Abstract
Summary: Aspergillus species are globally ubiquitous saprophytes found in a variety of ecological niches. Almost 200 species of aspergilli have been identified, less than 20 of which are known to cause human disease. Among them, Aspergillus fumigatus is the most prevalent and is largely responsible for the increased incidence of invasive aspergillosis (IA) in the immunocompromised patient population. IA is a devastating illness, with mortality rates in some patient groups reaching as high as 90%. Studies identifying and assessing the roles of specific factors of A. fumigatus that contribute to the pathogenesis of IA have traditionally focused on single-gene deletion and mutant characterization. In combination with recent large-scale approaches analyzing global fungal responses to distinct environmental or host conditions, these studies have identified many factors that contribute to the overall pathogenic potential of A. fumigatus. Here, we provide an overview of the significant findings regarding A. fumigatus pathogenesis as it pertains to invasive disease.
INTRODUCTION
Aspergillus species are ubiquitous, saprophytic fungi that play a significant role in global carbon and nitrogen recycling. Although their primary ecological niche is soil or decaying vegetation, aspergilli produce small, hydrophobic conidia that disperse easily into the air and can survive a broad range of environmental conditions. The genus Aspergillus, which includes almost 200 species, has a tremendous impact on public health both beneficially as the workhorse of industrial applications and negatively as plant and human pathogens (71). Several Aspergillus species are utilized for their rich enzymatic profile in the industrial production of foods and pharmaceuticals. For example, Aspergillus niger is used for the industrial production of citric acid, amylases, pectinases, phytases, and proteases; A. terreus is used for the cholesterol-lowering drug lovastatin; and A. oryzae is used for the fermentation of soybeans and rice into soy sauce and sake, respectively. Aspergilli also have a less reputable side in the agricultural industry. Aspergillus section Flavi, particularly A. flavus and A. parasiticus, can contaminate several common crops with aflatoxin, a highly toxic carcinogen with immunosuppressive properties (228, 230). The consumption of contaminated crops can cause serious illness or death and is a common problem in developing countries.
The Human Pathogen A. fumigatus
Among the human pathogenic species of Aspergillus, A. fumigatus is the primary causative agent of human infections, followed by A. flavus, A. terreus, A. niger, and the model organism, A. nidulans (54, 135). Aspergilli cause a wide range of human ailments depending on the immune status of the host (54, 107). In individuals with altered lung function such as asthma and cystic fibrosis patients, aspergilli can cause allergic bronchopulmonary aspergillosis, a hypersensitive response to fungal components. Noninvasive aspergillomas may form following repeated exposure to conidia and target preexisting lung cavities such as the healed lesions in tuberculosis patients. Invasive aspergillosis (IA) is perhaps the most devastating of Aspergillus-related diseases, targeting severely immunocompromised patients. Those most at risk for this life-threatening disease are individuals with hematological malignancies such as leukemia; solid-organ and hematopoietic stem cell transplant patients; patients on prolonged corticosteroid therapy, which is commonly utilized for the prevention and/or treatment of graft-versus-host disease in transplant patients; individuals with genetic immunodeficiencies such as chronic granulomatous disease (CGD); and individuals infected with human immunodeficiency virus (54, 97, 126, 133, 148, 162, 227). Mortality rates range from 40% to 90% in high-risk populations and are dependent on factors such as host immune status, the site of infection, and the treatment regimen applied (114). The severity and increased incidence of IA necessitate a better understanding of the interplay between host and fungus that contributes to A. fumigatus pathogenesis (130). Pathogenesis and virulence are terms used here in the context of altered host immune function, as this organism is inherently an opportunistic pathogen, and disease pathology and progression are the result of both fungal growth and the host response. In this review, we will thus discuss the pathogenic potential of A. fumigatus as a progression of the infectious life cycle within the context of these immunodeficiencies.
Invasive Aspergillosis
Infectious life cycle.
Aspergilli are predominantly saprophytes, growing on dead or decaying matter in the environment. The infectious life cycle of Aspergillus begins with the production of conidia (asexual spores) that are easily dispersed into the air, ensuring ubiquity in both indoor and outdoor environments (Fig. 1) (65, 137). The primary route of human infection is via the inhalation of these airborne conidia, followed by conidial deposition in the bronchioles or alveolar spaces. In healthy individuals, conidia that are not removed by mucociliary clearance encounter epithelial cells or alveolar macrophages, the primary resident phagocytes of the lung. Alveolar macrophages are primarily responsible for the phagocytosis and killing of Aspergillus conidia as well as the initiation of a proinflammatory response that recruits neutrophils (one type of polymorphonuclear cell [PMN]) to the site of infection. Conidia that evade macrophage killing and germinate become the target of infiltrating neutrophils that are able to destroy hyphae. The risk of developing IA results primarily from a dysfunction in these host defenses in combination with fungal attributes that permit A. fumigatus survival and growth in this pulmonary environment (176). Although other host responses have been associated with disease resistance, for this review, we will focus on fungal interactions with the primary innate components that are most important for fungal defense.
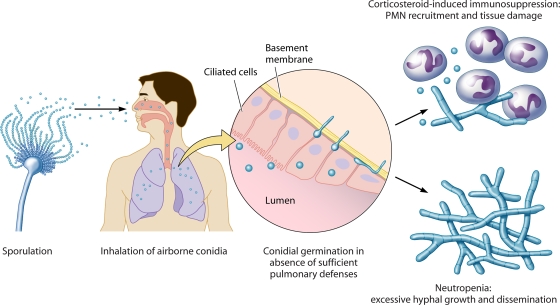
FIG. 1.
Infectious life cycle of A. fumigatus. Aspergillus is ubiquitous in the environment, and asexual reproduction leads to the production of airborne conidia. Inhalation by specific immunosuppressed patient groups results in conidium establishment in the lung, germination, and either PMN-mediated fungal control with significant inflammation (corticosteroid therapy) or uncontrolled hyphal growth with a lack of PMN infiltrates and, in severe cases, dissemination (neutropenia).
Risk factors and pathology.
The primary host immunodeficiencies that are responsible for the increased risk of IA are neutropenia and corticosteroid-induced immunosuppression, and the pathological consequences of IA under these immunosuppressive conditions differ, as described previously for patients and animal models (9, 17, 53, 192). Prolonged neutropenia is classically defined as the most dominant risk factor for IA and is often the result of highly cytotoxic therapies such as cyclophosphamide, which is used for transplant patients or those with hematological diseases. Cyclophosphamide, a DNA-alkylating agent, binds to DNA and interferes with cellular replication, depleting circulating white blood cells including neutrophils. In neutropenic patients and animal models of chemotherapy-induced neutropenia, IA is characterized by thrombosis and hemorrhage from rapid and extensive hyphal growth (41, 192). The lack of inflammatory infiltrates, despite the production of tumor necrosis factor alpha (TNF-α), results in low levels of inflammation. Without neutrophil recovery, angioinvasion and dissemination to other organs via the blood result.
A variety of nonneutropenic patients, most commonly those on corticosteroid therapy such as allogeneic transplant patients receiving corticosteroids for prophylaxis or treatment of graft-versus-host disease, are susceptible to IA, although the pathology of the disease is quite different. IA in these patients and nonneutropenic animal models is nonangioinvasive, characterized by limited fungal development with pyogranulomatous infiltrates, tissue necrosis, and excessive inflammation. Corticosteroids have significant consequences for phagocyte function, including but not limited to the impairment of phagocytosis, phagocyte oxidative burst, production of cytokines and chemokines, and cellular migration (reviewed in reference 116). Several studies have shown that corticosteroids impair the functional ability of phagocytes to kill A. fumigatus conidia and hyphae (37, 92, 132, 171, 172, 214). Despite the effects of steroids on innate immune cell function, neutrophils are recruited to the lung and prevent hyphal invasion but create an inflammatory environment that results in tissue injury. This exacerbated inflammatory response is generally regarded as being the cause of death, in contrast to the uncontrolled fungal growth observed in neutropenic hosts. The dramatic differences in both fungal development and host responses under each immunosuppressive regimen highlight the importance of studying Aspergillus pathogenesis within the context of host immune status and subsequent response to fungal infection.
Animal Models of Invasive Aspergillosis
Identification of the contribution of individual fungal components to overall pathogenicity requires the use of in vivo models of IA. Drosophila melanogaster (104, 115, 186) and Galleria mellonella (140, 163, 164, 166) have been applied to screen A. fumigatus mutants for virulence attributes owing to their ethical and financial advantages over the use of mammalian models. However, results should be interpreted with caution, and interesting phenotypes should be reevaluated using a more applicable animal model. For example, the difference in temperature (flies and worms, which are unable to grow at 37°C, are grown at 25°C) is known to affect multiple fungal characteristics including growth rate and toxin production (see below), and clearly, these models cannot be used to assess pathological outcomes of infection that are relevant to human infection. Indeed, a recent study highlights the need for caution in using Galleria; those authors found that melanin mutants known to be less virulent in mammalian studies (see below) were more virulent in the G. mellonella model (86). A variety of vertebrates including rats, rabbits, birds, and guinea pigs have been used, but mouse models predominate due to the availability of genetically defined species and reagents (42). Outbred mice are commonly chosen because of their cost compared to that of inbred strains, but sufficient numbers should be used to establish reproducibility due to the inherent genetic variability within populations. On the other hand, although inbred mice offer the advantage of genetic reproducibility, studies between individual inbred strains can be vastly different, such that comparisons of multiple inbred strains may benefit studies of fungal pathogenesis.
Specific genetic mouse models exist. CGD (p47phox−/−) or X-CGD (gp91phox−/−) mice display pathological consequences (such as peribronchiolar and alveolar necrosis) of A. fumigatus infection similar to those for humans with CGD and have been a useful model for studying aspergillosis in the context of this specific genetic disease (136, 161). The importance of pattern recognition receptors (PRRs) to fungal recognition and modulation of host responses has been clarified with the use of knockout mice, such as those for dectin-1 and several of the Toll-like receptors (TLRs) (142). Cytokine-deficient mice have also been used to demonstrate the contribution of cytokines to host resistance (such as TNF-α) or susceptibility (interleukin-10 [IL-10]) (33, 40, 158).
The most commonly used animal models of IA involve the induction of neutropenia or corticosteroid-induced immunosuppression to mimic human infection. Neutropenia may be induced by cyclophosphamide or other chemotherapeutic agents (antibody-mediated neutrophil depletion has also been used), whereas animals treated with corticosteroids represent the nonneutropenic model used to evaluate A. fumigatus pathogenesis in the context of inflammatory responses commonly observed in nonneutropenic patients. The use of specific drug or depletion regimens is known to influence survival, pathology, and other outcome parameters (191). Comparison of both models can help to differentiate the fungus-host interactions responsible for pathogenesis in unique patient populations. One of the most striking examples of this is in the case of gliotoxin mutants, which demonstrate wild-type virulence in a neutropenic model but reduced virulence in a nonneutropenic model, suggesting that gliotoxin may be important for pathogenicity only in the context of nonneutropenic hosts (Table 1).
TABLE 1.
Comparison of gliotoxin mutants in different animal models of IA
| Animal | Drug regimena | Inoculation method | Inoculum size (conidia) | Strain of origin | Relative virulence | Reference |
|---|---|---|---|---|---|---|
| BALB/c mouse | Cyclophosphamide, cortisone acetate | Intranasal | 3 × 104 | CEA17 | Similar to wild type | 103 |
| BALB/c mouse | Cyclophosphamide, cortisone acetate | Intranasal | 6 × 104 | ATCC 46645 | Similar to wild type | 103 |
| Outbred Swiss ICR mouse | Cyclophosphamide, cortisone acetate | Intranasal | 5 × 106 | Af293 | Similar to wild type | 25 |
| Outbred Swiss ICR mouse | Cyclophosphamide, cortisone acetate | Inhalation | 109/ml, 1 h | Af293 | Similar to wild type | 47 |
| BALB/c mouse | Cyclophosphamide, cortisone acetate | Inhalation | 109/ml, 1 h | Af293 | Similar to wild type | 186 |
| BALB/c mouse | Cortisone acetate | Intranasal | 5 × 106 | Af293 | Less than wild type | 186 |
| BALB/c mouse | Cortisone acetate | Inhalation | 109/ml, 1 h | Af293 | Less than wild type | 186 |
| BALB/c mouse | Cortisone acetate | Intranasal | 5 × 106 | B5233 | Less than wild type | 197 |
| 129/Sv | Cortisone acetate | Intranasal | 5 × 106 | B5233 | Less than wild type | 197 |
| D. melanogaster (Toll deficient) | NA | Needle puncture | 108/ml solution | Af293 | Less than wild type | 186 |
NA, not applicable.
Other variables to account for when establishing an appropriate animal model to assess fungal pathogenesis include the amount of fungal inoculum, route of infection, and outcome analyses (58). Conidial inoculation may be performed intratracheally, intranasally, intravenously, or via inhalation chamber. Intranasal inoculation is commonly used because of ease of handling, although chamber inhalation is potentially the most useful model in terms of both reproducibility and mimicking human infection (182, 190). Outcome analyses often chosen for assessing disease development include animal survival, organ pathology, host cellular responses, and fungal burden, all of which can be influenced by the variables described above (191).
The interaction of fungi with mammalian cells in vitro can be a useful complement to in vivo studies and can steer experiments toward the appropriate in vivo assays. For example, although a ΔgliZ gliotoxin mutant displayed virulence similar to that of a wild-type strain in the neutropenic mouse model, gliotoxin production did contribute to neutrophil apoptosis in vitro, supporting the observed virulence reduction of gliotoxin mutants in a nonneutropenic model and reduced neutrophil apoptosis at sites of infection (25, 186, 197). In vitro studies with primary mammalian cells and cell lines are frequently used to assess the role of specific fungal components during fungus-host cell interactions, although A. fumigatus mutants that display altered interactions with host cells in vitro do not always correlate with virulence defects in vivo, particularly when the only in vivo assessment made is animal mortality. This is in agreement with the multifactorial nature of A. fumigatus pathogenesis and emphasizes the significance of examining other outcomes of infection, such as histological analyses or fungal burden. Minor contributions of fungal components to overall pathogenicity may thus be characterized by studying the interaction of A. fumigatus with mammalian cells with further investigation in vivo to understand the pathogenesis of this disease. The use and careful interpretation of animal models and outcomes, as well as in vitro host systems, are thus essential to study the role of fungal and host elements in a disease setting that mimics human infection.
Biology of Aspergillus fumigatus
A genomic comparison of clinical and environmental isolates from diverse host sources and geographic locations suggested that any environmental strain of A. fumigatus may be pathogenic given an appropriate host (50). In comparison to other species, A. fumigatus displays a unique combination of basic traits that contribute to pathogenicity. The primary route of infection with Aspergillus is via the inhalation of airborne conidia and deposition in the bronchioles or alveolar spaces. The average size of A. fumigatus conidia (2 to 3 μm) is ideal for infiltrating deep into the alveoli, whereas larger conidia of other human pathogens including A. flavus and A. niger could be removed more easily by mucociliary clearance of the upper respiratory tract. Furthermore, A. fumigatus is more thermotolerant than other disease-causing species, growing well at 37°C and withstanding temperatures above 50°C, such as those encountered in decaying vegetation, a frequently inhabited niche. It has been speculated that growth at high temperatures may induce the expression of unique stress response genes that confer additional virulence benefits, although evidence for this theory is lacking.
Several studies suggested that the radial growth and germination rate of aspergilli at 37°C correlate with pathogenicity. The deletion of A. fumigatus genes involved in morphogenesis, including the regulatory subunit of the cyclic AMP-dependent protein kinase signaling gene pkaR and the ras family subhomologue rasB, resulted in reduced germination and growth rates in vitro, correlating with reduced virulence in a murine model of IA (67, 235). Mutants of the calcineurin pathway, which is involved in cellular stress responses and morphogenesis, are significantly impaired in growth, exhibiting defects in conidial germination and polarized hyphal growth at 37°C, and are significantly impaired in causing disease in multiple animal models of IA (48). Additionally, a comparison of A. fumigatus, A. flavus, and A. niger growths demonstrated a correlation between the germination rate and pathogenic prevalence. The germination rates of these species were similar at temperatures up to 30°C but differed at 37°C and 42°C (8). Interestingly, human sera, specifically albumin, enhance mycelial growth of several Aspergillus species in vitro and specifically promote conidial germination in A. fumigatus (170). It would appear, therefore, that one factor contributing to the pathogenicity of A. fumigatus is growth rate in vivo, specifically at 37°C. Indeed, the deletion of a gene involved in ribosome biogenesis, cgrA, in A. fumigatus had no effect on growth at 25°C or virulence in a Drosophila insect model (25°C) but was slower in radial growth at 37°C and was reduced in virulence in an animal model (37°C) (19, 23). These studies correlate the rate of growth at 37°C with virulence.
