Abstract
Cardiac myocytes rapidly proliferate during fetal life but exit the cell cycle soon after birth in mammals. Although the extent to which adult cardiac myocytes are capable of cell cycle reentry is controversial and species-specific differences may exist, it appears that for the vast majority of adult cardiac myocytes the predominant form of growth postnatally is an increase in cell size (hypertrophy) not number. Unfortunately, this limits the ability of the heart to restore function after any significant injury. Interst in novel regenerative therapies has led to the accumulation of much information on the mechanisms that regulate the rapid proliferation of cardiac myocytes in utero, their cell cycle exit in the perinatal period and the permanent arrest (terminal differentiation) in adult myocytes. The recent identification of cardiac progenitor cells capable of giving rise to cardiac myocyte-like cells has challenged the dogma that the heart is a terminally differentiated organ and opened new prospects for cardiac regeneration. In this review, we summarize the current understanding of cardiomyocyte cell cycle control in normal development and disease. In addition, we also discuss the potential usefulness of cardiomyocyte self-renewal as well as feasibility of therapeutic manipulation of the cardiac myocyte cell cycle for cardiac regeneration.
Keywords: heart, regeneration, cell cycle, stem cell, cardiac muscle
I. Introduction
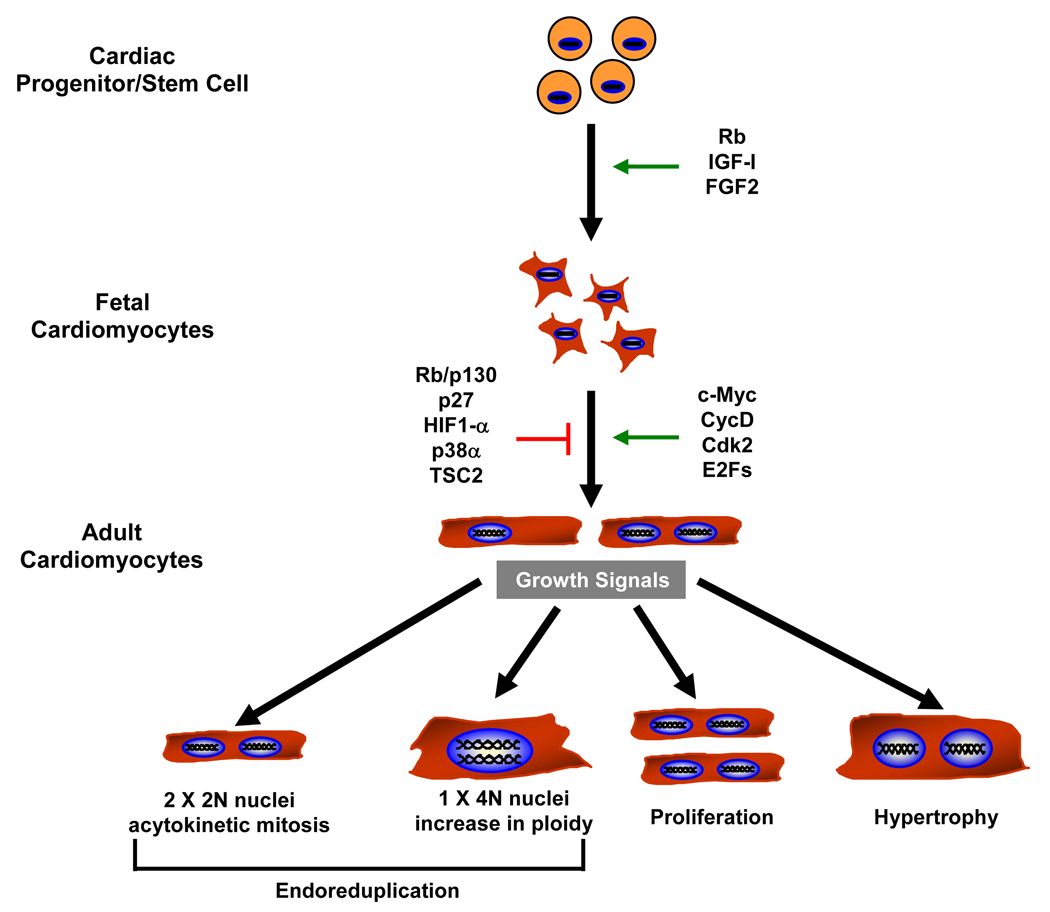
Adult cardiac myocytes represent a highly specialized and structured cell type; therefore, it is not surprising that complex and often overlapping systems have evolved to regulate cardiomyocyte growth. Cardiac myocytes rapidly proliferate during fetal life but in the perinatal period, proliferation ceases and myocytes undergo an additional round of DNA synthesis and nuclear mitosis without cytokinesis (acytokinetic mitosis) that leaves the majority of adult cardiac myocytes binucleated in most species (131,132). Typically, adult cardiac myocytes do not reenter the cell cycle when exposed to growth signals and further increases in cardiac mass are achieved through an increase in cell size or hypertrophy. Thus, cardiac myocytes display three developmentally determined forms of cell cycle control and growth; namely, proliferation, binucleation and hypertrophy.
Many mammalian tissues respond to injury by activating committed progenitor or stem cells or through proliferation of differentiated cells capable of reentering the cell cycle, although both have been felt to be quite limited in the heart until recently (21). Several groups have described the presence of a progenitor or stem cell that can differentiate into cardiac myocytes (56) but there is still much debate and confusion in the field regarding the exact identity of this cell and its potential for cardiac myocyte differentiation and myocardial repair (9,195). Nonetheless, most investigators would agree that adult cardiomyocytes have very limited potential for self renewal, which is inadequate to repair the heart after significant injury. The failure of the adult mammalian myocardium to reactivate the cell cycle has been postulated to be a primary limiting factor in restoring function to the damaged heart. Thus, although limited induction of DNA synthesis in the heart as a response to stress or other factors has been described (29,70) there is little evidence for cytoplasmic division or cytokinesis in mammals. In contrast, cardiomyocytes from lower vertebrates are capable of dividing postnatally (158,167). The mechanisms underlying these species differences are unknown.
One major limitation in the field has been the lack of adequate models or techniques in mammalian systems to truly mechanistically analyze cardiac myocyte proliferation and differentiate cell cycle reentry from its downstream consequences including increased ploidy, nuclear mitosis, cytokinesis and apoptosis. Currently no clonal cells lines exist that reproduce the normal developmental pattern of cardiac myocyte cell growth. Thus, investigators have been forced to utilize primary cell cultures despite the knowledge that there are many physiological and molecular differences between fetal or neonatal myocytes and post-mitotic adult myocytes. The fact that neonatal myocytes retain some proliferative potential and express endogenous cell cycle activators makes them a less than ideal model for studies designed to understand cell cycle regulation in the adult heart. Likewise, difficulties associated with culturing adult myocytes for extended periods and their tendency to dedifferentiate and regain proliferative potential also limits their usefulness for in vitro cell cycle analyses (39). Thus, although not without their own shortcomings, we have given special emphasis to in vivo studies using transgenics and gene targeting technologies. However, there are certain caveats that should be kept in mind when considering the data generated from genetically altered mouse models. Many of these transgenics have utilized constitutive, albeit cardiac-restricted, expression of genes implicated in cell cycle proliferation. This typically results in expression at developmental time points where the myocyte retains proliferative potential. We and others have shown in species as diverse as Drosophila and mouse that the effects of overexpression of some cell cycle proteins is dependent on whether it occurs in a cell retaining proliferative capacity versus a cell that is post mitotic (50). Further as we outline below, cell cycle reentry can have several outcomes, only one of which is cytokinesis, and even the documentation of mitotic figures does not necessarily imply proliferation given that cariac myocytes are well-known to be multinucleated. Despite these confounding factors, much useful information has been gained from generation and characterization of these genetically altered mice.
This chapter will focus on the progress that has been made in the field of cardiac growth control in mammals with an emphasis on several broad questions. What are the signals or molecules driving the rapid cardiac myocyte proliferation in utero, and what developmental mechanisms later block the capacity for proliferative growth? What potential does the heart have for regeneration? Do the same factors that regulate hyperplastic growth also mediate hypertrophic growth in adult post-mitotic myocytes? The answers to these questions have important implications not only for understanding cardiac development and disease but will also serve as a foundation towards manipulating cardiac growth for therapeutic benefit in the future.
II. Developmental Control of Cardiac Myocyte Proliferation
A. Temporal pattern of cardiac growth in mammals
Cardiac myocytes rapidly proliferate during fetal life but lose their ability to proliferate soon after birth; however, before terminal withdrawal from the cell cycle, cardiomyocytes undergo a final round of incomplete cell division, during which karyokinesis gets uncoupled from cytokinesis, resulting in binucleated cardiomyocytes. Analysis of cardiac myocyte proliferation during mouse development determined that cardiac myocyte DNA synthesis occurs in two distinct phases (213). The first occurs during fetal life, peaking at the earliest time points measured; where labeling indexes of 33% were observed in ventricles of embryonic day 12 fetuses. During this phase, karyokinesis and cytokinesis were matched, resulting in cardiac myocyte proliferation. The second phase occurred early in the neonatal period, peaking at day 4–6 postnatally. By contrast, in this phase, karyokinesis occurred in the absence of cytokinesis, resulting in binucleation of the ventricular myocytes. This process of nuclear division in the absence of cellular division is a specific form of endoreduplication known as acytokinetic mitosis. Interestingly, it was shown that these cardiomyocytes still assemble an actomyosin contractile ring in culture, but abscission no longer takes place (131). In mice and rats the accumulation of binucleated cardiomyocytes starts around day four and by the third postnatal week 85–90% of the cardiomyocytes are binucleated (41,213). This degree of binucleation varies somewhat between species, in pigs the percentage of binucleated cardiomyocytes can reach up to 32% (81) while in humans estimates have ranged from 25–57% (170,201). Not unexpectedly, this cell cycle exit is accompanied by a coordinated down regulation of positive cell cycle regulators and up regulation of Rb and cyclin dependent kinase inhibitors (Cdkl) p21 and p27, similar to that seen in skeletal muscle (114,180,236,236,237). The physiological significance of having cells that are binucleated is unclear but it has been postulated to be an adaptive response in metabolically active cells where the capacity to generate twice the RNA for protein synthesis might be advantageous.
The amount of DNA synthesis in the adult heart is a controversial subject, labeling experiments using tritiated thymidine or BrdU in rodents indicate that the number of cardiomyocytes entering cell cycle in the normal adult heart is very low (197,212). Tritiated thymidine incorporation assays revealed that only 0.005% of the ventricular cardiomyocytes show evidence of DNA synthesis in uninjured adult mice hearts (211). Although DNA synthesis does not seem to increase significantly in the injured mouse heart (0.004%) (175), a mitotic index of 0.015–0.08% has been reported in injured human myocardium (13,106). Thus, the intrinsic proliferative capacity of adult cardiomyocytes is quite low.
B. Expression and role of cell cycle regulators during cardiac development
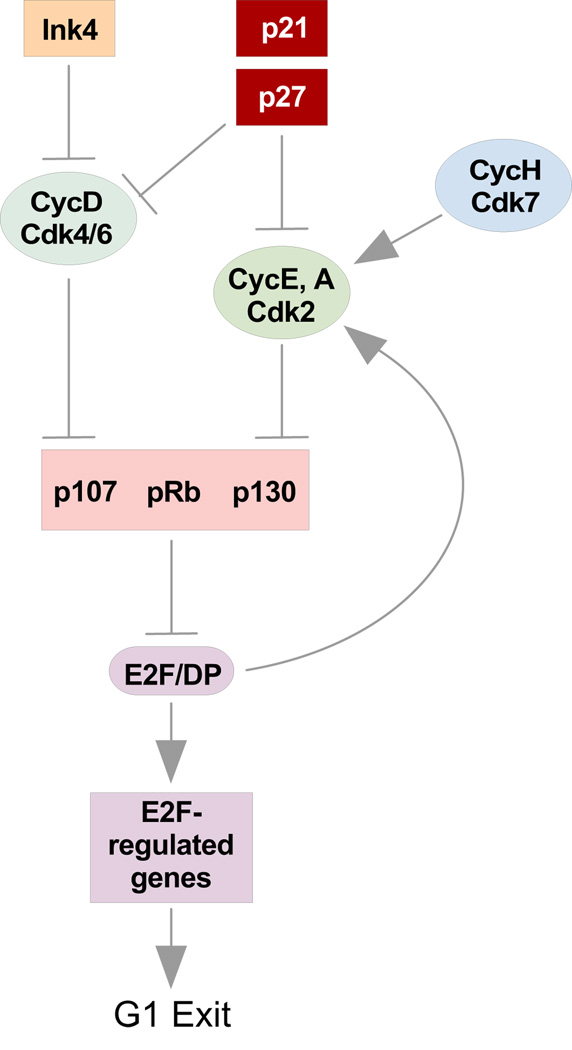
Cell cycle progression entails the tightly regulated transduction of mitogenic signals to cyclically expressed proteins known as cyclins and, hence, to their catalytically active targets, the cyclin-dependent protein kinases (Cdks) (Fig. 1). To ensure proper progression through each phase, cells have developed a series of orchestrated checkpoints that govern the different cyclin-Cdk complexes required for distinct cell cycle events. Their activities are regulated by CAK (cyclinH/Cdk7) and Cdk inhibitors (CKI) in both a positive and negative manner, respectively. Key regulators in the major cell cycle checkpoint in late G1, known as the restriction point in mammalian cells, include Cdk4 and Cdk6, which preferentially assemble into holoenzymes with cyclin D1, D2 or D3 (205). These complexes preferentially phosphorylate members of the Rb family (Rb, p107 and p130) leading to the release of E2F transcription factors. Cyclin E is mainly expressed at the G1-S transition where it enters into active complexes with its catalytic partner Cdk2 to accelerate the phosphorylation of the Rb proteins. Cyclin A and Cdk2 complexes play a major role in S phase, while cyclin B and Cdc2 are required during G2/M phase. E2F is required for the transcription of genes involved in mediating G1 exit and DNA synthesis (163).
Figure 1. Regulation of cardiac cell cycle.
Schematic diagram of the factors involved in cell cycle progression in cardiac myocytes
Cyclin/Cdk/Cdkls
The embryonic heart exhibits high levels of expression of cyclins involved in G1, S, G2 and M-phase like D1, D2, D3, A, B1 and E (22,73,109,250). Additionally, other genes required for DNA replication such as proliferating cell nuclear antigen (PCNA) and the cyclin-dependent kinases Cdc2, Cdk2, Cdk4 and Cdk6 are also highly expressed along with their associated kinase activities (Summarized in Table 1) (22,73,109,250). The relative importance of these factors remains largely unknown but development of the embryonic ventricle appears to be critically dependent on cyclin D expression. Mice lacking cyclins D1, D2, or D3 are viable and display only narrow and tissue specific phenotypes reflecting the highly overlapping pattern of expression of the three D-type cyclins (67). Although mice lacking a single cyclin D subtype did not demonstrate a cardiac phenotype, mice deficient for all three D-cyclins (cyclin D1−/−;D2−/−;D3−/−) died at mid/late gestation secondary to heart abnormalities and defective hematopoiesis (117). Mutant embryos displayed severely thinned ventricular walls, mainly affecting the compact zone and large ventricular septal defects. Interestingly, cell cycle kinetics were not abnormal in all tissues, suggesting a tissue-specific utilization of these proteins. Conversely, transgenic mice overexpressing cyclin D1, D2, or D3 in adult myocardium exhibited elevated rates of cardiomyocyte DNA synthesis at baseline in the adult hearts (175). Consistent with this finding, Cdk2−/−; Cdk4−/− mice die during embryogenesis around E15 as a result of heart defects (16). The loss of Cdk2 and Cdk4 caused hypophosphorylation of Rb, which led to repression of E2F target genes, like Cdc2 and cyclin A2. Hearts from these double mutant mice displayed reduced global size, enlargement of atria and thin ventricular walls. At the microscopic level, proliferation in some areas of the heart was decreased in mutant compared to wild-type mice. Interestingly, it has been shown that combined loss of Cdk4 and Cdk6 does not affect cell cycle initiation and progression, suggesting that Cdk2 compensates for the lack of cyclin D dependent kinases (144). These results argue for a specific role for CycD/Cdk4 complexes in normal cardiac development.
Table 1.
