Abstract
Hormonal replacement therapy (HRT) has recently been shown to increase the risk of cardiovascular events in women. However, it is not clear whether the adverse effect of HRT is related to dosage and/or the presence of progestin. Using a mouse model of myocardial infarction (MI), we studied the dose-effect of oestrogen replacement on mortality and cardiac remodelling and dysfunction post-MI in the absence of progestin. Six-week-old females were subjected to ovariectomy (OVX). A pellet containing a low, moderate or high dose of 17β-oestradiol (E2; 0.42, 4.2 or 18.8 μg day−1) or placebo was implanted subcutaneously on the day of OVX. Myocardial infarction was induced 8 weeks later, and cardiac morphology and function were evaluated 8 weeks after MI. We found that E2 at moderate and high doses adversely affected mortality. A low dose of E2 that restored plasma oestrogen close to physiological levels had no significant effect on mortality but tended to improve cardiac function and remodelling, associated with reduced fibrosis and increased capillary density. At the moderate dose, E2 exacerbated cardiac fibrosis, hypertrophy, dysfunction and dilatation, associated with liver and kidney enlargement and ascites. Protein kinase C and extracellular signal-regulated kinase were increased by MI but were not affected by E2. In summary, E2 at a low dose tended to be cardioprotective. At increased doses that raised plasma oestrogen far beyond the physiological level, E2 was detrimental to the heart. Our data suggest that dosage should be an important consideration when studying the effect of oestrogen replacement on the heart.
It has been established that the incidence of cardiovascular disease (CVD) and the attendant morbidity and mortality are much lower in premenopausal women compared with men of the same age (Isles et al. 1992; Brett & Madans, 1995; Barrett-Connor, 1997; Adams et al. 1999; Tamura et al. 1999). This gender advantage for women becomes far less or disappears with increased age and reduced oestrogen levels after menopause, suggesting that ovarian hormones, most likely oestrogen, protect women against CVD (Lerner & Kannel, 1986; Eaker et al. 1993). Abundant observational studies show that postmenopausal women who receive hormonal replacement therapy (HRT) have a lower incidence of CVD and cardiac death than thosewho do notreceive HRT. In thepast, HRThas commonly been used to prevent CVD in postmenopausal women (Stampfer et al. 1991; Wenger et al. 1993; Grodstein et al. 1997, 2000; Reis et al. 2000; Alexandersen et al. 2006). However, the cardioprotective benefits of HRT have recently been challenged by the Heart and Oestrogen/Progestin Replacement Study (HERS; Hulley et al. 1998, 2002) and the Women's Health Initiative (WHI; Rossouw et al. 2002; Hendrix et al. 2006), which showed an unfavourable effect of HRT on the risk and events of CVD in women. Although the data are convincing, there are other considerations that may have affected the outcome of HERS and WHI (Nishida et al. 2005; Bittner, 2006; Pines et al. 2006; Gambrell & Natrajan, 2006; Willett et al. 2006; Grodstein et al. 2006), such as composition of the drugs, dosage, time when HRT was initiated, age and pre-existing risk factors for CVD. Furthermore, it is not fully understood whether HRT slows the progression of heart failure in women once they develop myocardial infarction (MI). In animal studies, most data indicate that oestrogen has a cardioprotective role, which may involve inhibition of protein kinase C (PKC) activity and mitogen-activated protein kinase (MAPK) signalling (van Eickels et al. 2001; Pedram et al. 2005; Wang et al. 2006; Beer et al. 2007; Hunter et al. 2007). However, in other studies oestrogen either offered no cardioprotective effect or else worsened cardiac remodelling and dysfunction post-MI (Hügel et al. 1999; van Eickels et al. 2003). It is not known whether those conflicting results in animals are also related to oestrogen dosage.
Given the prevailing controversy over whether HRT is cardioprotective or detrimental, we hypothesized that the effects of oestrogen on the heart may be dose dependent; a low dose is cardioprotective, whereas a high dose may be harmful to the heart. To test this hypothesis, we used mice with surgically induced menopause (by ovariectomy) plus oestrogen replacement and studied the effects of oestrogen replacement at various doses on: (1) mortality post-MI; (2) cardiac morphology, remodelling and function; and (3) protein expression of PKC and extracellular signal-regulated kinase (ERK1/2) in the heart.
Methods
Animals
Four-week-old female C57BL/6J mice from Jackson Laboratories (Bar Harbor, ME, USA) were housed in an air-conditioned room with a 12 h–12 h light–dark cycle and given standard chow with free access to tap water. The study was approved by our Institutional Animal Care and Use Committee in accord with US National Institutes of Health guidelines.
Ovariectomy and oestrogen replacement therapy (ERT)
Between 1 and 2 weeks after introduction to their new environment, mice were anaesthetized with sodium pentobarbitone (50 mg kg−1, I.P.) and placed in the prone position. A bilateral ovariectomy (OVX) was performed via a pair of flank incisions (Cavasin et al. 2003, 2004). Pellets containing placebo or 17β-oestradiol (E2; 0.42, 4.2 or 18.8 μg day−1, 60 day release pellet; Innovative Research of America, Sarasota, FL, USA) were implanted subcutaneously at the back of the neck immediately after OVX. The pellets were replaced 8 weeks after OVX under anaesthesia (before MI). Sham OVX groups underwent the same procedure except that the ovaries were left intact. According to the literature, E2 has commonlybeen given at a dosage of 3.0−28.3 μg day−1 in animals (van Eickels et al. 2001, 2003; Pelzer et al. 2005; Tang et al. 2005; Iwakura et al. 2006; Babiker et al. 2006)
Induction of MI and evaluation of cardiac function
Eight weeks after OVX and ERT, mice were anaesthetized (sodium pentobarbitone, 50 mg kg−1, I.P.), and MI was induced by ligating the left anterior descending coronary artery (LAD) as described previously (Yang et al. 1999; Cavasin et al. 2003). Sham MI mice received the same open-chest operation except that the LAD was untied. There were six groups, as follows: (1) sham OVX + sham MI; (2) sham OVX + MI; (3) OVX + MI + placebo; (4) OVX + MI + low-dose E2 (0.42 μg day−1); (5) OVX + MI + moderate-dose E2 (4.2 μg day−1); and (6) OVX + MI + high-dose E2 (18.8 μg day−1).
