Abstract
Absence epilepsy is more prevalent in females, but reasons for this gender asymmetry are unknown. We reported previously that perinatal treatment of Long-Evans Hooded rats with the cholesterol synthesis inhibitor (CSI) AY9944 causes a life-long increase in EEG spike-wave discharges (SWDs), correlated with decreased expression of GABAA receptor subunit γ2 protein levels in thalamic reticular and ventrobasal nuclei (SS thalamus) (Li et al., 2006). In this study, we explored time course and gender different effects of perinatal AY9944 treatment on expression of GABAA receptor α1 and γ2 subunits in SS thalamus and SS cortex. Perinatal AY9944 treatment-induced decreases in GABAA γ2 receptor subunits in rat SS thalamus and increases in SS cortex are gender and age-specific. The findings suggest a mechanism for the higher prevalence of absence epilepsy in female patients.
Keywords: GABAA receptor subunits, Absence epilepsy, Cholesterol synthesis inhibitor, thalamic reticular and ventrobasal nuclei, Somatosensory cortex
Introduction
The γ2 subunit of GABAA receptors is highly expressed throughout the brain. Knockout of γ2 results in about a 94% reduction of benzodiazepine binding sites, while GABA binding sites are only slightly reduced (Pritchett et al., 1989). This suggests a crucial role of γ2 subunits in benzodiazepine responsiveness. Our previous studies of an acquired absence epilepsy model demonstrated that treatment of neonatal Long-Evans Hooded rats with a cholesterol synthesis inhibitor (CSI) resulted in enduring spike-wave discharges (SWDs), despite the limited neonatal treatment. Neurons acutely dissociated from CSI model thalamic nucleus reticularis displayed a specific decrease in sensitivity to benzodiazepine (Wu et al., 2004). We observed a reduction of GABAA receptor γ2 subunits in adult thalamic nucleus reticularis (nRt) and in the ventrobasal (VB) region (SS thalamus), but not in somatosensory cortex (SS cortex) (Li et al., 2006), suggesting that decreased γ2 subunit expression may play an important role in generation of thalamocortical SWDs in the acquired absence epilepsy model in rats. SWDs in rats given cholesterol synthesis inhibitors in the first weeks of life can be discerned as early as postnatal 21, and the abnormal discharges can last into adulthood (Cortez et al., 2001). To evaluate whether the alteration of GABAA receptor γ2 subunits is correlated with the onset of SWDs, we studied the effect of neonatal cholesterol synthesis inhibition on the normal development of GABAA receptor subunits during postnatal development. The goal of this study was to examine the effect of neonatal treatment with CSI on the time profile of the GABAA receptor subunit γ2 in SS cortex and SS thalamus, which are the two major brain areas presumably responsible for the SWDs in thalamocortical pathway (Inoue et al., 1993; Vergnes et al., 1990). The potential alteration of α1 subunits also was examined because the sites of benzodiazepines binding are between α1 and γ2 subunits (Sigel and Buhr, 1997; Sigel 2002). A related goal was to examine whether the neonatal treatment of CSI had gender different effects on GABAA receptor subunits. This is of interest because a higher prevalence of absence epilepsy occurs in female than male patients (Janz 1997). An abstract has reported some of the preliminary findings (Li et al., 2005).
Methods
Preparation of model animals
Experiments were carried out according to protocols approved by the Stanford Institutional Animal Care and Use Committee. Newborn Long-Evans Hooded rats were given injections of AY9944, suspended in olive oil, subcutaneously at postnatal days 1, 5, and 9, at dosages of 15 mg/kg of weight per injection. Neonatal rats were maintained on standard diets, including suckling with their mother. Each litter was split into those receiving AY9944 injections and those receiving olive oil control injections of equal volume.
Total RNA isolation, reverse transcription and real-time polymerase chain reaction (PCR)
Isolation of total RNA from SS thalamus (nRt-VB region) and SS cortex was performed at postnatal days 7 and 14, before the onset of SWDs to avoid the seizure-induced alteration of GABAa receptor subunits (Hwang et al., 2004), and at postnatal days 35 and 60, after sex characteristics have been established. The procedure was the same as previously described (Li et al., 2006). In brief, the isolation of total RNA was performed using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. The purity and concentration were determined spectrophotometrically. cDNA was prepared from total RNA using oligo primers and Superscript reverse transcriptase according to the accompanying protocol (Invitrogen, Carlsbad, CA, USA). Genomic DNA was eliminated by DNase I. For real-time PCR, the reactions were conducted by placing 10 μl of material into 96-well plates, using the Quantitect SYBR Green PCR kit (Applied Biosystems, Foster City, CA, USA). The PCR parameters were 94° C for 5 min, then 94° C for 30 s, 55° C for 30 s, 72° C for 60 s, repeating for 40 cycles. The amplification of synaptophysin RNA was used as an internal control. The data from real-time PCR experiments were analyzed by the comparative Ct method. The parameter Ct is derived for each cDNA sample and primer pair; Ct is an expression of amplification kinetics referring to the cycle at which log-phase amplification reaches a predetermined threshold. For a given sample, Ct values for synaptophysin RNA were subtracted from the Ct of each GABAA subunit to arrive at a ΔCt value. The mean ΔCt from all control animal reactions was then subtracted from the ΔCt for each control or treated sample to arrive at ΔΔCt. This parameter (ΔΔCt) reflects the fold-difference of over- or under-expression of GABAA subunits in CSI model rat tissue, relative to control according to the expression 2-ΔΔCt. In each experiment, samples were analyzed in triplicate for the indicated numbers of rats. Table 1 lists the sequence of primers used in the experiment.
