Abstract
As part of a study investigating commonalities between Prader-Willi syndrome (PWS — a genetic imprinting disorder) and early-onset obesity of unknown etiology (EMO) we measured total cerebral and cerebellar volume on volumetric MRI images. Individuals with PWS (n=16) and EMO (n=12) had smaller cerebellar volumes than a control group of 15 siblings (p=0.02 control vs. EMO; p=0.0005 control vs. PWS), although there was no difference among the groups in cerebral volume. Individuals with PWS and EMO also had impaired cognitive function: general intellectual ability (GIA): PWS 65 ± 25; EMO 81 ± 19; and Controls 112 ± 13 (p<0.0001 controls vs. PWS and controls vs. EMO). As both conditions are characterized by early-onset obesity and slowed cognitive development, these results raise the possibility that early childhood obesity retards both cerebellar and cognitive development.
Prader-Willi syndrome (PWS) is a genetic disorder which is associated with developmental delay and learning disabilities. Other associated clinical features include hypotonia, speech delay, language problems, endocrinopathies, behavioral and psychiatric problems, and neonatal failure to thrive followed by early-onset childhood obesity (Goldstone, 2004). PWS is an imprinted condition with approximately 70% of the cases due to a de novo deletion in the paternally inherited chromosome 15q11-q13 region, 25% from a maternal uniparental disomy (UPD) of chromosome 15, and the remaining 5% from either microdeletions or epimutations of the imprinting center in the 15q11-q13 region (i.e. imprinting defects) (Glenn et al, 1997; Nicholls and Knepper, 2001; Bittel and Butler, 2005). Structural brain abnormalities occurring with PWS are thought to be due to the loss of function of several paternally expressed genes in the PWS region. The MKRN3, MAGEL2, NDN, C15orf2, SNURF-SNRPN, and a cluster of 5 sno-RNA genes are all expressed in the brain (Bittel and Butler, 2005; Lee et al, 2005; Lee et al, 2003). The loss of some of these genes may result in misrouting of long projection axons, resulting in abnormalities of cortical development which are thought to be associated with cognitive delay in individuals with PWS (Lee et al, 2005).
Before MRI technology was available to allow correlation of structural brain findings with cognition, a retrospective study of individuals with PWS suggested that the degree of mental retardation in this syndrome can be modified by the extent of weight control early in life (i.e. those individuals with PWS who remained lean during early childhood did not have the decline in IQ noted in those who were obese during this time) (Crnic et al, 1980). This study raised the question as the extent to which environmental influences can modify the clinical phenotype in genetic syndromes. With current MRI technology it is now possible to investigate associations between brain structure, genotype, and phenotype in genetic disorders.
The clinical phenotype of individuals with PWS, including mental retardation, hypotonia, motor delay, and poor fine motor skills, support the idea that cerebellar development may be abnormal in these individuals. Many genetic disorders are associated with compromised cerebellar development (Steinlin, 2007). The cerebellum contains more than half of all of the neurons in the brain (Rapoport et al, 2000). In addition to being important for motor control, the cerebellum has connections to areas in the cerebrum which are relevant to cognition and behavior (Rapoport et al, 2000). Individuals with a variety of developmental disorders display abnormalities in cerebellar development as well as impaired cognition. Individuals with Down syndrome, fetal alcohol syndrome, Rett syndrome, Asperger syndrome, Bardet-Biedl syndrome, Joubert syndrome, and Fragile X syndrome all have decreased cerebellar volumes and lower general cognitive ability (GIA) compared to controls (Jernigan et al, 1993; Niccols, 2007; Belichenko et al, 2008; Huber, 2006; Steinlin, 2007).
Several autopsy studies of individuals with PWS have shown abnormalities in the white matter of the cerebellum and one found partial hypoplasia of the right cerebellar hemisphere (Hayashi et al, 1992; Miller et al, 2007). To date, there have been no systematic studies correlating cognitive scores with cerebellar volume in individuals with PWS. Therefore, we measured cerebral and cerebellar volume in an age-matched cohort of individuals with PWS, early-onset morbid obesity of unknown etiology (EMO), and the siblings from both of these groups who were participating in a study investigating the natural history of PWS. Given the reports of cerebellar pathology in other genetic syndromes, we hypothesized that individuals with PWS would have compromised cerebellar development compared to their sibling controls. Inclusion of the cohort with EMO and their siblings in this study was exploratory, since they were having an MRI scan as part of the larger study.
METHODS
Participants
The participants in all three groups in this study were between 4 to 24 years of age (Table 1). The subjects were individuals with PWS (n= 16; 6 females/10 males), individuals with EMO (n=12; 8 females/4 males), and the normal weight siblings from both groups (n=15; 10 females/5 males) who were participating in a study of the natural history of PWS and EMO. Participants for this study were selected from a larger group of individuals participating in the natural history study, with subjects under age 4 years and over age 24 being excluded from the current report due to the lack of appropriate age-matched controls. All of the subjects in this study also participated in our other published MRI studies. This case-control study was approved by the University of Florida Institutional Review Board, and all adult participants or guardians provided written informed consent and, where appropriate, participants provided assent.
