Abstract
Autophagy is an intracellular bulk degradation process whereby cytoplasmic proteins and organelles are degraded and recycled through lysosomes. In the heart, autophagy plays a homeostatic role at basal levels, and the absence of autophagy causes cardiac dysfunction and the development of cardiomyopathy. Autophagy is induced during myocardial ischemia and further enhanced by reperfusion. Although induction of autophagy during the ischemic phase is protective, further enhancement of autophagy during the reperfusion phase may induce cell death and appears to be detrimental. In this review we discuss the functional significance of autophagy and the underlying signaling mechanism in the heart during ischemia/reperfusion.
Introduction
Autophagy is an important intracellular bulk degradation process, whereby cytosolic proteins and organelles are sequestered by double membrane vesicles termed autophagosomes and delivered to lysosomes for degradation. 1, 2 Autophagy occurs at basal levels, thereby participating in homeostatic functions in cells, but can be further induced by stresses, such as nutrient depletion and ischemia/reperfusion (I/R), thereby mediating both protective and detrimental functions in cells. I/R induces multiple cellular conditions which favor autophagy. For example, energy starvation is one of the most established stimuli for induction of autophagy. Accompanying apoptosis, oxidative stress, endoplasmic reticulum (ER) stress and changes in the activity of the ubiquitin-proteasomal system (UPS) are intimately involved in the regulation of autophagy. Furthermore, the necessities of removing damaged macromolecules/organelles and even apoptotic cells, and initiating subsequent tissue remodeling may stimulate autophagy during and after I/R. Although induction of autophagy by I/R in mammalian organs, such as the heart, has been known for more than 30 years, the signaling mechanism by which I/R induces autophagy and the functional significance of autophagy during I/R have only just started to be unraveled recently, due to recent advancements in the elucidation of the molecular mechanism of autophagy and genetically altered mouse models.
Clinically, the heart is the organ most frequently affected by I/R. In the US, about 16 million people suffer from coronary heart disease and 8 million people have had myocardial infarction. 3 Thus, studying the function of autophagy in the heart during I/R has significant clinical implications. We have recently shown that autophagy is induced by I/R in the mouse heart in vivo. Ischemia stimulates autophagy through an AMP activated protein kinase (AMPK)-dependent mechanism, whereas reperfusion further stimulates autophagy through Beclin 1-dependent mechanisms. 4, 5 Unexpectedly, autophagy has distinct functions during ischemia and reperfusion: autophagy may be protective during ischemia, whereas it may be detrimental during reperfusion in the heart. In the current review, using the heart and cardiac myocytes therein, a highly I/R-sensitive organ and cell type, as model systems, we discuss the molecular mechanisms and the physiological significance of autophagy during I/R.
Role of autophagy in mediating cellular survival or death in the heart
Induction of autophagy in the heart was first reported in the 1970s. 6 The heart is one of the notable organs in mice where a drastic increase in autophagosomes is observed during starvation. 7 Autophagy might be more important in the terminally differentiated (and presumably long-lived) cell types, like cardiac myocytes, than in frequently renewing cell types because long-lived proteins and damaged organelles to be degraded are not diluted through cell proliferation. In addition, cardiac myocytes may need multiple layers of backup mechanisms to maintain ATP levels for continuous contraction. Before we discuss the specific function of autophagy during I/R, we briefly discuss the general function of autophagy in the heart at baseline and under stressed conditions. The recent development of genetically altered mouse models has provided valuable information regarding in vivo functions of autophagy in the heart. It is becoming clear that the baseline activity of autophagy is essential for the heart. Inducible deletion of autophagy related gene 5 (atg5) in the adult heart causes cardiac hypertrophy, chamber dilatation and contractile dysfunction, suggesting that autophagy under baseline conditions is a homeostatic mechanism to maintain the global cardiac structure and function in the adult heart. 8 These results are similar to those seen in the brain, where suppression of basal autophagy causes neurodegenerative diseases in mice, 9, 10 and suggest that the continuous clearance of cytosolic proteins through autophagy is essential for the survival of cardiac and neuronal cells. In mice deficient in lysosome-associated membrane protein-2 (lamp-2), an important constituent of the lysosomal membrane, extensive accumulation of autophagic vacuoles was observed in many organs, including the heart, presumably caused by attenuated degradation of autophagosomes. Cardiac function in lamp-2 deficient mice is reduced, mimicking the cardiac manifestation of Danon disease, the best known autophagic vacuolar myopathy caused by mutation in lamp-2. 11, 12
The role of autophagy in the heart under stress seems more complex. In a cardiomyopathic hamster model, autophagosome formation is increased in the heart, accompanied by increased expression of Rab7 and cathepsin D, which stimulate lysosomal fusion and degradation of autophagosomes. 13 Myocardial damage due to intoxication, infection or autoimmune responses also stimulates autophagy in the heart. 14
An important question here is whether the autophagosome formation is protective or detrimental in the heart under stress. Autophagy is upregulated immediately after birth, and mice deficient in atg5 die within 1 day of delivery with reduced amino acid concentrations and signs of energy depletion in the heart. The production of amino acids by autophagic degradation of ‘self’ proteins seems essential for the maintenance of energy homeostasis during neonatal starvation. 15 Heart-specific deletion of atg5 leads to the development of cardiac dysfunction and LV dilatation after transverse aortic constriction (TAC), 8 a well-established procedure for increasing afterload to the heart, suggesting that upregulation of autophagy is adaptive under pressure overload. In contrast, another report showed that suppression of autophagy caused by heterozygous deletion of beclin 1 slightly but significantly attenuated systolic dysfunction induced by TAC, whereas augmented expression of Beclin 1 in transgenic mouse hearts enhanced pathological hypertrophy and systolic dysfunction. These results suggest that autophagy during pressure overload could be detrimental under some experimental conditions. 16 The exact reason why these two reports lead to opposite conclusions is so far unknown. It is possible that Beclin 1 may have more complicated functions beyond autophagy. Importantly, however, autophagy might be suppressed below physiological levels in atg5 −/− mice. In contrast, in beclin 1+/− mice, the level of autophagy under increased afterload may be normalized and may not drop below normal, since only half of the beclin 1 genes is knocked out in these mice. Although this possibility needs to be tested experimentally, it is possible that reducing autophagy below normal may create an independent stress to the heart and, thus, the exacerbation of heart failure in atg5 −/− mice may not necessarily indicate that activation of autophagy at supra-normal levels is protective. Increasing lines of evidence suggest that cardiac myocytes die by multiple mechanisms, including autophagic cell death, in heart failure patients. 17–20 Kanaapen et al reported that autophagic cell death, characterized by the presence of granular cytoplasmic ubiquitin inclusions and absence of markers of apoptosis (TUNEL or cleaved caspase-3) or necrosis (C9, a marker of myocardial necrosis 21), is observed in 0.3% of cardiac myocytes in cardiomyopathy patients, 18 which is as high as the frequency of apoptotic cardiac myocytes observed in heart failure patients. 22 These results support the notion that autophagic cell death could be a major cause of programmed cell death in the heart under stress.