AIRWAY COLONIZATION
Inhalation of Aspergillus conidia is a common occurrence due to their ubiquitous presence in the environment; estimates suggest that the average person may inhale up to 200 conidia per day. In IA-susceptible patient populations, the mucosal defenses of the lung are compromised, leading to fungal colonization and growth.
Aspergillus Interactions with Soluble Lung Components
Following inhalation, A. fumigatus conidia immediately encounter the airway mucosa comprised of the fluid lining the respiratory tract and airway epithelia. This pulmonary fluid is comprised of mucus, proteins, lipids, ions, water, and other cellular secretions that contribute to the mucociliary clearance of inhaled particles or pathogens. Also within this complex fluid are opsonic PRRs that coat inhaled pathogens and contribute to host defense. Among these proteins are the collectins, a group of C-type lectin receptors secreted by type II cells and Clara cells that bind carbohydrate moieties in a calcium-dependent manner. Many pathogenic fungi, including A. fumigatus, have a carbohydrate-rich cell wall that can be recognized by the most common collectins, mannose-binding lectin (MBL) and the surfactant proteins SP-A and SP-D. In vitro, MBL, SP-A, and SP-D have been shown to bind and agglutinate A. fumigatus conidia as well as enhance the phagocytosis and killing of A. fumigatus conidia by macrophages and neutrophils (2, 3, 120, 144). MBL, SP-A, and SP-D were more recently found to activate complement (59). Collectins have been demonstrated to be important in vivo: MBL−/− and SP-D−/− mice exhibit increased susceptibility to IA, and recombinant MBL, SP-D, and SP-A have been used to enhance host defenses against aspergillosis in animal models (94, 121). Collectins may thus contribute to conidial clearance by enhancing complement activation, phagocytosis, and killing of conidia or aggregating conidia for other host defenses.
One of the earliest host responses to microorganisms is the activation of complement, a collection of serum proteins that recognize and bind conserved microbial constituents, resulting in opsonization or destruction. Although found predominantly in serum, complement components are present, albeit at lower levels, in bronchoalveolar fluid and have the potential to be involved in host defense against Aspergillus. Three complement pathways exist, converging at binding of C3 to the microbial surface. Early studies demonstrated that A. fumigatus conidia and hyphae bind C3. In comparison to other human pathogenic species, A. fumigatus as well as A. flavus bind fewer C3 molecules per unit of conidial surface (75). The majority of bound C3 is cleaved to iC3b, a ligand for phagocytic complement receptors; thus, A. fumigatus and A. flavus may be less susceptible to complement-mediated phagocytosis or phagocyte recognition (193, 194). Conidia and hyphae from several Aspergillus species also bind the alternative complement inhibitor factor H, its splice product FHR-1, and factor H family protein FFHL-1, preventing the activation of complement cascades (13, 213). Binding to the classical and lectin pathway inhibitor C4b-binding protein has been observed for A. fumigatus (213). Finally, A. fumigatus and A. flavus, but not A. niger, were found to produce a soluble complement-inhibitory factor, potentially lipid derived, that prevented alternative pathway activation (219, 221). It would thus appear that A. fumigatus and, to a lesser-known extent, A. flavus have defense mechanisms to inhibit or reduce complement activation. Thus, the ability of A. fumigatus to inhibit complement activation may contribute to the overall pathogenesis of this organism.
Other soluble components involved in Aspergillus defense include the pentraxin PTX3 and plasminogen. PTX3 is a soluble opsonin produced by phagocytes that facilitates microbial recognition (28). Mice deficient in PTX3 are susceptible to IA, which correlated with a reduced recognition of A. fumigatus conidia by phagocytes (69). A recent study implicated genetic variation in plasminogen, a component of the fibrolytic pathway, in susceptibility to IA (233). Plasminogen bound to A. fumigatus has also been detected, which, when cleaved into active plasmin, could enhance dissemination via its role in the degradation of the extracellular matrix (13).
Aspergillus Interaction with Respiratory Epithelia
Despite the importance of respiratory epithelia in initiating antimicrobial innate immune responses against many inhaled pathogens, few studies have examined the role of the airway epithelia in the host defense against Aspergillus (12, 127). As the first cells encountered by inhaled conidia, airway epithelia likely contribute to the overall immune response to A. fumigatus. Epithelial cells may secrete soluble antimicrobial compounds that play a direct role in airway defense. Members of the defensin family of antimicrobial peptides have broad-spectrum activity against multiple microbes and are produced by epithelial cells in vitro following incubation with A. fumigatus (1). In vitro, Aspergillus germinating conidia and hyphae, but not resting conidia, are recognized by host PRRs on epithelial cells and induce the production of cytokines and chemokines such as IL-6, TNF-α, and IL-8 (10, 14). Corticosteroid administration can eliminate this inflammatory response, questioning the function of epithelial cells in corticosteroid-treated patients at risk for IA (14). Epithelial cells likely assist in initiating proinflammatory responses against A. fumigatus, although their contribution is likely far less robust than that of the alveolar macrophage.
A. fumigatus conidia have been shown to bind and be engulfed by a variety of epithelial cells including tracheal epithelial cells, alveolar type II cells, human nasal epithelial cells, and the A549 lung epithelial cell line (66, 155, 223). Conidia engulfed by A549 epithelia enter acidic phagolysosomes and can be killed, although some conidia are able to germinate and exit both the phagolysosome and pneumocyte without evidence of pneumocyte damage (222, 223). A. fumigatus conidia are also able to inhibit drug- or TNF-α-induced apoptosis in primary epithelial cells and epithelial cell lines in vitro, although the in vivo implications of this are unknown (18).
Several fungally derived factors may contribute to the ability of A. fumigatus to bind and modulate the airway epithelium (Fig. 2). One factor contributing to A. fumigatus binding and uptake by epithelial cells is the presence of sialic acid residues on conidia (34, 51). Interestingly, pathogenic species of aspergilli, including A. fumigatus, display more conidial sialic acid than do nonpathogenic aspergilli (224). Adhesion to fibrinogen, the basement membrane glycoprotein laminin, and the extracellular matrix component fibronectin is also partially mediated by sialic acid residues and other proteins on the conidial surface (7, 30, 31, 34, 46, 202, 203). The significance of conidial binding to these components is linked to the fact that lung injury or distress is a risk factor for IA. Fibrinogen (fibrin after processing) and fibronectin attach to wounded surfaces such as the surface of damaged epithelia, and laminin is exposed upon epithelial injury or detachment. Sialic acid, and perhaps other conidial factors that bind to alveolar components, could thus contribute to pathogenicity by enhancing adhesion to and colonization of epithelia and components of injured tissue.
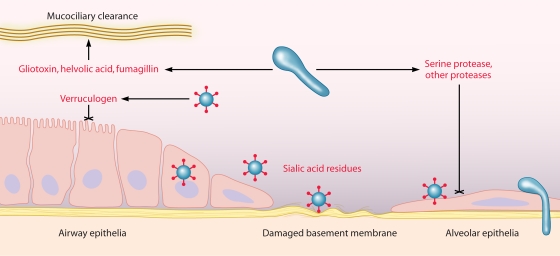
FIG. 2.
Interaction of A. fumigatus with respiratory epithelia. Following inhalation, A. fumigatus encounters airway epithelia (lining trachea, bronchi, and bronchioles), the mucus and fluid lining the upper respiratory tract, and, ultimately, the alveolar space. Fungal products (shown in red) may enhance colonization through tissue injury (cross-haired line) and attachment to epithelial cells or damaged basement membrane. Conidia may also germinate and invade the surrounding lung tissue via the basement membrane or following ingestion by epithelial cells.
A. fumigatus may facilitate colonization in otherwise healthy lung tissue via secreted products that alter epithelial function and viability. Culture filtrates from A. fumigatus strains have been shown to induce cell shrinkage, desquamation, and actin cytoskeleton rearrangement in A549 cells (93, 98). The activity of filtrates could be inhibited with serine and cysteine protease inhibitors, implicating protease activity. Indeed, when a specific serine protease, AF-ALF, was deleted, culture filtrates failed to induce actin cytoskeleton damage (98). Protease activity in A. fumigatus culture filtrates has also been linked to human nasal epithelial cell detachment and loss of focal contacts that may assist germinating hyphae in invading the lung tissue (98, 169, 201).
Human respiratory epithelial cell damage and slowed ciliary beat frequency from A. fumigatus culture filtrates and sputum samples obtained from patients with pulmonary aspergillosis have been linked to secondary metabolites, specifically gliotoxin and, at higher concentrations, fumagillin and helvolic acid (5, 6, 43). The tremorigenic metabolite verruculogen, produced in conidial and hyphal filtrates of many A. fumigatus strains, has also been implicated in modifying transepithelial resistance, hyperpolarization, and cytoplasmic vacuolization of human nasal epithelial cells in an air-liquid interface model of the airway epithelium (29, 96). Associated with conidia and hyphal elements, verruculogen could impact the airway epithelium during early infection, although production in vivo has yet to be observed (96). Thus, A. fumigatus is able to interfere with mucociliary clearance, bind respiratory epithelia and basement membrane proteins, and invade or damage epithelial cells to establish infection and potentially evade other host defenses.
Aspergillus and the Alveolar Macrophage
Macrophage responses to Aspergillus.
Alveolar macrophages are the primary resident phagocytic cells of the respiratory tract and a critical component of the host defense against Aspergillus conidia. Alveolar macrophages phagocytose Aspergillus conidia in an actin-dependent manner, a process mediated by the recognition of pathogen-associated molecular patterns by host cell PRRs. PRR engagement of A. fumigatus ligands generates a proinflammatory response characterized by the production of cytokines and chemokines that are important for host defense against this organism, including TNF-α, IL-1β, IL-6, IL-8, macrophage inflammatory protein 1α, and monocyte chemoattractant protein 1 (39, 131, 138, 158). TLR2 and TLR4 and the C-type lectin receptor dectin-1 are the most well-characterized PRRs involved in the recognition of A. fumigatus and the activation of host cells. In vitro studies have demonstrated that conidia and hyphae activate macrophages through TLR2 and TLR4, and TLR2 recognizes both conidial and hyphal morphologies, whereas TLR4 recognizes only the hypha form (143). Studies using TLR−/− mice also suggest an essential role for TLR4, and potentially TLR2, in vivo. In neutropenic models, TLR2−/− and TLR4−/− mice exhibit higher fungal burdens than wild-type mice (11, 15). Although TLR4−/− mice have lower survival rates than wild-type mice in these studies, contradicting results were demonstrated for TLR2−/− mice. The role of TLRs in nonneutropenic models has not been well studied, although TLR4 polymorphisms in allogeneic hematopoietic stem cell transplant patients have been associated with an increased risk for IA (22).
Unlike TLRs, dectin-1 is essential for the host defense against Aspergillus in both immunosuppressed and immunocompetent hosts (226). Dectin-1 is specific for the fungal carbohydrate β(1,3)-glucan, which is normally masked on resting A. fumigatus conidia by the proteinaceous hydrophobin layer. Following conidial swelling, β(1,3)-glucan becomes exposed and is present on swollen conidia, germlings, and hypha morphotypes (70, 77). Dectin-1-β(1,3)-glucan engagement results in phagocytosis, macrophage activation, and a strong induction of proinflammatory responses (35, 70, 77, 119, 189, 210). Thus, dectin-1, with additional contributions by TLRs, enables innate immune cells to phagocytose and kill conidia as well as elicit proinflammatory responses.
Alveolar macrophages kill conidia that have swollen within the phagolysosome with reactive oxygen species (ROS) and phagolysosomal acidification (83, 159, 220). Philippe et al. first demonstrated the role of ROS in macrophage-mediated conidial killing. In these experiments, alveolar macrophages from p47phox−/− CGD mice, or wild-type alveolar macrophages treated with chemical inhibitors of NADPH oxidase, were significantly impaired in their ability to kill A. fumigatus conidia (159). Although other studies suggested that NADPH-mediated oxidative responses do not contribute to alveolar macrophage killing of conidia, several factors (macrophage-to-conidium ratios, coincubation times, and animal strains and/or cell types tested) can lead to conflicting results (136, 177). It does appear that nonoxidative mechanisms contribute to conidial killing. Reactive nitrogen species are ineffective, although other candidates (antimicrobial peptides, for example) have yet to be tested (159). Overall, immunosuppressed patients who are susceptible to IA have reduced alveolar macrophage effector functions, either from corticosteroid-mediated suppression or from chemotherapy-induced depletion, resulting in the ability of A. fumigatus to escape macrophage killing.
A. fumigatus defenses. (i) Melanin.
In addition to masking β(1,3)-glucan and delaying macrophage activation, resting A. fumigatus conidia are resistant to macrophage killing (Fig. 3). The protective role of the pigment melanin against host defenses, specifically via scavenging ROS, has been described for many pathogenic fungi (87, 106). In Aspergillus, melanin provides the conidial pigment that has been used to distinguish between some species. In A. fumigatus, a white, pigmentless strain was first described by Jahn et al. following UV mutagenesis (88). Complementation of the wild-type phenotype using an A. fumigatus cosmid library identified a polyketide synthase gene, pksP, as being the source of pigment production (105). The white mutant displayed ultrastructural cell wall differences and increased susceptibility to the oxidants H2O2 and NaOCl in comparison to wild-type conidia (88). Additionally, white conidia induced greater monocyte and neutrophil production of ROS than did the wild type as a result of wild-type melanin scavenging ROS from the culture medium. Monocytes were able to kill more ingested mutant conidia than wild-type conidia, presumably via ROS-mediated mechanisms. In an animal model of systemic IA, the white conidia were less virulent, demonstrating for the first time the direct role of melanin in A. fumigatus pathogenesis. These studies implicate melanin as being an important contributor to pathogenesis as an ROS scavenger. It should be noted that the systemic animal model of IA does not fully recapitulate the infectious process of IA, as conidia are instilled directly into the blood via the tail vain as opposed to intranasally or intratracheally. Clearly, this administration could lead to a very different host response to A. fumigatus. Studies of another melanin mutant in an intranasal model (see below), however, support the role for melanin in A. fumigatus pathogenesis. One study also implicated the involvement of the cyanide-insensitive alternative oxidase in protecting conidia from macrophage ROS-mediated killing (the hyphal component not being explored) (123). In that study, however, RNA interference was used to knock down aox expression in a melanin mutant such that alternative oxidase may be important for protection against macrophage killing only in the context of a melanin deficiency.
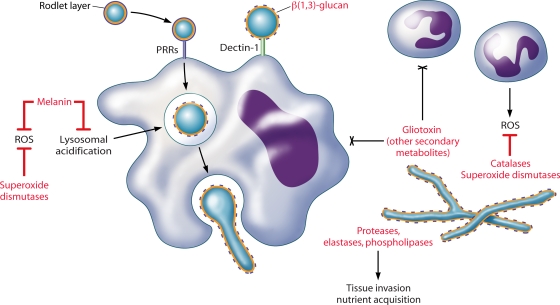
FIG. 3.
A. fumigatus interactions with phagocytes. Alveolar macrophages phagocytose inhaled conidia via PRRs. Conidial swelling (within or outside of the macrophage) releases the protective rodlet layer, exposing β(1,3)-glucan for recognition by dectin-1. Dectin-1-β(1,3)-glucan interactions are primarily responsible for the activation of macrophage proinflammatory responses, including conidial killing. Neutrophils attach to hyphae and degranulate, damaging hyphae by oxidative and nonoxidative mechanisms. Neutrophils may also aggregate conidia and prevent germination. Compromised phagocyte function is the primary risk factor for IA. Fungal products (shown in red) may contribute to fungal pathogenicity in these immunocompromised hosts by evading or modulating host defenses.
Additional studies indicated that human macrophage engulfment of pksP mutant conidia resulted in an increased acidification of the phagosome as a result of phagolysosomal fusion (89). The addition of chloroquine, which increases phagolysosomal fusion and pH, resulted in wild-type killing similar to that of the mutant. A functional pksP therefore prevented some level of phagolysosomal fusion, increasing conidial survival. Furthermore, that study implicated phagolysosome acidification as being a mechanism of conidial killing.
The initial discovery of pksP coincided with the identification by Tsai et al. and Watanabe et al. of another gene involved in pigment biosynthesis, arp1, a naphthopyrone synthase and homologue of scytalone dehydratase (206, 225). Mutants of arp1 were reddish-pink and bound fewer C3 molecules than wild-type conidia, implicating melanin or a pigmented intermediate synthesized by arp1 in the defense against host complement. Further study of conidial pigment biosynthesis in A. fumigatus identified a six-gene cluster involved in dihydroxynaphthalene-melanin biosynthesis, including pksP (also called alb1) (207). The deletion of alb1, similar to the observations by Jahn et al., led to a white phenotype and reduced virulence in mice (205). The alb1 mutants were also more susceptible to C3 binding, reinforcing the notion that melanin or melanin intermediates may prevent complement activation. Another melanin, pyomelanin, produced by the tyrosine degradation pathway, may also contribute to pathogenicity, as mutants showed an enhanced sensitivity to ROS (178). Overall, melanin appears to be a significant determinant of A. fumigatus pathogenesis by protecting conidia against multiple host defenses, particularly those of the alveolar macrophage.
