Abstract
Summary: Paragonimus species are highly evolved parasites with a complex life cycle that involves at least three different hosts, i.e., snails, crustaceans, and mammals. The adult forms of Paragonimus species reside and mate in the lungs of a variety of permissive mammalian hosts, including humans. Although human paragonimiasis is uncommonly encountered in North America, both autochthonous and imported disease may be encountered. Paragonimus kellicotti, the species endemic to North America, is a well-known pathogen in wild and domestic animals. Five patients with North American paragonimiasis have been reported in the recent medical literature. The biologic, clinical, radiologic, and laboratory features of paragonimiasis are reviewed, with emphasis on North American paragonimiasis whenever possible.
INTRODUCTION
The prevalence of many snail-borne and vector-borne parasitic diseases has been dramatically diminished in many areas where they were once common. However, the frequency for some food-borne parasitic diseases is increasing (113, 132, 133). Several of these may be directly linked to the dramatic increase in aquaculture, which is becoming very common in many Asian countries; this demonstrates how subtle changes in ecology, which may be economically driven, may contribute to an increased incidence of parasitic diseases (85). Just as the reemergence of tuberculosis has been associated with lapses in public health, so too paragonimiasis has also reemerged in areas of endemicity where public health measures of the past have also lapsed; however, novel reasons for regional increases in disease incidence have also been described (17, 26, 60, 113, 132, 133, 202).
A wide variety of trematodes, which are commonly known as flukes, may infect humans. These include a large number of intestinal trematodes (e.g., Fasiciolopsis), and the widely distributed and economically and medically important schistosomes. There is only one genus of trematode, however, whose members reside as adults in the lungs of terrestrial mammalian hosts. This is the genus Paragonimus. Paragonimus westermani is the most important and widely distributed of the many Paragonimus species that exist. This review will cover paragonimiasis in general but will comment particularly on the Paragonimus species of North America, P. kellicotti, whenever possible. This Paragonimus species is unknown to many health care providers because of the paucity of infections in humans. Only five patients with North American paragonimiasis have been reported in the medical literature since the mid-1980s (30, 49, 111, 138, 141). Paragonimiasis in the United States and Canada may also be due to imported disease. Travelers who have visited an area where the disease is endemic may returned infected, or an infected individual from an area of endemicity may immigrate to North America (123). Alternatively, paragonimiasis due to species not endemic to North America may also be contracted through the consumption of imported food that contains infective metacercariae.
Paragonimus westermani was initially discovered during the necropsy of a Bengal tiger at the Amsterdam zoological gardens, The Netherlands (57, 80). These parasites were sent to the Dutch zoologist Coenraad Kerbert, who described the organisms and compared them with other trematode species (57, 87). Similar trematodes had been observed in the otter and the Indian mongoose (57). This evaluation convinced Kerbert that this species had not been described, so he named it Distoma westermani, in honor of the zoo director, C. F. Westerman (57, 87). The next few years brought the discovery of human disease caused by this parasite.
The first described human infection was in a Portuguese resident in what is modern-day Taiwan (57). This patient, who died of an aortic aneurysm, underwent an autopsy that was performed by B. S. Ringer (57). Ringer shared his findings with P. Manson, who had cared for this patient during the previous year (57, 110). Subsequently, eggs from a Paragonimus species were independently noted in the respiratory specimens of two other patients. Erwin Baelz (1878, Tokyo, Japan) noted forms in the respiratory specimens of patients with hemoptysis, but he failed to recognize that these forms were eggs (12). Manson in 1880 described the eggs in the sputum of a patient with hemoptysis and correctly concluded that these were eggs from a parasite (57). The history of these and further discoveries concerning paragonimiasis and other helminthes of medical importance are delineated in detail in a excellent review, A History of Human Helminthology, by D. I. Grove (57).
Max Braun contributed to the early work in helminthology and established the genus Paragonimus in 1899, in which P. westermani was the type species (19, 21, 22, 112). The name Paragonimus is derived from the combination of two Greek words, “para” (on the side of) and “gonimos” (gonads or genitalia) (57, 112). There is a unique history of discovery and subsequent characterization of each Paragonimus species, most of which is beyond the scope of this text. Controversy surrounds the identity of the first Paragonimus species described in the Western Hemisphere. Diesing in 1850 described what he called Distoma rude in the lungs of the Brazilian otter, Pteronura brasiliensis (19, 172). The redescription of this organism by Braun in 1901 definitively placed this organism in the genus Paragonimus; he therefore called it P. rudis, although the particular species of this specimen remains debatable (21, 172). Although some have suggested that this trematode represented P. kellicotti, others contend that it was more likely another Paragonimus species, such as a species that is currently found in South America (19). There are inconsistencies regarding the distribution of P. kellicotti, with some contending that its range extends into South America, whereas others believe that it is restricted to North America. Comparative genomic studies will likely help define the geographic range of currently valid species over the next two decades. Regardless of the outcomes of these studies, the precise Paragonimus species that was originally discovered in the Western Hemisphere will remain a mystery; Paragonimus rudis is no longer a recognized Paragonimus species, although it may represent a senior synonym of another species.
Henry Ward and D. S. Kellicott are credited with the independent, near-simultaneous discovery of the only North American Paragonimus species in 1894. Ward first described the infection in a cat from Michigan, whereas Kellicott later that year recognized an infection in a dog, in Columbus, OH (86, 192). Although morphological differences between the North American species described by Ward and the species described by Kerbert from the tiger were appreciated, Ward tentatively identified the parasite as Distoma westermani. This was complicated by the possibility that the infected cat may have been imported from Asia as a pet (194). Subsequently, Ward and Hirsch determined that the North American species was a distinct Paragonimus species, which they generously named P. kellicotti (192, 193, 195). It was not until 16 years later that human cases of paragonimiasis in the Americas were described. Abend is credited with describing the first human case of paragonimiasis in North America (1). A number of patients with paragonimiasis were reported from 1910 through the late 1940s in both North and South America (1, 8, 45, 53, 61, 62, 94, 134, 172). Although some of these were probably caused by species endemic to the Americas, others were also likely instances of imported disease in Asian immigrants. Hereafter, I will not discuss further speculation regarding the species level differentiation of these more remote historical infections but rather will focus primarily on infections and advances in the late 20th and early 21st centuries.
TAXONOMY, DISTRIBUTION, AND EPIDEMIOLOGY
Taxonomy
The genus Paragonimus is in the phylum Platyhelminthes (i.e., flatworms) and the class Trematodea. The major features used to differentiate Paragonimus from other trematodes that cause significant human disease are the morphological differences in the adult, metacercariae and the metecercarial cyst, cercariae, and eggs. Most trematodes that cause human disease are hermaphroditic, whereas schistosomes are not, which is a feature that affords differentiation from this group. More recently, differentiation is being accomplished using molecular techniques. Throughout history as many as 40 species have been assigned to the genus Paragonimus. Many of these species, however, either are in contention or have been subsumed into an existing species. The taxonomic dispute concerning the status of individual species is not the focus of this article and is not covered further. There are eight or nine species that cause the majority of human infections (24, 102). The major species of medical importance and their geographic regions of endemicity are listed in Table 1. The precise number of species and the relationship between these species will become clearer once thorough genomic comparisons have been completed. Fortunately, such work is under way (72, 73).
TABLE 1.
The predominant Paragonimus species that cause human infections and their geographic distributiona
| Paragonimus species | Area(s) of endemicity |
|---|---|
| P. westermani | Asia, India, Philippines, New Guinea |
| P. miyazakiib | Japan |
| P. skrjabini | China, Southeast Asia |
| P. heterotremus | Thailand, China, Southeast Asia |
| P. hueitungensisc | China |
| P. uterobilateralis | West Africa |
| P. africanus | West Africa |
| P. kellicotti | North America |
| P. mexicanus | Central America, South America |
Paragonimus species are hermaphroditic, containing both ovaries and testes. The adult body is ovoid. The precise size and dimensions vary among species and also with the degree of contraction on fixation (112). These organisms measure 7.5 to 12 mm in length, 4 to 6 mm in width, and 3.5 to 5 mm in thickness (112). There is an oral terminal sucker, as well as a ventral sucker that is also known as an acetabulum. The digestive system consists of a truncated pharynx and esophagus that bifurcates early into paired ceca. The paired testes are deeply lobed; the ovary is on one side of the organism, whereas the tightly coiled uterus is on the opposite side (112). The body wall contains smooth muscle and is covered by a tegument. The tegument, in turn, is covered with variably scattered spines. When adult specimens are available, which in the clinical laboratory is extremely rare, the morphological features may be used for definitive identification. The most common features used for the identification of adult specimens include size and shape, the patterns of lobation of the ovary and testes, and the appearance of the cuticular spines (19, 35, 117). Although identification schemata that rely upon the evaluation of cuticular spines are considered problematic by some, they are useful at a minimum for determining taxonomic groupings. For example, groupings of tegumental spines are characteristic of the P. kellicotti group, whereas individual spines occur in the P. africanus group (112).
The morphology of the cercariae and metacercariae has also been studied extensively. Although parasitology experts may use some of these features to differentiate species based on stage-specific features, this is clearly beyond the skills of most medical parasitologists. To complicate matters, some of the morphological features present in these stages of the parasite are unstable and change with the growth of the organism. For example, although the number of flame cells has been reported to differ among species, these are also thought to be likely to increase in number as the cercaria matures, which therefore could lead to misclassification (19, 70, 71). In addition, differences in the sizes of metacercarial cysts and other morphological features may vary depending on the ploidy of the organism (i.e., diploid, triploid, or tetraploid forms of the same species) (180). Regardless of the outcome of these debates, the clinical parasitologist is very unlikely to encounter these forms of the parasite in the clinical laboratory. It is the eggs that are produced and released from the adults that are most likely to be present in a clinical specimen. Morphological diagnosticians (i.e., parasitologists, cytologists, and histopathologists) therefore should be most familiar with the eggs of the parasite. The eggs of Paragonimus may be present in respiratory specimens, tissue biopsy specimens, or stool samples.
Distribution
Paragonimus species are extremely successful parasites and are widely geographically distributed, with Paragonimus species being endemic in Asia, the Americas, and Africa (Table 1). Endemic Paragonimus species have yet to be reported from Europe, Australia, and Antarctica. Imported paragonimiasis, however, may occur in any region (125).
Certain species have a limited geographic distribution, whereas others, such as P. westermani, are more widely distributed. For example, P. miyazakii is endemic in Japan and P. heterotremus is endemic in Thailand, but P. westermani occurs in both of these locations (102, 132). Paragonimus kellicotti is the only Paragonimus species that is endemic in North America. Whether P. kellicotti extends into Central and South America remains controversial. Paragonimus mexicanus occurs in Central and South America. Paragonimus westermani is the most biologically successful and most widely distributed of the Paragonimus species (80, 107). The distribution of P. westermani ranges from Japan throughout Southeast Asia to India.
The distribution of Paragonimus species in part reflects the distribution of permissive animals that support infection (i.e., the natural distribution of the definitive and intermediate hosts) (47). The major faunas of North America that support the life cycle of Paragonimus kellicotti include domestic animals such as dogs and cats, as well as many wild animals (14, 86, 91, 139, 192). Bech-Nielsen et al. found P. kellicotti in 4.6% (3/65) of cats with respiratory tract disease in Louisiana (14). The wild animal hosts that support infection include skunks (Melphitis mephitis), red foxes (Vulpes vulpes), coyotes (Canis latrans), mink, (Mustela vison), and bobcats (Felis rufus); surprisingly, disease has been found to be rare in raccoons (Procyon lotor) (15, 16, 44, 140, 143, 169). Although this parasite is unknown to many physicians and medical microbiologists, it is well known to veterinarians and animal biologists in North America.
