Abstract
Potassium channels encoded by hERG (human ether-à-go-go-related gene) underlie the cardiac rapid delayed rectifier K+ current (IKr) and hERG mutations underpin clinically important repolarization disorders. Virtually all electrophysiological investigations of hERG mutations have studied exclusively the hERG1a isoform; however, recent evidence indicates that native IKr channels may be comprised of hERG1a together with the hERG1b variant, which has a shorter N-terminus. Here, for the first time, electrophysiological effects were studied of a gain-of-function hERG mutation (N588K; responsible for the ‘SQT1’ variant of the short QT syndrome) on current (IhERG1a/1b) carried by co-expressed hERG1a/1b channels. There were no significant effects of N588K on IhERG1a/1b activation or deactivation, but N588K IhERG1a/1b showed little inactivation up to highly positive voltages (⩽+80 mV), a more marked effect than seen for hERG1a expressed alone. IhERG1a/1b under action potential voltage-clamp, and the effects on this of the N588K mutation, also showed differences from those previously reported for hERG1a. The amplified attenuation of IhERG inactivation for the N588K mutation reported here indicates that the study of co-expressed hERG1a/1b channels should be considered when investigating clinically relevant hERG channel mutations, even if these reside outside of the N-terminus region.
Keywords: Channelopathy, hERG, hERG1a/1b, hERG1b, QT interval, Rapid delayed rectifier, Short QT syndrome
Introduction
In recent years a novel cardiac repolarization disorder called the short QT syndrome (SQTS) has been identified that is characterized by marked abbreviation of the QT interval on the electrocardiogram, changes in T wave morphology, poor rate adaptation of the QT interval and an increased risk of arrhythmia and sudden death [1–4]. Gain-of-function mutations in three potassium (K+) channel genes have been identified in SQTS patients: the SQT1 variant of the syndrome is linked to mutations to KCNH2, whilst SQT2 and SQT3 variants are associated, respectively, with mutations to KCNQ1 and KCNJ2 [5–7]. With a strong correlation between the SQTS and increased arrhythmia susceptibility [4,8], this syndrome provides an important opportunity to understand the link between accelerated cardiac repolarization and arrhythmogenesis; indeed, the SQTS has been proposed to provide a paradigm for understanding the role of cardiac K+ channels in ventricular fibrillation [9].
The first cardiac K+ current implicated in the SQTS was the rapid delayed rectifier (IKr), the channel α subunit of which is encoded by KCNH2 (alternative nomenclature hERG: human ether-à-go-go-related gene) [5,6]. IKr plays a critical role in ventricular action potential (AP) repolarization and, thereby, in determining the duration of the QT interval [10]. SQT1 patients were found to possess an asparagine to lysine substitution (N588K) in the S5-Pore linker region of the hERG channel protein [5,6], resulting in attenuation of a rapid C-type inactivation process that normally limits hERG current (IhERG) amplitude at positive voltages [11,12]. In turn, this is envisaged to enable greater repolarizing current to flow through SQT1 mutant than wild-type (WT) channels during cardiac APs [5,11–13].
Although studies conducted to-date have provided insight into the possible basis of abbreviated repolarization in SQT1 [5,11–13], there is some question as to their physiological relevance. This is because – in common with virtually all studies of recombinant hERG channels that have been conducted to-date – all information on the likely consequences for IKr kinetics of the N588K hERG mutation comes from studies of the hERG1a isoform [5,11–14]. Recent evidence, however, suggests that native cardiac IKr may not be comprised of hERG1a alone, but rather of hERG1a heteromerically expressed with an alternative transcript, hERG1b, an isoform with a truncated N-terminus [15,16]. The ERG1b splice variant was initially identified in both mouse and human hearts and is identical to the 1a isoform except for a truncated N-terminus [17,18]. It was subsequently proposed not to be expressed at the protein level in human heart [19]; however, the ERG1a and 1b isoforms have now been found to co-exist in several species and are co-localized in the T-tubules of ventricular myocytes [15]. They co-assemble to form functional heteromeric channels with altered kinetics (particularly in respect of deactivation) that may more closely recapitulate native IKr compared to ERG1a alone [15,16]. Very recent evidence of altered WT hERG channel gating for co-expressed hERG1a/1b [16,20] raises questions as to whether or not consequences for native IKr of disease-causing hERG mutations, including those that affect IhERG inactivation, can be considered to be adequately recapitulated by studying hERG1a alone. Consequently, the present study was conducted to determine the effects of the N588K hERG SQT1 mutation on co-expressed hERG1a/1b channels. This report shows for the first time that, despite the fact that the location of the S5-Pore linker in the hERG channel is distant from that of the N-terminus, the effects of the SQT1 N588K hERG mutation on IhERG carried by hERG1a/1b channels are more marked than those reported previously for hERG1a alone.
