Abstract
This study aimed to determine the predictors of increased risk of a second demyelinating event within the first year of an initial demyelinating event (IDE) suggestive of early multiple sclerosis (MS). Patients with MS or clinically isolated syndrome (CIS) seen at the UCSF MS Center within one year of the IDE were studied. Univariate and multivariate Cox models were used to analyze predictors of having a second event within 1 year of the IDE. Of 330 patients with MS/CIS, 111 had a second event within 1 year. Non-white race/ethnicity (HR = 2.39, 95% CI [1.58, 3.60], p < 0.0001) and younger age (HR for each 10-year decrease in age = 1.51, 95% CI [1.28, 1.80], p < 0.0001) were strongly associated with an increased risk of having a second event within one year of onset. Having a lower number of functional systems affected by the IDE was also associated with an increased risk of early second event (HR for every one less FS involved = 1.31, 95% CI [1.06, 1.61], p = 0.011). These results were similar after adjusting for treatment of the IDE with steroids and disease-modifying therapy. Non-white race/ethnicity, younger age, and a lower number of FS affected by the IDE are associated with a substantially increased hazard ratio for a second demyelinating event within 1 year. Since early relapse is predictive of worse long-term outcome, identifying and treating such patients after the IDE may be of benefit to them.
Keywords: Multiple sclerosis, Epidemiology, Clinical studies, Demyelinating diseases
Introduction
With the advent of disease-modifying therapies (DMTs) for relapsing remitting multiple sclerosis (RRMS), the scientific community has been under increasing pressure to improve the sensitivity of the diagnostic criteria for RRMS early in the disease course [1]. Furthermore, many experts urge the initiation of DMTs after the initial demyelinating event (IDE) in patients at high risk for subsequent attacks rather than waiting for dissemination in time based on MRI or clinical outcomes [2]. In practice, however, many patients do not start on DMTs after the first clinical event, in part due to delayed evaluation by a neurologist, uncertainty surrounding the diagnosis, or the patient’s resistance to beginning injectable treatment.
One common clinical problem in discussing the benefits of early treatment with patients is that it is difficult to predict which individuals are most at risk for relapse in the immediate future. Studies have identified prognostic factors for disability in the long-term (i.e. 10 or 20 years after disease onset) [3], predictors of relapse rate in patients with established MS [4–6], or magnetic resonance imaging (MRI) or other paraclinical features that increase the risk of conversion to MS [7–11], but few have evaluated clinical risk factors for early relapse in MS [12, 13].
We sought to explore potential predictors of increased risk of a second demyelinating event within 1 year of MS onset with the goal of confirming and extending findings of our previous report in a larger cohort of patients [13]. Since evidence suggests that early relapse is predictive of worse long-term outcomes [14–17], identifying patients most at risk for such may help clinicians to advise them about DMT.
Methods
This project was approved by the UCSF Committee on Human Research and was performed in accordance with the Declaration of Helsinki. Clinical and demographic information for all patients seen at the UCSF MS Center is entered into a Microsoft SQL server database (retrospectively when patients are first seen in clinic and then prospectively as patients return for follow-up visits). Regular follow-up visits typically occur every 6 months, and unscheduled visits occur if a patient has an exacerbation. We queried the database for all CIS and RRMS patients seen within the first year of disease onset between January 2000 and June 2007 [13, 18].
Patients were split into two groups to analyze the effects of race/ethnicity: white, non-Hispanics (referred to as whites in the rest of the manuscript) and non-whites (all others). Demyelinating events were defined as new or recurring neurologic symptoms referable to the central nervous system (CNS) lasting for at least 48 h after a remission of 30 days or more since the previous event. Pseudo-exacerbations were excluded. Based on clinical history and examination, each patient’s relapses were coded as occurring in the spinal cord, brainstem/cerebellum, optic nerve, or cerebrum. If the event involved at least two of these locations, it was considered polyregional. The number of functional systems (FS; e.g. sensory, motor, bladder/bowel) affected by the IDE was also calculated (possible range 1–7) [19]. Disease-modifying therapy (DMT) status was recorded. A patient was considered as being on DMT after he/she had at least 90 days of continuous treatment. This time period was used because it is an accepted length of time in which standard DMTs reach therapeutic effectiveness [20, 21].