Measurement of systolic blood pressure and cardiac function
Eight weeks after MI (16 weeks after OVX and ERT), systolic blood pressure (SBP) was measured in conscious mice using a non-invasive computerized tail-cuff system (BP-2000, Visitech, Apex, NC, USA). Cardiac geometry and function were measured with a Doppler echocardiographic system equipped with a 15 MHz linear transducer (Acuson c256, Mountain View, CA, USA) as described previously (Yang et al. 1999). Left ventricular systolic and diastolic dimension (LVDs and LVDd), systolic and diastolic cross-sectional area (LVAs and LVAd), fractional shortening (FS), ejection fraction (EF) and cardiac index (CI, i.e. cardiac output corrected for body weight) were evaluated. All studies were performed on conscious mice after several training sessions.
Histopathological study
After echocardiographic evaluation of cardiac function, mice were anaesthetized with sodium pentobarbitone (50 mg kg−1, I.P.); the chest was opened and the heart stopped at diastole by intraventricular injection of 15% KCl (50 μl). The heart, kidneys and liver were weighed to assess hypertrophy and congestion. Organ weight was corrected by body weight and expressed as the ratio of organ wet weight (in milligrams) to body weight (per 10 grams). After dissection, the left ventricle was sectioned transversely into four slices from apex to base. One slice was snap-frozen for Western blot and the other three were rapidly frozen in isopentane precooled in liquid nitrogen, then stored at −70°C for measurements of infarct size (IS), myocyte cross-sectional area (MCSA), interstitial collagen fraction (ICF) and capillary density as described previously (Liu et al. 1997). The MCSA, ICF and capillary density were studied only in the non-infarcted area. Mice with IS less than 20% were excluded.
Western blot of PKC and phosphorylated ERK (p-ERK1/2)
Heart tissue was homogenized in lysis buffer and centrifuged at 14 000g for 10 min; the supernatant was collected and protein content detected with a protein assay kit (Thermo Scientific, Rockford, IL, USA). Protein (65 μg) was separated out by 8% sodium dodecyl suphate-polyacrylamide gel electrophoresis and electrotransferred to an Immuno-Blot™ polyvinylidene difluoride membrane (Bio-Rad, CA, USA). Membranes were blocked with 5% non-fat milk and incubated overnight at 4°C with the primary antibody (anti-PKC, anti-p-ERK1/2 or anti-total ERK; Cell Signaling Technology, Boston, MA, USA), anti-β-actin (Santa Cruz Biotechnology, Hercules, CA, USA) and horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology). Blots were subjected to autoradiography (Enhanced Chemiluminescence Detection kit, Thermo Scientific). Results are expressed as the ratio of the density of specific bands to the corresponding β-actin or total-ERK1/2.
Measurement of plasma oestrogen and testosterone
Owing to high mortality in the moderate- and high-dose groups, additional mice in each group were used to determine plasma concentrations of oestrogen and testosterone. Blood samples were collected after 8 weeks of E2 or placebo and mice were killed without MI. Our previous study showed that MI does not have any significant influence on plasma oestrogen and testosterone levels (Cavasin et al. 2003). Oestrogen and testosterone were measured by enzyme immunoassay using a commercially available kit (Cayman Chemicals, Ann Arbor, MI, USA).
Statistical analysis
Data are expressed as means ± s.e.m. Differences in mortality rates between the various groups were compared using a log-rank test. Since morphological, functional and molecular biological parameters did not follow a normal distribution, comparisons were assessed using a non-parametric Mann–Whitney U test. The significance of the comparisons in each testing set was determined using Hochberg's method to adjust for multiple comparisons. Values of P < 0.05 were considered significant.
Results
Mortality after MI
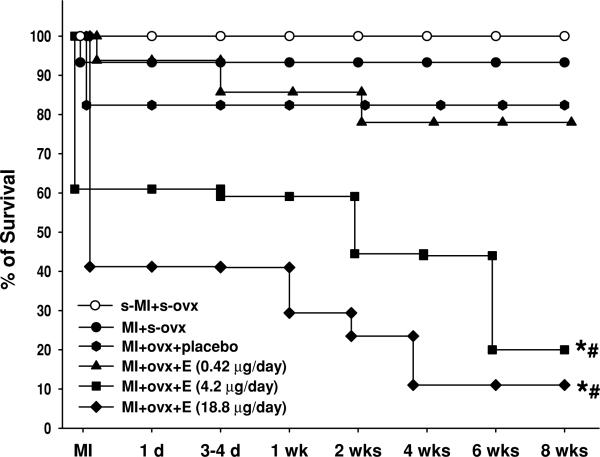
Out of the 15 sham OVX mice, one died on the first day of MI (6.6%) and 14 survived for the entire experimental period. Three of the 17 OVX mice given placebo died after MI (18%). In the low-dose E2 group, four out of 16 mice died (25%), which was not statistically different from placebo. With increased doses, E2 adversely affected mortality (Fig. 1): 33 out of 41 mice in the mid-dose group (80%) and 15 out of 17 mice in the high-dose group (89%) died. Since high-dose E2 (18.8 μg) caused drastically high mortality, this dose was discontinued and only mortality data were collected. Postmortem examination on mice given moderate- or high-dose E2 that died before the end of the study revealed marked heart, liver and kidney enlargement associated with hydronephropathy and fluid accumulation in the thoracic and abdominal cavities, indicating severe end-organ damage.
Figure 1. Effect of E2 at different doses on cumulative death rate in ovariectomized mice with MI.
The E2 replacement was started immediately after OVX. *P < 0.01 versus OVX + MI + placebo; #P < 0.01 versus OVX + MI + E2 (0.42 μg).
Organ weight
Myocardial infarction significantly increased total heart weight but had no significant influence on liver and kidney weight (Table 1). Ovariectomy did not increase heart weight further. The E2 at a low dose (0.42 μg) had no effect on heart weight, although kidney weight was higher than in the OVX + placebo group. A moderate dose of E2 (4.2 μg) increased both heart and kidney weight further compared with the placebo and low-dose E2 groups. This dose also tended to increase liver weight, although the difference did not reach statistical significance. Infarct size was similar among groups, although it tended to be larger in mice given a moderate dose of E2.
Table 1.