Activation of autophagy during ischemia and reperfusion in the heart
More than 30 years ago, Sybers et al reported that autophagosomes containing damaged organelles are observed in fetal mouse hearts in organ culture. 6 Formation of autophagosomes induced by transient (4 hours) deprivation of oxygen and glucose was accelerated by re-supply of them, a procedure similar to I/R. Interestingly, many cardiac cells recovered without residual injury after transient ischemia. The authors speculated that autophagy may play a role in the repair of sublethal injury. 6 Subsequently, Decker et al reported that hypoxic conditions for 40 minutes led to an increase in autophagic vacuoles, and that reperfusion after either 20 or 40 minutes of hypoxia induced a dramatic enhancement of autophagy in the perfused rabbit heart. 23 More recently, we and others showed that autophagy is observed in human and pig hearts under chronic myocardial ischemia, 24–26 as well as in mouse and rat hearts subjected to acute I/R. 4, 27 The extent of autophagy is enhanced during reperfusion compared with ischemia alone in the mouse heart. 4 Taken together, autophagy is induced by ischemia and further enhanced after reperfusion in the heart in vivo. In cultured neonatal and adult cardiac myocytes, hypoxia and reoxygenation (H/R) also induced autophagy in vitro, 28 indicating that induction of autophagy by I/R or H/R is cell autonomous in cardiac myocytes.
Upstream triggers of autophagy during I/R
Induction of autophagy by myocardial ischemia is likely to be triggered by depletion of cellular ATP. However, energy starvation may not be the primary mechanism of autophagy during reperfusion, when the lack of ATP is alleviated. Then what is the trigger of autophagy during reperfusion? First, production of reactive oxygen species (ROS) in mitochondria is dramatically amplified by a mechanism called ROS-induced ROS release when electron transport resumes during reperfusion. 29 ROS produces damaged proteins and organelles and lipid peroxidation in mitochondria, thereby playing an essential role in promoting autophagy caused by a wide variety of stresses, such as TNFa and NGF deprivation. 30, 31 Since autophagy can target catalase for degradation, which in turn causes accumulation of H2O2, autophagy and oxidative stress can initiate a positive feedback mechanism and cause autophagic cell death. 32 In addition, ROS directly affects the activity of the autophagy machinery. For example, H2O2 oxidizes a cysteine residue of HsAtg4, thereby inhibiting its cysteine protease activity and stimulating starvation-induced autophagy. 33 A recent review proposed intimate roles of ROS in mediating autophagosome formation through direct oxidative modifications of the autophagic machinery. 32
Second, cellular stresses impair the ability of the ER to confer posttranslational modifications and correct folding to nascent proteins, leading to accumulation of misfolded proteins in the ER, 34 a condition collectively called ER stress. ER stress induces compensatory responses, termed the unfolded protein response (UPR), including inhibition of protein translation, upregulation of chaperones, protein degradation, and induction of apoptosis. Accumulation of misfolded proteins induces autophagy through activation of ER stress sensors, such as PKR-like ER kinase (PERK) and inositol requiring enzyme 1 (IRE1), in mammalian cells. 35, 36Atg5 knockout mouse embryonic fibroblasts (MEF) are more vulnerable to ER stress than control MEF cells, exhibiting accumulation of misfolded proteins and cell death, suggesting that autophagy is protective against ER stress. 35, 36 Since the UPR is activated by I/R and failure in the heart, 37 ER stress triggered by I/R may contribute to increases in autophagy in the heart. Interestingly, autophagy induced by ER stress is generally adaptive. Whether such autophagy induced by ER stress is protective or maladaptive in the heart subjected to I/R remains to be clarified.