The severity of and recovery from the first event was determined by trained individuals as previously described [8, 13]. Briefly, mild IDE severity was defined as FS scores of 0–1 in 1–3 FS, but no higher than 1 or visual acuity (VA) better than or equal to 20/40, EDSS score range of 0–1.5 inclusive; moderate severity was defined as a score of at least 2, but not higher than 2 in one or two FS or four or more scores of 1 or VA of 20/50–20/190, EDSS score of 2.0–2.5 inclusive; severe was assigned for relapses exceeding prior criteria. IDE recovery was scored using the lowest EDSS and FS scores reported between two and 12 months after the attack. It was considered complete (no residual complaint, normal follow-up examination, all FS scores = 0, follow-up EDSS score = 0), fair (residual subjective complaint that does not impair activity, or at least one FS score of 1 at most or VA better or equal to 20/40, follow-up EDSS score = 1.0–1.5), or poor (residual deficits exceeding prior criteria).
Increased risk of early second event was analyzed using the Cox proportional hazards model. Hazard ratios (HRs) were generated with 95% confidence intervals (CIs) and p values. For comparability with a previous study [13], we performed the analyses including only events that occurred within 1 year of disease onset. Potential predictors included sex, race/ethnicity, age at onset, severity and recovery of the IDE, event location, monoregional versus polyregional presentation, steroid treatment for the first event, DMT status, and initial brain MRI scan results (normal vs. at least one T2-weighted hyperintense lesion), also chosen for comparability with the previous study [13]. Since DMT status changes with time, it was treated as a time-dependent covariate. Interactions between age, race, and DMT with subgroup (included in previous analysis vs. not) were evaluated. Tests of the proportional hazards assumption did not show strong evidence of any violations. For predictors that seemed to be important, we created Kaplan–Meier curves after generating a dichotomous predictor (for ease of presentation), when the original predictor was ordinal or continuous. We also performed sensitivity analyses that excluded the subjects who had been included in the previous cohort.
Results
Patient and event characteristics
We identified 330 patients (224 females) seen at the UCSF MS Center within a year of initial MS symptoms; mean follow-up was 759 ± 575 days (median 633 days, range 23–2,692 days). The mean age at IDE onset was 34 ± 12 years. Two hundred and sixty-seven (80.9%) patients were Caucasian; the remainder included 21 (6%) African Americans, 15 (5%) Asians, 14 (4%) Hispanic or Latinos, and 13 (4%) others (unknown). At onset, 301 (93%) of the 323 patients who had available brain imaging had an abnormal brain MRI.
Most IDEs (85%) were monoregional; event locations, severity, and recovery are presented in Table 1. Within the first year, 111 patients (34%) experienced a second event. Of the 219 patients who did not have a second event within a year, there was at least 1 year of follow-up for 134 (61%). The mean follow-up for the remaining 85 patients was 174 ± 94 days.
Table 1.
Characteristics of the initial demyelinating event
| Event characteristic | n = 330 |
|---|---|
| Location, n (%)a | |
| Spinal cord | 175 (53) |
| Brainstem/cerebellum | 110 (33) |
| Optic nerve | 86 (26) |
| Cerebrum | 10 (3) |
| Severity, n (%) | |
| Mild | 134 (41) |
| Moderate | 139 (42) |
| Severe | 57 (17) |
| Recovery, n (%)b | |
| Complete | 150 (46) |
| Fair | 118 (37) |
| Poor | 54 (17) |
aTotal does not add up to 100% since 48 patients (15%) had multiple sites affected
bIncomplete data for some individuals
One hundred and fifty-three (46%) patients received high dose steroid therapy for the IDE. DMT was initiated in 54% of patients (n = 177) during the entire follow-up period (Avonex 29%, Rebif 10%, Copaxone 8%, Betaseron 6%, other 1%); 105 began therapy within 1 year of the IDE. However, only nine patients who had a second event within a year of the first had at least 90 days of DMT before the second event (i.e. had an on-therapy relapse).