Effect of 17β-oestradiol (E2) replacement on organ weight and cardiac haemodynamics in mice with myocardial infarction
| Weight |
Haemodynamics |
|||||||
|---|---|---|---|---|---|---|---|---|
| Group | BW (g) | THW/BW (10−1) | Liver/BW (mg (10 g)−1) | Kidney/BW (mg (10 g)−1) | SBP (mmHg) | HR (beats min−1) | CI (ml min−1 (10 g)−1) | IS (%) |
| Sham OVX + sham MI | 24.4 ± 0.4 | 42.1 ± 1.2 | 426 ± 27 | 120 ± 6 | 107 ± 4 | 722 ± 6 | 11.8 ± 1.1 | – |
| Sham OVX + MI | 24.1 ± 0.4 | 56.7 ± 1.2a | 368 ± 18 | 113 ± 5 | 100 ± 3 | 711 ± 5 | 7.6 ± 0.3a | 42 ± 2 |
| OVX + MI + placebo | 27.5 ± 0.6 | 57.7 ± 3.8 | 430 ± 17 | 104 ± 3 | 96 ± 3 | 670 ± 12 | 6.2 ± 0.3b | 42 ± 2 |
| OVX + MI + E2 (0.42 μg day−1) | 25.1 ± 0.4 | 55.0 ± 2.4 | 437 ± 18 | 128 ± 2c | 110 ± 3 | 670 ± 13 | 8.6 ± 0.3c | 41 ± 3 |
| OVX + MI + E2 (4.2 μg day−1) | 24.4 ± 1.2 | 71.2 ± 2.3cd | 479 ± 25 | 140 ± 8.9c | 114 ± 5 | 543 ± 35cd | 5.5 ± 0.7d | 52 ± 4 |
Abbreviations: BW, body weight; THW, total heart weight; SBP, systolic blood pressure; HR, heart rate; CI, cardiac index; IS, infarct size; OVX, ovariectomy; MI, myocardial infarction; E2,17β-oestradiol.
P < 0.05 versus sham OVX + sham MI
P< 0.05 versus sham OVX + MI
P < 0.05 versus OVX + MI + placebo
P < 0.05 versus OVX + MI + E2 (0.42 μg day−1).
Cardiac function and remodelling
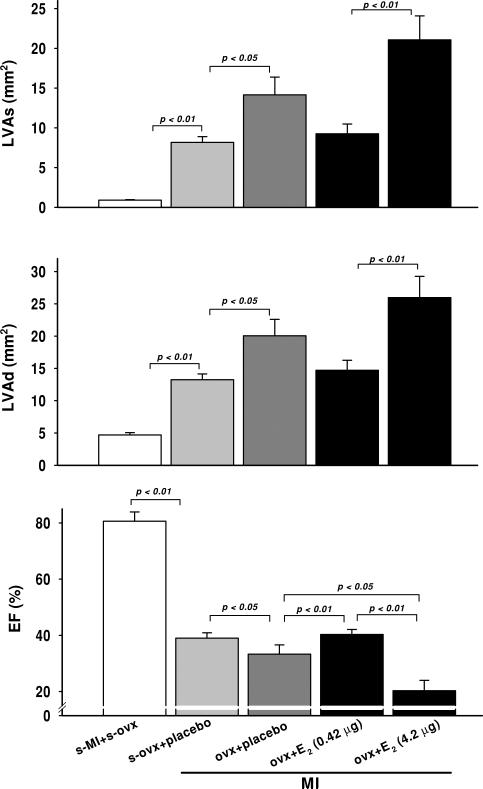
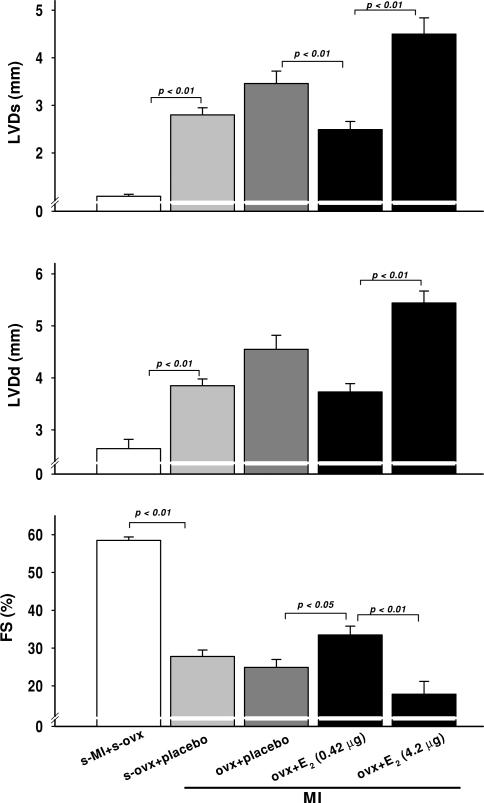
Systolic blood pressure was not affected by MI or E2. Heart rate (HR) did not change after MI with low-dose E2; however, it decreased significantly with mid-dose E2 (Table 1). We did not find this surprising, since we have often seen bradycardia in mice with severe heart failure. Myocardial infarction caused left ventricular dysfunction and dilatation, as evidenced by a decrease in cardiac index, EF and FS and an increase in left ventricular dimensions and area, which were more severe in the OVX + placebo group compared with sham OVX (Table 1 and Figs 2–4). A low dose of E2 increased FS and EF and tended to decrease left ventricular dilatation, indicating a cardioprotective effect of this dose. However, a moderate dose worsened cardiac function and remodelling, as indicated by a further decrease in CI, FS and EF and an increase in left ventricular dimensions and area (Table 1 and Figs 2–4).
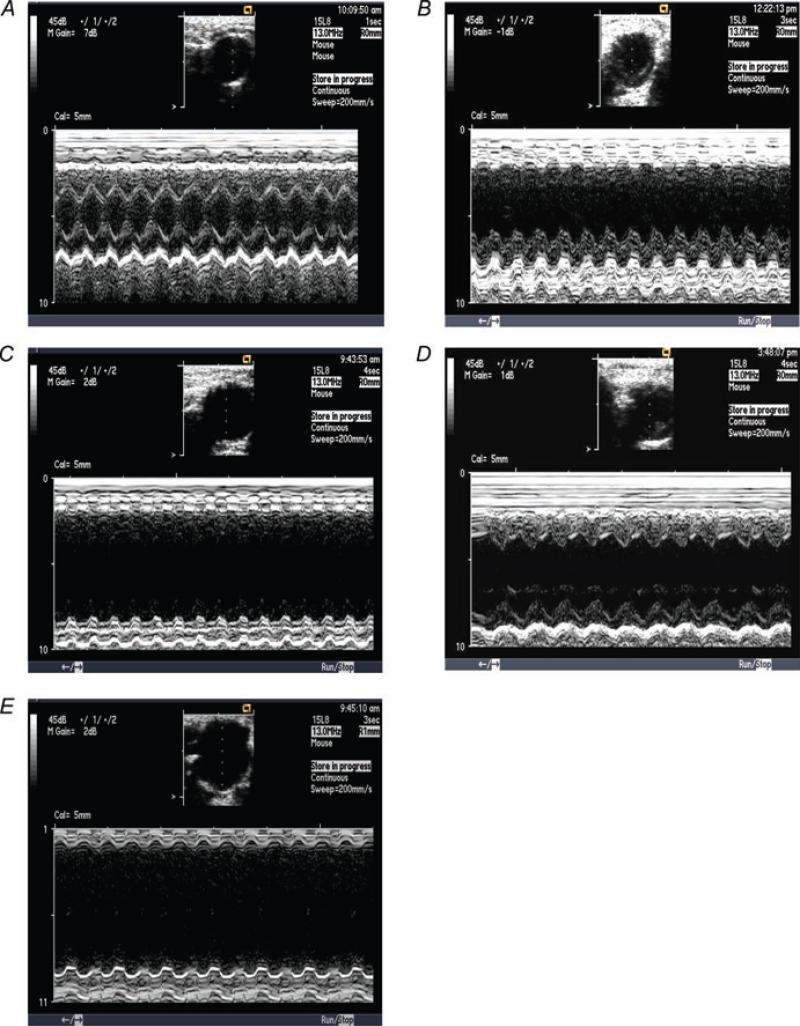
Figure 2. Representative echocardiograms showing left ventricular chamber dilatation and dysfunction after MI (B and C) as well as the effect of 17β-oestradiol (0.42 and 42 μg day−1) on left ventricular dilatation and dysfunction (D and E) in comparison with a normal mouse heart (A).
