Abstract
Although Glycogen (Glyc) is the main carbohydrate storage component, the role of Glyc in the brain during prolonged wakefulness is not clear. The aim of this study was to determine brain Glyc concentration ([ ]) and turnover time (τ) in euglycemic conscious and undisturbed rats, compared to rats maintained awake for 5 h.
To measure the metabolism of [1-13C]-labeled Glc into Glyc, 23 rats received a [1-13C]-labeled Glc solution as drink (10% weight per volume in tap water) ad libitum as their sole source of exogenous carbon for a “labeling period” of either 5 (n=13), 24 (n=5) or 48 h (n=5). Six of the rats labeled for 5 h were continuously maintained awake by acoustic, tactile and olfactory stimuli during the labeling period, which resulted in slightly elevated corticosterone levels. Brain [Glyc] measured biochemically after focused microwave fixation in the rats maintained awake (3.9±0.2 μmol/g, n=6) was not significantly different from that of the control group (4.0±0.1 μmol/g, n=7; t-test, P>0.5). To account for potential variations in plasma Glc isotopic enrichment (IE), Glyc IE was normalized by N-acetyl-aspartate (NAA) IE. A simple mathematical model was developed to derive brain Glyc turnover time as 5.3 h with a fit error of 3.2 h and NAA turnover time as 15.6 h with a fit error of 6.5 h, in the control rats. A faster τGlyc (2.9 h with a fit error of 1.2 h) was estimated in the rats maintained awake for 5 h.
In conclusion, 5 h of prolonged wakefulness mainly activates glycogen metabolism, but has minimal effect on brain [Glyc].
Keywords: brain metabolism, modeling, nuclear magnetic resonance
INTRODUCTION
Glycogen (Glyc) is the main carbohydrate storage component in the body and acts as a depot into which glucose (Glc) can be stored and rapidly released upon demand (Rao et al. 2006). Brain Glyc can be an emergency fuel reservoir during acute insulin-induced hypoglycemia (Choi et al. 2003, Gruetter 2003, Morgenthaler et al. 2006) or during hypoxia-ischemia (Allen et al. 2005, Gruetter 2003, Nordstrom & Siesjo 1978, Rossi et al. 2007). Moreover, lines of evidence indicate that brain Glyc can be tapped for fuel without overt or prolonged energy deficits (Gibbs et al. 2006, Gibbs et al. 2008, Gibbs et al. 2007). The analysis of the utilization and metabolic fate of brain Glyc will help understand its functional role in brain energetics (Dienel et al. 2007).
In the adult brain, Glyc is found mainly in astrocytes. Under resting conditions, brain Glyc level is stable and its turnover is slow (Choi et al. 2003, Watanabe & Passonneau 1973). It has been suggested that neurons, unlike astrocytes, have no energy reserves and may rely on astrocytes for the supply of energy during acute changes in energy demands (Pellerin & Magistretti 1994). It has been proposed that the abrupt energy demand for clearance of glutamate from the synaptic cleft and its conversion to glutamine create a stimulation of astrocytic glucose utilization (Dienel & Hertz 2005, Pellerin & Magistretti 1994). Moreover, it has been shown that glycogenolysis can increase substantially during brain activation (Cruz & Dienel 2002, Dienel & Cruz 2003, Dienel & Cruz 2004, Swanson 1992, Swanson et al. 1992). Glycogenolysis occurs in retina of the intact honeybee during light stimulation (Tsacopoulos & Evequoz 1980), in the chick brain during early memory consolidation (Gibbs et al. 2006) and in rat somato-sensory cortex in vivo during vibrissae stimulation (Swanson 1992, Swanson et al. 1992). On the other hand, it has been suggested that glial Glyc tends to accumulate during conditions of depressed neuronal activity (Cruz & Dienel 2002).
Increased glucose metabolism in astrocytes can be achieved by increasing glucose uptake through glucose transporters (GLUTs) or by glycogenolysis, and both processes are affected by adrenergic receptor stimulation (Sorg & Magistretti 1991, Subbarao & Hertz 1990). For example, it has been shown that adrenergic receptors, as well as Glyc, play an important role in memory formation in the chick (Hutchinson et al. 2008). Glycogenolysis in cultured astrocytes and brain slices assayed in vitro under normoglycemic conditions is initiated by many signals arising from neuronal activity, including the wide-spread locus coeruleus noradrenergic arousal-stress-response systems (Hertz et al. 2007, Magistretti et al. 1981). However, data obtained in primary cultures of astrocytes indicate that noradrenaline, in addition to its rapid (within minutes) glycogenolytic effects (Subbarao & Hertz 1990), trigger Glyc resynthesis beginning 60±90 min after application and reaching a maximum after 9 h (Sorg & Magistretti 1992). Similarly, sleep deprivation has been reported to elicit complex responses in Glyc synthesis and breakdown (Gip et al. 2002, Kong et al. 2002). It has been suggested that this may reflect an intricate and delicate balance between the ratio and rate of Glyc synthesis and degradation, influenced by stress, neuronal activity, and other factors (Gip et al. 2004). In mice cerebral cortex, an increase of protein targeting to glycogen mRNA and the decrease of both Glyc synthase and Glyc phosphorylase have been observed after 6 h of enforced waking (Petit et al. 2002). The temporal relationships between Glyc synthesis and degradation during activation are difficult to measure, but prevention of the decrease in CMRO2:CMRglc ratio during activation by propranolol, a β-adrenergic blocker, supports a role for Glyc turnover during brain activation (Schmalbruch et al. 2002). Recently, inhibition of Glyc phosphorylase during brief sensory stimulation has implied a role for Glyc in brain activation (Dienel et al. 2007). The increased Glyc turnover during activation complicates interpretation of standard approaches that assess concentration ([ ]) and labeling changes to evaluate roles or utilization of Glyc under various conditions (Dienel et al. 2007). Finally, a restricted set of neurotransmitters potentiate glycogenolysis (Magistretti 2006, Magistretti et al. 1981). They include norepinephrine, serotonin, and histamine that are released maximally during waking (Benington & Heller 1995).
