Abstract
Once-daily regimens of antiretroviral therapy are simpler than other regimens, but whether such regimens are associated with better adherence to treatment is controversial. We performed a meta-analysis of 11 randomized, controlled trials (total number of subjects, 3029), which revealed that the adherence rate was better with once-daily regimens (+2.9%; 95% confidence interval, 1.0%−4.8%; P < .003) than with twice-daily regimens. This modest effect was more pronounced at the time of treatment initiation and for regimens for which all medications were taken once per day.
Poor adherence to antiretroviral therapy increases the risk of incomplete viral suppression, disease progression, and death [1–3]. There has been important progress over the past decade in simplifying dosing frequency and pill burden [4]. Regimens have evolved from those involving administration of >25 pills 3 times per day to those involving 1 pill administered every day. Although some studies have found no difference in adherence rates for once-daily (quaque die in Latin) versus twice-daily (bis in die in Latin) regimens, the statistical power of these studies has generally been low. To synthesize existing data across studies, we conducted a meta-analysis that compared adherence and virologic suppression rates in patients receiving once-daily versus twice-daily regimens in randomized, controlled trials.
Methods
J.-J.P. and E.M.G. conducted searches of the PubMed database and recent HIV science conferences, both independently and in duplicate, to identify open-label, randomized, controlled trials that compared once-daily and twice-daily antiretroviral regimens given to HIV-1–infected subjects. A flow chart of the study is available from the authors. The once-daily regimens could contain twice-daily components, so long as adherence to the once-daily component was reported.
Because self-reported adherence may involve perceptions of perceived adherence efficacy that do not necessarily reflect adherence itself, we limited our review to studies that included pill counts or Medication Event Monitoring System [MEMS] measurements. The primary end point was the mean adherence rate, which was defined as follows: (total number of doses taken/total number of doses prescribed)× 100. The secondary end point was the percentage of subjects with HIV-1 RNA levels <50 copies/mL in the intent-to-treat, missing-equals-failure analysis. This data set was chosen for homogeneity, because it was consistently reported across the trials.
To compute effect sizes when data were incomplete, the following strategy was implemented: (1) contact the corresponding author; (2) estimate the SD on the basis of the sample size, median, and range [5] or on the basis of the sample size and P value; and (3) impute the SD reported in similar studies.
We used the mean weighted difference and 95% CI to assess adherence to treatment and the virologic control effect of once-daily versus twice-daily antiretroviral therapy regimens. A positive value indicated better adherence or virologic control for the once-daily antiretroviral regimen. DerSimonian and Laird random effects models were used to synthesize results across studies. A random effects model is based on the assumption that there is no “true” effect but, rather, a stochastic distribution of effects that produced the empirical values of the studies. These models are indicated if variations in sampling schemes could introduce heterogeneity to the result—in other words, if there is >1 intercept in the solution. The robustness of the overall primary end point result was assessed by exclusion sensitivity analysis.
Heterogeneity was assessed using the Cochran's Q test. This statistic was complemented with I2, which is the percentage of total variation across studies that is due to heterogeneity rather than chance. With consistent study results, the I2 equals 0. Publication bias was assessed using Eggers’ statistical test. Additional analyses were conducted to explore the reasons for heterogeneity in subgroup analyses, including (1) studies that included treatment-naive individuals initiating their first regimens of antiretroviral therapy versus those that included individuals with virologic suppression who entered a treatment-switch trial (prespecified before data collection), and (2) studies of regimens for which all components were administered once per day versus those with at least 1 twice-daily component (post-hoc). Statistical analysis was conducted with MIX software [6], and P values <.05 were considered to be statistically significant.
Results
Eleven studies [7–17], which included a total of 3029 subjects, were included in our meta-analysis. Descriptive data for each trial are provided in table 1. There was no evidence of publication bias (intercept, 0.8; 95% CI, −2.2 to 3.7; P=.58, by Eggers’ test) regarding the primary end point.
Table 1.