Methods
Maintenance of hERG expressing cell lines. WT hERG1b in pcDNA 3.1 was generously donated by Dr. Gail Robertson (University of Wisconsin). The construction of N588K hERG1a from WT hERG in pcDNA 3.0 vector has been described previously [12]. N588K hERG1b was made by replacement of the N-terminus in N588K 1a with that of WT hERG1b. This was achieved by 2-primer PCR of hERG1b (in pcDNA3.1) with the creation of a SalI restriction site (located in the S2 domain of hERG). The resulting PCR product was cut at SalI and HindIII sites and sub-cloned into the hERG1a vector (in pcDNA3.0). Chinese Hamster Ovary (CHO) cells were passaged using a non-enzymatic agent (Splitase, AutogenBioclear) and plated out onto small sterilised glass coverslips in 30 mm petri dishes containing Kaighn’s modification of Ham’s F12-K medium (Gibco), supplemented with 10% foetal bovine serum (Gibco) and 200 μg ml−1 gentamicin (Gibco). Prior to transfection, cells were plated out onto small sterilised glass coverslips. After 24 h cells were co-transfected with hERG and green fluorescent protein (in pCMX; donated by Dr. Jeremy Tavare) at a ratio of 2:1 using Lipofectamine LTX (Invitrogen), according to the manufacturer’s instructions. For the experiments performed using hERG1a and 1b co-expression, the cells were co-transfected with WT or N588K hERG1a and 1b at a co-expression ratio of 1:1. Following transfection, after 5–6 h incubation in serum-containing medium, medium was replaced. Cells were incubated at 37 °C for at least 1 day prior to electrophysiological study. Data from WT and N588K hERG1a expressed alone (Fig. 3) derive from CHO cells stably expressing WT or N588K hERG1a [12,21].
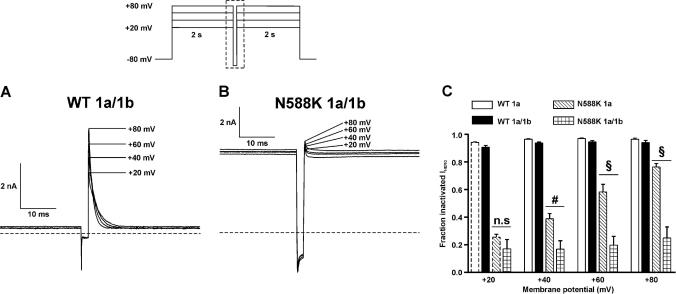
Fig. 3.
IhERG fractional inactivation. Representative current traces for WT (A) and N588K hERG1a/1b (B) that were elicited using the protocol shown in the inset (protocol not drawn to scale). The traces focus on the current profile during the second and third steps of the protocol, the area is indicated by the dashed box area in the protocol. The horizontal dashed line represents zero current. (C) The fraction of inactivated IhERG at different membrane potentials shown for WT 1a (n = 5 cells), WT 1a/1b (n = 5 cells), N588K 1a (n = 5 cells) and N588K hERG1a/1b (n = 6 cells). n.s denotes no statistical significance, # denotes statistical significance of P < 0.01 and § denotes statistical significance of P < 0.001. Mean ± SEM values for WT 1a and N588K 1a at +20 mV highlighted by dashed bars are identical to those in [22].
Electrophysiological recording. Data acquisition and recording methods used were identical to those described in previous studies of WT and N588K hERG1a from our laboratory [12,13,22]. Briefly, whole-cell voltage-clamp measurements were made at 37 °C with an external solution containing (in mM): 140 NaCl, 4 KCl, 2.5 CaCl2, 1 MgCl2, 10 Glucose and 5 HEPES (titrated to pH 7.45 with NaOH). The pipette dialysis solution contained (in mM): 130 KCl, 1 MgCl2, 5 EGTA, 5 MgATP and 10 HEPES (titrated to pH 7.2 with KOH). Pipette resistance ranged from 1.5–3.5 MΩ. Typically ∼80% series resistance could be compensated. The action potential (AP) waveforms used for ‘AP clamp’ experiments (Fig. 4) are identical to those described in [13].