In contrast to radiotracer studies, NMR studies to date have not detected activation-induced turnover and glycogenolysis (unpublished results). As in vivo NMR of glycogen requires the infusion of 13C-labeled Glc at high IE and thus necessitates anesthesia, we sought to use 13C isotopes, but applied to the awake rat brain. The goal of this study was to measure brain [Glyc] and turnover (directly) in euglycemic rats maintained awake for 5 h.
METHODS
Groups of animal studied
Four groups of rats (Sprague-Dawley rats, Charles River, France) were studied (n = 23; mean weight ± SEM: 247 ± 8 g). The first two groups of rats were labeled during 5 h (see below) and the effects of prolonged wakefulness on Glyc were studied. These groups consisted in the rats maintained awake during the labeling period (n = 6) and the corresponding control, i.e. undisturbed group (n = 7). With the two other groups of undisturbed rats, the ratio of Glyc isotopic enrichment (IE) over N-acetyl- aspartate (NAA) IE over time was modeled. The rats were therefore labeled for a longer period of time, either for 24 h (n = 5) or for 48 h (n = 5).
The study was performed in accordance with the local and federal guidelines and was approved by the local ethics committee.
Brain Glyc labeling
Rats were fasted overnight with free access to water before studies were performed. The following day the only nutrient they received was a 10% weight per volume [1-13C]-labeled Glc solution in tap water ad libitum for about 5 h (n = 13), 24 h (n = 5) or 48 h (n = 5). The amount of 13C-labeled solution ingested by each rat was estimated by subtracting the amount of liquid left in the bottle at the end of the experiment to the amount that had been proposed to each rat. All 23 rats ingested a significant amount (at least 1.9 g) of [1-13C]-labeled Glc solution during the labeling period. All of the rats labeled for 5h (n = 13) and most of the rats labeled for 24 h (n = 3) received a 99% 13C-enriched solution. The other rats labeled during 24 h (n = 2) and the rats labeled during 48 h (n = 5) received a 50% 13C-enriched solution as labeling solution, to minimize the cost of the experiment.
To avoid brain activation in the control group of rats, they were labeled in a quiet and familiar environment in a 12:12 h light-dark cycle (see under).
Arousal paradigm
The animals were kept at ambient temperature (~22°C ) under a 12:12 h light-dark cycle and adapted to these conditions for at least 5 days prior to the experiment. On the experimental day at 7:00 (light on), all rats (n = 13) received an identified amount of labeled Glc containing solution (see above). To minimize variability due to Glyc synthase and Glyc phosphorylase circadian variations (Petit et al. 2002), rat arousal always started at the same time. The rats maintained awake (n = 6) were moved into a separate room for about 5 h. They were constantly observed and whenever they exhibited signs of drowsiness or behavioral signs of falling asleep, they were aroused by acoustic stimuli, by tactile stimuli, by introducing objects into the cage, or by olfactory stimuli (such as being introduced to another cage with smells from other rats). These stimulations initially occurred approximately every half hour and were progressively increased up to 15 minute intervals.
Tissue analysis
At the end of each experiment, rats were anesthetized using isoflurane (isoflurane, Halocarbon laboratories, 5% for induction and 1.5% for maintenance while withdrawing blood and inserting the rat into the microwave) in oxygen (O2) gas. Blood was collected by tail bleed and immediately centrifuged to obtain plasma for subsequent analysis of [Glc] and [corticosterone] (see below). Then, the rats were sacrificed using a focused microwave fixation device irradiating the brain at 4 kW for 1.4–1.6 s (Gerling Applied Engineering, Inc., Modesto, CA, USA) as in previous studies (Morgenthaler et al. 2006, Lei et al. 2007, Morgenthaler et al. 2008). This procedure inactivates most brain enzymes before extraction or digestion, thereby minimizing possible in vitro Glyc loss (Kong et al. 2002). The anterior parts of the brain (excluding cerebellum) were dissected, and immediately placed into liquid nitrogen and manually reduced to powder with a pestle and mortar. Brain powder was stored at −80°C until further processing.
Glyc assay
Biochemical measurement of brain Glyc was performed on brain extracts as previously described (Cruz & Dienel 2002, Morgenthaler et al. 2006, Morgenthaler et al. 2008).
In vitro 1H-NMR: Glc IE, Glyc IE and NAA IE measurement
Brain Glc IE, Glyc IE and NAA IE were measured in vitro by 1H NMR as previously described (Morgenthaler et al. 2008), using a Bruker Avance-DRX 600 (14.1 T, 600 MHz) spectrometer (Bruker BioSpin SA, Fällanden, Switzerland).
Brain Glyc IE was calculated according to the following equation (1) after having processed the brain extracts with the Glyc assay so that the Glyc molecule was digested into Glc molecular units (Lei et al. 2007, Morgenthaler et al. 2008).
| (1) |
Total Glc (TotalGlc) represents brain Glc (BrainGlc) plus digested brain Glyc, i.e. measurement performed in samples subjected to amyloglucosidase digestion. Total Glc and brain Glc IE were obtained by high field 1H-spectroscopy, and brain [Glc] and [Glyc] were obtained by biochemical measurement.
Plasma analysis
Plasma [Glc] was determined using the same Analox instrument as above. Plasma [corticosterone] was measured by immunoassay (Correlate-EIA, corticosterone, Assay Designs, Ann Arbour, Michigan, USA).
