditions; then f12 = 0, f1 = 1 – f2 and r = −1. The correlation is equal to 0 when codes of two groups appear independently. Then f12 =f1f2 and r = 0.
3.3. Dependent competing risks model capturing negative correlation between causes of death
The simplest model describing the negative correlation between competing risks is the multivariate lognormal frailty model. We illustrate the properties of such a model in the bivariate case and provide an example of negative and positive correlations between causes of death in Section 4.
The outline of this model is as follows. Let µi(Zi,x) = Ziµ0i(x), i = 1, 2, be two random hazards in the dependent competing risk problem with two risks. Here µ0i(x) is the baseline hazard at age x and Zi, i = 1,2, are frailties, which are correlated random variables having the bivariate lognormal distribution:
Here m1, m2, , and ρ are means, variances and correlation coefficient for the logarithms of frailties (i.e., they specify the associated normal distribution). Means, variances and correlation coefficient for frailties Zi, i = 1, 2, are calculated from these parameters as follows:
Traditionally, it is assumed in frailty models that the life spans are conditionally independent given frailties and mz = mz1 = mz2 = 1. Simulation studies comparing different estimation strategies for such bivariate lognormal models are presented in Wienke et al. (2005). The bivariate lognormal frailty models, in contrast to the widely used gamma-frailty models (Yashin et al., 1995), allow for negative correlations between frailties as well as between life spans.
4. Results
4.1. Results of analyses of MCD data
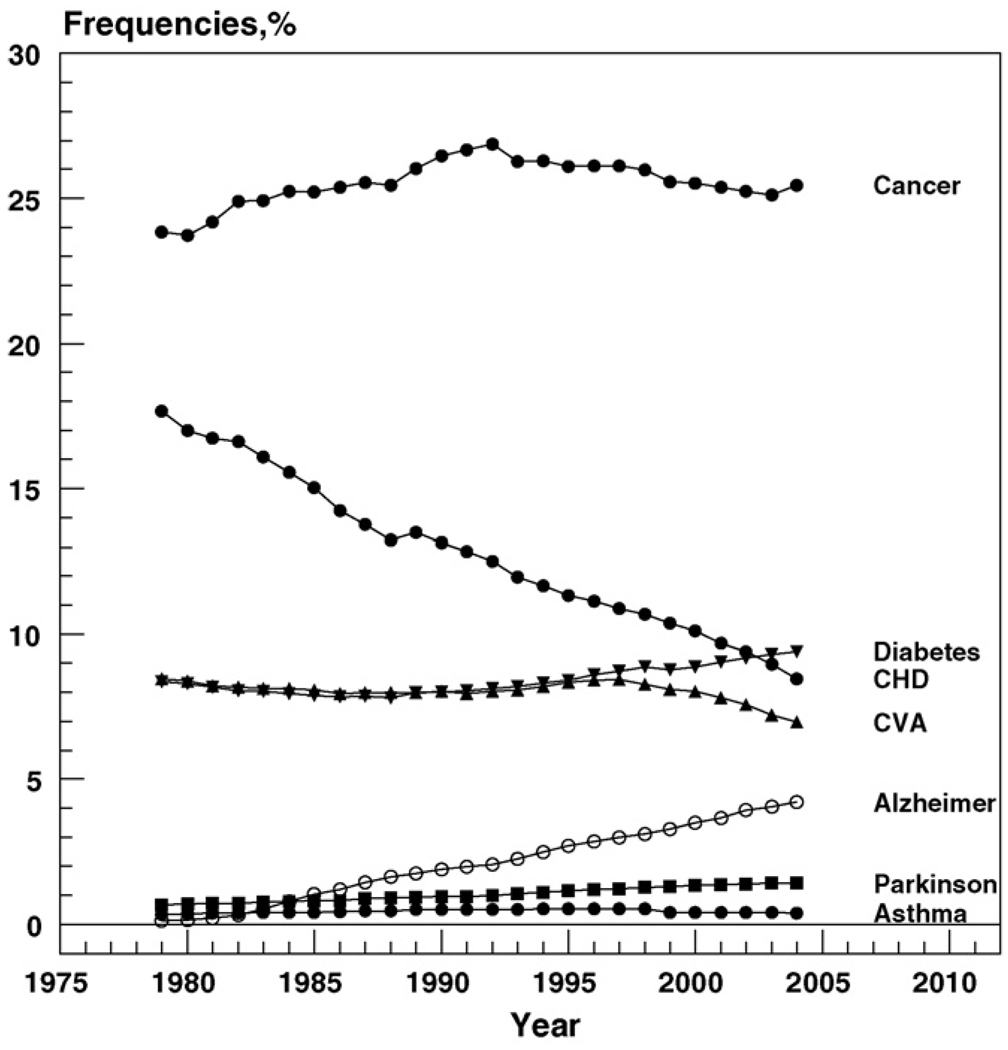
Fig. 1 shows the temporal trends in the proportion of deaths from diseases listed above in Section 3.1.
