Abstract
Chronic kidney disease (CKD) is common and associated with increased mortality in heart failure (HF). However, it is unknown whether the effect of CKD on mortality varies by left ventricular ejection fraction (LVEF). We evaluated the effect of CKD on mortality in systolic (LVEF ≤45%) and diastolic (LVEF >45%) HF patients. Of the 7788 patients in the Digitalis Investigation Group trial, 3527 (45%) had CKD (estimated glomerular filtration rate <60 ml/min/1.73m2). We calculated propensity score for CKD for each patient, using a multivariable logistic regression model (c statistic=0.76; post-match absolute standardized differences <5% for all 32 covariates). We matched 2399 pairs of patients with and without CKD with similar propensity scores. There were 757 (rate, 1,049/10,000 person-year) and 882 (rate, 1,282/10,000 person-year) deaths respectively in patients without and with CKD (hazard ratio=1.22, 95% confidence interval {CI}=1.09–1.36; p<0.0001). CKD-associated mortality was higher in diastolic HF (371 extra deaths/10,000 person-year; hazard ratio=1.71; 95% CI=1.21–2.41; p=0.002) than in systolic HF (214 extra deaths/10,000 person-year; hazard ratios =1.19; 95% CI =1.07–1.32; p=0.001), which was significant (adjusted p for interaction=0.034). There was a graded association between CKD-related deaths and LVEF. Hazard ratios (95% CI) for CKD-associated mortality for LVEF subgroups <35%, 35–55% and >55% were respectively 1.15 (1.02–1.29), 1.35 (1.11–1.64), and 2.33 (1.34–4.06). In conclusion, CKD-associated mortality was higher in diastolic than in systolic HF. Diastolic HF patients should be evaluated for CKD and the role of inhibitors of the renin-angiotensin system in these patients needs to be investigated.
Keywords: heart failure, chronic kidney disease, ejection fraction, and mortality
Chronic kidney disease (CKD) is common in heart failure (HF) and is associated with increased mortality in systolic HF or clinical HF with low left ventricular ejection fraction (LVEF).1–3 However, little is know about the effect of CKD in diastolic HF, or clinical HF with normal or near normal LVEF. In particular, it is unknown if the effect of CKD on mortality in HF varies by LVEF. HF patients with CKD often have higher burden of comorbidity, which in part explains their poor prognosis.1–3 To address this concern, we used propensity score methods to assemble a cohort of HF patients with and without CKD who were well-balanced in all measured baseline characteristics except for the presence of CKD.4–8 The objectives of our study were to determine whether the effect of CKD on HF outcomes would persist using propensity score methods, and to determine whether the effect of CKD on HF mortality varied by baseline ejection fraction.
Methods
This is a post-hoc propensity score analysis of the Digoxin Investigation Group (DIG) trial, which was conducted during 1991–1993 in the US and Canada. Detailed description of the rationale, design, and results of the DIG trial has been published previously.9 Of the 7788 ambulatory chronic HF patients in normal sinus rhythm, 6800 had systolic HF (LVEF<=45%) and 988 had diastolic HF (LVEF>45%). Patients with serum creatinine >2.5 mg/dL were excluded. Most patients were receiving diuretics (78%) and ACE inhibitors (93%). Data on beta-blockers were not collected. Data on baseline serum creatinine and LVEF were obtained for all 7788 participants. We estimated baseline glomerular filtration rate (GFR) using the Modification of Diet in Renal Disease (MDRD) formula,10 and defined CKD as calculated glomerular filtration rate(GFR) <60 ml/1.73 m2 body surface area.11 Recent (≤6 months) LVEF was acceptable if the patient’s clinical condition remained stable.12 When more than one technique was used to measure LVEF, angiographic or radionuclide techniques were preferred to echocardiogram. The primary outcome of this study was all-cause mortality during a median follow up of 38 months. Vital status was collected up to December 31, 1995 and was ascertained for 99% of the patients.13 We focus our current analysis to a subset of a pair of 2399 propensity-matched pairs of patients with or without CKD at baseline.