Epidemiology of Human Disease
Paragonimiasis is a zoonotic disease in which humans may act as definitive hosts. Although many species of Paragonimus may successfully reproduce and produce eggs in the human host, humans are not essential for the survival of this parasite, given the numerous definitive animal hosts that support infection (17). The prevalence of paragonimiasis throughout the world is difficult to ascertain. It was estimated in 1995 that approximately 20.7 million people may be infected with Paragonimus and that another 195 million were at risk for disease (112, 196). More recently, it has been estimated that 293 million people are at risk, whereas several million are actually infected (17, 85). Paragonimus species are found in tropical, subtropical, and temperate climates. They occur through East and South Asia, through sub-Saharan Africa, and in the Americas from Peru to Canada (17). Geographic regions that have or have had a high prevalence of disease include Cameroon, China, the Philippines, parts of Ecuador, parts of India, Japan, and Thailand (5, 32, 36, 108, 146, 157, 166, 167, 199, 202, 204). In 1984, the prevalence of paragonimiasis in the Philippines was estimated to be between 0.7 and 9.96% (27).
The success of this parasite is reliant upon the completion of the complex life cycle described below. Environmental factors, such as pollution, that adversely affect the population of either the first or second intermediate host will consequently affect the prevalence of disease. The prevalence of human paragonimiasis in many regions of endemicity has declined substantially in many areas over the past century. This has occurred for a number of reasons, but education concerning the risk of disease associated with the consumption of raw crustaceans and the use of raw animal products in folk medicine practices has been a major contributing factor. For example, the prevalence of disease in Korea was estimated to exceed 1.5 million people prior to educational efforts that focused on healthier eating habits, cessation of the use of crayfish juice in folk medicine, and more widespread pollution that adversely affected intermediate hosts (80, 88, 129). The prevalence of paragonimiasis in Korea was substantially reduced following the impact of these factors (80, 159). Although the prevalence of paragonimiasis has declined in many regions, areas of high endemicity still exist.
Paragonimiasis occurs in men and women and in both adults and children. Uchiyama et al. reviewed 104 patients with paragonimiasis from 1986 to 1998 and demonstrated that children as well as adults were infected and that there was no substantial difference in the male-to-female ratio (182). Ashitani et al. also reported paragonimiasis in children as well as adults and failed to demonstrate any obvious distributional differences with respect to sex (9). Some have suggested that children in some areas may be infected at a higher rate than adults. Furthermore, it is known that children are more likely to develop ectopic paragonimiasis, with cerebral paragonimiasis being the most severe form of ectopic disease (see below). In contrast to a near equivalent male-to-female ratio, Singh et al. reported that 90% (35/39) of the patients in Manipur, India, were male (167). In such reviews, however, the possibility of limited access to health care for women as an explanation for the observed difference has not been discussed.
Humans most commonly become infected through the ingestion of raw or undercooked crustaceans or products derived from these animals. These are consumed throughout the world for a variety of reasons. In many instances, it is because of local culinary preferences, practices, or customs. Eating raw or pickled crab meat is common in many parts of the world. For example, Singh et al. (167) reported that the vast majority (31/39) of patients they studied reported eating raw crab, whereas the remainder reported eating crab that was smoked or cooked. A number of culinary dishes have been linked with paragonimiasis. These include the Chinese and Korean dish “kejeng,” wherein live crustaceans are eaten with soybean sauce (80), and the Japanese dish “oboro-kiro,” wherein crab juice is added to bean paste (miso) soup (80, 158, 203). Paragonimiasis in Mexico has been associated with cerviche that contains uncooked crustaceans (Hugo Vicente Ralde, personal communication). Similarly, the Philippine specialty “kilano,” wherein raw crabs, citrus fruit juice, and coconut milk are taken with an alcoholic beverage, may cause infection (80). Likely the most well known culinary dish associated with paragonimiasis is the “drunken crab,” wherein uncooked crabs are pickled or preserved in rice wine and then eaten (80, 155). This type of preservation process does not kill the parasite. It is important to recognize that crabs imported from areas where paragonimiasis is endemic are available in specialty food markets in the United States. These crabs, which are either frozen or pickled, may contain viable metacercariae and have been associated with infections in the United States (115).
Folk medical practices and local customs or superstitious beliefs may also contribute to the transmission of this parasite. The ingestion of crayfish juice as a treatment for measles, as mentioned above, is/was a folk medicine treatment in Korea (206). It is a tribal belief in some areas of Cameroon, Africa, that the ingestion of raw crab will increase the fertility of a woman (80, 108). Both of these practices expose the person to infective metacercariae and have resulted in paragonimiasis.
Another means of acquiring paragonimiasis is through the ingestion of raw or incompletely cooked tissues of a paratenic host (described in detail below). Small paratenic hosts, such as rats in the Philippines, which serve as a food source for many larger animals, are thought to significantly contribute to the spread of disease (28). Although a naturally occurring paratenic host for P. kellicotti has not been described, the rat has been experimentally infected with this parasite and is a conceivable paratenic host (172). Swine also have been recognized as a paratenic host for Paragonimus species (118). The thorough cooking of tissues from any potential paratenic hosts (i.e., a carnivorous or omnivorous animal) and good cooking practices when these foods are processed are recommended.
North American paragonimiasis in humans is a rare disease. This is largely because the dietary habits of most North Americans do not include the consumption of raw or undercooked crayfish. Of the five patients with North American paragonimiasis reviewed here, all were male and none were children. These individuals had a propensity for hunting, camping, and “living off the land”; one consumed the raw crayfish in a “playful manner” (30, 100, 141) As indicated above, patients with paragonimiasis in North America may also have imported disease (i.e., infection with a species other than P. kellicotti). These people either immigrated while infected, became infected while traveling in an area of endemicity, or acquired infection through the ingestion of imported foods, all of which have been described (6, 23, 25, 76, 77, 109, 115, 179, 200).
LIFE CYCLE
Paragonimus species exist in nature in a complex life cycle that includes a mammal as the definitive host (i.e., the host in which the adult is found and in which sexual reproduction occurs) and snails and crustaceans as intermediate hosts. (47, 150, 161, 174). An understanding of the life cycle of Paragonimus is important for a thorough understanding of disease transmission and factors that affect the prevalence of disease in human and animal populations.
This description of the life cycle begins with the production and passage of fertilized, operculate eggs from sexually competent adult trematodes that reside within the lungs of the definitive mammalian host (Fig. 1); the possibility of eggs being produced by a single worm, particularly a triploid worm, also exists and is discussed in more detail below. The eggs are expectorated and either expelled or swallowed and passed in the feces. The eggs in fresh or brackish water eventually hatch and release a ciliated miracidium that migrates to and infects a permissive snail species, which is the first intermediate host. The snails that support the Paragonimus life cycle are usually in the families Pleuroceridae and Thiaridae, although in the Americas members of the families Hydrobiidae and Pomatiopsidae may also be involved (112). There are particular snail species in different parts of the world that support infections by endemic Paragonimus species. Paragonimus species benefit from a large variety of permissive snail species, in contrast to other trematodes, such as Schistosoma species.
FIG. 1.
Life cycle of Paragonimus. The paired adult flukes (not shown) that are present in the definitive mammalian host (e.g., the dog and cat) sexually reproduce and produce eggs (12 o'clock). Proceeding clockwise, the operculate egg, which is released into the respiratory tract, is eventually passed into the environment. A ciliated miracidium is released from each egg in freshwater. The miracidium infects a permissive snail species. After asexual reproduction within the snail, infective cercariae are released. These infect a crustacean host, such as the crab or crayfish represented here. The crustacean may also become infected if it eats an infected snail. The ingestion of the infected crab or crayfish by a permissive definitive host culminates in egg production from sexually competent adults, thereby completing the cycle. (Reprinted with the permission of The Cleveland Clinic Center for Medical Art & Photography © 2008. All rights reserved.)
Interactions between this stage of Paragonimus and other trematodes species within the snail may be competitive or symbiotic, depending on the species involved. The permissiveness of many snail species has been studied. For example, P. kellicotti has been shown to be able to infect Oncomelania hupensis nosophora, a Japanese snail, although these organisms do not coexist in the same geographic location. It appears, however, that there is a greater ability for Paragonimus species to infect the snail species that are naturally encountered in the same microenvironment. This is the case for P. ohirai and its preferred primary intermediate host, Angustassiminea parasitologica. Finally, an antagonistic relationship may exist within the snail between the local Paragonimus species and other infecting trematodes. This is the case between P. ohirai and S. japonicum in the snail Oncomelania nosophora.
In North America, the first intermediate host for P. kellicotti is Pomatiopsis lapidaria, an amphibious snail that ranges from the southeastern and midwestern United States into Ontario, Canada (143). The age of the snail is inversely proportional to the ability of the miracidium of P. kellicotti to infect the snail host, with smaller, immature snails being the most permissive to infections (170). Synergistic and competitive relationships between P. kellicotti and other locally encountered snail parasites have not been investigated.
Upon infection, a sporocyst is the structure that is formed in the hemocoel (i.e., the body cavity that contains blood) of the snail. The sporocysts are simple sac-like structures that contain germinal cells (172). Asexual reproduction ensues, forming first-generation rediae (i.e., a larval stage that occurs in the intermediate host). These occur in the lymphatic system of the snail, just proximal to the digestive system in infections caused by P. kellicotti (172). The first-generation rediae in turn also contain germinal cells and produce the second-generation rediae (172). The second-generation rediae produce the short-tailed cercariae, which emerge from the snail. An intricate analysis of the development of the sporocyst and the rediae of P. kellicotti was made by Ameel et al. in 1951, for those interested in further reading on this subject (4).
The cercaria, which contains an anterior stylet and a small tail, infects a crustacean, which is the second intermediate host. The crustacean may be infected by eating the tissues of an infected snail or by direct penetration of its tissues by the cercaria (156). Crabs and crayfish are common intermediate hosts, with 53 species of 21 genera known to support the Paragonimus life cycle (112). Crayfish, principally Cambarus spp., are the second intermediate host for P. kellicotti in North America. In addition to crustaceans, one group has reported that frogs (Rana boulengeri) may also contain encysted metacercariae of P. skrjabini and has shown that these were infective when fed to experimental cats and rats (198). After penetration or ingestion, the cercariae localize in a particular site in the body of the crustacean. The metacercariae of P. kellicotti localize to the heart and pericardium, whereas P. caliensis localizes to the hepatopancreas (172).
The life cycle of Paragonimus is perpetuated in nature in omnivorous and carnivorous crustacean-eating mammals, which are the definitive hosts. The definitive host usually becomes infected by eating raw or undercooked tissues from crustaceans that harbor infective metacercariae, which are essentially anatomically preadults. Definitive hosts are those mammals that are permissive for the development of adult flukes that are capable of sexual reproduction. This is in contrast to a paratenic host, in which the parasite does not develop to sexual maturity but rather remains viable but immature (see below). Humans typically enter this cycle through the ingestion of raw or undercooked crustaceans. A history of ingesting raw or undercooked crayfish was obtained from all of the patients with North American paragonimiasis (30, 49, 100, 111, 141). Similarly, patients with imported paragonimiasis usually have a history of eating crab, which in some instances was not cooked (6, 115).