(ii) Mediators of ROS defense.
Other molecules implicated in pathogenicity as scavengers of toxic ROS include rodlets and superoxide dismutases (SODs). Conidia are surrounded by a hydrophobic layer comprised of rodlet proteins. Of the two rodlet genes in A. fumigatus, rodA is solely responsible for rodlet production, and rodA mutants display increased susceptibility to alveolar macrophage killing (156). Rodletless conidia also induced a weak inflammatory response in a rat model of IA (183). Given that conidial swelling is essential for macrophage activation, the rodletless conidia may induce more rapid and robust macrophage activation and conidial elimination. The two eukaryotic SOD enzymes, Cu/Zn-SOD and Mn-SOD, have not been well studied for their role in A. fumigatus pathogenesis. Cu/Zn-SOD has been detected in the cell wall of conidia and hyphae, and SOD activity in culture filtrates has been demonstrated (73, 78). Although Cu/Zn-SOD is upregulated under low-iron conditions in vitro and sera from some patients with A. fumigatus infections react with Cu/Zn-SOD, a specific role in vivo has yet to be identified (38, 73, 79). McDonagh et al. found increased levels of Mn-SOD transcripts in response to neutrophils in vitro and during infection in a murine model of IA, implicating this SOD in oxidative stress defense (129).
Studies of rare hypervirulent mutants substantiate the contribution of oxidative stress resistance to pathogenicity. A disruption of the α(1,3)-glucan synthase gene ags3 led to increased virulence in an animal model of IA, correlating with resistance to oxidative stress in vitro, perhaps related to the increased melanin content or increased germination rate observed in this mutant (128). An unrelated mutant of oxylipin biosynthesis, in which the three cyclooxygenase genes in A. fumigatus were silenced by RNA interference, also demonstrated increased virulence correlating with increased resistance to H2O2 (208). The individual deletion of the cyclooxygenase genes did not yield an increase in virulence, although a loss of one gene, ppoC, resulted in greater resistance to H2O2 as well as aberrant conidium morphology and increased phagocytosis by macrophages (49). Finally, the deletion of the glycosylphosphatidylinositol-anchored protein ECM33 resulted in a hypervirulent strain with increased germination and resistance to cell wall-destabilizing agents (174). Intriguingly, the deletion of the ace2 gene, encoding a C2H2 zinc finger transcription factor, resulted in altered conidial pigmentation, cell wall organization, and resistance to H2O2 (63). The ace2 mutant was hypervirulent in a nonneutropenic mouse model but not in a neutropenic model, implicating resistance to neutrophil oxidative defenses. Transcripts of ags3, ecm33, and ppoC in this mutant were also examined and found to be lower than those of the wild type, suggesting that ACE2 regulation of these genes may contribute to some of the phenotypic similarities among these mutants.
ADAPTATION TO THE MAMMALIAN LUNG ENVIRONMENT
A. fumigatus germination and hyphal growth in the mammalian lung, following the survival of resident pulmonary defenses, require the activation of nutrient-sensing, acquisition, and biosynthetic pathways to obtain nutrients from the host environment. The study of metabolic pathways is essential for an understanding of A. fumigatus pathogenesis, as it allows the identification of host nutrient limitations and the reciprocal response pathways specifically utilized by A. fumigatus to adapt to this environmental niche.
Mammalian Tissue Degradation
The genome of A. fumigatus encodes an extensive arsenal of degradative enzymes supporting the ubiquitous growth of this fungus on plant matter in the environment (56). Although many of these enzymes are specific for plant cell wall components, it is likely that some fungal enzymes may be involved in pathogenesis, since filamentous growth in the mammalian host requires the breakdown of host tissue for nutrient acquisition and invasion (Fig. 3). When the need for protein secretion overwhelms the protein-folding capacity of the endoplasmic reticulum, the unfolded protein response (UPR) is initiated. This can occur under specific environmental stresses such as cell wall perturbation, thermal stress, and nutrient limitation. A recent study by Richie et al. revealed that a disruption of the UPR through the deletion of the hacA gene resulted in a mutant that is sensitive to various stresses, including growth on mammalian lung tissue (168). Moreover, this mutant was significantly impaired in three murine models of IA, implying the need for UPR to regulate the protein secretion pathways needed for tissue degradation and nutrient acquisition in vivo.
Since elastin represents a significant portion of lung tissue, the majority of studies of protease involvement in IA have focused on the role of Aspergillus elastases. Kothary et al. first described a correlation between elastase production and invasive disease (100). In that study, several elastase-producing and non-elastase-producing environmental strains of A. fumigatus were selected for an infection of cortisone-treated (nonneutropenic) mice. All of the elastase-producing strains were lethal, whereas almost two-thirds of the mice infected with non-elastase-producing strains survived. Higher fungal burdens and levels of tissue necrosis in the lungs of mice infected with elastase-producing strains were also observed, implicating elastase activity in fungal invasion and pathogenicity. Another study of clinical and environmental A. fumigatus isolates found that all strains from patients with IA displayed elastase activity but that more than one-third of environmental strains lacked elastase activity (21). Strains from patients with IA also displayed higher average elastase activities than strains from patients with aspergillomas or who were colonized with A. fumigatus, linking elastase activity to the pathogenicity of invasive disease.
The large number and functional redundancy of proteolytic enzymes have made it difficult to determine the significance of elastase activity, or of individual proteases, in pathogenicity. A serine protease, alp, was first identified as being one source of elastinolytic activity (165). Anti-alp antibodies were produced in sera of patients with aspergillosis, and Alp could be detected in infected human lung tissue, linking the production of this protease to infection. However, several studies have demonstrated that A. fumigatus alp (Afalp) mutants display no significant differences in virulence in cortisone-treated or neutropenic mice (134, 199). Additionally, a histological examination of pulmonary vasculature from patients with IA found a lack of elastinolysis of the blood vessel walls, which brings the contribution of elastase activity to IA into question (55). On the other hand, another elastolytic proteinase with strong homology to Afalp was identified by Kolattukudy et al., and an A. fumigatus mutant of this protease producing less than 10% of the total elastase activity of the wild type was significantly less virulent in irradiated (neutropenic) mice (99). This serine protease was localized to germinating conidia and hyphae in the lungs of neutropenic animals by immunogold electron microscopy (99, 125). In a similar manner, a metalloprotease with elastinolytic activity was localized to invading hyphae in the infected lungs of neutropenic animals. An aspartic proteinase purified from A. fumigatus that could degrade human elastin, type I and type III collagens, and fibronectin and was secreted by penetrating hyphae in a neutropenic model was also described (81, 108). Those studies revealed that one or more proteases with elastinolytic activity are produced during infection, although it is still unclear whether elastase activity contributes to pathogenesis. We suspect that the A. fumigatus proteases responsible for elastase activity are but one factor responsible for tissue degradation for nutrients and fungal invasion of host tissue. In a study of various Aspergillus sp. clinical isolates, A. fumigatus clinical isolates were found to have not only elastase activity but also acid proteinase and phospholipase activity, whereas strains of A. flavus had only elastase activity, and in A. niger, only phospholipase activity was detected (4). Thus, a combination of various proteolytic enzymes may contribute to the ability of A. fumigatus to degrade host tissue for nutritional acquisition and invasion.
Nutrient Biosynthesis and Acquisition
The nutritional needs of A. fumigatus during infection have classically been identified by studying the phenotypes of mutants in metabolic pathways. For example, infection studies with auxotrophic pyrG, pabaA, and lysF mutant strains demonstrated a requirement for fungal biosynthesis of uracil/uridine, folate, and lysine, respectively, to maintain survival and virulence in vivo (36, 52, 113, 175). To date, these are the only studies of auxotrophic strains that demonstrate the stringent need for the fungal biosynthesis of nutrients that are unavailable in the host.
Nitrogen metabolism also plays a role in the pathogenicity of A. fumigatus. Aspergilli can utilize a wide range of nitrogen sources and contribute significantly to global nitrogen recycling via nitrate assimilation, in which environmental nitrate is taken and converted into ammonium and subsequently glutamine and glutamate. Proteins that are involved in nitrate transport and processing are transcriptionally regulated by the areA gene locus. Hensel et al. found demonstrable growth differences between areA mutants and wild-type A. fumigatus when grown on poor nitrogen sources (74). Although areA mutants displayed virulence similar to that of wild-type strains in a neutropenic model of IA, a delayed-growth phenotype of the mutant was observed in the lung tissue. The reversion rate of areA-hygR transformants in vivo also suggested a beneficial role for areA regulation of nitrogen metabolism. In a separate study, Panepinto et al. identified the Ras-related protein RhbA based on transcriptional upregulation during the contact of A. fumigatus with human endothelial cells (153). rhbA mutants grew slower on poor nitrogen sources and displayed significantly reduced pathogenicity in a neutropenic model of IA. Examination of lung lesions further demonstrated a reduced growth of the rhbA mutant in vivo. It is apparent from these studies that versatility in nitrogen metabolism contributes to the overall pathogenicity of A. fumigatus.
In some cases, mutants of metabolic pathways that have no phenotype in animal models of IA can provide useful information regarding nutritional availability during infection. For A. fumigatus, growth on C2 compounds and fatty acids as sole sources of carbon and energy requires isocitrate lyase or malate synthase. However, mutants of either enzyme were fully virulent in animal models of IA, suggesting that A. fumigatus utilizes alternative carbon sources (147, 179). Other human pathogenic fungi upregulate isocitrate lyase, malate synthase, and β-oxidation enzymes upon phagocytosis by host macrophages, suggesting a glucose-poor, C2/fatty acid-rich environment. It was postulated that the ability to grow on lipids, also expected to be rich within the host cell, accounted for their fully virulent phenotype. However, these mutants also grew well on carbohydrate and amino acid sources of carbon. Considering that some conidia may not encounter alveolar macrophages, it is likely that multiple pathways of carbon assimilation can make up for the loss of the glyoxylate cycle. One candidate pathway is the methylcitrate cycle, which is required for fungal survival and pathogenicity (82, 122). In the degradation of amino acids for carbon assimilation, toxic propionyl coenzyme A accumulates within the cell, which is prevented by methylcitrate synthase. The deletion of methylcitrate synthase in A. fumigatus led to reduced growth and secondary metabolite production in culture, increased susceptibility to macrophage killing in vitro and in vivo, and reduced virulence in insect and murine models of IA (82, 122). It is unclear whether conidial killing was due to an accumulation of toxic propionyl coenzyme A independently of or in addition to susceptibility to host defenses. Nonetheless, carbon assimilation by A. fumigatus in vivo requires the methylcitrate cycle. These studies also suggest that A. fumigatus utilizes native or host protein degradation to obtain amino acids and other nutrients.
Amino acids may be obtained from degraded proteins or synthesized from carbon- and nitrogen-containing precursors. Under amino acid starvation conditions, the derepression of genes involved in amino acid biosynthesis occurs under the control of a conserved pathway regulated by the cpcA locus. The deletion of cpcA led to a less virulent strain in an animal inhalation model of IA, although lung histology showed no significant differences between the mutant and the wild type in terms of fungal growth or dissemination (101). Since the cpcA mutant was prototrophic, it was speculated that in vivo, an imbalance rather than a lack of amino acids results in cpcA activation. Thus, cpcA regulates amino acid biosynthetic pathways to provide the ideal nutrient conditions for growth in the mammalian lung.
Iron Acquisition
Iron is a necessary component of many biosynthetic pathways, acting as a cofactor in enzymatic reactions and as a catalyst in electron transport systems. However, the instability of free iron and sequestration in vivo by host defense mechanisms severely limit iron availability. For many human pathogens including A. fumigatus, the ability to acquire iron from the host is a necessary virulence determinant (Fig. 3). A. fumigatus utilizes two systems for iron acquisition: siderophore-mediated iron uptake and reductive iron assimilation (180). A. fumigatus produces four known siderophores, small ferric-iron-specific chelators. In Aspergillus, fusaricine C and triacetylfusaricine C are excreted to chelate extracellular iron, whereas ferricrocin and hydroxyferricrocin are involved in hyphal and conidial intracellular iron storage, respectively (62, 72). sidA was the first gene identified in the siderophore biosynthesis pathway and catalyzes the first step in all siderophore biosynthesis, the hydroxylation of l-ornithine (76). Mutants of sidA lacking both extracellular and intracellular iron storage were unable to grow or grew poorly under low-iron conditions or in human serum, displayed increased sensitivity to H2O2, and had a reduced growth rate (76, 180). Moreover, sidA mutants were avirulent in neutropenic and nonneutropenic mouse models of IA.
Schrettl et al. recently identified the iron-regulated genes involved in specific siderophore synthesis: sidC, sidD, sidF, and sidG (181). The deletion of any one of these genes in A. fumigatus attenuated virulence in a neutropenic model of IA, demonstrating the requirement for both extracellular and intracellular siderophores in iron acquisition and storage during infection. Characterization of the individual mutants suggested that intracellular siderophores regulated by sidC and sidG store iron for germination and resisting oxidative stress, both of which are important for pathogenesis. Moreover, sidD and sidF regulate the production of extracellular siderophores that promote hyphal growth, perhaps by acquiring iron from human transferrin as demonstrated in vitro. Although siderophore-independent mechanisms of iron assimilation are used by A. fumigatus to obtain iron in vitro, they do not appear to be essential for virulence. A mutant of ftrA, the iron permease involved in reductive iron assimilation, displayed a wild-type phenotype in vitro and retained full virulence in vivo (180). Thus, siderophore-mediated iron acquisition and intracellular storage are essential for A. fumigatus virulence.
In an attempt to gain global insight into the adaptation of A. fumigatus during early infection, McDonagh et al. performed a transcriptional analysis of A. fumigatus from the lungs of infected mice in a neutropenic model of IA (129). In agreement with in vitro and in vivo studies to date, genes upregulated during the initial adaptation to the lung, in comparison to iron-rich laboratory media, include siderophores and iron transport genes as well as genes involved in metal acquisition, nitrogen catabolism, lipid and amino acid catabolism, and carbohydrate transport. Taken as a whole, these studies demonstrate the versatility of nutrient sensing, acquisition, and biosynthesis that contribute to the overall pathogenic potential of A. fumigatus.
Additional Environmental Stresses
Besides nutritional acquisition, A. fumigatus must also adapt to environmental stresses such as temperature (discussed above), pH, and oxygen limitation. In an analysis of fungal adaptation to the mammalian lung during IA performed by McDonagh et al., genes differentially expressed in vivo (compared to laboratory media) correlated with genes expressed under alkaline conditions in vitro (pH 7), such as the pH regulator PacC (129). First identified in A. nidulans, PacC is a transcription factor that activates alkaline-expressed genes and represses acid-expressed genes when grown under alkaline conditions such as physiological pH (200). In a neutropenic model of IA, pacC was required for the full virulence of A. nidulans, implicating pH adaptation as being a pathogenic trait of Aspergillus (20). Adaptation to a hypoxic environment was also expected to be important for A. fumigatus pathogenicity. Inflammatory sites such as those found in Aspergillus-infected lung tissue amass recruited immune cells that metabolize available oxygen and obstruct blood vessels, creating an oxygen-depleted environment. Little is known about the genes involved in hypoxic adaptation in eukaryotic pathogens. A family of transcription factors, sterol-regulatory element-binding proteins, was first characterized in Schizosaccharomyces pombe, which mediates oxygen-dependent sterol synthesis and growth under hypoxic conditions (80). SrbA, a homologue of the S. pombe sterol-regulatory element-binding protein Sre1, was recently identified for A. fumigatus (229). The deletion of srbA led to altered sterol biosynthesis and cell wall morphology. Although growth and conidiation of the srbA mutant were unaffected under oxygen-replete conditions, the srbA mutant displayed altered hyphal growth and morphogenesis under hypoxic conditions and was avirulent in a neutropenic murine model of IA. In CGD mice, the srbA mutant was significantly less virulent than the wild type. Moreover, histological analysis of neutropenic lungs following infection demonstrated host clearance of the srbA mutant, leading to the hypothesis that A. fumigatus requires adaptation to hypoxic conditions to establish invasive infection. In contrast, McDonagh et al. were unable to demonstrate a connection between genes differentially expressed in the mammalian lung and under anaerobic conditions in vitro, although anaerobic conditions completely lack oxygen and are not a suitable comparison to hypoxia (129). Although no one has clearly addressed the availability of oxygen at sites of Aspergillus infection, hypoxia adaptation appears to be a critical component of the pathogenesis of other fungal pathogens including Candida albicans and Cryptococcus neoformans and deserves further investigation in A. fumigatus (64).