Third, the activities of multiple mechanisms of protein degradation in the heart, such as calpain, the UPS and autophagy, are affected by I/R. 38 It appears that the activity of each protein degradation mechanism seems affected by those of the others. For example, I/R stimulates calcium-activated proteases, such as calpain, 39–41 which in turn affects the activity of the UPS and autophagy in the heart. Loss of myocardial calpain activity due to overexpression of calpastatin induces suppression of protein ubiquitination, and causes dilated cardiomyopathy characterized by accumulation of protein aggregates and formation of autophagosomes in the heart. 42 It should be noted that autophagy is impaired by the absence of calpain in fibroblastic cell lines, suggesting that regulation of autophagy by calpain may be cell type-dependent. 43 Deletion of mitochondrial DNA causes inhibition of the UPS, which in turn activates autophagy, suggesting that autophagy may compensate for proteasome dysfunction. 44 These results suggest that other mechanisms of protein degradation may coordinately regulate autophagy during I/R.
Signaling mechanisms mediating autophagy during ischemia: critical roles of AMPK and HIF-1
A rapid decline in ATP/ADP with a subsequent increase in AMP levels during myocardial ischemia stimulates AMPK. 45 AMPK is the prime energy sensor in response to energy depletion. 46 Does AMPK play a critical role in mediating autophagy caused by ischemia? The yeast homolog, sucrose nonfermenting protein 1 (Snf1)/Snf4 complex, mediates autophagy during starvation, thereby mediating cell adaptation to a glucose-free environment. 47 Glucose deprivation-induced autophagy is inhibited in the presence of dominant negative AMPK in cultured cardiac myocytes. 4 Autophagy induced by myocardial ischemia is also diminished in mice overexpressing dominant negative AMPK. 4 These results suggest that AMPK plays an essential role in mediating autophagy in response to ischemia in cardiac myocytes. During ischemia, the ATP content in the heart was lower and LV end-diastolic pressure was higher in dominant negative AMPK mice than in wild type mice. 48 Furthermore, prolonged ischemia caused a larger myocardial infarction in the dominant negative AMPK mice, 5 suggesting that AMPK has a protective effect against ischemia. Although AMPK mediates cardioprotection through multiple mechanisms, including increases in glucose uptake, glycolysis, and fatty acid oxidation, 49 induction of autophagy may also contribute to the protective effects of AMPK during myocardial ischemia. At present, the signaling mechanism by which AMPK activates autophagy in the heart is unknown. Activation of AMPK causes phosphorylation of tuberous sclerosis complex 2 (TSC2), thereby leading to inhibition of mammalian target of rapamycin (mTOR), 46 a major negative regulator of autophagy. In anoxic cardiac myocytes and ischemic Langendorff perfused hearts, however, activation of AMPK leads to an inhibition of protein synthesis through phosphorylation of eukaryotic elongation factor-2 (eEF2) but not by inhibition of mTOR. 50 In fact, despite significant activation of AMPK by ischemia, we were unable to observe a significant drop in p70 S6 kinase (p70S6K) phosphorylation during ischemia, 4 suggesting that the activity of mTOR may not be significantly affected by myocardial ischemia in vivo. Since eEF2 kinase, which phosphorylates eEF2, regulates autophagy, ischemia-induced autophagy may be mediated through the AMPK-eEF2 kinase pathway rather than through the AMPK-induced inhibition of mTOR. 51 AMPK also stabilizes p27kip1, an inhibitor of the cyclin-cdk complex, through phosphorylation, which in turn prevents apoptosis but stimulates autophagy under stress, such as glucose starvation. 52 The molecular mechanism by which p27kip1 promotes autophagy is not well understood. Since the AMPK α2 subunit contains a putative nuclear localization signal, it is possible that DN-AMPK has direct effects upon the nuclear actions of AMPK, namely transcription of genes involved in autophagy. Recent evidence suggests that hypoxia inducible factor-1 (HIF-1) regulates autophagy and cell survival in chondrocytes. 53 Since HIF-1 is upregulated in the heart under ischemic conditions 54, and HIF-1 is activated by AMPK, 55 it will be interesting to examine the connection between AMPK and HIF-1 and the role of HIF-1 in mediating autophagy induced by myocardial ischemia.
Signaling mechanisms mediating autophagy during reperfusion: critical roles of Beclin 1 and BNIP3
Because AMPK is no longer activated after reperfusion, the further increase in autophagy during the reperfusion phase is unlikely to be mediated by AMPK-dependent mechanisms. Increases in autophagic activity in the reperfusion phase are accompanied by dramatic upregulation of Beclin 1 in the heart. Induction of autophagy during the reperfusion phase was significantly attenuated in beclin 1 +/− mice 4 Dramatic upregulation of Beclin 1 induced by reperfusion was also observed in brain and kidney cells. 56, 57 Collectively, upregulation of Beclin 1 may be responsible for increases in autophagy during reperfusion. 4 The signaling mechanism by which I/R dramatically upregulates Beclin 1 expression remains to be elucidated. TNFα induced upregulation of Beclin 1 and subsequent autophagy through the JNK pathway in vascular smooth muscle cells. 58 Since JNK is activated by reperfusion, 59–62 Beclin 1 may be upregulated by JNK during the reperfusion phase in the heart. Conversely, urocortin, a cardioprotective peptide, reduces the expression of Beclin 1 through phosphatidylinositol 3-kinase (PI3K)/Akt-dependent mechanisms, thereby suppressing autophagy in cultured cardiac myocytes. 28
Another mechanism mediating I/R-induced autophagy is BNIP3, a BH3-only protein. BNIP3 is dramatically induced by prolonged hypoxia, thereby mediating mitochondrial dysfunction and cell death in neonatal cardiac myocytes. 63 In HL-1 cells, a cardiac myocyte-like cell line, BNIP3 is both necessary and sufficient for induction of autophagy by I/R. 27 BNIP3 is also required for induction of autophagy by hypoxia in cancer cell lines. 64, 65 Since BNIP3 exists as an integral mitochondrial membrane protein, it may induce damage to the mitochondria, such as fragmentation, which in turn triggers autophagy. It is also possible that BNIP3 titrates Bcl-2 and/or Bcl-XL away from Beclin-1, thereby inducing autophagy. 65 Alternatively, BNIP3 directly binds Ras homolog enriched in brain (Rheb) and inhibits its GTPase activity, thereby inhibiting mTOR, 66 which would stimulate autophagy.