Table 1.
Subject demographics
| Control | EMO | PWS | |
|---|---|---|---|
| Gender (male/female) | 5/10 | 4/8 | 10/6 |
| Age (mean ± SD) | 12.08 ± 6.85 | 9.25 ± 3.78 | 16.53 ± 7.67 |
| P=0.2 con vs. EMO; |
p=0.06 con vs. PWS |
P=0.005 EMO vs. PWS |
|
| Height (cm) | 142.49 ± 30.41 |
141.31 ± 15.13 |
145.63 ± 25.37 |
| P=0.87 con vs. EMO |
P=0.65 con vs. PWS |
P=0.25 EMO vs. PWS |
|
| Weight (kg) | 43.01 ± 24.89 |
72.52 ± 24.27 |
68.29 ± 27.39 |
| P=0.003 con vs. EMO |
P=0.03 con vs. PWS |
P=0.59 EMO vs. PWS |
|
| BMI (kg/m2) | 19 | 36 | 29.7 |
| P<0.001 con vs. EMO |
P<0.001 con vs. PWS |
P0.07 EMO vs. PWS |
|
| BMI (SDS) | 0.49 ± 0.92 | 2.84 ± 0.61 | 1.82 ± 0.82 |
| P<0.001 con vs. EMO |
P<0.001 con vs. PWS |
P=0.002 EMO vs. PWS |
|
| Waist/Hip Ratio | 0.82 ± 0.14 | 0.99 ± 0.39 | 1.11 ± 0.36 |
| P=0.09 con vs. EMO |
P=0.004 con vs. PWS |
P=0.4 EMO vs. PWS |
Individuals with PWS were characterized by DNA methylation analysis at the 5′ SNURF-SNRPN locus and by fluorescence in situ hybridization (FISH) using the SNURF-SNRPN probe and a distal chromosome 15 control probe. If a subject was positive for PWS by DNA methylation analysis but had an intact 15q11-q13 region by FISH, then DNA polymorphism analysis was used to distinguish maternal UPD 15 from an imprinting defect (Glenn et al, 1997). Eleven individuals with PWS due to paternal deletion of the chromosomal 15q11-q13 region (six with a type I deletion, three with a type II deletion, and two with unique deletions) and 5 with maternal UPD 15 were identified to participate in this study.
Subjects with EMO were recruited based solely on a history of a body mass index (BMI) >95% for age and gender before the age of 4 years. All were still obese at the time of this study. None of the participants were identified as having any cognitive deficits prior to this study. Participants in the EMO group had a normal chromosomal, SNURF-SNRPN FISH and DNA methylation analysis for PWS (Goldstone, 2004), melanocortin 4-receptor mutation testing, and Fragile X DNA testing. Additionally, no subjects were found to be leptin deficient by commercial testing with radioimmunoassay (Nicholls Institute, California).
Testing
All subjects were given the standard cognitive and achievement battery of the Woodcock-Johnson Tests of Cognitive Abilities, Third Edition (WJIII-Cog) and the Woodcock-Johnson Tests of Achievement, Third Edition (WJIII-TA) (Woodcock et al, 2001). Only 6 individuals with PWS, 5 with EMO, and 11 controls had complete WJIII data (younger individuals are unable to complete the achievement portion of the WJIII -TA), but we were able to obtain an overall cognitive ability score (GIA) for all participants. Additionally, the parents and teachers of the participants were asked to rate their behavior using the Behavioral Assessment System for Children (BASC).
All subjects underwent head MRI scans in a 3T Siemens Allegra scanner (Siemens, Munich, Germany). Three dimensional anatomic images were obtained using an MPRAGE sequence (TR = 1780 ms, TE = 4.38 ms, flip angle = 8°) from 160 axial slices, with a matrix of 256×256, a field of view of 25.6 cm × 25.6 cm, and a slice thickness of 0.9∼1.3mm. Scans were processed using Functional MRI Brain Software Library (Smith et al, 2006) for brain extraction, rigid-body transformation, and alignment to standard Talairach space. At least two raters performed all measures, blinded to the identity and diagnosis of the individuals. Cerebral volume was measured by ascertaining the volume of each cerebral hemisphere by manually tracing the area around the hemisphere while avoiding the brainstem and cerebellum on every fifth sagital image laterally from the midline of the brain until cortex was no longer visible. The program recognized only gray or white matter, thus allowing for liberal tracing around the border of the dura and resulting in less than 5% variability between raters. Cerebellar volume was measured in a similar manner by manually tracing around the cerebellum while avoiding the cerebral hemisphere and the brainstem on every sixth sagital slice laterally from the midpoint of the cerebellum.