ASPERGILLUS AND THE NEUTROPHIL
Role of the Neutrophil
Neutropenia is a primary risk factor for IA. Animal models and in vitro studies have demonstrated that neutrophils attach to fungal hyphae and degranulate, resulting in fungal killing by oxidative and nonoxidative mechanisms (57, 111). Similarly to epithelial cells and macrophages, neutrophils utilize various PRRs, including TLRs and dectin-1, to recognize and respond to A. fumigatus (15, 16, 95, 226). In addition to oxidative attack mediated by NADPH oxidase, neutrophil granules contain a variety of antimicrobial compounds such as proteases, defensins, pentraxin-3, lysozyme, and lactoferrin (reviewed in reference 141). Although conidia are relatively resistant to neutrophil killing, recent evidence suggests that neutrophils contribute to conidial control by forming aggregates in the lung to inhibit conidial germination (27, 109, 110, 234). Inhibition was mediated by lactoferrin sequestration of iron, an essential nutrient for fungal growth in vivo (27, 234). The relevance of neutrophil defense against conidia has been argued given that neutrophil recruitment to the lung requires germinated conidia to elicit proinflammatory responses. However, in a patient setting, continuous or repeated exposure to conidial inocula is plausible; thus, neutrophils may encounter newly inhaled conidia following recruitment to the lung from a previous inoculum.
A. fumigatus is the leading cause of death in patients with CGD, although patients usually succumb to aspergillosis only under conditions of heavy inocula or following treatment with immunosuppressive drugs (68). CGD is a primary immunodeficiency characterized by mutations in components of NADPH oxidase, which produces superoxide anion and its metabolites including hydrogen peroxide (H2O2) and hypochlorous acid. Historically, the association of IA with CGD patients suggested a role of ROS in the control of A. fumigatus. However, CGD patients commonly present with hyperinflammation and acute lung injury. Recent studies revealed that superoxide is required for tryptophan catabolism, and the lack of this anion results in the IL-17-dependent hyperinflammatory phenotype that also contributes to the susceptibility of CGD patients to infection (173). Regardless, neutrophils from CGD patients and mouse models have been useful for studying the role of ROS in neutrophil-mediated hyphal killing.
Several studies have indicated the involvement of ROS in neutrophil-mediated hyphal killing by comparing normal human neutrophils to those from CGD patients as well as those with myeloperoxidase (MPO) deficiency. MPO catalyzes the formation of hypochlorous acid from H2O2, both of which are ROS produced by neutrophils. Neutrophils from patients with CGD and MPO deficiency were unable to kill A. fumigatus hyphae, in contrast to those from healthy patients (57, 167). The antihyphal activity could be reconstituted with H2O2, hydrochloric acid, or the addition of normal neutrophils (57, 167). Both p47phox−/− and MPO−/− mice are susceptible to infection with A. fumigatus, demonstrating that ROS contribute to murine control of A. fumigatus (161). CGD p47phox−/− mice exhibited a shorter survival time than MPO−/− mice, perhaps because H2O2 is a precursor for hypochlorous acid, and acute lung injury is already present from hyperinflammation at the initiation of infection (173).
A. fumigatus Hyphal Defenses
ROS scavengers.
As ROS scavengers, catalases may be important for A. fumigatus hyphal defense (Fig. 3). The deletion of the conidium-specific gene catA or the mycelium-specific catalase gene cat1 or cat2 had no effect on virulence in a neutropenic rat model of IA (101). Although the deletion of catA resulted in an increased susceptibility to H2O2 and neutrophil killing, catA conidia were killed similarly to the wild type by alveolar macrophages and were fully virulent in a neutropenic animal model of IA (23). It is possible that catA is not an efficient scavenger of H2O2 and that other scavengers (e.g., melanin or peroxidases) may limit the amount of damage done by macrophages. Regardless, it appears that catA does not contribute significantly to the overall pathogenesis of A. fumigatus. The deletion of either mycelial catalase resulted in a wild-type phenotype in vitro and in vivo (101). When both mycelial catalases were deleted, a slight increase in mycelial sensitivity to H2O2 was observed in vitro, and reduced virulence was observed in vivo, suggesting that mycelial catalases offer some amount of protection against neutrophil-mediated hyphal killing (101).
Some researchers have argued that superoxide and peroxide defenses do not play a significant role in A. fumigatus pathogenesis in an immunocompromised host. The deletion of Afyap1, a regulator of oxidative stress response genes, or the deletion of the putative AfYap1 binding partner AfSkn7 resulted in an increased susceptibility of these mutants to H2O2 but did not alter their virulence in a neutropenic model of IA (72, 76, 106). The Afyap1 mutant was killed to the same extent as the wild type by neutrophils in vitro and was unaffected by the addition of glutathione (a broad inhibitor of reactive oxygen and nitrogen intermediates) (76). Following challenge with H2O2, the Afyap1 mutant also demonstrated reduced catalase activity compared to that of the wild type (76). These observations suggested that defense against ROS was not important for pathogenesis. However, it is also possible that the catalase activities are sufficient or higher during incubation with neutrophils or in vivo than following H2O2 challenge and able to protect Afyap1 similarly to the wild type. Cat1 activity was reduced, but not absent, in the Afyap1 mutant. Considering that the deletion of both mycelial catalases was found to be necessary for the reduced virulence of A. fumigatus in vivo, this seems to be a plausible hypothesis (101). Alternatively, other unidentified ROS scavenging abilities could contribute to oxidative defense in the Afyap1 mutant. Another explanation for the similar virulence attributes of the Afyap1 mutant and the wild-type strain could be the specific immunosuppression model used. In some neutropenic models, the severity of immunosuppression results in such high susceptibility to A. fumigatus that minor contributions of fungal components cannot be established. In general, differences among animal models and fungal strains also make it difficult to compare data from many of these studies, as discussed above for gliotoxin mutants. These concerns are not specific to ROS defense and must be addressed by studies of A. fumigatus pathogenicity. Overall, ROS play a significant role in the host response to A. fumigatus, although the contribution of specific fungal ROS defenses to pathogenicity must be further investigated.
Secondary metabolites.
In addition to melanin, toxins represent a class of secondary metabolites that could contribute to A. fumigatus pathogenesis (Fig. 3 and Table 2). The biosynthesis of secondary metabolites among the aspergilli is quite diverse, and it has been hypothesized that the production of specific secondary metabolites by A. fumigatus in vivo contributes to its pathogenicity, particularly during hyphal growth. Among the known metabolites, the epipolythiodioxopiperazine toxin gliotoxin is abundantly produced by A. fumigatus and is the only toxin isolated in vivo from systemic aspergillosis and IA insect and animal models and from sera of patients with IA (112, 163). The biological activity of gliotoxin is based on an internal disulfide bridge that can bind and inactivate proteins via a sulfide:thiol exchange as well as ROS produced by redox cycling between oxidized and reduced forms of the toxin (61, 204, 217, 218).
TABLE 2.
A. fumigatus secondary metabolites implicated in pathogenesis
| Secondary metabolite | Fungal association(s) | Potential function in vivo | Reference(s) |
|---|---|---|---|
| Gliotoxin | Hyphae | Induction of host cell apoptosis | 25, 32, 45, 150, 160, 188, 195, 215, 216 |
| Epithelial cell damage and slowed ciliary beating | 6 | ||
| Inhibition of phagocytosis and oxidative burst | 60, 209, 232 | ||
| Inhibition of T-cell responses | 139, 188, 231 | ||
| Restrictocin | Hyphae | Inhibition of neutrophil-mediated hyphal damage | 84, 185 |
| Verruculogen | Conidia, hyphae | Affects transepithelial resistance and induces hyperpolarization, cytoplasmic vacuolization of epithelial cells | 29, 96 |
| Fumagillin | Hyphae | Epithelial cell damage and slowed ciliary beating; angiogenesis inhibitor | 5, 6, 43 |
| Helvolic acid | Hyphae | Epithelial cell damage and slowed ciliary beating | 5, 6, 43 |
| Ergot alkaloids | Conidia, hyphae | Unknown | 5, 6, 43 |
| Fumitremorgin | Unknown | Unknown | 124 |
| Afpes1 product | Unknown | Unknown | 164 |
One study examining the relative production of gliotoxin from clinical isolates of several Aspergillus species found that almost all A. fumigatus isolates produced gliotoxin (>95%), compared to other species (103). Gliotoxin was also produced at the highest concentrations in A. fumigatus, suggesting a link between gliotoxin production and the pathogenesis of this species. Studies of gliotoxin function in vitro have identified multiple immunosuppressive activities including an inhibition of macrophage phagocytosis, mitogen-activated T-cell proliferation, mast cell activation, and cytotoxic T-cell responses (60, 139, 146, 188, 231); suppression of immune cell reconstitution following sublethal irradiation (198); slowing of ciliary beat frequency and induction of epithelial cell damage (6); and apoptosis induction in lymphocytes, phagocytes, dendritic cells, liver cells, fibroblasts, and cancer cells (32, 45, 150, 160, 188, 195, 215, 216). Previously reported mechanisms of apoptosis induction include the induction of TNF-mediated cell death, activation of caspase-3 and ROS, inhibition of NF-κB activation, and activation of Bak, which in turn activates ROS production, mitochondrial pore formation, and cell death (149, 154, 236). Gliotoxin has also been shown to inhibit antigen presentation by monocytes and dendritic cells to effector T cells, limiting the subsequent expansion of an antigen-specific adaptive response. Furthermore, gliotoxin may prevent the formation of the NADPH oxidase complex in neutrophils (209, 232). Together, these studies reveal the broad nature of gliotoxin immunosuppression by preventing cellular effector functions or inducing cellular apoptosis.
The contribution of gliotoxin in vivo was first characterized by using mutants of two genes involved in gliotoxin biosynthesis, the transcriptional regulator gliZ and the nonribosomal peptide synthetase gliP. Mutants of either gene were found to be deficient in gliotoxin production (25, 47, 103, 186, 197). In a neutropenic murine model of IA, both gliZ and gliP mutants were found to be as virulent as wild-type strains despite showing reduced cytotoxicity or cellular inhibitory activity of culture filtrates (25, 47, 102). However, in cortisone-treated nonneutropenic mouse models, a reduced virulence of gliP mutant strains compared to that of the wild type was observed (186, 197). Furthermore, Spikes et al. found reduced neutrophil apoptosis at the site of fungal lesions in gliP mutant strain-infected mice (186). Interestingly, supernatants from a gliZ mutant cultured in vitro induced less PMN apoptosis than did supernatants from a wild-type strain (25). These analyses suggest that gliotoxin affects the contribution of neutrophils to host defense, likely via the induction of neutrophil apoptosis.
Specific roles for other toxins in the pathogenesis of IA have not been well defined. The deletion of the res gene to create mutants deficient in the ribonucleotoxin restrictocin did not have an effect on virulence or fungal burden in neutropenic models of IA (185). However, culture filtrates of restrictocin mutants were unable to suppress neutrophil-mediated hyphal damage, in contrast to filtrates from wild-type strains (84). Restrictocin may therefore be important under nonneutropenic conditions, but this has yet to be determined. It is possible that, as with other fungal components, the contribution of individual toxins to pathogenesis may be overlooked. In studies supporting the role for multiple toxins in virulence, Bok and Keller identified the first global regulator of secondary metabolite production, laeA (26). The deletion of laeA led to an almost complete loss of secondary metabolite production. Impaired virulence of the laeA mutant in a neutropenic model of IA correlated with increased uptake by macrophages and reduced killing of neutrophils by live hyphae or culture filtrates (24). The deletion of laeA in another pathogenic strain of A. fumigatus also resulted in reduced gliotoxin production and virulence in a nonneutropenic model of IA (196). Given that the laeA, but not the gliZ, mutant showed reduced virulence in a neutropenic animal model, those authors speculated that other toxins contribute to the pathogenesis of A. fumigatus. Indeed, microarray analysis of cultures grown in vitro revealed 13 secondary metabolite cluster loci regulated by laeA, almost all of which (97%) were suppressed under the conditions tested (157). Furthermore, microarray analysis of wild-type and laeA strains from murine lung following infection revealed a significant overlap in the regulation of genes, in particular secondary metabolite genes, by laeA compared to in vitro culture (129).
Candidate metabolites that may be involved in LaeA-regulated pathogenesis include clavine ergot alkaloids and the product of Afpes1 (164). Ergot alkaloids have been detected in conidial extracts of A. fumigatus as well as in broth cultures (44, 152, 187). One ergot alkaloid, festuclavine, could interfere with several mammalian regulatory systems via the ability to bind serotonin, dopamine, and α-adrenalin receptors (151). Although genes regulating the production of clavine ergot alkaloids have been identified for A. fumigatus (152, 211, 212), the role of these metabolites in the pathogenesis of IA has yet to be explored. AfPes1 is an atypical nonribosomal peptide synthetase with homology to nonribosomal peptide synthetases that produce siderophores or destruxins (164). However, since A. fumigatus produces no known destruxins and Afpes1 expression is regulated differently than siderophores, the gene product is expected to be a different compound (145, 164). The deletion of Afpes1 resulted in altered conidial surface morphology, hydrophobicity, and melanin production; an increased susceptibility to oxidative stress in vitro and killing by human neutrophils; and reduced virulence in a G. mellonella model of IA. The similarities between the Afpes1 and laeA mutants suggest a potential role for the Afpes1 metabolite in virulence.
Other LaeA-regulated toxins have been implicated in contributing to pathogenicity. Maiya et al. identified a gene cluster responsible for fumitremorgin biosynthesis in A. fumigatus, yet those authors could not detect fumitremorgin (or its precursors) under reported fumitremorgin-inducing conditions, and the role of this compound in pathogenesis has not been tested (124). Fumagillin, a potent angiogenesis inhibitor, is a sesquiterpene implicated in IA (85, 184). This metabolite could alter endothelial cell function associated with hyphal invasion (118). At high concentrations, fumagillin and the metabolite helvolic acid are cilioinhibitory, as mentioned previously (96). The genes required for helvolic acid synthesis have recently been identified (117), which should assist in genetically exploring the role of this metabolite in IA.
DISSEMINATION
A. fumigatus can disseminate throughout the lung and via the bloodstream to other organs. Growing hyphae that escape host defenses may invade the endothelial cell lining of blood vessels to gain access to the vasculature (Fig. 1). As mentioned previously, angioinvasion is commonly associated with neutropenia, as neutrophils are not available for hyphal killing and the control of fungal growth. Hyphal invasion occurs from the abluminal side to the luminal side of endothelial cells, inducing endothelial cell activation but little cell damage (90, 91). During this process, hyphal fragments can break off into the bloodstream and invade the endothelium at other sites, resulting in hematogenously disseminated disease. Invasion into other organs requires hyphal invasion from the luminal side to the abluminal side of endothelia, causing significant damage (90). In either case, angioinvasion results in thrombosis and infarction, creating an area of necrotic tissue that is well suited to fungal growth. To date, fungal products that contribute directly to the specific manipulation of endothelial cells are not well defined, although as noted above, angiogenesis-inhibitory properties associated with fumigillin render this metabolite a suspect. Live conidia induce more endothelial cell damage than do heat-killed conidia, suggesting the involvement of a secreted compound, whereas damage induced by germ tubes is independent of viability, implicating a fungal cell wall component (90). In contrast, hyphae induce greater endothelial cell activation than conidia, and endothelial cell activation may be independent of secondary metabolites (90, 91).
CONCLUSION
Aspergillus fumigatus is the most common etiological agent of human aspergillosis. Profoundly immunocompromised patients, particularly those with hematological malignancies or who have undergone transplantation, are at risk for the most severe of Aspergillus-caused infections, IA. Despite the breadth of studies of Aspergillus pathogenesis, there are few well-defined factors that contribute to A. fumigatus-related IA. Perhaps most important are the basic biological characteristics of this species. Small, easily airborne conidia access the lower respiratory tract, and A. fumigatus grows well at 37°C, with germination rates exceeding those of other species. In addition to these traits, A. fumigatus adapts to environmental conditions imposed within the mammalian lung. In immunosuppressed individuals at risk for IA, conidia colonize injured lung tissue or lung epithelia, evade macrophage killing, and degrade surrounding tissue to obtain or synthesize nutrients necessary for growth. Depending on the underlying host immune status, A. fumigatus may grow uncontrolled and disseminate (neutropenia) or be controlled by neutrophils and result in excessive inflammation (steroid-induced immunosuppression). The pathogenesis of A. fumigatus is multifactorial, and it is likely that many of the fungal attributes described in this review contribute to the prevalence of this particular species in IA. Mutant characterization and global analyses in response to conditions encountered in vivo have provided significant insight into the overall pathogenesis of A. fumigatus. In combination with these methods, cross-species comparisons and incorporation of host responses to Aspergillus infection could yield further insight into the complexity of A. fumigatus pathogenesis during IA.
