Abstract
Summary: An estimated 2.5 million children are currently living with HIV, the vast majority as a result of mother-to-child transmission. Prevention of perinatal HIV infection has been immensely successful in developed countries. A comprehensive package of services, including maternal and infant antiretroviral therapy, elective cesarean section, and avoidance of breast-feeding, has resulted in transmission rates of less than 2%. However, in developing countries, access to such services is often not available, as demonstrated by the fact that the vast majority of children with HIV live in Africa. Over the past few years, many developing nations have made great strides in improving access to much-needed services. Notably, in eastern and southern Africa, the regions most affected by HIV, mother-to-child-transmission coverage rates for HIV-positive women increased from 11% in 2004 to 31% in 2006. These successes are deserving of recognition, while not losing sight of the fact that much remains to be done; currently, an estimated 75% of pregnant women worldwide have an unmet need for antiretroviral therapy. Further work is needed to determine the optimal strategy for reducing perinatal transmission among women in resource-poor settings, with a particular need for reduction of transmission via breast-feeding.
INTRODUCTION
While the human immunodeficiency virus (HIV) epidemic has left virtually no region of the world unaffected, the disparities between rich and poor countries in HIV/AIDS prevention, diagnosis, and care are glaringly apparent. Not long ago the gap between resource-poor and wealthier nations seemed insurmountable. For many years the world failed to face the magnitude of the problem in those countries most affected and felt powerless over a perceived inability to mobilize the resources needed to provide services. While the situation has improved in recent years, to some degree, there are still significant disparities in access to comprehensive HIV services. The prevention of mother-to-child transmission (PMTCT) remains one area of HIV management where striking differences in care and treatment exist across the world.
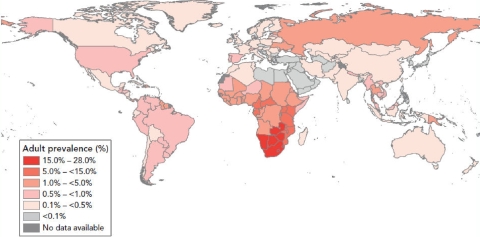
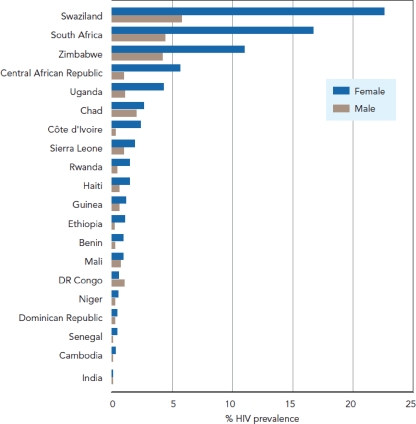
Two-thirds of the world's 33 million HIV-positive individuals live in sub-Saharan Africa (44) (Fig. 1) . In this region, women make up half of infected individuals, though their share of infections is increasing in several countries (44) (Fig. 2). Most are in their child-bearing and child-rearing years (47). HIV-positive women have a significant risk of transmitting the virus to their infants if effective interventions are not provided. Work in the United States and other developed nations has shown that mother-to-child transmission (MTCT) of HIV can be effectively interrupted with antiretroviral (ARV) therapy (ART); however, that capacity has yet to be fully realized in many poor countries of the world, most notably those in sub-Saharan Africa. The 2 million children currently living with HIV (44) speak to our inability to deliver those interventions everywhere. The problem is ongoing and severe; in 2007, an estimated 370,000 children became infected with HIV (44)—more than 1,000 children every day. Without care and treatment, more than half of them will die before their second birthday (95).
FIG. 1.
Thirty-three million people living with HIV in 2007. (Reprinted from reference 44 with permission.)
FIG. 2.
HIV prevalence among 15- to 24-year-olds, by sex, in selected countries, from 2005 to 2007. (Reprinted from reference 44 with permission.)
In the United States and other resource-rich countries where PMTCT services are widely available, perinatal infection is now a rare occurrence. Effective prevention of MTCT requires access to testing early and late in pregnancy and the ability to deliver ART to mothers and their infants. Prevention of transmission can be difficult in part because HIV transmission can occur during pregnancy, labor, and delivery or after birth through breast-feeding. Without any intervention, the risk of transmission ranges from 15 to 40% (28). Currently, almost 90% of the world's 2 million HIV-positive children reside in Africa (44).
While there are clear differences in the delivery of prevention services between the two continents, there remain some similarities. Although the scales of the epidemic are quite different, HIV continues to spread in both regions. In the United States, in spite of widespread testing and counseling services, prevention campaigns, and access to care and treatment, new infections continue to occur. The newly revised estimate from the Centers for Disease Control and Prevention (CDC) showed that more than 56,000 new infections occurred in 2006 (16). While almost half of those living with HIV in the United States are men who have sex with men, women account for 25% of the nation's HIV prevalence (15). As in the developing world, the proportion of women acquiring HIV has risen over the course of the epidemic and is largely related to heterosexual risk. The number of AIDS cases reported in women >13 years old in the United States increased from 7% in 1985 to 27% in 2006 (14). Furthermore, in the United States almost 75% of female infections are due to sex with high-risk partners (14). Even more concerning is the fact that a substantial proportion of infected individuals are unaware of their diagnosis; of the one million Americans living with the disease at the end of 2006, it is estimated that one in five is unaware of their infection (15). Clearly, the United States still has considerable work to do.
The magnitude of the problems and the need for care in Africa are daunting. Notable examples include South Africa, a nation of over 49 million (18), which remains the country with the largest number of HIV infections in the world, with an estimated 5.7 million individuals living with the disease in 2006 (44). The tiny nation of Swaziland has among the worst statistics; in 2006, the median HIV prevalence among women attending prenatal clinics was approaching 40%. A recent population-based survey in Swaziland found that 26% of adults ages 15 to 49 are infected with HIV—one in four people (47). While the United States has nowhere near the magnitude of disease as many of the nations of sub-Saharan Africa, both regions have faced some similar obstacles and failures. At the same time, many successful interventions have been made and progress continues in this area, with much to be learned from developments in both regions.
This review will highlight some of the successes and failures in the area of prevention of MTCT of HIV, focusing on the United States and Africa. In spite of the vast differences in disease burden and resources, there remain some similarities, especially regarding barriers to diagnosis, treatment, and prevention services. However, we know how to diagnose HIV infection in women, we know how to prevent most transmissions, and we know how to provide care for infants exposed to HIV at birth. Implementation of these practices, even if not perfect, has resulted in neonatal HIV infection becoming a rare event in most of the developed world. If appropriate diagnosis and care were provided to all women worldwide and if we were able to develop an effective means to prevent breast milk transmission, a newly infected HIV-positive infant could become as rare in Africa as it is in the United States today.
HISTORICAL OVERVIEW OF HIV IN THE UNITED STATES AND MTCT
After AIDS was first described in 1981, it was not long before the possibility of MTCT was realized (2, 13, 70, 76, 79). A series of case reports was published in Pediatrics in 1984, describing three female half-siblings whose mother was a prostitute and drug addict. The mother showed clinical and laboratory evidence indicative of AIDS. All three children had chronic candidal infections, and two of the three died from Pneumocystis jirovecii pneumonia (27). In the discussion the authors wrote, “there are several compelling reasons to suspect that these three half-siblings have an acquired immunodeficiency disease similar to what is being termed AIDS in adults” (27). They go on to state that while it is known that HIV can be transmitted via blood product transfusion, this case series of half-siblings suggests that another route may be across the placenta or through contact with cervical secretions. They concluded that “careful follow-up and observation of offspring from high-risk mothers should be done and the possibility of AIDS considered in such children with unusual clinical problems” (27). Today it is well known that vertical transmission of HIV can occur in utero, as a result of fetal exposure to HIV across the placenta or in the amniotic fluid; during the birth process via direct contact with blood or infected maternal cervical and vaginal secretions; and postnatally, via breast milk (56, 59, 61, 68, 71, 89, 90). Table 1 summarizes maternal and infant risk factors contributing to HIV transmission to the infant.
TABLE 1.
Maternal and infant risk factors contributing to HIV transmission to the infant
| Type of risk factor | Risk factors (reference[s]) |
|---|---|
| Maternal | High maternal viral load (24, 35, 42, 64, 71, 88), high vaginal/cervical shedding of HIV (42), low maternal CD4 count (24, 64, 68, 71, 89), maternal genital ulcer disease (42) |
| Infant | Prematurity (<34 wk) (56, 68) |
| In utero/intrapartum | Vaginal delivery with prolonged rupture of membranes (56, 61), chlorioamnionitis (56, 61), instrumentation (amniocentesis, invasive monitoring, etc.) (56) |
| Postpartum | Breast-feeding, especially nonexclusive or mixed feeding (25, 38, 43, 59); breast disease (mastitis, cracked/bleeding nipples) (42, 43) |
Significant progress has been made since the discovery of HIV in the early 1980s, specifically with regard to vertical transmission. The landmark study by the Pediatric AIDS Clinical Trials Group Protocol 076 (PACTG 076) showed that MTCT of HIV could be reduced by as much as two-thirds with a regimen of zidovudine prior to and during delivery and to the infant after delivery for 6 weeks (23). This randomized, double-blind, placebo-controlled trial enrolled 477 HIV-infected pregnant women from April 1991 to December 1993. Inclusion criteria were women with pregnancies between 14 to 34 weeks of gestation, CD4 lymphocyte counts of above 200, and no prior history of ART. Women on the zidovudine arm received both antepartum and intrapartum zidovudine until completion of delivery, and the infant received zidovudine for the first 6 weeks of life. The proportion of infants infected at 18 months in the zidovudine group was 8.3%, compared to 25.5% in the placebo group, corresponding to a 67.5% relative reduction in HIV transmission risk (23). Today, zidovudine is still a part of therapy for PMTCT in the United States and other resource-rich countries, though it is now generally provided as a component of highly active ART (HAART), and MTCT is now extremely rare in such countries, with transmission rates typically less than 2% (17, 28, 40, 41, 67, 72, 90, 91). It typically is impractical for resource-poor nations to adopt such a policy, however, owing to multiple factors, including prohibitive cost, complexity and length of the regimen, and inability to get women into prenatal care early.
In 1985, the CDC released its first set of recommendations for HIV testing of pregnant women. At the time, the virus known to cause AIDS was referred to as human T-lymphotropic virus type III/lymphadenopathy-associated virus (9). The CDC's recommendations pointed out that through counseling, HIV-positive women could delay pregnancy until more was known regarding perinatal transmission of the virus (9). Given the lack of treatment options available at the time, pregnancy prevention was one of few benefits to knowing one's status. Screening at this time was primarily risk-based, focusing on sex workers, intravenous drug users, and women from other countries with a higher burden of HIV disease (40).
It was not long, however, before it became apparent that risk-based screening was largely insufficient in the identification of substantial numbers of HIV-infected pregnant women. In response to PACTG 076, the CDC developed new recommendations for pregnant women with regard to HIV testing (40). In 1995, the U.S. Public Health Service recommended routine voluntary HIV counseling and testing for all pregnant women, “to address the increasing epidemic of HIV infection among women and their infants” (12). By 2001 the recommendations were revised to emphasize HIV testing as a routine part of prenatal care (10, 40). In spite of these new recommendations, the number of vertical transmissions remained unacceptably high, in part due to lack of timely diagnosis of pregnant women with HIV infection (many of whom received little or no prenatal care) (7, 11). In 2006, recommendations were further revised to make HIV testing a routine component of prenatal screening tests. A key element in the revised guidelines was the concept of “opt-out” testing, whereby testing is performed unless the patient declines (7). Specifically, the guidelines state that (i) HIV screening should be included in the routine panel of prenatal screening tests for all pregnant women; (ii) HIV screening is recommended after the patient is notified that testing will be performed unless the patient declines (opt-out screening); (iii) separate written consent for HIV testing should not be required, and general consent for medical care should be considered sufficient to encompass consent for HIV testing; and (iv) repeat screening in the third trimester is recommended in certain jurisdictions with elevated rates of HIV infection among pregnant women (7).