Statistical Analysis
This was a cross-sectional nonrandomized study examining cerebral and cerebellar volume in three different test groups: PWS, EMO, and sibling controls. Because siblings were of different ages than the probands, we were unable to perform a sibling pair analysis for each individual, so the sibling controls from both the PWS and EMO cohorts made up the normal weight control group. Initial descriptive statistics were calculated and are expressed as means, standard deviations, minimum, and maximum values. Analysis of variance (ANOVA) was used to evaluate potential differences between cerebral and cerebellar volume amongst the three groups. For each analysis, once an overall significant finding was observed, we then evaluated all pairwise comparisons, applying Bonferroni corrections to ensure an experiment-wide error level of 0.05. Finally, we used Pearson correlations to evaluate the strength of the relationship between each of the dependent measures. All comparisons were two-sided and the alpha level was set apriori to 0.05. All statistics were computed using SAS 9.13 (Cary, NC).
RESULTS
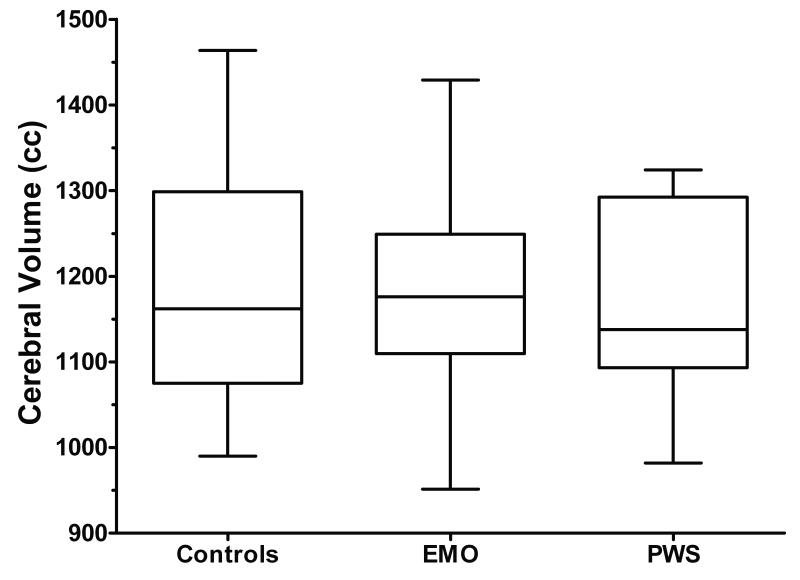
There were no significant differences in cerebral volume between the three groups (p=0.4, Figure 1). Mean cerebral volume was 1170 cc ± 111.5 for the PWS group, 1176cc ± 123.5 for the EMO group, and 1201 cc ± 136.4 for the sibling control group. Because of the small sample size we were unable to compare deletion subtypes or deletion vs. UPD within the PWS group.
Figure 1. Cerebral volume in control siblings, EMO, and PWS.
There are no differences in overall cerebral volume between normal weight control siblings, individuals with EMO, and those with PWS.
Boxplots graph data as a box representing statistical values. The bottom of the box indicates the 25th percentile, a line within the box marks the median, the cross within the box indicates the mean, and the top of the box indicates the 75th percentile. The whiskers above and below the box indicate the 90th and 10th percentiles.