Because HF patients with CKD were older and sicker (Table 1), propensity scores were used to reduce imbalance in baseline covariates between patients with and without CKD. The propensity score is the conditional probability of having an exposure (e.g. CKD) given a vector of measured covariates.4–8 We calculated the propensity score for CKD for each patient using a non-parsimonious multivariable logistic regression model (c statistic = 0.76), in which CKD (present or absent) was the outcome variable and all measured baseline patient characteristics listed in Table 1 were independent variables. We then used these derived propensity scores to match 2399 (68%) CKD patients with 2399 patients without CKD who had the similar propensity scores.14 The effectiveness of bias reduction after matching was assessed by standardized differences, expressed as a percentage of the pooled standard deviations.6,7,15 We supplemented these pre- and post-match selection bias assessments with Chi-square and student’s t-test or Wilcoxon rank-sum test as appropriate for categorical and continuous variables (Table 1).
Table 1.
Baseline characteristics of patients with and without chronic kidney disease, before and after propensity score matching
| Variables | Before Propensity Score Match |
P | After Propensity Score Match |
P | ||
|---|---|---|---|---|---|---|
| No CKD (n=4261) | CKD (n=3527) | No CKD (n= 2399) | CKD (n=2399) | |||
| Age (years) | 60.3 (±10.9) | 68.3 (±9.1) | <0.001 | 65.4 (±8.9) | 65.4 (±8.8) | 0.776 |
| Women | 827 (19.4%) | 1099 (31.2%) | <0.001 | 574 (23.9%) | 564 (23.5%) | 0.760 |
| Non-whites | 816 (19.2%) | 312 (8.8%) | <0.001 | 297 (12.4%) | 280 (11.7%) | 0.451 |
| Body mass index (kg/m2) | 27.6 (±5.5) | 26.9 (±5.2) | <0.001 | 27.2 (±5.2) | 27.2 (±5.2) | 0.801 |
| Duration of heart failure (months) | 15 | 18 | 0.049 | 15 | 18 | 0.232 |
| Primary cause of heart failure | ||||||
| Coronary ischemic | 2795 (65.6%) | 2565 (72.7%) | 1710 (71.3%) | 1699 (70.8%) | ||
| Hypertensive | 438 (10.3%) | 367 (10.4%) | <0.001 | 253 (10.5%) | 236 (9.8%) | 0.647 |
| Idiopathic | 697 (16.4%) | 414 (11.7%) | 302 (12.6%) | 327 (13.6%) | ||
| Others | 331 (7.8%) | 181 (5.1%) | 134 (5.6%) | 137 (5.7%) | ||
| Prior myocardial infarction | 2588 (60.7%) | 2320 (65.8%) | <0.001 | 1574 (65.6%) | 1553 (64.7%) | 0.525 |
| Current angina pectoris | 1104 (25.9%) | 1011 (28.7%) | 0.007 | 667 (27.8%) | 648 (27.0%) | 0.560 |
| Hypertension | 1887 (44.3%) | 1787 (50.7%) | <0.001 | 1140 (47.5%) | 1130 (47.1%) | 0.795 |
| Diabetes mellitus | 1123 (26.4%) | 1095 (31.0%) | <0.001 | 703 (29.3%) | 697 (29.1%) | 0.874 |
| Medications | ||||||
| Digoxin (pre-trial use) | 1865 (43.8%) | 1500 (42.5%) | 0.280 | 1002 (41.8%) | 1032 (43.0%) | 0.397 |
| Digoxin (trial use) | 2143 (50.3%) | 1746 (49.5%) | 0.495 | 1186 (49.4%) | 1204 (50.2%) | 0.624 |
| ACE inhibitors | 4009 (94.1%) | 3265 (92.6%) | 0.008 | 2235 (93.2%) | 2243 (93.5%) | 0.685 |
| Nitrates and hydralazine | 44 (1.0%) | 67 (1.9%) | 0.001 | 33 (1.4%) | 32 (1.3%) | >0.999 |
| Non-potassium-sparing diuretics | 3127 (73.4%) | 2949 (83.6%) | <0.001 | 1906 (79.4%) | 1902 (79.3%) | 0.915 |