Table 1. Oligonucleotide primers used for real-time PCR.
| Gene | 5′-primer (N=20) | 3′-primer (N=20) | Gene Bank Code |
|---|---|---|---|
| α1 | aaggacccatgacagtgctc | ggctcccttgtccactcata | NM183326 |
| γ2 | gacgatgaccactctcagca | acagtccttgccatccaaac | NM183327 |
| synaptophysin | actactcctcgtcggctgaa | acagggtccctcagttcctt | NM012664 |
Western blot analysis
The membrane fraction of SS thalamus or SS cortex protein was obtained according to the protocol provided by Upstate (Lake Placid, NY). All procedures were carried out at 0-4° C. A sample of 40 μg membrane protein from control or model brain tissue was electrophoresed on 10% Tris-HCl running gel and then transferred to nitro-cellulose membranes. The membranes were then blocked with 5% nonfat milk in TBS buffer for 2 hours at room temperature, and incubated with primary antibodies overnight at 4° C. The dilution of antibodies was 1:1000 for rabbit polyclonal anti-α1 (Upstate), and rabbit polyclonal anti-γ2 subunits (Chemicon International, Inc., Temicula, CA), and 1:2000 for mouse monoclonal anti-synaptophysin (Sigma). The membranes were then incubated with secondary antibodies (goat anti-rabbit, or goat anti-mouse IgG conjugated to horseradish peroxidase, 1: 20,000) for 2 hours at room temperature and developed with enhanced chemi-luminescence reagents (Amersham, UK). Quantify One (Bio-Rad, CA) was used to quantify the optical absorbency of Western blots. The relative protein expression of GABAA receptor subunits was calculated as the OD value of GABAA receptor subunit/OD value of synaptophysin. All data in figures and text were expressed as mean ± SEM. One-way ANOVA followed by Tukey's multiple comparison tests were used to asses the differences in the expression of α1 and γ2 subunits when more than two groups were compared. The differences between control and CSI model rats of the corresponding age in SS thalamus or SS cortex was analyzed using two-tailed Student t-tests. The data expressed were either normalized to control-fold changes in mRNA or to the ratio of target GABAA receptor subunit to synaptophysin in Western Blot measures, and these ratio-based data sets are not normally distributed. Therefore, we additionally analyzed logorithmically transformed data sets to confirm statistical significance. The level of significance criteria was set to p<0.05.
Results
Postnatal gender differences in the expression of GABAA subunits during development
The postnatal developmental changes in expression of GABAA α1 and γ2 subunit mRNAs in SS thalamus and SS cortex were examined in control rats. Expression of GABAA α1 and γ2 receptor subunit mRNA increased during postnatal development from p7 to p60, and significantly differed between SS thalamus and SS cortex as well as between males and females at ages p35 and p60.
Expression of GABAA α1 receptor subunit mRNA increased during postnatal development in SS thalamus (Fig. 1, upper left panel) from p7 to p60 (1.00 ± 0.04 vs. 12.39 ± 1.00, respectively). In SS cortex (Fig. 1, upper right panel), however, the most pronounced increase in expression of α1 subunit mRNA was between p7 and p35 (1.00 ± 0.04 vs. 7.37 ± 0.43). After p35, the expression of α1 mRNA level in SS cortex was relatively constant from p35 to p60 (7.37 ± 0.43 vs. 6.96 ± 0.61).
Figure 1.
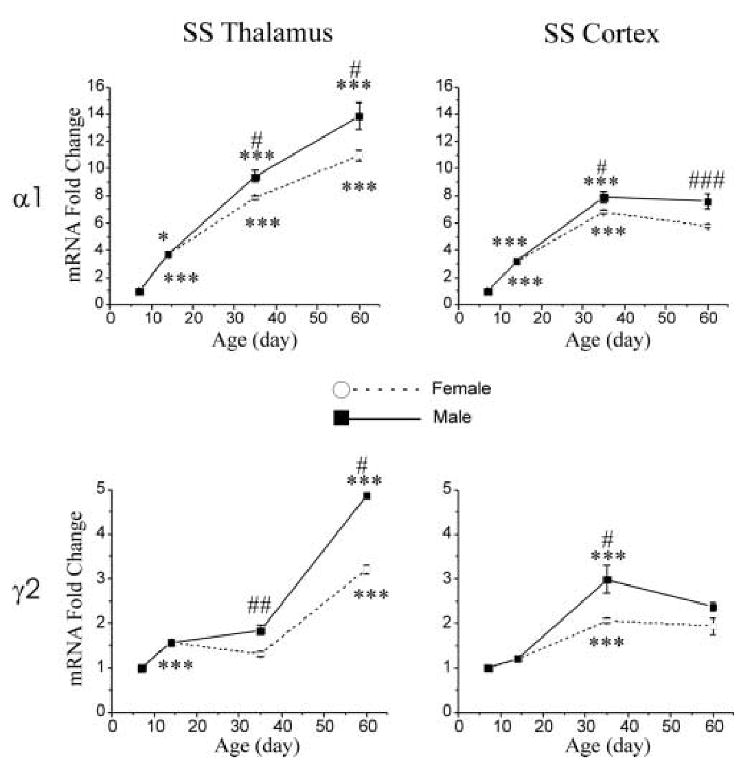
Gender different postnatal developmental changes in expression of GABAA receptor α1 and γ2 subunit mRNAs in the SS thalamus (left column), and SS cortex of control (right column) male (filled square) and female (open circle) rats. The values of p14-60 mRNAs are normalized to that of p7 mRNA. Data is expressed as mean ± SEM, n=4-5. One-way ANOVA, *, p < 0.05; **, p < 0.01; ***, p < 0.001, comparison between ages p7-14, p14-35 and p35-60. T-test, #, p < 0.05; ##, p < 0.01; ###, p < 0.001, comparison between male and female rats.