Expression patterns of cell cycle regulatory proteins during cardiac development.
| Expression Level | ||||||
|---|---|---|---|---|---|---|
| Gene | Function | Cell Cycle Phase | Fetal | Neonatal | Adult | References |
| Cell cycle mediators | ||||||
| Cyc D1 | Cdk4 and -6 partner | G1/S | ++ | + | − | (108,109,213) |
| Cyc D2 | Cdk4 and -6 partner | G1/S | ++ | + | − | (108,109,214) |
| Cyc D3 | Cdk4 and -6 partner | G1/S | ++ | + | − | (108,109,214) |
| Cyc E | Cdk2 partner | G1/S | + | + | − | (108,250) |
| Cyc A | Cdk2 partner | S and G2/M | ++ | + | − | (108,250) |
| Cyc B | Cdc2 partner | G2/M | ++ | + | − | (32,108,250) |
| Cdc2 | Promotes G2/M transit | G2/M | ++ | + | − | (108,109,136,214) |
| Cdk4 | Phosphorylates Rb family members | G1/S | ++ | + | − | (108,109,213) |
| Cdk2 | Phosphorylates Rb family members | G1/S | ++ | + | − | (108,109,214) |
| Cdk6 | Phosphorylates Rb family members | G1/S | ++ | + | − | (22) |
| Cell cycle inhibitors | ||||||
| p16 | Inhibits Cdk 4 and 6 activity | G1/S | nd | − | nd | (6), (114) |
| p18 | Inhibits Cdk 4 and 6 activity | G1/S | ++ | ++ | − | (23) |
| p21 | Inhibits Cdk 2, 4, and 6 activity | G1/S | + | + | ++ | (180) |
| p27 | Inhibits Cdk 2, 4, and 6 activity | G1/S | + | + | + | (114,180) |
| p57 | Inhibits Cdk 2, 4, and 6 activity | G1/S | ++ | − | − | (114,159) |
| p53 | Regulates cell cycle checkpoint | G1/S or G2M | nd | nd | − | (93) |
| p193 | Regulates G1/S checkpoint | G1/S | ++ | + | − | (229) |
| RB | Inhibits E2F activity | G1/S | − | + | ++ | (101,142) |
| p107 | Inhibits E2F activity | G1/S | ++ | + | − | (101,142) |
| p130 | Inhibits E2F activity | G1/S | + | ++ | + | (101,142) |
| TSC2 | Regulates p27 activity | G1/S | + | ++ | ++ | (213) |
| Transcription Factors | ||||||
| E2F1 | Transcription factor for cell cycle genes | G1-S-G2M | ++ | + | − | (142,235) |
| E2F3 | Transcription factor for cell cycle genes | G1-S-G2M | ++ | + | − | (235) |
| E2F4 | Transcription factor for differentiation | G0 | − | + | ++ | (142) |
| ++ | + | − | (235) | |||
| E2F5 | Transcription factor for differentiation | G0 | − | + | ++ | (142,235) |
| E2F6 | Transcription factor for cell cycle genes | G1-S | ++ | + | − | (155) |
| N-Myc | Transcription factor for cell cycle genes | G1-S-G2M | ++ | +/− | − | (156) |
| c-Myc | Transcription factor for cell cycle genes | G1-S-G2M | ++ | + | − | (142,202) |
++ refers to relatively stronger expression than +, − refers to not detected and nd, not determined.
The withdrawal of postnatal cardiomyocytes from the cell cycle is linked with a change in the expression pattern of many cell cycle regulatory molecules (Summarized in Table 1). The protein expression profiles of cyclins D1, D2, D3, A, B1 and E and their associated kinases are significantly downregulated in cardiomyocytes after birth compared to the levels observed in the embryonic heart. Moreover, the protein levels of cyclin A, B, D1, E and Cdc2 become undetectable by immunoblotting in adult cardiomyocytes (22,73,109,250). The downregulation in expression of cyclins and Cdks during normal development of cardiomyocytes has been shown to parallel a reciprocal upregulation of CdkIs. Two protein families exist to specifically inhibit Cdks. One family of Cdk inhibitors is specific for Cdk4/6 (the INK4 family, comprising p15, p16, p18, and p19), a second, the Cip/Kip family (p21, p27, and p57), has much broader activity inhibiting Cdk4/6 as well as Cdk2 and Cdc2 (205). INK4 proteins selectively inhibit the activity of cyclin D-dependent kinase by competitively binding to these kinases and thus preventing cyclin D interaction. Activity of cyclin E and cyclin A-dependent kinases are negatively regulated by the second CKI family including p21, p27 and p57 (206). Members of this family are also potent inhibitors of cyclin D-dependent kinases and thus have much broader substrate specificity than the INK4 family (184).
Expression patterns of Cdkls in the heart have been characterized by numerous investigators both during normal development and in diseased states. p16 and p18 of the INK4 family have been reported in the embryonic heart although levels in young adult hearts are low or undetectable (6,23,114). It has been suggested that there is a progressive increase in the percentage of p16 positive myocytes with age, which may represent not just quiescent but instead be a marker of cardiac myocyte senescence (107,118). In contrast, expression of Cip/Kip family members, p21 and p27, increase in cardiac myocytes in the perinatal period and reach high levels in adult myocardium (24,133). Expression at the transcriptional level of the third member of the Cip/Kip family, p57, has been reported (149), but the protein is detectable in the heart only at early stages in rat, although it persists throughout life in man (24). Both p21 and p27 are downregulated in cardiac myocytes in response to pathological stressors such as pressure overload although the significance of this finding remains to be determined (133).
Pocket proteins (Rb, p130, p107)
The primary target of G1 Cdks is the product of the Retinoblastoma susceptibility gene (Rb). This protein along with p107 and p130, comprise a family of proteins often called pocket proteins (38). All three family members are expressed in developing myocardium although the temporal pattern diverges considerably. Rb is scant or undetectable in fetal mouse myocardium at age E12.5, but is upregulated by the neonatal stage, and in adult, terminally differentiated cardiac tissue becomes the predominant pocket protein expressed (101,142). p107 is expressed in a pattern reciprocal to Rb, highest in the embryonic heart and lowest in adult. p130 expression peaks in the neonatal period and is subsequently downregulated and expressed at low levels in adult myocardium. These proteins are best known for their roles in inhibiting cell cycle progression through the regulation of E2F-responsive genes (42). In their hypophosphorylated form, Rb proteins bind to E2F complexes, recruiting transcriptional repressors such as HDACs or the Jumonji, a repressor that plays a critical role in embryonic heart development (104). Phosphorylation of Rb and its relatives by Cdk2 and −4, results in the release of E2F complexes, enabling them to activate transcription and trigger the expression of genes required for DNA synthesis and further cell cycle regulating molecules such as cyclin E, cyclin A, the mitotic kinase Cdc2 (p34/Cdk1) and E2F−1 itself.
There is accumulating evidence that Rb-proteins play a critical role in regulating cell cycle exit and possibly cardiac muscle differentiation. Rb-null embryos die at day 14.5 p.c. with widespread cell death and aberrant cell cycling in a variety of tissues (37,99,125) but apparently normal hearts. It was reported recently the Rb−/− embryonic stem cells displayed a delay in the expression of cardiac-specific transcription factors and subsequently cardiac differentiation. It was shown by other investigators that LEK1, a murine homolog of the cardiomyogenic factor 1, interacts with Rb, inhibiting its activity and allowing cardiac myocytes to proliferate despite the presence of Rb proteins during development (10). ES cells deficient in LEK1 recapitulated the delay in cardiac differentiation seen in Rb−/− ES cells (172).
Unlike Rb deficient mice, p107 and p130 nullizygous mice were initially reported as viable and phenotypically normally (43,126) but an additional level of complexity was added to the interpretation of pocket protein function by the report of a new p130-deficient mouse strain that although well tolerated in a C57BL/6J background, resulted in embryonic lethality in a Balbc/J background. These mice developed a thin-walled, hypoplastic myocardium with defective looping and chamber formation (124). While these experiments seem to suggest that p130 has a unique, strain-dependent role in cardiac development, p130-deficient mice created by separate investigators (43) do not display a similar phenotype in a Balbc/J background (Mao S, MacLellan WR., unpublished work). The explanation for this discrepancy remains to be determined although that the presence of a modifier gene, unique to original ES cells might explain this finding. We have demonstrated that Rb and p130 have overlapping functional roles in vivo to suppress cell cycle activators and maintain quiescence in postnatal cardiac muscle. Mice that are deficient in both Rb and p130 demonstrated a threefold increase in the heart weight-to-body weight ratio and showed increased numbers of bromodeoxyuridine- and phosphorylated histone H3-positive nuclei, consistent with persistent myocyte cycling (142). These data support the notion that Rb family members are critical for normal cardiac myocyte cell cycle exit but do not address the issue of whether they also mediate the inability of adult myocytes to divide in response to growth stimuli. Studies utilizing temporally regulated, cardiac-restricted Rb null are in progress to resolve this question.
C. Growth factors and cytokines
An exhaustive review of the numerous growth factors that have been implicated in mediating cardiac myocyte proliferation is beyond the scope of this review. Instead we will highlight those signaling pathways for which the most data exist to support a direct role in regulating cardiac growth.
Insulin-like growth factor-I
Insulin-like growth factor-I (IGF-I) is a single chain polypeptide that has a structural homology with proinsulin. IGF-I has insulin-like short term metabolic effects and growth factor-like long term effects on cell proliferation and differentiation of various cell types. IGF-I’s mitogenic activity is mediated primarily through binding the IGF-I receptor, also known as the type 1 IGF receptor (130). Mice carrying null mutations of the genes encoding IGF-I and/or IGF-I receptor demonstrate embryonic and postnatal growth retardation without a specific cardiac phenotype, and die perinatally of respiratory failure (11,138). Mice expressing reduced levels of IGF-I (~30% of normal) (129) survive to adulthood and have an intermediate size when compared to wild-type or IGF-I-deficient animals. Despite this finding they display normal LV mass when normalized for body weight, and normal adaptive hypertrophic response to increased hemodynamic load produced by supraaortic banding (129). Conversely, cardiac overexpression IGF-I in transgenic mice display increase in heart weight by 50% mediated by an increased number of cells in the heart (187). A similar phenotype was seen in mice lacking the IGF-II receptor, which normally functions to bind and inactivate IGF-I (120). This observation is consistent with in vitro evidence suggesting that IGF-I modulates myocyte proliferation rather than hypertrophy (105). However, a study has also been reported where overexpression of a local form of IGF-I in the hearts of transgenic mice specifically induced hypertrophic phenotype that eventually led to a reduced systolic performance (54). It is not clear if these discrepancies are related to differences in the temporal pattern of gene expression in the transgenics, levels of expression or simply technical differences. Certainly the signaling components downstream of the IGF-I receptor have been implicated in cardiac hypertrophy by a number of investigators and the IGF-PI3K-Akt-p70S6K signaling pathway has been shown to play an important role in regulating cardiac hypertrophy, viability and homeostasis (154). Overexpression of an activated form of PI3K catalytic subunit resulted in cardiac hypertrophy, while forced expression of a dominant negative PI3K produced smaller hearts and individual fibres (207). The role of Akt, the downstream effector, is less clear. Several investigators have demonstrated that overexpression of Akt in the heart results in increased heart size secondary to cardiac myocyte hypertrophy (44,45,208). In contrast, targeting Akt to the nucleus led to an increased number of cardiomyocytes, smaller in volume with enhanced ventricular function and myocyte contractility (194). However, since overexpressing a dominant-negative Akt during development did not affect cardiac myocyte size or number, Akt may not play a role in normal cardiac myocyte proliferation (207). Recently, it was reported that in IGF-I transgenic mice, cardiac stem cell division is increased, which is accompanied by enhanced telomerase activity, delayed senescence and preservation of a reservoir of functionally competent cardiac stem cells (227). Given that exogenous delivery of IGF-1 has been shown to have beneficial effects post-myocardial infarction (232), IGF-I may provide a useful tool to enhance myocardial repair after injury.
gp130-dependent signals
Accumulating evidence supports the concept that cytokines, particularly those that signal through receptor complexes containing the gp130 subunit play a key role in regulating cardiac myocyte proliferation. These cytokines, which include interleukin (IL)-6, IL-11, leukemia inhibitory factor (LIF), cardiotropin-1, ciliary neurotrophic factor and oncostatin M have been implicated in both normal and pathological cardiac growth (87,223). Mice deficient for these factors or their receptors (IL-6 (115), LIF (64,218), CNTF (148), IL-11Rα (189)) developed a plethora of developmental defects although none demonstrated specific cardiac defects. In contrast, germline deletion of gp130 results in embryonic lethality with a hypoplastic ventricle, suggesting gp130-dependent signals are critical for proliferative cardiac growth (249). Deleting gp130 in the perinatal period, likewise results in myocardial thinning presumably from a defect in cardiac myocyte proliferation or survival (18). Cardiac specific gp130-deficient mice develop normally with unremarkable appearing hearts at baseline although hemodynamic stress leads to the development of a dilated cardiomyopathy secondary to increased apoptosis (88). Thus, gp130-mediated signaling in cardiac myocytes is not necessary for normal cardiac development, but instead is necessary for myocyte survival during hypertrophic growth in the adult ventricle. This data raises the interesting possibility that the effects of gp130 deletion in utero are mediated indirectly, possibly through paracrine mechanisms by nonmyocytes in the developing ventricle.
Fibroblast growth factor
FGF-2 is a multifunctional protein that plays a vital role in regulating growth and differentiation of various cell types including cardiomyocytes. FGF-2 exerts many of its effects by binding to high affinity cell surface receptors (FGFR) of the tyrosine kinase family(65), among which the FGFR-1 isoform is the predominant in cardiomyocytes of all developmental stages (110). FGF-2 has been demonstrated to stimulate the proliferation of embryonic and neonatal cardiomyocytes in vitro (173), as well as cardiac stem cells (12). However, other groups have reported that FGF-2 does not control the number of stem cells but regulates their differentiation into cardiac myocytes (191). Blocking FGF signaling in the embryonic heart inhibits cardiac myocyte proliferation (152). Early attempts to directly assess the role of this receptor in cardiac development were hampered by the fact that germline FGFR1-deficient embryos display severe early growth defects (55,246). Ornitz et al. demonstrated recently that epicardial and endocardial FGF signaling is essential for myocardial proliferation and differentiation in vivo, acting redundantly through FGFR-1 and FGFR-2 (122). Embryos deficient for both FGFR-1 and FGFR-2 specifically in the myocardium displayed severe hypoplasia, enlarged atria and thinning of the ventricular wall (122). This confirms the functional requirement for both FGFR1 and FGFR2 in developing myocardium and the critical role of FGF signaling for myocardial proliferation.
D. Transcription Factors
E2Fs
E2F actually represent a family of transcription factors with eight members, E2F−1 through −8 (140). To bind DNA and activate transcription, E2Fs must form obligate heterodimers with members of a second family of transcription factors, DP-1 and −2. While relatively little is known regarding the specific roles of individual family members in the heart, based on structural and functional characteristics they are often subdivided into activator E2Fs (E2F−1, E2F−2 and E2F−3) and repressor E2Fs (E2F−4, E2F−5, E2F−6, E2F−7 and E2F−8). This classification is based upon differences in the ability of these overexpressed proteins to activate transcription or drive quiescent cells into the cell cycle, as well as on the phases of the cell cycle where the E2F proteins can be shown to be present at E2F-regulated promoters. The distinction between these two groups is not absolute. Repressor E2Fs can activate transcription when overexpressed and activator E2Fs can potentially form complexes with repressor proteins.
E2F−1, −2 and −3 share structurally similarity and are expressed in proliferating cells (127) and preferentially associate with Rb (84). In contrast, E2F−4 shares homology with E2F−5 and both are expressed primarily in quiescent and differentiating tissues (48). E2F−4 has been found in complexes with Rb, p107 and p130 (153). E2F−5 binds preferentially with p130 (86). E2F−6, −7, and −8 the newest members, also act as transcriptional repressors but their physiological role remains undetermined. Ectopic expression of E2F−1, −2 and −3 drives quiescent cells though G1 into S phase (53,102,183) but only E2F−1 induces apoptosis (220). In contrast, the repressive E2Fs, E2F−4 or E2F−5 promote differentiation (177) and are associated with the pocket protein-dependent downregulation of a number of genes involved in cell cycling including E2F−1 (75), cdc2 (226), b-myb (15). These results suggest that E2F family members and pocket proteins form a complex positive and negative regulatory network regulating proliferation and differentiation.
Limited in vivo data exists for a specific role of any particular E2F family member in cardiac development. E2F−1, E2F−3 and DP family members are downregulated from fetal to adult stages of ventricular development (142). Our data suggests that E2F−4 and −5 are upregulated in heart during development (142); however, others have reported that only E2F−4 is expressed in differentiated cardiac myocytes (235). Hence, the exact expression patern of E2F−4 in the heart remains unresolved, although upregulation E2F−4 and −5 has also been seen in skeletal muscle differentiation (46). Deleting E2F−1, −4 or −5 did not result in any discernable cardiac defect (247)(188) perhaps related to functional redundancy exists in this family (72,94,137). In contrast, deleting E2F−3 caused embryonic lethality in the majority of embryos but some mice survived to adulthood, eventually dying prematurely with signs of congestive heart failure (40). Unfortunately, the mechanism for the cardiac failure was never determined. Since the defects in E2F−3 null mice are distinct from those arising in E2F−1 mutant mice, it suggests that activating E2Fs must have unique biological functions in vivo. Since it required the combined loss of all three activating E2F family members (E2F−1, −2 and −3) to abolish the ability of cells to enter S phase and proliferate (242), it is not surprising that cardiac myocyte proliferation did not appear to be effected in any of the E2F knockouts. Overexpression of E2F−1, −2, −3 and −4 in neonatal cardiomyocytes can induce S-phase entry but increased expression of E2F−1 and E2F−3 induced apoptosis along with cell cycle progression (59). These results are similar to previous reports demonstrating E2F−1 induced DNA synthesis, and apoptosis in adult myocardium (3) and that E2F−1 is the family member most associated with apoptosis (72,199). Thus, the specific role of E2F members in cardiac development and proliferation remain unresolved.
Myc
Myc is the prototypical member of a family of sequence-specific DNA-binding proteins that are postulated to act as “third messengers” for ligand-dependent signals and are implicated in the regulation of growth in a variety of tissues (66). The Myc family, which includes c-Myc, N-Myc and L-Myc, are transcription factors of the basic helix-loop-helix-leucine zipper (bHLHZ) family of proteins that activate transcription as part of a heteromeric complex with a protein termed Max. Family members share a common binding motif and have been demonstrated to be capable of functionally complementing each other, suggesting the Myc gene family must have evolved to facilitate differential patterns of expression (145). Although there is a consensus that Myc is critical for normal development, the mechanism whereby it exerts this effect is controversial. Studies in vivo, using Myc transgenics and conditional knockouts of Myc, have concluded that Myc is critical for both proliferation and cellular growth (52,96) while others have implicated Myc in cellular division alone (228). In the heart, evidence exists to support a role for Myc in both cardiac myocyte division and hypertrophy as summarized below.