Biography
 Taylor Dagenais graduated from the University of Illinois, Urbana-Champaign, with a B.S. in Biochemistry in 2002. In 2007, she obtained her Ph.D. from the University of Wisconsin, Madison, following characterization of antigen-presenting cells during African trypanosomiasis. Currently in her third year as a postdoctoral fellow at the University of Wisconsin, Madison, her research interests are focused on the interactions of aspergilli with phagocytic cells of the immune system.
Taylor Dagenais graduated from the University of Illinois, Urbana-Champaign, with a B.S. in Biochemistry in 2002. In 2007, she obtained her Ph.D. from the University of Wisconsin, Madison, following characterization of antigen-presenting cells during African trypanosomiasis. Currently in her third year as a postdoctoral fellow at the University of Wisconsin, Madison, her research interests are focused on the interactions of aspergilli with phagocytic cells of the immune system.
 Nancy P. Keller is a professor in the Department of Medical Microbiology and Immunology and the Department of Bacteriology at University of Wisconsin, Madison. Her interest in fungal secondary metabolism dates back to her days in the Peace Corps in Lesotho (Africa), from first-hand knowledge of molded and toxic food supplies. Her current interests range from elucidation of virulence traits of human and plant pathogens to genome mining for fungal natural products. She received her Ph.D. from the Department of Plant Pathology at Cornell University in 1990 and was then employed as a postdoctoral research scientist with the USDA, followed by employment as an Assistant and Associate Professor in the Plant Pathology Department at Texas A&M University before moving to the Plant Pathology Department at University of Wisconsin.
Nancy P. Keller is a professor in the Department of Medical Microbiology and Immunology and the Department of Bacteriology at University of Wisconsin, Madison. Her interest in fungal secondary metabolism dates back to her days in the Peace Corps in Lesotho (Africa), from first-hand knowledge of molded and toxic food supplies. Her current interests range from elucidation of virulence traits of human and plant pathogens to genome mining for fungal natural products. She received her Ph.D. from the Department of Plant Pathology at Cornell University in 1990 and was then employed as a postdoctoral research scientist with the USDA, followed by employment as an Assistant and Associate Professor in the Plant Pathology Department at Texas A&M University before moving to the Plant Pathology Department at University of Wisconsin.
REFERENCES
- 1.Alekseeva, L., D. Huet, F. Femenia, I. Mouyna, M. Abdelouahab, A. Cagna, D. Guerrier, V. Tichanne-Seltzer, A. Baeza-Squiban, R. Chermette, J. P. Latge, and N. Berkova. 2009. Inducible expression of beta defensins by human respiratory epithelial cells exposed to Aspergillus fumigatus organisms. BMC Microbiol. 9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, M. J., R. Harbeck, B. Smith, D. R. Voelker, and R. J. Mason. 1999. Binding of rat and human surfactant proteins A and D to Aspergillus fumigatus conidia. Infect. Immun. 67:4563-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, M. J., D. R. Voelker, and R. J. Mason. 2001. Interactions of surfactant proteins A and D with Saccharomyces cerevisiae and Aspergillus fumigatus. Infect. Immun. 69:2037-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alp, S., and S. Arikan. 2008. Investigation of extracellular elastase, acid proteinase and phospholipase activities as putative virulence factors in clinical isolates of Aspergillus species. J. Basic Microbiol. 48:331-337. [DOI] [PubMed] [Google Scholar]
- 5.Amitani, R., T. Murayama, R. Nawada, W. J. Lee, A. Niimi, K. Suzuki, E. Tanaka, and F. Kuze. 1995. Aspergillus culture filtrates and sputum sols from patients with pulmonary aspergillosis cause damage to human respiratory ciliated epithelium in vitro. Eur. Respir. J. 8:1681-1687. [DOI] [PubMed] [Google Scholar]
- 6.Amitani, R., G. Taylor, E. N. Elezis, C. Llewellyn-Jones, J. Mitchell, F. Kuze, P. J. Cole, and R. Wilson. 1995. Purification and characterization of factors produced by Aspergillus fumigatus which affect human ciliated respiratory epithelium. Infect. Immun. 63:3266-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Annaix, V., J. P. Bouchara, G. Larcher, D. Chabasse, and G. Tronchin. 1992. Specific binding of human fibrinogen fragment D to Aspergillus fumigatus conidia. Infect. Immun. 60:1747-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araujo, R., and A. G. Rodrigues. 2004. Variability of germinative potential among pathogenic species of Aspergillus. J. Clin. Microbiol. 42:4335-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balloy, V., M. Huerre, J. P. Latge, and M. Chignard. 2005. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect. Immun. 73:494-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balloy, V., J. M. Sallenave, Y. Wu, L. Touqui, J. P. Latge, M. Si-Tahar, and M. Chignard. 2008. Aspergillus fumigatus-induced interleukin-8 synthesis by respiratory epithelial cells is controlled by the phosphatidylinositol 3-kinase, p38 MAPK, and ERK1/2 pathways and not by the Toll-like receptor-MyD88 pathway. J. Biol. Chem. 283:30513-30521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balloy, V., M. Si-Tahar, O. Takeuchi, B. Philippe, M. A. Nahori, M. Tanguy, M. Huerre, S. Akira, J. P. Latge, and M. Chignard. 2005. Involvement of Toll-like receptor 2 in experimental invasive pulmonary aspergillosis. Infect. Immun. 73:5420-5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bals, R., and P. S. Hiemstra. 2004. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur. Respir. J. 23:327-333. [DOI] [PubMed] [Google Scholar]
- 13.Behnsen, J., A. Hartmann, J. Schmaler, A. Gehrke, A. A. Brakhage, and P. F. Zipfel. 2008. The opportunistic human pathogenic fungus Aspergillus fumigatus evades the host complement system. Infect. Immun. 76:820-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellanger, A. P., L. Millon, K. Khoufache, D. Rivollet, I. Bieche, I. Laurendeau, M. Vidaud, F. Botterel, and S. Bretagne. 2009. Aspergillus fumigatus germ tube growth and not conidia ingestion induces expression of inflammatory mediator genes in the human lung epithelial cell line A549. J. Med. Microbiol. 58:174-179. [DOI] [PubMed] [Google Scholar]
- 15.Bellocchio, S., C. Montagnoli, S. Bozza, R. Gaziano, G. Rossi, S. S. Mambula, A. Vecchi, A. Mantovani, S. M. Levitz, and L. Romani. 2004. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 172:3059-3069. [DOI] [PubMed] [Google Scholar]
- 16.Bellocchio, S., S. Moretti, K. Perruccio, F. Fallarino, S. Bozza, C. Montagnoli, P. Mosci, G. B. Lipford, L. Pitzurra, and L. Romani. 2004. TLRs govern neutrophil activity in aspergillosis. J. Immunol. 173:7406-7415. [DOI] [PubMed] [Google Scholar]
- 17.Berenguer, J., M. C. Allende, J. W. Lee, K. Garrett, C. Lyman, N. M. Ali, J. Bacher, P. A. Pizzo, and T. J. Walsh. 1995. Pathogenesis of pulmonary aspergillosis. Granulocytopenia versus cyclosporine and methylprednisolone-induced immunosuppression. Am. J. Respir. Crit. Care Med. 152:1079-1086. [DOI] [PubMed] [Google Scholar]
- 18.Berkova, N., S. Lair-Fulleringer, F. Femenia, D. Huet, M. C. Wagner, K. Gorna, F. Tournier, O. Ibrahim-Granet, J. Guillot, R. Chermette, P. Boireau, and J. P. Latge. 2006. Aspergillus fumigatus conidia inhibit tumour necrosis factor- or staurosporine-induced apoptosis in epithelial cells. Int. Immunol. 18:139-150. [DOI] [PubMed] [Google Scholar]
- 19.Bhabhra, R., M. D. Miley, E. Mylonakis, D. Boettner, J. Fortwendel, J. C. Panepinto, M. Postow, J. C. Rhodes, and D. S. Askew. 2004. Disruption of the Aspergillus fumigatus gene encoding nucleolar protein CgrA impairs thermotolerant growth and reduces virulence. Infect. Immun. 72:4731-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bignell, E., S. Negrete-Urtasun, A. M. Calcagno, K. Haynes, H. N. Arst, Jr., and T. Rogers. 2005. The Aspergillus pH-responsive transcription factor PacC regulates virulence. Mol. Microbiol. 55:1072-1084. [DOI] [PubMed] [Google Scholar]
- 21.Blanco, J. L., R. Hontecillas, E. Bouza, I. Blanco, T. Pelaez, P. Munoz, J. Perez Molina, and M. E. Garcia. 2002. Correlation between the elastase activity index and invasiveness of clinical isolates of Aspergillus fumigatus. J. Clin. Microbiol. 40:1811-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bochud, P. Y., J. W. Chien, K. A. Marr, W. M. Leisenring, A. Upton, M. Janer, S. D. Rodrigues, S. Li, J. A. Hansen, L. P. Zhao, A. Aderem, and M. Boeckh. 2008. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N. Engl. J. Med. 359:1766-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boettner, D., N. Huebner, J. C. Rhodes, and D. S. Askew. 2001. Molecular cloning of Aspergillus fumigatus CgrA, the ortholog of a conserved fungal nucleolar protein. Med. Mycol. 39:517-521. [DOI] [PubMed] [Google Scholar]
- 24.Bok, J. W., S. A. Balajee, K. A. Marr, D. Andes, K. F. Nielsen, J. C. Frisvad, and N. P. Keller. 2005. LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot. Cell 4:1574-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bok, J. W., D. Chung, S. A. Balajee, K. A. Marr, D. Andes, K. F. Nielsen, J. C. Frisvad, K. A. Kirby, and N. P. Keller. 2006. GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect. Immun. 74:6761-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bok, J. W., and N. P. Keller. 2004. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot. Cell 3:527-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonnett, C. R., E. J. Cornish, A. G. Harmsen, and J. B. Burritt. 2006. Early neutrophil recruitment and aggregation in the murine lung inhibit germination of Aspergillus fumigatus conidia. Infect. Immun. 74:6528-6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bottazzi, B., C. Garlanda, A. Cotena, F. Moalli, S. Jaillon, L. Deban, and A. Mantovani. 2009. The long pentraxin PTX3 as a prototypic humoral pattern recognition receptor: interplay with cellular innate immunity. Immunol. Rev. 227:9-18. [DOI] [PubMed] [Google Scholar]
- 29.Botterel, F., C. Cordonnier, V. Barbier, L. Wingerstmann, M. Liance, A. Coste, E. Escudier, and S. Bretagne. 2002. Aspergillus fumigatus causes in vitro electrophysiological and morphological modifications in human nasal epithelial cells. Histol. Histopathol. 17:1095-1101. [DOI] [PubMed] [Google Scholar]
- 30.Bouchara, J. P., A. Bouali, G. Tronchin, R. Robert, D. Chabasse, and J. M. Senet. 1988. Binding of fibrinogen to the pathogenic Aspergillus species. J. Med. Vet. Mycol. 26:327-334. [PubMed] [Google Scholar]
- 31.Bouchara, J. P., M. Sanchez, A. Chevailler, A. Marot-Leblond, J. C. Lissitzky, G. Tronchin, and D. Chabasse. 1997. Sialic acid-dependent recognition of laminin and fibrinogen by Aspergillus fumigatus conidia. Infect. Immun. 65:2717-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braithwaite, A. W., R. D. Eichner, P. Waring, and A. Mullbacher. 1987. The immunomodulating agent gliotoxin causes genomic DNA fragmentation. Mol. Immunol. 24:47-55. [DOI] [PubMed] [Google Scholar]
- 33.Brieland, J. K., C. Jackson, F. Menzel, D. Loebenberg, A. Cacciapuoti, J. Halpern, S. Hurst, T. Muchamuel, R. Debets, R. Kastelein, T. Churakova, J. Abrams, R. Hare, and A. O'Garra. 2001. Cytokine networking in lungs of immunocompetent mice in response to inhaled Aspergillus fumigatus. Infect. Immun. 69:1554-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bromley, I. M., and K. Donaldson. 1996. Binding of Aspergillus fumigatus spores to lung epithelial cells and basement membrane proteins: relevance to the asthmatic lung. Thorax 51:1203-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown, G. D., J. Herre, D. L. Williams, J. A. Willment, A. S. Marshall, and S. Gordon. 2003. Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 197:1119-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown, J. S., A. Aufauvre-Brown, J. Brown, J. M. Jennings, H. Arst, Jr., and D. W. Holden. 2000. Signature-tagged and directed mutagenesis identify PABA synthetase as essential for Aspergillus fumigatus pathogenicity. Mol. Microbiol. 36:1371-1380. [DOI] [PubMed] [Google Scholar]
- 37.Brummer, E., A. Maqbool, and D. A. Stevens. 2001. In vivo GM-CSF prevents dexamethasone suppression of killing of Aspergillus fumigatus conidia by bronchoalveolar macrophages. J. Leukoc. Biol. 70:868-872. [PubMed] [Google Scholar]
- 38.Calera, J. A., S. Paris, M. Monod, A. J. Hamilton, J. P. Debeaupuis, M. Diaquin, R. Lopez-Medrano, F. Leal, and J. P. Latge. 1997. Cloning and disruption of the antigenic catalase gene of Aspergillus fumigatus. Infect. Immun. 65:4718-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cenci, E., A. Mencacci, A. Casagrande, P. Mosci, F. Bistoni, and L. Romani. 2001. Impaired antifungal effector activity but not inflammatory cell recruitment in interleukin-6-deficient mice with invasive pulmonary aspergillosis. J. Infect. Dis. 184:610-617. [DOI] [PubMed] [Google Scholar]
- 40.Cenci, E., A. Mencacci, C. F. d'Ostiani, G. Del Sero, P. Mosci, C. Montagnoli, A. Bacci, and L. Romani. 1998. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J. Infect. Dis. 178:1750-1760. [DOI] [PubMed] [Google Scholar]
- 41.Chiang, L. Y., D. C. Sheppard, F. N. Gravelat, T. F. Patterson, and S. G. Filler. 2008. Aspergillus fumigatus stimulates leukocyte adhesion molecules and cytokine production by endothelial cells in vitro and during invasive pulmonary disease. Infect. Immun. 76:3429-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clemons, K. V., and D. A. Stevens. 2005. The contribution of animal models of aspergillosis to understanding pathogenesis, therapy and virulence. Med. Mycol. 43(Suppl. 1):S101-S110. [DOI] [PubMed] [Google Scholar]
- 43.Cody, D. T., II, T. V. McCaffrey, G. Roberts, and E. B. Kern. 1997. Effects of Aspergillus fumigatus and Alternaria alternata on human ciliated epithelium in vitro. Laryngoscope 107:1511-1514. [DOI] [PubMed] [Google Scholar]
- 44.Cole, R. J., J. W. Kirksey, J. W. Dorner, D. M. Wilson, J. Johnson, Jr., D. Bedell, J. P. Springer, K. K. Chexal, J. Clardy, and R. H. Cox. 1977. Mycotoxins produced by Aspergillus fumigatus isolated from silage. Ann. Nutr. Aliment. 31:685-691. [PubMed] [Google Scholar]
- 45.Comera, C., K. Andre, J. Laffitte, X. Collet, P. Galtier, and I. Maridonneau-Parini. 2007. Gliotoxin from Aspergillus fumigatus affects phagocytosis and the organization of the actin cytoskeleton by distinct signalling pathways in human neutrophils. Microbes Infect. 9:47-54. [DOI] [PubMed] [Google Scholar]
- 46.Coulot, P., J. P. Bouchara, G. Renier, V. Annaix, C. Planchenault, G. Tronchin, and D. Chabasse. 1994. Specific interaction of Aspergillus fumigatus with fibrinogen and its role in cell adhesion. Infect. Immun. 62:2169-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cramer, R. A., Jr., M. P. Gamcsik, R. M. Brooking, L. K. Najvar, W. R. Kirkpatrick, T. F. Patterson, C. J. Balibar, J. R. Graybill, J. R. Perfect, S. N. Abraham, and W. J. Steinbach. 2006. Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot. Cell 5:972-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cramer, R. A., Jr., B. Z. Perfect, N. Pinchai, S. Park, D. S. Perlin, Y. G. Asfaw, J. Heitman, J. R. Perfect, and W. J. Steinbach. 2008. Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryot. Cell 7:1085-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dagenais, T. R., D. Chung, S. S. Giles, C. M. Hull, D. Andes, and N. P. Keller. 2008. Defects in conidiophore development and conidium-macrophage interactions in a dioxygenase mutant of Aspergillus fumigatus. Infect. Immun. 76:3214-3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Debeaupuis, J. P., J. Sarfati, V. Chazalet, and J. P. Latge. 1997. Genetic diversity among clinical and environmental isolates of Aspergillus fumigatus. Infect. Immun. 65:3080-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeHart, D. J., D. E. Agwu, N. C. Julian, and R. G. Washburn. 1997. Binding and germination of Aspergillus fumigatus conidia on cultured A549 pneumocytes. J. Infect. Dis. 175:146-150. [DOI] [PubMed] [Google Scholar]