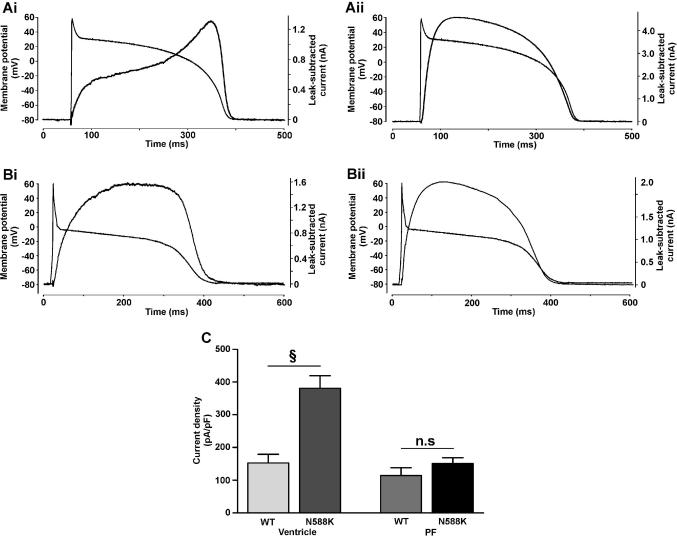
Fig. 4.
IhERG1a/1b profile during ventricular and Purkinje fibre AP waveforms. (A) Representative traces of WT (Ai) and N588K (Aii) IhERG1a/1b elicited by ventricular AP command waveform (shown overlain; n = 14 for both). (B) Representative traces of WT (Bi; n = 13) and N588K (Bii; n = 14) IhERG1a/1b elicited by a Purkinje fibre AP command waveform. (C) Plots of maximal current density for WT and N588K hERG1a/1b during ventricular and Purkinje fibre AP command waveforms. § denotes statistical significance of P < 0.001.
Data analysis and presentation. The numerical equation for voltage-dependence of activation is described in [12]. The use of a conventional ‘availability’ voltage-protocol to quantify the voltage-dependence of IhERG inactivation (e.g. [11,12]) is contingent upon the ability to inactivate fully IhERG with strong membrane depolarization. However, in this study N588K IhERG1a/1b could not be substantially inactivated by depolarizations as positive as +80 mV (Fig. 3) and so we adopted an approach to quantify inactivation similar to that used in a recent investigation of inactivation-attenuating hERG mutations [22]. For each of several test voltages (+20, +40, +60, +80 mV; Fig. 3) a 2 s depolarization (to activate/inactivate IhERG) was followed by a brief (2 ms) hyperpolarization to −100 mV (to relieve inactivation) and then inactivation was re-established by a 2 s depolarization to the same potential as the first step. The magnitude of the current transient during the third step of the protocol indicated IhERG at each voltage after pronounced recovery from inactivation, whilst the sustained current at the end of the first pulse was taken as representing current remaining after inactivation was complete at the relevant test voltage. The ratio of the two was used to assess the fraction of inactivated IhERG at each test voltage.
Data were analysed using Clampfit 8 (Axon Instruments), Excel 2002 and Prism v3 (Graphpad Inc.) software. Throughout, data are presented as the mean ± standard error of the mean (SEM). Statistical analysis was carried out using a Student’s t-test and one or two-way analysis of variance (ANOVA) as appropriate, with Bonferroni post-test using Prism v3 (Graphpad Inc.). P values of less than 0.05 were taken to be statistically significant.