The last recommendation applies to women living in areas with an HIV incidence of ≥17 cases per 100,000 person-years, as it is cost-effective and may result in significant MTCT reductions (7, 77). This criterion applies to several urban areas of the United States (essentially all of sub-Saharan Africa is above this threshold). While these recommendations are highly regarded by the medical profession, there are still barriers to overcome among politicians at the state level, where HIV testing laws are made. It is worth noting that a few states (New York and Connecticut) mandate testing of all newborns, and in North Carolina, as of January 2009, testing is mandated for all women with unknown HIV status at the time of labor and delivery (66). Unfortunately, however, the opt-out strategy has not been implemented in every state. The National HIV/AIDS Clinicians’ Consultation Center provides an updated compendium on HIV testing laws specific to each state, and guidelines can be downloaded at http://www.nccc.ucsf.edu/StateLaws/Index.html.
HISTORY OF HAART AND PMTCT SERVICES IN THE UNITED STATES
In the United States today, perinatal HIV infection has become a rare occurrence, and its prevention is considered a great public health success story. Studies have demonstrated that the maternal plasma HIV RNA level is the most important predictor of MTCT risk (24, 35, 42, 64, 71, 80). In women with replicating virus, other intrapartum factors affect the risk of transmission. Current PMTCT packages focus on risk reduction via methods that have proven to be of benefit, including combination ART during pregnancy for infected mothers, cesarean delivery, and avoidance of breast-feeding (40).
Elective cesarean delivery prior to rupture of membranes has been demonstrated to reduce the risk of HIV transmission by half compared to vaginal delivery (33). A meta-analysis of 15 prospective cohort studies in the United States and Europe reviewed data from over 8,000 mother-infant pairs, and after adjusting for multiple covariates (including receipt or not of ART, infant birth weight, and maternal stage of disease), the likelihood of HIV type 1 (HIV-1) transmission was lower with elective cesarean section (prior to rupture of membranes) than with other modes of delivery (57% efficacy) (33). The mechanism underlying this risk reduction is thought to be due to a reduction in the infant's exposure to contaminated maternal blood and vaginal and cervical secretions (33). It has also been demonstrated that maternofetal transfusion is lower in cases of scheduled cesarean section compared to vaginal delivery (50); whether “micro” blood transfusions from mother to infant during uterine contractions contribute to MTCT remains unclear.
In the United States and most other developed countries today, elective cesarean section is easily accessible, and it is often part of the prescribed package of PMTCT services for women with HIV. Current recommendations in the United States are for scheduled cesarean delivery at 38 weeks for women with an HIV viral load of >1,000 copies/ml at or near the time of delivery. This is irrespective of maternal ART (72). The guidelines go on to say that for women with a viral load of <1,000 copies/ml on ART, “data are insufficient to evaluate the potential benefit of cesarean delivery for prevention of perinatal transmission” (72), as it is known that among women not infected with HIV, maternal morbidity and mortality are greater with cesarean than with vaginal delivery. Thus, for women with very low or undetectable viral loads, decisions should be individualized based on discussion between the obstetrician and the mother (72).
Breast-feeding substantially increases the risk of MTCT of HIV-1 (26, 43). In the United States, complete avoidance of breast-feeding for women with HIV has been advised since the mid-1980s (9), when the specifics of MTCT were still being elucidated. In settings where avoidance of breast-feeding is feasible, affordable, and culturally acceptable (as in the United States), its utility as an intervention in PMTCT programs is obvious (73). As a result, breast-feeding among HIV-positive women in the United States is exceedingly uncommon (73).
HAART is recommended for all pregnant women, including women who do not require treatment for their own health, in order to optimally prevent perinatal transmission and minimize the risk of maternal development of resistance to ARVs, with the goal being for women to have undetectable levels of HIV-1 RNA (72). A minimum of three drugs is recommended even for women who would not otherwise require therapy (40, 72).
Today, PMTCT services are widespread in the United States, but treatment is complicated, is labor-intensive, and requires input from providers with expertise in HIV and pregnancy. Current recommendations for HIV-infected pregnant women have evolved considerably over the years. While HAART is recommended for all pregnant women, for those not already on HAART prior to pregnancy, consideration of delaying therapy until after the first trimester of pregnancy is generally regarded as prudent, given the potential for drug-related toxicities in the first trimester. Some ARV agents are avoided in pregnancy; efavirenz in particular should be avoided given its potential for teratogenicity early in pregnancy (19, 85), and nevirapine has the potential to cause severe hepatotoxicity in pregnant women with higher CD4+ cell counts (55, 72). Regimens containing zidovudine are favored when feasible (72). Furthermore, HIV-infected pregnant women should receive zidovudine as a continuous infusion during labor, with zidovudine prophylaxis provided to the infant within 12 h after birth and continuing for the first 6 weeks of life (72). It is noteworthy, however, that there are no data to suggest decreased efficacy in PMTCT when maternal zidovudine is given as an oral regimen (which is in fact what is done in much of the developing world). After pregnancy, women who do not meet criteria for treatment according to adult guidelines may be considered for cessation of therapy (72), although the long-term consequences of treatment initiation due to pregnancy with subsequent interruption remain unknown.
The 2008 Public Health Service Task Force Guidelines describe best treatment options for different clinical scenarios, depending on whether the expectant mother is treatment-naïve or experienced and whether HAART is recommended for her own optimal health (72). It is interesting to see that almost 15 years after PACTG 076, zidovudine is still a key component of therapy in the United States for PMTCT (40).
PMTCT IN AFRICA
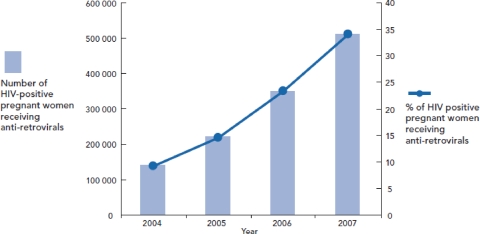
Recently revised estimates from the Joint United Nations Programme on HIV/AIDS (UNAIDS) shows that steady progress has been made in the provision of ART for pregnant women as part of PMTCT in low- and middle-income countries (44). As the primary advocate for comprehensive global action on the HIV epidemic around the world, UNAIDS’ mission involves leading and supporting an expanded response to HIV/AIDS, including prevention efforts, provision of care and support, and alleviation of the impact of the epidemic. At present, the UNAIDS Secretariat works in more than 80 countries worldwide (48a). Figure 3 illustrates the dramatic progress that has been made in recent years, with ART coverage of pregnant women increasing from only 9% in 2004 to 33% in 2007 (44).
FIG. 3.
Increase in ART coverage of HIV-infected women in low- and middle-income countries from 9% in 2004 to 33% in 2007. (Reprinted from reference 44 with permission.)
With the support of the U.S. President's Emergency Plan for AIDS Relief (PEPFAR), an estimated 6,000,000 pregnant women received PMTCT services in 2006, including counseling and testing for HIV and ART if the woman was found to be HIV positive. Over 500,000 received ART, which it is estimated resulted in the prevention of >100,000 newborn infections (71a, 95). Furthermore, through the Elizabeth Glaser Pediatric AIDS Foundation (one of the largest programs for the provision of PMTCT services), more than 2.6 million women accessed services over a 6.5-year period, with 75% of HIV-positive women receiving ARV prophylaxis (81).
Obviously, in order to effectively prevent MTCT of HIV, one must first identify all HIV-positive pregnant women. Testing must be simple, accurate, and inexpensive so that it can be provided to all pregnant women in settings where access to health care is not universal and acceptance of HIV testing is not automatic. In addition, once testing identifies a woman as being HIV positive, a comprehensive PMTCT package should include laboratory testing to determine the woman's disease status and her need for treatment for her own health, ART as clinically indicated, testing for the infant, and treatment for the infant. Such services are necessary but are costly, labor-intensive, and dependent on clinical expertise. In reality, most less developed countries rely heavily on clinical staging of disease severity as an indication to start therapy. While CD4 testing may be available, viral load testing is rarely an option.
Testing without intervention, or intervention without testing, is useless; each is dependent on the other to prevent MTCT. Across Africa, significant strides clearly have been made in each area, but each has its own barriers and to date, effective prevention of MTCT remains the exception rather than the rule.
HIV Testing and Counseling in Africa
While testing and counseling of pregnant women are now widespread in the United States and many other developed countries, much of the continent of Africa lags far behind. Surveys from 12 high-burden countries in sub-Saharan Africa reveal that a median of only 10% of women have been tested for HIV and know their status (96). Low levels of knowledge of HIV status translate into most individuals receiving testing only when they have become symptomatic and are often in the advanced stages of disease. One of the greatest challenges to improving the effectiveness of PMTCT programs is to increase the testing of pregnant women. While some women in the developing world seek medical care during pregnancy but do not deliver in a health care facility, there are also many women who present to health care facilities without having received prenatal care; strategies to test women in both of these settings are different but equally important in order to reach all women (96). Therefore, provision of HIV testing early in pregnancy and/or at the time of delivery for all women will require significant changes in the overall delivery of prenatal care for most women.
In recognition of this need, provider-initiated testing and counseling have been endorsed by the G8 leaders in 2005 and the United Nations General Assembly in 2006 as an essential step in working toward universal access to HIV care and treatment (96). As in the United States, UNAIDS and WHO recommend “opt-out” testing initiated by health care providers (96). Specifically, HIV testing is recommended (i) for all patients, irrespective of epidemic setting, whose clinical presentation might result from underlying HIV infection; (ii) as a standard part of medical care for all patients attending health facilities in generalized HIV epidemics; and (iii) more selectively in concentrated and low-level epidemics (96). Furthermore, the guidelines emphasize that antenatal, childbirth, and postpartum health services are considered a priority for the implementation of provider-initiated HIV testing (96).
Kenya is one country that has expanded its HIV testing and counseling services significantly over the last several years. In 2000, the government committed to the rapid expansion of services with a commitment to open 350 testing sites over four years, with five sites in each of Kenya's 70 districts. While rapid scale-up was achieved, with 680 voluntary counseling and testing sites at the end of 2005, an estimated 75% of Kenyans still did not know their HIV status (58). Furthermore, the number of new infections in Kenya appears to have risen over the last few years, with a prevalence of 6.7% in 2003 compared to an estimated 7.1 to 8.5% in 2007 (44).
Multiple perceived barriers have been identified as inhibiting participation in voluntary counseling and testing in sub-Saharan Africa: fear of actually testing positive and the psychological stress that this entails, concerns regarding the confidentiality of test results, and fear of social stigmatization (93). A small but significant number of HIV-positive women will experience domestic violence as a result of disclosing their status to their partner (46). Furthermore, practical barriers such as transportation and expenditures also play a considerable role in limiting access to testing (93).