- 52.D'Enfert, C., M. Diaquin, A. Delit, N. Wuscher, J. P. Debeaupuis, M. Huerre, and J. P. Latge. 1996. Attenuated virulence of uridine-uracil auxotrophs of Aspergillus fumigatus. Infect. Immun. 64:4401-4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Denning, D. W. 1996. Aspergillosis: diagnosis and treatment. Int. J. Antimicrob. Agents 6:161-168. [DOI] [PubMed] [Google Scholar]
- 54.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-803. [DOI] [PubMed] [Google Scholar]
- 55.Denning, D. W., P. N. Ward, L. E. Fenelon, and E. W. Benbow. 1992. Lack of vessel wall elastolysis in human invasive pulmonary aspergillosis. Infect. Immun. 60:5153-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Vries, R. P., and J. Visser. 2001. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol. Mol. Biol. Rev. 65:497-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diamond, R. D., and R. A. Clark. 1982. Damage to Aspergillus fumigatus and Rhizopus oryzae hyphae by oxidative and nonoxidative microbicidal products of human neutrophils in vitro. Infect. Immun. 38:487-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dixon, D. M., A. Polak, and T. J. Walsh. 1989. Fungus dose-dependent primary pulmonary aspergillosis in immunosuppressed mice. Infect. Immun. 57:1452-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dumestre-Perard, C., B. Lamy, D. Aldebert, C. Lemaire-Vieille, R. Grillot, J. P. Brion, J. Gagnon, and J. Y. Cesbron. 2008. Aspergillus conidia activate the complement by the mannan-binding lectin C2 bypass mechanism. J. Immunol. 181:7100-7105. [DOI] [PubMed] [Google Scholar]
- 60.Eichner, R. D., M. Al Salami, P. R. Wood, and A. Mullbacher. 1986. The effect of gliotoxin upon macrophage function. Int. J. Immunopharmacol. 8:789-797. [DOI] [PubMed] [Google Scholar]
- 61.Eichner, R. D., P. Waring, A. M. Geue, A. W. Braithwaite, and A. Mullbacher. 1988. Gliotoxin causes oxidative damage to plasmid and cellular DNA. J. Biol. Chem. 263:3772-3777. [PubMed] [Google Scholar]
- 62.Eisendle, M., M. Schrettl, C. Kragl, D. Muller, P. Illmer, and H. Haas. 2006. The intracellular siderophore ferricrocin is involved in iron storage, oxidative-stress resistance, germination, and sexual development in Aspergillus nidulans. Eukaryot. Cell 5:1596-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ejzykowicz, D. E., M. M. Cunha, S. Rozental, N. V. Solis, F. N. Gravelat, D. C. Sheppard, and S. G. Filler. 2009. The Aspergillus fumigatus transcription factor Ace2 governs pigment production, conidiation and virulence. Mol. Microbiol. 72:155-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ernst, J. F., and D. Tielker. 2009. Responses to hypoxia in fungal pathogens. Cell. Microbiol. 11:183-190. [DOI] [PubMed] [Google Scholar]
- 65.Falvey, D. G., and A. J. Streifel. 2007. Ten-year air sample analysis of Aspergillus prevalence in a university hospital. J. Hosp. Infect. 67:35-41. [DOI] [PubMed] [Google Scholar]
- 66.Filler, S. G., and D. C. Sheppard. 2006. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2:e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fortwendel, J. R., W. Zhao, R. Bhabhra, S. Park, D. S. Perlin, D. S. Askew, and J. C. Rhodes. 2005. A fungus-specific Ras homolog contributes to the hyphal growth and virulence of Aspergillus fumigatus. Eukaryot. Cell 4:1982-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gallin, J. I., and K. Zarember. 2007. Lessons about the pathogenesis and management of aspergillosis from studies in chronic granulomatous disease. Trans. Am. Clin. Climatol. Assoc. 118:175-185. [PMC free article] [PubMed] [Google Scholar]
- 69.Garlanda, C., E. Hirsch, S. Bozza, A. Salustri, M. De Acetis, R. Nota, A. Maccagno, F. Riva, B. Bottazzi, G. Peri, A. Doni, L. Vago, M. Botto, R. De Santis, P. Carminati, G. Siracusa, F. Altruda, A. Vecchi, L. Romani, and A. Mantovani. 2002. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 420:182-186. [DOI] [PubMed] [Google Scholar]
- 70.Gersuk, G. M., D. M. Underhill, L. Zhu, and K. A. Marr. 2006. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J. Immunol. 176:3717-3724. [DOI] [PubMed] [Google Scholar]
- 71.Gugnani, H. C. 2003. Ecology and taxonomy of pathogenic aspergilli. Front. Biosci. 8:s346-s357. [DOI] [PubMed] [Google Scholar]
- 72.Haas, H., M. Schoeser, E. Lesuisse, J. F. Ernst, W. Parson, B. Abt, G. Winkelmann, and H. Oberegger. 2003. Characterization of the Aspergillus nidulans transporters for the siderophores enterobactin and triacetylfusarinine C. Biochem. J. 371:505-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hamilton, A. J., M. D. Holdom, and L. Jeavons. 1996. Expression of the Cu,Zn superoxide dismutase of Aspergillus fumigatus as determined by immunochemistry and immunoelectron microscopy. FEMS Immunol. Med. Microbiol. 14:95-102. [DOI] [PubMed] [Google Scholar]
- 74.Hensel, M., H. N. Arst, Jr., A. Aufauvre-Brown, and D. W. Holden. 1998. The role of the Aspergillus fumigatus areA gene in invasive pulmonary aspergillosis. Mol. Gen. Genet. 258:553-557. [DOI] [PubMed] [Google Scholar]
- 75.Henwick, S., S. V. Hetherington, and C. C. Patrick. 1993. Complement binding to Aspergillus conidia correlates with pathogenicity. J. Lab. Clin. Med. 122:27-35. [PubMed] [Google Scholar]
- 76.Hissen, A. H. T., A. N. C. Wan, M. L. Warwas, L. J. Pinto, and M. M. Moore. 2005. The Aspergillus fumigatus siderophore biosynthetic gene sidA, encoding l-ornithine N5-oxygenase, is required for virulence. Infect. Immun. 73:5493-5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hohl, T. M., H. L. Van Epps, A. Rivera, L. A. Morgan, P. L. Chen, M. Feldmesser, and E. G. Pamer. 2005. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 1:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holdom, M. D., R. J. Hay, and A. J. Hamilton. 1995. Purification, N-terminal amino acid sequence and partial characterization of a Cu,Zn superoxide dismutase from the pathogenic fungus Aspergillus fumigatus. Free Radic. Res. 22:519-531. [DOI] [PubMed] [Google Scholar]
- 79.Holdom, M. D., B. Lechenne, R. J. Hay, A. J. Hamilton, and M. Monod. 2000. Production and characterization of recombinant Aspergillus fumigatus Cu,Zn superoxide dismutase and its recognition by immune human sera. J. Clin. Microbiol. 38:558-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hughes, A. L., B. L. Todd, and P. J. Espenshade. 2005. SREBP pathway responds to sterols and functions as an oxygen sensor in fission yeast. Cell 120:831-842. [DOI] [PubMed] [Google Scholar]
- 81.Iadarola, P., G. Lungarella, P. A. Martorana, S. Viglio, M. Guglielminetti, E. Korzus, M. Gorrini, E. Cavarra, A. Rossi, J. Travis, and M. Luisetti. 1998. Lung injury and degradation of extracellular matrix components by Aspergillus fumigatus serine proteinase. Exp. Lung Res. 24:233-251. [DOI] [PubMed] [Google Scholar]
- 82.Ibrahim-Granet, O., M. Dubourdeau, J. P. Latge, P. Ave, M. Huerre, A. A. Brakhage, and M. Brock. 2008. Methylcitrate synthase from Aspergillus fumigatus is essential for manifestation of invasive aspergillosis. Cell. Microbiol. 10:134-148. [DOI] [PubMed] [Google Scholar]
- 83.Ibrahim-Granet, O., B. Philippe, H. Boleti, E. Boisvieux-Ulrich, D. Grenet, M. Stern, and J. P. Latge. 2003. Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infect. Immun. 71:891-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ikegami, Y., R. Amitani, T. Murayama, R. Nawada, W. J. Lee, R. Kawanami, and F. Kuze. 1998. Effects of alkaline protease or restrictocin deficient mutants of Aspergillus fumigatus on human polymorphonuclear leukocytes. Eur. Respir. J. 12:607-611. [DOI] [PubMed] [Google Scholar]
- 85.Ingber, D., T. Fujita, S. Kishimoto, K. Sudo, T. Kanamaru, H. Brem, and J. Folkman. 1990. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 348:555-557. [DOI] [PubMed] [Google Scholar]
- 86.Jackson, J. C., L. A. Higgins, and X. Lin. 2009. Conidiation color mutants of Aspergillus fumigatus are highly pathogenic to the heterologous insect host Galleria mellonella. PLoS ONE 4:e4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jacobson, E. S. 2000. Pathogenic roles for fungal melanins. Clin. Microbiol. Rev. 13:708-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jahn, B., A. Koch, A. Schmidt, G. Wanner, H. Gehringer, S. Bhakdi, and A. A. Brakhage. 1997. Isolation and characterization of a pigmentless-conidium mutant of Aspergillus fumigatus with altered conidial surface and reduced virulence. Infect. Immun. 65:5110-5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jahn, B., K. Langfelder, U. Schneider, C. Schindel, and A. A. Brakhage. 2002. PKSP-dependent reduction of phagolysosome fusion and intracellular kill of Aspergillus fumigatus conidia by human monocyte-derived macrophages. Cell. Microbiol. 4:793-803. [DOI] [PubMed] [Google Scholar]
- 90.Kamai, Y., L. Y. Chiang, L. M. Lopes Bezerra, T. Doedt, A. S. Lossinsky, D. C. Sheppard, and S. G. Filler. 2006. Interactions of Aspergillus fumigatus with vascular endothelial cells. Med. Mycol. 44(Suppl. 1):S115-S117. [DOI] [PubMed] [Google Scholar]
- 91.Kamai, Y., A. S. Lossinsky, H. Liu, D. C. Sheppard, and S. G. Filler. 2009. Polarized response of endothelial cells to invasion by Aspergillus fumigatus. Cell Microbiol. 11:170-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kamberi, M., E. Brummer, and D. A. Stevens. 2002. Regulation of bronchoalveolar macrophage proinflammatory cytokine production by dexamethasone and granulocyte-macrophage colony-stimulating factor after stimulation by Aspergillus conidia or lipopolysaccharide. Cytokine 19:14-20. [DOI] [PubMed] [Google Scholar]
- 93.Kauffman, H. F., J. F. Tomee, M. A. van de Riet, A. J. Timmerman, and P. Borger. 2000. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J. Allergy Clin. Immunol. 105:1185-1193. [DOI] [PubMed] [Google Scholar]
- 94.Kaur, S., V. K. Gupta, S. Thiel, P. U. Sarma, and T. Madan. 2007. Protective role of mannan-binding lectin in a murine model of invasive pulmonary aspergillosis. Clin. Exp. Immunol. 148:382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kennedy, A. D., J. A. Willment, D. W. Dorward, D. L. Williams, G. D. Brown, and F. R. DeLeo. 2007. Dectin-1 promotes fungicidal activity of human neutrophils. Eur. J. Immunol. 37:467-478. [DOI] [PubMed] [Google Scholar]
- 96.Khoufache, K., O. Puel, N. Loiseau, M. Delaforge, D. Rivollet, A. Coste, C. Cordonnier, E. Escudier, F. Botterel, and S. Bretagne. 2007. Verruculogen associated with Aspergillus fumigatus hyphae and conidia modifies the electrophysiological properties of human nasal epithelial cells. BMC Microbiol. 7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kliasova, G. A., N. A. Petrova, E. N. Parovichnikova, L. N. Gotman, V. G. Isaev, E. A. Mikhailova, E. N. Ustinova, N. D. Khoroshko, E. S. Vishnevskaia, A. M. Kremenetskaia, S. K. Kravchenko, I. B. Kaplanskaia, A. A. Kokhno, S. A. Ptitsin, L. S. Liubimova, L. P. Mendeleeva, N. E. Mitish, G. M. Galstian, V. V. Ryzhko, A. V. Tochenov, and V. G. Savchenko. 2005. Invasive pulmonary aspergillosis. Ter. Arkh. 77:65-71. (In Russian.) [PubMed] [Google Scholar]
- 98.Kogan, T. V., J. Jadoun, L. Mittelman, K. Hirschberg, and N. Osherov. 2004. Involvement of secreted Aspergillus fumigatus proteases in disruption of the actin fiber cytoskeleton and loss of focal adhesion sites in infected A549 lung pneumocytes. J. Infect. Dis. 189:1965-1973. [DOI] [PubMed] [Google Scholar]
- 99.Kolattukudy, P. E., J. D. Lee, L. M. Rogers, P. Zimmerman, S. Ceselski, B. Fox, B. Stein, and E. A. Copelan. 1993. Evidence for possible involvement of an elastolytic serine protease in aspergillosis. Infect. Immun. 61:2357-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kothary, M. H., T. Chase, Jr., and J. D. Macmillan. 1984. Correlation of elastase production by some strains of Aspergillus fumigatus with ability to cause pulmonary invasive aspergillosis in mice. Infect. Immun. 43:320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krappmann, S., E. M. Bignell, U. Reichard, T. Rogers, K. Haynes, and G. H. Braus. 2004. The Aspergillus fumigatus transcriptional activator CpcA contributes significantly to the virulence of this fungal pathogen. Mol. Microbiol. 52:785-799. [DOI] [PubMed] [Google Scholar]
- 102.Kupfahl, C., T. Heinekamp, G. Geginat, T. Ruppert, A. Hartl, H. Hof, and A. A. Brakhage. 2006. Deletion of the gliP gene of Aspergillus fumigatus results in loss of gliotoxin production but has no effect on virulence of the fungus in a low-dose mouse infection model. Mol. Microbiol. 62:292-302. [DOI] [PubMed] [Google Scholar]
- 103.Kupfahl, C., A. Michalka, C. Lass-Florl, G. Fischer, G. Haase, T. Ruppert, G. Geginat, and H. Hof. 2008. Gliotoxin production by clinical and environmental Aspergillus fumigatus strains. Int. J. Med. Microbiol. 298:319-327. [DOI] [PubMed] [Google Scholar]
- 104.Lamaris, G. A., R. Ben-Ami, R. E. Lewis, and D. P. Kontoyiannis. 2008. Does pre-exposure of Aspergillus fumigatus to voriconazole or posaconazole in vitro affect its virulence and the in vivo activity of subsequent posaconazole or voriconazole, respectively? A study in a fly model of aspergillosis. J. Antimicrob. Chemother. 62:539-542. [DOI] [PubMed] [Google Scholar]
- 105.Langfelder, K., B. Jahn, H. Gehringer, A. Schmidt, G. Wanner, and A. A. Brakhage. 1998. Identification of a polyketide synthase gene (pksP) of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence. Med. Microbiol. Immunol. 187:79-89. [DOI] [PubMed] [Google Scholar]
- 106.Langfelder, K., M. Streibel, B. Jahn, G. Haase, and A. A. Brakhage. 2003. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 38:143-158. [DOI] [PubMed] [Google Scholar]
- 107.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee, J. D., and P. E. Kolattukudy. 1995. Molecular cloning of the cDNA and gene for an elastinolytic aspartic proteinase from Aspergillus fumigatus and evidence of its secretion by the fungus during invasion of the host lung. Infect. Immun. 63:3796-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Levitz, S. M., and R. D. Diamond. 1984. Killing of Aspergillus fumigatus spores and Candida albicans yeast phase by the iron-hydrogen peroxide-iodide cytotoxic system: comparison with the myeloperoxidase-hydrogen peroxide-halide system. Infect. Immun. 43:1100-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Levitz, S. M., and R. D. Diamond. 1985. Mechanisms of resistance of Aspergillus fumigatus conidia to killing by neutrophils in vitro. J. Infect. Dis. 152:33-42. [DOI] [PubMed] [Google Scholar]
- 111.Levitz, S. M., and T. P. Farrell. 1990. Human neutrophil degranulation stimulated by Aspergillus fumigatus. J. Leukoc. Biol. 47:170-175. [DOI] [PubMed] [Google Scholar]