Fig. 1.
Time trends in proportions of death from selected diseases from 1979 to 2004. Because of the large numbers, all standard errors are close to zero.
One can see from this figure that the frequency of death from CHD declined dramatically during the 25-year-period, and the decline was relatively steady during the entire interval. The proportion of deaths from stroke started to decline later, about 10 years ago. Proportions of deaths from diabetes and AD increased over time. Frequencies of death from cancer, asthma, and PD did not show a substantial trend at the selected time interval.
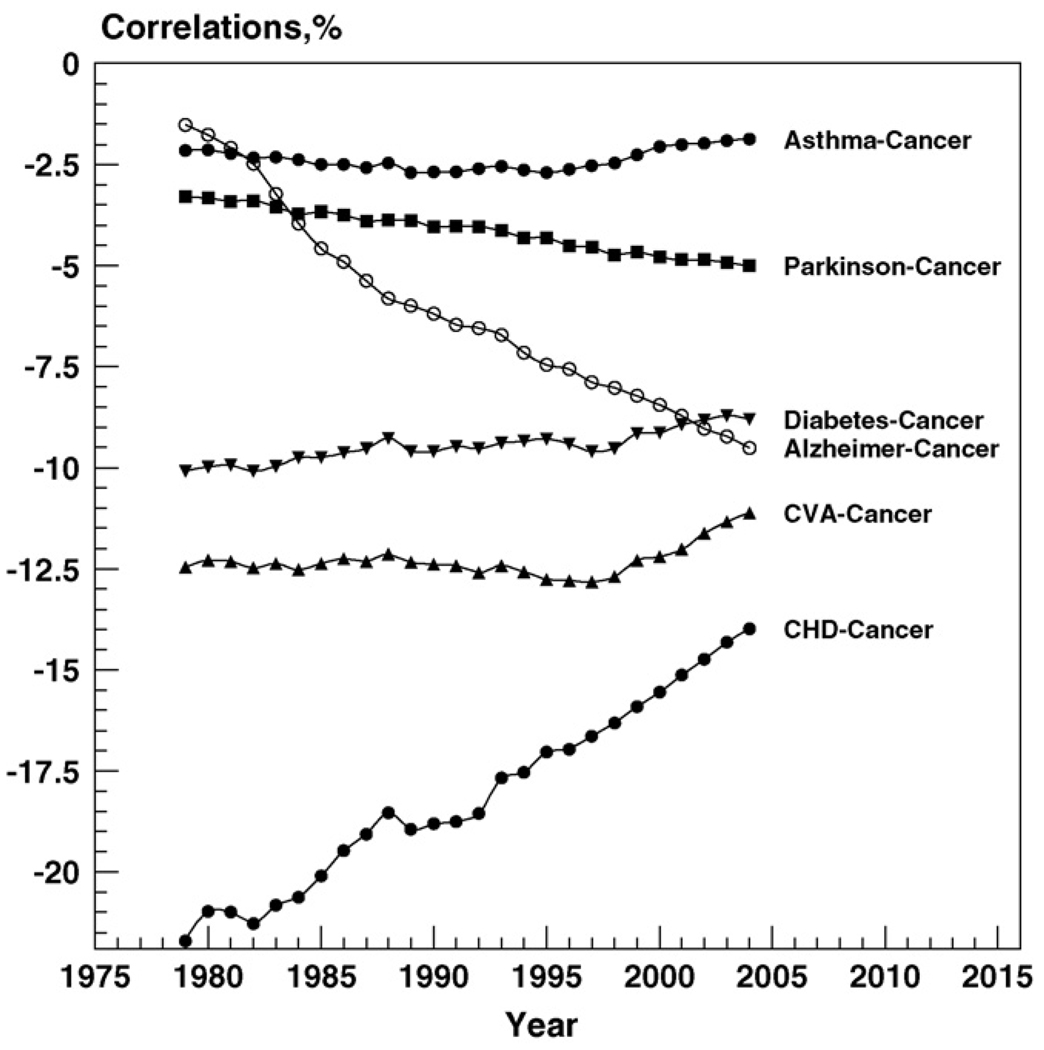
Fig. 2 shows negative correlations between cancer and selected diseases together with their time trends during 1979–2004. One can see from this figure that negative dependence was observed for asthma, PD, AD, diabetes, CVA, and CHD. For asthma and diabetes, this correlation remained relatively constant during the entire time interval. For CVA, this correlation was relatively constant until 1998. Then its absolute value started to decline. For PD, its absolute value slightly but steadily increased during the entire period. A much faster increase in the absolute value of correlation is observed for AD. The correlation between CHD and cancer has the highest absolute value. It also shows a substantial decline during the observational period.