Model of 13C incorporation into Glyc and NAA after [1-13C]-Glc ingestion
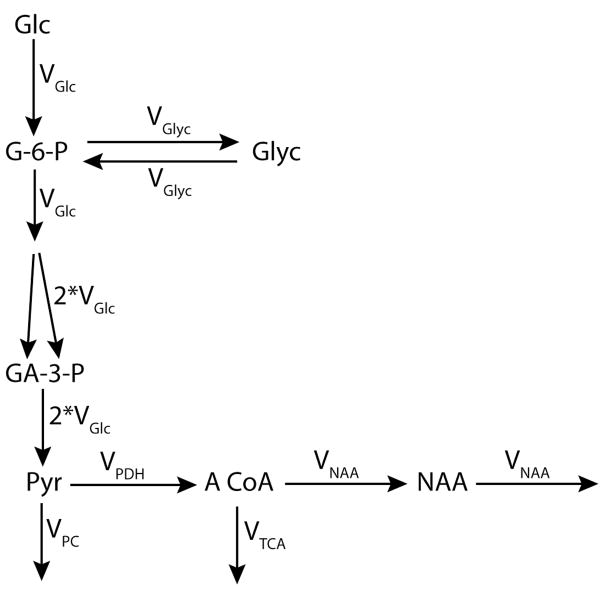
The chemical pathways of Glc that are involved in the 13C label incorporation into Glyc and NAA after [1-13C]-Glc ingestion are in figure 1. In this model, we only consider the chemical pools with a high concentration (NAA, Glyc) and the pools of the network localized at biochemical branch points (Fig. 1). The following mono-exponential equation (2) describes the IE of Glyc over time, where the turnover time (τ) of Glyc, τGlyc, is defined by Glyc /VGlyc (see appendix 1A):
| (2) |
Figure 1.
Chemical pathways used for Glc metabolism modeling. The mathematical model considers metabolic steady-state conditions and is simplified in order to consider only the chemical pools with high concentration, such as NAA and Glyc, as well as the pools located at chemical branch points. The carbon chain, composed of 6 carbons, is split into 2 chains of three carbons at the level of F-1,6-bisP resulting in the production of 2 molecules of GA-3-P. Therefore, the total flux of molecules (in micromol/g/min) is doubled. Subsequently to a labeling procedure with [1-13C]-labeled Glc, only one carbon in the F-1,6-bisP molecule is labeled, and only one of the two produced GA-3-P is labeled. Therefore, a dilution of the labeling by a factor of 2 is observed after F-1,6-bisP. Thus, the labeling flux remains the same as the one entering the pool of F-1,6-bisP (i.e. VGlc). Glc, Glyc, G-6-P, F-1,6-bisP, GA-3-P, Pyr, TCA, A CoA and NAA stand for glucose, glycogen, glucose-6-phosphate, fructose 1,6-bisphosphate, glyceraldehyde-3-phosphate, pyruvate, tricarboxylic acid cycle, Acetyl-CoA, and N-acetyl-aspartate, respectively. VGlc represents the rate of Glc metabolism via glycolysis and VGlyc the exchange rate with the Glyc molecule. VPDH represents the flux of pyruvate into acetyl-CoA catalysed by pyruvate dehydrogenase (PDH), and VPC the rest of the efflux from pyruvate to oxaloacetate thank to the pyruvate carboxylase (PC). The TCA cycle flux is represented by VTCA.
As VGlyc ≪ VGlc (Choi & Gruetter 2003, Watanabe & Passonneau 1973), there is a negligible influence of Glyc labeling on the labeling equations of pyruvate (Pyr) (see appendix 1B). Accordingly, this implies that the label incorporation of NAA is largely unaffected by glycogen metabolism, regardless of the specific NAA synthesis rate. Thus, we also obtain a mono-exponential time course for NAA IE (equation 3, where τNAA is defined by NAA/VNAA):
| (3) |
The factor takes into account the fact that the 6-carbon glucose is split into two 3- carbon units of which only one carbon is labeled. This labeling dilution implies that the maximum IE reachable for NAA is 50% of the IE of the infused Glc solution. And, the ratio of Glyc IE over NAA IE becomes (equation 4) by dividing equation 2 by 3:
| (4) |
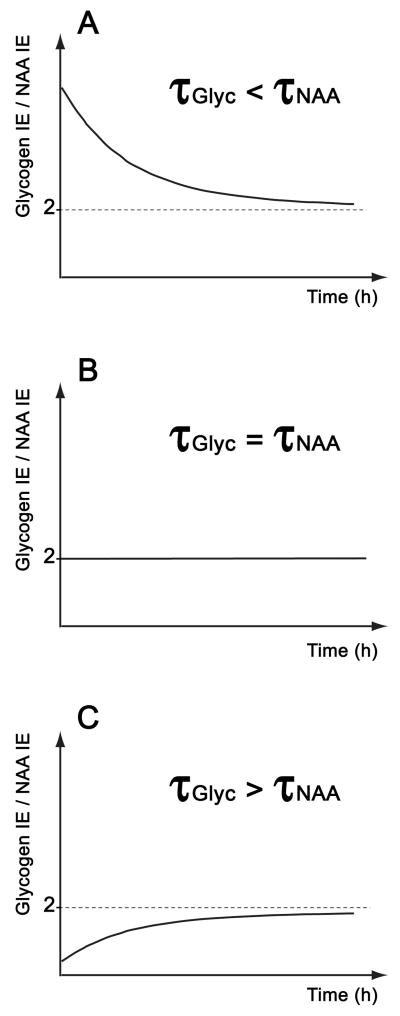
Finally, plotting Glyc IE/NAA IE over time suggests that there might be 3 different curves depending on τGlyc and τNAA times (Fig. 2).
Figure 2.
The 3 different curves that could be obtained by plotting Glyc IE/NAA IE over time using equation 4. These curves depend on the values of τGlyc and τNAA. The 3 different case figures are shown in A) if τGlyc < τNA, in B) If τGlyc = τNAA or in C) if τ Glyc > τNAA.