Increased expression of γ2 subunit mRNA in SS thalamus and SS cortex from P7 to P60 was relatively modest. The expression levels of γ2 subunit mRNA increased about four-fold in SS thalamus (Fig. 1, bottom left panel, γ2 mRNA-fold change 4.04 ± 0.55) and two-fold in SS cortex (Fig. 1, bottom right panel, γ2 mRNA-fold change 2.12 ± 0.17) during postnatal development from p7 to p60. In the SS thalamus, the most pronounced increase in expression of γ2 subunit mRNA was between p35 and p60, representing a 150% increase. In SS cortex, the most pronounced increased in expression of γ2 subunit mRNA levels was between p14 and p35: approximately 110%. The level of γ2 expression was relatively constant between p7 and p14, and between p35 and p60.
The expression of α1 and γ2 subunits in SS thalamus is significantly lower in females compared to males at p35 (α1: female 7.85 ± 0.13 vs. male 9.4 ± 0.37, p < 0.05; γ2: female 1.32 ± 0.05 vs. male 1.84 ± 0.09, p < 0.01) and p60 (α1: female 10.93 ± 0.39 vs. male 13.85 ± 0.88, p < 0.05; γ2: female 3.22 ± 0.09 vs. male 4.85 ± 0.4, p < 0.05). Such differences in expression of α1 and γ2 subunits between male and female rats were also observed in SS cortex at p35 (α1: female 6.79 ± 0.1 vs. male 7.93 ± 0.41, p < 0.05; γ2: female 2.06 ± 0.05 vs. male 3.04 ± 0.3, p < 0.05). At p60, the expression of α1 subunits in SS cortex was lower in females (female 5.77 ± 0.1 vs. male 8.15 ± 0.19, p < 0.001). The expression of γ2 subunits in SS cortex tended to be lower in females, but not at a level of statistical significance (female 1.94 ± 0.19 vs. male 2.3 ± 0.09, p = 0.14).
Comparison of GABAA receptor mRNA expression in control and CSI model tissue
Altered expression of α1 and γ2 subunit mRNAs in CSI model rats was modest but significantly differed from expression in their age-matched siblings for both female and male rats (Fig. 2). In general α1 and γ2 subunit mRNA was lower for model animals in thalamus, but higher in cortex. A developmental profile for the changes is evident in Fig. 2. At age p7, no difference in expression of α1 and γ2 subunit mRNAs was detected in SS thalamus and SS cortex between control and model rats. However, at age p14, the expression of both α1 and γ2 subunits mRNAs was lower in CSI model SS thalamus (α1 control 1.00 ± 0.04 vs. CSI model 0.81 ± 0.03, p < 0.01; γ2 control 1.00 ± 0.03 vs. CSI model 0.83 ± 0.04, p < 0.02). A trend towards lower levels of γ2 subunit mRNA levels in SS thalamus also was detected at p35, but a significant difference was not observed. Expression of α1 and γ2 subunits for control and model animals was at comparable levels in SS cortex. At p14, for control animals, α1 levels were 1.00 ± 0.05 vs. CSI model levels of 1.11 ± 0.04 (p = n.s). In control cortex, γ2 levels were 1.00 ± 0.04 vs. CSI model levels of 1.04 ± 0.10 (p = n.s.). The levels of α1 and γ2 subunit mRNAs in SS cortex at age p35 and p60 also did not differ in control and CSI model groups.
Figure 2.