Factors associated with early risk of second event
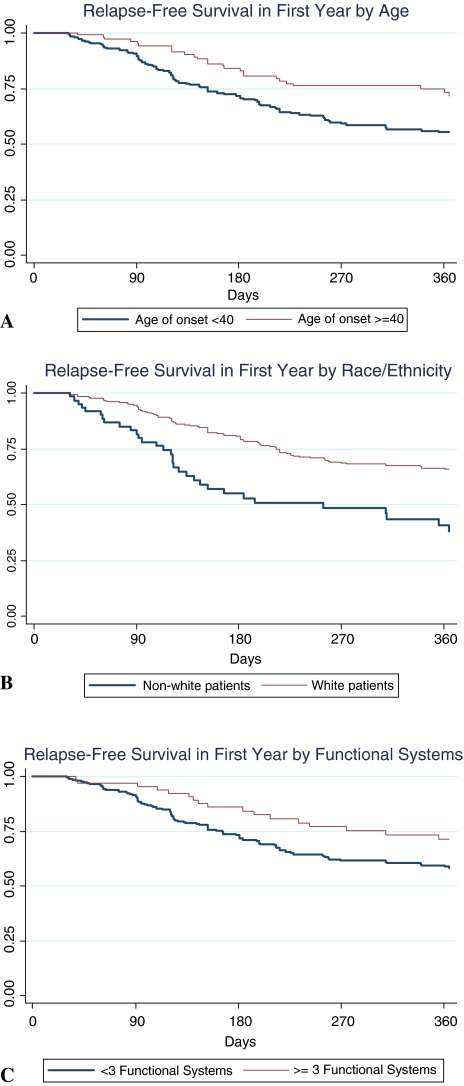
In the univariate Cox models, non-white race (HR = 2.39, 95% CI [1.58, 3.60], p < 0.0001) and younger age (HR for each 10-year decrease in age = 1.51, 95% CI [1.28, 1.80], p < 0.0001) were associated with a substantial increase in risk of a second event within the year after the IDE (Fig. 1). Fewer FS involved in the IDE predicted an increased risk of early second event (HR for each one less FS = 1.31, 95% CI [1.06, 1.61], p = 0.011) (Fig. 1). Fair versus complete IDE recovery conferred a reduction in the HR for a second event (HR = 0.76, 95% CI [0.50, 1.14], p = 0.180); poor versus complete recovery seemed to be associated with an even lower risk (HR = 0.57, 95% CI 0.31, 1.04], p = 0.069, although there was substantial overlap of the CIs. DMT status did not appear to substantially alter the HR for a second event within a year (HR = 0.98, 95% CI [0.48, 2.01], p = 0.96), but there was wide uncertainty around this estimate. First event severity, sex, location of onset, abnormal versus normal brain MRI, polyregional onset, and steroid treatment for the IDE did not appear to strongly influence the hazard of an early second event.
Fig. 1.
Relapse-free survival curves by age (a), race (b), and number of functional systems (c)
Non-white race, younger age, and a lower number of FS affected by the IDE remained strong and independent predictors of increased risk of a second event within 1 year in the multivariate model that included these predictors as well as steroid treatment for the IDE and DMT (Table 2).
Table 2.