Myc is expressed in embryonic ventricular myocytes and Myc-deficient mice die prematurely at E10.5 with cardiac defects (51). Myc-null embryos were generally smaller and retarded in development compared with their littermates suggesting a general role for Myc in cellular proliferation and displayed heart enlargement and pericardial effusions. Unfortunately, a detailed analysis of the cardiac phenotype was never performed. Therefore, no data exists at a cellular level as to the effects of the Myc deficiency on cardiac myocyte size or number. Likewise, whether the observed myocardial defects are due to a primary effect of Myc-deficiency on myocytes versus an indirect effect secondary to Myc’s effects on other organ systems is unknown. Conversely, transgenic mice that overexpressed Myc in the fetal myocardium developed ventricular enlargement secondary to myocyte hyperplasia (100). Despite the hyperplasia, post-natal DNA synthesis ceased earlier in transgenics than in wildtype mice (141). Although initially counterintuitive, it is now known that overexpression of proto-oncogenes such as Myc in primary cells can result in cell cycle arrest through the induction of p19ARF (69). These data support the notion that Myc is sufficient to mediate hyperplastic cardiac growth.
HIF-1α
Hypoxia inducible factor-1 (HIF-1), is a transcription factor complex consisting of α and β subunits that heterodimerize to form a functional complex, which activates or represses genes containing hypoxic response elements. HIF-1 is stabilized and activated in response to hypoxia and/or activation of specific signaling cascades. In contrast to HIF-1β which is constitutively expressed and functions also as a dimerizing partner for transcription factors not involved in hypoxia, HIF-1α is unique in its sensitivity and specificity to changes in oxygen levels. HIF-1α stability is regulated by the van Hippel-Lindau (VHL) tumor suppressor protein. In the absence of VHL, HIF-1α is not targeted for proteasomal degradation and thus stabilized (47,215). Deletion of HIF-1α resulted in developmental arrest and lethality by E11 in HIF-1α−/− embryos with multiple defects in cardiovascular development, including pericardial effusion and disorganized cardiac morphogenesis with myocardial hyperplasia and ventricular obstruction. The hyperplasia of cardiac myocytes resulted in a constriction between the ventricle and outflow tract of HIF-1α−/− embryos (97). There was also a reduction in vascularization that was attributed to decreased expression of VEGF, an angiogenic factor that is known to be induced by tissue hypoxia via HIF-1α. However, since the increase in cardiac cell number seen in HIF-1α−/− embryos was dramatically different than the hypoplastic myocardium described previously for VEGF+/− mice, the myocyte hyperplasia was unlikely secondary to impaired VEGF production or vasculogenesis. Instead it argues for a more direct role for HIF-1α in cardiac proliferation and morphogenesis.
Although numerous genetic studies have indicated the requirement of HIF-1α for hypoxia-induced growth arrest and activation of p21, a role in regulating development cell cycle arrest is a novel function of HIF-1α. The mechanism underlying HIF-1α-induced growth arrest has been elusive but it has been shown that even in the absence of a hypoxic signal, HIF-1α can induce cell cycle arrest by functionally counteracting Myc, thereby derepressing p21 (116). The HIF-1α-antagonism is mediated by displacing Myc binding from the p21 promoter. Interestingly, neither HIF-1α-transcriptional activity nor its DNA binding was essential for cell cycle arrest, indicating a divergent role for HIF-1α. Additional studies have also supported the hypothesis that stabilization of HIF-1α inhibits proliferation. For example, HIF-1α−/− tumors grow faster and become more invasive than its wildtype counterpart (44,230). Furthermore, HIF-1α stabilization not only fails to promote, but actually decreases tumor growth (108). HIF-1α, similar to Myc, has also has been implicated in playing a role in cardiac hypertrophy (36,112). A comprehensive analysis of the role of HIF-1α in cardiac proliferation and hypertrophy still needs to be performed, but given Myc’s critical role in mediating cardiac myocyte growth, HIF-1α may represent a novel endogenous antagonist.
E. Signaling Pathways
TSC1 & 2
Tuberous sclerosis complex (TSC) is an autosomal dominant tumor syndrome characterized by the appearance of tumor like growths that affect many organs including the heart. The syndrome often causes seizures, mental retardation and a variety of developmental disorders, including autism (119). The TSC disease-causing genes have been identified and encode for proteins called hamartin (234) and tuberin (1) respectively. Myocardial tumors are rare phenomena in humans but more than 50% of TSC patients show evidence of primary myocardial tumors (239). This propensity for cardiac tumor formation in TSC patients suggested that the TSC gene products could play an important role in the regulation of cardiomyocyte cell cycle. Eker rats, heterozygous for a germ line mutation in TSC2 (TSC2EK/+) are predisposed to renal carcinoma and animals homozygous for mutation (TSC2EK/EK) die in utero (171). Embryonic cardiomyocytes isolated from TSC2EK/EK embryos on E12.5 continued to actively proliferate and synthesize DNA after as many as eight passages in contrast to those from heterozygous or wild type embryos, which exited the cell cycle. Since TSC2EK/EK cardiomyocytes retained a highly differentiated phenotype similar to normal embryonic or neonatal rat cardiomyocytes, it was concluded that the TSC2 gene product is important specifically for normal cardiomyocyte cell cycle withdrawal and terminal differentiation. Overexpression of a mutant TSC2 in the hearts of transgenic mice that is predicted to block the growth inhibitory activity of the endogenous TSC2 resulted in normal cardiac development and cardiac myocyte cell cycle exit. Nonetheless, the level of cardiomyocyte DNA synthesis in transgenic mice was increased 35-fold above that of nontransgenic littermates in response to hypertrophic stimuli (174). TSC proteins are known to be positive regulators of the cyclin dependent kinase inhibitor p27, by inhibiting its degradation by the ubiquitin-proteasome pathway (193). Accordingly, it was assumed that aberrant trafficking of p27 might be the underlying cause of the altered cell cycle regulation observed in these transgenic mice but cytoplasmic sequestration of p27 was not seen in transgenic hearts (174). At present the mechanism underlying the enhanced DNA synthesis in mice expressing the modified TSC2 transgene is not clear. Furthermore, it is not known whether those cardiomyocytes synthesizing DNA did eventually undergo karyokinesis and/or cytokinesis. Identification of pathways activated in the responsive cells might uncover mechanisms to increase cell cycle reactivation to a point sufficient for regenerative growth of the heart.
p38 MAPK
In mammalian heart, mitogen-activated protein kinases (MAPK) signaling pathways have been hypothesized to regulate cardiomyocyte growth in response to diverse developmental signals (135). The MAPK signaling pathways consists of at least three prominent phosphorylation cascades terminating in the activation of extracellular signal regulated kinases (ERK), c-Jun NH2-terminal kinases (JNK), or p38 MAPKs. In cardiac myocytes, the ERK cascade is thought to be primarily activated in response to tyrosine kinase receptor and G-protein-coupled receptor (GPCR) activation while the JNK and p38 cascades are activated by both GPCR activation and stress signals. Of the four different p38 isoforms have been identified, the predominant isoform expressed in the adult heart is p38α, while p38β and p38γ are expressed at low levels, and p38δ is not expressed in heart (135,238). The major upstream activators of p38 MAPKs are MAPKKs including MKK3 MMK4 and MKK6, which directly phosphorylate the dual site in p38 MAPKs (Thr-Gly-Tyr). Substrates of p38 MAPKs include mainly other protein kinases and a growing list of transcription factors which includes MEF2, MAPKAPK2 and 3, ATF-2, ELK-1, Chop, TEF-1, C/EBPβ and Max (7,251). While it is known that p38 induces differentiation, its role in proliferation has also been recently recognized in many cell types (8,161,243). Depending on the cell type and stimulus, p38 MAPKs can have either a positive or negative influence on cell cycle progression (161). p38 appears to be required for proliferation of Swiss 3T3 cells induced by FGF-2 (143), for proliferation of haematopoietic cells induced by granulocyte colony stimulating factor (185) and for proliferation of erythropoietin-dependent cell line FD-EPO (160). On the other hand, in CCL39 and NIH 3T3 fibroblasts, p38 inhibits cell cycle progression at the G1/S transition, possibly by the inhibiting cyclin D1 expression (123). Recently it was suggested that p38 could also serve as a key negative regulator of the mammalian cardiomyocyte cell cycle. Genetic activation of p38 in vivo reduced fetal cardiomyocyte proliferation, whereas targeted disruption of p38α along with growth factor stimulation of cultured adult myocytes promoted cardiomyocyte cell cycle reentry. This study demonstrated a modest increase in mitotic cardiac nuclei index of 0.14% (62). However, in this study the adult myocytes were cultured in vitro for 12 days, which results in cardiac myocyte dedifferentiation and recovery of proliferative potential (39). More recently, the same authors demonstrated that treatment with FGF1 in combination with a p38 MAP kinase inhibitor after myocardial injury led to an increase in mitotic index in vivo suggesting a role for this pathway in maintaining terminal differentiation in addition to regulating cardiac cell cycle (61). Complicating, the interpretation of p38α’s role in adult myocardium, is a report that cardiac-specific transgenic mice expressing a dominant negative mutant p38α generates a hypertrophic rather than proliferative response in adult hearts (20). Thus, further characterization of p38α mutant mice and its molecular interaction with growth factor signaling will be necessary to clarify p38’s role in cardiomyocyte proliferation and terminal differentiation.
III. Regulation of Cardiac Myocyte Cell Cycle Exit
A. Adult cardiac myocytes are terminally differentiated
Many differentiated tissues undergo cell cycle arrest as part of their differentiation pathway but not all cells undergo permanent arrest and notable examples exist of highly specialized cell types having the capacity for regeneration (68). Terminal differentiation invariably involves two closely linked phenomena: permanent withdrawal from the cell cycle and cell type-specific differentiation characterized by the upregulation of a panel of tissue-specific genes. For instance fetal or neonatal rodent cardiac myocytes primarily express β-MHC and skeletal actin but as cardiac myocytes undergo the process of terminal differentiation they are downregulated and α-MHC and cardiac actin are upregulated in their stead (222). Consequently, although often used interchangeably in differentiated cell types, cell cycle exit and terminal differentiation are not synonymous. We define a terminally differentiated cell type, as one where the majority of cells do not reenter the cell cycle in response to mitogens or normal physiological stress.
Interestingly, although cell cycle reentry occurs rarely in the adult mouse myocytes in response to stress or injury to any significant degree (210) there is accumulating evidence that it likely does to a limited extent in the adult human heart (13). Consistent with this finding, data has existed for some time documenting increased DNA content per nuclei and nuclei per myocyte in cardiomyopathic human hearts (14,80,85). However, while restricted cell cycle reentry may occur in the injured human versus mouse hearts (1–4% (13) human vs. 0.0014% mouse (210) cardiac myocytes), the ultimate fate of these myocytes and whether species-specific differences really exist with respect to proliferative capacity remain unresolved. In contrast, in species such as newt where consensus exists on the capacity for myocardial regeneration, it has been demonstrated that mononucleated myocytes were more likely to successfully undergo cytokinesis than binucleated myocytes (150). When binucleated newt myocytes enter the cell cycle, it resulted in variably nucleated myocytes in the majority of cases as opposed to cytokinesis. Thus the reported difference in the percentage of mono- versus bi-nucleated cardiac myocytes between species (41,81,170,201,213) may account for some of the differences in potential for cell cycle reentry that have been reported. This may be due to the presence of a “tetraploid checkpoint” that arrests cells that fail to undergo cytokinesis in the following G1, which may be invoked in binucleated myocytes (221). Even in humans, the species with the highest percentage of mononucleated cardiac myocytes and reported cell cycle reentry, significant regeneration does not seem to occur after injury. Others have suggested the critical factor that determines adult cardiac myocyte proliferation is not the nucleation state, but rather its cellular size. It has been reported that myocytes smaller in size retain higher potential to reenter into the cell cycle than the fully differentiated myocytes in response to stress or injury in the adult heart (13,106,233).
Although Beltrami et al. concluded that their finding of mitotic nuclei in a minority of cardiac myocytes in the adult failing heart represented cardiac myocyte division (13), an equally plausible explanation is that the DNA synthesis or nuclear mitosis they observed simply resulted in endoreduplication (increased DNA per nuclei or increased nuclei per myocyte) as others have reported in human myocardium (85) without actual cardiac myocyte division. Consistent with this, studies subsequently have suggested that entrance of human cardiomyocytes into the cell cycle after myocardial infarction is transient, limited and that as opposed to cytokinesis and proliferation, it leads to endoreduplication (151). An increase in ploidy and nuclei per myocyte can also be seen in adult mouse myocardium after genetic manipulation (245). Endoreduplication may account for the discordance between the observed regenerative capacity of the heart after injury and that proposed based on pathological examination of cycling myocytes. Presently, no convincing evidence exists for the formation of a contractile ring in adult cardiac myocytes of any species, which would be a necessary requirement for any cytokinesis.
A third explanation for the observation of cycling myocytes in adult myocardium has also recently been proposed (192,231). Investigators have identified and characterized several cardiac progenitor cells capable of proliferating (12,168). However, once committed to the myocyte lineage, the progeny of a stem cell can only undergo three to four rounds of cell division before permanently withdrawing from the cell cycle (12). Thus, adult hearts, regardless of species, are likely composed of predominantly terminally differentiated myocytes that do not reenter the cell cycle; with a minority of myocytes or resident stem cells that are capable of some limited cell cycle reentry. Regardless, neither adult cardiac myocytes nor cardiac stem cells seem to possess the proliferative potential to regenerate the heart after injury such as myocardial infarction.
B. Basis for terminal differentiation in cardiac myocytes
Despite others and our work, the mechanisms underlying the cell cycle exit and the permanent growth arrest in cardiac muscle are poorly understood. Rb has been implicated in mediating not only cell cycle exit, but also the irreversibility of cell cycle arrest associated with terminal differentiation in various lineages including skeletal muscle (82), adipocytes (34) and macrophages (33). Early studies in vitro using fibroblasts induced to transdifferentiate into skeletal myocytes by overexpression of MyoD demonstrated that Rb−/− but not p107- or p130-null skeletal myocytes have a defect in cell cycle exit and maintenance of quiescence (164,165). Thus Rb appeared uniquely required for normal myogenic cell cycle control and full differentiation. These results were confirmed in vivo by deleting a floxed Rb allele either in proliferating myoblasts or after differentiation (92). Deleting Rb prior to myogenic differentiation with Myf5-Cre resulted in a severe defect in differentiation and apoptosis. If Rb was deleted after differentiation, the cells formed normal multinucleated myotubes that did not enter S-phase in response to serum stimulation. It was subsequently shown that serum did not induce DNA synthesis in differentiated myotubes even if all three pocket proteins had been removed (28). The authors concluded that Rb plays a crucial role in the switch from proliferation to differentiation in skeletal myocytes rather than maintenance of the terminally differentiated state. Studies using mouse inner ear hair cells, a terminally differentiated cell type, showed that Rb is required for maintaining quiescence in differentiated cells (146). Acute Rb loss resulted in fully differentiated hair cells of the inner ear reentering the cell cycle and proliferating. Thus Rb’s role in maintaining terminal differentiation may be tissue specific.
Cardiac-restricted Rb-deficient mice, where Rb was deleted in differentiated cardiac myocytes using a αMHC-driven Cre transgene, develop normally and do not display cardiac cell cycle defects even after physiological and pharmacological growth signals (142). Since Rb−/− embryonic stem cells display a delay in cardiac differentiation a developmental specific dependence on Rb may be operable in cardiac myocytes similar to skeletal muscle (172). Rb may be necessary for commitment and differentiation of cardiac myocytes but once differentiated it is dispensable. When the other pocket protein expressed in adult myocardium, p130, was also deleted the resultant Rb-p130-null mice displayed defects in cardiac myocyte cell cycle exit and differentiation (142) demonstrating that in cardiac muscle, Rb and p130 clearly have overlapping roles in mediating cardiac myocyte cell cycle exit. Whether Rb and p130 are also necessary for maintaining quiescence in cardiac myocytes has yet to be determined. These results likely explain the observation that cardiac-specific transgenic mice with increased Cdk4 (175,214,214) or Cdk2 (136) activity resulted in an increase in cardiac myocyte number and ongoing DNA synthesis in adult hearts since these maneuvers would be predicted to inactivate all pocket proteins not just Rb.
C. Regulation of cytokinesis
Cytokinesis is the final step of cell division. It is responsible for partitioning and separation of cytoplasm between daughter cells to complete mitosis (95). One strategy to study cytokinesis has been to focus on the regulation of the mechanical components responsible for contraction of the cleavage furrow-namely the actomyosin cytoskeleton. Actin has been postulated to act as scaffold onto which the rest of the cytokinesis machinery assembles. Apart from actin and myosin other possible key players such as small GTPases like RhoA and its effectors ROCK I and ROCK II, citron kinase, formin-homology proteins, GTPase Cdc42, Rac and septins have been identified in regulating the formation of the contractile ring (77). The formation of the contractile ring has been investigated in postnatal rat cardiomyocytes (131,132). However, these studies were restricted only to the expression and subcellular localization of F-actin and non-muscle myosin. In dividing neonatal myocytes, actin gets disassembled during early stages of mitosis and concentrates at the equator of the spindle during anaphase before finally forming an intensely staining, circumferential band in telophase. In contrast, cytoplasmic myosin evenly distributes in the cytoplasm as small spots, concentrates in association with the cortical membrane in the equator region in anaphase, forms a ring-like structure in telophase, and remains associated with adjacent membrane at the cleavage furrow until telophase (131). The study could demonstrate the assembly of actin and myosin at the contractile ring during the binucleation process and authors suggested that the molecules involved in the later part of cytokinesis may be responsible for the binucleation of cardiac myocytes during postnatal development. None of the other proteins involved in cytokinesis were analyzed with the exception of Polo-like kinase, a protein involved in spindle formation and chromosome segregation during mitosis. It was shown to be downregulated in the adult heart (76). More recently the localization of anillin, a known regulator of the cleavage furrow formation, was characterized in dividing versus binucleating cultured cardiomyocytes. It was reported that the failure to undergo abscission, which leads to binucleation is due to defective focussing of anillin in the mid-body region (63). Among the other regulators of cytokinesis, septins a family of cytoskeletal GTPases important for cytokinesis have been shown to demonstrate stage specific expression during heart development (5). Likewise, cardiomyocytes display a developmentally regulated expression of small Rho GTPases such as RhoA, Cdc42, Rac1, ROCK-I, ROCK-II, p-cofilin, that are coupled to the formation of actomyosin ring. High levels of these proteins were present in embryonic hearts where cytokinesis occurs but were downregulated perinatally as cardiac myocytes exit the cell cycle (Ahuja P, Ehler E., Submitted). The complex myofibrillar cytoarchitecture that develops postnatally, together with the fact that there is downregulation in the expression levels of proteins that regulate cell cycle and cytokinesis respectively, might contribute to uncoupling of karyokinetic and cytokinetic events as seen in the postnatal cardiomyocytes.