A, sham OVX + sham MI; B, sham OVX + MI + placebo; C, OVX + MI + placebo; D, OVX + MI + E2 (0.42 μg day−1); and E, OVX + MI + E2 (4.2 μg day−1).
Figure 4.
Effect of low (0.42 μg day−1) and moderate doses of 17β-oestradiol (42 μg day−1) on left ventricular systolic and diastolic cross-sectional area (LVAs and LVAd) and ejection fraction (EF) in OVX mice with MI
Cardiac histology
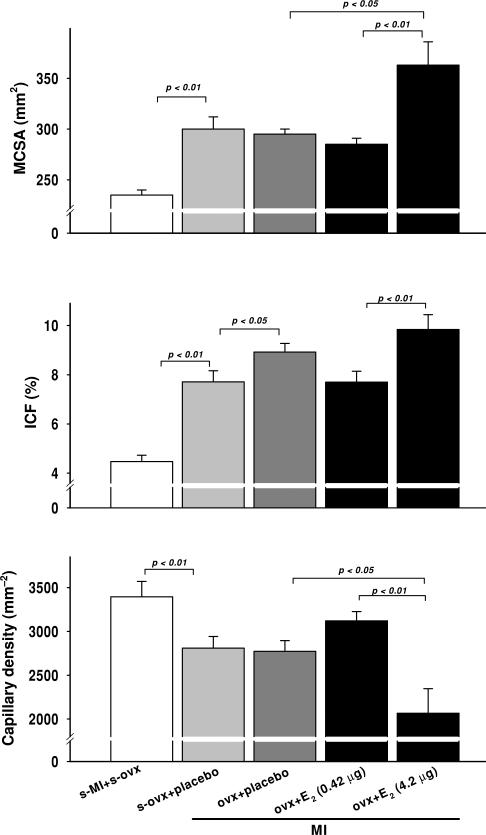
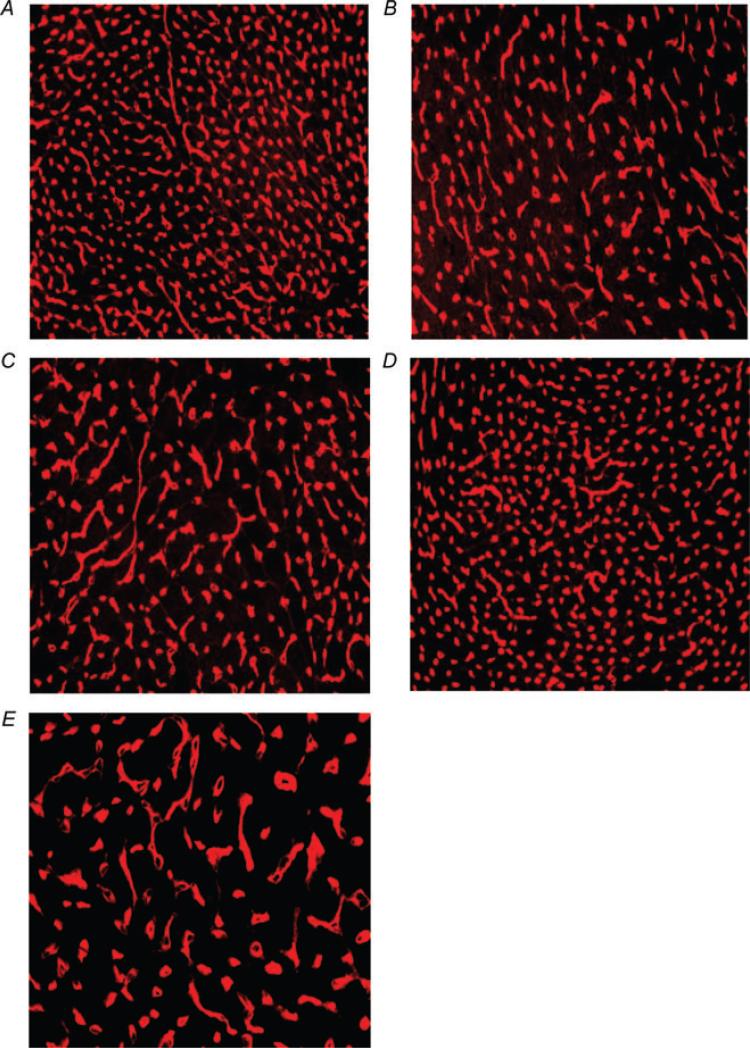
Myocardial infarction increased myocyte cross-sectional area, and this result was not influenced by OVX or low-dose E2. However, a moderate dose of E2 increased myocyte hypertrophy compared with either placebo or low-dose E2 (Fig. 5, top panel). Myocardial infarction also increased collagen deposition, which appeared to be enhanced by OVX and OVX + E2 at a moderate dose. A low dose tended to decrease interstitial collagen compared with placebo, although the difference did not reach statistical significance (Fig. 5, middle panel). Capillary density was significantly decreased as a result of MI, and this was not affected by OVX. A low dose of E2 tended to increase capillary density; however, at a moderate dose it reduced capillary density further (Fig. 5, bottom panel and Fig. 6).
Figure 5.
Effect of low (0.42 μg day−1) and moderate doses of 17β-oestradiol (42 μg day−1) on myocyte cross-sectional area (MCSA), interstitial collagen fraction (ICF) and capillary density in OVX mice with MI
Figure 6. Representative micrographs showing reduced capillary density (red staining) after MI (B and C) as well as the effect of 17β-oestradiol (0.42 and 42 μg day−1) on capillary density (D and E) in comparison with a normal mouse heart (A).