Interestingly, both Beclin 1 and BNIP3 are BH3 domain containing proteins and strongly inducible. Furthermore, they are localized at the ER and mitochondria, respectively, the intracellular organelles subjected to stress/damage under I/R, which would make them attractive sensors to initiate autophagy in response to I/R.
Role of autophagy in mediating survival or death during I/R
Early reperfusion of the ischemic myocardium plays an important role in minimizing myocardial tissue injury associated with acute myocardial ischemia. 67 However, the effects of reperfusion are complex and include some deleterious effects collectively referred to as reperfusion injury. 68 The reperfusion injury is manifest as acute heart failure, arrhythmia, and accelerated progression of cell death in certain critically injured myocytes. 69 Whether induction of autophagy during ischemia and reperfusion is physiological, and thereby protective, or detrimental, thereby mediating cell death, has just begun to be elucidated.
Autophagy induced by ischemia in cardiac myocytes is generally protective. Inhibition of autophagy during glucose deprivation, a condition mimicking ischemia, in cultured cardiac myocytes facilitates ATP depletion and cell death. 4 Inhibition of autophagy during acute myocardial ischemia in dominant negative AMPK mice was accompanied by cardiac injury and dysfunction. 4 Induction of autophagy allows the myocardium to recover from prolonged ischemia/hypoxia without significant myocardial injury. 23 How does autophagy play a protective role in the heart during ischemia? First, autophagy may provide a source of energy during hypoxia. Through degradation of the membrane lipids and proteins, autophagy generates free fatty acids and amino acids. Since a part of the ischemic risk area may still receive reduced levels of oxygen via diffusion from the border zone, acetyl-CoA converted from fatty acids and amino acids can be used by the cell to maintain mitochondrial ATP production through the tricarboxylic acid (TCA) cycle under hypoxia, if not complete ischemia. 1, 70 Second, autophagy mediates the removal of damaged protein aggregates in the heart. During ischemia, the function of the ubiquitin proteasome pathway is generally inhibited in the heart, 71 leading to accumulation of oxidized and ubiquitinated proteins. Autophagy may work as an alternative measure to scavenge and eliminate misfolded, polyubiquitinated protein aggregates during ischemia. 1, 70 Third, autophagy mediates the removal of damaged organelles in the heart. BNIP3-induced autophagosomes often contain fragmented mitochondria in cardiac myocytes, 27 suggesting that autophagy removes damaged mitochondria, which might otherwise trigger ROS-induced ROS release and generalized permeability transition pore opening.
Contrary to the generally protective effects of autophagy during ischemia, autophagy during I/R, especially at the reperfusion phase, may not necessarily be protective under some experimental conditions. For example, inhibition of H/R-induced autophagy by 3-methyladenine (3-MA) or knockdown of Beclin 1 leads to enhanced cardiac myocyte survival in vitro. 28 In addition, inhibition of autophagy during the reperfusion phase was accompanied by significant reduction in the size of myocardial infarction and cardiac myocyte apoptosis in beclin 1 +/− mice. 4 These data suggest that activation of autophagy during H/R or I/R may be detrimental, at least in some experimental conditions. What, then, would render autophagy detrimental in the heart during I/R?
First, hyperactivation of autophagy, which takes place under some conditions, could cause cell death. 72,70 Lysosomal enzymes are markedly upregulated in the heart after myocardial infarction. 73 Together with dramatic induction of Beclin 1 and BNIP3, it is expected that autophagy is enhanced to supra physiological levels during reperfusion.
Second, whether autophagy induces cell death or cell survival may be determined by the balance between Bcl-2 and Beclin 1. 74 The ability of Beclin 1 to promote autophagy is negatively regulated by interaction with Bcl-2. Thus, either expression or posttranslational modification of Bcl-2 would affect the activity of Beclin 1 and subsequent occurrence of autophagy. Theoretically, downregulation of Bcl-2 or upregulation of Bcl-2/Bcl-XL binding proteins, such as BNIP3, not only stimulates apoptosis but also activates autophagy through activation of Beclin 1. Thus, the combination of upregulation of Beclin 1 and BNIP3 and downregulation of Bcl-2 75 during the reperfusion phase would dramatically stimulate the activity of Beclin 1, thereby stimulating cell death.
Third, autophagy and apoptosis could be interconnected by common mediators. For example, Atg5 is cleaved by calpain, which is upregulated by I/R, and translocated to mitochondria, where it associates with the anti-apoptotic molecule, Bcl-xL, and induces cytochrome c release in Jurkat cells. 76 Upregulation of calpain during I/R may convert initial activation of autophagy by Atg5 to apoptosis through this mechanism. Atg5 is also reported to interact directly with Fas-associated protein with death domain (FADD) via the death domain, thereby stimulating autophagic cell death. 77 In the heart subjected to ischemia, induction of apoptosis is dramatically enhanced during the reperfusion phase. 78 Thus, concomitant activation of the apoptotic signaling mechanism during the reperfusion phase may influence the autophagy machinery upon myocardial cell death. It should be noted that more study is needed to clarify which mode of cell death is enhanced by the increased autophagy during reperfusion.