Results and discussion
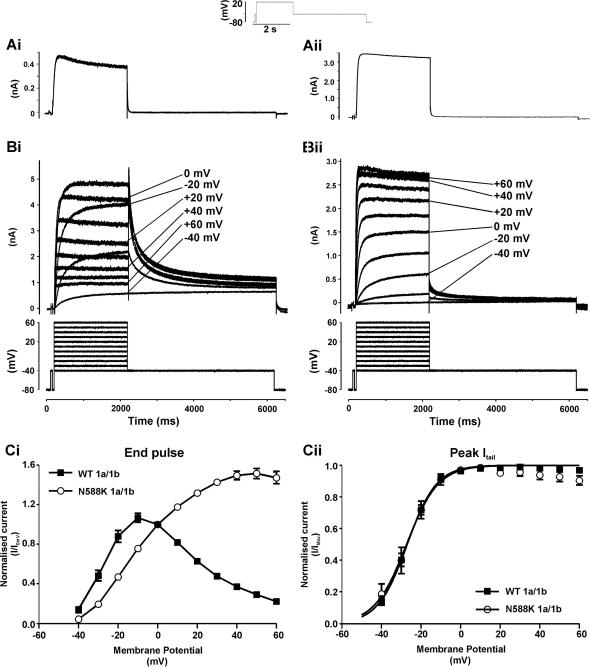
Fig. 1A shows representative records of IhERG elicited by a depolarizing voltage command to +20 mV from a holding potential of −80 mV (protocol shown as inset; [12,13,23]), for each of singly-expressed WT (Ai) and N588K (Aii) hERG1b channels. For both channels, IhERG1b amplitude during the depolarizing voltage command was much greater than the subsequent IhERG1b ‘tail’ visible on repolarization to −40 mV; tail current deactivation was also extremely rapid (with mono-exponential time-constants <30 ms for both WT and N588K hERG1b). The lack of a resurgent IhERG tail and extremely rapid deactivation time-course for WT IhERG1b indicates that, concordant with previous evidence [17,18], WT IhERG1b expressed alone does not recapitulate native IKr; therefore all further experiments were conducted using co-expressed hERG1a/1b. Fig. 1B shows IhERG families for each of WT (Bi) and N588K (Bii) IhERG1a/1b elicited by commands to a range of test voltages (Fig. 1B lower traces; cf. [12]). WT IhERG1a/1b increased progressively with depolarization up to ∼−10 mV and then declined at more positive potentials, whereas N588K IhERG1a/1b increased progressively up to ∼+40/+50 mV, declining only slightly at +60 mV. Fig. 1Ci illustrates this graphically for mean end-pulse current data. Resurgent IhERG1a/1b tails for WT hERG were evident, whilst for N588K IhERG1a/1b tails were markedly smaller than pulse currents following commands to positive voltages (Fig. 1Bii). Normalized peak IhERG1a/1b tail – voltage relations were plotted (Fig. 1Cii) to ascertain parameters describing voltage-dependent activation for the two channels (cf. [12]). The calculated half-maximal activation voltage (V0.5) values for WT and N588K IhERG1a/1b were, respectively, −27.0 ± 0.4 mV and −27.3 ± 1.1 mV, with corresponding k values of 7.4 ± 0.4 and 8.0 ± 1.0 mV (n = 8 cells each; P > 0.05 for both). These values are significantly more negative (P < 0.001) than those we have previously reported for WT and N588K IhERG1a alone, under identical recording conditions (−20.1 ± 0.1 and −22.4 ± 0.6 mV, respectively, [12] and cf. [20]). Collectively, the data in Fig. 1 indicate that, at 37 °C, the N588K mutation alters rectification of IhERG1a/1b, but without significantly altering the voltage-dependence of activation (cf. [12]).
Fig. 1.
I–V relations for WT and N588K IhERG1a/1b. (A) Traces of WT (Ai) and N588K (Aii) IhERG1b elicited by protocol shown in panel inset. (B) Traces of WT (Bi) and N588K (Bii) IhERG1a/1b elicited by 2 s depolarizations ranging from −40 to + 60 mV, from a holding potential of −80 mV. The repolarization step to −40 mV produced the resultant outward Itail. (C) Ci End-pulse currents normalised to the current observed at 0 mV and then plotted against membrane potential. Cii Plots of the peak Itails on repolarization to −40 mV against membrane potential for WT and N588K hERG1a/1b. For Cii data for each cell were normalised to the maximal Itail recorded; mean data were fitted with a Boltzmann equation to give V0.5 values of −27.0 ± 0.4 and −27.3 ± 1.1 mV for WT 1a/1b and N588K 1a/1b-hERG, respectively, with respective k values of 7.4 ± 0.4 and 8.0 ± 1.0 mV (n = 8 for each).