RESULTS
In order to measure brain [Glyc] and turnover in euglycemic rats after several hours of prolonged wakefulness, conscious rats were ingesting a [1-13C]-Glc solution (10% weight per volume) ad libitum. There was no significant difference in the amount of labeled Glc solution ingested by the rats maintained awake (mean Glc ingested ± SEM: 3.1 ± 0.4 g, n = 6) or the control rats (3.4 ± 0.4 g, n = 7; two-tailed t-test, P = 0.59).
Maintaining the rats awake for 5 h resulted in an increase in the plasma corticosterone levels ([mean] ± SEM: 97 ± 9 ng/mL, n = 6) compared to the control group (66 ± 21 ng/mL, n = 7) of rats (Table 1). This increase in corticosterone levels was not significant (two-tailed t-test, P = 0.21) due to one particular rat in the control group who had an unusually high corticosterone level of 160.3 ng/mL.
Table 1.
Effects of 5 h of prolonged wakefulness on rat plasma corticosterone levels and brain [Glyc]. Mean ± SEM are shown.
| Control rats (n=7) | Rats maintained awake (n=6) | |
|---|---|---|
| Plasma [corticosterone] (ng/mL) | 66 ± 21 | 97 ± 9 |
| Brain [Glyc] (μmol/g) | 4.0 ± 0.1 | 3.9 ± 0.1 |
Brain [Glyc] (Table 1) of the rats maintained awake for ~ 5 h (mean ± SEM: 3.9 ± 0.2 μmol/g, n = 6) was not significantly different from that of the control group (4.0 ± 0.1 μmol/g, n = 7; two-tailed t-test, P = 0.65).
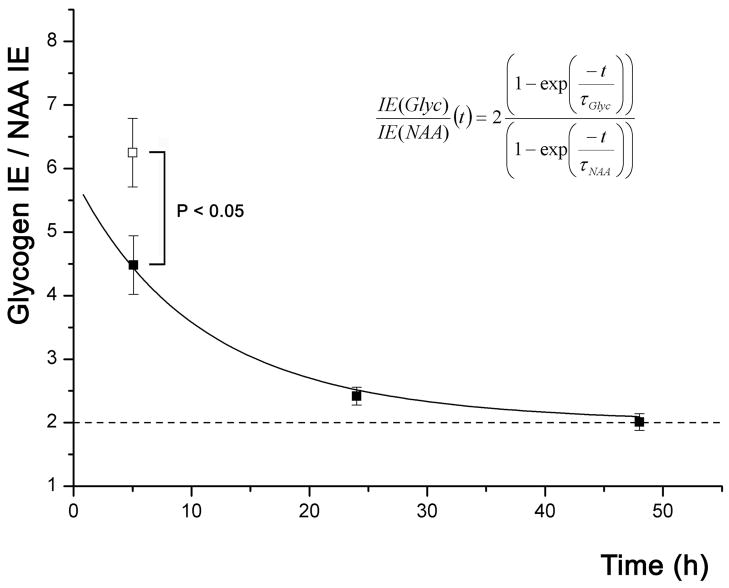
In order to evaluate changes in Glyc turnover due to prolonged wakefulness, NAA IE was used as an internal reference to minimize the impact of potential variations in the enrichment of plasma glucose (precursor). Figure 3 shows significant increase of Glyc IE/NAA IE in the “group of rats maintained awake” (mean ± SEM: 6.3 ± 0.5, n = 6) as compared to the control group of rats (4.5 ± 0.5, n = 7; two-tailed t-test, P = 0.03) after ~ 5 h of 13C-labeled Glc ingestion. The decrease of Glyc IE/NAA IE over time in control rats (Fig. 3) implied that the τGlyc was smaller than the τNAA, as shown from the case simulation in figure 2. As we show in appendix 2 (equation A17), the value of at time close to 0 provides an estimate of . At time t close to zero, the ratio of Glyc IE/NAA IE has to be higher than the value of the control rats labeled for 5 h (Fig. 3), i.e. 4.5, because is a decreasing function of time (case figure A in figure 2). This means that and consequently that τGlyc < τNAA. 44% of τNAA Furthermore, fitting the time course of using equation 4 allowed to estimate brain τGlyc and τNAA as 5.3 h with a fit error of 3.2 h and 15.6 h with a fit error of 6.5 h, respectively (Fig. 3). Finally, a faster τGlyc of 2.9 h (with a fit error of 1.2 h) was calculated in the rats maintained awake, assuming that τNAA was not altered by the arousal paradigm. This corresponds to an increase in Glyc turnover and it was calculated by isolating τGlyc in equation 4 using the mean measured ratio of (i. e. 6.3 ± 1.0 for the rats maintained awake) as well as the mean of the measurement time (4.5 h). It is worth noting that there was no significant difference in brain Glc IE of the control rats (mean ± SEM: 78 ± 7%, n = 7) as compared to the rats maintained awake for 5 h (83 ± 3%, n = 6; two-tailed t-test, P = 0.60).
Figure 3.
Graph representing Glyc IE/NAA IE over time. Shown are the mean values ± SEM for each group of rats (black squares: control rats, white square: rats maintained awake for 5 h). The ratio of brain Glyc IE over NAA IE is significantly increased (P<0.05) in the brain of the rats maintained awake for 5 h as compared to the corresponding control group (5 h labeled) of rats. Equation 4 is fitted to the data obtained from each individual control - undisturbed - rats. This plot enables to calculate a τ of 5.3 h with a fit error of 3.2 h and 15.6 h with a fit error of 6.5 h for Glyc and NAA, respectively.
DISCUSSION
The present study shows that maintaining rats awake for 5 h mainly activates Glyc turnover, but does not impact brain [Glyc] substantially.