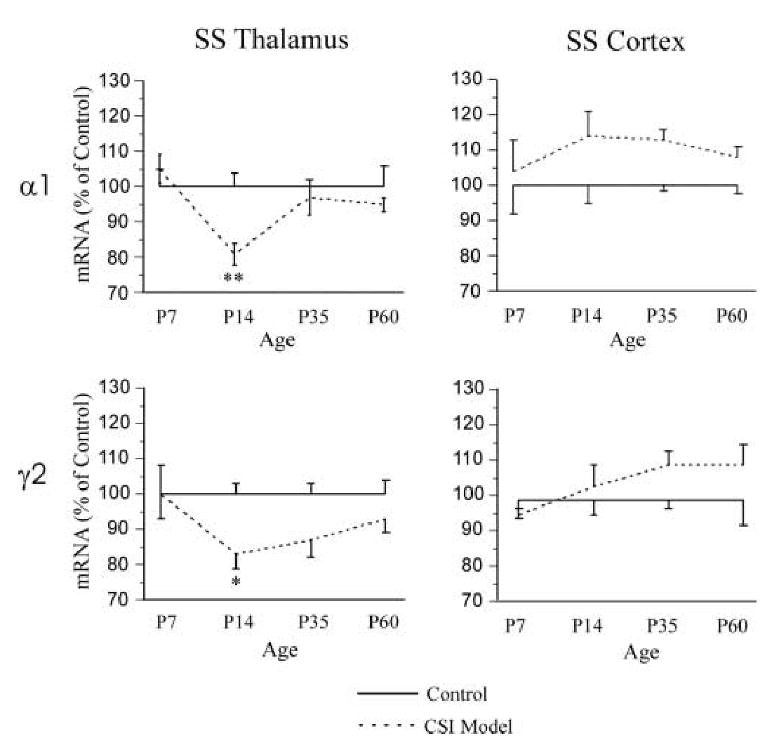
The expression of α1 and γ2 subunit mRNAs in the SS thalamus (left column) and SS cortex (right column) of ages p7-60 control (solid line, n=4-9 in each group) and CSI model (dash line, n=4-9 in each group) rats. One-way ANOVA, * p < 0.05; ** p < 0.01 between CSI model and age matched control groups.
Comparison of GABAA receptor protein expression control and CSI model tissue
Expression of α1 and γ2 subunit protein in CSI model SS thalamus and SS cortex was compared at p14, p35 and p60 to levels in age-matched control rats (combined female and male). No difference in expression of α1 subunit levels was detected from SS thalamus or SS cortex. However, a significant decline of γ2 subunit protein was detected from SS thalamus at p60 (control, 1.00 ± 0.04 vs. CSI model, 0.79 ± 0.05, p < 0.001). In contrast to the lower level of γ2 subunit protein in SS thalamus, an increase of γ2 subunit protein was observed in SS cortex at age p60 (control, 1.00 ± 0.03 vs. CSI model, 1.16 ± 0.04, p < 0.05). Figure 3 summarizes the results of α1 and γ2 Western Blots of membrane protein extracted from the SS thalamus and SS cortex at p14, p35 and p60.
Figure 3.
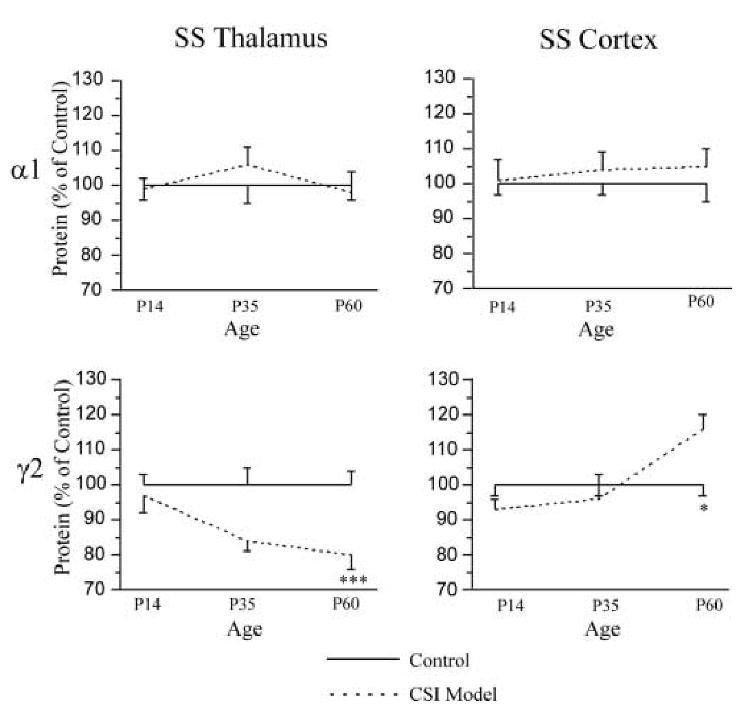
The expression of α1 and γ2 subunit protein in the SS thalamus (left column) and SS cortex (right column) of ages p14-60 control (solid line, n=8-16 in each group) and CSI model (dash line, n=8-16 in each group) rats. One-way ANOVA, * p < 0.05; *** p < 0.001 between CSI model and age matched control groups.