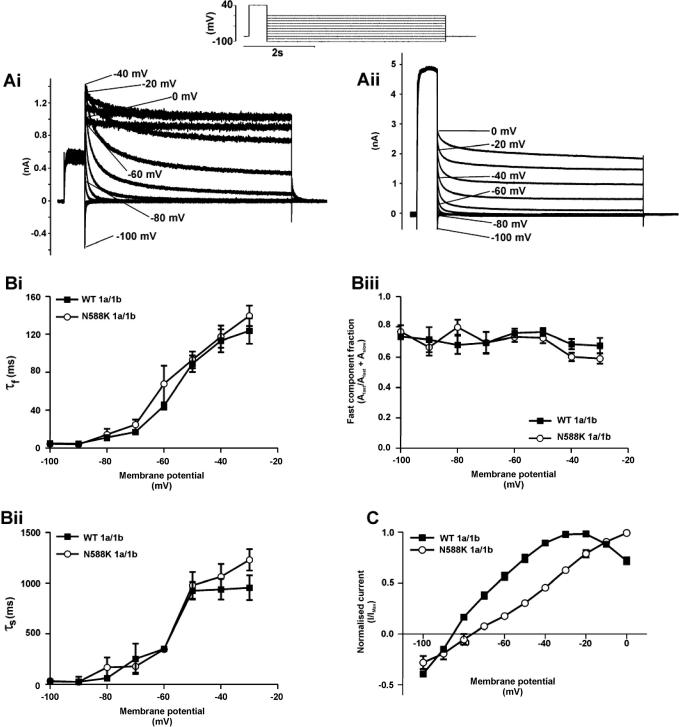
In order to compare IhERG deactivation between WT and N588K IhERG1a/1b the protocol shown above Fig. 2A (inset) was used [12] and the decline of IhERG1a/1b tails at different repolarization voltages was compared using bi-exponential tail current fitting. There was no significant difference between WT and mutant IhERG1a/1b in either fast (Fig. 2Bi) or slow (Fig. 2Bii) time-constants of deactivation, nor in the relative contributions of the fast/slow deactivating components (Fig. 2Biii; n = 7 cells each). The deactivation time-course for WT and N588K IhERG1a/1b was, however, faster than that of the corresponding hERG1a channels (data not shown), consistent with accelerated deactivation reported previously for WT hERG1a/1b [16,18,20]. A plot of the fully-activated current–voltage (I–V) relation derived from this protocol (Fig. 2C; cf. [12,24]) confirmed impaired rectification of N588K IhERG1a/1b compared to WT IhERG1a/1b, accompanied by a modest positive-shift in reversal potential (from −85.2 ± 0.4 mV to −78.7 ± 1.9 mV; n = 7 cells each) and relative pNa/pK ratio (from 0.0097 (WT) to 0.0201 (N588K)). These effects of the N588K hERG mutation are similar to those reported previously for hERG1a alone [12].
Fig. 2.
Deactivation and the fully activated I–V relation. (A) Representative traces of WT (Ai) and N588K (Aii) IhERG1a/1b elicited by the protocol shown in the inset. (B) Plots of the fast (Bi) and slow (Bii) time-constants of deactivation for WT and N588K hERG1a/1b against membrane potential (n = 7, for each). Biii shows proportion of deactivation that can be attributed to the fast component for WT and N588K hERG1a/1b (n = 7). (C) The peak Itail for WT and N588K hERG1a/1b plotted against the respective membrane potential and normalised to the peak outward Itail observed for each individual cell (n = 7).
Recent evidence suggests inactivation gating of WT IhERG1a/1b is altered compared to that of WT IhERG1a [16]. Given this, and also that the major reported effect of the N588K mutation on IhERG1a is on the voltage-dependence of inactivation [11,12], it was imperative to determine effects of the N588K mutation on IhERG1a/1b. The three-step protocol shown as an inset of Fig. 3A and B (see ‘Methods’ and [22]) was used to assess fractional inactivation of IhERG1a/1b. As shown in Fig. 3A, at each of the 4 positive membrane potentials examined, WT IhERG1a/1b was substantially inactivated. By contrast, however, N588K IhERG1a/1b exhibited only a small transient component at any voltage, with a large sustained component (Fig. 3B), indicative of comparatively little fractional inactivation at any of the voltages examined. Fig. 3C shows mean data for WT and N588K IhERG1a/1b, with mean data for WT and N588K IhERG1a also included for comparison. There was little difference between WT IhERG1a and IhERG1a/1b in fractional current inactivation at any voltage, whilst by contrast there was a very substantial difference at each voltage between N588K IhERG1a and IhERG1a/1b. Therefore, our data show that the inactivation-attenuating effects of the N588K mutation were markedly greater when co-expressed hERG1a and 1b were studied than when hERG1a alone was studied.