A recent study at a rural district hospital in Malawi sought to illuminate the reasons behind loss to follow-up among HIV-positive women in a PMTCT program. Fear of stigma and discrimination, lack of support from spouses, and inability to afford transportation costs were all cited. Furthermore, some women stated that they were not prepared for HIV testing and perceived it as being obligatory (8). One study participant was quoted as saying, “When you arrive for PMTCT you are given some information on the importance of PMTCT and then you are suddenly tested for HIV. We are not prepared for this”(8). Another look at a PMTCT program elsewhere in Malawi found an important reason that women actually went for HIV testing was to “confirm and protect.” Many women chose to go for HIV testing due to anxiety regarding their own HIV status, suspecting that they may be infected and if so, desiring to protect their children from infection (51).
The largest experience in Malawi with regard to testing of pregnant women comes from a retrospective analysis conducted over a 4-year period in the capital city of Lilongwe. Four antenatal clinics reaching a population of 20,000 pregnant women revealed that acceptance of HIV testing increased from 45% to 73% when rapid (same-day) testing was instituted. Furthermore, when opt-out testing was instituted, 99% of mothers agreed to being tested (65). In summary, testing for HIV is more widely available than in the past, though it still remains underutilized in many areas due to a number of factors. The first step to interrupt MTCT of HIV is identification of those at risk so that they may be provided with therapy and information to decrease the risk of infection in their infant.
Availability of ART for Pregnant Women in Africa
While combination ART successfully treats HIV-infected persons, the primary reason for administering these drugs during pregnancy if the pregnant woman is not immunocompromised and not in need of therapy for her own health is prophylaxis for the unborn child. ART is readily available for most persons with HIV in the United States and other resource-rich countries, though the same has certainly not been true for resource-poor countries, including most of sub-Saharan Africa. In 2005, only an estimated 15% of HIV-positive pregnant women worldwide received ARV prophylaxis (44, 46), and only rarely does the ART include HAART. As a consequence, children account for more than 10% of all new infections (95).
The majority of HIV-positive women reside in eastern and southern Africa. In 2005, eight countries in southern Africa had adult prevalence rates exceeding 15% (48). Not insignificantly, three countries in sub-Saharan Africa—Swaziland, Namibia, and South Africa—three of the most severely affected countries, doubled their maternal ART prophylaxis uptake among pregnant women (44, 95).
For the past decade the international community has increasingly recognized the need for improved service provision for women and children through PMTCT programs. The global scale-up of PMTCT has been slow, but has made important strides. At the end of 2004, more than 100 countries had established PMTCT programs. However, only one country in sub-Saharan Africa (Botswana) and only 16 countries in total had achieved national coverage (95).
Still, the successes are deserving of notation. In Botswana, where the government has made PMTCT services a priority, rates of HIV transmission to exposed babies have dropped to 4% (44). In South Africa, PMTCT coverage increased from 15% to 57% from 2004 to 2006 (44). Unfortunately, scale-up of services on a national level remains highly variable, and the international community still falls significantly short of adequate coverage, with more than 65% of women today still not receiving such services (44).
Introduction of Single-Dose Nevirapine for PMTCT in Resource-Poor Settings
Perhaps the most significant advance in preventing MTCT of HIV in resource-limited settings has been the documentation that “single-dose nevirapine” can be effective to prevent MTCT, based on the landmark HIVNET 012 study in Kampala, Uganda (36). The term “single dose” may be confusing, as the regimen is in fact two doses: one to the woman in labor and a second dose to the infant in the first 72 h of life. If the woman misses her intrapartum dose, the infant should receive two doses, one immediately after birth and the second dose at 24 to 48 h (62). The single-dose nevirapine regimen, as it results in therapeutic levels of nevirapine in the infant for at least 10 days (63), could provide protection to breast-fed infants in the first week or two of life, as breast-feeding is the nutritional norm in most of sub-Saharan Africa and other less developed countries (36).
HIVNET 012 enrolled 692 HIV-1-infected pregnant women and randomly assigned mother-infant pairs to receive a single dose of nevirapine (200 mg to mothers at onset of labor and 2 mg/kg to the infant within 72 h of birth) or zidovudine (at the onset of labor and every 3-h until delivery for the mother and 4 mg/kg twice daily for the infants in the first week of life). As is the norm in sub-Saharan Africa, the vast majority of babies were breast-fed, with >95% still being breast-fed at 14 to 16 weeks. The risk of transmission at this time point was 25.1% for the zidovudine arm and 13.1% for the nevirapine arm (P < 0.001), showing that a single dose of nevirapine given to the mother at the onset of labor and to the newborn within 72 h of birth lowered the risk of HIV transmission to the infant by 47% compared to a short course of zidovudine. Even more important, among this breast-fed population, HIV-free survival was demonstrated to be significantly longer for infants on the nevirapine arm than for those on the zidovudine regimen (36).
Based on HIVNET 012, single-dose nevirapine became a primary tool for PMTCT in resource-poor settings. Not only was it cost-effective, it was simple and deliverable (39). It was subsequently endorsed by the World Health Organization in 2000 (3, 94) and is currently still used for PMTCT in many resource-poor settings (94).
There is a cost to this simple regimen, however, in that single-dose nevirapine can induce the selection of HIV-1 resistance mutations in mothers and infants (3). While the extent of this problem is not yet fully elucidated, a number of studies have shown that a single dose of the drug, in part due to its long half-life, can promote drug resistance (28). This risk of resistance can be reduced by the addition of a 1-week “tail,” that is, having the mother continue nucleoside reverse transcriptase inhibitors for 1 week during the time that measurable amounts of nevirapine remain in her system (60).
Not long after the HIVNET 012 results were reported, it was demonstrated that combination regimens were more effective than single-drug regimens in reducing MTCT. The studies by Taha et al. in Malawi (83, 84) compared single-dose nevirapine plus a short course of zidovudine given to infants in the first week of life with a regimen of single-dose infant nevirapine alone in a breast-fed population. When mothers presented late for delivery and received no intrapartum nevirapine, there was significantly reduced HIV transmission at 4 to 8 weeks of life in those receiving two drugs (at 6 to 8 weeks, 7.7% in the nevirapine-plus-zidovudine group compared to 12.1% in the nevirapine-only group; P = 0.03) (83). However, when women did receive intrapartum nevirapine, the addition of a short course of zidovudine to single-dose nevirapine for infants did not decrease MTCT compared to nevirapine alone (at 6 to 8 weeks, transmission was 6.9% in the nevirapine-plus-zidovudine group compared to 6.5% in the nevirapine-only group; P = 0.88) (84).
Another study that supports an advantage of dual therapy in the prevention of MTCT comes from Thailand. Investigators conducted a randomized, double-blind trial with three treatment arms among 1,844 HIV-infected Thai women and their non-breast-fed infants. All women received antenatal zidovudine, and all babies received zidovudine in the first week of life. In addition, in one treatment arm mothers and infants received a single dose of nevirapine; in a second treatment arm, mothers and infants received nevirapine and placebo, respectively; and in a third arm, both mothers and infants received placebo. This “double-placebo” group was stopped early, as transmission rates were 6.3%, compared to 1.1% in the “double-nevirapine” group and 2.8% in the nevirapine-placebo group. Thus, this study demonstrated the high efficacy of adding maternal intrapartum nevirapine to oral zidovudine for further reduction in MTCT compared to zidovudine alone (53).
For HIV-positive women not qualifying for HAART initiation, the current preferred regimen for PMTCT in resource-poor settings, as recommended by the World Health Organization, is as follows: (i) for the woman, zidovudine beginning at 28 weeks gestation, followed by intrapartum zidovudine, lamivudine, and nevirapine and then by zidovudine plus lamivudine for 7 days postpartum, and (ii) for the infant, a single dose of nevirapine within 72 h of birth followed by a week-long course of zidovudine (94).
This regimen has been chosen both to reduce the incidence of MTCT of HIV and to decrease levels of HIV-1 RNA resistance (94). Not all countries will have the ability to immediately implement this regimen, and single-dose nevirapine will continue to be used. It has now been 2 years since the WHO recommendations, and it is clear that single-dose nevirapine still predominates. It should still, however, be considered “a short-term interim measure while steps are being taken to enable more effective regimens to be delivered” (94).
Other Interventions to Reduce MTCT
Other PMTCT services of proven benefit, such as elective cesarean section and avoidance of breast-feeding, are difficult to achieve in resource-limited settings. In many resource-poor countries, elective cesarean section is not feasible (82, 94), for reasons such as inadequate infrastructure and staffing and/or the lack of a skilled birth attendant during labor (51). In sub-Saharan Africa, a skilled birth attendant is present at fewer than half of all deliveries, which is a significant challenge to the successful implementation of PMTCT services (46). Furthermore, many women choose to give birth at home for social and cultural reasons. While the benefits of elective cesarean section for HIV-positive women is unclear for women on HAART and with undetectable maternal viral loads at the time of delivery (72, 90), women in resource-limited settings often do have replicating virus at the time of delivery; hence, there is the potential for benefit from availability of elective cesarean section.
The Breast-Feeding Dilemma
Breast-feeding in the developing world remains an important means of acquisition of HIV-1 infection for infants (25, 43). PMTCT programs report significantly lower rates of MTCT in non-breast-fed than in breast-fed populations (25). However, overall morbidity and mortality are often worsened by formula feeding, as breast milk replacement results in increased infant death from diarrhea and other infectious diseases where access to clean water and affordable replacement feeding are not guaranteed (46). For the majority of women in resource-poor countries, the tragic reality is that there is no choice but to breast-feed one's child; thus, breast-feeding remains an important route for contraction of HIV-1 for infants. Estimates from 2005 data suggest that 630,000 to 820,000 infants became infected with HIV, including 280,000 to 360,000 who became infected via breast milk (45).
Current recommendations for women in developing countries advise that formula feeding be used only when it is “acceptable, feasible, affordable, sustainable, and safe” (94). For many women, avoidance of breast-feeding is socially unacceptable and often not sustainable for financial and other reasons. Furthermore, “safe” formula feeding requires access to clean water, another formidable challenge for many in developing countries (94). Exclusive breast-feeding is associated with improved child survival (25). The WHO recommendations otherwise advise exclusive breast-feeding for the first 6 months of life and emphasize that providers should offer guidance and support to infected women, particularly when cessation of breast-feeding occurs (94). However, exclusive breast-feeding is rarely a cultural norm in African settings (29), as it is culturally appropriate and often expected to introduce simple foods and liquids along with breast milk very early in life.
As zidovudine has been shown to reduce MTCT risk when given during pregnancy (23, 53), it has also been studied for evidence of efficacy against breast milk transmission of HIV. The Mashi Study in Botswana randomized 1,200 HIV-infected pregnant women and their infants to compare the efficacy of breast-feeding with prolonged infant zidovudine therapy to formula feeding with short-course infant zidovudine therapy. All infants received single-dose nevirapine at birth and then were randomized to receive either zidovudine and breast-feeding for 6 months or zidovudine for 1 month with formula feeding. The risk of infant transmission at 7 months of age was higher in the breast-fed arm than in the formula-fed arm (9% versus 5.6%), though cumulative mortality at 7 months was higher in the formula-fed group. The 18-month HIV-free survival was similar for the two groups (88).