Characteristics of studies included in a meta-analysis of once-daily vs. twice-daily antiretroviral therapy regimens.
| Treatment regimen |
All components given once per daya | Duration of follow-up, weeks | Means of assessing adherence | ||||
|---|---|---|---|---|---|---|---|
| Study | Year | Once-daily regimen | Twice-daily regimen | Population or study type | |||
| Benson et al. [7] | 2004 | FTC, D4T or AZT, and an NNRTI or a PI | 3TC, D4T or AZT, and an NNRTI or PI | Switch | No | 48 | Pill count |
| Boyle et al. [8] | 2008 | D4T XR, 3TC, and EFV | NRTIs and a PI or NNRTI | Switch | Yes | 48 | MEMS |
| Eron et al. [9] | 2004 | LPV-RTV and NRTIs | LPV-RTV and NRTIs | Treatment-naive subjects | No | 48 | MEMS |
| Gallant et al. [10] | 2006 | TDF, FTC, and EFV | AZT, 3TC, and EFV | Treatment-naive subjects | Yes | 48 | Pill count |
| Kubota et al. [11] | 2006 | ABC, 3TC, and a third agent | ABC, 3TC, and a third agent | Treatment-naive subjects | No | 12 | Pill count |
| Molina et al. [12] | 2007 | LPV-RTV, TDF, and FTC | LPV-RTV, TDF, and FTC | Treatment-naive subjects | Yes | 96 | MEMS |
| Parienti et al. [13] | 2007 | NVP and NRTIs | NVP and NRTIs | Switch | No | 16 | MEMS |
| Porthsmouth et al. [14] | 2005 | D4T XR, 3TC, and EFV | D4T or AZT, 3TC, and EFV | Switch | Yes | 24 | MEMS |
| Rode et al. [15, 18] | 2008 | LPV-RTV, TDF, and FTC | LPV-RTV, TDF, and FTC | Initiation | Yes | 12 | MEMS |
| Ruane et al. [16] | 2006 | AZT, 3TC, ABC, and EFV | AZT, 3TC, ABC, and EFV | Switch | Yes | 24 | MEMS |
| Sosa et al. [17] | 2005 | ABC, 3TC, and a PI or NNRTI | ABC, 3TC, and a PI or NNRTI | Switch | No | 48 | Pill count |
NOTE. Drugs that were monitored for adherence are shown in boldface font. ABC, abacavir; AZT, zidovudine; D4T, stavudine; EFV, efavirenz; FTC, emtricitabine; LPV, lopinavir; MEMS, Medication Event Monitoring System; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitors; RTV, ritonavir; TDF, tenofovir; 3TC, lamivudine; XR, extended release.
In the once-daily regimen group.
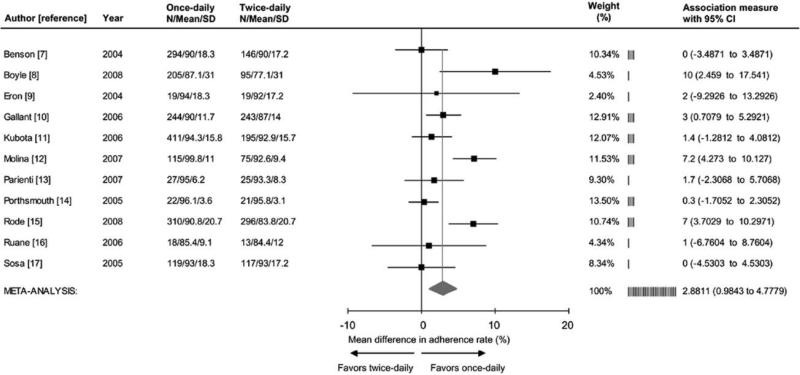
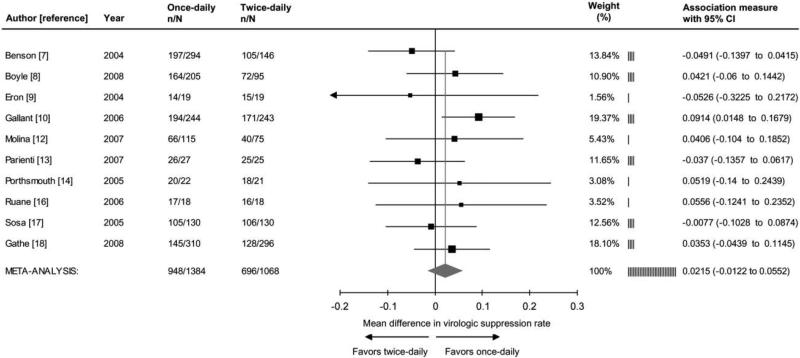
The meta-analysis revealed significantly better adherence (+2.9%; 95% CI, 1.0%−4.8%; P < .003) among recipients of once-daily regimens, compared with recipients of twice-daily regimens (figure 1). Better adherence to once-daily versus twice-daily regimens remained statistically significant in all 11 sensitivity analyses conducted, after we excluded 1 individual study (data not shown). Ten studies [7–10, 12–14, 16–18], which represented 2452 subjects, reported virologic end points. The meta-analysis found no significant difference in the proportion of subjects who achieved HIV RNA levels <50 copies/mL (+2.2%; 95% CI −1.2 to 5.5; P=.21) between recipients of once-daily versus twice-daily regimens (figure 2).