Our arousal paradigm was not very stressful. The rise in plasma corticosterone levels was lower than in other studies where rats were kept awake by gentle handling for a similar period of time (Gip et al. 2004). Moreover, no significant increase in either brain Glc (Gip et al. 2004) or plasma Glc levels (Dienel et al. 2007) was observed in our study (not shown). Glucocorticoids, such as corticosterone, are adrenal steroid hormones released systematically in response to stressors (Tobler et al. 1983). It has been shown that glucorcorticoids themselves seem to affect regional brain Glyc stores (Gip et al. 2004). In cultured astrocytes, glucocorticoids decrease Glyc stores (Allaman et al. 2004, Tombaugh et al. 1992). Similarly, adrenalectomy can either increase brain Glyc levels (Passonneau et al. 1971) or have no effect in the whole brain (Goldberg & O'Toole 1969) or cortex (Plaschke et al. 1996).
The present study did not reveal any significant decrease in brain [Glyc] among the rats that were maintained awake for ~ 5 h as compared to the control rats. It has been suggested that promotion of glycogenolysis during waking might provide a continual supplementary source of glucose to prevent transient impairment of astrocytic maintainance of the cellular milieu in the nervous system during waking behavior, thereby optimizing the capacity of the nervous system to respond to stimuli (Benington & Heller 1995). Significant decrease of 11% (Dienel et al. 2002, Madsen et al. 1999) to 22% (Dienel et al. 2002, Madsen et al. 1999) in [Glyc] after stimulation of sensory, motor and whisker-to-barrel pathways has been reported. In a more recent study, Dienel et al. (2007) observed a concentration decrease of 14% during a mixed-modal generalized sensory, acoustic and visual stimulation. Our study is consistent with a milder stimulus precluding changes in [Glyc] < ~8% over 4.5 hours (corresponding to a 95% confidence interval) which is in agreement with another study using a similar stimulation paradigm (Kong et al. 2002).
The calculated τNAA of 15.6 ± 6.5 h and τGlyc of 5.3 ± 3.2 h were comparable to previous reports (Choi & Gruetter 2003, Choi & Gruetter 2004, Moreno et al. 2001, Oz et al. 2006, Xin et al. 2007). A similar τNAA has been reported in anesthetized rats (Choi & Gruetter 2004) and in human (Moreno et al. 2001). A slightly longer τGlyc of about 10 h has been reported in anesthetized rats (Choi & Gruetter 2003). Moreover, we have previously shown that the slower turnover time of limit dextrin (Watanabe & Passonneau 1973) is sufficiently fast to lead to near-complete Glyc turnover in 24 h (Morgenthaler et al. 2008). The faster Glyc turnover in our control group of rats is consistent with τGlyc being affected by stimulation of the adrenergic system, largely suppressed by anesthesia (Dienel et al. 2007). However, it is of interest to note that in awake humans, τGlyc is about 1 day (Oz et al. 2006). As humans are well-known to generally have a slower brain energy metabolism than awake rats, this probably reflects species difference.
Our results suggest that prolonged wakefulness increases Glyc turnover. Although Glyc synthase and Glyc phosphorylase have opposite effects on Glyc content, a number of studies have shown parallel variations of these enzymes relative to changes in Glyc levels (David et al. 1990, Melendez et al. 1999, Mulmed et al. 1979, Petit et al. 2002). Moreover, a number of neurotransmitters that potentiate glycogenolysis, including norepinephrine, serotonin, and histamine are released maximally during waking (Benington & Heller 1995). Increases in Glyc turnover have been observed after shorter, but stronger stimulation paradigm (Dienel et al. 2007). It has been postulated that high Glyc turnover during sensory stimulation could arise if local neuronal activity triggers pulsatile Glyc degradation and re-synthesis, particularly in astrocytic fine processes that surround synaptic structures, as well as their soma and endfeet (Dienel et al. 2007). The increased Glyc turnover does not preclude small transient fluctuations in [Glyc], as such transient fluctuations in [Glyc] might result in increased label incorporation.
In the aforementioned derivation of about a twofold reduced τGlyc with arousal, we assumed that τNAA was not affected. While an increase in τNAA (reflecting reduced NAA metabolism) is rather unlikely in situations of increased brain metabolism, the effects of a potential decrease in τNAA were investigated. In appendix 3 equation A22, we show that the measurement of (at ~ 5 h in the control group of rats) can be used to estimate a minimum τNAA of 11.7 h. Replacing τNAA of 15.6 h with any value between 15.6 h and 11.7 h in equation 4 results in a τGlyc between 0 h and 2.9 h, thus shorter than the value for τNAA = 15.6 h. Therefore, a calculated value of τGlyc in case of a shorter τNAA would anyway be smaller than the value found with an assumption of unchanged τNAA. Subsequently, the difference in τGlyc between control and aroused rats would be even bigger than reported.
In conclusion, we show that a short period of prolonged wakefulness induced by mild sensory-motor stimulation results in an increase in the turnover of brain Glyc without substantially affecting its concentration.
Acknowledgments
We are grateful to Kai Uffmann for many helpful discussions and to Elsy Bays for her technical assistance. This work was supported by the “Centre d’Imagerie Biomédicale” (CIBM) of the University of Lausanne (UNIL), the Federal Institute of Technology in Lausanne (EPFL), the University of Geneva (UniGe), the “Centre Hospitalier Universitaire Vaudois” (CHUV) and the “Hôpitaux Universitaires Genevois” (HUG); and by the Leenards and the Jeantet Foundations; and by NIH grant R01NS042005.