Fig. 2.
Time trends in (negative) correlations between cancer and a number of other diseases between 1979 and 2004. Because of the large numbers, standard errors are close to zero.
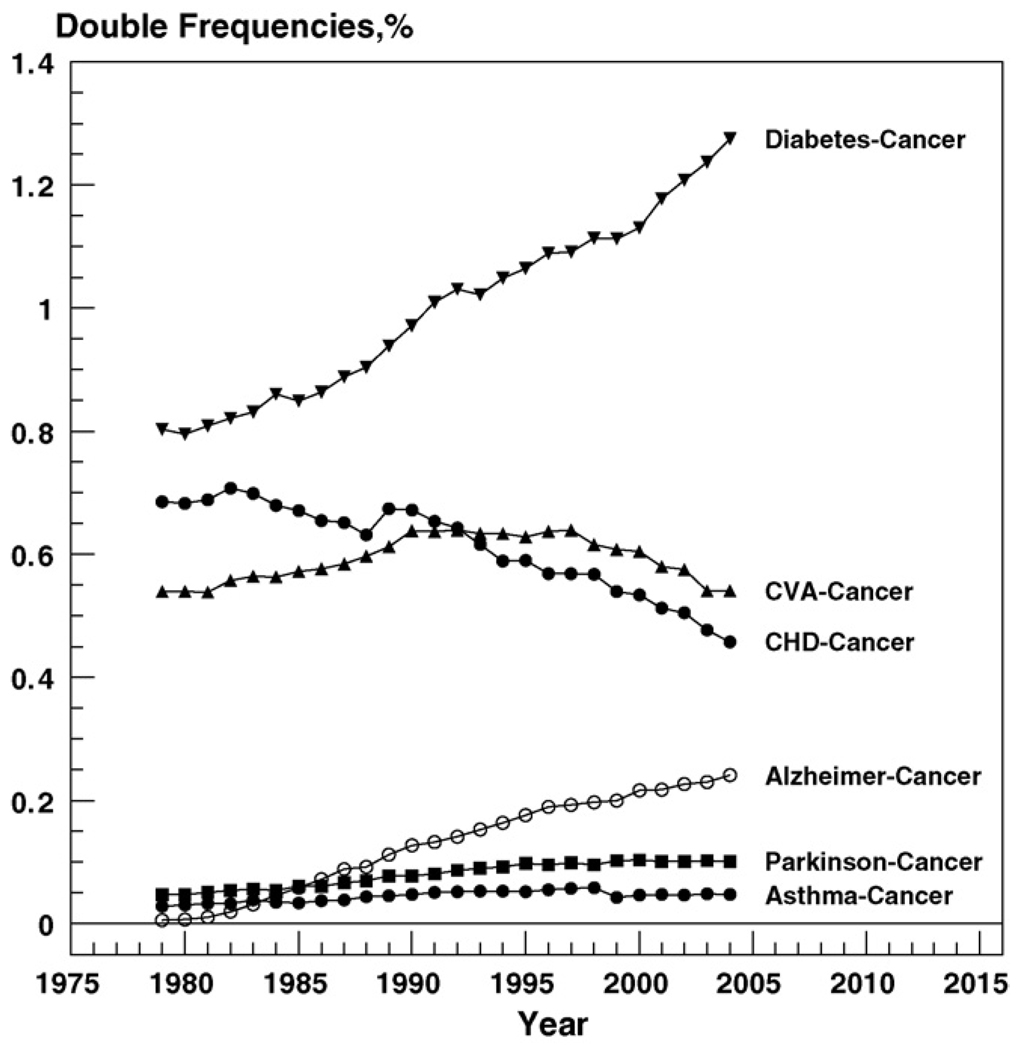
Fig. 3 shows values of joint frequencies of cancer and other diseases from the selected list, as well as their time trends.
Fig. 3.
Time trends in joint frequencies of death from cancer and selected diseases between 1979 and 2004. Because of the large numbers, standard errors are close to zero.
One can see from this figure that among six diseases negatively correlated with cancer, diabetes most frequently appears together with cancer in the death certificates and this frequency has a stable increasing trend. The joint frequency of cancer and AD was close to zero in 1979. Then it increased steadily at the entire time interval, reaching 0.25 in 2004. The joint frequencies of PD/cancer and asthma/cancer show a slight tendency to increase but remain small at the entire time interval. The frequency of CHD/cancer declined and CVA/cancer first increased and then declined.