Figure 1.
Forrest plot of the effect of once-daily versus twice-daily antiretroviral regimens on the rate of adherence. Adherence rate was defined as follows: (no. of taken doses/no. prescribed doses)×100. Cochrane Q test for heterogeneity: χ2=29.7; degrees of freedom, 10; P=<.001; I2=66.4%. Test for overall random effect: Z=2.98; P=<.003.
Figure 2.
Forrest plot of the effect of once-daily versus twice-daily antiretroviral regimens on viral suppression. Viral suppression was defined as a plasma RNA HIV level <50 copies/mL in the intent-to-treat analysis, with missing equals failure. In the study by Gathe et al. [18], data represent week 12 results among subjects with Medication Event Monitoring System evaluation (Dr. Rode, personal communication, December 2008). Week 48 results are 77% and 76% for once-daily and twice-daily regimens, respectively. Cochrane Q test for heterogeneity: χ2=8.2; degrees of freedom, 9; P=.43; I2=0.0%. Test for overall random effect: Z=1.25; P=.21. n, No. of subjects with viral suppression; N, total sample size.
Significant quantitative heterogeneity (I2=66.4%; 95% CI, 36.4−82.2) of the adherence effect size was explored in subgroup analyses. Among the 5 trials of treatment-naive subjects [9–12, 15] (1927 patients), use of once-daily regimens significantly improved adherence (+4.4%; 95% CI, 1.8%−7.0%; P < .001) and was associated with better virologic outcome (+5.7%; 95% CI, 0.7%−10.8%; P < .027). In contrast, among the 6 trials [7, 8, 13, 14, 16, 17] that involved treatment-experienced subjects (1102 patients), the adherence effect size was not statistically significant (+1.0%; 95% CI, −0.8 to 2.8; P=.29) and did not correspond to better virologic outcome (−0.7%; 95% CI, −5.2 to 3.8; P=.76).
Six studies [8, 10, 12, 14–16] investigated (1657 patients) a regimen that consisted entirely of medications with once-daily administration. In this subgroup, use of the once-daily regimens were associated with significantly improved adherence (+4.5%; 95% CI, 1.5%−7.5%; P < .004) and better virologic outcome (+5.7%; 95% CI, 1.4%−10.0%; P < .001). In contrast, in the 5 trials [7, 9, 11, 13, 17] (1372 patients) in which regimens with once-daily and twice-daily components were mixed, the adherence effect size was not significant (+0.9%; 95% CI −0.8% to 2.6%; P=.29), and treatment did not correspond with a better virologic outcome (−3.3%; 95% CI, −8.6% to 2.1%; P=.23).
Discussion
Our meta-analysis found that the rate of adherence to once-daily antiretroviral regimens was better (+2.9%) than the rate of adherence to twice-daily regimens. To our knowledge, this is the first attempt to quantify, in a meta-analysis, the effect of dosing schedule on adherence to anti-retroviral therapy. This effect was greater for patients who were initiating treatment than for those receiving stable therapy who were observed in “switch” studies. The smaller effect among recipients of stable treatment may be related to selection bias toward more-highly adherent patients in these studies, because all studies required an undetectable viral load before enrollment. Publication bias (i.e., the tendency for negative or inconclusive results to remain hidden and unpublished) was not likely for the primary end point, because the reporting of similar adherence rates between arms increases the internal validity of efficacy results. Random allocation of the dosing schedule and the use of objective measurements further support the internal validity of the adherence effect size estimate.
Although it was statistically significant, the 2.9% difference in adherence between once-daily and twice-daily regimens was modest. This difference in adherence did not correspond to an overall difference in rates of viral suppression among all studies. However, similar to the primary analysis of adherence, there was a difference in the rates of viral suppression that favored studies involving antiretroviral-naive patients over switch studies. Antiretroviral-naive patients may be more sensitive to differences in adherence because of lower rates of preexisting drug resistance. Furthermore, the association between adherence and viral suppression is likely to be more critical when rates of viral replication are high, such as at the time of initiation of a first antiretroviral regimen. There was also a difference in the rates of viral suppression that favored studies in which all medications were administered as a once-daily regimen. Administration of the entire regimen once per day may have led to better regimen adherence (and not just to the monitored medicine), leading to better virologic outcomes, than in regimens with more complicated dosing schedules.