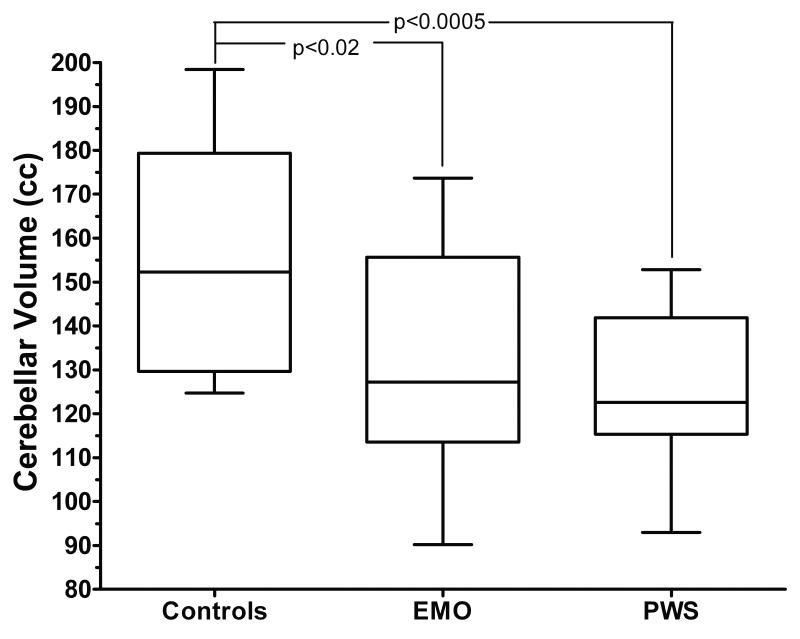
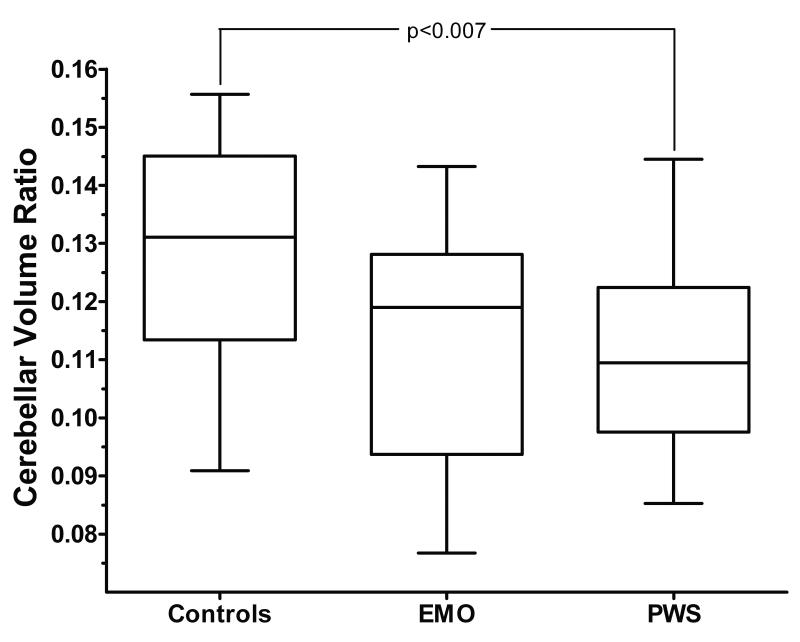
Both individuals with PWS (127.4 cc ± 16.9) and those with EMO (132.4 cc ± 26.6) had a significantly smaller cerebellar volume than the normal weight control siblings (157.9cc ± 25.9; p = 0.0005 sibling control vs. PWS and p=0.02 sibling control vs. EMO) (Figure 2 and 3). Additionally, individuals with PWS had a smaller cerebral/cerebellar volume ratio than the sibling controls (p=0.007 control vs. PWS) (Figure 4). Although subjects with EMO tended to have a smaller cerebral/cerebellar volume ratio as compared to the normal weight sibling control group, this difference was not statistically significant (p=0.07). Interestingly, there was no significant difference in cerebellar volume, or cerebral/cerebellar volume ratio between individuals with PWS and individuals with EMO.
Figure 2. Cerebellar volume in controls, EMO, and PWS.
Individuals with EMO and those with PWS have significantly smaller cerebellar volumes than the normal weight control siblings (p<0.02 EMO vs. controls; p<0.0005 PWS vs. controls). There is no significant difference in cerebellar volume between individuals with PWS and those with EMO.
Figure 3. Cerebral/cerebellar volume ratio.
Individuals with PWS have a smaller cerebral/cerebellar volume ratio than normal weight controls (p=0.007) and there is a trend toward a smaller ratio for individuals with EMO as compared to normal weight controls (p=0.07). There is no significant difference between individuals with PWS and those with EMO.
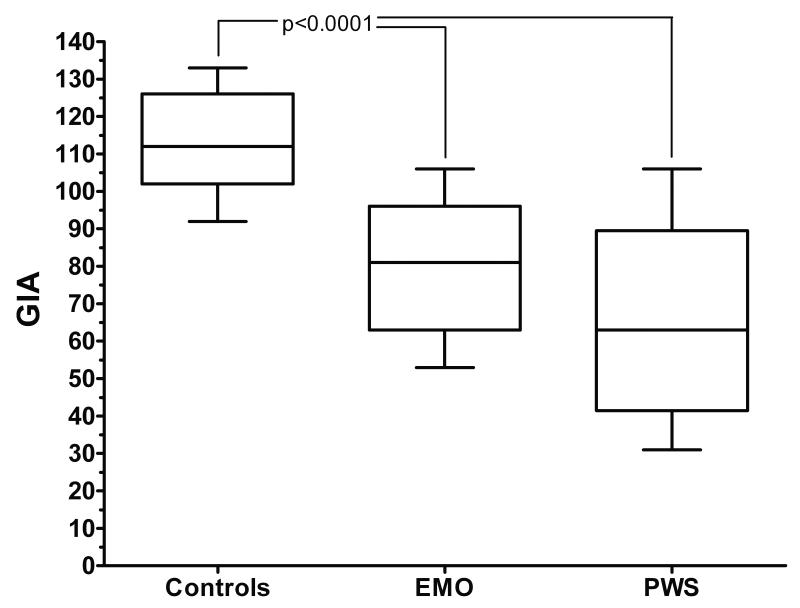
Figure 4. General Intellectual Ability (GIA) amongst groups.