In Malawi, the large phase III Post-Exposure Prophylaxis of Infants (PEPI) trial was recently completed, involving more than 3,000 HIV-exposed infants. This trial showed reduced postnatal MTCT among a breast-fed population receiving extended prophylaxis to 14 weeks of life. All infants received single-dose nevirapine at birth plus twice-daily zidovudine for 1 week. Infants were then randomized to one of three groups: a control arm (no further therapy), an extended-nevirapine group with daily nevirapine until 14 weeks of life, and an extended-dual-prophylaxis group with both nevirapine and zidovudine until 14 weeks of life. All infants were breast-fed. At 9 months, the rates of infant HIV infection were significantly lower in the extended-therapy groups than in the control arm (5.2% in the extended-nevirapine group [P < 0.001] and 6.4% in the extended-dual-prophylaxis group [P = 0.002], compared to 10.6% in the control group). The two extended-therapy groups did not differ significantly (52).
The Six-Week Extended Dose Nevirapine (SWEN) study conducted in Ethiopia, India, and Uganda did not show a significant reduction in HIV transmission risk at 6 months when nevirapine was used for only the first 6 weeks of life. The SWEN study randomized >2,000 infants to receive either single-dose nevirapine at birth or a single dose followed by a 5-mg daily regimen beginning on day 8 of life and continuing to 6 weeks of age. All infants were breast-fed. At 6 weeks of age, 54 babies in the single-dose group and 25 in the extended-dose group were HIV infected (relative risk, 0.54; P = 0.009). At 6 months, however, there was no statistically significant difference between the two groups, with 87 babies in the single-dose group and 62 in the extended-dose group infected (relative risk, 0.80; 95% confidence interval, 0.58 to 1.10; P = 0.16). These data suggest that a course longer than 6 weeks may be more effective in preventing breast milk transmission where safe replacement feeding is not an option (5).
Whether breast-feeding is “exclusive” or “mixed” has also been shown to be of particular importance in the risk of MTCT. Exclusive breast-feeding has been found to have a significantly lower transmission risk than mixed feeding (25, 38). A large Zimbabwean study found that the introduction of animal milk or solid foods before the age of 3 months was associated with a fourfold greater risk of perinatal HIV transmission at 6 months compared to exclusive breast-feeding (38). A later study in South Africa found that infants born to HIV-positive women who were breast-fed but also received solids (generally in the form of porridge or cereal) were more than 10 times as likely to acquire HIV infection as those infants who were exclusively breast-fed (25). Furthermore, the exclusively breast-fed infants in this study had a cumulative 3-month mortality of 6.1%, compared to 15.1% in infants given replacement feeds (25). This increased risk of HIV transmission with mixed feeding is thought to be due to a disruption in the integrity of the intestinal mucosa, which is normally protected by breast milk (25), allowing HIV to more readily penetrate these microabrasions.
Other factors associated with the risk of transmission via breast-feeding include maternal viral load and CD4+ count, as well as some clinical parameters. Women with increased HIV-1 RNA in plasma and breast milk have an increased risk of transmitting the virus to their infants through breast-feeding. Low maternal CD4+ count is also independently associated with an increased breast-feeding transmission risk. Clinical factors such as maternal breast health (mastitis or cracked nipples) and infant oral candidiasis also increase transmission risk (43). The longer the duration of breast-feeding, the greater the cumulative risk of postnatal HIV transmission (26, 43). Finally, it appears that breast milk viral load is significantly higher after rapid weaning and thus may pose an increased transmission risk to infants who are weaned but then resume breast-feeding at a later time (87). While new studies continue to shed light on this subject, the lack of accurate estimates of the risk of HIV acquisition via breast-feeding combined with an ever-changing body of literature regarding optimal feeding practices has left many women and their medical providers confused and unsure regarding the best infant feeding options.
RESISTANCE AND DRUG TOXICITIES
While the provision of ARVs to HIV-infected women and their babies has played a major role in reducing MTCT, new problems related to drug toxicities and ART resistance have begun to emerge and need to be addressed. ART toxicity in infants has certainly been questioned and in some instances proven, and regardless, this warrants further study. Infant toxicity risks depend on the duration of exposure for the baby (including in utero and during the neonatal period) and the type and number of drugs (91). No studies yet have shown an increased risk of congenital malformations or childhood cancers associated with ARV drug exposure in utero (91). Hematologic abnormalities, most commonly associated with zidovudine exposure, are well described (32, 91).
Signs of mitochondrial toxicity in infants exposed to long-term ARV regimens have also been described, though other groups have failed to find this effect, and this issue continues to be debated. A case report published in 1998 describes an infant born to an HIV-infected female who was exposed to zidovudine in utero and was on standard prophylactic zidovudine from birth; on day 9 of life, she developed a sudden onset of respiratory distress with severe metabolic acidosis and a dramatic rise in serum lactate. Other common causes for lactic acidosis (hypoxia, shock, etc) were excluded on a clinical basis, and the abnormal lab and clinical findings returned to baseline within 24 h of stopping zidovudine therapy (78). Subsequently, a retrospective analysis of symptoms of mitochondrial dysfunction in infants of HIV-infected women in France reported eight children with mitochondrial dysfunction, all of whom had been exposed to zidovudine (with four exposed to zidovudine-lamivudine). Five of the children presented with neurologic symptoms, most notably seizures, and two of these subsequently died. The other children were asymptomatic but had significant laboratory abnormalities, including elevated lactate, transaminases, and/or pancreatic lipase. All children were treated after birth with either zidovudine or zidovudine-lamivudine (6). Following this review, the French Perinatal Cohort examined over 2,500 infants exposed to ARVs and found evidence for mitochondrial toxicity in 12 children, most of whom had cognitive delay and abnormal magnetic resonance imaging findings. Eleven of 12 showed a profound deficit in one of the respiratory chain complexes. The estimated 18-month incidence was 0.26%, compared to the general figure of 0.01% in the general pediatric population (4). Other similar cases of hyperlactatemia and/or mitochondrial dysfunction have been reported elsewhere in Europe and Canada (1, 69).
More recently, a prospective study conducted among women-infant pairs in Cote d'Ivoire compared infants exposed to nucleoside analogs (zidovudine or zidovudine-lamivudine) in utero to infants with nevirapine exposure only. Serum lactate levels were measured at 4, 6, and 12 weeks of life, and investigators found no difference in the 3-month period prevalence of hyperlactatemia between the two groups. Furthermore, of those with elevated serum lactate levels, none were symptomatic (30). The data to date suggests that there is possibly a very small risk of mitochondrial dysfunction in babies with nucleoside reverse transcriptase inhibitor exposure. The sensitivity and specificity of elevated lactate as a marker for mitochondrial dysfunction in such instances remain unclear.
The fact that ART resistance may occur in both women and infants following prophylactic ART (as in the case of PMTCT) is well established, but how this should affect management remains highly controversial. Single-dose nevirapine still remains the primary option for PMTCT of HIV-1 in resource-limited countries. As such, its use can result in viral resistance mutations which may compromise future ART regimens for mothers and/or their babies (21, 31, 60). As more women receive single-dose nevirapine for PMTCT, over time many of them are presenting a second time for prevention, leading to a recent analysis of the effectiveness of single-dose nevirapine when used in consecutive pregnancies (57). Several studies are worthy of mention here; Table 2 highlights several of the more recent trials. The Botswana Mashi trial showed that women who received single-dose nevirapine as part of PMTCT had higher rates of virologic failure than women without nevirapine exposure when ART was initiated within 6 months of delivery (54). However, whether this “virologic failure” is clinically significant is not entirely clear. Jourdain et al. found that Thai women who received intrapartum nevirapine compared to placebo were more likely to “fail” therapy with a nevirapine-containing regimen when that regimen was begun within 6 months of the nevirapine exposure (49). “Failure” was defined as the inability to achieve viral suppression of <50 copies at 6 months after starting ART. Again, a failure in terms or clinical or immunological response is not described. Thus, resistance certainly has been shown to occur, though its impact on subsequent therapy is not yet entirely elucidated.
TABLE 2.
Selected key trials affecting PMTCT policy over the past decadea
| Trial/site (reference) | Details
|
Results | |||||
|---|---|---|---|---|---|---|---|
| General | Antenatal | Intrapartum | Postpartum
|
Infant feeding | |||
| Mother | Baby | ||||||
| ZDV ± NVP/Thailand (53) | n = 1,844 Thai women; double-blind RCT; 3 arms | All women on ZDV 300 mg BID beginning at 28 wk | All women on ZDV 300 mg every 3 h, plus: arm 1, NVP 200 mg × 1; arm 2, placebo; arm 3, NVP 200 mg × l | No ARVs | All infants received 2 mg/kg ZDV every 6 h × 1 wk, plus: arm 1, NVP 6 mg × 1 at 48-72 h; arm 2, placebo; arm 3, placebo | Formula | Placebo-placebo group stopped early; transmission rate 6.3%, compared to 1.1 % in NVP-NVP group and 2.8% in NVP-placebo group |
| Infant PEP, NVP vs NVP + ZDV (NVAZ trial)/Malawi (83) | n = 1,119 babies; randomized, phase 3, open-label trial | No ARVs | No NVP (women presenting late for delivery) | No ARVs | Arm 1, NVP 2 mg/kg × 1 immediately after birth; arm 2, NVP 2 mg/kg × 1 immediately after birth + ZDV 4 mg/kg BID × 1 wk | Breast | In babies HIV uninfected at birth, at 6-8 wk transmission was 7.7% in NVP + ZDV group and 12.1 % in NVP-only group (36% protective efficacy) |
| Infant PEP, NVP vs NVP + ZDV (NVAZ trial)/Malawi (84) | Infants born to 894 women; randomized, phase 3, open-label trial | No ARVs | NVP 200 mg × 1 | No ARVs | Arm 1, NVP 2 mg/kg × 1 at 48-72 h; arm 2, NVP 2 mg/kg × 1 at 48-72 h + ZDV 4 mg/kg BID × 1 wk | Breast | In babies HIV uninfected at birth, at 6-8 wk transmission was 6.9% in NVP + ZDV group and 6.5% in NVP-only group (when mothers received intrapartum NVP, addition of short-course ZDV to NVP for infants did not decrease MTCT compared to NVP alone) |
| Infant PEP and NVP resistance/Malawi (NVAZ trial) (31) | 95 infants infected with HIV; plasma collected at 6-8 wk of age; genotyping in 78 samples | No ARVs | Arm 1, NVP 200 mg × 1; arm 2, NVP 200 mg × 1; arm 3, no NVP; arm 4, no NVP | No ARVs | Arm 1, NVP 2 mg/kg × 1 immediately after birth; arm 2, NVP 2 mg/kg × 1 immediately after birth + ZDV 4 mg/kg BID × 1 wk; arm 3, NVP 2 mg/kg × 1 immediately after birth; arm 4, NVP 2 mg/kg × 1 immediately after birth + ZDV 4 mg/kg BID × 1 wk | Breast | Resistance in infants: arm 1, 87%; arm 2, 57%; arm 3, 74%; arm 4, 27% (NVP resistance lower with no maternal NVP); transmission rates comparable in all groups at 6-8 wk of life except arm 3 (no maternal NVP and infant single-dose NVP) (range, 14.1-16.3%) |