The metacercariae excyst after the definitive host has ingested infected crustacean tissues. External cues produced within the mammalian gastrointestinal tract signal excystation (17). All of the external stimuli have not been determined, but bile salts have been shown to stimulate the excystation of the metacercariae of P. ohirai (64). Excystment and migration of the parasite are accomplished in part through the production of cysteine proteases. The metacercariae of P. kellicotti have been found to excyst rapidly in the digestive tract of a definitive host (172). The infectivity depends on the definitive host species involved. For example, the infectivity of P. kellicotti in cats has been found to be 66% (171). The juvenile form of the parasite, following excystation, penetrates through the small intestine and enters the peritoneal cavity. Less commonly, some species may penetrate through the stomach. Gastrointestinal penetration is usually complete with hours, depending on the infecting species, the host, and a variety of other factors. It has been demonstrated in experimental animals that this penetration is accomplished within 2 hours by P. kellicotti (172). The next phase, migration, also varies depending on the infecting species and the host. Some species remain in the abdominal cavity for a period of time, whereas others migrate to the abdominal wall and remain there for approximately a week prior to returning to the abdominal cavity (19, 82, 172, 189). Sogandares-Bernal and Seed described Paragonimus kellicotti as usually being found in the liver and under the peritoneum in animal models, but this remains controversial (171, 172). The preadults of P. kellicotti remain in this location for 2 to 3 weeks in infected animals (172). This stage of infection has never been observed in humans infected by P. kellicotti. Thereafter, migration through the diaphragm ensues, sometimes with liver penetration, depending on the species. These organisms migrate into the pleural space. The preferred route for the migration of P. kellicotti in animal models is through the muscular portion or central aponeurosis of the diaphragm (172).
There is evidence that the immature adult that is present in the pleural space may remain there until a suitable mate arrives. Interesting experiments have been performed with P. kellicotti wherein a single infective metacercarium was fed to cats (i.e., one metacercarium per cat). This resulted in an immature adult in the pleural space and a failure of this organism to produce a pulmonary cyst and eggs. If, however, a second feeding of infected tissues was given to introduce another metacercarium, then the two organisms paired, migrated into the lung, formed a cyst, and produced eggs (172). However, the immature adult can wait for a sexual partner only for a certain period of time. Sogandares-Bernal found that if 12 weeks or more elapsed before the introduction of the second parasite, then the ability of the immature worms to find each other and produce a cyst diminished by 50% (171, 172). Alternatively, if only a single trematode is present, then it may begin to wander, producing visceral larva migrans.
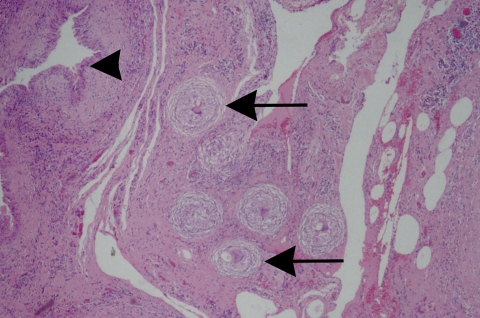
Although Paragonimus species are hermaphroditic, most do not self-fertilize. The preferred method of reproduction is pairing and cross-fertilization. Mates pair in the pleural cavity, penetrate into the lung, and form a cyst. Therefore, the cysts for most species of Paragonimus usually contain a pair of adult flukes, but sometimes more than two worms are present. Two worms are usually present in the cysts produced by P. kellicotti (2, 172). As with other species, however, more than two worms per cyst may be detected in severe infections where there is a large worm burden (2, 171). The cysts or cavities are thought by some to be produced by localized necrosis of bronchioles (98, 99). These measure up to 2 cm in diameter and are produced by the host response to the release of parasite products, including eggs (17, 39). The cysts in infections caused by P. kellicotti measure 1 to 1.5 cm in diameter in animal models and are spherical/round to ovoid (2, 54, 99, 172). The cyst lining consists of simple cuboidal nonciliated epithelial cells (43, 83, 92). The ultrastructural changes of the cyst have been reported elsewhere in great detail (172).
An exception to this mate-pairing rule is P. westermani, which may exist commonly as either a diploid or triploid variant (17). A rare tetraploid variant of P. westermani has even been described. The triploid variant can effectively self-fertilize, so a single worm may be present in cysts. The fact that a single P. westermani worm may produce a cyst and release fertilized eggs may in part explain the biologic success of this Paragonimus species.
The survival of Paragonimus within the cyst in the host is the result of a set of fascinating adaptations, which are just beginning to be understood. The parasite produces products that result in what has been termed “a zone of immune privilege” (17, 116). This occurs, in part, as the result of the action of 27- and 28-kDa enzymes released from the parasite that cleave human immunoglobulin G (IgG) (17, 42). The degradation of IgG in turn results in the diminution of IgG-induced eosinophil degranulation and production/release of superoxide (160). Eosinophils become apoptotic in response to worm excretory/secretory products, which in turn results in a substantial downregulation of the local inflammatory response (116).
The time to the start of egg production ranges from 28 to 111 days (reviewed in reference 19). The time to egg production varies depending on the Paragonimus species causing the infection and the species of the infected host. The development of eggs by P. kellicotti has been described in detail by Beaver et al. in 1964 (13). The cyst in which the adult parasites exist consists of a mixture of residual pulmonary parenchyma, cells of the inflammatory response, fibrous tissue, and entrapped eggs that have been released from the adult. Rupture of the cyst into a patent airspace (i.e., bronchus or bronchiole) releases eggs into the tracheobronchial tree and is the cause of hemoptysis. The eggs are then expectorated and either expelled or swallowed and passed in the feces. The release of the eggs into the environment completes the cycle. The life cycle of Paragonimus was first demonstrated in its entirety in 1920 by Ando, when infected snails were exposed to crabs that were subsequently fed to dogs (7, 112). The life cycle of P. kellicotti was described by Ameel in 1934 (2, 172).
There is, however, another method by which a definitive host may become infected. This is through the ingestion of raw or undercooked infected tissues from a paratenic host. Paratenic hosts are animals, usually mammals, which are not permissive for the development of sexually competent adults. Within paratenic hosts, the metacercariae undergo minimal maturation into juveniles and remain latent, often within the muscles of the host. When the raw or undercooked tissues of an infected paratenic host are eaten by a definitive (permissive) host, then the latent juvenile trematode reactivates and completes its usual migration and maturation into adulthood. Although most paratenic hosts are mammals, there is some evidence that birds, such as ducks and geese, may also serve as paratenic hosts. Paratenic hosts are thought to be the source of infection for large carnivores, such as tigers, since these animals do not eat crustaceans. Human infections may also result from the consumption of tissues from paratenic hosts. Wild boars are known paratenic hosts that have been reported to cause paragonimiasis in Japanese hunters in Kyushu (118, 122). Paratenic hosts for P. kellicotti likely exist in nature, but none has been described. However, it has been experimentally demonstrated that common rats may be paratenic hosts for P. kellicotti (172). Rats are thought to be important paratenic hosts in the Philippines where paragonimiasis is endemic (28). None of the reported human cases of North American paragonimiasis were suspected to be due to the ingestion of tissues from a paratenic host. However, a recent patient with North American paragonimiasis seen in Illinois and not yet reported in the medical literature denied travel, crayfish consumption, or eating imported foods. This patient did, however, report eating raw, locally caught fish, which raises the possibility of a heretofore unreported paratenic host for P. kellicotti, but this remains speculative (Joan Barenfanger, personal communication).
PARAGONIMIASIS
Clinical Features of Paragonimiasis
Paragonimiasis has been previously known as Oriental lung fluke, pulmonary distomatosis, and benign endemic hemoptysis, among other names. The clinical findings of paragonimiasis have been extensively reviewed in several excellent articles that are recommended to those interested in further reading on the subject (19, 172, 201, 202). In addition to these reviews, which cover the subject matter broadly, there are a number of reviews that focus on paragonimiasis in particular geographic regions (e.g., infections by P. heterotremus in Thailand). Representative reviews of paragonimiasis in specific geographic regions include reviews from the Philippines, (27, 50), Korea (39, 158), parts of China (36), Japan (126, 182), Vietnam (48, 51), Central and South America (148, 186-188), Liberia (149), and Thailand (185, 190).
Human disease usually occurs after the consumption of raw or insufficiently cooked crustaceans that harbor infective metacercariae. A cursory review of the literature may depict paragonimiasis as a severe disease. This is likely because patients with severe disease are more likely to be reported in the medical literature, particularly in case report formats. In most instances, however, Paragonimus causes very limited morbidity, and it rarely causes death. For this reason, it has been called “benign endemic hemoptysis” in the past. This relatively benign disease course reflects how highly adapted this parasite is to certain mammalian hosts (i.e., to permissive mammalian hosts). Many patients are asymptomatic or have subclinical disease and are unaware of the infection, whereas others have mild to moderate symptoms for many years before they come to medical attention, if they ever come to medical attention at all (20). Regardless of the mild nature of paragonimiasis in many patients, Paragonimus remains an important cause of morbidity throughout many parts of the world, with occasional deaths directly caused by this parasite.
Paragonimiasis is an infrequently encountered disease, particularly in areas with a low prevalence of human disease (e.g., North America), and is therefore not often considered when formulating an initial differential diagnosis. To complicate matters for the diagnosis of the individual patient, neither the clinical nor the radiologic features of paragonimiasis are unique or pathognomonic (65, 132). The diagnosis, therefore, relies upon the clinical recognition of the possibility of this infection, supportive radiologic findings, and diagnostic laboratory evidence of infection.
Paragonimiasis may be categorized as acute paragonimiasis (i.e., the manifestations that follow relatively soon after infection), chronic pleuropulmonary paragonimiasis (i.e., the manifestations of established pulmonary disease), and ectopic paragonimiasis (i.e., the manifestations that result from the presence of the parasite in a location other than the lungs). The clinical findings for the individual patient reflect the stage and type of disease. The acute phase is produced to some degree in all patients, since this phase is associated with the gastrointestinal penetration and initial migration of the parasite. However, many patients who come to medical attention at the chronic pleuropulmonary stage of disease may not recall or were unaware of the transient symptoms present during the early stage of infection (i.e., they were asymptomatic or had subclinical acute paragonimiasis). In one review that included serologic studies to determine if patients were infected, as many as 20% of patients with paragonimiasis were reportedly asymptomatic (182). The subsequent disease manifestations following the acute phase of disease (i.e., the subtypes of chronic pleuropulmonary and ectopic paragonimiasis) depend on host factors, as well as the species of Paragonimus causing the infection. The main features of acute, chronic pleuropulmonary, and ectopic paragonimiasis are discussed below.
Acute Paragonimiasis (Early-Stage Disease)
The acute or early stage of paragonimiasis consists of the disease manifestations that follow the ingestion of the infective metacerariae and their migration to the pleural space. The very early portion of this stage of infection occurs between 2 and 15 days following the ingestion event. Abdominal pain, fever, and diarrhea are possible early manifestations of infection and are more common in patients with heavy worm burdens (208). In contrast, patients are often asymptomatic early in the infection process or may have subclinical disease (80). Fever, chest pain, fatigue, and urticaria may follow. Fever has been noted to occur in only 10 to 20% of patients early in the disease process (80). Eosinophilia accompanies these clinical findings (see “General Laboratory Findings” below). The initial manifestations of gastrointestinal penetration are nonspecific, so a definitive diagnosis is not generally possible at this stage.
Some of these very early manifestations of acute disease have been documented for two of the patients with North American paragonimiasis. One patient had a 3-day course of watery diarrhea following the ingestion of the infected crayfish (49). Another patient developed a fever to 40°C and malaise within 2 weeks of the ingestion event; the interpretation of the early clinical findings for this patient, however, was complicated by the high likelihood of a concomitant acute Epstein-Barr virus infection (138).