Individuals with PWS and EMO have a lower GIA than their normal weight control siblings (p<0.0001). There is no significant difference between the individuals with PWS and those with EMO.
We were able to evaluate general intellectual ability (GIA) in all individuals who had MRI measurements. We found significant differences in GIA (normal population range = 85 to 115) between both individuals with PWS and those with EMO as compared to the sibling control group, as we have previously reported in a larger sample that overlaps with this one (Miller et al, 2006). The mean GIA for the group with PWS was 65 ± 25 (p<0.0001 vs. controls), while it was 81 ± 19 for those with EMO (p<0.0001 vs. controls) and 112 ± 13 for the normal weight sibling controls (Figure 4). There was no significant difference in the GIA for individuals with PWS vs. EMO. Cerebral volume and cerebellar volume were not significantly correlated with GIA within either the PWS (r=-0.30; p=0.33; r=0.32; p=0.29) or EMO (r=0.23; p=0.50; r=0.16; p=0.63) groups. Similarly, cerebral/cerebellar volume ratio was not significantly correlated with GIA within either the PWS (r=0.48; p=0.10) or EMO (r=0.08; p=0.82) groups.
In the 6 individuals with PWS who were able to complete the entire WJIII there was a moderately strong correlation between cerebral volume and working memory scores (r=0.79; p=0.06; Table 2). None of the correlations between cerebellar volume and cerebral/cerebellar volumes of individuals with PWS and the subtests of the WJIII reached statistical significance (Table 2). In the 5 individuals with EMO who were able to complete the entire cognitive and achievement test there was a trend toward significant correlations between cerebral volume and several of the subtests on the WJIII (Table 2). However, the correlations between cerebellar volume or cerebral/cerebellar volume ratio and WJIII scores did not reach statistical significance in this small group of individuals with EMO. In contrast, in the 11 sibling controls who were able to complete the entire WJIII, several correlations between cerebral volume, cerebellar volume, and cerebral/cerebellar volume ratio and WJIII subtest scores reached statistical significance (Table 2). Interestingly, eight of the individuals with EMO also had significant speech delay, which was seen in all of the individuals with PWS in this study but in none of the controls.
Table 2.
Correlations between MRI measurements and WJIII scores for subjects who were able to complete the WJIII
| Verbal Ability+ |
Thinking Ability+ |
Cognitive Efficiency+ |
Working Memory+ |
TIA†‡ | Reading Fluency‡ |
Writing Fluency‡ |
Passage Comp. ‡ |
|
|---|---|---|---|---|---|---|---|---|
|
Cerebral
Volume |
||||||||
|
|
||||||||
| PWS (n=6) |
r=0.76; p=0.47 |
r=0.14; p=0.80 |
r=0.67; p=0.15 |
r=0.79; p=0.06 |
r=0.44; p=0.38 |
r=-0.14; p=0.80 |
r=0.05; p=0.92 |
r=0.17; p=0.74 |
|
| ||||||||
| EMO (n=5) |
r=0.84; p=0.08 |
r=0.23; p=0.71 |
r=0.16; p=0.79 |
r-0.06; p=0.92 |
r=0.83; p=0.08 |
r=0.86; p=0.06 |
r=0.77; p=0.13 |
r=0.85; p=0.07 |
|
| ||||||||
| Controls (n=11) |
r=-0.65; p=0.03* |
r=-0.11; p=0.74 |
r=0.002; p=0.99 |
r=-0.23; p=0.49 |
r=-0.48; p=.13 |
r=-0.49; p=0.12 |
r=0.004; p=0.99 |
r=-0.37; p=0.27 |
|
| ||||||||
|
Cerebellar
Volume |
||||||||
|
|
||||||||
| PWS (n=6) |
r=0.37; p=0.48 |
r=0.35; p=0.50 |
r=-0.35; p=0.49 |
r=-0.11; p=0.82 |
r=0.39; p=0.45 |
r=0.66; p=0.15 |
r=0.24; p=0.65 |
r=0.28; p=0.59 |
|
| ||||||||
| EMO (n=5) |
r=0.44; p=0.46 |
r=0.63; p=0.25 |
r=0.004; p=.99 |
r=-0.17; p=0.78 |
r=0.36; p=0.55 |
r=0.21; p=0.74 |
r=0.10; p=0.88 |
r=0.31; p=0.62 |
|
| ||||||||
| Controls (n=11) |
r=-0.90; p=0.0002* |
r=-0.40; p=0.23 |
r=-0.23; p=0.48 |
r=-0.49; p=0.13 |
r=-0.79; p=0.004* |
r=-0.71; p=0.02 |
r=0.001; p=0.99 |
r=-0.71; p=0.02* |
|
| ||||||||
|
Cerebral/
cerebellar volume ratio |
||||||||
|
|
||||||||
| PWS (n=6) |
r=0.15; p=0.78 |
r=0.25; p=0.63 |
r=-0.70; p=0.12 |
r=-0.53; p=0.27 |
r=0.14; p=0.79 |
r=0.71; p=0.11 |
r=0.21; p=0.68 |
r=0.17; p=.75 |
|
| ||||||||
| EMO (n=5) |
r=0.11; p=0.86 |
r=0.62; p=0.27 |
r=-0.09; p=0.89 |
r=-0.24; p=0.70 |
r=0.02; p=0.97 |
r=-0.18; p=0.77 |
r=-0.25; p=0.69 |
r=-0.05; p=0.93 |
|
| ||||||||
| Controls (n=11) |
r=-0.29; p=0.39 |
r=-0.60; p=0.05 |
r=-0.61; p=0.05 |
r=-0.77; p=0.006* |
r=-0.35; p=0.29 |
r=-0.28; p=0.40 |
r=-0.21; p=0.53 |
r=-0.36; p=0.28 |
Sub-tests on the WJIII-cog
Total Achievement Score
Sub-tests on the WJIII-TA
P<0.05