One longstanding theory to explain the lack of cytokinesis in adult cardiac myocytes is the presence of highly organized mature myofibrils in the adult cardiomyocytes which physically prevent cell division. Since cells must disassemble their cytoskeletal filaments prior to entering cell division, disassembly of the cytoarchitecture in adult cardiac myocytes would presumably negatively impact myocyte contractile function although this seems to occur in fetal cardiomyocytes, which completely disassemble the myofibrils prior to dividing (4). This disassembly occurs in two steps with Z-disk and thin-filament-associated proteins getting disassembled before disassembly of the thick (myosin) filaments. Thus, the cellular shape and cell-cell contacts of dividing myocytes remains similar to non-dividing cells, which may be necessary for uninterrupted function of the working myocardium. In adult cardiomyocytes the presence of stable, highly ordered and functional myofibrils may physically prevent cell division. It is known that during progressive differentiation from the embryonic to a adult stage, there is a gradual increase in the size, number and complexity of organization of myofibrils in ventricular cardiomyocytes is observed (60,89,196). In contrast, atrial cardiomyocytes are smaller in size, about 40% poorer in myofibrils and appear less differentiated. Interestingly, they also retain a higher ability to regenerate both in vivo and in vitro (198,217), which might explain why atrial tumors were more common in transgenic mice expressing a fusion of atrial natriuretic factor and SV−40 T-antigen (111). Thus, the impediment to complete cytokinesis in adult cardiac myocytes remains speculative but sarcomeric structure likely plays an important role.
IV. Cell Cycle Regulators in Cardiac Hypertrophy
During development, cell cycle progression is tightly coupled to the accumulation of cell mass (cell growth) to ensure that cell size is constant (162). In contrast, in many human diseases cell growth can become uncoupled from proliferation resulting in hypertrophic growth. Since cardiac hypertrophy is associated with such negative outcomes, much effort has been focused on characterizing the intracellular signal transduction pathways that are associated with cardiac hypertrophy (58). The link between this process and cell cycle progression, as well as whether the same factors that regulate hyperplastic growth also mediate hypertrophic growth in adult post-mitotic myocytes has been largely ignored. We will review the data linking the aforementioned cell cycle regulators in regulating cardiac myocyte hypertrophy.
A. Cyclin/Cdk/Cdkls
Hypertrophic stimulation of adult myocardium is accompanied by the upregulation of G1 cyclin/Cdks (134,179) and reciprocal downregulation of Cdkls (133). Some investigators have interpreted this data as evidence of post-mitotic cardiac myocytes reentering the cell cycle and undergoing proliferative growth. Increasing data is accumulating that proteins classically thought to be involved in cell cycle regulation also play a critical role in the control of cellular growth. In Drosophila, CycD/Cdk4 regulates cell size and number, however, the effect of CycD/Cdk4 overexpression varied depending on the cell type (50). CycD/Cdk4 stimulates cell growth in post-mitotic cells but proliferation in cells capable of cell cycle reentry (50). This CycD/Cdk4-induced cell growth was dependent on a gene encoding the mitochondrial ribosomal protein, mRpL12 (74). In the absence of mRpL12, cells demonstrated reduced growth and mitochondrial activity suggesting that CycD/Cdk4 controls cell growth via a mitochondrial-dependent pathway. Recently the same group showed that the orthologous CycD/Cdk4 mammalian complex can stimulate growth in Drosophila through a very similar pathway compared to the fly complex (32). A number of reports have implicated CycD/Cdk4 in regulating cardiac hypertrophy in mammalian cells as well, although the downstream effectors have not been identified (25,224). Although forced expression of CycD2 led to cell cycle activation and not hypertrophy in cardiac myocytes(26,175) this might be related to the fact, that analogous to the Drosophila model (50), the transgene expression in this study began during fetal development when cardiomyocytes are able to proliferate. Inhibiting G1-Cyclin/Cdk activity in the adult, post-mitotic cardiac myocytes blocks hypertrophic growth (166,225). Consistent with this finding, our laboratory has demonstrated that Myc-induced cardiac hypertrophy is attenuated in CycD2 null mice (252).
A novel mechanism to control cardiac hypertrophy through a novel class of Cdks has recently been proposed (200). This Cdk9 can interact directly with the core transcriptional machinery by phosphorylating the carboxyl-terminal domain (CTD) of RNA pol II, increasing its transcriptional activity. Cdk9 activity was increased in a number of in vitro and in vivo models of cardiac hypertrophy (200). Cdk9 kinase associates with Cyclin T1 or T2 and is a component of the transcription positive-acting complex pTEFb, which facilitates the transition from abortive to productive transcription elongation by phosphorylating the CTD of RNA pol II. In these hypertrophic models, Cdk9 activation was not related to changes in the level of Cdk9 or cyclin T. Instead it involved the dissociation of 7SK small nuclear RNA (snRNA), an endogenous inhibitor, from the Cdk 9 complex. In culture, dominant-negative Cdk9 blocked ET-1-induced hypertrophy, whereas an anti-sense inhibition of 7SK snRNA provoked spontaneous cell growth. In transgenic mice, activation of Cdk9 activity via cardiac-specific overexpression of cyclin T1 is sufficient to provoke hypertrophy. Together, these findings implicate Cdk9 activity as a novel regulator of cardiac hypertrophy.
B. c-Myc
Myc has been implicated in regulating growth, differentiation, apoptosis and metabolism in a wide variety of organisms and cell types (176). It is one of the few factors implicated in controlling both cell size and number (219,228). This is of importance in the heart since Myc is expressed in embryonic ventricular myocytes and is upregulated in adult myocardium in response to virtually all hypertrophic stimuli (98,216). Creation of transgenic mice which overexpress Myc in myocardium has led to an evolution in the postulated role of this transcription factor in mediating cardiac hypertrophy. Initially generated transgenic mice overexpressing Myc did not display baseline cardiac myocyte hypertrophy but hypertrophic growth in adult cardiac myocytes was potentiated in response to some (T3), but not all (isoproterenol) agonists (190). This led to investigators questioning the importance of Myc in regulating cardiac growth. To readdress this issue, we created mice where Myc could be inducibly activated, specifically in adult myocardium. Our results demonstrate that Myc activation is sufficient to induce hypertrophic growth in adult myocardium even in the absence of G1 exit (245). Conversely, we have recently demonstrated that Myc-deficient adult hearts have attenuated stress-induced hypertrophic growth, secondary to a reduction in cell growth of individual myocytes (252). The view that Myc can mediate cellular growth is also supported by the fact that decreased expression of a homolog of c-Myc, dMyc, in drosophila reduces cell proliferation and cell size (103). In contrast, dMyc overexpression leads to increase in cell size without affecting proliferation rate.
Myc is known to regulate multiple candidate genes implicated with cell growth and metabolism (49,78). The mechanisms by which Myc regulates cellular growth are less clear, nevertheless, it is interesting to note that the genes responsible for its ability to promote the cell cycle have also been implicated in regulating cell size under certain circumstances. Myc activation in the heart is accompanied by the upregulation of cyclin D2 and cyclin dependent kinase Cdk2 and Cdk4 activities which are important for cell cycle progression (245). To explore the dependence of Myc-induced cell growth on CycD2, we created bigenic mice where Myc can be selectively activated in CycD2-null adult myocardium. Myc-dependent hypertrophic growth and cell cycle reentry is blocked in CycD2-deficient hearts (252). In contrast to Myc-induced DNA synthesis, hypertrophic growth was independent of Cdk2 activity. These data suggest that Myc is required for a normal hypertrophic response and that its growth promoting effects are also mediated through a CycD2-dependent pathway. Myc has also been implicated in directly regulating activity of the components of the biosynthetic apparatus. Cardiac hypertrophy is accompanied by a rise in transcription by RNA polymerase (pol) III, which produces essential ribosomal components, including 5S rRNA and tRNAs (79). This increase in transcription is achieved by changes in both the activity and level of the essential pol III-specific transcription factor TFIIIB. Given that small molecule inhibitors of Myc currently exist (248), targeting of a transcription factor like c-Myc might provide a novel therapeutic approach for inhibiting the development of cardiac hypertrophy and thereby preventing the onset of heart failure.
The mechanisms whereby Myc is activated in cardiac hypertrophy are not clear but both Myc and cyclin D1 can be activated by β-catenin (83,209). β-Catenin is a multifunctional protein that can act in the cytoplasm to link cadherins to the actin cytoskeleton or enter the nucleus and function as a transactivator (240). In the absence of Wnt signaling, free β-catenin is phosphorylated by glycogen synthase kinase-3β (GSK-3β) and rapidly targeted for proteasomal degradation. Numerous hypertrophic signals stimulate a cascade that inhibits β-catenin degradation (58). Stabilization of β-catenin is associated with its translocation to the nucleus, where it interacts with members of the lymphoid enhancer factor (LEF)/T- cell factor (TCF) and activates specific target genes such as Myc and CycD. β-Catenin is upregulated in stress-induced hypertrophy and targeted deletion of β-catenin in the heart blunts hypertrophy in response to pathological stress (35). Deletion of β-catenin blunts expression of Myc in response to pressure overload directly linking this signaling pathway to Myc in the regulation of hypertrophic growth in the heart.
C. E2F
Very little is known about the potential roles of these transcription factors in cardiac hypertrophy. E2F−4 and −5 are the predominant E2F family members expressed in adult cardiac myocytes (142,235). Recently, expression patterns of E2F−6 and E2F−6b in rat cardiomyocytes were characterized and it was shown that E2F−6 protein is downregulated too developmentally like other E2F proteins and is upregulated during the development of cardiac hypertrophy (155). Although, induction of E2F−1, −3, and −4 was observed in neonatal cardiomyocytes treated with serum or phenylephrine, these results should be interpreted with caution given that cultures of neonatal cardiac myocytes are not truly terminally differentiated and it is unknown whether these changes also occur in adult myocardium in vivo subjected to a physiological hypertrophic stress. Nonetheless, it was also shown that inhibition of E2F abrogates the development of cardiac hypertrophy (235). Inhibiting E2F activity with a specific peptide that blocks E2F-DP heterodimerization prevented the induction of hypertrophic markers like ANF and BNP, reduced the increase in myocyte size and inhibited protein synthesis in the cardiomyocytes stimulated with serum and phenylephrine (235). The mechanism, whereby E2F might participate in the hypertrophic response is speculative. In skeletal muscle hypertrophy, a subset of E2F−1 target genes involved in protein synthesis, cytoskeletal organization, and mitochondrial function but not G1 exit have been shown to be upregulated during a hypertrophic response (90). Interestingly, cytochrome c oxidase subunits IV, V, and VIIc are up-regulated during the hypertrophic response and at least cytochrome c oxidase IV is a direct transcriptional target of E2F−1 (90). Given that cardiac hypertrophy is accompanied by an increase in mitochondrial number and activity (244), E2Fs may play an important role in mediating mitochondrial biogenesis and function enabling myocytes to cope with the increased energy demands of the hypertrophic state.
V. Potential for Cardiac Myocyte Self-renewal
Many mammalian tissues respond to injury by activating committed progenitor cells or stem cells or through proliferation of differentiated cells such as liver or endothelial cells (21). In contrast, adult mammalian cardiomyocytes have very limited potential for self renewal. On the other hand, it has been known for some time that cardiomyocytes from lower vertebrates are capable of dividing postnatally and regenerating myocardium after injury (17,150,158,167).
A. Cardiac regeneration in nonmammalian models
Amphibians, such as newt, were the first adult vertebrates identified that are capable of regenerating their organs. This ability to regenerate large sections of the body is widespread in Metazoan phylogeny, and this discovery was an important feature of the emergence of experimental biology in the eighteenth century (57). Although the process of tissue regeneration unfold in a different manner in the heart, limbs or tail of the adult newt, they all depend on the plasticity of the remaining differentiated cells after the tissue injury. Zebrafish can also regenerate the heart and other organs like fins, retina and spinal cord, which has facilitated the application of modern molecular biology techniques to study this phenomenon (182). After the removal of the apical region of the ventricle, the heart in both newt and zebrafish seals by contraction around the clot. Newt and zebrafish adult cardiomyocytes re-enter the cell cycle and divide in a zone that surrounds the clot (167,182). If the animal is injected with labeled thymidine, to identify those cells that are in S-phase, approximately 10% of the cardiomyocytes in this region are labeled in a one-day period. More recent studies suggest that although cardiomyocytes can enter S phase, more than half of these cells stably arrest at either entry to mitosis or during cytokinesis, similar to what is seen in mammalian cardiomyocytes (17). Only a third of the cardiac myocytes entering the cell cycle progress through mitosis and enter successive cell divisions. This suggests proliferative potential is retained in only a subset of cardiomyocytes and that regulation of proliferation in the majority is similar to that described for their mammalian counterparts, as they arrest during mitosis or cytokinesis.
The molecular mechanisms that underlie the difference in cardiomyocyte proliferative potential between these species and mammals are unknown. Interestingly, a variant strain of Zebrafish with a mutation in checkpoint kinase called Mps1, fails to regenerate its heart after ventricular resection and develops scars in the damaged myocardium (182). These studies suggest that injury stimulates a proliferative response in these species which can lead to myocardial regeneration. No similar response to injury has been observed in the mammalian hearts. It is likely that with the evolution several organs, including the heart lost the ability to regenerate with a corresponding increase in the intricacy of patterning and function. Defining the molecular basis of regeneration in these unique non-mammalian model systems may illuminate basic insights into cardiomyocyte regeneration. Similar to newt and zebrafish, a unique regenerative response was also documented in the MRL mouse strain after a cryoinjury to the myocardium (128). The MRL mouse strain has a dramatically enhanced capacity to heal surgical wounds, a complex trait that maps to at least seven genetic loci. When these mice were subjected to cardiac injury, scarring was markedly reduced and cardiomyocyte mitotic index increased 10-fold in MRL mice compared with C57BL/6 mice. Several other groups have since reported that heart regeneration in the MRL mouse either does not occur or is much more limited than reported previously by Heber-Katz and colleagues in response to experimental myocardial injury (2,169).
B. Cardiac progenitor cells resident in the adult heart
The dogma that the heart is a postmitotic non-regenerating organ has recently been challenged. Multiple groups have independently described a resident cardiac stem cell or progenitor cell population with the capacity to differentiate into cardiac myocytes (12,121,168). The first endogenous cardiac stem cell reported was a Lin−;c-kit+ that was reported to differentiate into cells that are phenotypically indistinguishable from cardiomyocytes in vivo (12). In addition, these clones may also differentiate into smooth muscle cells and endothelial cells, indicative of their possible pluripotency. When injected into the border zone of hearts with new infarcts, cardiac c-kit+ cells led to bands of regenerating myocardium, contributed to endothelium and vascular smooth muscle, and improved the function of the heart (12). Although reported to be present in human myocardium as well (231,233), significant regeneration is not observed following myocardial infarction. Therefore, either the endogenous c-kit positive cardiac stem cells are not responsive to local growth signals or are unable to migrate and differentiate in response to infarction.
An additional independent adult mouse heart-derived cardiac progenitor cell expressing stem cell antigen 1(Sca-1+) (168). Based on immunophenotyping, these cells appear to be distinct from c-kit+ stem cells in the heart. When subjected to 5-azacytidine treatment cardiac Sca-1+ cells activate several cardiac-specific genes in vitro. When injected intravenously into mice six hours after myocardial infarction, engrafted Sca-1+ donor cells expressed cardiac markers, suggesting that they differentiate into cardiac myocytes in vivo. Approximately one half of the donor derived cells fused with host cardiomyocytes and 50% differentiated without fusion. It remains to be determined whether cardiac Sca-1+ cells have restricted developmental potential to only differentiate into cardiomyocytes or whether they will be able to differentiate into other cell types similar to c-kit+ cells. Another cardiac-derived subpopulation with progenitor potential, that likely overlaps with the Sca-1+ cells is a rare population of cells termed side population (SP) cells (147,178). They were isolated from mouse hearts based on their ability to exclude Hoechst dye, which was shown to be dependent on the expression of the ABCG2 transport protein, a member of the family of ATP-binding cassette (ABC) transporters (147). These cells are present throughout cardiac development, also express Sca-1+, but are rare and their ability to differentiate into cardiomyocytes and contribute to functional repair of the damaged myocardium needs to be fully evaluated.
Most recently, it was demonstrated that a subpopulation of cells in the anterior pharynx expresses the homeobox gene islet-1 (isl1) (27). During development, isl1 + cells contribute to formation of the outflow tract, the atria and the right ventricle (27). Expression of isl1 is lost when these cells differentiate into cardiomyocytes. Some isl1+ cells have been identified in the mature hearts of newborn rodents and humans where they remain undifferentiated (121). These cells fail to express stem cell antigen 1 (Sca-1), CD31, or c-kit, though they express Nkx 2.5 and GATA4. It was demonstrated that these cells could differentiate into cardiomyocytes both in vivo and in vitro (121). It is not known whether isl1+ cells exist in the adult heart beyond the early postnatal period. Moreover, the capacity of isl1+ cardiac progenitors to home to damaged myocardial tissue and form functional myocytes remains to be determined.