| Infant feeding and ZDV (Mashi study)/Botswana (88) | n = 1,200 HIV-infected pregnant women; RCT | All women on ZDV 300 mg BID from 34 wk | Arm 1, ZDV 300 mg every 3 h; arm 2, ZDV 300 mg every 3 h + NVP 200 mg × 1 | No ARVs | Arm 1, NVP × 1 at birth plus ZDV × 6 mo; arm 2, NVP × 1 at birth plus ZDV × 1 mo | Arm 1, breast; arm 2, formula | Risk of infant transmission at 7 mo higher in breast-fed ZDV group than in formula-fed group (9% vs 5.6%); cumulative mortality at 7 mo was higher in formula-fed group than in breast-fed ZDV group (9.3% vs 4.9%); 18-mo HIV-free survival similar among two groups |
| Six-week extended-dose NVP for infants (SWEN study)/Ethiopia, India, Uganda (5) | Three coordinated but separate RCTs; combined analysis | No ARVs | All women received NVP 200 mg × 1 | No ARVs | Arm 1, NVP 2 mg/kg × 1 at birth; arm 2, NVP 2 mg/kg × 1 at birth, followed by NVP 5 mg daily days 8-42 | Breast | At 6 wk of age, for the infants on prolonged NVP, risk of HIV infection was 2.5% vs 5.3% in single-dose NVP infants (P = 0.009); at 6 mo, risk of postnatal transmission in the extended-NVP group was 6.9% vs 9% in the single-dose NVP group (P = 0.16); overall mortality at 6 mo was 3.6% in the single-dose group compared to 1.1% in the extended-dose group (P = 0.02) |
| Postexposure prophylaxis for infants (PEPI trial)/Malawi (52) | Open-label phase 3 RCT; n = 3,016 infants | No ARVs | NVP × 1 (unless late presenter) | No ARVs (referred for treatment if CD4 < 200) | Arm 1, NVP 2 mg/kg × 1 + ZDV 4 mg/kg BID × 1 wk (NVAZ trial); arm 2, ZDV 4 mg/kg BID × 7 days + NVP × 14 wk; arm 3, ZDV 4 mg/kg BID × 14 wks + NVP × 14 wk | Breast | At 9 mo, MTCT in NVAZ group was 10.0%, compared to 6.4% for extended-ZDV/NVP and 5.2% extended-NVP groups (40 and 51% reductions, respectively); no significant differences between two extended-prophylaxis groups |
| CBV + NVP to reduce NNRTI resistance/South Africa (60) | RCT, open label; n = 228 infants, 226 mothers | No ARVs | Arm 1, NVP 200 mg × 1; arm 2, NVP 200 mg × 1 + CBV every 12 h; arm 3, NVP 200 mg × 1 + CBV every 12 h | Arm 1, no ARVs; arm 2, CBV BID × 4 days; arm 3, CBV BID × 7 days | Arm 1, NVP 2 mg/kg × 1; arm 2, NVP 2 mg/kg × 1 + ZDV/lamivudine × 4 days; arm 3, NVP 2 mg/kg × 1 + ZDV/lamivudine × 7 days | Breast and formula | Arm 1 stopped early; overall MTCT rate was 9.2% in utero and 10.5% at 6 wk; NNRTI resistance in 7/9 infants in single-dose NVP group, 1/8 in 4-day dual-therapy group, and 0/7 in 7-day dual-therapy group |
| Tenofovir and emtricitabine to reduce viral resistance (TD2)/Zambia (20, 21) | n = 397 women; 355 infants at 6 weeks postpartum; open-label RCT | ZDV from 32 wk | Arm 1, NVP 200 mg × 1; arm 2, NVP 200 mg × 1 + 300 mg tenofovir/200 mg emtricitabine (Truvada) × 1 | NVP 2 mg/kg × 1 + ZDV 4 mg/kg BID × 1 wk | Breast | Primary evaluation, among women with viral loads >2,000 copies/ml, 30% had NNRTI resistance mutations in the single-dose NVP group, compared to 14% in the Truvada group; secondary evaluation, Truvada was not significantly associated with decreased MTCT (at 6 wk, 1.6% infants infected from Truvada group compared to 2.8% in single-dose NVP group; P = 0.67) | |
| NVP resistance following single-dose NVP in PMTCT (Mashi study)/Botswana (54) | n = 218 women; n = 47 HIV-infected infants (20 with HIV RNA at 24 mo); prospective observational cohort nested within RCT | ZDV from 32 wk | All women received ZDV, and in addition: arm 1, NVP 200 mg × 1; arm 2, placebo | NVP-based HAART for CD4 < 200 or AIDS-defining illness | Arm 1, NVP × 1 at 48-72 h; arm 2, placebo (subsequently modified based on external data to provide single-dose NVP to all infants) | Formula with 1 mo ZDV or breast with 6 mo ZDV | Of 60 women starting ART within 6 mo of delivery, 10/24 (41.7%) had virologic failure in single-dose NVP group compared to 0/36 in placebo group; of 158 women starting ART > 6 mo after delivery, failure rates did not differ, with 8% in single-dose NVP group and 12% in placebo group (P = 0.39); of 47 infected infants,12 died before ART and 5 died after ART; of 25 remaining, virologic failure was 9% vs 77% in placebo vs single-dose NVP groups, respectively, at both 6 mo and 12 mo |
| Effectiveness of NVP in successive pregnancies for PMTCT/South Africa, Cote d'lvoire (57) | Two prospective cohorts; n = 120 (S. Africa) | No ARVs | South Africa: NVP 200 mg × 1 on two occasions in subsequent pregnancies | No ARVs | NVP 2 mg/kg × 1 at 48-72 h | Arm 1, breast; arm 2, formula | Median delivery interval for two cohorts was 21 and 26 mo; effectiveness not reduced by prior exposure (South Africa, 11.1% MTCT rates for both pregnancies) |
| Zambia Exclusive Breastfeeding Study (ZEBS) with abrupt vs routine weaning (87) | n = 297 HIV-infected women | No ARVs | NVP 200 mg × 1 | No ARVs | NVP 2 mg/kg × 1 at 48 h | Arm 1, exclusive breast-feeding with abrupt weaning (24 h) at 4 mo postpartum; arm 2, exclusive breast-feeding to 6 mo with introductory weaning foods thereafter | Of 71 breast milk samples analyzed, 21/31 in early-weaning group had detectable HIV RNA compared to 17/40 in women still breast-feeding (68% vs 42.5%) |
| MTCT during exclusive breast-feeding in first 6 mo of life/South Africa (25) | 2,722 women (n = 1372 HIV infected) | No ARVs | NVP 200 mg × 1 | No ARVs | NVP 2 mg/kg × 1 at 48 h | Exclusive breast-feeding | Median exclusive breast-feeding was 159 days; cumulative MTCT was 14.1% by 6 wk of age and 19.5% by 6 mo of age; MTCT also associated with maternal CD4 < 200 and birth wt < 2,500 g; for infants uninfected at 6 wk, MTCT was 1.1% after 1 mo and 4% after 5 mo’ infants with mixed feeding had almost 11 × increased MTCT than exclusively breast-fed infants |
For key prior studies, see reference 90. Abbreviations: ZDV, zidovudine; NVP, nevirapine; CBV, Combivir; NNRTI, nonnucleoside reverse transcriptase inhibitor; RCT, randomized controlled trial; BID, twice a day.
Other drugs and their potential for reducing resistance continue to be investigated. A Zambian study recently showed that Truvada (tenofovir-emtricitabine), given to the mother as a single dose at delivery, reduced resistance to nonnucleoside reverse transcriptase inhibitors by half at 6 weeks after delivery (21). However, the addition of Truvada to short-course zidovudine and peripartum nevirapine did not further reduce perinatal transmission rates in this setting (20). Finally, other studies have shown that a “tail” of nonnucleoside reverse transcriptase inhibitors given for at least 1 week after a dose of nevirapine does reduce the frequency of detection of nevirapine resistance mutations (60).
COMPARISON OF TWO CONTINENTS AND LESSONS LEARNED
What can we learn from experiences both in the West, where access to successful prevention and interventions is widespread, and on the continent of Africa, where increasing access to lifesaving interventions has only just begun? A look at the WHO's comprehensive strategic approach to PMTCT is helpful to determine whether programs are on track and, if not, what changes need to be made. While the focus of this review is prevention of HIV transmission from mothers to infants, this is just one of the four key components outlined by WHO, each of which is worthy of review: (i) primary prevention of HIV infection among women of childbearing age; (ii) prevention of unintended pregnancies among women living with HIV; (iii) prevention of HIV transmission from mothers living with HIV to their infants; and (iv) care, treatment, and support for mothers living with HIV, their children, and their families (94, 95).
Primary prevention of HIV infection in adults is of paramount importance to addressing the epidemic, and progress continues to be inadequate worldwide. The fact that more than 56,000 new infections occurred in the United States in 2006 is a sobering reminder that the epidemic is far from over, even among the most developed countries (16). While homosexual and bisexual men are most heavily affected in the United States, women comprise 25% of all those living with the disease, with the majority of them being in their childbearing years, and a significant number remain unaware of their status (15).
In several African nations, women carry more of the disease burden than men (95). For sub-Saharan Africa overall, women constitute >50% of those living with HIV (44). Central to primary prevention of HIV infection is having access to testing, counseling, and prevention services. However, in sub-Saharan Africa, only 12% of men and 10% of women knew their HIV status in 2006 (34). Furthermore, there are many who still lack basic, accurate information on how to avoid infection. The global goal of ensuring comprehensive knowledge of HIV among young people by 2010 is far from being met, with surveys from 64 countries showing an estimated 40% or fewer possessing such information (44).
Prevention of unintended pregnancies is an important component of the WHO's strategic approach for PMTCT but tends to receive the least attention. Preventing unintended pregnancies can have an important effect on reducing the number of infants born with HIV (74). Previous studies demonstrated that current levels of contraceptive use in sub-Saharan Africa have prevented more than 170,000 unintended HIV-positive births annually—more than 400 HIV-positive children each day (74, 75). However, there remain an estimated 120 million couples worldwide with an unmet need for contraception (22, 34) and, therefore, thousands of unintended HIV-positive births occurring each year (74).
The care and treatment for mothers living with HIV and their families have also received little attention. While women in most developed countries have access to HAART for treatment and PMTCT, developing countries have significant ground left to cover. The identification of pregnant women with HIV should be used as an entry point for the entire family to access HIV testing and, if necessary, long-term follow-up and treatment (95). Without strong referral links between PMTCT services and HIV treatment programs for infected women and their children, many will fail to receive adequate care (34), ultimately leading to poorer maternal health and increased risks of transmission to future children.
Clearly, resource-rich countries such as the United States and much of Europe have been better able to control the HIV epidemic, particularly with regard to the prevention of vertical transmissions. Due to a dearth of resources and inadequate infrastructure, the majority of poor countries remain with great challenges. The scale-up of PMTCT comes with major programmatic challenges even for those countries with obvious commitment and decent access to maternal and child health services (46).
While the stark contrast between rich and poor countries in their success of PMTCT of HIV is largely due to a difference in resources, there are many barriers to effective prevention and treatment which appear to be universal. HIV testing remains a challenge for developed and less-developed countries alike. The fact that one in five HIV-positive Americans is unaware of their diagnosis (15) speaks to our inadequacies in achieving universal testing, counseling, and prevention services in this country. Fear of disclosure, regardless of country or cultural setting, is often a significant barrier to receiving testing and treatment.
CONCLUSION
In conclusion, PMTCT has made great strides, particularly in the United States and other developed countries, such that the birth of children with HIV in such countries today has become a rare event. As the leading risk factor for MTCT is maternal plasma HIV RNA load (24, 35, 42, 64, 71, 80), the priority is decreasing the viral load to undetectable levels with ARVs by the time of delivery. With a combination of comprehensive services available for all pregnant women, including universal provision of testing, ARV prophylaxis during pregnancy, and caesarean section delivery prior to labor and rupture of membranes (if indicated), along with safe replacement feeding and ARVs for the infant in the neonatal period, HIV transmission can be reduced to less than 2% (17, 28, 40, 41, 67, 72, 90, 91).