4.2. Dependent competing risks model capturing negative correlation between causes of death: simulation study
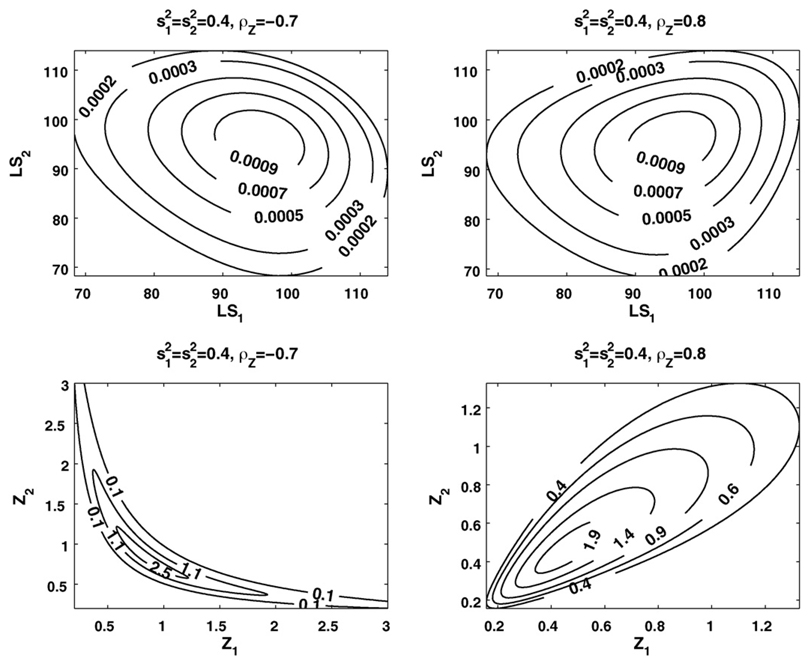
Examples of positive and negative correlations between frailties and between life spans for a hypothetical population are presented in Fig. 4 (we used the Gompertz baseline hazard rates µ01(x) = µ02(x) = µ0(x) = aebx with parameters a and b typical of human mortality data).
Fig. 4.
Examples of positive and negative correlations between frailties (Z) and between life spans (LS) in the bivariate lognormal frailty model with different frailty distributions.
One can see from this figure that in case of positive correlation between frailties the bivariate distributions of both frailty and life spans are spread along with the main diagonal. However, in case of negative correlation these distributions are spread in the directions opposite from the diagonal. These effects are better pronounced in the frailty distributions than in the life span ones.
5. Discussion
5.1. Importance of revealing negative correlations among causes of death
The negative correlation between cancer and a number of causes of death described above indicates that individuals susceptible to cancer might in principle be less susceptible to some other diseases. This link suggests a possibility of getting an additional (indirect) contribution to the longevity increase resulting from eradication or reduction mortality from cancer. Indeed, those individuals whose lives were saved from cancer may turn out to be more resistant to other diseases, which would result in an additional longevity increase. The reality is, however, more complicated because of often confronting effects of treatment of one disease on susceptibility to other disease(s) among survivors. This problem is well known to specialists in the dependent competing risk: the effect of reduction or elimination of selected risk on other risks dependsonhow such reduction or elimination is performed.
Studying trade-offs among diseases is also important for understanding factors affecting human longevity. The presence of such trade-offs indicates that an increase in longevity may happen not only due to affecting the rates of physiological aging but also due to the effects of changing balance between diseases, for which mortality risks are negatively correlated. This situation indicates the need for more careful studies of the roles of aging per se and aging associated diseases in connection between cancer and longevity.
5.2. Cancer and aging or cancer and other diseases?
The results of this study suggest that the connection between cancer and longevity discussed above may be mediated by trade-offs between cancer and other diseases, which do not directly involve any basic mechanism of aging per se. In humans, it could, for example, result from a trade-off between vulnerabilities to cancer and CHD or stroke as related to the differential effect of apoptosis on risks of/mortality from these diseases. For instance, in case of stroke neurons die from a damage caused by brain ischemia and the inherently higher intensity of apoptosis would increase the number of dead neurons. As a result, there will be more destroyed areas in the brain and a greater probability of paralysis or death from stroke. In case of cancer, the inherently higher cellular susceptibility to apoptosis would, conversely, mean a lower risk of the disease because fewer cells will survive damage leading to malignant transformation.