Abbreviations
- F-1
6-bisP, fructose 1,6-bisphosphate
- GA-3-P
glyceraldehyde-3-phosphate
- Glc
glucose
- Glyc
glycogen
- IE
isotopic enrichment
- NAA
N-acetyl- aspartate
- SEM
standard error of mean
- τ
turnover time
APPENDIX
1A Enrichment of Glyc following exogenous administration of [1-13C]-labeled Glc
To measure the IE enrichments of Glyc, we consider a standard step function as input for the [Glc], as well as a negligible amount of label at t = 0 (13C natural abundance being ~ 1.1%). Moreover, we model a situation of steady-state, i.e. absolute concentrations remain constant over time. For example, no change in [Glyc] implies equal Glyc synthesis and degradation fluxes (represented by VGlyc in Fig. 1). Since the concentration of glucose-6-phosphate (G-6-P) is low compared to Glyc, the labeling equation of Glyc corresponds to the labeling equation of a single pool system, in which the precursor is glucose and the product is Glyc. The rate of label incorporation into G-6-Pis given by:
| (A1) |
Where the names without 13 represent the total amount of molecules (labeled + unlabeled). From this we can isolate the IE of G-6-P:
| (A2) |
For Glyc, the labeling equation is the following:
| (A3) |
Inserting equation A2 into equation A3, and rearranging the terms yields to equation A4:
| (A4) |
Assuming that the temporal change of the isotopic enrichment of pools with a much smaller concentration can be neglected compared with pools with higher concentrations (Uffmann & Gruetter 2007).
| (A5) |
Equation A4 is simplified to:
| (A6) |
Equation A6 shows that the effective labeling flux of Glyc is a composite flux of VGlc and VGlyc. Nevertheless, glycolysis flux VGlc is typically much faster than Glyc synthesis flux VGlyc (VGlyc≪VGlc) (Choi & Gruetter 2003, Watanabe & Passonneau 1973). Consequently, equation A6 is simplified to:
| (A7) |
Thus, the labeling equation of Glyc, solution of equation (A7), is:
| (A8) |
where and .
1B Enrichment of NAA following injection of [1-13C]-labeled Glc
The carbon chain, composed of 6 carbons, is split into 2 chains of three carbons at the level of fructose 1,6-bisphosphate (F-1,6-bisP) resulting in the production of 2 molecules of glyceraldehydes 3-phosphate (GA-3-P). Thus, one F-1,6-bisP molecule produces 2 molecules of GA-3-P. Therefore, the total flux of molecules (in micromol/g/min) is doubled (Fig. 1). Nevertheless, since only one carbon is labeled in the F-1,6-bisP, only one of the two produced GA-3-P is labeled and a dilution of the labeling by a factor of 2 is observed after F-1,6-bisP. Thus, the labeling flux remains the same as the one entering the pool of F-1,6-bisP (i.e. VGlc).
The labeling of glucose-6-phosphate(G-6-P) is the following:
| (A9) |
The time evolution of 13Glyc, given by equation (A8), can be explicitly replaced in equation (A9). The solution for G-6-P labeling is then:
| (A10) |
Using the initial condition 13G-6-P (t=0) = 0.The term A in equation (A10) is the free turnover part that would characterize G-6-P if the pool of glycogen would not exist. The term B is a transitional part which represents the temporary dilution of G-6-P through glycogen, until glycogen reached its labeling steady-state corresponding to the IE of glucose. As the glycolysis flux VGlc is much smaller than the glycogen flux VGlyc (dilution of G-6-P) (Choi & Gruetter 2003, Watanabe & Passonneau 1973), the second term B is negligible and the expression of the labeling is a simple free exponential turnover (term A). This can be seen from the fact that the coefficient in front of the parentheses in term B approaches zero for VGlc ≫ VGlyc (not shown). Therefore, the rate of labeling of G-6-P is largely independent of the metabolic rate of glycogen. This will have a consequence on the labeling of the following metabolites, and notably NAA.
After this simplification, we have the following effective labeling equation of G-6-P:
| (A11) |
The labeling equation for GA-3-P is the following:
| (A12) |
All the intermediates of the glycolysis are assumed to have a much smaller concentration than the NAA, and thus a smaller temporal change in IE, i.e.:
| (A13) |
With this assumption, we obtain a simplified differential equation for NAA labeling, similar to the one found for glycogen:
| (A14) |
The solution of equation (A14), assuming 13NAA (t=0) = 0, has the following time evolution:
| (A15) |
where .
We can see that the factor ½ is only affecting the steady-state enrichment and not the dynamics of the exponential turnover i.e., the characteristic time constant is still .
2 Relation between τGlycand τNAA
When time tends to zero, the following first order Taylor expansion can be used:
| (A16) |
From this we calculate that:
| (A17) |
3 Lower bound for τNAA
From the labeling equation (4), we consider a measured value at a time point texp (in our case ~5h). For simplicity of notation, we substitute by exp M.