In most of sub-Saharan Africa, the situation differs dramatically. While transmission risk factors remain the same, the existence of fewer options for women, including access to HAART during pregnancy, elective cesarean sections, and safe and culturally acceptable alternatives to breast-feeding, means that rates of MTCT remain much higher. Successes over the past decade are notable and deserving of recognition. There still remain, however, an estimated two-thirds of pregnant HIV-positive women in low- and middle-income countries with an unmet need for ARVs during pregnancy (44).
Further work is needed in particular to determine the optimal means to reduce transmission through breast milk, which remains a dilemma for HIV-positive women (46) especially in sub-Saharan Africa. Current strategies being studied include longer-term HAART for HIV-positive breast-feeding women, longer-term therapy for babies after delivery, or vaccines (37). At present, the best options seem to be exclusive breast-feeding for the first 6 months of life, with particular avoidance of mixed feeding. However, counseling, public education, and ongoing support of women who, until now, have received very mixed messages regarding optimal infant nutrition are sorely needed.
The target set forth by the United Nations General Assembly Special Session on HIV/AIDS in 2001 is to reach 80% of pregnant women in need with PMTCT services by 2010. Over the last few years, several of the countries hardest hit by the HIV epidemic have proven that scale-up of PMTCT services is possible (46). With continued effort from the research and care community, the existence of newly infected HIV-positive infants and children can become a historic discussion rather than a pressing clinical dilemma.
Biography
 Ann M. Buchanan (M.D., M.P.H., D.T.M.&H.) is currently a fellow in pediatric infectious diseases at Duke University Medical Center. Prior to beginning fellowship, she spent 2 years in sub-Saharan Africa working for the Baylor International Pediatric AIDS Initiative, first in Malawi and subsequently in Tanzania. Her fellowship research will focus on the prevalence of mycobacteremia among HIV-infected children in Tanzania. Dr. Buchanan holds an M.P.H. in Maternal and Child Health and a Diploma in Tropical Medicine and Hygiene. Her primary research interests include HIV and tuberculosis coinfection, as well as mother-to-child transmission of HIV.
Ann M. Buchanan (M.D., M.P.H., D.T.M.&H.) is currently a fellow in pediatric infectious diseases at Duke University Medical Center. Prior to beginning fellowship, she spent 2 years in sub-Saharan Africa working for the Baylor International Pediatric AIDS Initiative, first in Malawi and subsequently in Tanzania. Her fellowship research will focus on the prevalence of mycobacteremia among HIV-infected children in Tanzania. Dr. Buchanan holds an M.P.H. in Maternal and Child Health and a Diploma in Tropical Medicine and Hygiene. Her primary research interests include HIV and tuberculosis coinfection, as well as mother-to-child transmission of HIV.
 Coleen K. Cunningham (M.D.) is board certified in pediatric infectious diseases and has been active in clinical care, research, and teaching in this area for more than 15 years. She is currently Chief of Infectious Diseases in the Department of Pediatrics and co-fellowship program director. Dr. Cunningham maintains a broad interest in pediatric infectious diseases, but her research focus is in the treatment of HIV infection in children, prevention of perinatal transmission of HIV, and vaccines to prevent HIV infection in children and adolescents. She heads the Vaccine Committee of the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT). More recently, she developed an interest in international health and is assisting in several pediatric studies being conducted in Africa.
Coleen K. Cunningham (M.D.) is board certified in pediatric infectious diseases and has been active in clinical care, research, and teaching in this area for more than 15 years. She is currently Chief of Infectious Diseases in the Department of Pediatrics and co-fellowship program director. Dr. Cunningham maintains a broad interest in pediatric infectious diseases, but her research focus is in the treatment of HIV infection in children, prevention of perinatal transmission of HIV, and vaccines to prevent HIV infection in children and adolescents. She heads the Vaccine Committee of the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT). More recently, she developed an interest in international health and is assisting in several pediatric studies being conducted in Africa.
REFERENCES
- 1.Alimenti, A., D. R. Burdge, G. S. Ogilvie, D. M. Money, and J. C. Forbes. 2003. Lactic acidemia in human immunodeficiency virus-uninfected infants exposed to perinatal antiretroviral therapy. Pediatr. Infect. Dis. J. 22:782-789. [DOI] [PubMed] [Google Scholar]
- 2.Ammann, A. J. 1983. Is there an acquired immune deficiency syndrome in infants and children? Pediatrics 72:430-432. [PubMed] [Google Scholar]
- 3.Arrive, E., M. L. Newell, D. K. Ekouevi, M. L. Chaix, R. Thiebaut, B. Masquelier, V. Leroy, P. V. Perre, C. Rouzioux, and F. Dabis. 2007. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int. J. Epidemiol. 36:1009-1021. [DOI] [PubMed] [Google Scholar]
- 4.Barret, B., M. Tardieu, P. Rustin, C. Lacroix, B. Chabrol, I. Desguerre, C. Dollfus, M. J. Mayaux, and S. Blanche. 2003. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS 17:1769-1785. [DOI] [PubMed] [Google Scholar]
- 5.Bedri, A., B. Gudetta, A. Isehak, S. Kumbi, S. Lulseged, Y. Mengistu, A. V. Bhore, R. Bhosale, V. Varadhrajan, N. Gupte, J. Sastry, N. Suryavanshi, S. Tripathy, F. Mmiro, M. Mubiru, C. Onyango, A. Taylor, P. Musoke, C. Nakabiito, A. Abashawl, R. Adamu, G. Antelman, R. C. Bollinger, P. Bright, M. A. Chaudhary, J. Coberly, L. Guay, M. G. Fowler, A. Gupta, E. Hassen, J. B. Jackson, L. H. Moulton, U. Nayak, S. B. Omer, L. Propper, M. Ram, V. Rexroad, A. J. Ruff, A. Shankar, and S. Zwerski. 2008. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet 372:300-313. [DOI] [PubMed] [Google Scholar]
- 6.Blanche, S., M. Tardieu, P. Rustin, A. Slama, B. Barret, G. Firtion, N. Ciraru-Vigneron, C. Lacroix, C. Rouzioux, L. Mandelbrot, I. Desguerre, A. Rotig, M. J. Mayaux, and J. F. Delfraissy. 1999. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet 354:1084-1089. [DOI] [PubMed] [Google Scholar]
- 7.Branson, B. M., H. H. Handsfield, M. A. Lampe, R. S. Janssen, A. W. Taylor, S. B. Lyss, and J. E. Clark. 2006. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recommend. Rep. 55:1-17; quiz CE1-4. [PubMed] [Google Scholar]
- 8.Bwirire, L. D., M. Fitzgerald, R. Zachariah, V. Chikafa, M. Massaquoi, M. Moens, K. Kamoto, and E. J. Schouten. 2008. Reasons for loss to follow-up among mothers registered in a prevention-of-mother-to-child transmission program in rural Malawi. Trans. R. Soc. Trop. Med. Hyg. 102:1195-1200. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 1985. Recommendations for assisting in the prevention of perinatal transmission of human T-lymphotropic virus type III/lymphadenopathy-associated virus and acquired immunodeficiency syndrome. MMWR Morb. Mortal. Wkly. Rep. 34:721-726, 731-732. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 2001. Revised recommendations for HIV screening of pregnant women. MMWR Recommend. Rep. 50:63-85; quiz CE1-19a2-CE6-19a2. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2006. Twenty-five years of HIV/AIDS—United States, 1981-2006. MMWR Morb. Mortal. Wkly. Rep. 55:585-589. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 1995. U.S. Public Health Service recommendations for human immunodeficiency virus counseling and voluntary testing for pregnant women. MMWR Recommend. Rep. 44:1-15. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. 1982. Unexplained immunodeficiency and opportunistic infections in infants—New York, New Jersey, California. MMWR Morb. Mortal. Wkly. Rep. 31:665-667. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. 2007. HIV/AIDS surveillance in women. Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/hiv/topics/surveillance/resources/slides/women/index.htm.
- 15.Centers for Disease Control and Prevention. 5 May 2008, posting date. New estimates of U.S. HIV prevalence, 2006. Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/hiv/topics/surveillance/resources/slides/women/index.htm.
- 16.Centers for Disease Control and Prevention. 11 September 2008, posting date. New HIV incidence estimates: CDC responds. Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/hiv/topics/surveillance/resources/factsheets/response.htm.
- 17.Centers for Disease Control and Prevention. November 2007, posting date. Reducing HIV transmission from mother-to-child: an opt-out approach to HIV screening. Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/hiv/topics/perinatal/resources/factsheets/opt-out.htm.
- 18.Central Intelligence Agency. 12 May 2009, posting date. The 2008 World Factbook. Central Intelligence Agency, McLean, VA. http://www.cia.gov/library/publications/the-world-factbook/.