Gender difference in expression of GABAA receptor subunits
To examine whether the CSI model had gender-specific effects on the expression of GABAA receptor subunits, we analyzed female and male rats separately at p35 and p60. Figure 4 shows the results of α1 and γ2 Western blots of membrane protein extracted from female and male SS thalamus and SS cortex at p35 and p60. A reduction of expression of α1 subunit protein level was detected in p60 female SS thalamus (control 1.00 ± 0.04 vs. CSI model 0.79 ± 0.04, p < 0.02), but not in the male SS thalamus and SS cortex. Expression of GABAA receptor γ2 subunit protein was significantly reduced in the female p35 and p60 SS thalamus (p35: control, 1.00 ± 0.07 vs. CSI model, 0.82 ± 0.04, p < 0.02; p60: control 1.00 ± 0.06 vs. CSI Model 0.71 ± 0.05, p < 0.01) but not in the male SS thalamus (p35: control, 1.00 ± 0.04 vs. CSI model, 0.97 ± 0.04, p= 0.67; p60: control, 1.00 ± 0.04 vs. CSI model 0.93 ± 0.06, p = 0.25). In contrast, the expression of γ2 subunit protein in CSI model SS cortex was increased in both females and males at p60, compared to age-matched control SS cortex (Fig. 4, bottom, right panels, female γ2 control 1.00 ± 0.06 vs. CSI model 1.15 ± 0.06, p < 0.03; male γ2 control 1.00 ± 0.02 vs. CSI model 1.21 ± 0.06, p < 0.02).
Figure 4.
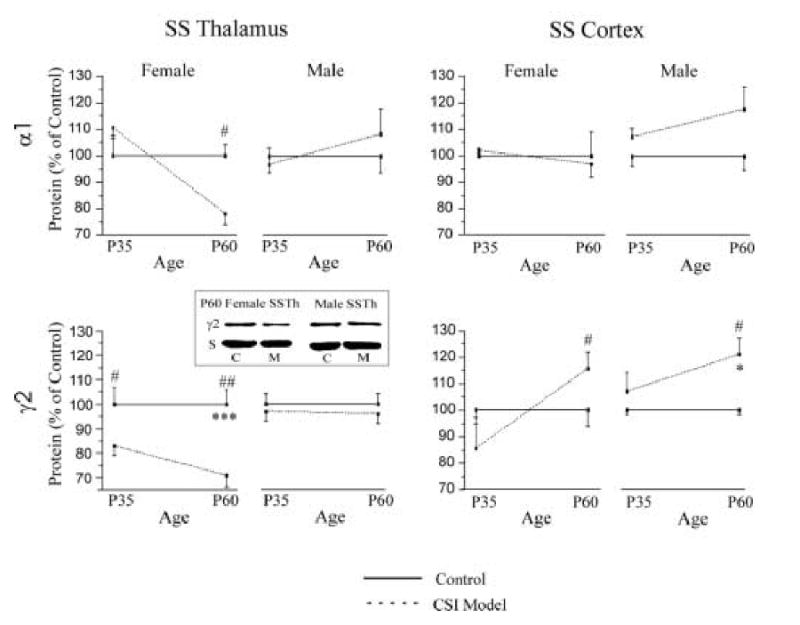
The expression of α1 and γ2 subunit protein in SS thalamus (left column) and SS cortex (right column) at p35 and p60 male and female control (solid line, n=4-8 in each group) and CSI model (dash line, n=4-8 in each group) rats. The insert is a representative Western blot sample of SS thalamic γ2 subunits obtained from control (C) and model (M) p60 female and male rats. S: synaptophysin. One way ANOVA, * p < 0.05; *** p < 0.001 between control and age matched CSI model groups. T-test, #, p < 0.05; ##, p < 0.01 between control and age matched CSI model groups.
Discussion
This study shows that perinatal treatment with the cholesterol synthesis inhibitor AY9944 induces subunit-specific and age-specific changes in GABAA receptor subunits in rat SS thalamus and SS cortex. Furthermore, alterations in GABAA receptor subunits in CSI model rats are much more prominent in female rats. The changes in thalamic γ2 subunits in our studies correspond in time to the approximate period of emergence of abnormal spike-waves rhythms in the EEGs of rats with acquired epilepsy, and also with the gender-predominance of absence epilepsy in female patients, discussed below.
The developmental time course of gene expression for GABAA receptor α1 and γ2 subunits was evaluated by using quantitative real time PCR methods to measure the amount of mRNA at four postnatal ages from p7 to p60. The expression of GABAA α1 and γ2 subunits is regional and temporal specific during brain development (Golshani et al., 1997; Laurie et al., 1992; Yu et al., 2006). We have demonstrated that, from p7 to p60, GABAA receptor α1 subunit mRNAs are highly expressed, and significantly increase during postnatal development in both SS cortex and thalamic VB nucleus, consistent with prior studies (Golshani et al., 1997; Laurie et al., 1992; Yu et al., 2006). Few previous studies systematically investigated changes in expression of γ2 subunits in cortex and thalamus during postnatal age from p7 to p60. For example, Laurie et al., measured from embryonic time to p12 and in adult male rats and found that the expression of γ2 subunits reached a moderate level by birth, which continued into maturity in cortex (Laurie et al., 1992). Yu et al., reported no change in expression of γ2 subunit protein in SS cortex during p30 to p60 (Yu et al., 2006). We found no significant change in expression of γ2 subunits in SS cortex from p7 to p14, and from p35 to p60, which agrees with the above results; in addition we found a slight increase in expression of γ2 subunits in SS cortex from p14 to p35, which has not been reported by others. The expression of γ2 subunits in SS thalamus also moderately increased with brain maturation (Figure 1), this result differs from others, which found the expression of γ2 subunits in thalamus reached a peak before birth, decreased after birth and was very weak or undetectable in adult (Laurie et al., 1992). However, the same research group and others demonstrated a fairly strong expression of γ2 subunits in adult thalamus (Pirker et al., 2000; Wisden et al., 1992), which favors our findings.