If our hypothesis that autophagy during myocardial ischemia is protective whereas that during reperfusion phase is detrimental is true, autophagy should be stimulated in the middle of ischemia but must be inhibited during reperfusion in patients. Such phase-dependent contrasting biological functions between ischemia and reperfusion are not without precedent. For example, activation of AMPK is protective during ischemia because it stimulates glycolytic ATP production. However, continued activation of AMPK during reperfusion is detrimental because increased fatty acid oxidation induces uncoupling between glycolysis and glucose oxidation, resulting in cytosolic proton production and intracellular acidosis. 49 It should be noted, however, that whether induction of autophagy during I/R is salutary or detrimental may be affected by the length/extent of initial ischemia. In fact, induction of autophagy during reoxygenation is protective in cardiac myocyte-like HL1 cells. 79 If the level of initial ischemia is modest, activation of autophagy at reperfusion may also be modest and thus protective. More investigations are needed to apply our knowledge regarding autophagy to clinical treatment to reduce I/R injury in patients.
Conclusions
Autophagy has both protective and detrimental effects for the heart subjected to I/R. Autophagy in the ischemic and reperfusion phases may be mediated by different cellular mechanisms and possess distinct functional significance (Figure 1). However, elucidating the causative role of autophagy in mediating cell survival or cell death during I/R has been challenged by difficulties in manipulating autophagy to physiological levels without affecting other cellular responses in animal models. For example, complete knock down or overexpression alone may introduce independent artifacts. Development of new mouse models which allow us to precisely control the activity of autophagy would be useful. Ideally, the speed of such manipulation should be fast enough so that we are able to distinguish the role of autophagy during ischemia and reperfusion. There are multiple connections between the apoptotic and autophagic processes, and even evolutionarily conserved autophagy genes may possess autophagy-independent functions, such as in apoptosis and necrosis. Comparing responses to I/R in multiple mouse models with genetic alterations in the autophagic machinery would be helpful to identify which phenotype is specifically regulated by autophagy.
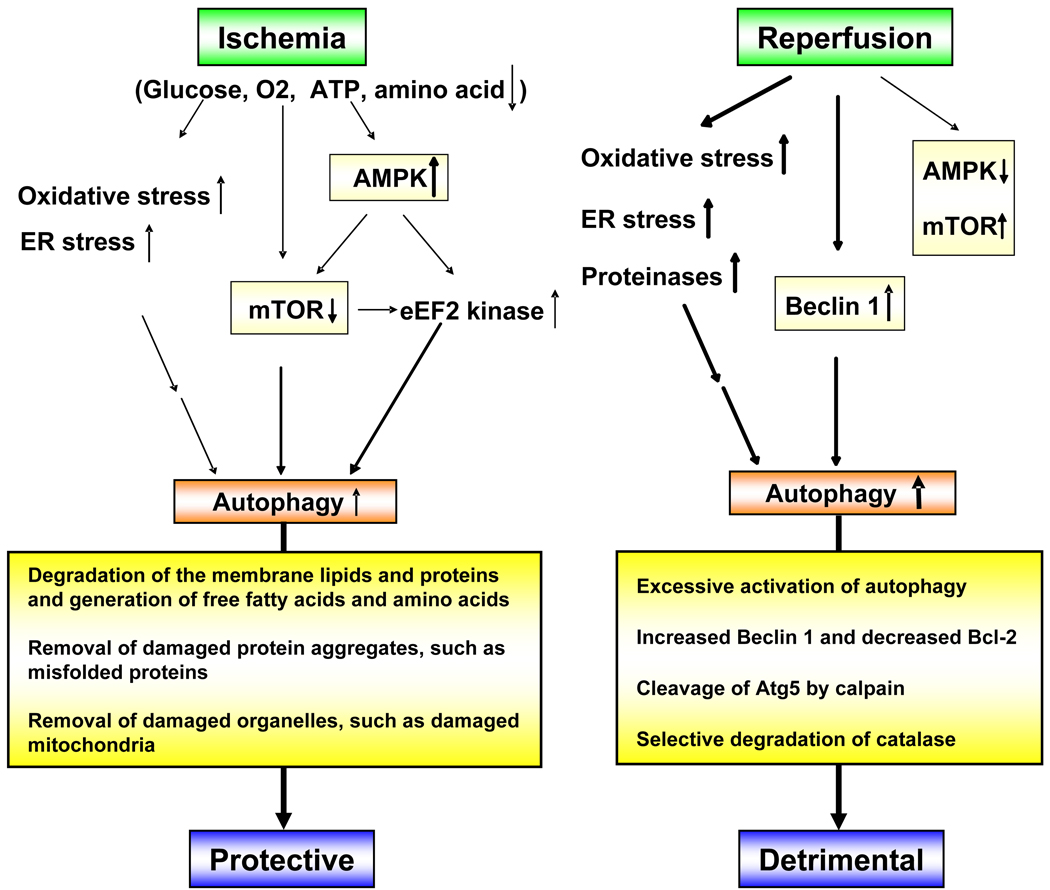
Figure 1.