This data argues that the postnatal heart has one or more populations of resident stem or progenitor cells that might be utilized to regenerate the heart after injury. Since it is unlikely that multiple stem cells exist, it will be important in the future to determine the heirarachy of these various cells. It is also apparent that even if a cardiac stem cell exists; these cells, by themselves are not capable of mounting a robust response to repopulate damaged myocardium as is seen in the newt and zebrafish. Clinical use of these progenitor cells will require a better understanding of the signals involved in the activation of their proliferation and migration to the site of injury or alternatively, isolating and expanding them in vitro before reintroducing them into the damaged myocardium.
VI. Potential for Therapeutic Manipulation of Cardiac Myocyte Cell Cycle
An alternative approach to the use of stem cells for cardiac repair is to reactive the proliferative potential of existing differentiated cardiac myocytes as is seen in lower vertebrates. Investigators have taken a number of approaches to genetically manipulate key cell cycle regulators to promote cell cycle progression. Since proliferating cardiac myocytes express high levels and activity of cell cycle promoting factors such as cyclins D1, E, A and B, Cdk2, Cdk4/6, and Cdc2 as well as E2F family members and low levels of the Cdk inhibitors p21 and p27 (22,180), attempts to induce cardiomyocyte proliferation have focused on overexpressing cell cycle regulatory factors to promote cell cycle progression. A number of studies have shown that constitutive expression of fundamental cell cycle regulators can stimulate DNA synthesis in cardiomyocytes and in some cases lead to complete genomic replication and karyokinesis but cytokinesis remains an elusive goal in the adult heart. The earliest studies overexpressed viral oncoproteins such as adenovirus E1A and SV40 large T antigen (SV40) to override cell cycle checkpoints. Targeted expression of SV40 T-Ag was sufficient to induce sustained cycling of cardiomyocytes both in embryonic and adult heart (71,203). De novo expression of E1A or its downstream effector, E2F−1, although activating DNA synthesis resulted in widespread apoptosis that limits its usefulness as a regeneration strategy (113).
Inducible activation of c-Myc in adult myocardium resulted in cell cycle reentry and nuclear mitosis without evidence of apoptosis but whether cytokinesis occurred is questionable (100). In another study, transgenic hearts that expressed high levels of Cdk2 mRNA showed significantly increased levels of Cdk4 and cyclins A, D3, and E and augmented DNA synthesis in the adult animal but limited ongoing DNA synthesis (136). Over-expression of CycD1, D2 or D3 in transgenic mice was sufficient to stimulate DNA synthesis in adult myocardium under baseline conditions although differences in biological activity were uncovered after cardiac injury (175). In mice expressing cyclin D1 or D3 there was a reduction in the level of cardiomyocyte DNA synthesis after injury. In contrast, cardiac injury in mice expressing cyclin D2 did not alter cardiomyocyte cell cycle. Since infarct size was similar at seven days but decreased by about 30% in CycD2 overexpressing mice at 150 days when compared to nontransgenic littermates, the authors concluded that regeneration had occurred. It should be noted however, that given at least a subset of the cardiac myocytes in the CycD2 hearts had never undergone terminal differentiation, it will be important to determine whether de novo expression of CycD2 in adult myocardium using inducible transgenics or gene therapy has a similar effect. Elevated DNA synthesis and mitotic index were also demonstrated in cyclin A2 overexpressing transgenic mice (31). This group of investigators also demonstrated that delivery of a cyclin A2 expressing adenoviral vector immediately post-infarct enhanced cardiac function post injury (241). Recently, another study demonstrated that treatment with FGF1 in combination with a p38 MAP kinase inhibitor after myocardial injury could improve cardiac function, reduce scarring and induce mitosis in cardiac myocytes (61). This and the other examples outlined above are very promising approaches to regenerate myocardium; however, the results should be interpreted with caution. In many cases, the effects of the genetic or therapeutic maneuver on infarct size were not determined or alternatively, a reduction in infarct size was reported. Thus, it is not clear how much of the beneficial effects of the therapy were related to a reduction in infarct size versus true regeneration. Likewise, it will be important in future studies to document evidence of true cytokinesis where regeneration is thought to have occurred and either delay therapy with potential therapeutics to a time post-MI where infarct size is not affected or utilize inducible transgenics.
VII. Summary
Investigators and clinicians have been faced with the therapeutic challenge that, regardless of your believe on the proliferative potential of the adult cardiac myocyte or the presence of stem cells capable of differentiating into cardiac myocytes, the heart has little, if any, potential for regeneration after injury. This limits the adaptive response to the increased hemodynamic stress following cardiac injury primarily to cardiac hypertrophy and leads to alterations in the expression of a panel of genes that have been proposed to contribute to the eventual deterioration in cardiac function that leads to congestive heart failure. Consequently, investigators searched for strategies to increase myocardial mass after injury through increasing myocyte number versus cell size. The field of cardiac regeneration has exploded since the identification of stem and progenitor cells capable of differentiating into cardiac myocytes. While much research presently focuses on the identification, expansion and therapeutic use of these cells, results from human studies thus far have been mixed and the ability of the cells to actually differentiate in cardiac myocytes questioned (157).
Nonetheless, it is likely, given the limited proliferative potential of cardiac myocytes or stem cells (12) that an understanding of the regulation of the cardiac cell cycle will be necessary for the field of cardiac regeneration to continue to advance regardless of whether it is to therapeutically reactivate cell cycle progression in existing cardiac myocytes or to establish methodologies to expand cardiac stem cells without losing their differentiation potential. This review has attempted to summarize the dramatic advances that have been made in our understanding of cardiac myocyte cell cycle control in recent years. Despite these advances, major hurdles remain before manipulating the cardiac cell cycle could be used therapeutically such as lack of cytokinesis in adult myocytes and the challenge that maneuvers to induce cell cycle reactivation in adult myocytes is often accompanied by unwanted cell death.
Figure 2. Control of cardiac cell cycle.
Schematic representation of the factors influencing cardiac myocyte cell cycle progression and proliferation at different developmental stages. The potential outcomes of growth factor stimulation of adult cardiac myocytes namely proliferation, endoreduplication and hypertrophy in cardiac myocytes are also shown.
Table 2.
Genetic manipulations affecting cardiomyocyte proliferation/DNA synthesis
| Gene | Modifications done | Observed Phenotype | References |
|---|---|---|---|
| Cell cycle mediators | |||
| Cyc D1/D2/D3 | Transgenic mouse under the control of α-MHC promoter | Increased multinucleation and DNA synthesis in adult cardiomyocytes | (26,175,214) |
| Cyc A2 | Transgenic mouse under the control of α-MHC promoter | Postnatal hyperplasia but with no evidence of cytokinesis | (31) |
| Cyc B1/Cdc2 | Adenoviral transfer in neonatal and adult rat cardiomyocytes | Increased cell number | (122)] |
| Cdk2 | Transgenic mouse under the control of α-MHC promoter | Increased DNA and PCNA synthesis in adult cardiomyocytes | (136) |
| Cell cycle inhibitors | |||
| p27 | Knockout mouse model | Increased heart size, cardiomyocyte number, DNA and PCNA synthesis in adult cardiomyocytes | (181) |
| p53 | Adenoviral transfer in neonatal rat cardiomyocytes | Activates mitochondrial death pathway and promotes apoptosis | (186) |
| p38α MAPK | Conditional knockout mouse model using Cre/Lox system | Promotes complete division in adult cardiomyocytes | (62) |
| RB/p130 | Double knockout mouse model using Cre/Lox system | Increased heart size, BrdU and His H3 positive adult cardiomyocytes | (142) |
| TSC2 | Homozygous mutation at TSC2 locus in Eker rat embryos | Sustained proliferation of cardiomyocytes from mutant embryos in vitro | (171) |
| TSC2 | Targeted expression of a dominant negative TSC2 cDNA under the control of α-MHC promoter | Increased DNA synthesis in isoproterenol induced hypertrophy in adult cardiomyocytes | (174) |
| Transcription Factors | |||
| E2F1 | Adenoviral transfer in adult rat cardiomyocytes | Increased DNA synthesis and apoptosis | (3) |
| E2F1/in p53 KO | Adenoviral transfer in adult mouse cardiomyocytes | Increased DNA synthesis and apoptosis | (3) |
| E2F2/E2F4 | Adenoviral transfer in neonatal mouse cardiomyocytes | Induces S-phase entry | (59) |
| c-Myc | Transgenic mouse under the control of α-MHC promoter | Hyperplastic growth of heart during embryonic stage and accelerated hypertrophic growth during neonatal stage | (100,141) |
| c-Myc | Transgenic mouse under the control of α-MHC promoter | Increased cell cycle re-entry with increase in multinucleation and ploidy G1-S-G2M | (245) |
| Jumonji | Knockout mouse model | Increase in the number of mitotic cardiomyocytes | (104) |
| Growth Factors | |||
| FGF-2 | DNA transfection in neonatal rat cardiomyocyte cultures | Increased DNA synthesis and cell number | (169) |
| FGFR-1 | DNA transfection in neonatal rat cardiomyocyte cultures | Increased DNA synthesis and cell number | (204) |
| FGFR-1/FGFR-2 | Double knockout mouse model using Cre/Lox system | Severe hypoplasia, enlarged atria and thinning of the ventricular wall | (19) |
| IGF-I | Transgenic mouse under the control of α-MHC promoter | Increased number of cardiomyocytes | (187) |
| TGFβRIIΔKD/FGF-2 | Adenoviral transfer in neonatal rat cardiomyocytes | Increased DNA synthesis | (204) |
| TGFβ type-IR-ALK5 | Transgenic mouse under the control of α-MHC promoter | Linear, dilated, hypoplastic heart tube | (30) |
| Tumor virus Oncoproteins | |||
| SV40-TAg | Transgenic mouse under the control of ANF promoter | Mice developed atrial tumors and arrhythmias | (217) |
| SV40-TAg | Transgenic mouse under the control of protamine promoter | Mice developed cardiac rhabdomyosarcomas | (233) |
| SV40-TAg | Transgenic mouse under the control of α-MHC promoter | Developed atrial and ventricular tumors | (111) |
| SV40-TAg | Adenoviral transfer in neonatal rat cardiomyocytes | Increase in the number of mitotic cardiomyocytes | (203) |
| Temperature sensitive (tsA58) mutant of SV40-TAg | Retroviral delivery in adult rat cardiomyocytes | Increase in the number of mitotic cardiomyocytes | (107) |
| E1A | Adenoviral transfer in embryonic rat cardiomyocytes | Increased DNA synthesis and apoptosis | (139) |
| E1A/E1B | Adenoviral transfer in neonatal rat cardiomyocytes | Increased DNA synthesis without apoptosis | (113) |
| E1A | Adenoviral transfer in embryonic rat cardiomyocytes | Increased DNA synthesis and apoptosis | (194) |
| E1A/E1B and hCDC5 | Adenoviral transfer in neonatal rat cardiomyocytes | Promotes G2/M progression and complete division | (91) |
Acknowledgments
This work was supported by gifts from the Laubisch Fund Endowment as well as NIH grants R01 HL 70748, P01 HL080111 and AHA grant 0340087N to W.R.M. We are grateful to Elisabeth Ehler and Yibin Wang for reading the manuscript and helpful discussions.
Reference List
- 1.Identification and characterization of the tuberous sclerosis gene on chromosome 16. The European Chromosome 16 Tuberous Sclerosis Consortium. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 2.Abdullah I, Lepore JJ, Epstein JA, Parmacek MS, Gruber PJ. MRL mice fail to heal the heart in response to ischemia-reperfusion injury. Wound.Repair Regen. 2005;13:205–208. doi: 10.1111/j.1067-1927.2005.130212.x. [DOI] [PubMed] [Google Scholar]
- 3.Agah R, Kirshenbaum LA, Abdellatif M, Truong LD, Chakraborty S, Michael LH, Schneider MD. Adenoviral delivery of E2F-1 directs cell cycle reentry and p53-independent apoptosis in postmitotic adult myocardium in vivo. J.Clin.Invest. 1997;100:2722–2728. doi: 10.1172/JCI119817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahuja P, Perriard E, Perriard JC, Ehler E. Sequential myofibrillar breakdown accompanies mitotic division of mammalian cardiomyocytes. J.Cell Sci. 2004;117:3295–3306. doi: 10.1242/jcs.01159. [DOI] [PubMed] [Google Scholar]
- 5.Ahuja P, Perriard E, Trimble W, Perriard JC, Ehler E. Probing the role of septins in cardiomyocytes. Exp.Cell Res. 2006;312:1598–1609. doi: 10.1016/j.yexcr.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Akli S, Zhan S, Abdellatif M, Schneider MD. E1A can provoke G1 exit that is refractory to p21 and independent of activating cdk2. Circ.Res. 1999;85:319–328. doi: 10.1161/01.res.85.4.319. [DOI] [PubMed] [Google Scholar]
- 7.Ambrosino C, Iwata T, Scafoglio C, Mallardo M, Klein R, Nebreda AR. TEF-1 and C/EBPbeta are major p38alpha MAPK-regulated transcription factors in proliferating cardiomyocytes. Biochem.J. 2006;396:163–172. doi: 10.1042/BJ20051502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambrosino C, Nebreda AR. Cell cycle regulation by p38 MAP kinases. Biol.Cell. 2001;93:47–51. doi: 10.1016/s0248-4900(01)01124-8. [DOI] [PubMed] [Google Scholar]
- 9.Anversa P, Leri A, Kajstura J, Nadal-Ginard B. Myocyte growth and cardiac repair. J.Mol.Cell Cardiol. 2002;34:91–105. doi: 10.1006/jmcc.2001.1506. [DOI] [PubMed] [Google Scholar]
- 10.Ashe M, Pabon-Pena L, Dees E, Price KL, Bader D. LEK1 is a potential inhibitor of pocket protein-mediated cellular processes. J.Biol.Chem. 2004;279:664–676. doi: 10.1074/jbc.M308810200. [DOI] [PubMed] [Google Scholar]
- 11.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 12.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 13.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N. Engl. J. Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 14.Beltrami CA, Di Loreto C, Finato F, Yan SM. DNA Content in End-Stage Heart Failure. Adv.Clin.Path. 1997;1:59–73. [PubMed] [Google Scholar]
- 15.Bennett JD, Farlie PG, Watson RJ. E2F binding is required but not sufficient for repression of B-myb transcription in quiescent fibroblasts. Oncogene. 1996;13:1073–1082. [PubMed] [Google Scholar]
- 16.Berthet C, Klarmann KD, Hilton MB, Suh HC, Keller JR, Kiyokawa H, Kaldis P. Combined loss of Cdk2 and Cdk4 results in embryonic lethality and Rb hypophosphorylation. Dev. Cell. 2006;10:563–573. doi: 10.1016/j.devcel.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Bettencourt-Dias M, Mittnacht S, Brockes JP. Heterogeneous proliferative potential in regenerative adult newt cardiomyocytes. J. Cell Sci. 2003;116:4001–4009. doi: 10.1242/jcs.00698. [DOI] [PubMed] [Google Scholar]
- 18.Betz UAK, Bloch W, van den Broek M, Yoshida K, Taga T, Kishimoto T, Addicks K, Rajewsky K, Muller W. Postnatally induced inactivation of gp130 in mice results in neurological, cardiac, hematopoietic, immunological, hepatic, and pulmonary defects. J.Exp.Med. 1998;188:1955–1965. doi: 10.1084/jem.188.10.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bicknell KA, Coxon CH, Brooks G. Forced expression of the cyclin B1-CDC2 complex induces proliferation in adult rat cardiomyocytes. Biochem.J. 2004;382:411–416. doi: 10.1042/BJ20031481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, Braunwart J, Glascock BJ, Klevitsky R, Kimball TF, Hewett TE, Molkentin JD. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J.Clin.Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- 22.Brooks G, Poolman RA, McGill CJ, Li JM. Expression and activities of cyclins and cyclin-dependent kinases in developing rat ventricular myocytes. J.Mol.Cell Cardiol. 1997;29:2261–2271. doi: 10.1006/jmcc.1997.0471. [DOI] [PubMed] [Google Scholar]