In order to assess the physiological consequences of the N588K mutation for co-expressed hERG1a and 1b, AP clamp experiments were performed. Fig. 4A shows representative currents for WT (Fig. 4Ai) and N588K (Fig. 4Aii) IhERG1a/1b, elicited by a human ventricular AP command [13]. For WT IhERG1a/1b there was little current immediately following the AP upstroke, with current developing progressively throughout the AP plateau, declining during the terminal repolarization phase. Mean data from 14 cells showed that maximal WT IhERG1a/1b during AP repolarization occurred at −22.3 ± 1.0 mV. This value is significantly more positive than that we have recently reported for WT IhERG1a during an identical AP command (−37.6 ± 1.7 mV; [13]) and the earlier peak IhERG1a/1b is consistent with faster deactivation together with accelerated activation and recovery from inactivation of IhERG1a/1b recently reported [16]. For N588K IhERG1a/1b, current increased rapidly following the AP upstroke and peaked early during the AP plateau. Current then declined, with a relatively rapid decline during the final AP repolarization phase. Peak N588K IhERG1a/1b occurred at +27.1 ± 0.2 mV (n = 14), which compares with a value of +24.6 ± 0.6 mV for IhERG1a during the same waveform [13] (P < 0.001). Current density plots for peak repolarizing IhERG1a/1b (Fig. 4C) show a markedly greater maximal repolarizing current for N588K than for WT IhERG1a/1b during a ventricular AP command waveform (P < 0.001).
For hERG1a, a differential effect of the N588K mutation on current during ventricular and Purkinje fibre APs has been suggested to contribute toward U wave formation and a pro-arrhythmic substrate in SQT1 [11,12]. Therefore, AP clamp experiments with a Purkinje fibre (PF) AP command [12] were also performed (Fig. 4B). For both WT (Bi) and N588K (Bii) IhERG1a/1b, current during the PF AP was bow-shaped, peaking at −17.4 ± 3.0 mV (n = 13) and −9.0 ± 0.3 mV (n = 14; P < 0.001), respectively, (this compares with −45.6 ± 1.6 and −14.8 ± 0.3 mV for WT and N588K IhERG1a during the same PF waveform under identical conditions [12]). Fig. 4C shows that peak repolarization current densities for both WT and N588K conditions during the PF AP were significantly lower than those during the ventricular AP command, and that the N588K mutation produced only a small increase in maximal repolarizing IhERG1a/1b amplitude, that was not statistically significant (P > 0.05). Thus, effects of the N588K mutation on current during the PF AP were small for co-expressed hERG1a/1b (smaller than for hERG1a alone under identical conditions [12]), consistent with enhanced heterogeneity in repolarizing IKr between ventricular and PF APs in SQT1.
To-date there is comparatively little information available on the effects of clinically relevant hERG mutations on hERG1a/1b heteromers [16]. This is the first study to have investigated hERG1a/1b heteromeric channels in the context of the short QT syndrome and it is only the second that provides detailed information on biophysical properties of WT IhERG1a/1b, at a physiologically relevant temperature. Our findings support the notion that the contribution to ventricular repolarization of current through heteromeric WT hERG1a/1b channels differs from that of hERG1a alone [16]. More remarkably, they demonstrate for the first time a marked difference in the inactivation-attenuating effect of a pathologically relevant hERG mutation when heteromeric hERG1a/1b channels are studied. N-terminal deletion has been demonstrated to slow hERG1a inactivation [25] and the amplified effect of the N588K mutation on fractional inactivation of IhERG1a/1b seen here may reflect synergy between the mutation’s disruption of the role of the S5-Pore linker in the inactivation process and the potential for reduced stabilization of inactivation due to the fact that 1a/1b heteromers have fewer N-terminal contacts to interact with the internal S4–S5 linker [16,25]. Whilst it may be intuitively obvious that the study of hERG1b-specific mutations [16] or N-terminal long QT syndrome (LQTS) mutations (∼20% of hERG1a-linked LQTS mutations occur in the N-terminus [26]) warrants the study of hERG1a/1b heteromeric channels, until now there may not have been a clear rationale to adopt this approach when studying mutations that are distant from the N-terminus. The present study provides a clear demonstration that such mutations cannot be assumed to have identical effects on hERG1a/1b to those found for hERG1a alone. Consequently, it may be prudent to incorporate more widely the study of hERG1a/1b heteromeric channels when investigating biophysical consequences of hERG mutations in both the LQTS and SQTS.
Acknowledgment
This study was funded by the British Heart Foundation (PG/06/139).