| (A18) |
Thus,
| (A19) |
Which necessarily implies that
| (A20) |
| (A21) |
| (A22) |
Since M > 2, equation A22 can be computed. Thus, the knowledge of a single measurement (average) point makes it possible to find a lower bound to the value of τNAA, in the case of a decreasing Glyc IE/NAA IE curve (see figure 2A) (in the other situation as schematized in figure 2C, a similar calculation could be done for τGlyc). Neglecting the error on the measured time, we obtain the following uncertainty on the lower limit of τNAA using noise error propagation calculation:
| (A23) |
In the group of aroused rats, we have M = 6.3 ± 1 and texp = 4.5 h, which gives a lower limit of τNAA of 11.7 ±1.6 h.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allaman I, Pellerin L, Magistretti PJ. Glucocorticoids modulate neurotransmitter-induced glycogen metabolism in cultured cortical astrocytes. J Neurochem. 2004;88:900–908. doi: 10.1046/j.1471-4159.2003.02235.x. [DOI] [PubMed] [Google Scholar]
- Allen NJ, Karadottir R, Attwell D. A preferential role for glycolysis in preventing the anoxic depolarization of rat hippocampal area CA1 pyramidal cells. J Neurosci. 2005;25:848–859. doi: 10.1523/JNEUROSCI.4157-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- Choi IY, Gruetter R. In vivo 13C NMR assessment of brain glycogen concentration and turnover in the awake rat. Neurochemistry international. 2003;43:317–322. doi: 10.1016/s0197-0186(03)00018-4. [DOI] [PubMed] [Google Scholar]
- Choi IY, Gruetter R. Dynamic or inert metabolism? Turnover of N-acetyl aspartate and glutathione from D-[1-13C]glucose in the rat brain in vivo. J Neurochem. 2004;91:778–787. doi: 10.1111/j.1471-4159.2004.02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Seaquist ER, Gruetter R. Effect of hypoglycemia on brain glycogen metabolism in vivo. J Neurosci Res. 2003;72:25–32. doi: 10.1002/jnr.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz NF, Dienel GA. High glycogen levels in brains of rats with minimal environmental stimuli: implications for metabolic contributions of working astrocytes. J Cereb Blood Flow Metab. 2002;22:1476–1489. doi: 10.1097/01.WCB.0000034362.37277.C0. [DOI] [PubMed] [Google Scholar]
- David M, Petit WA, Laughlin MR, Shulman RG, King JE, Barrett EJ. Simultaneous synthesis and degradation of rat liver glycogen. An in vivo nuclear magnetic resonance spectroscopic study. J Clin Invest. 1990;86:612–617. doi: 10.1172/JCI114752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Ball KK, Cruz NF. A glycogen phosphorylase inhibitor selectively enhances local rates of glucose utilization in brain during sensory stimulation of conscious rats: implications for glycogen turnover. J Neurochem. 2007;2(102):466–478. doi: 10.1111/j.1471-4159.2007.04595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Neighborly interactions of metabolically-activated astrocytes in vivo. Neurochemistry international. 2003;43:339–354. doi: 10.1016/s0197-0186(03)00021-4. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Nutrition during brain activation: does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochemistry international. 2004;45:321–351. doi: 10.1016/j.neuint.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Hertz L. Astrocytic contributions to bioenergetics of cerebral ischemia. Glia. 2005;50:362–388. doi: 10.1002/glia.20157. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Wang RY, Cruz NF. Generalized sensory stimulation of conscious rats increases labeling of oxidative pathways of glucose metabolism when the brain glucose-oxygen uptake ratio rises. J Cereb Blood Flow Metab. 2002;22:1490–1502. doi: 10.1097/01.WCB.0000034363.37277.89. [DOI] [PubMed] [Google Scholar]
- Ghajar JB, Plum F, Duffy TE. Cerebral oxidative metabolism and blood flow during acute hypoglycemia and recovery in unanesthetized rats. J Neurochem. 1982;38:397–409. doi: 10.1111/j.1471-4159.1982.tb08643.x. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Anderson DG, Hertz L. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia. 2006;54:214–222. doi: 10.1002/glia.20377. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Bowser DN, Hutchinson DS, Loiacono RE, Summers RJ. Memory Processing in the Avian Hippocampus Involves Interactions between beta-Adrenoceptors, Glutamate Receptors, and Metabolism. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.5. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Lloyd HG, Santa T, Hertz L. Glycogen is a preferred glutamate precursor during learning in 1-day-old chick: Biochemical and behavioral evidence. J Neurosci Res. 2007;85:3326–3333. doi: 10.1002/jnr.21307. [DOI] [PubMed] [Google Scholar]
- Gip P, Hagiwara G, Ruby NF, Heller HC. Sleep deprivation decreases glycogen in the cerebellum but not in the cortex of young rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R54–59. doi: 10.1152/ajpregu.00735.2001. [DOI] [PubMed] [Google Scholar]
- Gip P, Hagiwara G, Sapolsky RM, Cao VH, Heller HC, Ruby NF. Glucocorticoids influence brain glycogen levels during sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1057–1062. doi: 10.1152/ajpregu.00528.2003. [DOI] [PubMed] [Google Scholar]
- Goldberg ND, O’Toole AG. The properties of glycogen synthetase and regulation of glycogen biosynthesis in rat brain. J Biol Chem. 1969;244:3053–3061. [PubMed] [Google Scholar]