A, sham OVX + sham MI; B, sham OVX + MI + placebo; C, OVX + MI + placebo; D, OVX + MI + E2 (0.42 μg day−1); and E, OVX + MI + E2 (4.2 μg day−1)
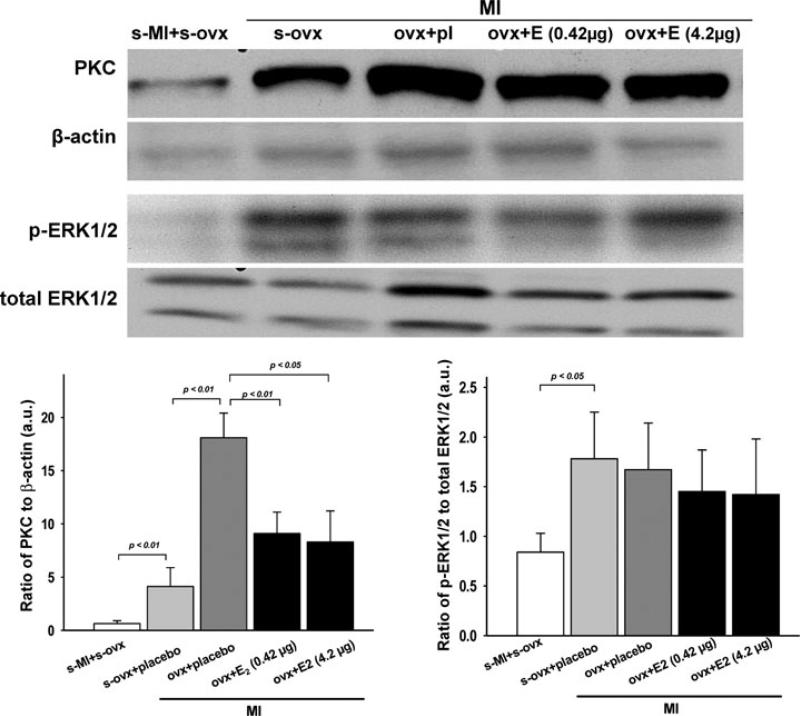
Phosphorylation of PKC and ERK
Protein expression of PKC was significantly increased in OVX mice after MI. Low-dose E2 decreased PKC expression. Unexpectedly, PKC expression was also lower in mice given a moderate dose of E2 (Fig. 7, left panel). Phosphorylation of ERK1/2 was increased by MI. Ovariectomy with or without E2 had no effect on p-ERK1/2 expression compared with sham OVX mice with MI(Fig. 7, right panel).
Figure 7.
Effect of low (0.42 μg day−1) and moderate doses of 17β-oestradiol (42 μg day−1) on phosphorylation of PKC and ERK1/2 in cardiac tissue
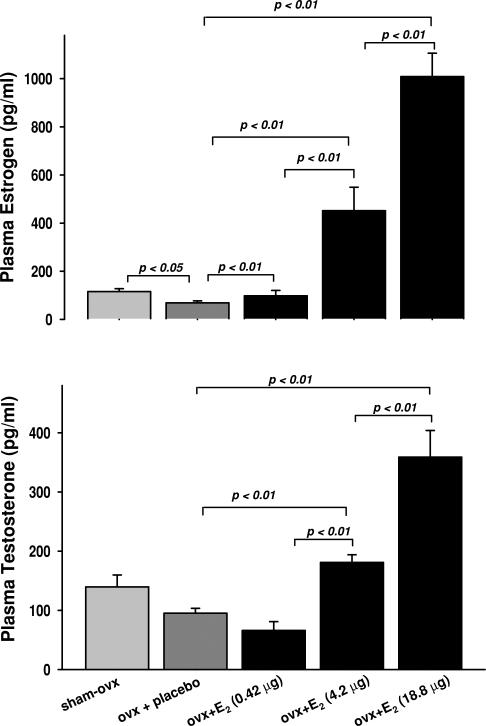
Plasma levels of oestrogen and testosterone
Plasma oestrogen was significantly reduced by OVX. Low-dose E2 (0.42 μg) raised it to around the same level as sham OVX mice, while 4.2 μg induced a fourfold increase compared with sham OVX. High-dose E2 (18.8 μg) increased plasma oestrogen approximately 8.5-fold (Fig. 8, top panel). Ovariectomy tended to reduce plasma testosterone compared with the sham OVX group, and this result was not affected by low-dose E2. A moderate dose caused an approximately twofold increase in testosterone compared with OVX + placebo, while a high dose further increased it by 3.5-fold (Fig. 8, bottom panel).
Figure 8.
Effect of OVX and low (0.42 μg day−1) and moderate doses of 17β-oestradiol (42 μg day−1) on plasma oestrogen and testosterone levels
Discussion
Using a mouse model of surgically induced menopause (OVX) followed by MI, we examined the dose-dependent effects of oestrogen replacement on mortality, cardiac function and remodelling post-MI. We found that: (1) oestrogen adversely affected mortality at moderate and high doses (4.2 and 18.8 μg day−1) but notata low dose (0.42 μg); (2) at a low dose, 17β-oestradiol tended to improve cardiac function and remodelling, as evidenced by better FS and EF and less severe left ventricular dilatation, but at a moderate dose (4.2 μg), oestrogen exacerbated cardiac hypertrophy, dysfunction and remodelling, associated with liver enlargement and hydronephrosis; and (3) moderate and high doses of oestrogen adversely affected cardiac remodelling and function and raised plasma oestrogen far above physiological levels, associated with a significant increase in plasma testosterone. Oestrogen did not appear to affect blood pressure; however, at a high dose it significantly decreased heart rate. We believe this is the first report demonstrating that the adverse effect of oestrogen replacement on the heart in mice post-MI is dose related.