- 112.Lewis, R. E., N. P. Wiederhold, M. S. Lionakis, R. A. Prince, and D. P. Kontoyiannis. 2005. Frequency and species distribution of gliotoxin-producing Aspergillus isolates recovered from patients at a tertiary-care cancer center. J. Clin. Microbiol. 43:6120-6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liebmann, B., T. W. Muhleisen, M. Muller, M. Hecht, G. Weidner, A. Braun, M. Brock, and A. A. Brakhage. 2004. Deletion of the Aspergillus fumigatus lysine biosynthesis gene lysF encoding homoaconitase leads to attenuated virulence in a low-dose mouse infection model of invasive aspergillosis. Arch. Microbiol. 181:378-383. [DOI] [PubMed] [Google Scholar]
- 114.Lin, S. J., J. Schranz, and S. M. Teutsch. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 32:358-366. [DOI] [PubMed] [Google Scholar]
- 115.Lionakis, M. S., and D. P. Kontoyiannis. 2005. Fruit flies as a minihost model for studying drug activity and virulence in Aspergillus. Med. Mycol. 43(Suppl. 1):S111-S114. [DOI] [PubMed] [Google Scholar]
- 116.Lionakis, M. S., and D. P. Kontoyiannis. 2003. Glucocorticoids and invasive fungal infections. Lancet 362:1828-1838. [DOI] [PubMed] [Google Scholar]
- 117.Lodeiro, S., Q. Xiong, W. K. Wilson, Y. Ivanova, M. L. Smith, G. S. May, and S. P. Matsuda. 2009. Protostadienol biosynthesis and metabolism in the pathogenic fungus Aspergillus fumigatus. Org. Lett. 11:1241-1244. [DOI] [PubMed] [Google Scholar]
- 118.Lopes Bezerra, L. M., and S. G. Filler. 2004. Interactions of Aspergillus fumigatus with endothelial cells: internalization, injury, and stimulation of tissue factor activity. Blood 103:2143-2149. [DOI] [PubMed] [Google Scholar]
- 119.Luther, K., A. Torosantucci, A. A. Brakhage, J. Heesemann, and F. Ebel. 2007. Phagocytosis of Aspergillus fumigatus conidia by murine macrophages involves recognition by the dectin-1 beta-glucan receptor and Toll-like receptor 2. Cell. Microbiol. 9:368-381. [DOI] [PubMed] [Google Scholar]
- 120.Madan, T., P. Eggleton, U. Kishore, P. Strong, S. S. Aggrawal, P. U. Sarma, and K. B. Reid. 1997. Binding of pulmonary surfactant proteins A and D to Aspergillus fumigatus conidia enhances phagocytosis and killing by human neutrophils and alveolar macrophages. Infect. Immun. 65:3171-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Madan, T., K. B. Reid, M. Singh, P. U. Sarma, and U. Kishore. 2005. Susceptibility of mice genetically deficient in the surfactant protein (SP)-A or SP-D gene to pulmonary hypersensitivity induced by antigens and allergens of Aspergillus fumigatus. J. Immunol. 174:6943-6954. [DOI] [PubMed] [Google Scholar]
- 122.Maerker, C., M. Rohde, A. A. Brakhage, and M. Brock. 2005. Methylcitrate synthase from Aspergillus fumigatus. Propionyl-CoA affects polyketide synthesis, growth and morphology of conidia. FEBS J. 272:3615-3630. [DOI] [PubMed] [Google Scholar]
- 123.Magnani, T., F. M. Soriani, V. de Paulo Martins, A. C. de Freitas Policarpo, C. A. Sorgi, L. H. Faccioli, C. Curti, and S. A. Uyemura. 2008. Silencing of mitochondrial alternative oxidase gene of Aspergillus fumigatus enhances reactive oxygen species production and killing of the fungus by macrophages. J. Bioenerg. Biomembr. 40:631-636. [DOI] [PubMed] [Google Scholar]
- 124.Maiya, S., A. Grundmann, S. M. Li, and G. Turner. 2006. The fumitremorgin gene cluster of Aspergillus fumigatus: identification of a gene encoding brevianamide F synthetase. Chembiochem 7:1062-1069. [DOI] [PubMed] [Google Scholar]
- 125.Markaryan, A., I. Morozova, H. Yu, and P. E. Kolattukudy. 1994. Purification and characterization of an elastinolytic metalloprotease from Aspergillus fumigatus and immunoelectron microscopic evidence of secretion of this enzyme by the fungus invading the murine lung. Infect. Immun. 62:2149-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marr, K. A., R. A. Carter, M. Boeckh, P. Martin, and L. Corey. 2002. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 100:4358-4366. [DOI] [PubMed] [Google Scholar]
- 127.Martin, T. R., and C. W. Frevert. 2005. Innate immunity in the lungs. Proc. Am. Thorac. Soc. 2:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maubon, D., S. Park, M. Tanguy, M. Huerre, C. Schmitt, M. C. Prevost, D. S. Perlin, J. P. Latge, and A. Beauvais. 2006. AGS3, an alpha(1-3)glucan synthase gene family member of Aspergillus fumigatus, modulates mycelium growth in the lung of experimentally infected mice. Fungal Genet. Biol. 43:366-375. [DOI] [PubMed] [Google Scholar]
- 129.McDonagh, A., N. D. Fedorova, J. Crabtree, Y. Yu, S. Kim, D. Chen, O. Loss, T. Cairns, G. Goldman, D. Armstrong-James, K. Haynes, H. Haas, M. Schrettl, G. May, W. C. Nierman, and E. Bignell. 2008. Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog. 4:e1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McNeil, M. M., S. L. Nash, R. A. Hajjeh, M. A. Phelan, L. A. Conn, B. D. Plikaytis, and D. W. Warnock. 2001. Trends in mortality due to invasive mycotic diseases in the United States, 1980-1997. Clin. Infect. Dis. 33:641-647. [DOI] [PubMed] [Google Scholar]
- 131.Mehrad, B., R. M. Strieter, and T. J. Standiford. 1999. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J. Immunol. 162:1633-1640. [PubMed] [Google Scholar]
- 132.Meier-Osusky, I., G. Schoedon, F. Blauer, M. Schneemann, and A. Schaffner. 1996. Comparison of the antimicrobial activity of deactivated human macrophages challenged with Aspergillus fumigatus and Listeria monocytogenes. J. Infect. Dis. 174:651-654. [DOI] [PubMed] [Google Scholar]
- 133.Mikulska, M., A. M. Raiola, B. Bruno, E. Furfaro, M. T. Van Lint, S. Bregante, A. Ibatici, V. Del Bono, A. Bacigalupo, and C. Viscoli. Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transplant., in press. [DOI] [PubMed]
- 134.Monod, M., S. Paris, J. Sarfati, K. Jaton-Ogay, P. Ave, and J. P. Latge. 1993. Virulence of alkaline protease-deficient mutants of Aspergillus fumigatus. FEMS Microbiol. Lett. 106:39-46. [DOI] [PubMed] [Google Scholar]
- 135.Morgan, J., K. A. Wannemuehler, K. A. Marr, S. Hadley, D. P. Kontoyiannis, T. J. Walsh, S. K. Fridkin, P. G. Pappas, and D. W. Warnock. 2005. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med. Mycol. 43(Suppl. 1):S49-S58. [DOI] [PubMed] [Google Scholar]
- 136.Morgenstern, D. E., M. A. Gifford, L. L. Li, C. M. Doerschuk, and M. C. Dinauer. 1997. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J. Exp. Med. 185:207-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Morris, G., M. H. Kokki, K. Anderson, and M. D. Richardson. 2000. Sampling of Aspergillus spores in air. J. Hosp. Infect. 44:81-92. [DOI] [PubMed] [Google Scholar]
- 138.Morrison, B. E., S. J. Park, J. M. Mooney, and B. Mehrad. 2003. Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis. J. Clin. Investig. 112:1862-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mullbacher, A., and R. D. Eichner. 1984. Immunosuppression in vitro by a metabolite of a human pathogenic fungus. Proc. Natl. Acad. Sci. USA 81:3835-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mylonakis, E. 2008. Galleria mellonella and the study of fungal pathogenesis: making the case for another genetically tractable model host. Mycopathologia 165:1-3. [DOI] [PubMed] [Google Scholar]
- 141.Nauseef, W. M. 2007. How human neutrophils kill and degrade microbes: an integrated view. Immunol. Rev. 219:88-102. [DOI] [PubMed] [Google Scholar]
- 142.Netea, M. G., G. Ferwerda, C. A. van der Graaf, J. W. Van der Meer, and B. J. Kullberg. 2006. Recognition of fungal pathogens by Toll-like receptors. Curr. Pharm. Des. 12:4195-4201. [DOI] [PubMed] [Google Scholar]
- 143.Netea, M. G., A. Warris, J. W. Van der Meer, M. J. Fenton, T. J. Verver-Janssen, L. E. Jacobs, T. Andresen, P. E. Verweij, and B. J. Kullberg. 2003. Aspergillus fumigatus evades immune recognition during germination through loss of Toll-like receptor-4-mediated signal transduction. J. Infect. Dis. 188:320-326. [DOI] [PubMed] [Google Scholar]
- 144.Neth, O., D. L. Jack, A. W. Dodds, H. Holzel, N. J. Klein, and M. W. Turner. 2000. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect. Immun. 68:688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Neville, C., A. Murphy, K. Kavanagh, and S. Doyle. 2005. A 4′-phosphopantetheinyl transferase mediates non-ribosomal peptide synthetase activation in Aspergillus fumigatus. Chembiochem 6:679-685. [DOI] [PubMed] [Google Scholar]
- 146.Niide, O., Y. Suzuki, T. Yoshimaru, T. Inoue, T. Takayama, and C. Ra. 2006. Fungal metabolite gliotoxin blocks mast cell activation by a calcium- and superoxide-dependent mechanism: implications for immunosuppressive activities. Clin. Immunol. 118:108-116. [DOI] [PubMed] [Google Scholar]
- 147.Olivas, I., M. Royuela, B. Romero, M. C. Monteiro, J. M. Minguez, F. Laborda, and J. R. De Lucas. 2008. Ability to grow on lipids accounts for the fully virulent phenotype in neutropenic mice of Aspergillus fumigatus null mutants in the key glyoxylate cycle enzymes. Fungal Genet. Biol. 45:45-60. [DOI] [PubMed] [Google Scholar]
- 148.Pagano, L., C. Girmenia, L. Mele, P. Ricci, M. E. Tosti, A. Nosari, M. Buelli, M. Picardi, B. Allione, L. Corvatta, D. D'Antonio, M. Montillo, L. Melillo, A. Chierichini, A. Cenacchi, A. Tonso, L. Cudillo, A. Candoni, C. Savignano, A. Bonini, P. Martino, and A. Del Favero. 2001. Infections caused by filamentous fungi in patients with hematologic malignancies. A report of 391 cases by GIMEMA Infection Program. Haematologica 86:862-870. [PubMed] [Google Scholar]
- 149.Pahl, H. L., B. Krauss, K. Schulze-Osthoff, T. Decker, E. B. Traenckner, M. Vogt, C. Myers, T. Parks, P. Warring, A. Muhlbacher, A. P. Czernilofsky, and P. A. Baeuerle. 1996. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-kappaB. J. Exp. Med. 183:1829-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pan, X. Q., and J. Harday. 2007. Electromicroscopic observations on gliotoxin-induced apoptosis of cancer cells in culture and human cancer xenografts in transplanted SCID mice. In Vivo 21:259-265. [PubMed] [Google Scholar]
- 151.Panaccione, D. G. 2005. Origins and significance of ergot alkaloid diversity in fungi. FEMS Microbiol. Lett. 251:9-17. [DOI] [PubMed] [Google Scholar]
- 152.Panaccione, D. G., and C. M. Coyle. 2005. Abundant respirable ergot alkaloids from the common airborne fungus Aspergillus fumigatus. Appl. Environ. Microbiol. 71:3106-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Panepinto, J. C., B. G. Oliver, T. W. Amlung, D. S. Askew, and J. C. Rhodes. 2002. Expression of the Aspergillus fumigatus rheb homologue, rhbA, is induced by nitrogen starvation. Fungal Genet. Biol. 36:207-214. [DOI] [PubMed] [Google Scholar]
- 154.Pardo, J., C. Urban, E. M. Galvez, P. G. Ekert, U. Muller, J. Kwon-Chung, M. Lobigs, A. Mullbacher, R. Wallich, C. Borner, and M. M. Simon. 2006. The mitochondrial protein Bak is pivotal for gliotoxin-induced apoptosis and a critical host factor of Aspergillus fumigatus virulence in mice. J. Cell Biol. 174:509-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Paris, S., E. Boisvieux-Ulrich, B. Crestani, O. Houcine, D. Taramelli, L. Lombardi, and J. P. Latge. 1997. Internalization of Aspergillus fumigatus conidia by epithelial and endothelial cells. Infect. Immun. 65:1510-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Paris, S., J. P. Debeaupuis, R. Crameri, M. Carey, F. Charles, M. C. Prevost, C. Schmitt, B. Philippe, and J. P. Latge. 2003. Conidial hydrophobins of Aspergillus fumigatus. Appl. Environ. Microbiol. 69:1581-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Perrin, R. M., N. D. Fedorova, J. W. Bok, R. A. Cramer, J. R. Wortman, H. S. Kim, W. C. Nierman, and N. P. Keller. 2007. Transcriptional regulation of chemical diversity in Aspergillus fumigatus by LaeA. PLoS Pathog. 3:e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Phadke, A. P., and B. Mehrad. 2005. Cytokines in host defense against Aspergillus: recent advances. Med. Mycol. 43(Suppl. 1):S173-S176. [DOI] [PubMed] [Google Scholar]
- 159.Philippe, B., O. Ibrahim-Granet, M. C. Prevost, M. A. Gougerot-Pocidalo, M. Sanchez Perez, A. Van der Meeren, and J. P. Latge. 2003. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect. Immun. 71:3034-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Piva, T. J. 1994. Gliotoxin induces apoptosis in mouse L929 fibroblast cells. Biochem. Mol. Biol. Int. 33:411-419. [PubMed] [Google Scholar]
- 161.Pollock, J. D., D. A. Williams, M. A. Gifford, L. L. Li, X. Du, J. Fisherman, S. H. Orkin, C. M. Doerschuk, and M. C. Dinauer. 1995. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 9:202-209. [DOI] [PubMed] [Google Scholar]
- 162.Post, M. J., C. Lass-Floerl, G. Gastl, and D. Nachbaur. 2007. Invasive fungal infections in allogeneic and autologous stem cell transplant recipients: a single-center study of 166 transplanted patients. Transpl. Infect. Dis. 9:189-195. [DOI] [PubMed] [Google Scholar]
- 163.Reeves, E. P., C. G. Messina, S. Doyle, and K. Kavanagh. 2004. Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella. Mycopathologia 158:73-79. [DOI] [PubMed] [Google Scholar]
- 164.Reeves, E. P., K. Reiber, C. Neville, O. Scheibner, K. Kavanagh, and S. Doyle. 2006. A nonribosomal peptide synthetase (Pes1) confers protection against oxidative stress in Aspergillus fumigatus. FEBS J. 273:3038-3053. [DOI] [PubMed] [Google Scholar]
- 165.Reichard, U., S. Buttner, H. Eiffert, F. Staib, and R. Ruchel. 1990. Purification and characterisation of an extracellular serine proteinase from Aspergillus fumigatus and its detection in tissue. J. Med. Microbiol. 33:243-251. [DOI] [PubMed] [Google Scholar]
- 166.Renwick, J., P. Daly, E. P. Reeves, and K. Kavanagh. 2006. Susceptibility of larvae of Galleria mellonella to infection by Aspergillus fumigatus is dependent upon stage of conidial germination. Mycopathologia 161:377-384. [DOI] [PubMed] [Google Scholar]
- 167.Rex, J. H., J. E. Bennett, J. I. Gallin, H. L. Malech, and D. A. Melnick. 1990. Normal and deficient neutrophils can cooperate to damage Aspergillus fumigatus hyphae. J. Infect. Dis. 162:523-528. [DOI] [PubMed] [Google Scholar]
- 168.Richie, D. L., L. Hartl, V. Aimanianda, M. S. Winters, K. K. Fuller, M. D. Miley, S. White, J. W. McCarthy, J. P. Latge, M. Feldmesser, J. C. Rhodes, and D. S. Askew. 2009. A role for the unfolded protein response (UPR) in virulence and antifungal susceptibility in Aspergillus fumigatus. PLoS Pathog. 5:e1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Robinson, B. W., T. J. Venaille, A. H. Mendis, and R. McAleer. 1990. Allergens as proteases: an Aspergillus fumigatus proteinase directly induces human epithelial cell detachment. J. Allergy Clin. Immunol. 86:726-731. [DOI] [PubMed] [Google Scholar]
- 170.Rodrigues, A. G., R. Araujo, and C. Pina-Vaz. 2005. Human albumin promotes germination, hyphal growth and antifungal resistance by Aspergillus fumigatus. Med. Mycol. 43:711-717. [DOI] [PubMed] [Google Scholar]
- 171.Roilides, E., K. Uhlig, D. Venzon, P. A. Pizzo, and T. J. Walsh. 1993. Enhancement of oxidative response and damage caused by human neutrophils to Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect. Immun. 61:1185-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Roilides, E., K. Uhlig, D. Venzon, P. A. Pizzo, and T. J. Walsh. 1993. Prevention of corticosteroid-induced suppression of human polymorphonuclear leukocyte-induced damage of Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect. Immun. 61:4870-4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Romani, L., F. Fallarino, A. De Luca, C. Montagnoli, C. D'Angelo, T. Zelante, C. Vacca, F. Bistoni, M. C. Fioretti, U. Grohmann, B. H. Segal, and P. Puccetti. 2008. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature 451:211-215. [DOI] [PubMed] [Google Scholar]