Improvements in treatment adherence for once-daily versus twice-daily regimens in switch studies were marginal and statistically nonsignificant. However, because the switch studies reviewed here required subjects to have an undetectable viral load, it is unclear whether simplification from twice-daily to once-daily regimens would improve adherence to a greater extent in less-adherent patients. Among individuals with viral suppression, switches in the reverse direction (from once-daily to twice-daily regimens) are sometimes necessary for management of toxicities. These data suggest that a switch from once-daily to twice-daily treatment regimens may not result in adherence problems or loss of virologic efficacy.
There are several limitations to our study. The differences we observed could have been due to the drugs themselves, rather than how often they were taken each day. Most studies had relatively short follow-up periods and recruited patients who were highly adherent to treatment in clinical trial settings. The impact of dosing frequency on adherence and virologic outcome may differ in less-adherent populations found in routine clinical practice. The effect of dosing frequency may also be different with longer follow-up periods, because adherence wanes over time [19, 20]. Finally, because only the adherence rate for the once-daily or twice-daily regimen component was measured, the potential for differential drug exposure, which increases the risk of resistance [21, 22], was not evaluated.
The availability of once-daily combination antiretroviral regimens represents a considerable advancement, which has been welcomed by patients [23]. On the basis of our findings, we conclude that once-daily dosing improved adherence, particularly at treatment initiation and if all of the medications were administered once per day. Furthermore, these effects were compatible with better virologic outcome in selected subgroups. However, physicians should be aware that the objective impact of once-daily versus twice-daily dosing on adherence rates is modest. Because adherence to medication regimens is a complex behavior with multiple factors at play, efforts to improve adherence should not be restricted to prescription of once-daily medications [24, 25]. Other factors, including tolerability, potency, and potential risk of resistance, given the patient's individual adherence pattern, are important considerations in selecting the optimal regimen for each patient.
Acknowledgments
We thank Dr. Andrew K. Cheng (Gilead Sciences) and Dr. Richard A. Rode (Abbott) for providing additional data from their studies.
Financial support. Côte de Nacre University Hospital (research grant to J.-J.P.), National Institute of Alcohol Abuse and Alcoholism (K-24 015287 to E.M.G.) and the National Institutes of Health (career development award AI067063 to D.R.B.).
Footnotes
Potential conflicts of interest. J.-J.P. has received prior grant support from Abbott and Boehringer-Ingelheim. D.R.B. has received prior grant support from Abbott, Bristol-Myers Squibb, and Gilead Sciences. R.V. and E.M.G.: no conflicts.
References
- 1.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–3. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 3.Wood E, Hogg RS, Yip B, Harrigan PR, O'Shaughnessy MV, Montaner JS. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350 × 109 cells/L. Ann Intern Med. 2003;139:810–6. doi: 10.7326/0003-4819-139-10-200311180-00008. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett JA, Fath MJ, Demasi R, et al. An updated systematic overview of triple combination therapy in antiretroviral-naive HIV-infected adults. AIDS. 2006;20:2051–64. doi: 10.1097/01.aids.0000247578.08449.ff. [DOI] [PubMed] [Google Scholar]
- 5.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KG. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benson CA, van der Horst C, Lamarca A, et al. A randomized study of emtricitabine and lamivudine in stably suppressed patients with HIV. AIDS. 2004;18:2269–76. doi: 10.1097/00002030-200411190-00007. [DOI] [PubMed] [Google Scholar]
- 8.Boyle BA, Jayaweera D, Witt MD, Grimm K, Maa JF, Seekins DW. Randomization to once-daily stavudine extended release/lamivudine/efavirenz versus a more frequent regimen improves adherence while maintaining viral suppression. HIV Clin Trials. 2008;9:164–76. doi: 10.1310/hct0903-164. [DOI] [PubMed] [Google Scholar]
- 9.Eron JJ, Feinberg J, Kessler HA, et al. Once-daily versus twice-daily lopinavir/ritonavir in antiretroviral-naive HIV-positive patients: a 48-week randomized clinical trial. J Infect Dis. 2004;189:265–72. doi: 10.1086/380799. [DOI] [PubMed] [Google Scholar]