Results of the multivariate analysis for predictors of early second event
| Predictors | Hazard ratio | 95% CI | p value |
|---|---|---|---|
| Age (10-year decrease) | 1.46 | 1.22, 1.74 | <0.0001 |
| Race/ethnicity (non-white vs. white) | 2.06 | 1.34, 3.17 | 0.0010 |
| Functional systems (per one less) | 1.32 | 1.05, 1.67 | 0.020 |
| Treatment of first attack with steroids | 0.87 | 0.58, 1.32 | 0.51 |
| Treatment with DMT | 1.01 | 0.48, 2.16 | 0.97 |
DMT disease-modifying therapy
We then performed a sensitivity analysis, excluding the 176 patients who had been included in a prior analysis [13]. Only the 154 seen after January 1, 2005 were included in this evaluation. The univariate analyses demonstrate that non-white race (HR = 2.11, 95% CI [1.10, 4.03], p = 0.025) and younger age (HR for 10-year decrease in age = 1.69, 95% CI [1.33, 2.14], p < 0.0001) were still very strong predictors of increased risk of an early second event. That the p value for non-white race is weaker reflects the decreased sample size, which also predicts widened CIs. While the number of FS was no longer as strong a predictor, the trend was in a similar direction (HR for every one less FS involved = 1.16, 95% CI [0.90, 1.51], p = 0.25). Poor versus complete recovery from the IDE was still predictive of a lower risk for another event within a year (HR = 0.48, 95% CI [0.20, 1.17], p = 0.11).
Tests for interaction of age or race/ethnicity with the two subgroups did not produce strong evidence for any meaningful interactions. On the other hand, the test for interaction of DMT and the two cohorts was statistically significant (p = 0.019). In the new cohort of patients, DMT was associated with an increased HR for second event within 1 year (HR = 2.65, 95% CI [1.04, 6.76], p = 0.041), whereas treatment with DMT in the previous cohort was associated with a reduced risk of early second event (HR = 0.41, 95% CI [0.13, 1.36], p = 0.14).
Discussion
The results reported herein support that non-white race/ethnicity, younger age, and fewer FS associated with the IDE substantially increase the risk for early relapse in patients with CIS.
In previous studies, African American patients had more rapid disease progression and/or were more likely to be disabled than were Caucasians, suggesting that the long-term course of MS may be more aggressive in the former group, although specific predictors of such outcomes have not been fully characterized [22–25]. Here, we included not only African Americans, but also Asians, Hispanics or Latinos, and patients of other racial/ethnic backgrounds as “not white” and demonstrated that non-white race/ethnicity was associated with a higher risk of early second event, independent of age or treatment status. This finding is worrisome given that more activity in the first year of disease onset has been associated with a poorer long-term prognosis [14–17]. While some groups reported no difference in the percentage of African American versus white patients who were treated with DMT [24, 25], it is unknown if there are racial disparities in early initiation of DMT, i.e. after the first demyelinating event. Certainly other racial disparities in care have been documented in MS [26]. More analyses are needed to determine if the non-white individuals in our cohort experience a more aggressive long-term disease course as well as to explore whether there are systematic differences in treatment initiation.
That age is independently associated with early risk of relapse is consistent with previous studies showing that new gadolinium-enhancing lesions are less likely to develop in older patients than in younger patients [27, 28]. The finding that older patients are less likely to have an early second event after the IDE may relate to the same factors that are responsible for the different onset ages, whether genetic, biologic or both. Since younger patients are more at risk for an early second event, the urgency of starting therapy may be greater for a 25-year-old than a 55-year-old individual, since the risk of early relapse is more than four times higher for the former compared to the latter patient. However, the ability of DMT to delay or prevent the second event has not been studied specifically in high-risk patients.
Having a higher number of FS involved or a poor or moderate recovery from the IDE was associated with a lower risk of early relapse. Perhaps patients who have more concurrent or destructive demyelinating lesions are more prone to temporarily suppress biologic disease processes compared with those with less aggressive disease onset. Alternatively, having more CNS territory involved in the IDE or poor recovery may lead to masking of subtle subsequent exacerbations, particularly if they occurred in similar anatomic areas, such that the apparent relationship between these predictors and time to next relapse is explained by missed second events.