| Potassium-sparing diuretics | 272 (6.4%) | 324 (9.2%) | <0.001 | 190 (7.9%) | 184 (7.7%) | 0.788 |
| Potassium supplement | 1106 (26.0%) | 1093 (31.0%) | <0.001 | 683 (28.5%) | 688 (28.7%) | 0.873 |
| Symptoms and signs (current) | ||||||
| Dyspnea at rest | 902 (21.2%) | 803 (22.8%) | 0.093 | 523 (21.8%) | 527 (22.0%) | 0.917 |
| Dyspnea on exertion | 3131 (73.5%) | 2731 (77.4%) | <0.001 | 1818 (75.8%) | 1818 (75.8%) | >0.999 |
| Jugular venous distension | 497 (11.7%) | 523 (14.8%) | <0.001 | 310 (12.9%) | 326 (13.6%) | 0.523 |
| Third heart sound | 965 (22.6%) | 881 (25.0%) | 0.017 | 566 (23.6%) | 571 (23.8%) | 0.892 |
| Pulmonary râles | 646 (15.2%) | 655 (18.6%) | <0.001 | 392 (16.3%) | 409 (17.0%) | 0.536 |
| Lower extremity edema | 826 (19.4%) | 807 (22.9%) | <0.001 | 502 (20.9%) | 503 (21.0%) | >0.999 |
| NYHA functional class | ||||||
| I | 678 (15.9%) | 425 (12.0%) | 282 (11.8%) | 346 (14.4%) | ||
| II | 2438 (57.2%) | 1806 (51.2%) | <0.001 | 1364 (56.9%) | 1283 (53.5%) | 0.658 |
| III | 1093 (25.7%) | 1194 (33.9%) | 714 (29.8%) | 705 (29.4%) | ||
| IV | 52 (1.2%) | 102 (2.9%) | 39 (1.6%) | 65 (2.7%) | ||
| Heart rate (per minute), mean (±SD) | 78.6 (±12.6) | 78.1 (±12.6) | 0.133 | 78.4 (±12.2) | 78.0 (±12.5) | 0.325 |
| Blood pressure, systolic (mm Hg) | 126.0 (±19.6) | 129.0 (±21.4) | <0.001 | 127.7 (±20.0) | 127.7 (±21.0) | 0.905 |
| Blood pressure, diastolic (mm Hg) | 75.8 (±11.3) | 74.5 (±11.3) | 0.01 | 75.0 (±11.4) | 75.0 (±11.4) | 0.951 |
| Chest radiograph findings | ||||||
| Cardiothoracic ratio > 0.5 | 2473 (31.8%) | 2217 (62.9%) | 0.001 | 1460 (60.9%) | 1421 (59.2%) | 0.263 |
| Pulmonary congestion | 562 (13.2%) | 547 (15.5%) | 0.004 | 348 (14.5%) | 348 (14.5%) | >0.999 |
| Serum creatinine (mg/dL), | 1.06 (±0.18) | 1.55 (±0.37) | <0.001 | 1.04 (±0.17) | 1.55 (±0.35) | <0.001 |
| Serum potassium (mEq/L) | 4.31 (±0.43) | 4.38 (±0.45) | <0.001 | 4.35 (±0.40) | 4.35 (±0.45) | 0.871 |
| Ejection fraction (%) | 31.8 (±12.3) | 32.2 (±12.9) | 0.379 | 31.9 (±12.5) | 32.0 (±12.6) | 0.789 |
ACE = angiotensin-converting enzyme, NYHA = New York Heart Association
Initially, we estimated the effect of CKD on mortality in propensity-matched patients using matched Cox regression analyses. Then, to test for potential interactions between CKD and diastolic HF, we calculated absolute risk differences and then formally tested for interactions using Mantel–Haenszel tests of homogeneity.16 To account for duration of follow up, we calculated rates of death per 10,000 person-years of follow up. In addition, we also tested for first-order interactions between CKD and diastolic HF in bivariate and multivariable Cox proportional hazards models, in which CKD, diastolic HF and their interactions were entered as covariates.. Because of a significant interaction between CKD and diastolic HF, we performed separate Cox regression analyses in patients with systolic and diastolic HF. Kaplan-Meier survival analyses and log-rank tests were used to compare cumulative mortality in patients with (1) systolic HF-no CKD, (2) systolic HF-CKD, (3) diastolic HF-no CKD, and (4) diastolic HF-CKD.