References
- 1.Gussak I., Brugada P., Brugada J., Wright R.S., Kopecky S.I., Chaitman B.R., Bjeerregaard P. Idiopathic short QT interval: a new clinical syndrome? Cardiology. 2000;94:99–102. doi: 10.1159/000047299. [DOI] [PubMed] [Google Scholar]
- 2.Gaita F., Giustetto C., Bianchi F., Wolpert C., Schimpf R., Riccardi R., Grossi S., Richiardi E., Borggrefe M. Short QT syndrome: a familial cause of sudden death. Circulation. 2003;108:965–970. doi: 10.1161/01.CIR.0000085071.28695.C4. [DOI] [PubMed] [Google Scholar]
- 3.Schimpf R., Wolpert C., Gaita F., Giustetto C., Borggrefe M. Short QT syndrome. Cardiovasc. Res. 2005;67:357–366. doi: 10.1016/j.cardiores.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 4.Maury P., Extramiana F., Sbragia P., Giustetto C., Schimpf R., Duparc A., Wolpert C., Denjoy I., Delay M., Borggrefe M., Gaita F. Short QT syndrome. Update on a recent entity. Arch. Cardiovasc. Dis. 2008;101:779–786. doi: 10.1016/j.acvd.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Brugada R., Hong K., Dumaine R., Cordeiro J., Gaita F., Borggrefe M., Menendez T.M., Brugada J., Pollevick G.D., Wolpert C., Burashnikov E., Matsuo K., Wu Y.S., Guerchicoff A., Bianchi F., Giustetto C., Schimpf R., Brugada P., Antzelevitch C. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation. 2004;109:30–35. doi: 10.1161/01.CIR.0000109482.92774.3A. [DOI] [PubMed] [Google Scholar]
- 6.Hong K., Bjeerregaard P., Gussak I., Brugada R. Short QT syndrome and atrial fibrillation caused by mutation in KCNH2. J. Cardivasc. Electophysiol. 2005;16:394–396. doi: 10.1046/j.1540-8167.2005.40621.x. [DOI] [PubMed] [Google Scholar]
- 7.Bellocq C., van Ginneken A.C., Bezzina C.R., Alders M., Escande D., Mannens M.M., Baro I., Wilde A.A. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation. 2004;109:2394–2397. doi: 10.1161/01.CIR.0000130409.72142.FE. [DOI] [PubMed] [Google Scholar]
- 8.Giustetto C., Di M.F., Wolpert C., Borggrefe M., Schimpf R., Sbragia P., Leone G., Maury P., Anttonen O., Haissaguerre M., Gaita F. Short QT syndrome: clinical findings and diagnostic-therapeutic implications. Eur. Heart J. 2006;27:2440–2447. doi: 10.1093/eurheartj/ehl185. [DOI] [PubMed] [Google Scholar]
- 9.Cerrone M., Noujaim S., Jalife J. The short QT syndrome as a paradigm to understand the role of potassium channels in ventricular fibrillation. J. Int. Med. 2006;259:24–38. doi: 10.1111/j.1365-2796.2005.01582.x. [DOI] [PubMed] [Google Scholar]
- 10.Mitcheson J.S., Sanguinetti M.C. Biophysical properties and molecular basis of cardiac rapid and slow delayed rectifier K channels. Cell. Physiol. Biochem. 1999;9:201–216. doi: 10.1159/000016317. [DOI] [PubMed] [Google Scholar]
- 11.Cordeiro J.M., Brugada R., Wu Y.S., Hong K., Dumaine R. Modulation of IKr inactivation by mutation N588K in KCNH2: a link to arrhythmogenesis in short QT syndrome. Cardiovasc. Res. 2005;67:498–509. doi: 10.1016/j.cardiores.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 12.McPate M.J., Duncan R.S., Milnes J.T., Witchel H.J., Hancox J.C. The N588K-HERG K+ channel mutation in the ‘short QT syndrome’: mechanism of gain-in-function determined at 37 °C. Biochem. Biophys. Res. Commun. 2005;334:441–449. doi: 10.1016/j.bbrc.2005.06.112. [DOI] [PubMed] [Google Scholar]
- 13.McPate M.J., Zhang H., Ideniran I., Cordeiro J.M., Witchel H.J., Hancox J.C. Comparative effects of the short QT N588K mutation at 37 °C on hERG K+ channel current during ventricular, Purkinje fibre and atrial action potentials: an action potential clamp study. J. Physiol. Pharmacol. 2009;60:23–41. [PubMed] [Google Scholar]