- 23.Burton PB, Raff MC, Kerr P, Yacoub MH, Barton PJ. An intrinsic timer that controls cell-cycle withdrawal in cultured cardiac myocytes. Dev.Biol. 1999;216:659–670. doi: 10.1006/dbio.1999.9524. [DOI] [PubMed] [Google Scholar]
- 24.Burton PB, Yacoub MH, Barton PJ. Cyclin-dependent kinase inhibitor expression in human heart failure. A comparison with fetal development. Eur.Heart J. 1999;20:604–611. doi: 10.1053/euhj.1998.1231. [DOI] [PubMed] [Google Scholar]
- 25.Busk PK, Bartkova J, Strom CC, Wulf-Andersen L, Hinrichsen R, Christoffersen TE, Latella L, Bartek J, Haunso S, Sheikh SP. Involvement of cyclin D activity in left ventricle hypertrophy in vivo and in vitro. Cardiovasc.Res. 2002;56:64–75. doi: 10.1016/s0008-6363(02)00510-2. [DOI] [PubMed] [Google Scholar]
- 26.Busk PK, Hinrichsen R, Bartkova J, Hansen AH, Christoffersen TE, Bartek J, Haunso S. Cyclin D2 induces proliferation of cardiac myocytes and represses hypertrophy. Exp.Cell Res. 2005;304:149–161. doi: 10.1016/j.yexcr.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 27.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev.Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camarda G, Siepi F, Pajalunga D, Bernardini C, Rossi R, Montecucco A, Meccia E, Crescenzi M. A pRb-independent mechanism preserves the postmitotic state in terminally differentiated skeletal muscle cells. J. Cell Biol. 2004;167:417–423. doi: 10.1083/jcb.200408164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capasso JM, Bruno S, Cheng W, Li P, Rodgers R, Darzynkiewicz Z, Anversa P. Ventricular loading is coupled with DNA synthesis in adult cardiac myocytes after acute and chronic myocardial infarction in rats. Cir.Res. 1992;71:1379–1389. doi: 10.1161/01.res.71.6.1379. [DOI] [PubMed] [Google Scholar]
- 30.Charng MJ, Frenkel PA, Lin Q, Yamada M, Schwartz RJ, Olson EN, Overbeek P, Schneider MD, Yumada M. A constitutive mutation of ALK5 disrupts cardiac looping and morphogenesis in mice. Dev.Biol. 1998;199:72–79. doi: 10.1006/dbio.1998.8905. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhry HW, Dashoush NH, Tang H, Zhang L, Wang X, Wu EX, Wolgemuth DJ. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J.Biol.Chem. 2004;279:35858–35866. doi: 10.1074/jbc.M404975200. [DOI] [PubMed] [Google Scholar]
- 32.Chen HW, Yu SL, Chen WJ, Yang PC, Chien CT, Chou HY, Li HN, Peck K, Huang CH, Lin FY, Chen JJ, Lee YT. Dynamic changes of gene expression profiles during postnatal development of the heart in mice. Heart. 2004;90:927–934. doi: 10.1136/hrt.2002.006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen PL, Riley DJ, Chen-Kiang S, Lee WH. Retinoblastoma protein directly interacts with and activates the transcription factor NF-IL6. Proc.Natl.Acad.Sci.U.S.A. 1996;93:465–469. doi: 10.1073/pnas.93.1.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen PL, Riley DJ, Chen Y, Lee WH. Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes Dev. 1996;10:2794–2804. doi: 10.1101/gad.10.21.2794. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Shevtsov SP, Hsich E, Cui L, Haq S, Aronovitz M, Kerkela R, Molkentin JD, Liao R, Salomon RN, Patten R, Force T. The beta-catenin/T-cell factor/lymphocyte enhancer factor signaling pathway is required for normal and stress-induced cardiac hypertrophy. Mol.Cell Biol. 2006;26:4462–4473. doi: 10.1128/MCB.02157-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chun YS, Hyun JY, Kwak YG, Kim IS, Kim CH, Choi E, Kim MS, Park JW. Hypoxic activation of the atrial natriuretic peptide gene promoter through direct and indirect actions of hypoxia-inducible factor-1. Biochem.J. 2003;370:149–157. doi: 10.1042/BJ20021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clarke AR, Maandag ER, van Roon M, van der Lugt NM, van der Valk M, Hooper ML, Berns A, te RH. Requirement for a functional Rb-1 gene in murine development. Nature. 1992;359:328–330. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- 38.Classon M, Salama S, Gorka C, Mulloy R, Braun P, Harlow E. Combinatorial roles for pRB, p107, and p130 in E2F-mediated cell cycle control. Proc.Natl.Acad.Sci.U.S.A. 2000;97:10820–10825. doi: 10.1073/pnas.190343497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claycomb WC, Bradshaw HD., Jr. Acquisition of multiple nuclei and the activity of DNA polymerase alpha and reinitiation of DNA polymerase alpha and reinitiation of DNA replication in terminally differentiated adult cardiac muscle cells in culture. Dev.Biol. 1983;99:331–337. doi: 10.1016/0012-1606(83)90283-x. [DOI] [PubMed] [Google Scholar]
- 40.Cloud JE, Rogers C, Reza TL, Ziebold U, Stone JR, Picard MH, Caron AM, Bronson RT, Lees JA. Mutant mouse models reveal the relative roles of E2F1 and E2F3 in vivo. Mol.Cell Biol. 2002;22:2663–2672. doi: 10.1128/MCB.22.8.2663-2672.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clubb FJJ, Bishop SP. Formation of binucleated myocardial cells in the neonatal rat. An index for growth hypertrophy. Lab.Invest. 1984;50:571–577. [PubMed] [Google Scholar]
- 42.Cobrinik D. Pocket proteins and cell cycle control. Oncogene. 2005;24:2796–2809. doi: 10.1038/sj.onc.1208619. [DOI] [PubMed] [Google Scholar]
- 43.Cobrinik D, Lee MH, Hannon G, Mulligan G, Bronson RT, Dyson N, Harlow E, Beach D, Weinberg RA, Jacks T. Shared role of the pRB-related p130 and p107 proteins in limb development. Genes Dev. 1996;10:1633–1644. doi: 10.1101/gad.10.13.1633. [DOI] [PubMed] [Google Scholar]
- 44.Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J., Jr. Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc.Natl.Acad.Sci.U.S.A. 2002;99:12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cook SA, Matsui T, Li L, Rosenzweig A. Transcriptional effects of chronic Akt activation in the heart. J.Biol.Chem. 2002;277:22528–22533. doi: 10.1074/jbc.M201462200. [DOI] [PubMed] [Google Scholar]
- 46.Corbeil HB, Whyte P, Branton PE. Characterization of transcription factor E2F complexes during muscle and neuronal differentiation. Oncogene. 1995;11:909–920. [PubMed] [Google Scholar]
- 47.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dagnino L, Fry CJ, Bartley SM, Farnham P, Gallie BL, Phillips RA. Expression patterns of the E2F family of transcription factors during mouse nervous system development. Mech.Dev. 1997;66:13–25. doi: 10.1016/s0925-4773(97)00083-x. [DOI] [PubMed] [Google Scholar]
- 49.Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol.Cell Biol. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Datar SA, Jacobs HW, de la Cruz AF, Lehner CF, Edgar BA. The Drosophila cyclin D-Cdk4 complex promotes cellular growth. EMBO J. 2000;19:4543–4554. doi: 10.1093/emboj/19.17.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davis AC, Wims M, Spotts GD, Hann SR, Bradley A. A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev. 1993;7:671–682. doi: 10.1101/gad.7.4.671. [DOI] [PubMed] [Google Scholar]
- 52.de Alboran IM, O'Hagan RC, Gartner F, Malynn B, Davidson L, Rickert R, Rajewsky K, DePinho RA, Alt FW. Analysis of C-MYC function in normal cells via conditional gene-targeted mutation. Immunity. 2001;14:45–55. doi: 10.1016/s1074-7613(01)00088-7. [DOI] [PubMed] [Google Scholar]
- 53.DeGregori J, Leone G, Miron A, Jakoi L, Nevins JR. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc. Natl. Acad. Sci. U. S. A. 1997;94:7245–7250. doi: 10.1073/pnas.94.14.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delaughter MC, Taffet GE, Fiorotto ML, Entman ML, Schwartz RJ. Local insulin-like growth factor I expression induces physiologic, then pathologic, cardiac hypertrophy in transgenic mice. FASEB J. 1999;13:1923–1929. doi: 10.1096/fasebj.13.14.1923. [DOI] [PubMed] [Google Scholar]
- 55.Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994;8:3045–3057. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- 56.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J.Clin.Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dinsmore CE. The foundations of contemporary regeneration research: historical perspectives. Monogr Dev.Biol. 1992;23:1–27. [PubMed] [Google Scholar]
- 58.Dorn GWI, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J.Clin.Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ebelt H, Hufnagel N, Neuhaus P, Neuhaus H, Gajawada P, Simm A, Muller-Werdan U, Werdan K, Braun T. Divergent siblings: E2F2 and E2F4 but not E2F1 and E2F3 induce DNA synthesis in cardiomyocytes without activation of apoptosis. Circ.Res. 2005;96:509–517. doi: 10.1161/01.RES.0000159705.17322.57. [DOI] [PubMed] [Google Scholar]
- 60.Ehler E, Rothen BM, Hammerle SP, Komiyama M, Perriard JC. Myofibrillogenesis in the developing chicken heart: assembly of Z-disk, M-line and the thick filaments. J.Cell Sci. 1999;112(Pt 10):1529–1539. doi: 10.1242/jcs.112.10.1529. [DOI] [PubMed] [Google Scholar]
- 61.Engel FB, Hsieh PC, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc.Natl.Acad.Sci.U.S.A. 2006;103:15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engel FB, Schebesta M, Keating MT. Anillin localization defect in cardiomyocyte binucleation. J.Mol.Cell Cardiol. 2006;41:601–612. doi: 10.1016/j.yjmcc.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 64.Escary JL, Perreau J, Dumenil D, Ezine S, Brulet P. Leukaemia inhibitory factor is necessary for maintenance of haematopoietic stem cells and thymocyte stimulation. Nature. 1993;363:361–364. doi: 10.1038/363361a0. [DOI] [PubMed] [Google Scholar]
- 65.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Evan GI, Littlewood TD. The role of c-myc in cell growth. Curr.Opin.Genet.Dev. 1993;3:44–49. doi: 10.1016/s0959-437x(05)80339-9. [DOI] [PubMed] [Google Scholar]
- 67.Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- 68.Fausto N. Liver regeneration. J.Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 69.Felsher DW, Zetterberg A, Zhu J, Tlsty T, Bishop JM. Overexpression of MYC causes p53-dependent G2 arrest of normal fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10544–10548. doi: 10.1073/pnas.190327097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferrans VJ, Rodriguez ER. Evidence of myocyte hyperplasia in hypertrophic cardiomyopathy and other disorders with myocardial hypertrophy? Z.Kardiol. 1987;76(Suppl 3):20–25. [PubMed] [Google Scholar]
- 71.Field LJ. Atrial natriuretic factor-SV40 T antigen transgenes produce tumors and cardiac arrhythmias in mice. Science. 1988;239:1029–1033. doi: 10.1126/science.2964082. [DOI] [PubMed] [Google Scholar]
- 72.Field SJ, Tsai FY, Kuo F, Zubiaga AM, Kaelin WGJ, Livingston DM, Orkin SH, Greenberg ME. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996;85:549–561. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- 73.Flink IL, Oana S, Maitra N, Bahl JJ, Morkin E. Changes in E2F complexes containing retinoblastoma protein family members and increased cyclin-dependent kinase inhibitor activities during terminal differentiation of cardiomyocytes. J.Mol.Cell.Cardiol. 1998;30:563–578. doi: 10.1006/jmcc.1997.0620. [DOI] [PubMed] [Google Scholar]
- 74.Frei C, Galloni M, Hafen E, Edgar BA. The Drosophila mitochondrial ribosomal protein mRpL12 is required for Cyclin D/Cdk4-driven growth. EMBO J. 2005;24:623–634. doi: 10.1038/sj.emboj.7600523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Furukawa Y, Iwase S, Kikuchi J, Nakamura M, Yamada H, Matsuda M. Transcriptional repression of the E2F-1 gene by interferon-alpha is mediated through induction of E2F-4/pRB and E2F-4/p130 complexes. Oncogene. 1999;18:2003–2014. doi: 10.1038/sj.onc.1202500. [DOI] [PubMed] [Google Scholar]
- 76.Georgescu SP, Komuro I, Hiroi Y, Mizuno T, Kudoh S, Yamazaki T, Yazaki Y. Downregulation of polo-like kinase correlates with loss of proliferative ability of cardiac myocytes. J.Mol.Cell Cardiol. 1997;29:929–937. doi: 10.1006/jmcc.1996.0334. [DOI] [PubMed] [Google Scholar]
- 77.Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 78.Gomez-Roman N, Grandori C, Eisenman RN, White RJ. Direct activation of RNA polymerase III transcription by c-Myc. Nature. 2003;421:290–294. doi: 10.1038/nature01327. [DOI] [PubMed] [Google Scholar]
- 79.Goodfellow SJ, Innes F, Derblay LE, MacLellan WR, Scott PH, White RJ. Regulation of RNA polymerase III transcription during hypertrophic growth. EMBO J. 2006;25:1522–1533. doi: 10.1038/sj.emboj.7601040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goodman LC, Epling S, Kelly S, Lee S, Fishbein MC. DNA flow cytometry of myocardial cell nuclei in paraffin-embedded, human autopsy, cardiac tissue. Am.J.Cardiovasc.Pathol. 1990;3:55–59. [PubMed] [Google Scholar]
- 81.Grabner W, Pfitzer P. Number of nuclei in isolated myocardial cells of pigs. Virchows Arch.B Cell Pathol. 1974;15:279–294. doi: 10.1007/BF02889344. [DOI] [PubMed] [Google Scholar]
- 82.Gu W, Schneider JW, Conderstil G, Kaushai S, Mahdavi V, Nadal-Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell. 1993;72:309–324. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- 83.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 84.Helin K, Harlow E. Heterodimerization of the transcription factors E2F-1 and DP-1 is required for binding to the adenovirus E4 (ORF6/7) protein. J.Virology. 1993;7:1850–1861. doi: 10.1128/jvi.68.8.5027-5035.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herget GW, Neuburger M, Plagwitz R, Adler CP. DNA content, ploidy level and number of nuclei in the human heart after myocardial infarction. Cardiovasc.Res. 1997;36:45–51. doi: 10.1016/s0008-6363(97)00140-5. [DOI] [PubMed] [Google Scholar]
- 86.Hijmans EM, Voorhoeve PM, Beijersbergen RL, van 't Veer LJ, Bernards R. E2F-5, a new E2F family member that interacts with p130 in vivo. Mol. Cell Biol. 1995;15:3082–3089. doi: 10.1128/mcb.15.6.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine.Growth Factor.Rev. 1997;8:241–252. doi: 10.1016/s1359-6101(98)80005-1. [DOI] [PubMed] [Google Scholar]
- 88.Hirota H, Chen J, Betz UAK, Rajewsky K, Gu Y, Ross JJ, Muller W, Chien KR. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1999;97:189–198. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- 89.Hirschy A, Schatzmann F, Ehler E, Perriard JC. Establishment of cardiac cytoarchitecture in the developing mouse heart. Dev.Biol. 2006;289:430–441. doi: 10.1016/j.ydbio.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 90.Hlaing M, Spitz P, Padmanabhan K, Cabezas B, Barker CS, Bernstein HS. E2F-1 regulates the expression of a subset of target genes during skeletal myoblast hypertrophy. J.Biol.Chem. 2004;279:43625–43633. doi: 10.1074/jbc.M408391200. [DOI] [PubMed] [Google Scholar]
- 91.Huang SS, Huang JS. TGF-beta control of cell proliferation. J. Cell Biochem. 2005;96:447–462. doi: 10.1002/jcb.20558. [DOI] [PubMed] [Google Scholar]
- 92.Huh MS, Parker MH, Scime A, Parks R, Rudnicki MA. Rb is required for progression through myogenic differentiation but not maintenance of terminal differentiation. J. Cell Biol. 2004;166:865–876. doi: 10.1083/jcb.200403004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huh NE, Pasumarthi KB, Soonpaa MH, Jing S, Patton B, Field LJ. Functional abrogation of p53 is required for T-Ag induced proliferation in cardiomyocytes. J.Mol.Cell Cardiol. 2001;33:1405–1419. doi: 10.1006/jmcc.2001.1403. [DOI] [PubMed] [Google Scholar]
- 94.Humbert PO, Rogers C, Ganiatsas S, Landsberg RL, Trimarchi JM, Dandapani S, Brugnara C, Erdman S, Schrenzel M, Bronson RT, Lees JA. E2F4 is essential for normal erythrocyte maturation and neonatal viability. Mol. Cell. 2000;6:281–291. doi: 10.1016/s1097-2765(00)00029-0. [DOI] [PubMed] [Google Scholar]
- 95.Hyams JS. Cytokinesis: the great divide. Trends Cell Biol. 2005;15:1. doi: 10.1016/j.tcb.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 96.Iritani BM, Eisenman RN. c-Myc enhances protein synthesis and cell size during B lymphocyte development. Proc.Natl.Acad.Sci.U.S.A. 1999;96:13180–13185. doi: 10.1073/pnas.96.23.13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Izumo S, Nadal-Ginard B, Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc.Natl.Acad.Sci.U.S.A. 1988;85:339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 100.Jackson T, Allard MF, Sreenan CM, Doss LK, Bishop SP, Swain JL. The c-myc proto-oncogene regulates cardiac development in transgenic mice. Mol.Cell Biol. 1990;10:3709–3716. doi: 10.1128/mcb.10.7.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang Z, Zacksenhaus E, Gallie BL, Phillips RA. The retinoblastoma gene family is differentially expressed during embryogenesis. Oncogene. 1997;14:1789–1797. doi: 10.1038/sj.onc.1201014. [DOI] [PubMed] [Google Scholar]