To determine if there was a graded relationship with LVEF and CKD related mortality, we categorized patients by LVEF <35%, 35–55% and >55%.17 We combined LVEF 35–45% and 46–55% into one group, as these subgroups have been shown to be prognostically similar.18 Finally, we tested for interaction using LVEF as a continuous variable.
To determine if the effects of CKD-related mortality also varied in subgroups, we performed subgroup analyses by age, sex, race, HF etiology, NYHA functional class, presence of diabetes, treatment with ACE inhibitors, diuretics, and digoxin, adjusting for propensity scores. In addition, we also tested for first-order interaction between CKD and each of these covariates, in all patients, adjusting for all covariates16. All statistical tests were evaluated using a two-tailed 95% confidence level. All data analyses were performed using SPSS for Windows version 14 (SPSS Inc. Chicago, IL).
Results
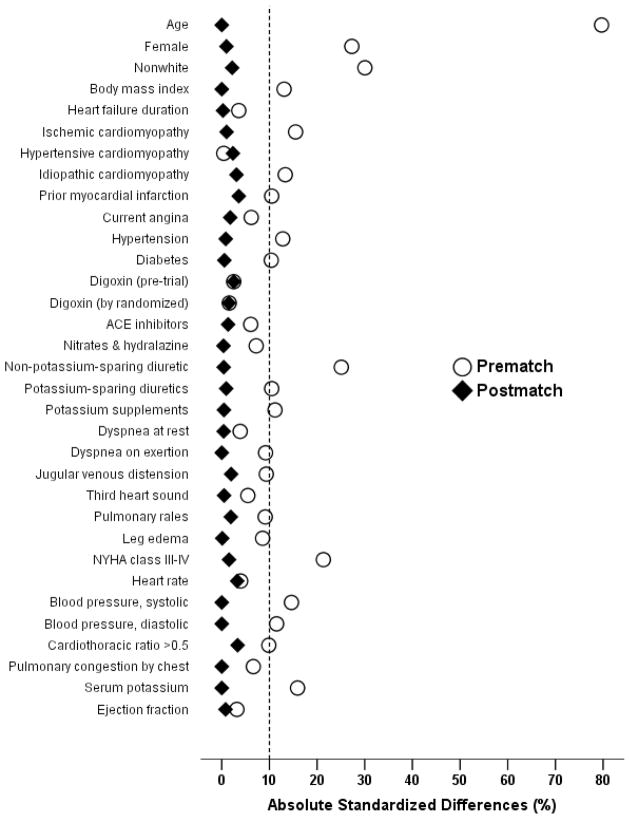
Before matching, 45% of patients had CKD. The prevalence of CKD among systolic and diastolic HF patients were respectively 49% and 45% (p=0.01). After matching, CKD and no-CKD patients were similar in all 32 baseline covariates (Table 1 and Figure 1). Before matching, mean propensity score was significantly higher for patients with CKD (0.56) than those without CKD (0.37), with a standardized difference of 98% in linear propensity score. After matching, mean propensity scores for patients with and without CKD were 0.48, with a standardized difference of 0.1% in linear propensity score. Absolute standardized differences for all measured covariates were reduced to <5% in the post-match cohort, suggesting significant bias reduction (Figure 1).
Figure 1.
Absolute standardized differences in baseline covariates between patients with and without chronic kidney disease, before and after propensity score matching (post-match standardized difference <5% indicate excellent covariate balance).