Hypothetical scheme describing the mechanism of autophagy under ischemia and reperfusion. Under ischemia, O2 and nutrient supplies are decreased and the intracellular ATP level is reduced, causing activation of AMPK. These stimuli lead to autophagy, which may be protective. On the other hand, upon reperfusion, activation of AMPK is no longer observed. Instead, expression of Beclin 1 is markedly upregulated. Simultaneously, increased oxidative stress and ER stress may occur. These stimuli induce autophagy, which could be detrimental.
We speculate that induction of well-controlled autophagy, as well as suppression of inappropriate autophagy, should protect cardiac myocytes from cell death during I/R in the heart. Ischemic heart disease is one of the most common health problems in the western world. Elucidating the specific cellular mechanism allowing activation of autophagy at an appropriate level and with appropriate timing may lead to the development of novel cardioprotective strategies for ischemic heart disease.
Acknowledgement
We thank Daniela Zablocki for critical reading of the manuscript. This work was supported in part by U.S. Public Health Service Grants HL59139, HL67724, HL67727, HL69020, and HL73048 and by American Heart Association 0340123N.
Abbreviations
- I/R
ischemia-reperfusion
- ER
endoplasmic reticulum
- AMPK
AMP activated kinase
- Atg
autophagy related gene
- LAMP
Lysosome associated membrane protein
- TAC
transverse aortic constriction
- H/R
hypoxia and reoxygenation
- ROS
reactive oxygen species
- UPR
unfolded protein response
- PERK
PKR-like ER kinase
- IRE1
inositol requiring enzyme 1
- UPS
ubiquitin proteasome system
- MEF
mouse embryonic fibroblasts
- Snf
sucrose nonfermenting protein
- TSC2
tuberous sclerosis complex
- mTOR
mammalian target of rapamycin
- eEF2
eukaryotic elongation factor-2
- p70S6K
p70 S6 kinase
- HIF-1
hypoxia inducible factor-1
- PI3K
phosphatidylinositol 3-kinase
- Rheb
Ras homologue enriched in brain
- TCA
tricarboxylic acid
- 3-MA
3-methyladenine
- FADD
Fas-associated protein with death domain
References
- 1.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klionsky DJ, Ohsumi Y. Vacuolar import of proteins and organelles from the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:1–32. doi: 10.1146/annurev.cellbio.15.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 4.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 5.Takagi H, Matsui Y, Hirotani S, Sakoda H, Asano T, Sadoshima J. AMPK mediates autophagy during myocardial ischemia in vivo. Autophagy. 2007;3:405–407. doi: 10.4161/auto.4281. [DOI] [PubMed] [Google Scholar]
- 6.Sybers HD, Ingwall J, DeLuca M. Autophagy in cardiac myocytes. Recent Adv Stud Cardiac Struct Metab. 1976;12:453–463. [PubMed] [Google Scholar]
- 7.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 9.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 10.Mizushima N, Hara T. Intracellular quality control by autophagy: how does autophagy prevent neurodegeneration? Autophagy. 2006;2:302–304. doi: 10.4161/auto.2945. [DOI] [PubMed] [Google Scholar]
- 11.Nishino I, Fu J, Tanji K, Yamada T, Shimojo S, Koori T, Mora M, Riggs JE, Oh SJ, Koga Y, Sue CM, Yamamoto A, Murakami N, Shanske S, Byrne E, Bonilla E, Nonaka I, DiMauro S, Hirano M. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R, Janssen PM, Blanz J, von Figura K, Saftig P. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406:902–906. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- 13.Miyata S, Takemura G, Kawase Y, Li Y, Okada H, Maruyama R, Ushikoshi H, Esaki M, Kanamori H, Li L, Misao Y, Tezuka A, Toyo-Oka T, Minatoguchi S, Fujiwara T, Fujiwara H. Autophagic cardiomyocyte death in cardiomyopathic hamsters and its prevention by granulocyte colony-stimulating factor. Am J Pathol. 2006;168:386–397. doi: 10.2353/ajpath.2006.050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akazawa H, Komazaki S, Shimomura H, Terasaki F, Zou Y, Takano H, Nagai T, Komuro I. Diphtheria toxin-induced autophagic cardiomyocyte death plays a pathogenic role in mouse model of heart failure. J Biol Chem. 2004;279:41095–41103. doi: 10.1074/jbc.M313084200. [DOI] [PubMed] [Google Scholar]
- 15.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 16.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, Bauer EP, Klovekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 18.Knaapen MW, Davies MJ, De Bie M, Haven AJ, Martinet W, Kockx MM. Apoptotic versus autophagic cell death in heart failure. Cardiovasc Res. 2001;51:304–312. doi: 10.1016/s0008-6363(01)00290-5. [DOI] [PubMed] [Google Scholar]
- 19.Kostin S, Pool L, Elsasser A, Hein S, Drexler HC, Arnon E, Hayakawa Y, Zimmermann R, Bauer E, Klovekorn WP, Schaper J. Myocytes die by multiple mechanisms in failing human hearts. Circ Res. 2003;92:715–724. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- 20.Shimomura H, Terasaki F, Hayashi T, Kitaura Y, Isomura T, Suma H. Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Jpn Circ J. 2001;65:965–968. doi: 10.1253/jcj.65.965. [DOI] [PubMed] [Google Scholar]