Correspondingly, the lower activity of apoptotic signal would mean more neurons surviving brain ischemia and the higher risk of malignant transformation. It was shown that neurons die from apoptosis in oxygen-deprived brains, and the lower activity of the apoptotic signal leads to better survival of neurons after the stroke-induced ischemia (Barinaga, 1998). Recent results of experimental animal studies of effects of histone deacetylase inhibitors as well as other compounds for treatment of acute stroke suggest that the medicated suppression of apoptosis may significantly improve survival and recovery after stroke (Harrison, 2007; Kim et al., 2007; Fisher et al., 2006). Preliminary results of a trial of the acute stroke treatment with DP-b99, a D-Pharm company compound (which protects primary cortical neurons from cell death induced by ischemia), demonstrated that such treatment may double the chances of the patients to recover from ischemic stroke (Rosenberg et al., 2005; D-Pharm, 2007). On the other side, the lower apoptotic activity has been shown to increase resistance of malignant tumors to anti-cancer therapy (Haffty and Glazer, 2003).
5.3. Time trends in negative correlation between cancer and other diseases
We suggested differential activity of apoptosis in tissues as one possible explanation for the negative correlation between cancer and CHD or stroke. One could also explain the observed weakening of this negative correlation over time within the same concept. It is possible that those who survived stroke or MI are more resistant to apoptosis and therefore more vulnerable to cancer as compared with deceased. If so, then improving survival from stroke and MI might contribute to the decline (in the absolute value) of the correlation between deaths from cancer and CHD over time shown in Fig. 2. It may happen as well that cancer treatment, which commonly induces apoptosis (e.g., with chemo or radiation therapy), increases the risk of stroke and CHD among cancer survivors and thus contributes to the weakening of the negative correlation between the diseases. The decline in the absolute value of negative correlation may also be the result of increased survival, because when individuals live longer, the chances of acquiring additional co-morbidity increase.
Besides the pairs cancer-CHD and cancer-CVA, the most prominent temporal trend in our study is observed for correlation between cancer and AD. An over time strengthening in this negative correlation could happen, for example, if cancer treatment (e.g., with cytostatic drugs) is someway protective against AD.
Our analyses show that mortality from cancer has positive correlation with some diseases as well. The presence of such correlation may mask the effects of reducing mortality from cancer on the total mortality and life expectancy for certain treatment strategies. However, as we mentioned before, the presence of positive correlation between causes of death does not exclude the possibility of a treatment, which will reduce mortality from both diseases. The search for a common susceptibility factor of external or internal origin could be the key for development of respective treatment strategy.
It is likely that during the life course a human organism undergoes the influence of factors capable of promoting both positive and negative correlation among diseases. In which way the co-morbidity pattern will be developing probably depends on duration and levels of exposure to these factors during certain periods of the individual’s life course. Studying these factors and mechanisms of their action will help better understand regularities of aging related decline in human health/well-being/survival status, and give a proper evaluation of consequences of interventions aiming at improving it.
5.4. Dependent competing risks capturing negative correlation between causes of death: modeling perspectives
Testing this hypothesis on connection of diseases at the epidemiological level requires the dependent competing risk model. Using such model, Rothenberg (1994) established that contribution of the CHD mortality decline to the increase in cancer mortality has been small and does not account for the increasing age-specific risk of cancer among older persons. Llorca and Delgado-Rodriguez (2001) used the Markov chain model to analyze interrelation between CVD, CHD, and cancer in Spanish females. They found that declines in CVD and CHD mortality did not have an impact on cancer mortality. Although the absence of positive correlation between cancer and other selected diseases did not explain time trends in cancer rates for the old and oldest old adults, it suggested the possibility of increase in the estimate of gain in life expectancy by searching for a negative correlation between death from cancer and from other causes.
Note that even in the simple case of the bivariate correlated frailty models described above one has several alternative ways to affect marginal distribution of life span (which will further affect mortality from respective cause). One deals with the reduction of respective baseline hazard. The other involves transformation of frailty distribution. These two strategies of reducing mortality rate from one cause will produce different effects on mortality rate from the other cause (Yashin and Iachine, 1996). This model may be appropriate for illustration of the effects of dependence between competing risks. However, it is too simplified to be used for evaluation of consequences of disease prevention and treatment. More sophisticated models of dependent competing risks, which include the effects of changes in physiological and other variables affecting risks of diseases, have to be used. The use and further extension of the stochastic process model of human mortality and aging for analyzing effects of dependence between diseases and respective causes of death (Yashin et al., 1986) looks as a promising alternative.