Our results demonstrate an interesting gender specific changes in GABAA receptor α1 and γ2 subunits during development at p35 and p60. We found the expression of α1 and γ2 subunits in SS cortex and SS thalamus consistently to be lower in females compared to males. Increasing evidence (Majewska et al., 1986, 1990) suggests that neurosteroids and other sex hormones are important neuronal modulators for GABAA receptors, but, no previous studies have systematically reported the different expression of GABAA receptor subunits in adult male and female brains; almost all studies of GABAA receptor subunit distributions focused on male rats only or gave no indication of sex (Laurie et al., 1992; Pirker et al., 2000; Wisden et al., 1992; Yu et al. 2006).
We previously showed that perinatal treatment with AY9944 leads to a decrease in GABAA γ2 subunit protein levels in adult rat SS thalamus (Li et al., 2006). In the present study, we further show that thalamic γ2 subunit protein levels are lower for model animals than for controls by postnatal day 60, and are trending lower by age 35 days after birth. For female rats, showing a greater reduction of γ2 subunit protein levels, a statistically significant reduction in expression of both mRNA (23% reduction) and protein levels (18% reduction) in the SS thalamus was detected by age 35.
The mRNA signals for γ2 subunits in CSI model SS thalamus were lower in model animals by p14, which is before the onset age of SWDs in CSI model rats at 3-6 weeks (Cortez et al., 2001). Diminished expression of γ2 subunit mRNA persisted to p35 in female rats, then returned to control levels at p60 for female rats. These results suggest that decreased γ2 subunit mRNA expression in SS thalamus may play an important role in emergence of thalamocortical SWDs in the acquired absence epilepsy model rats, probably through both transcriptional and post-translational pathways.
In contrast to the reduction of expression of GABAA receptor γ2 subunits in SS thalamus, γ2 protein levels increased slightly in CSI model rat SS cortex at p60, suggesting that the regulation of GABAA receptor subunits by perinatal AY9944 treatment is region specific. The increase in γ2 subunits in SS cortex was not observed in adult CSI model rats (Li et al., 2006), nor at p35 (see Fig. 3). Benzodiazepines bind in the cleft between the α1 and γ2 subunits of the GABAA receptors. Since the α1 subunits in SS cortex are unchanged, the increased γ2 subunits may account for the increased benzodiazepine binding. The physiological significance of the reciprocal changes in γ2 subunits between the SS thalamus and the SS cortex at certain ages is not clear. One possibility is that the increase in expression of γ2 subunits in SS cortex may be a compensatory effect for decreased expression of γ2 subunits in the SS thalamus, because decreased γ2 subunits in SS thalamus could increase excitatory inputs from thalamus to cortex. An alternative speculation is that cortical changes result from ongoing exposure to thalamocortical spike-waves. Our data do not allow us to identify the mechanism of reciprocal subunit changes in thalamus and cortex. The third possibility is that the regulation of GABAA receptor subunits by neurosteroids is time, region and subunit specific in brain (Follesa et al., 2004). When progesterone, pregnanolone and allopregnanolone concentrations are lowered in brain by administration of ethinyl estrodiol and levnorgestrel, γ2 subunit mRNA and protein levels in cortex and hippocampus increase; in contrast, the α1, α4, and β1-3 subunits are not affected by this treatment (Follesa 2002).
In addition to a decreased expression of γ2 subunits in SS thalamus, we also detected a significant decrease in expression of α1 subunit mRNAs at p14 (Figure 2), before the onset of SWDs, suggesting that the AY9944 treatment caused changes in GABAA subunits in this CSI model rats, rather than the subsequent absence seizures. Furthermore, the observation that neither α1 nor γ2 subunits changed in SS cortex at this age suggest the pathological changes are likely to start at SS thalamus in this particular model. A decreased expression of α1 protein levels at p60 female thalamus also was detected in the present study (Figure 4). This reduction in expression of α1 protein was not observed at p35 or p60 male thalamus. Taken together with the gender specific modulation of γ2 subunits in SS thalamus at p35 and p60, our results suggest that sex hormones may pay a role in regulation of GABAA receptor subunit levels.
Gender specific behaviors are known to be determined by steroid sex hormones, such as androgen and estrogen, during critical periods of early perinatal development (Hutchison, 1997). Perinatal CSI block lowers brain levels of cholesterol, and decreased cholesterol in turn reduces brain levels of neurosteroids and other sex hormones (Baulieu et al., 2001; Tsutsui et al., 2000). Cholesterol is metabolized to pregnenolone, then by 3-beta-hydroxysteroid dehydrogenase, to progesterone (Chan et al. 2000). If AY9944 is administered to pregnant rats, then both cholesterol and progesterone levels in brain are reduced as early as 24 hours after treatment, with progesterone falling to 40% of controls (Kolf-Clauw et al. 1996).