It has been well established that premenopausal women are less prone to CVD than men, probably owing to endogenous oestrogen (Isles et al. 1992; Brett & Madans, 1995; Barrett-Connor, 1997; Adams et al. 1999; Tamura et al. 1999). However, data on the outcome of HRT are conflicting (Stampfer et al. 1991; Wenger et al. 1993; Grodstein et al. 1997, 2000; Reis et al. 2000; Shlipak et al. 2001; Alexandersen et al. 2006). We could find no good explanation as to why young or premenopausal women are protected against CVD as shown in observational studies, whereas HRT did not provide protection to postmenopausal women in clinical trials. One consideration should be dose, which has not been well established. Grodstein et al. (2000) showed that oestrogen at 0.3 mg day−1 decreased major coronary events in healthy women, whereas at 0.625 mg and in combination with progestin, oestrogen increased the risk of stroke. Genant et al. (1997) reported that a low dose of esterified oestrogen (0.3 mg) provided better bone and lipid benefits than a higher dose. Various doses of oestrogen (from 3 to 83.3 μg day−1) have been used in animal studies. Depending on the study, oestrogen was reported to be either beneficial, ineffective or detrimental to the heart (Hügel et al. 1999; van Eickels et al. 2003; Pelzer et al. 2005; Iwakura et al. 2006; Hamada et al. 2006; Babiker et al. 2006; Beer et al. 2007). For example, Beer and co-workers recently showed that high-dose oestrogen (83.3 μg day−1) prevented development of heart failure post-MI in rats (Beer et al. 2007), whereas Hügel and co-workers reported that oestrogen replacement (11.1 μg day−1, which restored plasma oestrogen to physiological levels) had no effect on remodelling post-MI in rats (Hügel et al. 1999). Using FVB mice, Iwakura et al. (2006) reported that oestrogen at 18.8 μg day−1 enhanced functional recovery after MI. However, we used the same dose and clearly observed a detrimental effect in C57BL/6J mice with MI. Using a lower dose (4 μg day−1), van Eickels et al. (2003) showed that oestrogen increased mortality post-MI, which was confirmed by the present study. Although we have no clear answer to these conflicting data, differences in species and strains may be important factors. Furthermore, it is not known whether these differing data are dose related, since in almost all animal studies only a single dose of oestrogen was used. To overcome these limitations, in the present study we tested the effects of three doses of oestrogen (0.42, 4.2 and 18.8 μg day−1) on mortality, cardiac remodelling and function post-MI. We found that at a low dose, oestrogen tended to be cardioprotective and mortality was significantly lower than at high doses. Most mice died during the first week after MI, mainly as a result of cardiac rupture. It is known that after MI, the infarcted region of the heart undergoes a dynamic healing and repair process. Delayed or heightened inflammatory responses that adversely affect infarct healing could be a significant cause of cardiac rupture (Ertl & Frantz, 2005; Gao et al. 2005). With higher doses, oestrogen significantly increased mortality and worsened cardiac dysfunction and remodelling. Moreover, the high dose we used (18.8 μg day−1) not only damaged the heart but also caused liver enlargement and ascites, which may well be related to the severe heart failure, while hydronephropathy may be due to uterine hypertrophy, which causes urinary tract obstruction. However, whether these pathological changes also reflect the toxic effects of high-dose oestrogen needs to be evaluated further, since that was not the focus of the present study.
Among those studies showing that oestrogen is cardioprotective, many have demonstrated that it can inhibit activation of PKC and the MAPK signalling cascade, including ERK1/2, Jun-N terminal kinase (JNK) and p38 MAPKsboth in vivo and in vitro (van Eickels et al. 2001; Pedram et al. 2005; Wang et al. 2006; Hunter et al. 2007). In the present study, we measured PKC and ERK1/2 MAPK expression to determine whether the effect of oestrogen is linked to inhibition or activation of these protein kinases. In agreement with most previous studies, we found that PKC was increased after MI. Upregulation of PKC was further enhanced by OVX but suppressed by oestrogen replacement. It is rational to speculate that the beneficial cardiac effect of low-dose oestrogen we observed may be related to inhibition of PKC activity. However, we do not have a good explanation as to why a moderate dose also reduced PKC expression, since it was clearly harmful to the heart and other organs. This could be related to the fact that we only measured total PKC, not separate PKC isoforms. It has been shown that in the end-stages of heart failure, some PKC isoforms are upregulated whereas others decline (Koide et al. 2003; Simonis et al. 2007; Hunter et al. 2007). We do not know whether this is the case with oestrogen replacement in the present study. We also did not measure translocation of PKC; thus, we do not know whether this differs between the two doses used here. Alternatively, our data may indicate that changes in PKC are not responsible for the detrimental effect of oestrogen. Expression of p-ERK1/2 was increased by MI; however, this increase was not affected by OVX with or without oestrogen replacement. These data do not seem to support a role of ERK signalling in mediating the effect of oestrogen on cardiac remodelling and dysfunction in mice post-MI.
We measured plasma oestrogen and testosterone and found that oestrogen was significantly reduced but not depleted by OVX. In postmenopausal women, when ovaries cease to produce oestrogen, extragonadal sites become major sources of oestradiol production via aromatization (Simpson et al. 2001; Simpson & Davis, 2001). Thus, it is not surprising that circulating oestrogen levels were detectable in the OVX mice in our study. Low-dose oestrogen replacement restored plasma oestrogen to a level similar to intact females, and at this dose oestrogen tended to be cardioprotective. However, moderate and high doses that brought plasma oestrogen far above physiological levels were obviously detrimental to the heart. These data may suggest that whether oestrogen is protective or detrimental to the heart is largely dependent on the dose and its circulating levels. Testosterone was not significantly influenced by OVX per se or OVX plus low-dose oestrogen. Surprisingly, moderate and high doses significantly increased plasma testosterone, though the mechanism responsible for such an increase in testosterone by exogenous oestrogen is not known. Since mice with high levels of testosterone had increased mortality and decreased cardiac function and remodelling (Cavasin et al. 2003), we cannot exclude the possibility that the adverse effects we observed with high-dose oestrogen were directly linked to increased plasma testosterone.
Limitations of the study
Model of surgically induced menopause. Menopause is a slowly progressive process of oestrogen depletion, whereas surgically induced OVX causes sudden depletion. Thus this model is not perfect for mimicking naturally occurring menopause, even though a low dose of oestrogen brought plasma oestrogen close to physiological levels compared with intact females.
Dosage of oestrogen.Three doses of oestrogen were studied, but morphological and functional studies were completed only in the low- and moderate-dose groups. The high dose only allowed us to observe mortality, since nearly all mice in this group died before the end of the experiment. We could say this dose is excessive, and thus no further study is needed. However, it is debatable whether the low dose we used was low enough, since mortality in the low-dose group still tended to be high and hypertrophy did not differ from placebo, although cardiac function was improved. A lower dose may be needed in future studies to confirm that oestrogen dosage is an important determinant of its effects on the heart. Also, the effect of oestrogen on infarct healing should be evaluated in future studies.
This study focused on mortality and functional evaluation. The underlying molecular and cellular mechanisms responsible for the actions of oestrogen on the heart, either protective or detrimental, were not emphasized and need to be tested in the future.
Conclusion
In summary, our data showed that oestrogen can be either cardioprotective or detrimental, largely depending on the dose. A low dose tended to be beneficial; however, at high doses that drastically increased plasma oestrogen it was detrimental. These results suggest that dosage should be an important consideration when studying the effects of oestrogen replacement on the heart.