The manifestations of the latter aspects of the acute phase of disease are caused by the presence of the immature, migrating forms in the pleural cavity. Pleuritic chest pain and pleural effusions may be seen at this stage. Uchiyama et al. have described pleural manifestations as predominating early in the disease process, whereas pulmonary parenchymal lesions predominate later in the course of disease, which is consistent with the life cycle of this parasite (182). Manifestations of pleura-based disease, which occur relatively early in the course of an infection, have also been documented for two of the patients with North American paragonimiasis (30, 49, 138). One of these patients developed dyspnea, left pleuritic chest pain, and a left-sided pneumothorax approximately a month after the initiation of infection. Concurrently, he had a white blood cell (WBC) count of 6,900 × 109 cells/mm3 with left-shifted hematopoiesis (59% band forms), but only 6% eosinophils. The fever and pneumothorax spontaneously resolved for this patient, whereas the pleuritic chest pain, dyspnea, fatigue, and malaise persisted. One month later, the patient developed diffuse lymphadenopathy and eosinophilia that peaked at 38%. This patient subsequently developed pleural thickening and pleural effusions, which most likely heralded the beginning of the chronic phase of disease. Another patient with North American paragonimiasis who had a pleural component early in the course of disease (i.e., approximately 2 months after infection) had a complete blood count of 8,000 × 109 cells/mm3 with 25% eosinophils (49).
The differential diagnosis of acute paragonimiasis is extensive and consists of diseases that produce overlapping signs and symptoms. The presence of fever, abdominal pain, and diarrhea in the very early phase of infection raises the possibility of the sundry causes of gastroenteritis. These include viral and bacterial causes of acute gastroenteritis, such as caliciviruses and the common bacterial pathogens, such as Salmonella, Shigella, Vibrio, and Campylobacter species, among others. Gastrointestinal parasites, such as Giardia, should also be considered. The latter aspects of the early stage of infection (i.e., the pleural manifestations with eosinophila) raise the possibility of paragonimiasis, but other migrating parasites (e.g., Ascaris) and other infectious diseases (e.g., a parapneumonic effusion) must also be considered.
Chronic Pleuropulmonary Paragonimiasis (Late-Stage Disease)
The chronic or the later stage of pleuropulmonary paragonimiasis occurs when the worms migrate to their final destination in the pulmonary parenchyma, adult worms are paired in a cyst, and fertilized eggs are produced. An exception to this occurs when the infection is caused by a triploid variant of P. westermani, wherein a single worm occupies a cyst and parthenogenically produces viable eggs (17). The clinical manifestations of disease are directly related to the pathological process associated with this stage of infection. These depend to a large degree on the location of the parasitic cyst, the number of cysts produced, and any associated sequelae. The earliest aspect of chronic paragonimiasis begins with the migration of the parasites from the pleura to the location where the cyst will be formed. In many instances, the cyst is just under the pleural surface. The migrating worms may cause bronchiectasis, interstitial pneumonitis, transient hemorrhage, or bronchopneumonia to varying degrees (80, 129).
The location of the parasite may be predominantly parenchymal or predominately pleural, or both locations may be affected (i.e., pleuropulmonary). The eggs of the parasite must reach the environment in order to complete the parasitic cycle, as described above. Therefore, the preferred location for the adult worms to encyst and form eggs is near an airspace, so that the fertilized eggs may exit in the respiratory secretions. When this occurs a parenchyma-based lesion is formed.
Cough and recurrent hemoptysis are often the predominant clinical findings for patients with chronic paragonimiasis. The most common clinical finding for 39 patients with proven paragonimiasis from Manipur, India, were recurrent hemoptysis (94.5%; 37/39), cough (61.5%; 24/39), and pleuritic chest pain (61.5%; 24/39); approximately one-quarter of patients had fever (23.1%; 9/39) and crepitation (28.2%; 11/39) (167). The cough was often exacerbated by physical strain in many of the patients (42.6%), which has also been reported by others (52, 167). Less common findings included weakness (10%; 4/39), rhonchi (5.1%; 2/39), hoarseness (10.3%; 4/39), and breathlessness (5.1%; 2/39) (167). Chronic bronchitis and wheezing have been reported by others (32, 80). In another study, Im et al. similarly found that the most common clinical findings were cough (63%; 45/71), blood-tinged sputum (61%; 43/71), dyspnea (38%; 27/71), and chest pain (38%; 27/71) (66). Several authors have reviewed the common clinical manifestations of patients with paragonimiasis; the most common clinical findings and the ranges of frequencies of these findings from these studies are the are summarized in Table 2 (32, 66, 77, 80, 158, 167). An unusual (and disturbing) finding that may occur on rare occasions is the expectoration of an intact adult worm (190). Some of the complications of parenchymal paragonimiasis include gross hemorrhage and bacterial superinfection, which may lead to pneumonia or a pulmonary abscess (95).
TABLE 2.
Clinical manifestations of paragonimiasis: a collation of findings from five studiesa
| Clinical feature | Frequency (%)
|
Comments | |
|---|---|---|---|
| Avg | Range | ||
| Cough | 83 | 62-100 | Cough may be exacerbated by physical strain |
| Hemoptysis | 70 | 61-95 | The rusty discoloration of the sputum is caused not only by hemosiderin but also because of the presence of the tan- to brown-pigmented Paragonimus eggs; the sputa of these patients have been classically described as resembling “iron filings” (107) |
| Chest pain or discomfort | 65 | 38-94 | A predominance of pleuritic pain suggests a prominent pleural component; if only pleural disease is present, eggs will not be present in the sputum or stool |
| Dyspnea | 42 | 5-53 | |
| Fever and/or chills | 37 | 11-67 | These may occur as occasional febrile episodes, with what appears to be spontaneous resolution (80) |
| Asymptomatic | 2 | 0-8 | Others have reported that as many as 20% of patients with paragonimiasis may be asymptomatic; the number of asymptomatic infected individuals may be underrepresented in this summary, since all the clinical findings summarized here were diagnosed with disease (i.e., these were clinical studies, rather than epidemiologic surveys that employed serology or skin testing) |
Based on data from reference 80. These studies do not include infections by P. kellicotti, as a series of patients with North American paragonimiasis has not been described. The frequency ranges and average frequencies of these clinical findings are derived from the review of 411 patients examined in five studies. The range denotes the highest and lowest percentages of patients with a given clinical finding from these five studies, whereas the average frequency is the total number of patients with the symptom divided by the total number of patients examined. A more detailed summary of the five studies has been produced by F. T. Kagawa (80). Other important symptoms not presented in this table that may be present in patients with paragonimiasis include weakness, hoarseness, and breathlessness. Physical signs such as crepitation (i.e., a crackling sound heard upon auscultation of the lungs) and rhonchi (i.e., a coarse rattling sound heard upon auscultation of the lungs) may be present. The diagnosis should be suspected if the patient is from an area of endemicity, particularly if he or she has a history of eating crab or crayfish.
Although the hemoptysis is a classic sign of paragonimiasis, it may be absent for a few reasons. Foremost, there may be a low worm burden and limited pulmonary destruction and bleeding. This is difficult to prove in an infected human but has been studied in animal models. Cats that were experimentally infected with Paragonimus and that had ≤15 worms did not have hemoptysis (172). The absence of hemoptysis may also occur if the parasite has aberrantly migrated to an ectopic location or if the patient has solely pleura-based disease, as described below.
When the parasitic cyst is produced near the pleura and the eggs exit into the pleural space, which is a dead end for the parasite (i.e., is nonproductive for the completion of the life cycle), then pleura-based disease is produced. Eggs released into the pleural space become entrapped in the parietal and visceral pleurae and elicit a substantial inflammatory response. The inflammatory mediators and the presence of foreign material (i.e., eggs) cause edema and effusions. This inflammatory process resolves into fibrosis that entraps and restricts the lungs. The signs and symptoms are generally those of any patient with lung entrapment (e.g., shortness of breath). Parenchyma- versus pleura-based disease, however, is not necessarily an either/or phenomenon. In many instances, there are features of both parenchyma- and pleura-based disease, so this stage of infection is generally referred as pleuropulmonary or chronic pleuropulmonary paragonimiasis. Communications between the pleural space and patent airways, which may be produced through cyst maturation or rupture, cause a pneumothorax. Hemorrhage into the airspace causes hemoptysis, whereas hemorrhage into the pleural space results in a hemothorax. Pleural effusions, fibrosis, and pneumo- or hemothoracies are common in patients with a pleural component of disease. The common signs and symptoms, such as shortness of breath because of lung compression, associated with a hemo- or pneumothorax of whatever etiology will also be present in patients with paragonimiasis who have these manifestations of disease.
Uchiyama et al. reviewed 104 patients with paragonimiasis and found that only 50% had eggs in their sputum or bronchoalveolar lavage specimens (182). The possibility of pleura-based paragonimiasis, wherein eggs are released into the pleural space and fail to reach the outside environment, is important to recognize in patients suspected of having paragonimiasis. In that study, all but two of the patients were confirmed to have paragonimiasis by serologic methods, which indicates the importance of serologic testing of patients who are clinically suspected to have paragonimiasis but who do not have eggs present in their stool or respiratory specimens (131) (see “Antibody detection” below).
Four of the five patients with North American paragonimiasis had a predominant pleural component of disease. All of these patients had pleural effusions, whereas two had pneumothoraces (30, 49, 100, 138). One of these patients, who had abundant eggs present in the pleural peel and recurrent pneumothoraces, did not have a history of hemoptysis. In this patient, the primary communication would seem to have been between the parasitic cyst and the pleural space (49).
Cough and hemoptysis, particularly when in conjunction with cavitary changes (i.e., cyst production), raise the possibility of tuberculosis. Furthermore, many of the geographic regions where Paragonimus is endemic also have high rates of tuberculosis. It is not surprising, then, that paragonimiasis is often misdiagnosed as tuberculosis. Some have actually referred to paragonimiasis as “nonresponsive tuberculosis” (17). Fifty-nine percent of the patients in the series reported by Singh et al. were misdiagnosed and treated for tuberculosis before the correct diagnosis was made (167). It has been estimated that between 50 and 70% of patients with paragonimiasis may initially be thought to have tuberculosis and erroneously receive antituberculous therapy (76, 80, 129, 167). This has also been described by other authors (164, 165). There are a number of important sequelae associated with the misdiagnosis of tuberculosis. Foremost, patients who are misdiagnosed and treated for tuberculosis are not appropriately treated for the disease that they have, which can therefore progress. In addition, they are unnecessarily exposed to therapeutics to which they may develop side effects and/or may experience drug interactions. The failure of clinical improvement, due to misdiagnosis and erroneous treatment, may discourage family members and friends of the patients from seeking medical attention for similar symptoms, since the medical therapy appears to be ineffective (101, 130). This is especially problematic if the friend or family member actually has tuberculosis, given the communicable and more progressive nature of tuberculosis. Finally, antituberculous drugs are much more expensive than the pharmacologic agents used to treat paragonimiasis. The inappropriate use of expensive therapeutics places an unnecessary economic burden on communities that often are already resource limited. The presence of bilateral pulmonary disease, which occurs in many patients with paragonimiasis, is a clue that the disease process is not likely tuberculosis, which far less commonly affects both lungs (158). In addition, patients with paragonimiasis are often otherwise in apparently good health, which is in contrast with patients with tuberculosis (97, 112). The routine use of a variety of simple laboratory techniques, such as acid-fast stains for respiratory specimens and Paragonimus serologic studies, is an effective means for achieving the correct diagnosis for these patients.
None of the patients with North American paragonimiasis were misdiagnosed as having tuberculosis. This may be because of the relatively low incidence of tuberculosis in the United States. However, the challenge of differentiating tuberculosis from paragonimiasis has occurred in the United States when imported paragonimiasis has been encountered in immigrants (76, 77, 200). There have been similar instances of misdiagnoses of paragonimiasis as tuberculosis in Mexico, where the disease is caused by P. mexicanus (Hugo Vicente Ralde, personal communication).