- 174.Romano, J., G. Nimrod, N. Ben-Tal, Y. Shadkchan, K. Baruch, H. Sharon, and N. Osherov. 2006. Disruption of the Aspergillus fumigatus ECM33 homologue results in rapid conidial germination, antifungal resistance and hypervirulence. Microbiology 152:1919-1928. [DOI] [PubMed] [Google Scholar]
- 175.Sandhu, D. K., R. S. Sandhu, Z. U. Khan, and V. N. Damodaran. 1976. Conditional virulence of a p-aminobenzoic acid-requiring mutant of Aspergillus fumigatus. Infect. Immun. 13:527-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schaffner, A., H. Douglas, and A. Braude. 1982. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J. Clin. Investig. 69:617-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schaffner, A., H. Douglas, A. I. Braude, and C. E. Davis. 1983. Killing of Aspergillus spores depends on the anatomical source of the macrophage. Infect. Immun. 42:1109-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schmaler-Ripcke, J., V. Sugareva, P. Gebhardt, R. Winkler, O. Kniemeyer, T. Heinekamp, and A. A. Brakhage. 2009. Production of pyomelanin, a second type of melanin, via the tyrosine degradation pathway in Aspergillus fumigatus. Appl. Environ. Microbiol. 75:493-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schobel, F., O. Ibrahim-Granet, P. Ave, J. P. Latge, A. A. Brakhage, and M. Brock. 2007. Aspergillus fumigatus does not require fatty acid metabolism via isocitrate lyase for development of invasive aspergillosis. Infect. Immun. 75:1237-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schrettl, M., E. Bignell, C. Kragl, C. Joechl, T. Rogers, H. N. Arst, Jr., K. Haynes, and H. Haas. 2004. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J. Exp. Med. 200:1213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schrettl, M., E. Bignell, C. Kragl, Y. Sabiha, O. Loss, M. Eisendle, A. Wallner, H. N. Arst, Jr., K. Haynes, and H. Haas. 2007. Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 3:1195-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sheppard, D. C., G. Rieg, L. Y. Chiang, S. G. Filler, J. E. Edwards, Jr., and A. S. Ibrahim. 2004. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 48:1908-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shibuya, K., M. Takaoka, K. Uchida, M. Wakayama, H. Yamaguchi, K. Takahashi, S. Paris, J. P. Latge, and S. Naoe. 1999. Histopathology of experimental invasive pulmonary aspergillosis in rats: pathological comparison of pulmonary lesions induced by specific virulent factor deficient mutants. Microb. Pathog. 27:123-131. [DOI] [PubMed] [Google Scholar]
- 184.Sin, N., L. Meng, M. Q. Wang, J. J. Wen, W. G. Bornmann, and C. M. Crews. 1997. The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc. Natl. Acad. Sci. USA 94:6099-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Smith, J. M., J. E. Davies, and D. W. Holden. 1993. Construction and pathogenicity of Aspergillus fumigatus mutants that do not produce the ribotoxin restrictocin. Mol. Microbiol. 9:1071-1077. [DOI] [PubMed] [Google Scholar]
- 186.Spikes, S., R. Xu, C. K. Nguyen, G. Chamilos, D. P. Kontoyiannis, R. H. Jacobson, D. E. Ejzykowicz, L. Y. Chiang, S. G. Filler, and G. S. May. 2008. Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J. Infect. Dis. 197:479-486. [DOI] [PubMed] [Google Scholar]
- 187.Spilsbury, J. F., and S. Wilkinson. 1961. The isolation of festuclavine and two new clavine alkaloids from Aspergillus fumigatus. Fres. J. Chem. Soc. 5:2085-2091. [Google Scholar]
- 188.Stanzani, M., E. Orciuolo, R. Lewis, D. P. Kontoyiannis, S. L. Martins, L. S. St. John, and K. V. Komanduri. 2005. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood 105:2258-2265. [DOI] [PubMed] [Google Scholar]
- 189.Steele, C., R. R. Rapaka, A. Metz, S. M. Pop, D. L. Williams, S. Gordon, J. K. Kolls, and G. D. Brown. 2005. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 1:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Steinbach, W. J., D. K. Benjamin, Jr., S. A. Trasi, J. L. Miller, W. A. Schell, A. K. Zaas, W. M. Foster, and J. R. Perfect. 2004. Value of an inhalational model of invasive aspergillosis. Med. Mycol. 42:417-425. [DOI] [PubMed] [Google Scholar]
- 191.Stephens-Romero, S. D., A. J. Mednick, and M. Feldmesser. 2005. The pathogenesis of fatal outcome in murine pulmonary aspergillosis depends on the neutrophil depletion strategy. Infect. Immun. 73:114-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Stergiopoulou, T., J. Meletiadis, E. Roilides, D. E. Kleiner, R. Schaufele, M. Roden, S. Harrington, L. Dad, B. Segal, and T. J. Walsh. 2007. Host-dependent patterns of tissue injury in invasive pulmonary aspergillosis. Am. J. Clin. Pathol. 127:349-355. [DOI] [PubMed] [Google Scholar]
- 193.Sturtevant, J., and J. P. Latge. 1992. Participation of complement in the phagocytosis of the conidia of Aspergillus fumigatus by human polymorphonuclear cells. J. Infect. Dis. 166:580-586. [DOI] [PubMed] [Google Scholar]
- 194.Sturtevant, J. E., and J. P. Latge. 1992. Interactions between conidia of Aspergillus fumigatus and human complement component C3. Infect. Immun. 60:1913-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Suen, Y. K., K. P. Fung, C. Y. Lee, and S. K. Kong. 2001. Gliotoxin induces apoptosis in cultured macrophages via production of reactive oxygen species and cytochrome c release without mitochondrial depolarization. Free Radic. Res. 35:1-10. [DOI] [PubMed] [Google Scholar]
- 196.Sugui, J. A., J. Pardo, Y. C. Chang, A. Mullbacher, K. A. Zarember, E. M. Galvez, L. Brinster, P. Zerfas, J. I. Gallin, M. M. Simon, and K. J. Kwon-Chung. 2007. Role of laeA in the regulation of alb1, gliP, conidial morphology, and virulence in Aspergillus fumigatus. Eukaryot. Cell 6:1552-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sugui, J. A., J. Pardo, Y. C. Chang, K. A. Zarember, G. Nardone, E. M. Galvez, A. Mullbacher, J. I. Gallin, M. M. Simon, and K. J. Kwon-Chung. 2007. Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot. Cell 6:1562-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sutton, P., N. R. Newcombe, P. Waring, and A. Mullbacher. 1994. In vivo immunosuppressive activity of gliotoxin, a metabolite produced by human pathogenic fungi. Infect. Immun. 62:1192-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tang, C. M., J. Cohen, T. Krausz, S. Van Noorden, and D. W. Holden. 1993. The alkaline protease of Aspergillus fumigatus is not a virulence determinant in two murine models of invasive pulmonary aspergillosis. Infect. Immun. 61:1650-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tilburn, J., S. Sarkar, D. A. Widdick, E. A. Espeso, M. Orejas, J. Mungroo, M. A. Penalva, and H. N. Arst, Jr. 1995. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J. 14:779-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tomee, J. F., A. T. Wierenga, P. S. Hiemstra, and H. K. Kauffman. 1997. Proteases from Aspergillus fumigatus induce release of proinflammatory cytokines and cell detachment in airway epithelial cell lines. J. Infect. Dis. 176:300-303. [DOI] [PubMed] [Google Scholar]
- 202.Tronchin, G., J. P. Bouchara, G. Larcher, J. C. Lissitzky, and D. Chabasse. 1993. Interaction between Aspergillus fumigatus and basement membrane laminin: binding and substrate degradation. Biol. Cell 77:201-208. [DOI] [PubMed] [Google Scholar]
- 203.Tronchin, G., K. Esnault, G. Renier, R. Filmon, D. Chabasse, and J. P. Bouchara. 1997. Expression and identification of a laminin-binding protein in Aspergillus fumigatus conidia. Infect. Immun. 65:9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Trown, P. W., and J. A. Bilello. 1972. Mechanism of action of gliotoxin: elimination of activity by sulfhydryl compounds. Antimicrob. Agents Chemother. 2:261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tsai, H. F., Y. C. Chang, R. G. Washburn, M. H. Wheeler, and K. J. Kwon-Chung. 1998. The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J. Bacteriol. 180:3031-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tsai, H. F., R. G. Washburn, Y. C. Chang, and K. J. Kwon-Chung. 1997. Aspergillus fumigatus arp1 modulates conidial pigmentation and complement deposition. Mol. Microbiol. 26:175-183. [DOI] [PubMed] [Google Scholar]
- 207.Tsai, H. F., M. H. Wheeler, Y. C. Chang, and K. J. Kwon-Chung. 1999. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 181:6469-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tsitsigiannis, D. I., J. W. Bok, D. Andes, K. F. Nielsen, J. C. Frisvad, and N. P. Keller. 2005. Aspergillus cyclooxygenase-like enzymes are associated with prostaglandin production and virulence. Infect. Immun. 73:4548-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tsunawaki, S., L. S. Yoshida, S. Nishida, T. Kobayashi, and T. Shimoyama. 2004. Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase. Infect. Immun. 72:3373-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Underhill, D. M., E. Rossnagle, C. A. Lowell, and R. M. Simmons. 2005. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106:2543-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Unsold, I. A., and S. M. Li. 2005. Overproduction, purification and characterization of FgaPT2, a dimethylallyltryptophan synthase from Aspergillus fumigatus. Microbiology 151:1499-1505. [DOI] [PubMed] [Google Scholar]
- 212.Unsold, I. A., and S. M. Li. 2006. Reverse prenyltransferase in the biosynthesis of fumigaclavine C in Aspergillus fumigatus: gene expression, purification, and characterization of fumigaclavine C synthase FGAPT1. Chembiochem 7:158-164. [DOI] [PubMed] [Google Scholar]
- 213.Vogl, G., I. Lesiak, D. B. Jensen, S. Perkhofer, R. Eck, C. Speth, C. Lass-Florl, P. F. Zipfel, A. M. Blom, M. P. Dierich, and R. Wurzner. 2008. Immune evasion by acquisition of complement inhibitors: the mould Aspergillus binds both factor H and C4b binding protein. Mol. Immunol. 45:1485-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Waldorf, A. R., S. M. Levitz, and R. D. Diamond. 1984. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J. Infect. Dis. 150:752-760. [DOI] [PubMed] [Google Scholar]
- 215.Waring, P. 1990. DNA fragmentation induced in macrophages by gliotoxin does not require protein synthesis and is preceded by raised inositol triphosphate levels. J. Biol. Chem. 265:14476-14480. [PubMed] [Google Scholar]
- 216.Waring, P., T. Khan, and A. Sjaarda. 1997. Apoptosis induced by gliotoxin is preceded by phosphorylation of histone H3 and enhanced sensitivity of chromatin to nuclease digestion. J. Biol. Chem. 272:17929-17936. [DOI] [PubMed] [Google Scholar]
- 217.Waring, P., N. Newcombe, M. Edel, Q. H. Lin, H. Jiang, A. Sjaarda, T. Piva, and A. Mullbacher. 1994. Cellular uptake and release of the immunomodulating fungal toxin gliotoxin. Toxicon 32:491-504. [DOI] [PubMed] [Google Scholar]
- 218.Waring, P., A. Sjaarda, and Q. H. Lin. 1995. Gliotoxin inactivates alcohol dehydrogenase by either covalent modification or free radical damage mediated by redox cycling. Biochem. Pharmacol. 49:1195-1201. [DOI] [PubMed] [Google Scholar]
- 219.Washburn, R. G., D. J. DeHart, D. E. Agwu, B. J. Bryant-Varela, and N. C. Julian. 1990. Aspergillus fumigatus complement inhibitor: production, characterization, and purification by hydrophobic interaction and thin-layer chromatography. Infect. Immun. 58:3508-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Washburn, R. G., J. I. Gallin, and J. E. Bennett. 1987. Oxidative killing of Aspergillus fumigatus proceeds by parallel myeloperoxidase-dependent and -independent pathways. Infect. Immun. 55:2088-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Washburn, R. G., C. H. Hammer, and J. E. Bennett. 1986. Inhibition of complement by culture supernatants of Aspergillus fumigatus. J. Infect. Dis. 154:944-951. [DOI] [PubMed] [Google Scholar]
- 222.Wasylnka, J. A., and M. M. Moore. 2003. Aspergillus fumigatus conidia survive and germinate in acidic organelles of A549 epithelial cells. J. Cell Sci. 116:1579-1587. [DOI] [PubMed] [Google Scholar]
- 223.Wasylnka, J. A., and M. M. Moore. 2002. Uptake of Aspergillus fumigatus conidia by phagocytic and nonphagocytic cells in vitro: quantitation using strains expressing green fluorescent protein. Infect. Immun. 70:3156-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wasylnka, J. A., M. I. Simmer, and M. M. Moore. 2001. Differences in sialic acid density in pathogenic and non-pathogenic Aspergillus species. Microbiology 147:869-877. [DOI] [PubMed] [Google Scholar]
- 225.Watanabe, A., I. Fujii, H. Tsai, Y. C. Chang, K. J. Kwon-Chung, and Y. Ebizuka. 2000. Aspergillus fumigatus alb1 encodes naphthopyrone synthase when expressed in Aspergillus oryzae. FEMS Microbiol. Lett. 192:39-44. [DOI] [PubMed] [Google Scholar]
- 226.Werner, J. L., A. E. Metz, D. Horn, T. R. Schoeb, M. M. Hewitt, L. M. Schwiebert, I. Faro-Trindade, G. D. Brown, and C. Steele. 2009. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J. Immunol. 182:4938-4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wiederhold, N. P., R. E. Lewis, and D. P. Kontoyiannis. 2003. Invasive aspergillosis in patients with hematologic malignancies. Pharmacotherapy 23:1592-1610. [DOI] [PubMed] [Google Scholar]
- 228.Wild, C. P. 2007. Aflatoxin exposure in developing countries: the critical interface of agriculture and health. Food Nutr. Bull. 28:S372-S380. [DOI] [PubMed] [Google Scholar]
- 229.Willger, S. D., S. Puttikamonkul, K. H. Kim, J. B. Burritt, N. Grahl, L. J. Metzler, R. Barbuch, M. Bard, C. B. Lawrence, and R. A. Cramer, Jr. 2008. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog 4:e1000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Williams, J. H., T. D. Phillips, P. E. Jolly, J. K. Stiles, C. M. Jolly, and D. Aggarwal. 2004. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 80:1106-1122. [DOI] [PubMed] [Google Scholar]
- 231.Yamada, A., T. Kataoka, and K. Nagai. 2000. The fungal metabolite gliotoxin: immunosuppressive activity on CTL-mediated cytotoxicity. Immunol. Lett. 71:27-32. [DOI] [PubMed] [Google Scholar]
- 232.Yoshida, L. S., S. Abe, and S. Tsunawaki. 2000. Fungal gliotoxin targets the onset of superoxide-generating NADPH oxidase of human neutrophils. Biochem. Biophys. Res. Commun. 268:716-723. [DOI] [PubMed] [Google Scholar]
- 233.Zaas, A. K., G. Liao, J. W. Chien, C. Weinberg, D. Shore, S. S. Giles, K. A. Marr, J. Usuka, L. H. Burch, L. Perera, J. R. Perfect, G. Peltz, and D. A. Schwartz. 2008. Plasminogen alleles influence susceptibility to invasive aspergillosis. PLoS Genet. 4:e1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zarember, K. A., J. A. Sugui, Y. C. Chang, K. J. Kwon-Chung, and J. I. Gallin. 2007. Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. J. Immunol. 178:6367-6373. [DOI] [PubMed] [Google Scholar]
- 235.Zhao, W., J. C. Panepinto, J. R. Fortwendel, L. Fox, B. G. Oliver, D. S. Askew, and J. C. Rhodes. 2006. Deletion of the regulatory subunit of protein kinase A in Aspergillus fumigatus alters morphology, sensitivity to oxidative damage, and virulence. Infect. Immun. 74:4865-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhou, X., A. Zhao, G. Goping, and P. Hirszel. 2000. Gliotoxin-induced cytotoxicity proceeds via apoptosis and is mediated by caspases and reactive oxygen species in LLC-PK1 cells. Toxicol. Sci. 54:194-202. [DOI] [PubMed] [Google Scholar]