Neurosteroids such as allopregnanolone decrease neurogenesis in rat hippocampus, with a differential effect for female and male rats (Galea et al. 2006). Differences, although relatively small, are seen between female and male rats in ability of progesterone to influence muscimol binding at, and chloride flux through, the GABAA receptor (Wilson and Biscardi 1997). Therefore, it is plausible to speculate that cholesterol synthesis inhibition could lead to decreased γ2 subunits of the GABAA receptor, different for female and male rats, although the precise mechanism for this sequence of events remains unknown.
Clinical absence epilepsy is more prevalent in females than males (Janz, 1997; Wisniewski, 1991). Previous studies in the AY9944 CSI model showed that female rats had up to three-fold more spike-wave discharges (SWDs) per hour and twice the SWDs duration per seizure than did male rats (Persad, et al., 2002). Our results provide a possible mechanism for this female prevalence of EEG spike-waves. Assuming that the findings in the CSI model can be extended to other animal models and to clinical epilepsy, then the predilection of EEG spike-waves for females may result in part from differential regulation of GABAA receptor subunits in males and females.
This study has several interpretive limitations. As with all animal models, questions arise about applicability to clinical disorders. The CSI model is designed to model absence provoked by a perinatal injury. Block of cholesterol synthesis provides such an injury in our model, but it rarely is encountered in patients. When a clinical condition does demonstrate problems with cholesterol trafficking (e.g., Niemann-Pick, Type C) or cholesterol synthesis (e.g., Smith-Lemli-Opitz disease), then seizures do sometimes result (Brady et al. 1989; Fink et al. 1989). Whether our GABAA subunit changes would be seen with other types of neonatal insults remains uncertain. Additionally, correlation of γ2 subunit changes with approximate time of onset of spike-waves in model rats cannot be taken to imply causation. Both could result from other, unexplored factors.
Other interpretive problems derive from the relatively global measurements of regional mRNAs and proteins. We did not distinguish nRt from VB regions of thalamus, but always analyzed these regions in a conjoint tissue sample. nRt and VB play different physiological roles in thalamus (Scheibel, 1997; Zhang et al., 1997), and the profile of GABAA receptor subunit changes with development might also be different. Similarly, we did not distinguish global tissue levels of subunit protein or mRNA from levels associated specifically with neurons, or with neuronal membranes. Such an analysis might show different subunit developmental profiles. The γ2 subunits come in two different splice variants, a shorter γ2S fragment and a longer γ2L fragment (Whiting et al., 1990). Tracking of γ2 splice variants in different regions, ages and genders might contribute further insights to our basic observations, but we lacked specific antibodies to make this distinction.
In conclusion, our findings support the hypothesis that absence seizures acquired by perinatal block of cholesterol synthesis are associated with reduced γ2 subunits of the GABAA receptor in the SS thalamus, especially in female rats. Further work will be required to determine the mechanisms of these changes and to judge their applicability to the clinical arena.
Acknowledgments
Dr. Fisher was supported by the Maslah Saul MD Chair and the James and Carrie Anderson Fund for Epilepsy Research. Dr. Li was supported by the Susan Horngren Fund and the Epilepsy Foundation. Dr. Huguenard was supported by NIH RO1 NS34774. We gratefully acknowledge Wyeth-Ayerst for supplying the AY-9944 drug for the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baulieu EE, Robel P, Schumacher M. Neurosteroids: beginning of the story. Int Rev Neurobiol. 2001;46:1–32. doi: 10.1016/s0074-7742(01)46057-0. [DOI] [PubMed] [Google Scholar]
- Brady RO, Filling-Katz MR, Barton NW, Pentchev PG. Niemann-Pick disease types C and D. Neurol Clin. 1989;7:75–88. [PubMed] [Google Scholar]
- Chan JR, Rodriguez-Waitkus PM, Ng BK, Liang P, Glaser M. Progesterone synthesized by Schwann cells during myelin formation regulates neuronal gene expression. Mol Biol Cell. 2000;11:2283–9. doi: 10.1091/mbc.11.7.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez MA, McKerlie C, Snead OC. A model of atypical absence seizures: EEG, pharmacology, and developmental characterization. Neurology. 2001;56:341–9. doi: 10.1212/wnl.56.3.341. [DOI] [PubMed] [Google Scholar]
- Fink JK, Filling-Katz MR, Sokol J, Cogan DG, Pikus A, Sonies B, Soong B, Pentchev PG, Comly ME, Brady RO. Clinical spectrum of Niemann-Pick disease type C. Neurology. 1989;39:1040–9. doi: 10.1212/wnl.39.8.1040. [DOI] [PubMed] [Google Scholar]
- Follesa P, Porcu P, Sogliano C, Cinus M, Biggio F, Mancuso L, Mostallino MC, Paoletti AM, Purdy RH, Biggio G, Concas A. Changes in GABAA receptor gamma 2 subunit gene expression induced by long-term administration of oral contraceptives in rats. Neuropharmacology. 2002;42:325–36. doi: 10.1016/s0028-3908(01)00187-3. [DOI] [PubMed] [Google Scholar]