5.5. Dependence among diseases affects time trends in human health and survival
The last century was characterized by a persistent increase in survival in populations of the developed world. These changes resulted from improvements in the quality of nutrition, living conditions, the progress in medical technology and health care. The effects of these improvements on population health are, however, complicated. For example, the mortality rate from CHD substantially (about 3 fold) declined during the second half of the last century. The overall cancer mortality, however, increased until 1990s and only slightly declined afterwards in the U.S. (CDC, 2007). The decline in the incidence rate for CHD first appeared in 1950s. Only several decades later, the decline became visible for the cancer incidence rate (Sytkowski et al., 1996; IARC, 1965–2003).
Despite a substantial progress in understanding factors and mechanisms responsible for such a difference in trends, many important details remain unclear. The discordance of time trends in characteristics describing the two major human health disorders was initially explained by existence of common susceptibility factors. In particular, it was hypothesized that individuals, whose lives were saved from cardio-vascular death, remain more susceptible to cancer than other individuals in the population. Although it has long been recognized that existing trends and dependence between other diseases and cancer can affect trends in cancer morbidity and mortality, no detailed analyses of cancer trends have been performed that account for this possibility. Comprehensive analyses of this problem would require better understanding molecular biological and physiological mechanisms connecting different pathologies.
Acknowledgements
This work was supported by grants 5P01AG008761, R01AG028259, and R01AG027019 from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
References
- Anisimov VN. Carcinogenesis and Aging. vol. 1 and 2. Boca Raton: CRC Press; 1987. [Google Scholar]
- Anisimov VN. Physiological function of pineal gland (gerontological aspect) Russian Physiol. J. 1998;83:1–10. [Google Scholar]
- Anisimov VN, Khavinson VK, Mikhalski AI, Yashin AI. Effect of synthetic thymic and pineal peptides on biomarkers of ageing, survival and spontaneous tumour incidence in female CBA mice. Mech. Ageing Dev. 2001;122(1):41–68. doi: 10.1016/s0047-6374(00)00184-6. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Khavinson VKh, Zavarzina NYu, Zabezhinski MA, Zimina OA, Popovich IG, Shtylik AV, Malinin VV, Morozov VG, Arutjunyan AV, Oparina TI, Prokopenko VM. Effect of peptide bioregulators and melatonin on biomarkers of aging, life span and tumor development in mice. Adv. Gerontol. 2000;4:88–96. [Google Scholar]
- Barinaga M. Stroke-damaged neurons may commit cellular suicide. Science. 1998;281(5381):1302–1303. doi: 10.1126/science.281.5381.1302. [DOI] [PubMed] [Google Scholar]
- Bauer JH, Helfand SL. New tricks of an old molecule: lifespan regulation by p53. Aging Cell. 2006;5(5):437–440. doi: 10.1111/j.1474-9726.2006.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer JH, Poon PC, Glatt-Deeley H, Abrams JM, Helfand SL. Neuronal expression of p53 dominant-negative proteins in adult Drosophila melanogaster extends lifespan. Curr. Biol. 2005;15:2063–2068. doi: 10.1016/j.cub.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Blackwell BN, Bucci TJ, Hart RW, Turturro A. Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol. Pathol. 1995;23(5):570–582. doi: 10.1177/019262339502300503. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cancer and aging: yin, yang, and p53. Sci. Aging Knowledge Environ. 2002;1:pe1. doi: 10.1126/sageke.2002.1.pe1. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cancer and ageing: rival demons? Nat. Rev. Cancer. 2003;3(5):339–349. doi: 10.1038/nrc1073. [DOI] [PubMed] [Google Scholar]
- CDC. Trends in Health and Aging. Mortality. 2007 http://209.217.72.34/aging/ReportFolders/ReportFolders.aspx (as of Jan. 2008)
- Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- Conti S, Farchi G, Masocco M, Toccaceli V, Vichi M. The impact of the major causes of death on life expectancy in Italy. Int. J. Epidemiol. 1999;28(5):905–910. doi: 10.1093/ije/28.5.905. [DOI] [PubMed] [Google Scholar]
- Dilman VM, Anisimov VN. Effect of treatment with phenofromin, dyphenyl-hydantoin or l-DOPA on life span and tumor incidence in C3H/Sn mice. Gerontology. 1980;26:241–245. doi: 10.1159/000212423. [DOI] [PubMed] [Google Scholar]