On the BASC assessment by parents and teachers, only the teacher rating of aggressive behavior was found to negatively correlate with cerebral/cerebellar volume ratio in controls (r=-0.70; p=0.02) with a trend toward negative correlation with cerebellar volume (r=-0.61; p=0.06) in this group as well. A correlation between parental assessment of social skills and cerebral/cerebellar ratio was seen in the individuals with PWS (r=0.80; p=0.03), but no correlations between behavioral problems and cerebellar size were observed. As PWS has a unique behavioral phenotype including temper outbursts, self-injurious behaviors such as skin-picking, compulsivity, and hyperphagia, we compared these behaviors amongst the three groups. None of the sibling controls in this study had any behaviors that were similar to PWS. Seven of the individuals with EMO had problems with temper outbursts, three had skin-picking or other self-injurious behaviors (all females), four had issues of compulsivity, and five had hyperphagia. Three of the EMO patients were classified as being “PWS-like” with all of the above-mentioned behavioral features, but negative genetic testing and lack of clinical phenotype for PWS.
DISCUSSION
The clinical phenotype of PWS includes both motor and language delay, as well as cognitive impairment, similar to that seen in other genetic neurodevelopmental syndromes which are associated with a decreased cerebellar volume, such as Down syndrome (Jernigan et al, 1993). We found that individuals with PWS had a smaller cerebellar volume, as well as a smaller cerebral/cerebellar ratio, than the sibling control group. Interestingly, the participants with EMO, who do not share an identifiable genetic condition, also had smaller cerebellar volumes than the sibling control group, and had no significant differences in cerebellar volume or cerebral/cerebellar ratio as compared to individuals with PWS. The individuals with PWS and EMO also had significantly lower GIA scores (an estimate of IQ) than their normal weight control siblings, but no significant relationship was found between cognitive scores and the cerebral or cerebellar volume in either the PWS or EMO group, possibly due to the small sample size or restriction of age range.
Neuronal growth and development is governed by interactions between genes and the environment. In PWS, the loss of several paternally expressed genes which are expressed in the brain, including MKRN3, MAGEL2, NDN, C15orf2, SNURF-SNRPN, and a cluster of 5 sno-RNA genes (Bittel and Butler, 2005; Lee et al, 2005; Lee et al, 2003) likely plays a role in abnormal neuronal growth and development. By contrast, the individuals with EMO who participated in this study had no identifiable genetic abnormalities. Interestingly, we observed no differences in cerebral or cerebellar volume between the individuals with PWS and those with EMO.
As both the EMO and PWS groups had small cerebellar volumes, we looked for any commonalities between these two groups that differentiated them from the sibling controls. The EMO group was phenotypically diverse, which decreased the likelihood that any single genetic factor was solely responsible for the similar findings. Indeed, the only shared feature between individuals with PWS and those with EMO, which was not shared by the sibling controls, was presence of obesity during childhood.
Studies have shown that obesity is associated with abnormalities in brain tissue composition and function (Whitmer et al, 2005). It is known from animal studies that the morphology of the dendrites and the size of the Purkinje cells in the cerebellum are affected by characteristics of the environment (Floeter and Greenough, 1997). Additionally, acquired atrophy of the cerebellar vermis is seen in individuals with early childhood environmental insults (e.g. prematurity and fetal alcohol syndrome) (Steinlin, 2007). Hypotheses as to how excess adipose tissue could impair the development of the Purkinje cells or cause atrophy of the vermis in the cerebellum include: the abnormal lipid metabolism in obesity resulting in abnormal accumulation of lipids in the brain, the free fatty acid excess associated with obesity resulting in pathological lipid metabolism in the brain, or the inflammatory cytokines produced by excess adipose tissue damaging the cells in the cerebellum (Sriram et al, 2002; Whitmer et al, 2005). It has been shown that chronic inflammation and hypoxia from obstructive sleep apnea (both of which are associated with obesity) cause damage to the Purkinje cells in the cerebellum (Elenkov, 2008; Pae et al, 2005). Further longitudinal studies are required to identify which, if any, of these mechanisms are related to the small cerebellar volume in individuals with PWS and EMO.