- 19.Chersich, M. F., M. F. Urban, F. W. Venter, T. Wessels, A. Krause, G. E. Gray, S. Luchters, and D. L. Viljoen. 2006. Efavirenz use during pregnancy and for women of child-bearing potential. AIDS Res. Ther. 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chi, B. H., N. Chintu, R. A. Cantrell, C. Kankasa, G. Kruse, F. Mbewe, M. Sinkala, P. J. Smith, E. M. Stringer, and J. S. Stringer. 2008. Addition of single-dose tenofovir and emtricitabine to intrapartum nevirapine to reduce perinatal HIV transmission. J. Acquir. Immune Defic. Syndr. 48:220-223. [DOI] [PubMed] [Google Scholar]
- 21.Chi, B. H., M. Sinkala, F. Mbewe, R. A. Cantrell, G. Kruse, N. Chintu, G. M. Aldrovandi, E. M. Stringer, C. Kankasa, J. T. Safrit, and J. S. Stringer. 2007. Single-dose tenofovir and emtricitabine for reduction of viral resistance to non-nucleoside reverse transcriptase inhibitor drugs in women given intrapartum nevirapine for perinatal HIV prevention: an open-label randomised trial. Lancet 370:1698-1705. [DOI] [PubMed] [Google Scholar]
- 22.Cleland, J., S. Bernstein, A. Ezeh, A. Faundes, A. Glasier, and J. Innis. 2006. Family planning: the unfinished agenda. Lancet 368:1810-1827. [DOI] [PubMed] [Google Scholar]
- 23.Connor, E. M., R. S. Sperling, R. Gelber, P. Kiselev, G. Scott, M. J. O'Sullivan, R. VanDyke, M. Bey, W. Shearer, R. L. Jacobson, et al. 1994. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N. Engl. J. Med. 331:1173-1180. [DOI] [PubMed] [Google Scholar]
- 24.Cooper, E. R., M. Charurat, L. Mofenson, I. C. Hanson, J. Pitt, C. Diaz, K. Hayani, E. Handelsman, V. Smeriglio, R. Hoff, and W. Blattner. 2002. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J. Acquir. Immune Defic. Syndr. 29:484-494. [DOI] [PubMed] [Google Scholar]
- 25.Coovadia, H. M., N. C. Rollins, R. M. Bland, K. Little, A. Coutsoudis, M. L. Bennish, and M. L. Newell. 2007. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet 369:1107-1116. [DOI] [PubMed] [Google Scholar]
- 26.Coutsoudis, A., F. Dabis, W. Fawzi, P. Gaillard, G. Haverkamp, D. R. Harris, J. B. Jackson, V. Leroy, N. Meda, P. Msellati, M. L. Newell, R. Nsuati, J. S. Read, and S. Wiktor. 2004. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J. Infect. Dis. 189:2154-2166. [DOI] [PubMed] [Google Scholar]
- 27.Cowan, M. J., D. Hellmann, D. Chudwin, D. W. Wara, R. S. Chang, and A. J. Ammann. 1984. Maternal transmission of acquired immune deficiency syndrome. Pediatrics 73:382-386. [PubMed] [Google Scholar]
- 28.Dao, H., L. M. Mofenson, R. Ekpini, C. F. Gilks, M. Barnhart, O. Bolu, and N. Shaffer. 2007. International recommendations on antiretroviral drugs for treatment of HIV-infected women and prevention of mother-to-child HIV transmission in resource-limited settings: 2006 update. Am. J. Obstet Gynecol. 197:S42-S55. [DOI] [PubMed] [Google Scholar]
- 29.Doherty, T., M. Chopra, L. Nkonki, D. Jackson, and T. Greiner. 2006. Effect of the HIV epidemic on infant feeding in South Africa: “When they see me coming with the tins they laugh at me”. Bull. W. H. O. 84:90-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekouevi, D. K., R. Toure, R. Becquet, I. Viho, C. Sakarovitch, F. Rouet, B. Towne-Gold, P. Fassinou, V. Leroy, S. Blanche, F. Dabis, et al. 2006. Serum lactate levels in infants exposed peripartum to antiretroviral agents to prevent mother-to-child transmission of HIV. Pediatrics 118:e1071-e1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eshleman, S. H., D. R. Hoover, S. E. Hudelson, S. Chen, S. A. Fiscus, E. Piwowar-Manning, J. B. Jackson, N. I. Kumwenda, and T. E. Taha. 2006. Development of nevirapine resistance in infants is reduced by use of infant-only single-dose nevirapine plus zidovudine postexposure prophylaxis for the prevention of mother-to-child transmission of HIV-1. J. Infect. Dis. 193:479-481. [DOI] [PubMed] [Google Scholar]
- 32.European Collaborative Study. 2004. Levels and patterns of neutrophil cell counts over the first 8 years of life in children of HIV-1-infected mothers. AIDS 18:2009-2017. [DOI] [PubMed] [Google Scholar]
- 33.European Mode of Delivery Collaboration. 1999. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet 353:1035-1039. [DOI] [PubMed] [Google Scholar]
- 34.Eyakuze, C., D. A. Jones, A. M. Starrs, and N. Sorkin. 2008. From PMTCT to a more comprehensive AIDS response for women: a much-needed shift. Dev. World Bioeth. 8:33-42. [DOI] [PubMed] [Google Scholar]
- 35.Garcia, P. M., L. A. Kalish, J. Pitt, H. Minkoff, T. C. Quinn, S. K. Burchett, J. Kornegay, B. Jackson, J. Moye, C. Hanson, C. Zorrilla, J. F. Lew, et al. 1999. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N. Engl. J. Med. 341:394-402. [DOI] [PubMed] [Google Scholar]
- 36.Guay, L. A., P. Musoke, T. Fleming, D. Bagenda, M. Allen, C. Nakabiito, J. Sherman, P. Bakaki, C. Ducar, M. Deseyve, L. Emel, M. Mirochnick, M. G. Fowler, L. Mofenson, P. Miotti, K. Dransfield, D. Bray, F. Mmiro, and J. B. Jackson. 1999. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 354:795-802. [DOI] [PubMed] [Google Scholar]
- 37.HIV Prevention Trials Network 027. 2008, posting date. A phase I study to evaluate the safety and immunogenicity of ALVAC-HIV vCP1521 in infants born to HIV-1-infected women in Uganda. http://www.hptn.org/research_studies/HPTN027.asp.
- 38.Iliff, P. J., E. G. Piwoz, N. V. Tavengwa, C. D. Zunguza, E. T. Marinda, K. J. Nathoo, L. H. Moulton, B. J. Ward, and J. H. Humphrey. 2005. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS 19:699-708. [DOI] [PubMed] [Google Scholar]
- 39.Jackson, J. B., P. Musoke, T. Fleming, L. A. Guay, D. Bagenda, M. Allen, C. Nakabiito, J. Sherman, P. Bakaki, M. Owor, C. Ducar, M. Deseyve, A. Mwatha, L. Emel, C. Duefield, M. Mirochnick, M. G. Fowler, L. Mofenson, P. Miotti, M. Gigliotti, D. Bray, and F. Mmiro. 2003. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet 362:859-868. [DOI] [PubMed] [Google Scholar]
- 40.Jamieson, D. J., J. Clark, A. P. Kourtis, A. W. Taylor, M. A. Lampe, M. G. Fowler, and L. M. Mofenson. 2007. Recommendations for human immunodeficiency virus screening, prophylaxis, and treatment for pregnant women in the United States. Am. J. Obstet Gynecol. 197:S26-32. [DOI] [PubMed] [Google Scholar]
- 41.Jaspan, H. B., and R. F. Garry. 2003. Preventing neonatal HIV: a review. Curr. HIV Res. 1:321-327. [DOI] [PubMed] [Google Scholar]
- 42.John, G. C., R. W. Nduati, D. A. Mbori-Ngacha, B. A. Richardson, D. Panteleeff, A. Mwatha, J. Overbaugh, J. Bwayo, J. O. Ndinya-Achola, and J. K. Kreiss. 2001. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J. Infect. Dis. 183:206-212. [DOI] [PubMed] [Google Scholar]
- 43.John-Stewart, G., D. Mbori-Ngacha, R. Ekpini, E. N. Janoff, J. Nkengasong, J. S. Read, P. Van de Perre, and M. L. Newell. 2004. Breast-feeding and transmission of HIV-1. J. Acquir. Immune Defic. Syndr. 35:196-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joint United Nations Programme on HIV/AIDS. August 2008, posting date. 2008 report on the global AIDS epidemic. UNAIDS, Geneva, Switzerland. http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.
- 45.Joint United Nations Programme on HIV/AIDS. December 2005, posting date. AIDS Epidemic Update. UNAIDS/WHO, Geneva, Switzerland. http://data.unaids.org/Publications/IRC-pub06/epi_update2005_en.pdf.
- 46.Joint United Nations Programme on HIV/AIDS, United Nations Children's Fund, World Health Organization. April 2008, posting date. Children and AIDS: second stocktaking report. UNICEF, New York, NY. http://www.unicef.org/publications/index_43451.html.
- 47.Joint United Nations Programme on HIV/AIDS/World Health Organization. 19 November 2007, posting date. AIDS epidemic update. UNAIDS/WHO. http://www.unaids.org/en/KnowledgeCentre/HIVData/EpiUpdate/EpiUpdArchive/2007/.
- 48.Joint United Nations Programme on HIV/AIDS/World Health Organization. 16 April 2008, posting date. Sub-Saharan Africa AIDS epidemic update regional summary. UNAIDS/WHO, Geneva, Switzerland. http://www.unaids.org/en/KnowledgeCentre/HIVData/EpiUpdate/EpiUpdArchive/2007/.
- 48a.Joint United Nations Programme on HIV/AIDS. 2009, posting date. About UNAIDS. UNAIDS, Geneva, Switzerland. http://www.unaids.org/en/AboutUNAIDS/default.asp.
- 49.Jourdain, G., N. Ngo-Giang-Huong, S. Le Coeur, C. Bowonwatanuwong, P. Kantipong, P. Leechanachai, S. Ariyadej, P. Leenasirimakul, S. Hammer, and M. Lallemant. 2004. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N. Engl. J. Med. 351:229-240. [DOI] [PubMed] [Google Scholar]
- 50.Kaneda, T., K. Shiraki, K. Hirano, and I. Nagata. 1997. Detection of maternofetal transfusion by placental alkaline phosphatase levels. J. Pediatr. 130:730-735. [DOI] [PubMed] [Google Scholar]
- 51.Kasenga, F., A. K. Hurtig, and M. Emmelin. 2008. HIV-positive women's experiences of a PMTCT programme in rural Malawi. Midwifery [Epub ahead of print.] doi: 10.1016/j.midw.2008.04.007. [DOI] [PubMed]
- 52.Kumwenda, N. I., D. R. Hoover, L. M. Mofenson, M. C. Thigpen, G. Kafulafula, Q. Li, L. Mipando, K. Nkanaunena, T. Mebrahtu, M. Bulterys, M. G. Fowler, and T. E. Taha. 2008. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N. Engl. J. Med. 359:119-129. [DOI] [PubMed] [Google Scholar]
- 53.Lallemant, M., G. Jourdain, S. Le Coeur, J. Y. Mary, N. Ngo-Giang-Huong, S. Koetsawang, S. Kanshana, K. McIntosh, and V. Thaineua. 2004. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N. Engl. J. Med. 351:217-228. [DOI] [PubMed] [Google Scholar]
- 54.Lockman, S., R. L. Shapiro, L. M. Smeaton, C. Wester, I. Thior, L. Stevens, F. Chand, J. Makhema, C. Moffat, A. Asmelash, P. Ndase, P. Arimi, E. van Widenfelt, L. Mazhani, V. Novitsky, S. Lagakos, and M. Essex. 2007. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N. Engl. J. Med. 356:135-147. [DOI] [PubMed] [Google Scholar]
- 55.Lyons, F., S. Hopkins, B. Kelleher, A. McGeary, G. Sheehan, J. Geoghegan, C. Bergin, F. M. Mulcahy, and P. A. McCormick. 2006. Maternal hepatotoxicity with nevirapine as part of combination antiretroviral therapy in pregnancy. HIV Med. 7:255-260. [DOI] [PubMed] [Google Scholar]
- 56.Mandelbrot, L., M. J. Mayaux, A. Bongain, A. Berrebi, Y. Moudoub-Jeanpetit, J. L. Benifla, N. Ciraru-Vigneron, J. Le Chenadec, S. Blanche, and J. F. Delfraissy. 1996. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohorts. Am. J. Obstet Gynecol. 175:661-667. [DOI] [PubMed] [Google Scholar]
- 57.Martinson, N. A., D. K. Ekouevi, F. Dabis, L. Morris, P. Lupodwana, B. Tonwe-Gold, P. Dhlamini, R. Becquet, J. G. Steyn, V. Leroy, I. Viho, G. E. Gray, and J. A. McIntyre. 2007. Transmission rates in consecutive pregnancies exposed to single-dose nevirapine in Soweto, South Africa and Abidjan, Cote d'Ivoire. J. Acquir. Immune Defic. Syndr. 45:206-209. [DOI] [PubMed] [Google Scholar]
- 58.Marum, E., M. Taegtmeyer, and K. Chebet. 2006. Scale-up of voluntary HIV counseling and testing in Kenya. JAMA 296:859-862. [DOI] [PubMed] [Google Scholar]
- 59.Mayaux, M. J., S. Blanche, C. Rouzioux, J. Le Chenadec, V. Chambrin, G. Firtion, M. C. Allemon, E. Vilmer, N. C. Vigneron, J. Tricoire, et al. 1995. Maternal factors associated with perinatal HIV-1 transmission: the French Cohort Study: 7 years of follow-up observation. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 8:188-194. [PubMed] [Google Scholar]
- 60.McIntyre, J. A., N. Martinson, G. E. Gray. 2005. Single dose nevirapine combined with a short course of combivir for prevention of mother to child transmission of HIV-1 can significantly decrease the subsequent development of maternal and infant resistant virus. Antivir. Ther. 10:S4. [Google Scholar]
- 61.Minkoff, H., D. N. Burns, S. Landesman, J. Youchah, J. J. Goedert, R. P. Nugent, L. R. Muenz, and A. D. Willoughby. 1995. The relationship of the duration of ruptured membranes to vertical transmission of human immunodeficiency virus. Am. J. Obstet. Gynecol. 173:585-589. [DOI] [PubMed] [Google Scholar]
- 62.Mirochnick, M., A. Dorenbaum, S. Blanchard, C. K. Cunningham, R. D. Gelber, L. Mofenson, M. Culnane, and J. L. Sullivan. 2003. Predose infant nevirapine concentration with the two-dose intrapartum neonatal nevirapine regimen: association with timing of maternal intrapartum nevirapine dose. J. Acquir. Immune Defic. Syndr. 33:153-156. [DOI] [PubMed] [Google Scholar]
- 63.Mirochnick, M., K. Nielsen-Saines, J. H. Pilotto, J. Pinto, E. Jimenez, V. G. Veloso, T. Parsons, D. H. Watts, J. Moye, L. M. Mofenson, M. Camarca, and Y. Bryson. 2008. Nevirapine concentrations in newborns receiving an extended prophylactic regimen. J. Acquir. Immune Defic. Syndr. 47:334-337. [PubMed] [Google Scholar]
- 64.Mofenson, L. M., J. S. Lambert, E. R. Stiehm, J. Bethel, W. A. Meyer III, J. Whitehouse, J. Moye, Jr., P. Reichelderfer, D. R. Harris, M. G. Fowler, B. J. Mathieson, G. J. Nemo, et al. 1999. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. N. Engl. J. Med. 341:385-393. [DOI] [PubMed] [Google Scholar]
- 65.Moses, A., C. Zimba, E. Kamanga, J. Nkhoma, A. Maida, F. Martinson, I. Mofolo, G. Joaki, J. Muita, A. Spensley, I. Hoffman, and C. M. van der Horst. 2008. Prevention of mother-to-child transmission: program changes and the effect on uptake of the HIVNET 012 regimen in Malawi. AIDS 22:83-87. [DOI] [PubMed] [Google Scholar]
- 66.National HIV/AIDS Clinicians’ Consultation Center. 17 March 2009, posting date. Compendium of State HIV Testing Laws—2009. University of California, San Francisco. http://www.nccc.ucsf.edu/StateLaws/Index.html.