Figure 3.
Effect of low (0.42 μg day−1) and moderate doses of 17β-oestradiol (42 μg day−1) on left ventricular systolic and diastolic dimensions (LVDs and LVDd) and fractional shortening (FS) in OVX mice with MI
Acknowledgements
This work was supported by National Institutes of Health/National Heart, Lung and Blood Institute Grant HL-078951 (X.-P.Y.) and Henry Ford Health System institutional funds (X.-P.Y.).
References
- Adams KF, Jr, Sueta CA, Gheorghiade M, O'Connor CM, Schwartz TA, Koch GG, Uretsky B, Swedberg K, McKenna W, Soler-Soler J, Califf RM, for the FIRST Investigators Gender differences in survival in advanced heart failure. Insights from the FIRST study. Circulation. 1999;99:1816–1821. doi: 10.1161/01.cir.99.14.1816. [DOI] [PubMed] [Google Scholar]
- Alexandersen P, Tankó LB, Bagger YZ, Qin G, Christiansen C. The long-term impact of 2−3 years of hormone replacement therapy on cardiovascular mortality and atherosclerosis in healthy women. Climacteric. 2006;9:108–118. doi: 10.1080/13697130600647743. [DOI] [PubMed] [Google Scholar]
- Babiker FA, Lips D, Meyer R, Delvaux E, Zandberg P, Janssen B, van Eys G, Grohé C, Doevendans PA. Estrogen receptor β protects the murine heart against left ventricular hypertrophy. Arterioscler Thromb Vasc Biol. 2006;26:1524–1530. doi: 10.1161/01.ATV.0000223344.11128.23. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation. 1997;95:252–264. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- Beer S, Reincke M, Kral M, Callies F, Ströer H, Dienesch C, Steinhauer S, Ertl G, Allolio B, Neubauer S. High-dose 17β-estradiol treatment prevents development of heart failure post-myocardial infarction in the rat. Basic Res Cardiol. 2007;102:9–18. doi: 10.1007/s00395-006-0608-1. [DOI] [PubMed] [Google Scholar]
- Bittner V. Menopause and cardiovascular risk. Cause or consequence? J Am Coll Cardiol. 2006;47:1984–1986. doi: 10.1016/j.jacc.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Brett KM, Madans JH. Long-term survival after coronary heart disease. Comparisons between men and women in a national sample. Ann Epidemiol. 1995;5:25–32. doi: 10.1016/1047-2797(94)00037-t. [DOI] [PubMed] [Google Scholar]
- Cavasin MA, Sankey SS, Yu AL, Menon S, Yang XP. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;284:H1560–H1569. doi: 10.1152/ajpheart.01087.2002. [DOI] [PubMed] [Google Scholar]
- Cavasin MA, Tao Z, Menon S, Yang XP. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci. 2004;75:2181–2192. doi: 10.1016/j.lfs.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Eaker ED, Chesebro JH, Sacks FM, Wenger NK, Whisnant JP, Winston M. Cardiovascular disease in women. Circulation. 1993;88:1999–2009. doi: 10.1161/01.cir.88.4.1999. [DOI] [PubMed] [Google Scholar]
- Ertl G, Frantz S. Healing after myocardial infarction. Cardiovasc Res. 2005;66:22–32. doi: 10.1016/j.cardiores.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Gambrell RD, Jr, Natrajan PK. Moderate dosage estrogen-androgen therapy improves continuation rates in postmenopausal women: impact of the WHI reports. Climacteric. 2006;9:224–233. doi: 10.1080/13697130600643437. [DOI] [PubMed] [Google Scholar]
- Gao X-M, Xu Q, Kiriazis H, Dart AM, Du XJ. Mouse model of post-infarct ventricular rupture: time course, strain- and gender-dependency, tensile strength, and histopathology. Cardiovasc Res. 2005;65:469–477. doi: 10.1016/j.cardiores.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Genant HK, Lucas J, Weiss S, Akin M, Emkey R, McNaney-Flint H, Downs R, Mortola J, Watts N, Yang HM, Banav N, Brennan JJ, Nolan JC. Low-dose esterified estrogen therapy: effects on bone, plasma estradiol concentrations, endometrium, and lipid levels. Estratab/Osteoporosis Study Group. Arch Intern Med. 1997;157:2609–2615. doi: 10.1001/archinte.157.22.2609. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt) 2006;15:35–44. doi: 10.1089/jwh.2006.15.35. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Stampfer MJ, Colditz GA, Willett WC, Manson JE, Joffe M, Rosner B, Fuchs C, Hankinson SE, Hunter DJ, Hennekens CH, Speizer FE. Postmenopausal hormone therapy and mortality. N Engl J Med. 1997;336:1769–1775. doi: 10.1056/NEJM199706193362501. [DOI] [PubMed] [Google Scholar]
- Hamada H, Kim MK, Iwakura A, Ii M, Thorne T, Qin G, Asai J, Tsutsumi Y, Sekiguchi H, Silver M, Wecker A, Bord E, Zhu Y, Kishore R, Losordo DW. Estrogen receptors α and β mediate contribution of bone marrow-derived endothelial progenitor cells to functional recovery after myocardial infarction. Circulation. 2006;114:2261–2270. doi: 10.1161/CIRCULATIONAHA.106.631465. [DOI] [PubMed] [Google Scholar]
- Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BC, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, Buring JE, Criqui MH, Herrington D, Lynch JK, Rapp SR, Torner J, for the WHI Investigators Effects of conjugated equine estrogen on stroke in the Women's Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- Hügel S, Reincke M, Strömer H, Winning J, Horn M, Dienesch C, Mora P, Schmidt HH, Allolio B, Neubauer S. Evidence against a role of physiological concentrations of estrogen in post-myocardial infarction remodeling. J Am Coll Cardiol. 1999;34:1427–1434. doi: 10.1016/s0735-1097(99)00368-x. [DOI] [PubMed] [Google Scholar]
- Hulley S, Furberg C, Barrett-Connor E, Cauley J, Grady D, Haskell W, Knopp R, Lowery M, Satterfield S, Schrott H, Vittinghoff E, Hunninghake D, for the HERS Research Group Noncardiovascular disease outcomes during 6.8 years of hormone therapy. Heart and Estrogen/Progestin Replacement Study follow-up (HERS II). JAMA. 2002;288:58–66. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E, for the Heart and Estrogen/Progestin Replacement Study (HERS) Research Group Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Hunter JC, Kostyak JC, Novotny JL, Simpson AM, Korzick DH. Estrogen deficiency decreases ischemic tolerance in the aged rat heart: roles of PKCδ, PKCε, Akt, and GSK3β. Am J Physiol Regul Integr Comp Physiol. 2007;292:R800–R809. doi: 10.1152/ajpregu.00374.2006. [DOI] [PubMed] [Google Scholar]