- 21.Doran JP, Howie AJ, Townend JN, Bonser RS. Detection of myocardial infarction by immunohistological staining for C9 on formalin fixed, paraffin wax embedded sections. J Clin Pathol. 1996;49:34–37. doi: 10.1136/jcp.49.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 23.Decker RS, Wildenthal K. Lysosomal alterations in hypoxic and reoxygenated hearts. I. Ultrastructural and cytochemical changes. Am J Pathol. 1980;98:425–444. [PMC free article] [PubMed] [Google Scholar]
- 24.Elsasser A, Vogt AM, Nef H, Kostin S, Mollmann H, Skwara W, Bode C, Hamm C, Schaper J. Human hibernating myocardium is jeopardized by apoptotic and autophagic cell death. J Am Coll Cardiol. 2004;43:2191–2199. doi: 10.1016/j.jacc.2004.02.053. [DOI] [PubMed] [Google Scholar]
- 25.Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, Yang G, Matsui Y, Sadoshima J, Vatner SF. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A. 2005;102:13807–13812. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan L, Sadoshima J, Vatner DE, Vatner SF. Autophagy: a novel protective mechanism in chronic ischemia. Cell Cycle. 2006;5:1175–1177. doi: 10.4161/cc.5.11.2787. [DOI] [PubMed] [Google Scholar]
- 27.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA, Gustafsson AB. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 28.Valentim L, Laurence KM, Townsend PA, Carroll CJ, Soond S, Scarabelli TM, Knight RA, Latchman DS, Stephanou A. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol. 2006;40:846–852. doi: 10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- 29.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 30.Djavaheri-Mergny M, Amelotti M, Mathieu J, Besancon F, Bauvy C, Souquere S, Pierron G, Codogno P. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J Biol Chem. 2006;281:30373–30382. doi: 10.1074/jbc.M602097200. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, Lenardo MJ. Reactive oxygen species regulate autophagy through redox-sensitive proteases. Dev Cell. 2007;12:484–485. doi: 10.1016/j.devcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17:422–427. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. Embo J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 35.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 37.Glembotski CC. Endoplasmic reticulum stress in the heart. Circ Res. 2007;101:975–984. doi: 10.1161/CIRCRESAHA.107.161273. [DOI] [PubMed] [Google Scholar]
- 38.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 39.Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem. 2001;276:30724–30728. doi: 10.1074/jbc.M103701200. [DOI] [PubMed] [Google Scholar]
- 40.Chen M, Won DJ, Krajewski S, Gottlieb RA. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem. 2002;277:29181–29186. doi: 10.1074/jbc.M204951200. [DOI] [PubMed] [Google Scholar]
- 41.Khalil PN, Neuhof C, Huss R, Pollhammer M, Khalil MN, Neuhof H, Fritz H, Siebeck M. Calpain inhibition reduces infarct size and improves global hemodynamics and left ventricular contractility in a porcine myocardial ischemia/reperfusion model. Eur J Pharmacol. 2005;528:124–131. doi: 10.1016/j.ejphar.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 42.Galvez AS, Diwan A, Odley AM, Hahn HS, Osinska H, Melendez JG, Robbins J, Lynch RA, Marreez Y, Dorn GW., 2nd Cardiomyocyte degeneration with calpain deficiency reveals a critical role in protein homeostasis. Circ Res. 2007;100:1071–1078. doi: 10.1161/01.RES.0000261938.28365.11. [DOI] [PubMed] [Google Scholar]
- 43.Demarchi F, Bertoli C, Copetti T, Tanida I, Brancolini C, Eskelinen EL, Schneider C. Calpain is required for macroautophagy in mammalian cells. J Cell Biol. 2006;175:595–605. doi: 10.1083/jcb.200601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alemi M, Prigione A, Wong A, Schoenfeld R, DiMauro S, Hirano M, Taroni F, Cortopassi G. Mitochondrial DNA deletions inhibit proteasomal activity and stimulate an autophagic transcript. Free Radic Biol Med. 2007;42:32–43. doi: 10.1016/j.freeradbiomed.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dolinsky VW, Dyck JR. Role of AMP-activated protein kinase in healthy and diseased hearts. Am J Physiol Heart Circ Physiol. 2006;291:H2557–H2569. doi: 10.1152/ajpheart.00329.2006. [DOI] [PubMed] [Google Scholar]
- 46.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z, Wilson WA, Fujino MA, Roach PJ. Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol Cell Biol. 2001;21:5742–5752. doi: 10.1128/MCB.21.17.5742-5752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ, Tian R. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- 49.Tian R, Balschi JA. Interaction of insulin and AMPK in the ischemic heart: another chapter in the book of metabolic therapy? Circ Res. 2006;99:3–5. doi: 10.1161/01.RES.0000233142.26369.f6. [DOI] [PubMed] [Google Scholar]
- 50.Horman S, Beauloye C, Vertommen D, Vanoverschelde JL, Hue L, Rider MH. Myocardial ischemia and increased heart work modulate the phosphorylation state of eukaryotic elongation factor-2. J Biol Chem. 2003;278:41970–41976. doi: 10.1074/jbc.M302403200. [DOI] [PubMed] [Google Scholar]
- 51.Wu H, Yang JM, Jin S, Zhang H, Hait WN. Elongation factor-2 kinase regulates autophagy in human glioblastoma cells. Cancer Res. 2006;66:3015–3023. doi: 10.1158/0008-5472.CAN-05-1554. [DOI] [PubMed] [Google Scholar]
- 52.Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, Slingerland JM, Mills GB. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 53.Bohensky J, Shapiro IM, Leshinsky S, Terkhorn SP, Adams CS, Srinivas V. HIF-1 regulation of chondrocyte apoptosis: induction of the autophagic pathway. Autophagy. 2007;3:207–214. doi: 10.4161/auto.3708. [DOI] [PubMed] [Google Scholar]