In addition to tuberculosis, other causes of lung disease, such as bacterial pneumonia, lung abscess, and echinococcosis, must also be considered in the differential diagnosis of pleuropulmonary paragonimiasis. The presence of eosinophila and an elevated IgE level raise the possibility of a number of other parasitic diseases that are more common than paragonimiasis in North America. These include strongyloidiasis, ascariasis, toxocariasis, and ancylostomiasis (20). Additionally, certain fungal infections of the lungs, particularly coccidioidomycosis, may be associated with an eosinophilic infiltrate (20). Elevated IgE levels and eosinophilia may also be seen in patients with bronchopulmonary aspergillosis (20). Finally, noninfectious causes, such as Churg-Strauss syndrome (i.e., an autoimmune vasculitis that predominantly involves blood vessels of the lungs), must also be considered in patients with pulmonary disease, eosinophilia, and elevated IgE levels (20).
Ectopic Paragonimiasis
Aberrant migration is known to occur with Paragonimus, as with many other helminthes. It has been suggested that aberrant migration is more likely to occur in heavy infections (9, 126). The migrating immature parasites may come to rest in a variety of organs. The brain, unfortunately, is the primary site of ectopic paragonimiasis. It is estimated that thousands of cases of cerebral paragonimiasis occurred in Korea through the 1960s, prior to the implementation of effective control measures; fortunately, now fewer than 30 cases are estimated to occur each year in Korea (17, 38). Less commonly, ectopic paragonimiasis has been reported to involve the breast (55), adrenal gland (58), heart and mediastinum (148), and genital organs (202). Ectopic paragonimiasis has been reported as an uncommon cause of infertility secondary to fallopian tube obstruction in women and of marked swelling of the scrotum in men (59, 151).
Cerebral paragonimiasis has primarily two manifestations. A minority of patients present with the signs and symptoms of a meningitis or meningoencephalitis due to the migration of the worm (93, 112). Alternatively, the majority of patients have an expansive, space-occupying lesion in the brain (93). Not surprisingly, patients with cerebral paragonimiasis have a much poorer prognosis than patients with pleuropulmonary disease. Patients with cerebral paragonimiasis present with a variety of signs and symptoms, which depend to a certain extent upon which areas of the brain are involved. Headache, vomiting, and seizures are common. Seventy percent of patients will exhibit personality changes and a decline of cognitive function (112). Fifteen percent of patients with cerebral paragonimiasis will go into a coma (112). Death occurs through herniation caused by the increase in intracranial pressure, as occurs with other space-occupying lesions. Although the brain is not the target organ for these parasites, eggs are commonly produced (Fig. 2). Cerebral paragonimiasis is more common in children than in adults for unknown reasons. The vast majority (90%) of patients with cerebral paragonimiasis are <30 years old, with 75% presenting before the age of 20 (93). The mean age of patients with cerebral paragonimiasis is 15 years (93). The age of the patient seems to contribute to the aberrancy of worm migration in some way; in addition to cerebral paragonimiasis, increased hepatic involvement has also been noticed in children (17). When considering cerebral paragonimiasis, one must consider all of the other causes of meningoencephalitis and space-occupying brain lesions in the differential diagnosis.
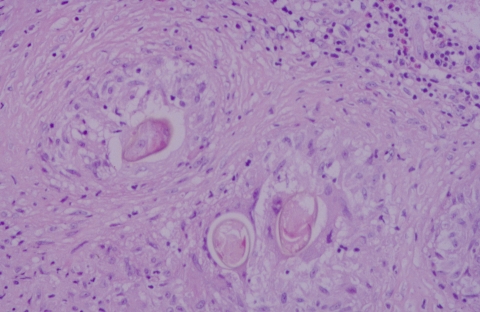
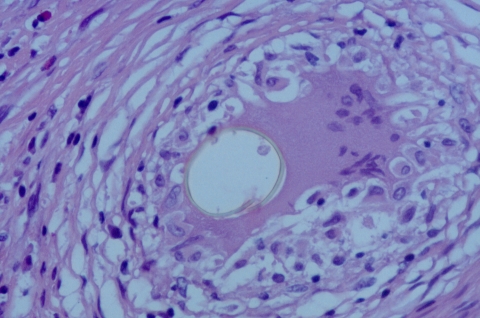
FIG. 2.
Several eggs the brain of a patient with cerebral paragonimiasis caused by P. mexicanus. Eggs in histologic sections are often distorted by the granulomatous response, as demonstrated here. Hematoxylin and eosin staining was used. Magnification, ×400.
In addition to the brain, the skin is a common site for ectopic lesions of paragonimiasis. Ashitani et al. reported that patients with paragonimiasis may have multifocal skin lesions, as well as lung lesions (9). All of the factors associated with aberrant parasite migration have not been determined, but it is likely that both host and parasite factors are involved. As described above, some mammals are not permissive for the development of the adult stage for certain Paragonimus species. The parasite remains immature in these hosts, but it may migrate. Similarly, some Paragonimus species have been found to be more likely to aberrantly migrate in humans than others. It is well known that P. skrjabini (previously known as P. szechuanensis) is more likely than other Paragonimus species to aberrantly migrate in humans, with reported loci of infection that include the brain, skin, peritoneal cavity, and eye (137). Paragonimus skrjabini has been described to produce subcutaneous nodules in 30 to 60% of infected people (75, 112, 199). The host-parasite interaction between humans and P. skrjabini is clearly different than that between humans and more commonly encountered Paragonimus species, and interestingly, this Paragonimus species is found in less populated areas. The crabs that harbor the metacercariae of P. skrjabini live at high elevations in mountains streams.
It is important to remember that patients with ectopic paragonimiasis may also have a pulmonary component. Patients who are suspected to have cerebral paragonimiasis or an ectopic infection in another site should have chest radiographic studies, respiratory and stool specimen examination for parasite eggs, and serologic studies for Paragonimus and any other suspected parasite.
RADIOLOGIC FEATURES OF PARAGONIMIASIS
The radiologic findings in patients who have contracted North American paragonimiasis are too few to consider in a series, but some radiographic images have been reported in the literature (Fig. 3 and 4). The radiologic features that have been described in patients with North American paragonimiasis are similar to those caused by infections due to other Paragonimus species. Therefore, the radiologic findings that may be seen in patients with paragonimiasis are reviewed.
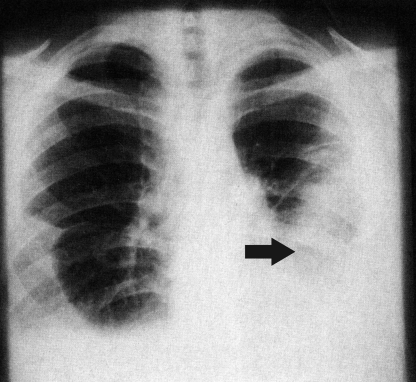
FIG. 3.
Chest roentgenogram of a patient with North American paragonimiasis. The roentgenogram demonstrates bilateral pleural effusions and an infiltrate in the lower lobe of the left lung (arrow). (Reprinted from reference 49 with permission of the publisher.)
FIG. 4.
CT scan of a patient with North American paragonimiasis. This patient with North American paragonimiasis has bilateral pleural effusions and thickening of the left pleura with possible cystic change (arrow). (Reprinted from reference 49 with permission of the publisher.)
As with the clinical findings, the radiologic features seen in patients with paragonimiasis reflect the stage of disease and the anatomic location of the parasite. Pulmonary lesions are pleural or parenchymal, or both may be present. Aberrant migration of the parasite to ectopic sites (i.e., the brain or the skin) may demonstrate radiologic changes in these locations, depending on the sensitivity of the imaging technique that is used. The radiologic findings that may be seen in patients with pleuropulmonary and cerebral paragonimiasis are discussed below.
Pleuropulmonary Paragonimiasis
When the worm penetrates into the pleural cavity and subsequently into the lung early in the disease process, it often produces a pleural effusion. This effusion is often small, and limited imaging has been done in humans at this stage of disease. Occasionally, however, a massive exudative effusion may occur at this stage (77, 80). The penetration of the lung parenchyma and the subsequent migration result in a worm tract. This appears as a linear streak or streaks and localized airspace consolidation in the chest radiograph. The burrow tracts are 0.5 to 1.0 cm in diameter (80). These are more clearly demonstrated using computed tomography (CT) or magnetic resonance imaging (MRI) scans. Although the burrow tracts are not the most common sign, if discovered they strongly support the diagnosis of paragonimiasis (80, 167). These are most readily identified when they run perpendicular to airways, with which they may be confused. These radiologic changes correlate with the focal hemorrhage and local tissue damage caused by parasite migration. When the worms cease to migrate, a nodule or cystic lesion is formed. Cysts formed in the pleura or in the distal aspects of the lungs near the pleura are responsible for pleural manifestations of chronic disease, such as pneumothorax and effusions. Pleura-based lesions, whether due to the presence of early migratory forms or to later chronic disease, have been described in up to 70% of patients with paragonimiasis (65, 80, 158).
Uchiyama et al. have described the radiologic findings for 104 patients with paragonimiasis who were examined from 1986 to 1998 (132, 182). Forty-five percent of these patients had a unilateral nodular or cavitary lesion, whereas 10% had a pleuroparenchymal complex. Thirty-four percent had unilateral pleurisy, whereas approximately 5% had bilateral disease. Two patients had both pulmonary and cutaneous lesions. Similarly, Im et al. retrospectively reviewed the features of 71 patients from South Korea who had paragonimiasis (66). Disease was confirmed in these patients by the detection of eggs and/or positive serologic studies. Most patients (83%; 59/71) had some type of pulmonary abnormality on chest radiograph. The parasite demonstrated no preference for either lung. The most common finding in this study was focal patchy or segmental lobar airspace consolidation, which occurred in 52% (37/71) of the patients. Peripheral linear shadows (2 to 4 mm by 2 to 7 mm) were seen in 41% (29/71) of the patients. Sixty-one percent (43/71) of the patients had some type of pleural abnormality, with 37% (26/71) demonstrating effusion, 17% (12/71) a hydropneumothroax, and 7% (5/71) pleural thickening. Seventeen percent (12/71) of the patients had bilateral pleural effusions. The most common radiologic findings for patients with pleuropulmonary paragonimiasis are summarized in Table 3.
TABLE 3.
The most common radiologic features of pleuropulmonary paragonimiasisa
| Radiographic feature | Frequency (%)
|
|
|---|---|---|
| Avg (no. with finding/no. examined for finding) | Range | |
| Consolidation | 58 (123/211) | 52-68 |
| Pleural effusions | 51 (108/211) | 10-66 |
| Cystic lesions | 32 (67/211) | 13-46 |
| Linear streaks | 25 (53/211) | 3-41 |
| Nodules | 20 (43/211) | 8-25 |
| Pleural thickening | 16 (33/211) | 7-28 |
| Ring shadow | 14 (19/135) | 3-23 |
| Calcified lesions | 6 (4/64) | 4-8 |
| Adenopathy | 3 (1/39) | Not available; only a single study reported on adenopathy |
| Normal | 8 (11/140) | 5-13 |
Based on data from reference 80. These studies do not include infections by P. kellicotti, as a series of patients with North American paragonimiasis has not been described. The presence of consolidation, pleural effusions, cystic lesions, linear streaking, nodules, and pleural thickening is summarized for 211 patients from four studies. Fewer studies recorded the presence of a ring shadow, a calcified lesion, adenopathy, or a normal chest radiograph; the number of patients for whom these findings were described is the denominator given for each finding. The range denotes the highest and lowest percentage of patients with a given radiologic finding from the studies where the finding was documented; the average frequency is the total number of patients with the radiologic finding divided by the total number of patients examined for the finding. A more detailed summary of the five studies has been produced by F. T. Kagawa (80).