- 10.Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–60. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 11.Kubota M, Cohen C, Scribner A, et al. Short-term safety and tolerability of ABC/3TC administered once-daily (QD) compared with the separate components administered twice-daily (BID): results from ESS101822 (ALOHA) [abstract H-1904].. Program and abstracts of the 46th International Conference on Antimicrobial Agents and Chemotherapy (San Francisco).; Washington, DC: American Society for Microbiology. 2006. p. 298. [Google Scholar]
- 12.Molina JM, Podsadecki TJ, Johnson MA, et al. A lopinavir/ritonavir-based once-daily regimen results in better compliance and is non-inferior to a twice-daily regimen through 96 weeks. AIDS Res Hum Retroviruses. 2007;23:1505–14. doi: 10.1089/aid.2007.0107. [DOI] [PubMed] [Google Scholar]
- 13.Parienti JJ, Massari V, Reliquet V, et al. Effect of twice-daily nevirapine on adherence in HIV-1-infected patients: a randomized controlled study. AIDS. 2007;21:2217–22. doi: 10.1097/QAD.0b013e3282eff388. [DOI] [PubMed] [Google Scholar]
- 14.Portsmouth SD, Osorio J, McCormick K, Gazzard BG, Moyle GJ. Better maintained adherence on switching from twice-daily to once-daily therapy for HIV: a 24-week randomized trial of treatment simplification using stavudine prolonged-release capsules. HIV Med. 2005;6:185–90. doi: 10.1111/j.1468-1293.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 15.Rode RA, Marsh T, Naylor C, Cohen D, Podsadecki TJ. Adherence with lopinavir/ritonavir (LPV/r) tablet and soft-gel capsule (SGC)–based antiretroviral regimens and predictors of early treatment compliance [abstract P-170].. Program and abstracts of the 9th Internation Congress on Drug Therapy in HIV Infection (Glasgow).; 2008. Available at: http://www.jiasociety.org/content/11/S1/P170. [Google Scholar]
- 16.Ruane P, Lang J, DeJesus E, et al. Pilot study of once-daily simplification therapy with abacavir/lamivudine/zidovudine and efavirenz for treatment of HIV-1 infection. HIV Clin Trials. 2006;7:229–36. doi: 10.1310/hct0705-229. [DOI] [PubMed] [Google Scholar]
- 17.Sosa N, Hill-Zabala C, Dejesus E, et al. Abacavir and lamivudine fixed-dose combination tablet once daily compared with abacavir and lamivudine twice daily in HIV-infected patients over 48 weeks ( ESS30008, SEAL). J Acquir Immune Defic Syndr. 2005;40:422–7. doi: 10.1097/01.qai.0000184859.24071.bd. [DOI] [PubMed] [Google Scholar]
- 18.Gathe J, de Silva BA, Loufty M, et al. Study M05−730 primary efficacy results at week 48: phase 3, randomized, open-label study of lopinavir/ritonavir (LPV/r) tablets once-daily (QD) versus twice-daily (BID), co-administered with tenofovir DF (TDF) + emtricitabine (FTC) in antiretroviral-naive (ARV) HIV-1 infected subjects [abstract 775].. Program and abstracts of the the 15th Conference on Retroviruses and Opportunistic Infections (Boston).; Alexandria, VA: Foundation for Retrovirology and Human Health. 2008. [Google Scholar]
- 19.Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus–infected persons in clinical trials. Clin Infect Dis. 2002;34:1115–21. doi: 10.1086/339074. [DOI] [PubMed] [Google Scholar]
- 20.Gardner EM, Burman WJ, Maravi ME, Davidson AJ. Durability of adherence to antiretroviral therapy on initial and subsequent regimens. AIDS Patient Care STDS. 2006;20:628–36. doi: 10.1089/apc.2006.20.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parienti JJ, Massari V, Descamps D, et al. Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis. 2004;38:1311–6. doi: 10.1086/383572. [DOI] [PubMed] [Google Scholar]
- 22.Gardner EM, Sharma S, Peng G, et al. Differential adherence to combination antiretroviral therapy is associated with virological failure with resistance. AIDS. 2008;22:75–82. doi: 10.1097/QAD.0b013e3282f366ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone VE, Jordan J, Tolson J, Miller R, Pilon T. Perspectives on adherence and simplicity for HIV-infected patients on antiretroviral therapy: self-report of the relative importance of multiple attributes of highly active antiretroviral therapy (HAART) regimens in predicting adherence. J Acquir Immune Defic Syndr. 2004;36:808–16. doi: 10.1097/00126334-200407010-00007. [DOI] [PubMed] [Google Scholar]
- 24.Petersen ML, Wang Y, van der Laan MJ, Guzman D, Riley E, Bangsberg DR. Pillbox organizers are associated with improved adherence to HIV antiretroviral therapy and viral suppression: a marginal structural model analysis. Clin Infect Dis. 2007;45:908–15. doi: 10.1086/521250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simoni JM, Pearson CM, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load: a meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S23–35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]