- 54.Kido M, Du L, Sullivan CC, Li X, Deutsch R, Jamieson SW, Thistlethwaite PA. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol. 2005;46:2116–2124. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 55.Lee M, Hwang JT, Lee HJ, Jung SN, Kang I, Chi SG, Kim SS, Ha J. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J Biol Chem. 2003;278:39653–39661. doi: 10.1074/jbc.M306104200. [DOI] [PubMed] [Google Scholar]
- 56.Chien CT, Shyue SK, Lai MK. Bcl-xL Augmentation Potentially Reduces Ischemia/Reperfusion Induced Proximal and Distal Tubular Apoptosis and Autophagy. Transplantation. 2007;84:1183–1190. doi: 10.1097/01.tp.0000287334.38933.e3. [DOI] [PubMed] [Google Scholar]
- 57.Rami A, Langhagen A, Steiger S. Focal cerebral ischemia induces upregulation of Beclin 1 and autophagy-like cell death. Neurobiol Dis. 2008;29:132–141. doi: 10.1016/j.nbd.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Jia G, Cheng G, Gangahar DM, Agrawal DK. Insulin-like growth factor-1 and TNF-alpha regulate autophagy through c-jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth cells. Immunol Cell Biol. 2006;84:448–454. doi: 10.1111/j.1440-1711.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- 59.Armstrong SC. Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;61:427–436. doi: 10.1016/j.cardiores.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 60.Bogoyevitch MA, Gillespie-Brown J, Ketterman AJ, Fuller SJ, Ben-Levy R, Ashworth A, Marshall CJ, Sugden PH. Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart. p38/RK mitogen-activated protein kinases and c-Jun N-terminal kinases are activated by ischemia/reperfusion. Circ Res. 1996;79:162–173. doi: 10.1161/01.res.79.2.162. [DOI] [PubMed] [Google Scholar]
- 61.Engelbrecht AM, Niesler C, Page C, Lochner A. p38 and JNK have distinct regulatory functions on the development of apoptosis during simulated ischaemia and reperfusion in neonatal cardiomyocytes. Basic Res Cardiol. 2004;99:338–350. doi: 10.1007/s00395-004-0478-3. [DOI] [PubMed] [Google Scholar]
- 62.Meijer AJ, Codogno P. Signalling and autophagy regulation in health, aging and disease. Mol Aspects Med. 2006;27:411–425. doi: 10.1016/j.mam.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 63.Regula KM, Ens K, Kirshenbaum LA. Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ Res. 2002;91:226–231. doi: 10.1161/01.res.0000029232.42227.16. [DOI] [PubMed] [Google Scholar]
- 64.Azad MB, Chen Y, Henson ES, Cizeau J, McMillan-Ward E, Israels SJ, Gibson SB. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy. 2007:4. doi: 10.4161/auto.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol. 2007;27:6229–6242. doi: 10.1128/MCB.02246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y, Wang Y, Kim E, Beemiller P, Wang CY, Swanson J, You M, Guan KL. Bnip3 Mediates the Hypoxia-induced Inhibition on Mammalian Target of Rapamycin by Interacting with Rheb. J Biol Chem. 2007;282:35803–35813. doi: 10.1074/jbc.M705231200. [DOI] [PubMed] [Google Scholar]
- 67.Simoons ML, Serruys PW, van den Brand M, Res J, Verheugt FW, Krauss XH, Remme WJ, Bar F, de Zwaan C, van der Laarse A, et al. Early thrombolysis in acute myocardial infarction: limitation of infarct size and improved survival. J Am Coll Cardiol. 1986;7:717–728. doi: 10.1016/s0735-1097(86)80329-1. [DOI] [PubMed] [Google Scholar]
- 68.Kukan M. Emerging roles of proteasomes in ischemia-reperfusion injury of organs. J Physiol Pharmacol. 2004;55:3–15. [PubMed] [Google Scholar]
- 69.Zolk O, Schenke C, Sarikas A. The ubiquitin-proteasome system: focus on the heart. Cardiovasc Res. 2006;70:410–421. doi: 10.1016/j.cardiores.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 70.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 71.Powell SR. The ubiquitin-proteasome system in cardiac physiology and pathology. Am J Physiol Heart Circ Physiol. 2006;291:H1–H19. doi: 10.1152/ajpheart.00062.2006. [DOI] [PubMed] [Google Scholar]
- 72.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 73.Mayanskaya SD, Mayanskaya NN, Efremov AV, Yakobson GS. Activity of lysosomal apparatus in rat myocardium during experimental coronary and noncoronary myocardial damage. Bull Exp Biol Med. 2000;129:530–532. doi: 10.1007/BF02434867. [DOI] [PubMed] [Google Scholar]
- 74.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 75.Grunenfelder J, Miniati DN, Murata S, Falk V, Hoyt EG, Kown M, Koransky ML, Robbins RC. Upregulation of Bcl-2 through caspase-3 inhibition ameliorates ischemia/reperfusion injury in rat cardiac allografts. Circulation. 2001;104:I202–I206. doi: 10.1161/hc37t1.094833. [DOI] [PubMed] [Google Scholar]
- 76.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 77.Pyo JO, Jang MH, Kwon YK, Lee HJ, Jun JI, Woo HN, Cho DH, Choi B, Lee H, Kim JH, Mizushima N, Oshumi Y, Jung YK. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem. 2005;280:20722–20729. doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- 78.Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res. 1998;82:1111–1129. doi: 10.1161/01.res.82.11.1111. [DOI] [PubMed] [Google Scholar]
- 79.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]