Im et al. (66) found that 46% (33/71) of the patients had cystic lesions, which measured between 5 and 30 mm in diameter. One side of the cyst may appear thicker than the opposite side, which is thought to be due to the presence of the worm or paired worms pressed against the wall of the cyst. These lesions were associated with either focal airspace consolidation in (52%; 17/33) or thin-walled ring shadows (48%; 16/33). Of interest regarding disease progression, 36% (9/25) of the patients who had focal airspace consolidation but no cystic changes upon initial evaluation eventually developed cystic changes over the next 8 months. Four patients demonstrated the so-called “soap bubble” appearance (also see below), which is caused by the juxtaposition of several cysts. Although only 6% (4/71) of patients in this series demonstrated the soap bubble appearance on chest radiographs, it has been described in up to 30% of patients by others (158). The cystic nature of nodules present in the chest radiograph of patients with paragonimiasis may be demonstrated with CT or MRI scans (65, 66, 80). Finally, after the worm dies, the cyst shrinks due to continued fibrosis and scarring, and at this point it may calcify (80, 158, 167).
A variety of radiologic findings have been described for the patients with North American paragonimiasis. The historically first patient described in this review had a self-resolving pneumothorax early in disease and then subsequently developed bilateral pleural thickening, bilateral pleural effusions, right-sided lymphadenopathy, and a right-sided pulmonary infiltrate. The second patient described, who had parenchymal disease, had several chest roentenograms, all of which were interpreted as normal; nonspecific linear streaking, however, was noted on one. This finding is known to be present in some patients with paragonimiasis. The third patient described had bilateral pleural effusions and a left lung infiltrate. The next patient initially had a right-sided pneumothorax and subsequently developed bilateral pneumothoracies, a right-sided pleural effusion, and a cavitary lesion in the right lower lobe. The most recently reported patient with North American paragonimiasis had a pleural effusion, even though his disease was considered remote and inactive. Even though the number of patients with North American paragonimiasis is limited, the radiologic findings from these patients demonstrate most of the features described for pleuropulmonary paragonimiasis elsewhere.
Lymphadenopathy is apparently an uncommon finding in patients with paragonimiasis. Singh et al. (167), in a review of 39 patients with paragonimiasis from Manipur, India, found only 3% of the patients to have adenopathy documented by chest roentgenography. Similarly, the presence of significant lymphadenopathy in patients with North American paragonimiasis appears to be unusual. Diffuse lymphadenopathy was present in one patient, but this person also likely had a concomitant Epstein-Barr virus infection (138).
Cerebral Paragonimiasis
The brain, more particularly the cerebral cortex of the brain, is the second most common site of parasite localization after the lung (63, 79, 90). The radiologic findings of acute and chronic cerebral paragonimiaisis have been described, with the latter state being more thoroughly studied (Table 4). The changes in acute disease are less specific than those found in chronic disease but are important to recognize, since the disease is still progressing and further brain injury may be curtailed with appropriate therapy. Cerebral paragonimiasis caused by P. kellicotti has not been described in humans, but this likely would occur if the infection were more widespread. We have had the opportunity to examine tissues from a patient from Columbia with cerebral paragonimiasis caused by P. mexicanus (see “Pathologic Findings” below) (Fig. 2). Although cerebral paragonimiasis has been reported from Central and South America, it has been studied predominantly in the Eastern Hemisphere.
TABLE 4.
The radiologic features of cerebral paragonimiasis
| Stage of disease | Results with the following imaging technique:
|
|
|---|---|---|
| Skull film | CT/MRI | |
| Early | Not of value | Conglomerated, multiple ring-shaped enhancements with a variable degree of surrounding edema, which appear as a cluster that resembles grapes; a minority of patients may have a solitary ring-shaped lesion; the nodules of early cerebral paragonimiasis have iso- or hypointense centers with a hyperintense periphery (T1-weighted image) or with an iso- to hypointense periphery compared with the center (unenhanced T2-weighted image); localized areas of hemorrhage may also be seen with both CT and MRI |
| Late | Four types of intracranial calcifications: type I, punctuate, amorphous calcified deposits that occasionally contain calcified trabeculae; type II, a spotty arrangement of round nodular calcifications (5-7 mm in diam) with poor demarcation; type III, a solitary, round, well-defined cystic calcification (10-20 mm in diam); type IV, congregated, multiple, round-to-oval, cystic calcifications that have a hypodense center in comparison with the periphery (7-30 mm in diam) (these clustered, calcified cysts are thought to resemble “soap bubbles”) | CT scans demonstrate multiple round or nodular densely calcified areas that correlate with the “soap bubble” or type IV calcifications on skull plain films; T1-weighted images are nodules with peripheral low density and central hyperintensity compared with the intensity of the gray matter; T2-weighted images have peripheral regions of low intensity and areas of central high intensity; CT scan may also show large low-density areas, surrounding or connected with the calcified area, and may also demonstrate ventricular dilatation and widening of the cortical sulci; MRI clearly demonstrates the lesions described above but may also show areas of surrounding gliosis and changes in the cortical sulci more clearly than with the CT scans |
Early cerebral paragonimiasis.
The findings for early cerebral paragonimiasis are not as well described as those for chronic disease, but sporadic reports and small series have been issued (11, 31, 33, 96, 145). Patients with early cerebral paragonimiasis are more difficult to recognize, since calcifications that are readily detected by plain skull films are absent (96). Some form of advanced imaging such as CT or MRI is necessary to detect the early lesions of cerebral paragonimiasis, since plain skull films are not useful.
Cha et al. have retrospectively reviewed the CT findings from seven patients with early cerebral paragonimiasis (31). The diagnosis of paragonimiasis in these patients was established based on clinical findings, dietary history, and a positive Paragonimus-specific IgG enzyme-linked immunosorbent assay (ELISA) of the cerebrospinal fluid or serum. Surgical and histopathological verification of infection was also accomplished for all seven patients. Conglomerated, multiple ring-shaped enhancements with a variable degree of surrounding edema were present in 55% of the patients with early disease. These lesions consisted of an aggregate of three to five ring-shaped enhancements, which imparted an appearance of “grape clusters.” These are thought to be the precursor of the characteristic “soap bubble” lesions, which are the calcified lesions that appear later in the course of disease. However, 20% of the patients in this study had only a solitary ring-shaped lesion. The nodules of early cerebral paragonimiasis had iso- or hypointense centers with a hyperintense periphery when examined as contrast-enhanced T1-weighted images. Conversely, the unenhanced T2-weighted image demonstrated lesions in which the walls or the peripheral aspect of the ring lesions had an iso- to hypointense appearance compared with the center, which demonstrated an increased intensity. Localized areas of hemorrhage, possibly secondary to recent worm migration, could also be seen with both CT and MRI but were best visualized with MRI.
Chronic cerebral paragonimiasis.
Findings using plain skull films, CT scans, and MRI studies have been described for patients with chronic cerebral paragonimiasis (Fig. 5 and 6); the plain skull film is the type of study that is most likely to be available in resource-limited areas and adequately demonstrates the characteristic cerebral calcifications (56, 79, 135, 136, 183). These consist of multiple, round to oval areas of intracranial calcifications. Oh has described four types of intracranial calcifications in cerebral paragonimiasis (135). These are punctuate and amorphous calcified deposits that occasionally contain calcified trabeculae (type I); a spotty arrangement of round nodular calcifications (5 to 7 mm in diameter) with poor demarcation (type II); a solitary, round, well-defined cystic calcification (10 to 20 mm in diameter) (type III); and congregated, multiple, round to oval, cystic calcifications that have a hypodense center in comparison with the periphery (7 to 30 mm in diameter) (type IV) (136). The type IV lesion is the lesion that consists of the clustered, calcified cysts and is thought to resemble “soap bubbles,” as described above. The presence of a type IV lesion is thought to be pathognomonic of chronic cerebral paragonimiasis in the appropriate clinical setting (Fig. 5 and 6).
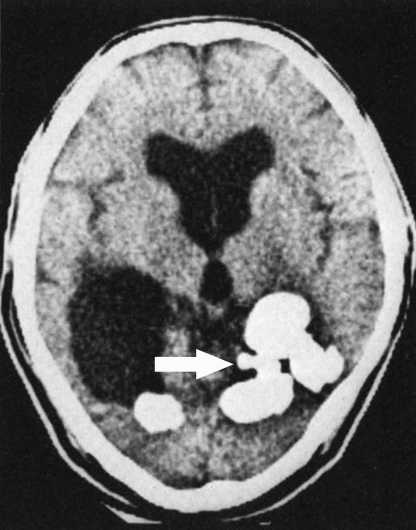
FIG. 5.
Plain skull film of a patient with chronic cerebral paragonimiasis. A type IV lesion, which is thought to resemble “soap bubbles,” is demonstrated (arrow). (Reprinted from reference 183 with kind permission of Springer Science and Business Media.)
FIG. 6.
Clustered parasitic cysts/elements (arrow) are well-delineated in this CT scan of a patient with cerebral paragonimiasis. (Reprinted from reference 183 with kind permission of Springer Science and Business Media.)
The CT findings for patients with chronic cerebral paragonimiasis may show multiple round or nodular densely calcified areas, which correlate with the type IV calcifications on plain skull films. In addition, large low-density areas, surrounding or connected with the calcified area, may be seen. Depending of the location of the lesion, there may be ventricular dilatation and widening of the cortical sulci (183). The multiple calcified nodules seen on CT scans appear as nodules with peripheral low density and central hyperintensity compared with the intensity of the gray matter in T1-weighted images. Peripheral low-intensity and central high-intensity lesions are seen in T2-weighted images (79). MRI adds evidence of surrounding gliosis and changes in the cortical sulci, which are more clearly delineated with this technology than with the CT scans.
LABORATORY DIAGNOSIS OF PARAGONIMIASIS
General Laboratory Findings
The general laboratory findings, although nonspecific, are often suggestive of an infection by a helminthic parasite. These finding would be supportive of the presumptive diagnosis of paragonimiasis made on the basis of clinical and radiologic findings. Eosinophilia is a common finding in patients with paragonimiasis, with approximately two-thirds having >500 × 109 eosinophils/mm3 (80, 132). Singh et al. reported that 62% (24/39) of the patients in their series had eosinophila, which ranged from an absolute eosinophil count of 650 × 109 to 4,000 × 109/mm3 (167). The average number of eosinophils in the blood is 0.20 × 109/mm3 (i.e., 2.7%), and the normal reference range is 0 to 0.45 × 109/mm3 (124). Rarely, hypereosinophilia may occur, with eosinophil counts exceeding 80,000 × 109 cells/mm3 (81). IgE levels are also frequently elevated in patients with paragonimiasis. Eosinophilia and/or an increased IgE were present in 80% of the 104 patients reviewed by Uchiyama et al. (132, 182). Approximately one-third will have leukocytosis (i.e., a WBC count of >10,000/mm3) (132, 158, 167). The converse is equally important to recognize, as approximately two-thirds of the patients with paragonimiasis will have leukocyte counts of less than 10,000 cells/mm3 (32, 108). Between 25 and 33% will have another nonspecific indicator of infection present, such as an elevated erythrocyte sedimentation rate (32, 132, 167). Eosinophilia is most pronounced in the latter aspect of the early phase of infection, when the migrating immature forms are present in the pleura (128).
Eosinophils and Charcot-Leyden crystals may be found in the respiratory secretions and stools of patients with paragonimiasis (40, 141). Although nonspecific, these should prompt an extensive search for parasite elements and may necessitate requesting additional specimens and ordering serologic studies. Eosinophils and Charcot-Leyden crystals, although highly suggestive of a parasitic infection, may also be seen as a response to the presence of fungi in certain instances. Eosinophils and Charcot-Leyden crystals are commonly present in the mucus of patients with chronic allergic fungal sinusitis. Similarly, eosinophils may be present in the cerebrospinal fluid of patients with meningitis due to Coccidioides immitis. Charcot-Leyden crystals and eosinophils, which are frequently degranulated, may also be found in the stools of patients with food allergies. Finally, neutrophils and macrophages may also be present in respiratory specimens from patients with paragonimiasis (40, 141). These cellular elements, in addition to eosinophils, have been seen in the respiratory specimens from a patient with North American paragonimiasis (141).