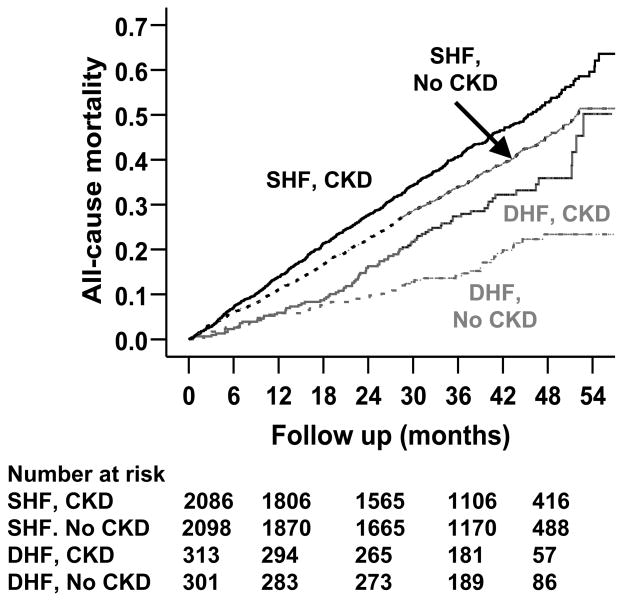
During a median 38 months of follow up, 1639 (34%) patients died from all causes. The Kaplan Meier plots for all-cause mortality for systolic and diastolic HF by CKD are displayed in Figure 2. CKD was associated with increased mortality in both systolic and diastolic HF (Table 2). However, CKD-related mortality was higher in those with diastolic HF. Mortality due to all causes occurred in 757 patients without CKD during 7,216 years of follow up (1,049/10,000 person years) and 882 patients with CKD during 6,877 years (1,282/10,000 person years) of follow up (hazard ratio=1.22, 95% confidence interval=1.09–1.36; p<0.0001; Table 2).
Figure 2.
Kaplan-Meier plots for cumulative risk of all-cause death associated with chronic kidney disease (CKD) (SHF=systolic heart failure or heart failure with ejection fraction ≤ 45% and DHF=diastolic heart failure or heart failure with ejection fraction >45%)
Table 2.
Chronic kidney disease (CKD), ejection fraction (EF) and all-cause mortality in heart failure (HF)
| Death/total follow up in year (Death rate Per 10,000 person-years of follow-up) |
Rate difference (Per 10,000 Person- Years) | Hazard ratio (95% CI) | Adjusted* hazard ratio (95% CI) | |||
|---|---|---|---|---|---|---|
| All patients | No CKD | CKD | ||||
| All (N=4798) | 1639 / 14093 (1163) | 757 / 7216 (1049) | 882 / 6877 (1282) | 233 | 1.22 (1.09 – 1.36); p<0.0001 | 1.26 (1.12 – 1.42); p<0.0001 |
| EF <=45% (N=4184) | 1500 / 12152 (1234) | 705 / 6238 (1130) | 795 / 5914 (1344) | 214 | 1.19 (1.07 – 1.32); p=0.001 | 1.19 (1.07 – 1.32); p=0.001 |
| EF >45% (N=614) | 139 / 1941 (716) | 52 / 978 (532) | 87 / 963 (903) | 371 | 1.71 (1.21 – 2.41); p=0.002 | 1.74 (1.23 – 2.45); p=0.002 |
| EF <35% (N=2963) | 1164 / 8417 (1383) | 558 / 4328 (1289) | 606 / 4090 (1482) | 192 | 1.15 (1.02 – 1.29); p=0.019 | 1.14 (1.02–1.28); p=0.027 |
| EF 35–55% (N=1585) | 416 / 4879 (853) | 181 / 2485 (728) | 235 / 2395 (981) | 253 | 1.35 (1.11 – 1.64); p=0.002 | 1.40 (1.15 – 1.70); p=0.001 |
| EF >55% (N=250) | 59 / 796 (741) | 18 / 404 (446) | 41 / 392 (1046) | 600 | 2.33 (1.34 – 4.06); p=0.002 | 2.25 (1.28 – 3.92); p=0.005 |
Adjusted for age, sex, race, body mass index, HF duration, HF etiology, prior myocardial infarction, current angina pectoris, hypertension, diabetes mellitus, use of digoxin (pre-trial), angiotensin-converting enzyme inhibitors, diuretics, and combination of hydralazine and nitrates, current dyspnea at rest and dyspnea on exertion, heart rate, systolic and diastolic blood pressure, current jugular venous distension, third heart sound, pulmonary râles, and lower extremity edema, NYHA functional class, pulmonary congestion and cardiothoracic ratio >0.5 by chest x-ray, serum potassium, and ejection fraction.