The findings of small cerebellar volume in individuals with PWS and EMO is an intriguing preliminary finding that suggests that certain parts of the developing brain may be sensitive to the metabolic perturbations associated with excess adipose tissue. We found low-to-moderate correlations between cerebral volume, cerebellar volume, and GIA in individuals with PWS, similar to what has been described in other neurodevelopmental disorders. We also saw no relationship between these variables in our participants with EMO. Our study is limited, however, by our small sample size and lack of ability to exactly match sibling pairs, as well as by the qualitative nature of the scans. Although the siblings of each of these groups served as controls in this study, it was impossible to age and gender-match each proband to their sibling to exclude the role of familial factors in our findings. Additionally, we were unable to determine the directionality of the relationship between cerebellar hypoplasia and body weight in this study due to its cross-sectional nature. One confounding factor in this study may be the wide age range studied, although cerebellar volume should be stable by age 4 years. Lastly, the lack of gender matching between groups is another possible limitation of this study, as males typically have a larger cerebral and cerebellar volume than females, but each individual with PWS or EMO had at least one sex and age-matched control. Longitudinal studies will need to be done to better understand the relationship between the development of early-onset obesity and cerebellar volume, cerebral/cerebellar volume ratio, and cognitive outcome.
In conclusion, we found that individuals with PWS and EMO had small cerebellar volume and low GIA compared to their normal weight control siblings. Due to the genetic differences between PWS and EMO, it is unlikely that a single genetic factor is responsible. The most parsimonious explanation is that early childhood obesity alone may cause damage to the developing brain, thus adding to the public health concern surrounding the epidemic of obesity in childhood and further emphasizing the need for early intervention. Additionally, as first suggested in 1980 by Crnic et al, some aspects of cognitive performance in PWS may be preserved by preventing the early onset of obesity that is typical of this condition (Crnic et al, 1980).
Acknowledgements
Funding Support provided by: Lawson Wilkins Pediatric Endocrine Society (JM); National Institutes of Health 1K24 HD01361 (DJD); 1U54 RR019478 (DJD and JLM); 1R21 NS045518 (YL); General Clinical Research Center M01 RR00082 from the National Center for Research Resources, University of Florida; and the Hayward Foundation (DJD). The authors would like to thank Tony Goldstone and Paul Kuipers for their assistance with the genetic testing.
References
- Belichenko NP, Belichenko PV, Li HH, Mobley WC, Francke U. Comparative study of brain morphology in Mecp2 mutant mouse models of Rett syndrome. J Comp Neuro. 2008 May 1;508(1):184–95. doi: 10.1002/cne.21673. [DOI] [PubMed] [Google Scholar]
- Bittel DC, Butler MG. Prader-Willi syndrome: clinical genetics, cytogenetics and molecular biology. Expert Rev Mol Me. 2005 Jul 25;7(14):1–20. doi: 10.1017/S1462399405009531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crnic KA, Sulzbacher S, Snow J, Holm VA. Preventing mental retardation associated with gross obesity in the Prader-Willi syndrome. Pediatrics. 1980 Nov;66(5):787–9. [PubMed] [Google Scholar]
- Elenkov IJ. Neurohormonal-cytokine interactions: implications for inflammation, common human diseases, and well-being. Neurochem Int. 2008 Jan;52(1-2):40–51. doi: 10.1016/j.neuint.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Floeter MK, Greenough WT. Cerebellar plasticity: modification of Purkinje cells by differential rearing in monkeys. Science. 1979 Oct;206(12):227–229. doi: 10.1126/science.113873. [DOI] [PubMed] [Google Scholar]
- Glenn CG, Driscoll DJ, Thomas PY, Nicholls RD. Genomic imprinting: potential function and mechanisms revealed by the Prader-Willi and Angelman syndromes. Mol Hum Reprod. 1997;3:321–32. doi: 10.1093/molehr/3.4.321. [DOI] [PubMed] [Google Scholar]