- 102.Johnson DG, Cress WD, Jakoi L, Nevins JR. Oncogenic capacity of the E2F1 gene. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12823–12827. doi: 10.1073/pnas.91.26.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnston LA, Prober DA, Edgar BA, Eisenman RN, Gallant P. Drosophila myc regulates cellular growth during development. Cell. 1999;98:779–790. doi: 10.1016/s0092-8674(00)81512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jung J, Kim TG, Lyons GE, Kim HR, Lee Y. Jumonji regulates cardiomyocyte proliferation via interaction with retinoblastoma protein. J.Biol.Chem. 2005;280:30916–30923. doi: 10.1074/jbc.M414482200. [DOI] [PubMed] [Google Scholar]
- 105.Kajstura J, Cheng W, Reiss K, Anversa P. The IGF-1-IGF-1 receptor system modulates myocyte proliferation but not myocyte cellular hypertrophy in vitro. Exp.Cell Res. 1994;215:273–283. doi: 10.1006/excr.1994.1343. [DOI] [PubMed] [Google Scholar]
- 106.Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P. Myocyte proliferation in end-stage cardiac failure in humans. Proc.Natl.Acad.Sci.U.S.A. 1998;95:8801–8805. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kajstura J, Pertoldi B, Leri A, Beltrami CA, Deptala A, Darzynkiewicz Z, Anversa P. Telomere shortening is an in vivo marker of myocyte replication and aging. Am.J.Pathol. 2000;156:813–819. doi: 10.1016/S0002-9440(10)64949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kang MJ, Kim JS, Chae SW, Koh KN, Koh GY. Cyclins and cyclin dependent kinases during cardiac development. Mol.Cells. 1997;7:360–366. [PubMed] [Google Scholar]
- 109.Kang MJ, Koh GY. Differential and dramatic changes of cyclin-dependent kinase activities in cardiomyocytes during the neonatal period. J.Mol.Cell Cardiol. 1997;29:1767–1777. doi: 10.1006/jmcc.1997.0450. [DOI] [PubMed] [Google Scholar]
- 110.Kardami E, Liu L, Kishore S, Pasumarthi B, Doble BW, Cattini PA. Regulation of basic fibroblast growth factor (bFGF) and FGF receptors in the heart. Ann.N.Y.Acad.Sci. 1995;752:353–369. doi: 10.1111/j.1749-6632.1995.tb17444.x. [DOI] [PubMed] [Google Scholar]
- 111.Katz EB, Steinhelper ME, Delcarpio JB, Daud AI, Claycomb WC, Field LJ. Cardiomyocyte proliferation in mice expressing alpha-cardiac myosin heavy chain-SV40 T-antigen transgenes. Am.J.Physiol. 1992;262:H1867–H1876. doi: 10.1152/ajpheart.1992.262.6.H1867. [DOI] [PubMed] [Google Scholar]
- 112.Kim CH, Cho YS, Chun YS, Park JW, Kim MS. Early expression of myocardial HIF-1alpha in response to mechanical stresses: regulation by stretch-activated channels and the phosphatidylinositol 3-kinase signaling pathway. Circ.Res. 2002;90:E25–E33. doi: 10.1161/hh0202.104923. [DOI] [PubMed] [Google Scholar]
- 113.Kirshenbaum LA, Schneider MD. Adenovirus E1A represses cardiac gene transcription and reactivates DNA synthesis in ventricular myocytes, via alternative pocket protein-and p300-binding domains. J.Biol.Chem. 1995;270:7791–7794. doi: 10.1074/jbc.270.14.7791. [DOI] [PubMed] [Google Scholar]
- 114.Koh KN, Kang MJ, Frith-Terhune A, Park SK, Kim I, Lee CO, Koh GY. Persistent and heterogenous expression of the cyclin-dependent kinase inhibitor, p27KIP1, in rat hearts during development. J.Mol.Cell Cardiol. 1998;30:463–474. doi: 10.1006/jmcc.1997.0611. [DOI] [PubMed] [Google Scholar]
- 115.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 116.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kozar K, Ciemerych MA, Rebel VI, Shigematsu H, Zagozdzon A, Sicinska E, Geng Y, Yu Q, Bhattacharya S, Bronson RT, Akashi K, Sicinski P. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 118.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J.Clin.Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kwiatkowski DJ. Tuberous sclerosis: from tubers to mTOR. Ann.Hum.Genet. 2003;67:87–96. doi: 10.1046/j.1469-1809.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 120.Lau MM, Stewart CE, Liu Z, Bhatt H, Rotwein P, Stewart CL. Loss of the imprinted IGF2/cation-independent mannose 6-phosphate receptor results in fetal overgrowth and perinatal lethality. Genes Dev. 1994;8:2953–2963. doi: 10.1101/gad.8.24.2953. [DOI] [PubMed] [Google Scholar]
- 121.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J, Ornitz DM. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev.Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 123.Lavoie JN, L'Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J.Biol.Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 124.LeCouter JE, Kablar B, Whyte PF, Ying C, Rudnicki MA. Strain-dependent embryonic lethality in mice lacking the retinoblastoma-related p130 gene. Development. 1998;125:4669–4679. doi: 10.1242/dev.125.23.4669. [DOI] [PubMed] [Google Scholar]
- 125.Lee EYHP, Chang CY, Hu N, Wang YCJ, Lai CC, Herrup K, Lee WH, Bradley A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992;359:288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- 126.Lee MH, Williams BO, Mulligan G, Mukai S, Bronson RT, Dyson N, Harlow E, Jacks T. Targeted disruption of p107: functional overlap between p107 and Rb. Genes Dev. 1996;10:1621–1632. doi: 10.1101/gad.10.13.1621. [DOI] [PubMed] [Google Scholar]
- 127.Lees JA, Saito M, Vidal M, Valentine M, Look T, Harlow E, Dyson N, Helin K. The retinoblastoma protein binds to a family of E2F transcription factors. Mol. Cell Biol. 1993;13:7813–7825. doi: 10.1128/mcb.13.12.7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Leferovich JM, Bedelbaeva K, Samulewicz S, Zhang XM, Zwas D, Lankford EB, Heber-Katz E. Heart regeneration in adult MRL mice. Proc.Natl.Acad.Sci.U.S.A. 2001;98:9830–9835. doi: 10.1073/pnas.181329398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lembo G, Rockman HA, Hunter JJ, Steinmetz H, Koch WJ, Ma L, Prinz MP, Ross JJ, Chien KR, Powell-Braxton L. Elevated blood pressure and enhanced myocardial contractility in mice with severe IGF-1 deficiency. J.Clin.Invest. 1996;98:2648–2655. doi: 10.1172/JCI119086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.LeRoith D, Helman L. The new kid on the block(ade) of the IGF-1 receptor. Cancer Cell. 2004;5:201–202. doi: 10.1016/s1535-6108(04)00054-6. [DOI] [PubMed] [Google Scholar]
- 131.Li F, Wang X, Bunger PC, Gerdes AM. Formation of binucleated cardiac myocytes in rat heart: I. Role of actin-myosin contractile ring. J.Mol.Cell Cardiol. 1997;29:1541–1551. doi: 10.1006/jmcc.1997.0381. [DOI] [PubMed] [Google Scholar]
- 132.Li F, Wang X, Gerdes AM. Formation of binucleated cardiac myocytes in rat heart: II. Cytoskeletal organisation. J.Mol.Cell Cardiol. 1997;29:1553–1565. doi: 10.1006/jmcc.1997.0403. [DOI] [PubMed] [Google Scholar]
- 133.Li JM, Brooks G. Downregulation of cyclin-dependent kinase inhibitors p21 and p27 in pressure-overload hypertrophy. Am.J.Physiol. 1997;273:H1358–H1367. doi: 10.1152/ajpheart.1997.273.3.H1358. [DOI] [PubMed] [Google Scholar]
- 134.Li JM, Poolman RA, Brooks G. Role of G1 phase cyclins and cyclin-dependent kinases during cardiomyocyte hypertrophic growth in rats. Am.J.Physiol. 1998;275:H814–H822. doi: 10.1152/ajpheart.1998.275.3.H814. [DOI] [PubMed] [Google Scholar]
- 135.Liang Q, Molkentin JD. Redefining the roles of p38 and JNK signaling in cardiac hypertrophy: dichotomy between cultured myocytes and animal models. J.Mol.Cell Cardiol. 2003;35:1385–1394. doi: 10.1016/j.yjmcc.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 136.Liao HS, Kang PM, Nagashima H, Yamasaki N, Usheva A, Ding B, Lorell BH, Izumo S. Cardiac-specific overexpression of cyclin-dependent kinase 2 increases smaller mononuclear cardiomyocytes. Circ.Res. 2001;88:443–450. doi: 10.1161/01.res.88.4.443. [DOI] [PubMed] [Google Scholar]
- 137.Lindeman GJ, Dagnino L, Gaubatz S, Xu Y, Bronson RT, Warren HB, Livingston DM. A specific, nonproliferative role for E2F-5 in choroid plexus function revealed by gene targeting. Genes Dev. 1998;12:1092–1098. doi: 10.1101/gad.12.8.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 139.Liu Y, Kitsis RN. Induction of DNA synthesis and apoptosis in cardiac myocytes by E1A oncoprotein. J.Cell Biol. 1996;133:325–334. doi: 10.1083/jcb.133.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Logan N, Graham A, Zhao X, Fisher R, Maiti B, Leone G, La Thangue NB. E2F-8: an E2F family member with a similar organization of DNA-binding domains to E2F-7. Oncogene. 2005;24:5000–5004. doi: 10.1038/sj.onc.1208703. [DOI] [PubMed] [Google Scholar]
- 141.Machida N, Brissie N, Sreenan C, Bishop SP. Inhibition of cardiac myocyte division in c-myc transgenic mice. J.Mol.Cell Cardiol. 1997;29:1895–1902. doi: 10.1006/jmcc.1997.0427. [DOI] [PubMed] [Google Scholar]
- 142.MacLellan WR, Garcia A, Oh H, Frenkel P, Jordan MC, Roos KP, Schneider MD. Overlapping roles of pocket proteins in the myocardium are unmasked by germ line deletion of p130 plus heart-specific deletion of Rb. Mol.Cell Biol. 2005;25:2486–2497. doi: 10.1128/MCB.25.6.2486-2497.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maher P. p38 mitogen-activated protein kinase activation is required for fibroblast growth factor-2-stimulated cell proliferation but not differentiation. J.Biol.Chem. 1999;274:17491–17498. doi: 10.1074/jbc.274.25.17491. [DOI] [PubMed] [Google Scholar]
- 144.Malumbres M, Sotillo R, Santamaria D, Galan J, Cerezo A, Ortega S, Dubus P, Barbacid M. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118:493–504. doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 145.Malynn BA, de Alboran IM, O'Hagan RC, Bronson R, Davidson L, DePinho RA, Alt FW. N-myc can functionally replace c-myc in murine development, cellular growth, and differentiation. Genes Dev. 2000;14:1390–1399. [PMC free article] [PubMed] [Google Scholar]
- 146.Mantela J, Jiang Z, Ylikoski J, Fritzsch B, Zacksenhaus E, Pirvola U. The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development. 2005;132:2377–2388. doi: 10.1242/dev.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev.Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 148.Masu Y, Wolf E, Holtmann B, Sendtner M, Brem G, Thoenen H. Disruption of the CNTF gene results in motor neuron degeneration. Nature. 1993;365:27–32. doi: 10.1038/365027a0. [DOI] [PubMed] [Google Scholar]
- 149.Matsuoka S, Edwards MC, Bai C, Parker S, Zhang P, Baldini A. p51KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995;9:650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- 150.Matz DG, Oberpriller JO, Oberpriller JC. Comparison of mitosis in binucleated and mononucleated newt cardiac myocytes. Anat.Rec. 1998;251:245–255. doi: 10.1002/(SICI)1097-0185(199806)251:2<245::AID-AR14>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 151.Meckert PC, Rivello HG, Vigliano C, Gonzalez P, Favaloro R, Laguens R. Endomitosis and polyploidization of myocardial cells in the periphery of human acute myocardial infarction. Cardiovasc.Res. 2005;67:116–123. doi: 10.1016/j.cardiores.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 152.Mima T, Ueno H, Fischman DA, Williams LT, Mikawa T. Fibroblast growth factor receptor is required for in vivo cardiac myocyte proliferation at early embryonic stages of heart development. Proc.Natl.Acad.Sci.U.S.A. 1995;92:467–471. doi: 10.1073/pnas.92.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moberg K, Starz MA, Lees JA. E2F-4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol. Cell Biol. 1996;16:1436–1449. doi: 10.1128/mcb.16.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Molkentin JD, Dorn GW., II Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu.Rev.Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 155.Movassagh M, Bicknell KA, Brooks G. Characterisation and regulation of E2F-6 and E2F-6b in the rat heart: a potential target for myocardial regeneration? J.Pharm.Pharmacol. 2006;58:73–82. doi: 10.1211/jpp.58.1.0009. [DOI] [PubMed] [Google Scholar]
- 156.Mugrauer G, Alt FW, Ekblom P. N-myc proto-oncogene expression during organogenesis in the developing mouse as revealed by in situ hybridization. J.Cell Biol. 1988;107:1325–1335. doi: 10.1083/jcb.107.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J.Am.Coll.Cardiol. 2006;47:1777–1785. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 158.Nag AC, Healy CJ, Cheng M. DNA synthesis and mitosis in adult amphibian cardiac muscle cells in vitro. Science. 1979;205:1281–1282. doi: 10.1126/science.472744. [DOI] [PubMed] [Google Scholar]
- 159.Nagahama H, Hatakeyama S, Nakayama K, Nagata M, Tomita K, Nakayama K. Spatial and temporal expression patterns of the cyclin-dependent kinase (CDK) inhibitors p27Kip1 and p57Kip2 during mouse development. Anat.Embryol. (Berl) 2001;203:77–87. doi: 10.1007/s004290000146. [DOI] [PubMed] [Google Scholar]
- 160.Nagata Y, Takahashi N, Davis RJ, Todokoro K. Activation of p38 MAP kinase and JNK but not ERK is required for erythropoietin-induced erythroid differentiation. Blood. 1998;92:1859–1869. [PubMed] [Google Scholar]
- 161.Nebreda AR, Porras A. p38 MAP kinases: beyond the stress response. Trends Biochem.Sci. 2000;25:257–260. doi: 10.1016/s0968-0004(00)01595-4. [DOI] [PubMed] [Google Scholar]
- 162.Neufeld TP, Edgar BA. Connections between growth and the cell cycle. Curr.Opin.Cell Biol. 1998;10:784–790. doi: 10.1016/s0955-0674(98)80122-1. [DOI] [PubMed] [Google Scholar]
- 163.Nevins JR. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 164.Novitch BG, Mulligan GJ, Jacks T, Lassar AB. Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J.Cell.Biol. 1996;135:441–456. doi: 10.1083/jcb.135.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Novitch BG, Spicer DB, Kim PS, Cheung WL, Lassar AB. pRb is required for MEF2-dependent gene expression as well as cell-cycle arrest during skeletal muscle differentiation. Curr.Biol. 1999;9:449–459. doi: 10.1016/s0960-9822(99)80210-3. [DOI] [PubMed] [Google Scholar]
- 166.Nozato T, Ito H, Watanabe M, Ono Y, Adachi S, Tanaka H, Hiroe M, Sunamori M, Marum F. Overexpression of cdk Inhibitor p16INK4a by adenovirus vector inhibits cardiac hypertrophy in vitro and in vivo: a novel strategy for the gene therapy of cardiac hypertrophy. J.Mol.Cell Cardiol. 2001;33:1493–1504. doi: 10.1006/jmcc.2001.1412. [DOI] [PubMed] [Google Scholar]
- 167.Oberpriller JO, Oberpriller JC, Matz DG, Soonpaa MH. Stimulation of proliferative events in the adult amphibian cardiac myocyte. Ann.N.Y.Acad.Sci. 1995;752:30–46. doi: 10.1111/j.1749-6632.1995.tb17404.x. [DOI] [PubMed] [Google Scholar]
- 168.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc.Natl.Acad.Sci.U.S.A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Oh YS, Thomson LE, Fishbein MC, Berman DS, Sharifi B, Chen PS. Scar formation after ischemic myocardial injury in MRL mice. Cardiovasc.Pathol. 2004;13:203–206. doi: 10.1016/j.carpath.2004.03.610. [DOI] [PubMed] [Google Scholar]
- 170.Olivetti G, Cigola E, Maestri R, Corradi D, Lagrasta C, Gambert SR, Anversa P. Aging, cardiac hypertrophy and ischemic cardiomyopathy do not affect the proportion of mononucleated and multinucleated myocytes in the human heart. J.Mol.Cell Cardiol. 1996;28:1463–1477. doi: 10.1006/jmcc.1996.0137. [DOI] [PubMed] [Google Scholar]
- 171.Pajak L, Jin F, Xiao GH, Soonpaa MH, Field LJ, Yeung RS. Sustained cardiomyocyte DNA synthesis in whole embryo cultures lacking the TSC2 gene product. Am.J. Physiol. 1997;273:H1619–H1627. doi: 10.1152/ajpheart.1997.273.3.H1619. [DOI] [PubMed] [Google Scholar]
- 172.Papadimou E, Menard C, Grey C, Puceat M. Interplay between the retinoblastoma protein and LEK1 specifies stem cells toward the cardiac lineage. EMBO J. 2005;24:1750–1761. doi: 10.1038/sj.emboj.7600652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pasumarthi KB, Kardami E, Cattini PA. High and low molecular weight fibroblast growth factor-2 increase proliferation of neonatal rat cardiac myocytes but have differential effects on binucleation and nuclear morphology.Evidence for both paracrine and intracrine actions of fibroblast growth factor-2. Circ.Res. 1996;78:126–136. doi: 10.1161/01.res.78.1.126. [DOI] [PubMed] [Google Scholar]
- 174.Pasumarthi KB, Nakajima H, Nakajima HO, Jing S, Field LJ. Enhanced cardiomyocyte DNA synthesis during myocardial hypertrophy in mice expressing a modified TSC2 transgene. Circ Res. 2000;86:1069–1077. doi: 10.1161/01.res.86.10.1069. [DOI] [PubMed] [Google Scholar]
- 175.Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ.Res. 2005;96:110–118. doi: 10.1161/01.RES.0000152326.91223.4F. [DOI] [PubMed] [Google Scholar]