In systolic HF, 705 (1,130/10,000 person-year) and 795 (1,344/10,000 person-year) patients respectively without and with CKD died (hazard ratio=1.19, 95% confidence interval=1.07–1.32; p=0.001; Table 2). In diastolic HF, in contrast, 52 (532/10,000 person-year) and 87 (903/10,000 person-year) patients respectively without and with CKD died (hazard ratio=1.71, 95% confidence interval=1.21–2.41; p=0.002; Table 2). These difference in CKD-related death in systolic and diastolic HF were significant (adjusted p for interaction=0. 034).
There was a graded increase in the risk of CKD-related mortality among HF patients with LVEF <35%, 35–55% and >55% (Table 2). When LVEF was used as a continuous variable, there was a significant increase in CKD-related death with increase in LVEF (adjusted p for interaction =0.004). When we repeated our analysis in the full (pre-match) cohort of 7788 patients, we noted similar results. The associations of CKD with all-cause mortality in various subgroups of patients are displayed in Figure 3. There were no significant interactions between CKD and any of these covariates (except LVEF).
Figure 3.
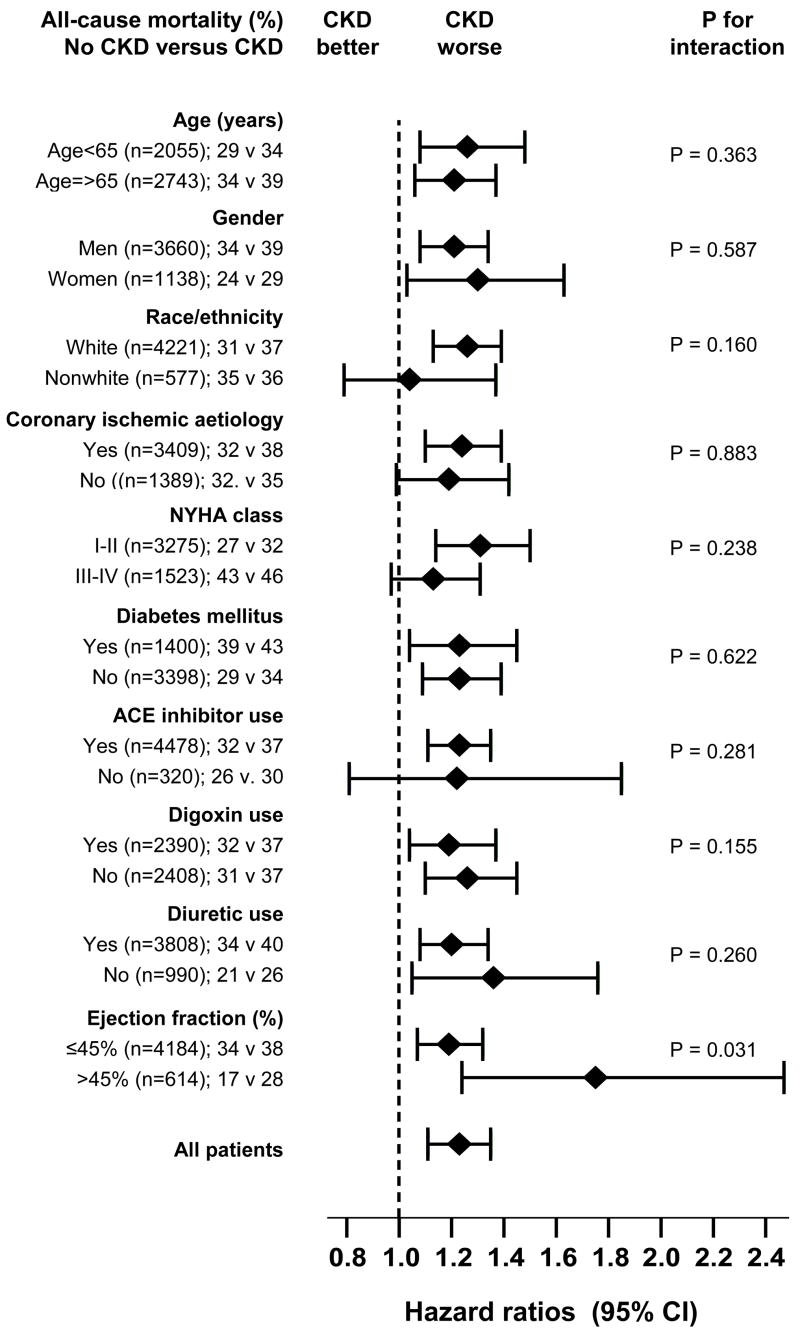
Hazard ratios and 95% confidence intervals for mortality associated with chronic kidney disease (CKD) in subgroups of patients with heart failure (ACEI=angiotensin-converting enzyme inhibitor, DM=diabetes, EF=ejection fraction, NYHA=New York Heart Association)
Discussion
There are 2 key findings of the current study: first, in a propensity score matched cohort of ambulatory chronic HF patients receiving diuretics and ACE inhibitors, presence of CKD was associated with increased risk of all-cause mortality, and second, CKD-associated mortality was worse in diastolic HF patients than in those with systolic HF. CKD is common in HF and is associated with increased mortality. However, our finding that CKD-associated mortality is higher in diastolic HF is new. This is important because as the US population ages, the prevalence of diastolic HF is likely to increase in the coming decades.