- 67.Newell, M. L. 2006. Current issues in the prevention of mother-to-child transmission of HIV-1 infection. Trans. R. Soc. Trop. Med. Hyg. 100:1-5. [DOI] [PubMed] [Google Scholar]
- 68.Newell, M. L., D. T. Dunn, C. S. Peckham, A. E. Semprini, G. Pardi, et al. 1996. Vertical transmission of HIV-1: maternal immune status and obstetric factors. AIDS 10:1675-1681. [PubMed] [Google Scholar]
- 69.Noguera, A., C. Fortuny, E. Sanchez, R. Artuch, M. A. Vilaseca, C. Munoz-Almagro, J. Pou, and R. Jimenez. 2003. Hyperlactatemia in human immunodeficiency virus-infected children receiving antiretroviral treatment. Pediatr. Infect. Dis. J. 22:778-782. [DOI] [PubMed] [Google Scholar]
- 70.Oleske, J., A. Minnefor, R. Cooper, Jr., K. Thomas, A. dela Cruz, H. Ahdieh, I. Guerrero, V. V. Joshi, and F. Desposito. 1983. Immune deficiency syndrome in children. JAMA 249:2345-2349. [PubMed] [Google Scholar]
- 71.Pitt, J., D. Brambilla, P. Reichelderfer, A. Landay, K. McIntosh, D. Burns, G. V. Hillyer, H. Mendez, and M. G. Fowler. 1997. Maternal immunologic and virologic risk factors for infant human immunodeficiency virus type 1 infection: findings from the Women and Infants Transmission Study. J. Infect. Dis. 175:567-575. [DOI] [PubMed] [Google Scholar]
- 71a.President's Emergency Plan for AIDS Relief. 2007. The power of partnerships: third annual report to Congress on PEPFAR. PEPFAR, Washington, DC.
- 72.Public Health Service Task Force. 8 July 2008, posting date. Recommendations for use of antiretroviral drugs in pregnant HIV-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. Public Health Service, Washington, DC. http://aidsinfo.nih.gov/contentfiles/PerinatalGL001020.pdf.
- 73.Read, J. S. 2006. Prevention of mother-to-child transmission of HIV, p. 107-133. In S. L. Zeichner and J. S. Read (ed.), Handbook of pediatric HIV care. Cambridge University Press, Cambridge, United Kingdom.
- 74.Reynolds, H. W., B. Janowitz, R. Wilcher, and W. Cates. 2008. Contraception to prevent HIV-positive births: current contribution and potential cost savings in PEPFAR countries. Sex. Transm. Infect. 84(Suppl. 2):49-53. [DOI] [PubMed] [Google Scholar]
- 75.Reynolds, H. W., M. J. Steiner, and W. Cates, Jr. 2005. Contraception's proved potential to fight HIV. Sex. Transm. Infect. 81:184-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rubinstein, A., M. Sicklick, A. Gupta, L. Bernstein, N. Klein, E. Rubinstein, I. Spigland, L. Fruchter, N. Litman, H. Lee, and M. Hollander. 1983. Acquired immunodeficiency with reversed T4/T8 ratios in infants born to promiscuous and drug-addicted mothers. JAMA 249:2350-2356. [PubMed] [Google Scholar]
- 77.Sansom, S. L., D. J. Jamieson, P. G. Farnham, M. Bulterys, and M. G. Fowler. 2003. Human immunodeficiency virus retesting during pregnancy: costs and effectiveness in preventing perinatal transmission. Obstet. Gynecol. 102:782-790. [DOI] [PubMed] [Google Scholar]
- 78.Scalfaro, P., J. J. Chesaux, P. A. Buchwalder, J. Biollaz, and J. L. Micheli. 1998. Severe transient neonatal lactic acidosis during prophylactic zidovudine treatment. Intensive Care Med. 24:247-250. [DOI] [PubMed] [Google Scholar]
- 79.Scott, G. B., B. E. Buck, J. G. Leterman, F. L. Bloom, and W. P. Parks. 1984. Acquired immunodeficiency syndrome in infants. N. Engl. J. Med. 310:76-81. [DOI] [PubMed] [Google Scholar]
- 80.Shaffer, N., R. Chuachoowong, P. A. Mock, C. Bhadrakom, W. Siriwasin, N. L. Young, T. Chotpitayasunondh, S. Chearskul, A. Roongpisuthipong, P. Chinayon, J. Karon, T. D. Mastro, R. J. Simonds, et al. 1999. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Lancet. 353:773-780. [DOI] [PubMed] [Google Scholar]
- 81.Spensley, A., T. Sripipatana, A. N. Turner, C. Hoblitzelle, J. Robinson, and C. Wilfert. 2009. Preventing mother-to-child transmission of HIV in resource-limited settings: the Elizabeth Glaser Pediatric AIDS Foundation experience. Am. J. Public Health 99:631-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stanton, C. K., and S. A. Holtz. 2006. Levels and trends in cesarean birth in the developing world. Stud. Fam. Plann. 37:41-48. [DOI] [PubMed] [Google Scholar]
- 83.Taha, T. E., N. I. Kumwenda, A. Gibbons, R. L. Broadhead, S. Fiscus, V. Lema, G. Liomba, C. Nkhoma, P. G. Miotti, and D. R. Hoover. 2003. Short postexposure prophylaxis in newborn babies to reduce mother-to-child transmission of HIV-1: NVAZ randomised clinical trial. Lancet 362:1171-1177. [DOI] [PubMed] [Google Scholar]
- 84.Taha, T. E., N. I. Kumwenda, D. R. Hoover, S. A. Fiscus, G. Kafulafula, C. Nkhoma, S. Nour, S. Chen, G. Liomba, P. G. Miotti, and R. L. Broadhead. 2004. Nevirapine and zidovudine at birth to reduce perinatal transmission of HIV in an African setting: a randomized controlled trial. JAMA 292:202-209. [DOI] [PubMed] [Google Scholar]
- 85.Taylor, G. P., and N. Low-Beer. 2001. Antiretroviral therapy in pregnancy: a focus on safety. Drug Saf. 24:683-702. [DOI] [PubMed] [Google Scholar]
- 86.Reference deleted.
- 87.Thea, D. M., G. Aldrovandi, C. Kankasa, P. Kasonde, W. D. Decker, K. Semrau, M. Sinkala, and L. Kuhn. 2006. Post-weaning breast milk HIV-1 viral load, blood prolactin levels and breast milk volume. AIDS 20:1539-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thior, I., S. Lockman, L. M. Smeaton, R. L. Shapiro, C. Wester, S. J. Heymann, P. B. Gilbert, L. Stevens, T. Peter, S. Kim, E. van Widenfelt, C. Moffat, P. Ndase, P. Arimi, P. Kebaabetswe, P. Mazonde, J. Makhema, K. McIntosh, V. Novitsky, T. H. Lee, R. Marlink, S. Lagakos, and M. Essex. 2006. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA 296:794-805. [DOI] [PubMed] [Google Scholar]
- 89.Thomas, P. A., J. Weedon, K. Krasinski, E. Abrams, N. Shaffer, P. Matheson, M. Bamji, A. Kaul, D. Hutson, K. T. Grimm, et al. 1994. Maternal predictors of perinatal human immunodeficiency virus transmission. Pediatr. Infect. Dis. J. 13:489-495. [DOI] [PubMed] [Google Scholar]
- 90.Thorne, C., and M. L. Newell. 2003. Mother-to-child transmission of HIV infection and its prevention. Curr. HIV Res. 1:447-462. [DOI] [PubMed] [Google Scholar]
- 91.Thorne, C., and M. L. Newell. 2007. Safety of agents used to prevent mother-to-child transmission of HIV: is there any cause for concern? Drug Saf. 30:203-213. [DOI] [PubMed] [Google Scholar]
- 92.Reference deleted.
- 93.Vermeer, W., A. E. Bos, J. Mbwambo, S. Kaaya, and H. P. Schaalma. 2009. Social and cognitive variables predicting voluntary HIV counseling and testing among Tanzanian medical students. Patient Educ. Couns. 75:135-140. [DOI] [PubMed] [Google Scholar]
- 94.World Health Organization. 2006, posting date. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants in resource-limited settings: toward universal access. WHO, Geneva, Switzerland. http://www.who.int/hiv/pub/guidelines/pmtct/en/.
- 95.World Health Organization. 1 November 2007, posting date. Guidance on global scale-up of the prevention of mother-to-child transmission of HIV: towards universal access for women, infants and young children and eliminating HIV and AIDS among children. WHO, Geneva, Switzerland. http://www.who.int/hiv/pub/guidelines/pmtct_scaleup2007/en/.
- 96.World Health Organization/Joint United Nations Programme on AIDS. May 2007, posting date. Guidance on provider-initiated HIV testing and counseling in health facilities. WHO, Geneva, Switzerland. http://www.who.int/hiv/pub/guidelines/pitc2007/en/.