- Isles CG, Hole DJ, Hawthorne VM, Lever AF. Relation between coronary risk and coronary mortality in women of the Renfrew and Paisley survey: comparison with men. Lancet. 1992;339:702–706. doi: 10.1016/0140-6736(92)90599-x. [DOI] [PubMed] [Google Scholar]
- Iwakura A, Shastry S, Luedemann C, Hamada H, Kawamoto A, Kishore R, Zhu Y, Qin G, Silver M, Thorne T, Eaton L, Masuda H, Asahara T, Losordo DW. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation. 2006;113:1605–1614. doi: 10.1161/CIRCULATIONAHA.105.553925. [DOI] [PubMed] [Google Scholar]
- Koide Y, Tamura K, Suzuki A, Kitamura K, Yokoyama K, Hashimoto T, Hirawa N, Kihara M, Ohno S, Umemura S. Differential induction of protein kinase C isoforms at the cardiac hypertrophy stage and congestive heart failure stage in Dahl salt-sensitive rats. Hypertens Res. 2003;26:421–426. doi: 10.1291/hypres.26.421. [DOI] [PubMed] [Google Scholar]
- Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111:383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- Liu Y-H, Yang XP, Sharov VG, Nass O, Sabbah HN, Peterson E, Carretero OA. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptors. J Clin Invest. 1997;99:1926–1935. doi: 10.1172/JCI119360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Okumura Y, Fujimoto S, Shiraishi I, Itoi T, Hamaoka K. Adoptive transfer of macrophages ameliorates renal fibrosis in mice. Biochem Biophys Res Commun. 2005;332:11–16. doi: 10.1016/j.bbrc.2005.04.083. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Aitkenhead M, Levin ER. Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem. 2005;280:26339–26348. doi: 10.1074/jbc.M414409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzer T, Loza PA, Hu K, Bayer B, Dienesch C, Calvillo L, Couse JF, Korach KS, Neyses L, Ertl G. Increased mortality and aggravation of heart failure in estrogen receptor-β knockout mice after myocardial infarction. Circulation. 2005;111:1492–1498. doi: 10.1161/01.CIR.0000159262.18512.46. [DOI] [PubMed] [Google Scholar]
- Pines A, Sturdee DW, Birkhäuser M, on behalf of the International Menopause Society More data on hormone therapy and coronary heart disease: comments on recent publications from the WHI and Nurses’ Health Study. Climacteric. 2006;9:75–76. doi: 10.1080/13697130600655415. [DOI] [PubMed] [Google Scholar]
- Reis SE, Holubkov R, Young JB, White BG, Cohn JN, Feldman AM. Estrogen is associated with improved survival in aging women with congestive heart failure: analysis of the vesnarinone studies. J Am Coll Cardiol. 2000;36:529–533. doi: 10.1016/s0735-1097(00)00738-5. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Andersen GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford FA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women's Health Initiative investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Shlipak MG, Angeja BG, Go AS, Frederick PD, Canto JG, Grady D, for the National Registry of Myocardial Infarction-3 investigators Hormone therapy and in-hospital survival after myocardial infarction in postmenopausal women. Circulation. 2001;104:2300–2304. doi: 10.1161/hc4401.98414. [DOI] [PubMed] [Google Scholar]
- Simonis G, Briem SK, Schoen SP, Bock M, Marquetant R, Strasser RH. Protein kinase C in the human heart: differential regulation of the isoforms in aortic stenosis or dilated cardiomyopathy. Mol Cell Biochem. 2007;305:103–111. doi: 10.1007/s11010-007-9533-3. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Clyne C, Speed C, Rubin G, Bulun S. Tissue-specific estrogen biosynthesis and metabolism. Ann N Y Acad Sci. 2001;949:58–67. doi: 10.1111/j.1749-6632.2001.tb04002.x. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Davis SR. Minireview: aromatase and the regulation of estrogen biosynthesis–some new perspectives. Endocrinology. 2001;142:4589–4594. doi: 10.1210/endo.142.11.8547. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses health study. N Engl J Med. 1991;325:756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- Tamura T, Said S, Gerdes AM. Gender-related differences in myocyte remodeling in progression to heart failure. Hypertension. 1999;33:676–680. doi: 10.1161/01.hyp.33.2.676. [DOI] [PubMed] [Google Scholar]
- Tang AC, Nakazawa M, Romeo RD, Reeb BC, Sisti H, McEwen BS. Effects of long-term estrogen replacement on social investigation and social memory in ovariectomized C57BL/6 mice. Horm Behav. 2005;47:350–357. doi: 10.1016/j.yhbeh.2004.10.010. [DOI] [PubMed] [Google Scholar]
- van Eickels M, Grohó C, Cleutjens JP, Janssen BJ, Wellens HJ, Doevendans PA. 17β-Estradiol attenuates the development of pressure-overload hypertrophy. Circulation. 2001;104:1419–1423. doi: 10.1161/hc3601.095577. [DOI] [PubMed] [Google Scholar]
- van Eickels M, Patten RD, Aronovitz MJ, Alsheikh-Ali A, Gostyla K, Celestin F, Grohe C, Mendelsohn ME, Karas RH. 17-β-Estradiol increases cardiac remodeling and mortality in mice with myocardial infarction. J Am Coll Cardiol. 2003;41:2084–2092. doi: 10.1016/s0735-1097(03)00423-6. [DOI] [PubMed] [Google Scholar]
- Wang M, Crisostomo P, Wairiuko GM, Meldrum DR. Estrogen receptor-α mediates acute myocardial protection in females. Am J Physiol Heart Circ Physiol. 2006;290:H2204–H2209. doi: 10.1152/ajpheart.01219.2005. [DOI] [PubMed] [Google Scholar]
- Wenger NK, Speroff L, Packard B. Cardiovascular health and disease in women. N Engl J Med. 1993;329:247–256. doi: 10.1056/NEJM199307223290406. [DOI] [PubMed] [Google Scholar]
- Willett WC, Manson JE, Grodstein F, Stampfer MJ, Colditz GA. Re: “combined postmenopausal hormone therapy and cardiovascular disease: toward resolving the discrepancy between observational studies and the women's health initiative clinical trial”. Am J Epidemiol. 2006;163:1067–1068. doi: 10.1093/aje/kwj156. [DOI] [PubMed] [Google Scholar]
- Yang X-P, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol Heart Circ Physiol. 1999;277:H1967–H1974. doi: 10.1152/ajpheart.1999.277.5.H1967. [DOI] [PubMed] [Google Scholar]