- Donehower L. Does p53 affect organismal aging? J. Cell. Physiol. 2002;192:23–33. doi: 10.1002/jcp.10104. [DOI] [PubMed] [Google Scholar]
- D-Pharm announcement. 2007 DP-b99 Phase IIb trial results presented at the European Stroke Conference; 2007. http://www.dpharm.com/news.asp?year=2007&type=0&mode=1&id=91. [Google Scholar]
- Dumont P, Leu JI, Della Pietra AC, III, George DL, Murphy M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat. Genet. 2003;33:357–365. doi: 10.1038/ng1093. [DOI] [PubMed] [Google Scholar]
- Fisher M, Dávalos A, Rogalewski A, Schneider R, Ringelstein EB, Schäbitz W-R. Toward a multimodal neuroprotective treatment of stroke. Stroke. 2006;37(4):1129–1136. doi: 10.1161/01.STR.0000209330.73175.34. [DOI] [PubMed] [Google Scholar]
- García-Cao I, García-Cao M, Martín-Caballero J, Criado LM, Klatt P, Flores JM, Weill JC, Blasco MA, Serrano M. Super p53′ mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO. 2002;21(22):6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovski MD, Weisman NY, Arbeev KG, Ukraintseva SV, Yashin AI. Decrease in the lgl tumor suppressor dose in Drosophila increases survival and longevity in stress conditions. Exp. Gerontol. 2006;41(9):819–827. doi: 10.1016/j.exger.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Haffty BG, Glazer PM. Molecular markers in clinical radiation oncology. Oncogene. 2003;22:5915–5925. doi: 10.1038/sj.onc.1206704. [DOI] [PubMed] [Google Scholar]
- Harrison C. Neurological diseases: new avenues for stroke treatment. Nat. Rev. Drug Discov. 2007;6:520. [Google Scholar]
- Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu. Rev. Med. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- IARC (The International Agency for Research on Cancer [France]) IARC Sci. Publ. Volumes I-VIII. Lyon: IARC; 1965–2003. Cancer Incidence in Five Continents. [Google Scholar]
- Ivy GO, Rick JT, Murphy MP. Effects of l-deprenyl on manifestations of aging in the rat and dog. Ann. N.Y. Acad. Sci. 1994;717:45–59. doi: 10.1111/j.1749-6632.1994.tb12072.x. [DOI] [PubMed] [Google Scholar]
- James SJ, Muskhelishvili L, Gaylor DW, Turturro A, Hart R. Upregulation of apoptosis with dietary restriction: implications for carcinogenesis and aging. Environ. Health Perspect. 1998;106(Suppl 1):307–312. doi: 10.1289/ehp.98106s1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyfitz N. What difference would it make if cancer were eradicated? An examination of the taeuber paradox. Demography. 1977;14(4):411–418. [PubMed] [Google Scholar]
- Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J. Pharmacol. Exp. Ther. 2007;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- Kitani K, Kanai S, Carrillo MC, Ivy GH. (−)Deprenyl increases the life span as well as activities of superoxide dismutase and catalase but not of glutathione peroxidase in selective brain regions in Fisher rats. Ann. N.Y. Acad. Sci. 1994;717:60–71. doi: 10.1111/j.1749-6632.1994.tb12073.x. [DOI] [PubMed] [Google Scholar]
- Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- Lemaire J. The cost of firearm deaths in the United States: reduced life expectancies and increased insurance costs. J. Risk Insurance. 2005;72(4):679–683. [Google Scholar]
- Lim DS, Vogel H, Willerford DM, Sands AT, Platt KA, Hasty P. Analysis of ku80-mutant mice and cells with deficient levels of p53. Mol. Cell. Biol. 2000;20:3772–3780. doi: 10.1128/mcb.20.11.3772-3780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca J, Delgado-Rodriguez M. Competing risks analysis using Markov chains: impact of cerebrovascular and ischaemic heart disease in cancer mortality. Int. J. Epidemiol. 2001;30:99–101. doi: 10.1093/ije/30.1.99. [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402(6759):309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- Ørsted DD, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. Tumor suppressor p53 Arg72Pro polymorphism and longevity, cancer survival, and risk of cancer in the general population. J. Exp. Med. 2007;204(6):1295–1301. doi: 10.1084/jem.20062476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantanelli L, Zaia A, Rossolini G. Influence of l-deprenyl treatment on mouse survival kinetics. Ann. N.Y. Acad. Sci. 1994;717:72–78. doi: 10.1111/j.1749-6632.1994.tb12074.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg G, Angel I, Kozak A. Clinical pharmacology of DP-b99 in healthy volunteers: first administration to humans. Br. J. Clin. Pharmacol. 2005;60(1):7–16. doi: 10.1111/j.1365-2125.2005.02378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg RB. Competing mortality and progress against cancer. Epidemiology. 1994;5(2):197–203. doi: 10.1097/00001648-199403000-00010. [DOI] [PubMed] [Google Scholar]