One perplexing finding of our study is that while for the overall cohort, DMT did not appear to substantially change the risk of an early second event, there was an interaction between this predictor and when patients were first seen (from January 2000 to January 2005 vs. January 2005 to June 2007). In the more recent subgroup, DMT use was associated with an increased HR for early second event. These findings suggest that there are factors not captured in this study that make the two groups different. Such covariates could be due to differences in the patient population or due to changes in patterns of recommended treatments. Perhaps practitioners in our group have become more selective with respect to which patients should receive early treatment by using clinical or paraclinical data, such as brain MRI characteristics, that we did not measure in this study. For example, if physicians tend to initiate DMT in patients who have a higher burden of disease on brain MRI, this would lead to a false impression that the risk of relapse is higher on treatment when in fact; such patients were already at a higher risk of early second event [7, 9–11]. Further, only a small number of patients are driving this finding since only nine patients (three in the earlier and six in the later cohort), who had a second event within a year of the IDE actually began DMT before it occurred.
There are some limitations to our study. As our cohort size was moderate, addressing this research question in a larger cohort would be desirable, as it would provide more accurate estimates and permit evaluations of more predictors simultaneously in larger multivariate models. Also, it would be valuable to evaluate a larger population of non-white individuals, such that further determination of whether all, or only some, racial backgrounds predispose patients to an increased risk of an early second event. Since this is a clinical cohort, MRI scans were available for most patients at the time of the initial clinical visit, but as imaging protocols were not standardized nor were images systematically stored in the radiology database at UCSF, specific characteristics of the scans could not be analyzed for this study. A confirmation study would also be helpful; performing such a study in a large, multi-racial cohort with standardized, longitudinal imaging and clinical follow-up would be one good way to confirm our findings while exploring MRI characteristics as covariate predictors of early second event. Incorporating genetic studies, such as HLA haplotype and gene expression, might also help to increase our ability to understand which patients are at greatest risk of early relapse and to elucidate the mechanism by which factors that have already been identified, such as non-white race and younger age, confer risk to individuals who possess them.
Acknowledgments
This study was supported in part by a Clinical Research Fellowship Award from the Partners MS Center, a National Multiple Sclerosis Society Sylvia Lawry Fellowship Award, the Nancy Davis Foundation, and NMSS RG-3692A. Statistical analysis for this publication was made possible by NIH/NCRR UCSF-CTSI (grant number UL1 RR024131). Dr. Waubant has received research support from Biogen Idec, Genentech Inc, Pfizer, and Sanofi-Aventis, and honorarium for one educational presentation from Teva and Biogen.
Conflicts of interest statement There are no conflicts of interest.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Polman CH, Reingold SG, Edan G et al (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria”. Ann Neurol 58:840–846 [DOI] [PubMed]
- 2.Frohman E, Havrdova E, Lublin F et al (2006) Most patients with multiple sclerosis or clinically isolated demyelinating syndrome should be treated at time of diagnosis. Arch Neurol 63:614–619 [DOI] [PubMed]
- 3.Ramsaransing GSM, DeKeyser J (2007) Predictive value of clinical characteristics for “benign” multiple sclerosis. Eur J Neurol 14:885–889 [DOI] [PubMed]
- 4.Held U, Heigenhauser L, Shang C et al (2005) Predictors of relapse rate in MS clinical trials. Neurology 65:1769–1773 [DOI] [PubMed]