- Gruetter R. Glycogen: the forgotten cerebral energy store. J Neurosci Res. 2003;74:179–183. doi: 10.1002/jnr.10785. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hutchinson DS, Summers RJ, Gibbs ME. Energy metabolism and memory processing: Role of glucose transport and glycogen in responses to adrenoceptor activation in the chicken. Brain Res Bull. 2008;76:224–234. doi: 10.1016/j.brainresbull.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD. Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci. 2002;22:5581–5587. doi: 10.1523/JNEUROSCI.22-13-05581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Morgenthaler F, Yue T, Gruetter R. Direct validation of in vivo localized 13C MRS measurements of brain glycogen. Magn Reson Med. 2007;57:243–248. doi: 10.1002/mrm.21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen PL, Cruz NF, Sokoloff L, Dienel GA. Cerebral oxygen/glucose ratio is low during sensory stimulation and rises above normal during recovery: excess glucose consumption during stimulation is not accounted for by lactate efflux from or accumulation in brain tissue. J Cereb Blood Flow Metab. 1999;19:393–400. doi: 10.1097/00004647-199904000-00005. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Morrison JH, Shoemaker WJ, Sapin V, Bloom FE. Vasoactive intestinal polypeptide induces glycogenolysis in mouse cortical slices: a possible regulatory mechanism for the local control of energy metabolism. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:6535–6539. doi: 10.1073/pnas.78.10.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez R, Melendez-Hevia E, Canela EI. The fractal structure of glycogen: A clever solution to optimize cell metabolism. Biophys J. 1999;77:1327–1332. doi: 10.1016/S0006-3495(99)76982-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A, Ross BD, Bluml S. Direct determination of the N-acetyl-L-aspartate synthesis rate in the human brain by (13)C MRS and [1-(13)C]glucose infusion. J Neurochem. 2001;77:347–350. doi: 10.1046/j.1471-4159.2001.t01-1-00282.x. [DOI] [PubMed] [Google Scholar]
- Morgenthaler FD, Koski DM, Kraftsik R, Henry PG, Gruetter R. Biochemical quantification of total brain glycogen concentration in rats under different glycemic states. Neurochemistry international. 2006;48:616–622. doi: 10.1016/j.neuint.2005.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler FD, van Heeswijk RB, Xin L, Laus S, Frenkel H, Lei H, Gruetter R. Non-invasive quantification of brain glycogen absolute concentration. J Neurochem. 2008;107:1414–1423. doi: 10.1111/j.1471-4159.2008.05717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulmed LN, Gannon MC, Gilboe DP, Tan AW, Nuttall FQ. Glycogen synthase, synthase phosphatase, and phosphorylase response to glucose in somatostatin-pretreated intact rats. Diabetes. 1979;28:231–236. doi: 10.2337/diab.28.3.231. [DOI] [PubMed] [Google Scholar]
- Nordstrom CH, Siesjo BK. Effects of phenobarbital in cerebral ischemia. Part I: cerebral energy metabolism during pronounced incomplete ischemia. Stroke. 1978;9:327–335. doi: 10.1161/01.str.9.4.327. [DOI] [PubMed] [Google Scholar]
- Oz G, Seaquist ER, Kumar A, Criego AB, Benedict LE, Rao JP, Henry PG, Van de Moortele PF, Gruetter R. Human Brain Glycogen Content and Metabolism: Implications on its Role in Brain Energy Metabolism. Am J Physiol Endocrinol Metab. 2006 doi: 10.1152/ajpendo.00424.2006. [DOI] [PubMed] [Google Scholar]
- Passonneau JV, Brunner EA, Molstad C, Passonneau R. The effects of altered endocrine states and of ether anaesthesia on mouse brain. J Neurochem. 1971;18:2317–2328. doi: 10.1111/j.1471-4159.1971.tb00187.x. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit JM, Tobler I, Allaman I, Borbely AA, Magistretti PJ. Sleep deprivation modulates brain mRNAs encoding genes of glycogen metabolism. Eur J Neurosci. 2002;16:1163–1167. doi: 10.1046/j.1460-9568.2002.02145.x. [DOI] [PubMed] [Google Scholar]
- Plaschke K, Muller D, Hoyer S. Effect of adrenalectomy and corticosterone substitution on glucose and glycogen metabolism in rat brain. J Neural Transm. 1996;103:89–100. doi: 10.1007/BF01292619. [DOI] [PubMed] [Google Scholar]
- Rao J, Oz G, Seaquist ER. Regulation of cerebral glucose metabolism. Minerva Endocrinol. 2006;31:149–158. [PubMed] [Google Scholar]
- Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nature neuroscience. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalbruch IK, Linde R, Paulson OB, Madsen PL. Activation-induced resetting of cerebral metabolism and flow is abolished by beta-adrenergic blockade with propranolol. Stroke. 2002;33:251–255. doi: 10.1161/hs0102.101233. [DOI] [PubMed] [Google Scholar]
- Sorg O, Magistretti PJ. Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain research. 1991;563:227–233. doi: 10.1016/0006-8993(91)91538-c. [DOI] [PubMed] [Google Scholar]
- Sorg O, Magistretti PJ. Vasoactive intestinal peptide and noradrenaline exert long-term control on glycogen levels in astrocytes: blockade by protein synthesis inhibition. J Neurosci. 1992;12:4923–4931. doi: 10.1523/JNEUROSCI.12-12-04923.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao KV, Hertz L. Effect of adrenergic agonists on glycogenolysis in primary cultures of astrocytes. Brain research. 1990;536:220–226. doi: 10.1016/0006-8993(90)90028-a. [DOI] [PubMed] [Google Scholar]
- Swanson RA. Physiologic coupling of glial glycogen metabolism to neuronal activity in brain. Can J Physiol Pharmacol. 1992;70(Suppl):S138–144. doi: 10.1139/y92-255. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MM, Sagar SM, Sharp FR. Sensory stimulation induces local cerebral glycogenolysis: demonstration by autoradiography. Neuroscience. 1992;51:451–461. doi: 10.1016/0306-4522(92)90329-z. [DOI] [PubMed] [Google Scholar]
- Tobler I, Murison R, Ursin R, Ursin H, Borbely AA. The effect of sleep deprivation and recovery sleep on plasma corticosterone in the rat. Neurosci Lett. 1983;35:297–300. doi: 10.1016/0304-3940(83)90333-6. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Yang SH, Swanson RA, Sapolsky RM. Glucocorticoids exacerbate hypoxic and hypoglycemic hippocampal injury in vitro: biochemical correlates and a role for astrocytes. J Neurochem. 1992;59:137–146. doi: 10.1111/j.1471-4159.1992.tb08884.x. [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M, Evequoz V. [The effect of light on glycogen turnover in the retina of the honeybee drone (author's transl)] Klin Monatsbl Augenheilkd. 1980;176:519–521. doi: 10.1055/s-2008-1057490. [DOI] [PubMed] [Google Scholar]
- Uffmann K, Gruetter R. Mathematical modeling of (13)C label incorporation of the TCA cycle: the concept of composite precursor function. J Neurosci Res. 2007;85:3304–3317. doi: 10.1002/jnr.21392. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Passonneau JV. Factors affecting the turnover of cerebral glycogen and limit dextrin in vivo. J Neurochem. 1973;20:1543–1554. doi: 10.1111/j.1471-4159.1973.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Xin L, Gambarota G, Mlynarik V, Gruetter R. Proton T(2) relaxation time of J-coupled cerebral metabolites in rat brain at 9.4 T. NMR Biomed. 2007 doi: 10.1002/nbm.1205. [DOI] [PubMed] [Google Scholar]