Examination of pleural fluid specimens from patients with paragonimiasis will often disclose an exudate (i.e., exudates have a fluid-to-total protein ratio of >0.5 and a fluid-to-serum lactate dehydrogenase [LDH] ratio of >0.6). Shim et al. described protein levels of >3 g/dl and LDH levels of >1,000 IU/ml in the pleural fluid samples from 95% and 84% of patients with paragonimiasis, respectively (158). IgE levels may also be elevated in this fluid (202). Pleural fluid is an excellent specimen to test for the presence of anti-Paragonimus specific antibody. Although numerous eggs may be seen entrapped in the fibrous tissue that is removed during decortication (Fig. 7), they are usually not seen in the pleural fluid. Leukocytes, eosinophils, and Charcot-Leyden crystals are commonly seen in the pleural fluid; these crystals are birefringent and may be easily visualized using plane-polarized light (76, 78). The examination of the pleural fluid from one patient with North American paragonimiasis demonstrated a WBC count of 2,900/μl, 93% of which were eosinophils. The pleural fluid in this patient had pH 7.2, a LDH level of 4,431 IU/liter, and a glucose concentration of 5 mg/dl.
FIG. 7.
The excised pleura from a patient with North American paragonimiasis demonstrates mesothelial hyperplasia (arrowhead), an acute and chronic inflammatory cell infiltrate with eosinophils, and eggs of P. kellicotti that are entrapped in nonnecrotizing granulomas that are beginning to be surrounded by concentric fibrosis (arrows). Hematoxylin and eosin staining was used. Magnification, ×40.
Pathological Findings
The pathological findings in the tissue are dependent on the stage of infection. However, in humans the infection usually does not come to clinical attention until it is well established (i.e., chronic pleuropulmonary or cerebral paragonimiasis). The finding of a migrating immature worm in tissue while it is migrating is highly unlikely and if it occurred would likely be serendipitous and due to the removal of tissue for another reason. Small foci of hemorrhage are present in the areas where the excysted metacercariae penetrate through the wall of the digestive tract. This has been demonstrated in experimental animals and would also be expected to occur in humans (172, 202). The continued migration of the immature parasite through the tissues is accomplished by a complex set of processes that are not completely understood but involve tissue degradation due to parasite-derived proteases. Focal hemorrhage and WBC infiltration may be seen in the areas of recent parasite migration (80, 107). A histologic examination of the tissues does not usually occur at this stage of infection in humans. It conceivably could occur, however, if the patient was suspected to have an Anisakis infection and underwent endoscopy as a means to retrieve the worm, and a biopsy was performed.
The diagnosis of paragonimiasis, particularly in North America where the incidence of disease is very low, may not be considered initially, or if it is considered it may be ranked low in the differential diagnosis of the probable causes of disease. Therefore, it may be the histopathologist who first examines tissue or the cytologist/cytopathologist who examines respiratory specimen preparations that contain the eggs or other parasite elements. This was the case for all of the patients with North American paragonimiasis (Table 5). In each instance, paragonimiasis was not initially suspected. In many instances, the history of eating uncooked or undercooked crustaceans was not given, nor was it elicited during the initial history and physical examination.
TABLE 5.
Salient features of five patients with North American paragonimiasis
| Feature | Data for:
|
||||
|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
| Yr reported (reference) | 1984 (138), 1986 (111) | 2000 (141) | 2002 (49) | 2003 (30) | 2007 (100) |
| Stage of disease | Acute/active | Acute/active | Acute/active | Acute/active | Remote/inactive |
| Age (yr)/sex | 19/male | 21/male | 18/male | 35/male | 71/male |
| Geographic location | Missouri | Oklahoma | Michigan | Oklahoma | Iowa/Nebraska |
| Travel to areas where other Paragonimus species are endemic | Not given, but unlikely | No | No | No | No |
| Crayfish ingestion; prepn | Yes, one; raw | Yes, one event but more than one crayfish consumed; undercooked | Yes, one; raw | Yes, routine consumption; prepn methods unknown | Yes, routine consumption; prepn method unknown |
| Early signs/symptoms | Fever, fatigue, and malaise, with progression to include chills, cough, diaphoresis, and temp to 104°F; further progression to continued fever, bilateral pleuritic pain, and a self-resolving unilateral mild pneumothorax | Not documented; asymptomatic to subclinical | Watery diarrhea (3 days) following ingestion of crayfish | None documented | None documented |
| Later signs/symptoms | Cough and abdominal pain (approx 4 mo postinfection); hemoptysis (approx 6 mo postinfection) | Morning hemoptysis for six mo prior to presentation; febrile (37.9°C) at initial presentation but subsequently afebrile | Headache, fatigue, dyspnea on exertion, and wt loss; tachypnea; dullness to percussion and absent breath sounds in lower 2/3 of the chest, bilaterally | Hemoptysis, cough, and recurrent pneumothoracies | Progressive shortness of breath; this and other symptoms, such as fever and hypotension, were secondary to sepsis and bacterial seeding of the pleural fluid |
| Radiologic findings | A self-resolving unilateral pneumothorax developed within the first mo; bilateral pleural thickening and effusions developed by the second mo; bilateral pleural effusions, with right hilar adenopathy, and a right upper lobe infiltrate were seen by the fourth mo | Several chest radiographs were described as normal, but one was noted to have “nonspecific linear streaking” | Bilateral pleural effusions, with a left lung infiltrate | First presentation, right-sided pneumothorax; second presentation, CT scan demonstrated a cavitary mass in right lower lobe, bilateral pneumothoracies, and a right-sided pleural effusion | Pleural effusion, right sided |
| Diagnostic procedure | Sputum cytology | Bronchoalveolar lavage, cytology | Decortication with histologic examination | Decortication with histologic examination | Effusion/empyema drainage |
| Laboratory findings | Neutrophilia with a left shift was present to various degrees throughout the course of infection; eosinophila was present at 6% (1 mo postinfection), 38% (2 mo postinfection), and 8% (4 mo postinfection); pleural fluid was exudative (LDH, 3,952 IU/ml; WBC count, 2,180/mm3); eggs in sputum (6 mo postinfection); stool exam was negative for eggs | WBC count, 6,800 cells/mm3; 9.8% “mid cells,” which are the cellular component that includes eosinophils | WBC count, 8,000 with 25% eosinophils; pleural fluid analysis showed pH 7.2, LDH level of 4,431 IU/liter, glucose level of 5 mg/dl, red blood cell count of 5,000/μl, WBC count of 2,900/mm3 with 93% eosinophils | Marked leukocytosis (specifics not provided) | The laboratory findings present, such as the leukocyte count in the pleural fluid, were thought to be associated with the viscus perforation, sepsis, and empyema |
| Serology | Complement fixation, 1:64 | Not performed | Enzyme immunoassay IgG, 1:128 | Not performed | Immunoblotting, performed at CDC and negative |
| Surgery required | No | No | Yes; video-assisted thoracoscopic decortication | Yes; thoracoscopic decortication | No, thorocentesis |
| Pathological and parasitologic findings | Cytology demonstrated several golden-brown eggs; cells present consisted of polymorphonuclear leukocytes and histiocytes admixed with cellular debris; no apparent eosinophila | Cytology demonstrated numerous golden-brown eggs; cells present included numerous macrophages, neutrophils, and eosinophils | Eosinophilic pleuritis with polarizable birefringent eggs within granulomas; macrophages and eosinophils infiltrated the lung parenchyma; eosinophilic pneumonia | Diffuse pleural thickening; reactive mesothelial cells, granulation tissue; mixed inflammatory cell infiltrate consisting of lymphocytes, plasma cells, and focally abundant eosinophils; nonnecrotizing granulomas, granulation tissue, and hyalinizing fibrosis with associated parasite eggs; eggs stained with Gomori's methenamine silver but not with periodic acid-Schiff stain | Degenerate adult forms with discernible remnants of the anterior sucker, portions of the genital organs, and possible ova |
| Egg morphology | Eggs were operculate, regularly ovoidal, and widest centrally between the operculum and the abopercular end; eggs measured 85 by 55 μm (avg); the shell was 1-2 μm thick except at the aboperculum, where it was 3-4 μm and had a terminal projection; identification confirmed at Armed Forces Institute of Pathology | Eggs were operculate and broadest centrally; the distinctive opercular ridges or “shoulders” were readily recognized; The eggs measured 83 by 57.7 μm (avg), with an avg shell thickness of 3 μm; The eggshell was birefringent; the shell was slightly thicker at the abopercular end; identification confirmed at Armed Forces Institute of Pathology | Eggs were ovoid, broadest centrally, birefringent, and operculate; many were distorted by the inflammatory response | Eggs were ovoid, broadest centrally, birefrringent, and operculate; avg size, 80 by 54.6 μm; avg shell thickness, 2.4 μm; many eggs were tangentially sectioned or distorted by the inflammatory response/fibrosis | Possible degenerate eggs present |
| Antiparasitic therapy | Praziquantel, 2-day therapy (25 mg/kg orally, three times a day) | Praziquantel, 2-day therapy (25 mg/kg orally, three times a day) | Initial course, praziquantel, 2-day therapy (25 mg/kg orally, three times a day); second course, same drug dose but given for 7 days | Praziquantel, 2-day therapy (25 mg/kg orally, three times a day) | None given, because of rapid demise secondary to bacterial infection |
| Outcome | Cure | Cure | Eventual cure; patient required a second surgery and was given a second course of antiparasitic therapy for a longer duration (well at 9 mo follow-up) | Cure (well at 24 mo) | Death, not directly due to parasitic infection; however, the parasite-induced pleural effusion served as a site of loculated fluid that became infected and thereby secondarily contributed to mortality |
The later stages of pleuropulmonary paragonimiasis consist predominantly of parenchymal nodules and cysts, pleural-based lesions, or both. Nodules most likely represent immature cysts. The walls of the cysts, which contain the adult parasites, become progressively fibrotic with time (40). After the worms die, the cyst contracts and becomes a scar that contains residual eggs entrapped in the fibrous tissue and may calcify (129, 158).
If nodules rather than cysts are seen by radiologic imaging, then surgical excision may proceed to exclude the possibility of malignancy. In such circumstances, the adult or portions of the adult worm may be seen in the excisional biopsy specimen of the nodule or cyst. It may difficult to taxonomically identify the adult fluke in histologic sections of excised tissues, since the worm is not sectioned in a perfect longitudinal or cross-section. However, if the adult is not too degenerate, it should be possible identify it as a trematode. Expert helminthologists may be able to determine the precise identification using features such as cuticular spine morphology, but this is beyond the expertise of most pathologists and clinical parasitologists. For practical purposes, the determination of the presence of a trematode in the lung is sufficient for the provisional diagnosis of paragonimiasis. There are, however, some clues that the organism present may be a Paragonimus species. The first clue would be the size or estimated size of the worm. It is far larger than the stages of other helminthes that occur in the lungs (i.e., the larvae of Strongyloides stercoralis and other geohelminthes that transmigrate the lung as part of their normal life cycle). Paragonimus lacks any structures that resemble the protoscoleces of Echinococcus species, which also produces parasitic lung disease. The presence of cuticular spines is another helpful clue, as is the presence of the host-derived fibrous capsule or cyst that contains the worm. When adults are seen, careful inspection may disclose the presence of two worms. This, however, is not always the case, as the single, larger triploid variants of P. westermani may inhabit a cyst, produce eggs parthenogenically, and cause disease (17).