- 176.Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nat.Rev.Cancer. 2002;2:764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- 177.Persengiev SP, Kondova II, Kilpatrick DL. E2F4 actively promotes the initiation and maintenance of nerve growth factor-induced cell differentiation. Mol.Cell Biol. 1999;19:6048–6056. doi: 10.1128/mcb.19.9.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31-but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ.Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 179.Poolman RA, Brooks G. Expressions and activities of cell cycle regulatory molecules during the transition from myocyte hyperplasia to hypertrophy. J.Mol.Cell Cardiol. 1998;30:2121–2135. doi: 10.1006/jmcc.1998.0808. [DOI] [PubMed] [Google Scholar]
- 180.Poolman RA, Gilchrist R, Brooks G. Cell cycle profiles and expressions of p21CIP1 AND P27KIP1 during myocyte development. Int.J.Cardiol. 1998;67:133–142. doi: 10.1016/s0167-5273(98)00320-9. [DOI] [PubMed] [Google Scholar]
- 181.Poolman RA, Li JM, Durand B, Brooks G. Altered expression of cell cycle proteins and prolonged duration of cardiac myocyte hyperplasia in p27KIP1 knockout mice. Circ.Res. 1999;85:117–127. doi: 10.1161/01.res.85.2.117. [DOI] [PubMed] [Google Scholar]
- 182.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 183.Qin XQ, Livingston DM, Kaelin WG, Jr, Adams PD. Deregulated transcription factor E2F-1 expression leads to S-phase entry and p53-mediated apoptosis. Proc.Natl.Acad.Sci.U.S.A. 1994;91:10918–10922. doi: 10.1073/pnas.91.23.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Quelle DE, Ashmun RA, Hannon GJ, Rehberger PA, Trono D, Richter KH, Walker C, Beach D, Sherr CJ, Serrano M. Cloning and characterization of murine p16INK4a and p15INK4b genes. Oncogene. 1995:635–645. [PubMed] [Google Scholar]
- 185.Rausch O, Marshall CJ. Cooperation of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways during granulocyte colony-stimulating factor-induced hemopoietic cell proliferation. J.Biol.Chem. 1999;274:4096–4105. doi: 10.1074/jbc.274.7.4096. [DOI] [PubMed] [Google Scholar]
- 186.Regula KM, Kirshenbaum LA. p53 activates the mitochondrial death pathway and apoptosis of ventricular myocytes independent of de novo gene transcription. J.Mol.Cell Cardiol. 2001;33:1435–1445. doi: 10.1006/jmcc.2001.1405. [DOI] [PubMed] [Google Scholar]
- 187.Reiss K, Cheng W, Ferber A, Kajstura J, Li P, Li B, Olivetti G, Homcy CJ, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice. Proc.Natl.Acad.Sci.U.S.A. 1996;93:8630–8635. doi: 10.1073/pnas.93.16.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rempel RE, Saenz-Robles MT, Storms R, Morham S, Ishida S, Engel A, Jakoi L, Melhem MF, Pipas JM, Smith C, Nevins JR. Loss of E2F4 activity leads to abnormal development of multiple cellular lineages. Mol. Cell. 2000;6:293–306. doi: 10.1016/s1097-2765(00)00030-7. [DOI] [PubMed] [Google Scholar]
- 189.Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat.Med. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- 190.Robbins RJ, Swain JL. C-myc protooncogene modulates cardiac hypertrophic growth in transgenic mice. Am.J.Physiol. 1992;262:H590–H597. doi: 10.1152/ajpheart.1992.262.2.H590. [DOI] [PubMed] [Google Scholar]
- 191.Rosenblatt-Velin N, Lepore MG, Cartoni C, Beermann F, Pedrazzini T. FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J. Clin.Invest. 2005;115:1724–1733. doi: 10.1172/JCI23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rosenthal N. High hopes for the heart. N.Engl.J. Med. 2001;344:1785–1787. doi: 10.1056/NEJM200106073442311. [DOI] [PubMed] [Google Scholar]
- 193.Rosner M, Freilinger A, Hengstschlager M. The tuberous sclerosis genes and regulation of the cyclin-dependent kinase inhibitor p27. Mutat.Res. 2006 doi: 10.1016/j.mrrev.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 194.Rota M, Boni A, Urbanek K, Padin-Iruegas ME, Kajstura TJ, Fiore G, Kubo H, Sonnenblick EH, Musso E, Houser SR, Leri A, Sussman MA, Anversa P. Nuclear targeting of Akt enhances ventricular function and myocyte contractility. Circ.Res. 2005;97:1332–1341. doi: 10.1161/01.RES.0000196568.11624.ae. [DOI] [PubMed] [Google Scholar]
- 195.Rubart M, Field LJ. Cardiac regeneration: repopulating the heart. Annu.Rev.Physiol. 2006;68:29–49. doi: 10.1146/annurev.physiol.68.040104.124530. [DOI] [PubMed] [Google Scholar]
- 196.Rumyantsev PP. Interrelations of the proliferation and differentiation processes during cardiac myogenesis and regeneration. Int.Rev.Cytol. 1977;51:186–273. 186–273. [PubMed] [Google Scholar]
- 197.Rumyantsev PP, Borisov A. DNA synthesis in myocytes from different myocardial compartments of young rats in norm, after experimental infarction and in vitro. Biomed.Biochim.Acta. 1987;46:S610–S615. [PubMed] [Google Scholar]
- 198.Rumyantsev PP, Marakjan VO. Reactive synthesis of DNA and mitotiv division in atrial heart muscle cells following ventricle infarction. Experientia. 1968;24:1234–1235. doi: 10.1007/BF02146641. [DOI] [PubMed] [Google Scholar]
- 199.Saavedra HI, Wu L, de Bruin A, Timmers C, Rosol TJ, Weinstein M, Robinson ML, Leone G. Specificity of E2F1, E2F2, and E2F3 in mediating phenotypes induced by loss of Rb. Cell Growth Differ. 2002;13:215–225. [PubMed] [Google Scholar]
- 200.Sano M, Abdellatif M, Oh H, Xie M, Bagella L, Giordano A, Michael LH, DeMayo FJ, Schneider MD. Activation and function of cyclin T-Cdk9 (positive transcription elongation factor-b) in cardiac muscle-cell hypertrophy. Nat.Med. 2002;8:1310–1317. doi: 10.1038/nm778. [DOI] [PubMed] [Google Scholar]
- 201.Schmid G, Pfitzer P. Mitoses and binucleated cells in perinatal human hearts. Virchows Arch.B Cell Pathol.Incl.Mol.Pathol. 1985;48:59–67. doi: 10.1007/BF02890115. [DOI] [PubMed] [Google Scholar]
- 202.Schneider MD, Payne PA, Ueno H, Perryman MB, Roberts R. Dissociated expression of c-myc and a fos-related competence gene during cardiac myogenesis. Mol.Cell Biol. 1986;6:4140–4143. doi: 10.1128/mcb.6.11.4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sen A, Dunnmon P, Henderson SA, Gerard RD, Chien KR. Terminally differentiated neonatal rat myocardial cells proliferate and maintain specific differentiated functions following expression of SV40 large T antigen. J.Biol.Chem. 1988;263:19132–19136. [PubMed] [Google Scholar]
- 204.Sheikh F, Hirst CJ, Jin Y, Bock ME, Fandrich RR, Nickel BE, Doble BW, Kardami E, Cattini PA. Inhibition of TGFbeta signaling potentiates the FGF-2-induced stimulation of cardiomyocyte DNA synthesis. Cardiovasc.Res. 2004;64:516–525. doi: 10.1016/j.cardiores.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 205.Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 206.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 207.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000;19:2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase B promotes organ growth in transgenic mice. Mol.Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc.Natl.Acad.Sci.U.S.A. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Soonpaa MH, Field LJ. Assessment of cardiomyocyte DNA synthesis during hypertrophy in adult mice. Am.J.Physiol. 1994;266:H1439–H1445. doi: 10.1152/ajpheart.1994.266.4.H1439. [DOI] [PubMed] [Google Scholar]
- 211.Soonpaa MH, Field LJ. Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. Am.J. Physiol. 1997;272:H220–H226. doi: 10.1152/ajpheart.1997.272.1.H220. [DOI] [PubMed] [Google Scholar]
- 212.Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ.Res. 1998;83:15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 213.Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ. Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. 1996;271:H2183–H2189. doi: 10.1152/ajpheart.1996.271.5.H2183. [DOI] [PubMed] [Google Scholar]
- 214.Soonpaa MH, Koh GY, Pajak L, Jing S, Wang H, Franklin MT, Kim KK, Field LJ. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J. Clin. Invest. 1997;99:2644–2654. doi: 10.1172/JCI119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 216.Starksen NF, Simpson PC, Bishopric N, Coughlin SR, Lee WM, Escobedo JA, Williams LT. Cardiac myocyte hypertrophy is associated with c-myc protooncogene expression. Proc.Natl.Acad.Sci.U.S.A. 1986;83:8348–8350. doi: 10.1073/pnas.83.21.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Steinhelper ME, Lanson NAJ, Dresdner KP, Delcarpio JB, Wit AL, Claycomb WC, Field LJ. Proliferation in vivo and in culture of differentiated adult atrial cardiomyocytes from transgenic mice. Am.J. Physiol. 1990;259:H1826–H1834. doi: 10.1152/ajpheart.1990.259.6.H1826. [DOI] [PubMed] [Google Scholar]
- 218.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 219.Stocker H, Hafen E. Genetic control of cell size. Curr.Opin.Genet.Dev. 2000;10:529–535. doi: 10.1016/s0959-437x(00)00123-4. [DOI] [PubMed] [Google Scholar]
- 220.Strom DK, Cleveland JL, Chellappan S, Nip J, Hiebert SW. E2F-1 and E2F-3 are functionally distinct in their ability to promote myeloid cell cycle progression and block granulocyte differentiation. Cell Growth Differ. 1998;9:59–69. [PubMed] [Google Scholar]
- 221.Stukenberg PT. Triggering p53 after cytokinesis failure. J.Cell Biol. 2004;165:607–608. doi: 10.1083/jcb.200405089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Subramaniam A, Jones WK, Gulick J, Wert S, Neumann J, Robbins J. Tissue-specific regulation of the α-myosin heavy chain gene promoter in transgenic mice. J.Biol.Chem. 1991;266:24613–24620. [PubMed] [Google Scholar]
- 223.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu.Rev.Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. 797–819. [DOI] [PubMed] [Google Scholar]
- 224.Tamamori-Adachi M, Ito H, Nobori K, Hayashida K, Kawauchi J, Adachi S, Ikeda MA, Kitajima S. Expression of cyclin D1 and CDK4 causes hypertrophic growth of cardiomyocytes in culture: a possible implication for cardiac hypertrophy. Biochem.Biophys.Res.Commun. 2002;296:274–280. doi: 10.1016/s0006-291x(02)00854-9. [DOI] [PubMed] [Google Scholar]
- 225.Tamamori M, Ito H, Hiroe M, Terada Y, Marumo F, Ikeda MA. Essential roles for G1 cyclin-dependent kinase activity in development of cardiomyocyte hypertrophy. Am.J.Physiol. 1998;275:H2036–H2040. doi: 10.1152/ajpheart.1998.275.6.H2036. [DOI] [PubMed] [Google Scholar]
- 226.Tommasi S, Pfeifer GP. In vivo structure of the human cdc2 promoter: release of a p130-E2F-4 complex from sequences immediately upstream of the transcription initiation site coincides with induction of cdc2 expression. Mol.Cell Biol. 1995;15:6901–6913. doi: 10.1128/mcb.15.12.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ.Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 228.Trumpp A, Refaeli Y, Oskarsson T, Gasser S, Murphy M, Martin GR, Bishop JM. c-Myc regulates mammalian body size by controlling cell number but not cell size. Nature. 2001;414:768–773. doi: 10.1038/414768a. [DOI] [PubMed] [Google Scholar]
- 229.Tsai SC, Pasumarthi KB, Pajak L, Franklin M, Patton B, Wang H, Henzel WJ, Stults JT, Field LJ. Simian virus 40 large T antigen binds a novel Bcl-2 homology domain 3-containing proapoptosis protein in the cytoplasm. J.Biol.Chem. 2000;275:3239–3246. doi: 10.1074/jbc.275.5.3239. [DOI] [PubMed] [Google Scholar]
- 230.Tsujita Y, Muraski J, Shiraishi I, Kato T, Kajstura J, Anversa P, Sussman MA. Nuclear targeting of Akt antagonizes aspects of cardiomyocyte hypertrophy. Proc.Natl.Acad.Sci.U.S.A. 2006;103:11946–11951. doi: 10.1073/pnas.0510138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Urbanek K, Quaini F, Tasca G, Torella D, Castaldo C, Nadal-Ginard B, Leri A, Kajstura J, Quaini E, Anversa P. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc.Natl.Acad.Sci.U.S.A. 2003;100:10440–10445. doi: 10.1073/pnas.1832855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ.Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 233.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc.Natl.Acad.Sci.U.S.A. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den OA, Halley D, Young J, Burley M, Jeremiah S, Woodward K, Nahmias J, Fox M, Ekong R, Osborne J, Wolfe J, Povey S, Snell RG, Cheadle JP, Jones AC, Tachataki M, Ravine D, Sampson JR, Reeve MP, Richardson P, Wilmer F, Munro C, Hawkins TL, Sepp T, Ali JB, Ward S, Green AJ, Yates JR, Kwiatkowska J, Henske EP, Short MP, Haines JH, Jozwiak S, Kwiatkowski DJ. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 235.Vara D, Bicknell KA, Coxon CH, Brooks G. Inhibition of E2F Abrogates the Development of Cardiac Myocyte Hypertrophy. J.Biol.Chem. 2003;278:21388–21394. doi: 10.1074/jbc.M212612200. [DOI] [PubMed] [Google Scholar]
- 236.Walsh K, Perlman H. Cell cycle exit upon myogenic differentiation. Curr.Opin.Genet Dev. 1997;7:597–602. doi: 10.1016/s0959-437x(97)80005-6. [DOI] [PubMed] [Google Scholar]
- 237.Wang J, Nadal-Ginard B. Regulation of cyclins and p34CDC2 expression during terminal differentiation of C2C12 myocytes. Biochem.Biophys.Res.Commun. 1995;206:82–88. doi: 10.1006/bbrc.1995.1012. [DOI] [PubMed] [Google Scholar]
- 238.Wang Y, Huang S, Sah VP, Ross J, Jr, Brown JH, Han J, Chien KR. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J.Biol.Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 239.Watson GH. Cardiac rhabdomyomas in tuberous sclerosis. Ann.N.Y.Acad.Sci. 1991;615:50–57. doi: 10.1111/j.1749-6632.1991.tb37747.x. [DOI] [PubMed] [Google Scholar]
- 240.Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20:1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- 241.Woo YJ, Panlilio CM, Cheng RK, Liao GP, Atluri P, Hsu VM, Cohen JE, Chaudhry HW. Therapeutic delivery of cyclin A2 induces myocardial regeneration and enhances cardiac function in ischemic heart failure. Circulation. 2006;114:I206–I213. doi: 10.1161/CIRCULATIONAHA.105.000455. [DOI] [PubMed] [Google Scholar]
- 242.Wu L, Timmers C, Maiti B, Saavedra HI, Sang L, Chong GT, Nuckolls F, Giangrande P, Wright FA, Field SJ, Greenberg ME, Orkin S, Nevins JR, Robinson ML, Leone G. The E2F1–3 transcription factors are essential for cellular proliferation. Nature. 2001;414:457–462. doi: 10.1038/35106593. [DOI] [PubMed] [Google Scholar]
- 243.Wu Z, Woodring PJ, Bhakta KS, Tamura K, Wen F, Feramisco JR, Karin M, Wang JY, Puri PL. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol.Cell Biol. 2000;20:3951–3964. doi: 10.1128/mcb.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Xia Y, Buja LM, Scarpulla RC, McMillin JB. Electrical stimulation of neonatal cardiomyocytes results in the sequential activation of nuclear genes governing mitochondrial proliferation and differentiation. Proc.Natl.Acad.Sci.U.S.A. 1997;94:11399–11404. doi: 10.1073/pnas.94.21.11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Xiao G, Mao S, Baumgarten G, Serrano J, Jordan MC, Roos KP, Fishbein MC, MacLellan WR. Inducible Activation of c-Myc in Adult Myocardium In Vivo Provokes Cardiac Myocyte Hypertrophy and Reactivation of DNA Synthesis. Circ.Res. 2001;89:1122–1129. doi: 10.1161/hh2401.100742. [DOI] [PubMed] [Google Scholar]
- 246.Yamaguchi TP, Harpal K, Henkemeyer M, Rossant J. fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev. 1994;8:3032–3044. doi: 10.1101/gad.8.24.3032. [DOI] [PubMed] [Google Scholar]
- 247.Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson NJ. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell. 1996;85:537–548. doi: 10.1016/s0092-8674(00)81254-4. [DOI] [PubMed] [Google Scholar]
- 248.Yin X, Giap C, Lazo JS, Prochownik EV. Low molecular weight inhibitors of Myc-Max interaction and function. Oncogene. 2003;22:6151–6159. doi: 10.1038/sj.onc.1206641. [DOI] [PubMed] [Google Scholar]
- 249.Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, Fujiwara H, Hirata M, Yamagami T, Nakahata T, Hirabayashi T, Yoneda Y, Tanaka K, Wang WZ, Mori C, Shiota K, Yoshida N, Kishimoto T. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc.Natl.Acad.Sci.U.S.A. 1996;93:407–411. doi: 10.1073/pnas.93.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yoshizumi M, Lee WS, Hsieh CM, Tsai JC, Li J, Perrella MA, Patterson C, Endege WO, Schlegel R, Lee ME. Disappearance of cyclin A correlates with permanent withdrawal of cardiomyocytes from the cell cycle in human and rat hearts. J.Clin.Invest. 1995;95:2275–2280. doi: 10.1172/JCI117918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 252.Zhong W, Mao S, Tobis S, Angelis E, Jordan MC, Roos KP, Fishbein MC, de Alboran IM, MacLellan WR. Hypertrophic growth in cardiac myocytes is mediated by Myc through a Cyclin D2-dependent pathway. EMBO J. 2006;25:3869–3879. doi: 10.1038/sj.emboj.7601252. [DOI] [PMC free article] [PubMed] [Google Scholar]