- 5.Hirst C, Ingram G, Pearson O et al (2008) Contribution of relapses to disability in multiple sclerosis. J Neurology 255:280–287 [DOI] [PubMed]
- 6.Young PJ, Lederer C, Eder K et al (2006) Relapses and subsequent worsening of disability in relapsing-remitting multiple sclerosis. Neurology 67:804–808 [DOI] [PubMed]
- 7.Iannucci G, Tortorella C, Rovaris M et al (2000) Prognostic value of MR and magnetization transfer imaging findings in patients with clinically isolated syndromes suggestive of multiple sclerosis at presentation. AJNR 21:1034–1038 [PMC free article] [PubMed]
- 8.Masjuan J, Alvarez-Cermeno JC, Garcia-Barragan N et al (2006) Clinically isolated syndromes: a new oligoclonal band test accurately predicts conversion to MS. Neurology 28:576–578 [DOI] [PubMed]
- 9.Morrissey SP, Miller DH, Kendall BE et al (1993) The significance of brain magnetic resonance imaging abnormalities at presentation with clinically isolated syndromes suggestive of multiple sclerosis. Brain 116:135–146 [DOI] [PubMed]
- 10.O’Riordan JI, Thompson AJ, Kingsley DP et al (1998) The prognostic value of brain MRI in clinically isolated syndromes of the CNS. A 10-year follow-up. Brain 121:495–503 [DOI] [PubMed]
- 11.Brex PA, O’Riordan JI, Miszkiel KA et al (1999) Multisequence MRI in clinically isolated syndromes and the early development of MS. Neurology 53:1184–1190 [DOI] [PubMed]
- 12.Achiron A, Borak Y (2000) Multiple sclerosis—from probable to definite diagnosis. Arch Neurol 57:974–979 [DOI] [PubMed]
- 13.West T, Wyatt M, High A et al (2006) Are initial demyelinating event recovery and time to second event under differential control? Neurology 67:809–813 [DOI] [PubMed]
- 14.Weinshenker BG, Bass B, Rice GP et al (1989) The natural history of multiple sclerosis: a geographically based study. 2. Predictive value of the early clinical course. Brain 112:1419–1428 [DOI] [PubMed]
- 15.Pittock SJ, Mayr WT, McClelland RL et al (2004) Change in MS-related disability in a population-based cohort. Neurology 62:51–59 [DOI] [PubMed]
- 16.Confavreux C, Vukusix S, Adeleine P (2003) Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain 12:770–782 [DOI] [PubMed]
- 17.Amato MP, Ponziani G, Bartolozzi ML et al (1999) A prospective study on the natural history of multiple sclerosis: clues to the conduct and interpretation of clinical trials. J Neurol Sci 168:96–106 [DOI] [PubMed]
- 18.Deen S, Bacchetti P, High A et al (2008) Predictors of the location of the multiple sclerosis relapse. J Neurol Neurosurg Psychiatry 79:1190–1193 [DOI] [PubMed]
- 19.Kurtzke J (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452 [DOI] [PubMed]
- 20.Comi G, Filippi M, Wolinsky J (2001) European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging-measured disease activity and burden in patients with relapsing multiple sclerosis. Ann Neurol 49:290–297 [DOI] [PubMed]
- 21.Waubant E, Goodkin DE, Sloan R et al (1999) A pilot study of MRI activity before and during interferon beta-1a therapy. Neurology 53:874–876 [DOI] [PubMed]
- 22.Cree BAC, Khan O, Bourdette D et al (2004) Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology 63:2039–2045 [DOI] [PubMed]
- 23.Kaufman MD, Johnson SK, Moyer D et al (2003) Multiple sclerosis: severity and progression rate in African Americans compared to whites. Am J Phys Med Rehabil 82:582–590 [DOI] [PubMed]
- 24.Marrie RA, Cutter G, Tyry T et al (2006) Does multiple sclerosis-associated disability differ between races? Neurology 66:1235–1240 [DOI] [PubMed]
- 25.Weinstock-Guttman B, Jacobs LD, Brownscheidle CM et al (2003) Multiple sclerosis characteristics in African American patients in the New York State Multiple Sclerosis Consortium. Mult Scler 9:293–298 [DOI] [PubMed]
- 26.Shabas D, Heffner M (2005) Multiple sclerosis management for low-income minorities. Mult Scler 11:635–640 [DOI] [PubMed]
- 27.Tortorella C, Bellacosa A, Paolicell D et al (2005) Age-related gadolinium-enhancement of MRI brain lesions in multiple sclerosis. J Neurol Sci 239:95–99 [DOI] [PubMed]
- 28.Filippi M, Wolinsky JS, Sormani MP et al (2001) Enhancement frequency decreases with increasing age in relapsing-remitting multiple sclerosis. Neurology 56:422–423 [DOI] [PubMed]