- Goldstone AP. Prader-Willi syndrome: advances in genetics, pathophysiology and treatment. Trends Endocrinol Metab. 2004 Jan-Feb;15(1):12–20. doi: 10.1016/j.tem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Itoh M, Kabasawa Y, Hayashi H, Satoh J, Morimatsu Y. A neuropathological study of a case of the Prader-Willi syndrome with an interstitial deletion of the proximal long arm of chromosome 15. Brain Dev. 1992 Jan;14(1):58–62. doi: 10.1016/s0387-7604(12)80281-6. [DOI] [PubMed] [Google Scholar]
- Huber KM. The fragile X-cerebellum connection. Trends Neurosci. 2006 Apr;29(4):183–5. doi: 10.1016/j.tins.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Bellugi U, Sowell E, Doherty S, Hesselink JR. Cerebral morphologic distinctions between Williams and Down syndromes. Arch Neurol. 1993 Feb;50(2):186–91. doi: 10.1001/archneur.1993.00540020062019. [DOI] [PubMed] [Google Scholar]
- Lee S, Walker CL, Wevrick R. Prader-Willi syndrome transcripts are expressed in phenotypically significant regions of the developing mouse brain. Gene Expr Patterns. 2003 Oct;3(5):599–609. doi: 10.1016/s1567-133x(03)00113-3. [DOI] [PubMed] [Google Scholar]
- Lee S, Walker CL, Karten B, Kuny SL, Tennese AA, O’Neill MA, Wevrick R. Essential role for the Prader-Willi syndrome protein necdin in axonal outgrowth. Hum Mol Genet. 2005 Mar 1;14(5):627–37. doi: 10.1093/hmg/ddi059. [DOI] [PubMed] [Google Scholar]
- Miller JL, Couch JA, Schmalfuss I, He G, Liu Y, Driscoll DJ. Intracranial abnormalities detected by three-dimensional magnetic resonance imaging in Prader-Willi syndrome. Am J Med Genet A. 2007 Mar 1;143(5):476–83. doi: 10.1002/ajmg.a.31508. [DOI] [PubMed] [Google Scholar]
- Miller J, Kranzler J, Liu Y, Schmalfuss I, Theriaque DW, Shuster JJ, Hatfield A, Mueller OT, Goldstone AP, Sahoo T, Beaudet AL, Driscoll DJ. Neurocognitive findings in Prader-Willi syndrome and early-onset morbid obesity. J Pediatr. 2006 Aug;149(2):192–8. doi: 10.1016/j.jpeds.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Moldrich RX, Dauphinot L, Laffaire J, Rossier J, Potier MC. Down syndrome gene dosage imbalance on cerebellum development. Prog Neurobiol. 2007 Jun;82(2):87–94. doi: 10.1016/j.pneurobio.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Pae EK, Chien P, Harper RM. Intermittent hypoxia damages cerebellar cortex and deep nuclei. Neurosci Lett. 2005 Feb 28;375(2):123–8. doi: 10.1016/j.neulet.2004.10.091. [DOI] [PubMed] [Google Scholar]
- Niccols A. Fetal alcohol syndrome and the developing socio-emotional brain. Brain Cogn. 2007 Oct;65(1):135–42. doi: 10.1016/j.bandc.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Nicholls RD, Knepper JL. Genome organization, function and imprinting in Prader-Willi and Angelman syndromes. Annu. Rev. Genomics Hum.Genet. 2001;2:153–75. doi: 10.1146/annurev.genom.2.1.153. [DOI] [PubMed] [Google Scholar]
- Rapoport M, van Reekum R, Mayberg H. The role of the cerebellum in cognition and behavior: a selective review. J Neuropsychiatry Clin Neurosci. 2000 Spring;12(2):193–8. doi: 10.1176/jnp.12.2.193. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader M Zaheer, Matthews P, Behrens TEJ. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Sriram K, Benkovic SA, Miller DB, O’Callaghan JP. Obesity exacerbates chemically induced neurodegeneration. Neuroscience. 2002;115(4):1335–46. doi: 10.1016/s0306-4522(02)00306-8. [DOI] [PubMed] [Google Scholar]
- Steinlin M. The cerebellum in cognitive processes: supporting studies in children. Cerebellum. 2007;6(3):237–41. doi: 10.1080/14734220701344507. [DOI] [PubMed] [Google Scholar]
- Titomanlio L, De Brasi D, Romano A, Genesio R, Diano AA, Del Giudice E. Partial cerebellar hypoplasia in a patient with Prader-Willi syndrome. Acta Paediatr. 2006 Jul;95(7):861–3. doi: 10.1080/08035250500527307. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and the future risk of dementia. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson Psycho-Educational Battery III. Riverside Publishing; Itasca, IL: 2001. [Google Scholar]
- Yamada K, Matsuzawa H, Uchiyama M, Kwee IL, Nakada T. Brain developmental abnormalities in Prader-Willi syndrome detected by diffusion tensor imaging. Pediatrics. 2006 Aug;118(2):e442–8. doi: 10.1542/peds.2006-0637. [DOI] [PubMed] [Google Scholar]