- Sheldon WG, Bucci TJ, Hart RW, Turturro A. Age-related neoplasia in a lifetime study of ad libitum-fed and food-restricted B6C3F1 mice. Toxicol. Pathol. 1995;23(4):458–476. doi: 10.1177/019262339502300403. [DOI] [PubMed] [Google Scholar]
- Stallard E. Underlying and multiple cause mortality at advanced ages: United States 1980–1998. North Am. Actuarial J. 2002;6(3):64–87. [Google Scholar]
- Sytkowski PA, D’Agostino RB, Belanger A, Kannel WB. Sex and time trends in cardiovascular disease incidence and mortality: the Framingham Heart Study, 1950–1989. Am. J. Epidemiol. 1996;143(4):338–350. doi: 10.1093/oxfordjournals.aje.a008748. [DOI] [PubMed] [Google Scholar]
- ThyagaRajan S, Quadri SK. l-Deprenyl inhibits tumor growth, reduces serum prolactin, and suppresses brain monoamine metabolism in rats with carcinogen-induced mammary tumors. Endocrine. 1999;10:225–232. doi: 10.1007/BF02738621. [DOI] [PubMed] [Google Scholar]
- ThyagaRajan S, Meites J, Quadri SK. Deprenyl reinitiated estrous cycles, reduces serum prolactin, and decreases the incidence of mammary and pituitary tumors in old acyclic rats. Endocrinology. 1995;136:1103–1110. doi: 10.1210/endo.136.3.7867565. [DOI] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Hee Park S, Thompson T, Karsenty G, Bradley A, Donehower LA. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- Ukraintseva SV, Yashin AI. Individual aging and cancer risk: how are they related? Demographic Res. 2003a;9(8):163–196. [Google Scholar]
- Ukraintseva SV, Yashin AI. Opposite phenotypes of cancer and aging arise from alternative regulation of common signaling pathways. Ann. N.Y. Acad. Sci. 2003b;1010:489–492. doi: 10.1196/annals.1299.089. [DOI] [PubMed] [Google Scholar]
- Ukraintseva SV, Yashin AI. Cancer as “Rejuvenescence”. Ann. N.Y. Acad. Sci. 2004;1019:200–205. doi: 10.1196/annals.1297.032. [DOI] [PubMed] [Google Scholar]
- Ukraintseva SV, Yashin AI. Economic Progress as Cancer Risk Factor: Part II. Why is Overall Cancer Risk Higher in More Developed Countries? Max Planck Institute for Demographic Research Working Paper series, WP-2005–022. 2005 ( http://www.demogr.mpg.de/papers/working/wp-2005-022.pdf) [Google Scholar]
- van Heemst D, Mooijaart SP, Beekman M, Schreuder J, de Craen AJ, Brandt BW, Slagboom PE, Westendorp RG. Long Life study group. Variation in the human TP53 gene affects old age survival and cancer mortality. Exp. Gerontol. 2005;40(1–2):11–15. doi: 10.1016/j.exger.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Vogel H, Lim DS, Karsenty G, Finegold M, Hasty P. Deletion of Ku86 causes early onset of senescence in mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:10770–10775. doi: 10.1073/pnas.96.19.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford R. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215(4538):1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- WHO, ICD-10 on-line, version. Cerebrovascular Diseases (I60–I69) 2007 http://www.who.int/classifications/apps/icd/icd10online/?gi60.htm+i64.
- Wienke A, Arbeev KG, Locatelli I, Yashin AI. A comparison of different bivariate correlated frailty models and estimation strategies. Math. Biosci. 2005;198:1–13. doi: 10.1016/j.mbs.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Yashin AI, Iachine IA. Population Studies of Aging #18. Denmark: Odense University; 1996. Surprising Dynamics of Hazards in the Dependent Competing Risks Problem: The Case of Correlated Frailty. [Google Scholar]
- Yashin AI, Manton K, Stallard E. Dependent competing risks: a stochastic process model. J. Math. Biol. 1986;24:119–140. doi: 10.1007/BF00275995. [DOI] [PubMed] [Google Scholar]
- Yashin AI, Vaupel JW, Iachine IA. Correlated individual frailty: an advantageous approach to survival analysis of bivariate data. Math. Population Stud. 1995;5(2):145–159. doi: 10.1080/08898489509525394. [DOI] [PubMed] [Google Scholar]
- Yashin AI, Ukraintseva SV, De Benedictis G, Anisimov VN, Butov AA, Arbeev K, Jdanov DA, Boiko SI, Begun AZ, Bonafe M, Franceschi C. Have the oldest old adults ever been frail in the past? A hypothesis that explains modern trends in survival. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:B432–B442. doi: 10.1093/gerona/56.10.b432. [DOI] [PubMed] [Google Scholar]