- 14.Grunnet M., Diness T.G., Hansen R.S., Olesen S.P. Biophysical characterization of the short QT mutation hERG-N588K reveals a mixed gain-and loss-of-function. Cell. Physiol. Biochem. 2008;22:611–624. doi: 10.1159/000185545. [DOI] [PubMed] [Google Scholar]
- 15.Jones E.M., Roti Roti E.C., Wang J., Robertson G.A. Cardiac IKr channels minimally comprise hERG 1a and 1b subunits. J. Biol. Chem. 2004;279:44690–44694. doi: 10.1074/jbc.M408344200. [DOI] [PubMed] [Google Scholar]
- 16.Sale H., Wang J., O’Hara T.J., Tester D.J., Phartiyal P., He J.Q., Rudy Y., Ackerman M.J., Robertson G.A. Physiological properties of hERG 1a/1b heteromeric currents and a hERG 1b-specific mutation associated with long-QT syndrome. Circ. Res. 2008;103:e81–e95. doi: 10.1161/CIRCRESAHA.108.185249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lees-Miller J.P., Kondo C., Wang L., Duff H.J. Electrophysiological characterization of an alternatively processed ERG K+ channel in mouse and human hearts. Circ. Res. 1997;81:719–726. doi: 10.1161/01.res.81.5.719. [DOI] [PubMed] [Google Scholar]
- 18.London B., Trudeau M.C., Newton K.P., Bayer A.K., Copeland N.G., Gilbert D.J., Jenkins N.A., Satler C.A., Robertson G.A. Two isoforms of the mouse ether-a-go-go related gene coassemble form channels with properties similar to the rapidly activating component of the cardiac delayed rectifier K current. Circ. Res. 1997;81:870–878. doi: 10.1161/01.res.81.5.870. [DOI] [PubMed] [Google Scholar]
- 19.Pond A.L., Scheve B.K., Benedict A.T., Petrecca K., van Wagoner D.R., Shrier A., Nerbonne J.M. Expression of distinct ERG proteins in rat, mouse, and human heart. Relation to functional IKr channels. J. Biol. Chem. 2000;275:5997–6006. doi: 10.1074/jbc.275.8.5997. [DOI] [PubMed] [Google Scholar]
- 20.Larsen A.P., Olesen S.P., Grunnet M., Jespersen T. Characterization of hERG1a and hERG1b potassium channels – a possible role for hERG1b in the IKr current. Pflugers Arch. 2008;456:1137–1148. doi: 10.1007/s00424-008-0476-7. [DOI] [PubMed] [Google Scholar]
- 21.Milnes J.T., Crociani O., Arcangeli A., Hancox J.C., Witchel H.J. Blockade of HERG potassium currents by fluvoxamine: incomplete attenuation by S6 mutations at F656 or Y652. Br. J. Pharmacol. 2003;139:887–898. doi: 10.1038/sj.bjp.0705335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McPate M.J., Duncan R.S., Hancox J.C., Witchel H.J. Pharmacology of the short QT syndrome N588K-hERG K+ channel mutation: differential impact on selected class I and class III antiarrhythmic drugs. Br. J. Pharmacol. 2008;155:957–966. doi: 10.1038/bjp.2008.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McPate M.J., Duncan R.S., Witchel H.J., Hancox J.C. Disopyramide is an effective inhibitor of mutant HERG K+ channels involved in variant 1 short QT syndrome. J. Mol. Cell. Cardiol. 2006;41:563–566. doi: 10.1016/j.yjmcc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Hancox J.C., Levi A.J., Witchel H.J. Time course and voltage dependence of expressed HERG current compared with native ‘rapid’ delayed rectifier K current during the cardiac ventricular action potential. Pflugers Arch. Eur. J. Physiol. 1998;436:843–853. doi: 10.1007/s004240050713. [DOI] [PubMed] [Google Scholar]
- 25.Wang J., Trudeau M.C., Zappia A.M., Robertson G.A. Regulation of deactivation by an amino terminal domain in HERG potassium channels. J. Gen. Physiol. 1998;112:637–647. doi: 10.1085/jgp.112.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson G.A., Jones E.M., Wang J. Gating and assembly of heteromeric hERG1a/1b channels underlying IKr in the heart Novartis. Found. Symp. 2005;266:4–15. [PubMed] [Google Scholar]