Increased CKD-related mortality is unlikely to be explained by differences in baseline covariates between patients with and without CKD as after matching, all measured covariates, including age, HF etiology, NYHA class, LVEF, and medication use, were well balanced with absolute standardized differences at <5% (Figure 1). Our finding of increased all-cause mortality associated with CKD in a wide spectrum of HF patients is consistent with data published by other investigators.1–3 However, in those studies, there were significant imbalances among baseline covariates between patients with and without CKD (similar to our pre-match cohort, Table 1), raising the possibility of residual bias despite multivariable regression-based risk adjustment. However, in the current analysis, we confirm that the effect of CKD on mortality in HF persist even when HF patients with and without CKD are matched by propensity scores for CKD.
HF and CKD share a number of etiological factors including diabetes and hypertension. The high prevalence of CKD in HF may therefore be related to pre-existence of these common risk factors.11 In addition, patients with HF often develop cardiorenal syndrome, which is due to low effective glomerular filtration pressure caused by low cardiac output. It is noteworthy that the prevalence of CKD in diastolic HF was as high as that in systolic HF. This might in part be due to more advanced age in diastolic HF patients. Mean age of diastolic HF patients was 3 years older than those with systolic HF (p<0.0001). Among HF patients 75 years and older, the prevalence of CKD has been reported to be higher in diastolic HF than in systolic HF.19 However, mechanisms of worsening kidney function in diastolic HF are less well understood.
An intriguing finding of the current analysis is that CKD-associated mortality was higher in diastolic HF than in systolic HF. In addition, there was a graded-response relation, with higher CKD-related mortality occurring with increasing LVEF. It is possible that CKD in systolic HF was not truly intrinsic kidney disease, but was due to reversible reduction in GFR associated with use of ACE inhibitors. However, in our matched analysis, 93% patients in each group were receiving ACE inhibitors. It is also possible that CKD in more elderly diastolic HF patients was intrinsic in nature and was also in more advanced stages. Advanced CKD is associated with vascular calcification, anemia and hypovitaminosis D, all of which are associated with increased risk of death. Another explanation might be that the prognosis in systolic HF was worse than that in diastolic HF and thus relatively less affected by CKD. There is emerging evidence that use of ACE inhibitors is associated with reduced mortality in systolic HF patients with CKD.2,20,21 However, little is known about the effects of ACEI inhibitors or angiotensin receptor blockers in diastolic HF patients with CKD. The effect of these drugs in diastolic HF patients with CKD needs to be determined in a randomized clinical trial, or a well-designed prospective follow up study.