- Galea LA, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–32. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Golshani P, Truong H, Jones EG. Developmental expression of GABA(A) receptor subunit and GAD genes in mouse somatosensory barrel cortex. J Comp Neurol. 1997;383:199–219. doi: 10.1002/(sici)1096-9861(19970630)383:2<199::aid-cne7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hutchison JB. Gender-specific steroid metabolism in neural differentiation. Cell Mol Neurobiol. 1997;17(6):603–26. doi: 10.1023/a:1022581902880. [DOI] [PubMed] [Google Scholar]
- Hwang IK, Park S, An S, Yoo K, Kim D, Jung J, Won MH, Choi S, Kwon O, Kang T. GABAA, not GABAB, receptor shows subunit-and spatial-specific alterations in the hippocampus of seizure prone gerbils. Brain Res. 2004;1003:98–107. doi: 10.1016/j.brainres.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Inoue M, Duysens J, Vossen JM, Coenen AM. Thalamic multiple-unit activity underlying spike-wave discharges in anesthetized rats. Brain Res. 1993;612:35–40. doi: 10.1016/0006-8993(93)91641-5. [DOI] [PubMed] [Google Scholar]
- Janz D. The idiopathic generalized epilepsies of adolescence with childhood and juvenile age of onset. Epilepsia. 1997;38:4–11. doi: 10.1111/j.1528-1157.1997.tb01073.x. [DOI] [PubMed] [Google Scholar]
- Kolf-Clauw M, Chevy F, Wolf C, Siliart B, Citadelle D, Roux C. Inhibition of 7-dehydrocholesterol reductase by the teratogen AY9944: a rat model for Smith-Lemli-Opitz syndrome. Teratology. 1996;54:115–25. doi: 10.1002/(SICI)1096-9926(199609)54:3<115::AID-TERA1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–72. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Kraus A, Wu J, Huguenard JR, Fisher RS. Selective changes in thalamic and cortical GABA(A) receptor subunits in a model of acquired absence epilepsy in the rat. Neuropharmacology. 2006;51:121–128. doi: 10.1016/j.neuropharm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Demirgoren S, Spivak CE, London ED. The neurosteroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABAA receptor. Brain Res. 1990;526(1):143–6. doi: 10.1016/0006-8993(90)90261-9. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232(4753):1004–7. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Martin JV, Williams DB. Benzodiazepine binding varies with stage of estrous cycle in unwashed membranes from mouse brain. Life Sci. 1995;57:1903–1909. doi: 10.1016/0024-3205(95)02177-k. [DOI] [PubMed] [Google Scholar]
- Persad V, Cortez MA, Carter Snead O., III A chronic model of atypical absence seizures: studies of developmental and gender sensitivity. Epilepsia Res. 2002;48:111–119. doi: 10.1016/s0920-1211(01)00319-9. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunohistochemical distribution of 13 subunitsin the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–5. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Scheibel AB. The thalamus and neuropsychiatric illness. J Neuropsychiatry Clin Neurosci. 1997;9(3):342–53. doi: 10.1176/jnp.9.3.342. [DOI] [PubMed] [Google Scholar]
- Sigel E. Mapping of the benzodiazepine recognition site on GABA(A) receptors. Curr Top Med Chem. 2002;2:833–9. doi: 10.2174/1568026023393444. [DOI] [PubMed] [Google Scholar]
- Sigel E, Buhr R. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci. 1997;18:425–9. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Ukena K, Usui M, Sakamoto H, Takase M. Novel brain function: biosynthesis and actions of neurosteroids in neurons. Neurosci Res. 2000;36(4):261–73. doi: 10.1016/s0168-0102(99)00132-7. [DOI] [PubMed] [Google Scholar]
- Vergnes M, Marescaux C, Depaulis A. Mapping of spontaneous spike and wave discharges in Wistar rats with genetic generalized non-convulsive epilepsy. Brain Res. 1990;523:87–91. doi: 10.1016/0006-8993(90)91638-w. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Biscardi R. Influence of gender and brain region on neurosteroid modulation of GABA responses in rats. Life Sci. 1997;60:1679–91. doi: 10.1016/s0024-3205(97)00110-0. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, Diencephalon, Mesencephalon. J Neurosci. 1992;12(3):1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski KE, Segan SM, Miezejeski CM, Sersen EA, Rudelli RD. The Fra (X) syndrome: neurological, electrophysiological, and neuropathological abnormalities. Am J Med Genet. 1991;38:476–480. doi: 10.1002/ajmg.1320380267. [DOI] [PubMed] [Google Scholar]
- Wu J, Ellsworth K, Ellsworth M, Schroeder KM, Smith K, Fisher RS. Abnormal benzodiazepine and zinc modulation of GABA(A) receptors in an acquired absence epilepsy model. Brain Res. 2004;1013:230–40. doi: 10.1016/j.brainres.2004.03.075. [DOI] [PubMed] [Google Scholar]
- Yu Z, Wang W, Fritschy J, Witte OW, Redecker C. Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res. 2006;1099:73–81. doi: 10.1016/j.brainres.2006.04.118. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Huguenard JR, Prince DA. GABAA receptor-mediated Cl- currents in rat thalamic reticular and relay neurons. J Neurophysiol. 1997;78(5):2280–6. doi: 10.1152/jn.1997.78.5.2280. [DOI] [PubMed] [Google Scholar]