Our study has several potential limitations. The results might be affected by unmeasured or hidden covariates, or by incomplete or inexact matching. To address concerns about loss of data due to incomplete matching, we analyzed data from all 7788 patients, using both direct regression adjustment for propensity score, and stratification based on quintiles of propensity scores, and found similar results. The DIG trial enrolled predominantly white, male, and relatively younger HF patients with normal sinus rhythm and mild to moderate CKD. We defined CKD using GFR estimated by the MDRD formula, which might have underestimated GFR in HF patients without CKD and may have misclassified CKD patients as having no CKD.22 The rate of use of beta-blocker was probably low among DIG participants as beta-blockers were not yet approved for use in systolic HF. However, the relative infrequency of beta-blocker use in the era of the DIG trial should not affect the association of CKD with mortality as beta-blockers are equally efficacious in HF patients with or without CKD. So, the greater use of beta-blockers would have lowered the overall mortality rate in our study, but should not have affected the associations of CKD with mortality in patients with systolic and diastolic HF. This would only have biased our findings if beta-blockers were more beneficial in HF patients with CKD than without CKD. However, there is no evidence to suggest this interaction.
In conclusion, CKD was associated with increased mortality in HF and CKD-associated mortality was higher in diastolic than in systolic HF. Diastolic HF patients should be evaluated for CKD and the role of inhibitors of renin-angiotensin system in these patients needs to be investigated.
Acknowledgments
Funding/Support: Dr. Ahmed is supported by the National Institutes of Health through grants from the National Institute on Aging (1-K23-AG19211-04) and the National Heart, Lung, and Blood Institute (1-R01-HL085561-01 and P50-HL077100).
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Shlipak MG, Smith GL, Rathore SS, Massie BM, Krumholz HM. Renal function, digoxin therapy, and heart failure outcomes: evidence from the digoxin intervention group trial. J Am Soc Nephrol. 2004;15:2195–2203. doi: 10.1097/01.ASN.0000135121.81744.75. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed A, Kiefe CI, Allman RM, Sims RV, DeLong JF. Survival benefits of angiotensin-converting enzyme inhibitors in older heart failure patients with perceived contraindications. J Am Geriatr Soc. 2002;50:1659–1666. doi: 10.1046/j.1532-5415.2002.50457.x. [DOI] [PubMed] [Google Scholar]
- 3.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109:1004–1009. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Asso. 1984;79:516–524. [Google Scholar]
- 5.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 6.Ahmed A, Rich MW, Love TE, Lloyd-Jones DM, Aban IB, Colucci WS, Adams KF, Gheorghiade M. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27:178–186. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed A, Husain A, Love TE, Gambassi G, Dell’italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed A, Perry GJ, Fleg JL, Love TE, Goff DC, Jr, Kitzman DW. Outcomes in ambulatory chronic systolic and diastolic heart failure: A propensity score analysis. Am Heart J. 2006;152:956–66. doi: 10.1016/j.ahj.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 12.The Digitalis Investigation Group. Protocol: Trial to evaluate the effect of digitalis on mortality n heart failure. NHLBI. 1991:1–36. [Google Scholar]
- 13.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–730. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Levesque R. Macro. In: Levesque R, editor. SPSS® Programming and Data Management, 2nd Edition. A Guide for SPSS® and SAS® Users. Chicago, IL: SPSS Inc; [Last access date: June 4, 2005]. Available online at: http://www.spss.com/spss/data_management_book.htm. [Google Scholar]
- 15.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 16.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–186. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 17.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–327. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 18.Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, Portnay EL, Marshalko SJ, Radford MJ, Krumholz HM. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–742. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 19.Kerzner R, Gage BF, Freedland KE, Rich MW. Predictors of mortality in younger and older patients with heart failure and preserved or reduced left ventricular ejection fraction. Am Heart J. 2003;146:286–290. doi: 10.1016/S0002-8703(03)00151-0. [DOI] [PubMed] [Google Scholar]
- 20.Frances CD, Noguchi H, Massie BM, Browner WS, McClellan M. Are we inhibited? Renal insufficiency should not preclude the use of ACE inhibitors for patients with myocardial infarction and depressed left ventricular function. Archives of Internal Medicine. 2000;160:2645–2650. doi: 10.1001/archinte.160.17.2645. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed A, Love TE, Sui X, Rich MW. Effects of ACE inhibitors in systolic heart failure patients with chronic kidney disease: A propensity score analysis. J Card Failure. 2006;12:499–506. doi: 10.1016/j.cardfail.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]