The eggs are much more likely to be seen in the histologic examination of excised tissues and cytologic preparations of respiratory specimens than is the adult form of Paragonimus. Unfortunately, the eggs in histologic sections are often compressed and distorted (Fig. 2). Tissue processing and histologic sectioning may fracture the eggs (Fig. 8 and 9). If only a few eggs are present and they are distorted, then there is a limited likelihood of identifying the species of the infecting Paragonimus. There are a number of features of the eggs that support their being from a Paragonimus species. The size and shape of the eggs are of primary importance, but it is important to remember that shrinkage occurs with fixation and processing. The detection of an operculum is particularly useful in limiting the differential diagnosis. Only a limited number of helminthic parasites produce operculate eggs, and none of these produce pulmonary infections, except for Paragonimus. The operculum will not be discernible in every egg, particularly in histologic sections, wherein the eggs will be sectioned in a variety of planes and distorted by the inflammatory response. However, close inspection may demonstrate an egg with an opened operculum. Or, if sectioning is in the longitudinal axis of the eggs, then slits that are evidence of an operculum may be seen, as demonstrated well in one report (30). The eggs of Paragonimus species are birefringent and will polarize when exposed to plane-polarized light (Fig. 9). This is helpful in differentiating them from ectopically located eggs of Schistosoma species, which are not birefringent. In addition, the eggs of schistosomes are not operculate, and the eggs of S. mansoni and S. haematobium have a lateral spine and a terminal spine, respectively. The size of the eggs, their shape, and the presence of the opercular ridges are useful characteristics for differentiating the eggs of Paragonimus from the eggs of other helminthes that contain opercula.
FIG. 8.
A distorted, fractured egg of Paragonimus kellicotti, which has been phagocytosed by a giant cell, was one among many seen in the fibrotic, inflamed pleural tissue (also see Fig. 7). Hematoxylin and eosin staining was used. Magnification, ×400.
FIG. 9.
The eggs of Paragonimus are birefringent when exposed to plane-polarized light. This is the same microscopic field as shown in Fig. 8. Hematoxylin and eosin staining and plane-polarized light exposure were used. Magnification, ×400.
The truest morphology of the eggs is present in cytologic or parasitologic preparations of respiratory specimens or, if the eggs were swallowed, in the microscopic examination of the feces (Fig. 10 and 11). The morphological features of the eggs may be useful for suggesting or even identifying the Paragonimus species present, if a sufficient number of eggs are present for examination (141). The presence of shoulders on the eggs are evidence of an operculum (Fig. 10). When such eggs are present in the stool (see below), the shoulders or opercular ridges are useful for differentiating Paragonimus eggs from the large operculate eggs of other trematodes, such as Fasciola. The particular size, shape, and other morphological features of the eggs of P. kellicotti, and certain other Paragonimus species, which are useful for species identification are described below.
FIG. 10.
The operculate egg of P. kellicotti may be discovered only in the cytologic preparation, as was the case with this patient. Note the excellent preservation of the egg morphology. Egg size, 83 by 58 μm. Papanicolaou staining and oil immersion were used. Magnification, ×500.
FIG. 11.
An egg of Paragonimus westermani in a fecal specimen. This egg is broadest nearer to the opercular end of the egg (upper right aspect of image) and has an abopercular thickening of the eggshell, which are features not prominent in P. kellicotti. Iodine preparation was used. Magnification, ×400. (Courtesy of Lynne Garcia, reproduced with permission.)
The capsule of the cyst consists of a mixture of fibrosis in various stages of organization and granulomas. Nonnecrotizing granulomas are often formed in response to eggs (Fig. 7). Eggs may be seen entrapped in giant cells within the granulomas (Fig. 8). The concentric rings of fibrous tissue may be seen around the egg(s) and the associated granulomas (Fig. 7) (30). A variably dense infiltrate of eosinophils will also be present. Elsewhere in the lung, an eosinophilic pneumonia or eosinophilic abscesses (Fig. 12) may occur near the location of the parasite.
FIG. 12.
An eosinophilic abscess (arrows), granulomatous inflammation, and fibrosis replace the pulmonary parenchyma in this patient with North American paragonimiasis. Hematoxylin and eosin staining was used. Magnification, ×40.
When the pleurae are involved, there is marked, dense fibrosis and associated mesothelial hyperplasia (Fig. 7). A variety of inflammatory cells, including eosinophils, are admixed with the fibrous tissue. The eggs, as described above, become entrapped in nonnecrotizing granulomas and subsequently in fibrous tissue. It has been suggested that pleural biopsies are generally not helpful and show only chronic inflammation with eosinophils (76). This is likely secondary to sampling error, since the excision of the fibrotic pleura contains entrapped eggs, albeit scattered through the tissue (Fig. 7). Transbronchial biopsies are similarly limited by sampling error, but occasionally patients have been diagnosed by this method (144).
The five patients with North American paragonimiasis reported in the recent medical literature demonstrate the full range of the pathological findings described. Two of the patients had recurrent hemoptysis, and the diagnosis was made based on the finding of characteristic operculate eggs in respiratory specimens (141). Numerous eosinophils, neutrophils, and macrophages were also present and highly suggested a parasitic infection. These examples demonstrate the need for the cytotechnologist and cytopathologists to recognize and question unusual elements in the respiratory specimens so that further investigations may be undertaken. Cytologists should be able to recognize Paragonimus eggs, since, as in these cases, the possibility of a parasitic disease may not have occurred to the clinician and the respiratory specimen may not have been submitted specifically for a parasitologic examination.
Three of the patients with North American paragonimiasis had predominantly pleura-based lesions. One patient was not diagnosed until years after active infection, when the pleural effusion became infected due to an unrelated process and degenerate adult trematodes were found. Two of the other patients required decortication because of extensive pleural fibrosis and recurrent pneumothoracies. These patients were diagnosed based on the histopathological findings, which included eggs present in the pleural peel. Eggs were not sought in the respiratory tract secretions or stool specimens from these patients prior to surgery, because paragonimiasis was not suspected. The forms present in one of these specimens were initially thought to possibly represent endospores or immature spherules of Coccidioides immitis (30). Fortunately, the primary histopathologist recognized the need for consultation, since the treatment of these diseases requires different therapeutic agents. These examples demonstrate the importance of the histopathologist being aware of unusual elements in the excised specimen and consulting an individual with expertise in parasitology and/or infectious disease pathology.
The findings of ectopic paragonimiasis depend on the location of the parasite and whether the parasite matures and produces eggs. If the immature Paragonimus species does not mature and subsequently migrates, then cutaneous and/or visceral larva migrans will be produced. The immature worm is not usually present in biopsy specimens from patients with larva migrans, but rather residual necrosis and an inflammatory response are seen. These nonspecific findings are evidence of the worm migration track. However, when an ectopic infection occurs in the brain, eggs may be produced. Paired worms may produce viable eggs, although these never reach the environment. Conversely, Miyazaki et al. have shown that even individual diploid worms may produce eggs, but the eggs are nonviable (121). The presence of eggs in the brain results in nonnecrotizing granulomas and fibrosis (Fig. 2). The eggs are characterized as described above for pulmonary infections. Lesions in the brain readily calcify with age, and necrosis may fill the residual cyst (Fig. 13). Finally, “egg emboli” have been described in anatomic locations distant from the lung and are thought to be due to transposition of eggs by the lymphatics and/or the bloodstream (172, 202).
FIG. 13.
Calcification and fibrosis are common histologic finding in patients with chronic cerebral paragonimiasis. This infection was caused by P. mexicanus. Hematoxylin and eosin staining was used. Magnification, ×40.
Parasitologic Features
The diagnosis of paragonimiasis has classically been established through the demonstration of eggs in sputum and/or feces by microscopy (102, 202). Unfortunately, the microscopic examination of respiratory specimens and stool specimens is not very sensitive. In general, the examination of a single sputum specimen has a sensitivity of between 30 and 40% (32, 80, 89, 158, 167). In one study, Nakamura-Uchiyama et al. found that only 10% (3/30) of patients with paragonimiasis caused by P. westermani had eggs in their sputa (128). The low sensitivity of this assay has caused some investigators to analyze six or more sputum specimens when using this method to screen patients for paragonimiasis (32, 167). The examination of multiple sputum specimens increases the sensitivity to between 54 and 89% (32, 80, 89, 158, 167). Others have sought to examine a 24-sputum collection, with some success (36). One research group has suggested a periodicity of egg release, and they suggest that sputum should be collected from 5 a.m. to 9 a.m. for analysis for patients suspected to be infected by P. uterobilateralis (10). Recommendations for the optimal times for specimen collection from patients infected by other species have not been made. The eggs of a Paragonimus species are most likely to be found if the patient has diffuse pulmonary infiltrates and cavities on chest roentenogram and hemoptysis (77, 80, 158).
The sensitivity of a stool examination is inferior to that of a sputum examination, with the sensitivity of a single stool examination being in the range of 11 to 15% (89, 159). The examination of three stool specimens raises the sensitivity to 25% (167). In one study, it was found that although the stool examination was positive in only 25.6% of patients, 60% of these patients were 10 years old or less; this is consistent with the fact that children are more likely to swallow than to expectorate respiratory secretions (162). Cabrera et al. have suggested that a stool examination may be superior to a sputum examination for the diagnosis of paragonimiasis in the young and the very old (27). In occasional instances eggs have been found in the stool but not in the sputum. Therefore, if paragonimiasis is suspected on a clinical or radiologic basis, a stool examination for parasite eggs should be performed in conjunction with an examination of respiratory secretions, even though the diagnostic yield of a stool examination is low. The microscopic examinations of the stool and sputum, however, are not helpful in assisting with the diagnosis of purely extrapulmonary disease. However, patients with ectopic disease may also have a pulmonary component. Therefore, even if ectopic disease is suspected (e.g., cerebral paragonimiasis), an examination of respiratory or stool specimens is warranted.
The sensitivity of sputum and stool microscopic examination for the detection of the eggs of P. kellicotti is unknown, since so few human infections occur and in all instances of documented disease the diagnosis was not suspected and these types of specimens were not submitted for a parasitologic examination.
In some instances, the species identification for Paragonimus can be made with a high degree of certainty based predominantly on egg morphology. The important features of egg morphology include egg size, the location of greatest width with respect to the diameter of the egg, the presence of abopercular thickening, and the character of the eggshell (i.e., smooth versus pitted or dimpled). For example, the eggs of P. kellicotti can be definitively differentiated by size from those of P. mexicanus, which is the species with the most likely geographic overlap. The eggs of P. kellicotti average 91.22 ± 3.60 μm in length (range, 82.3 to 99.8 μm) with a mean width of 56.70 ± 1.78 μm (range, 54.3 to 61.3 μm), whereas the eggs of P. mexicanus average 74.11 ± 3.28 μm in length (range, 64.8 to 78.8 μm) with a mean width of 44.45 ± 1.97 μm (range, 38.5 to 45.5 μm) (119, 120, 172). The thickness of the shell of the egg of these species is also distinct. The eggshells of P. kellicotti average 2.27 ± 0.26 μm in thickness (range, 1.68 to 2.68 μm), whereas the eggshell thickness of P. mexicanus averages 1.17 ± 0.19 μm (range, 0.67 to 1.34 μm) (120, 172). These features are important for differentiating P. mexicanus from P. kellicotti, which have a similar egg shape (i.e., broadest centrally) that is distinctly different from that of P. westermani, which is broadest near the operculum and has more distinct abopercular thickening.
The eggs of P. westermani may be encountered in the respiratory specimens or biopsied tissues from immigrants. These measure 80 by 120 μm but rarely exceed 80 μm in histologic sections (112). The eggs are brown, ovoid, and operculate. The shape of the egg tapers from the broadest aspect, which is between the central aspect (i.e., the equator) of the egg and the opercular end, to the narrowest aspect near the abopercular end (Fig. 11). The shell measures approximately 2 μm, except where it is thickened at the abopercular end and measures approximately 4 μm in thickness (112).
Although the purist may desire to achieve a definitive identification through morphological features alone, a pragmatic approach would include obtaining epidemiologic information, particularly about where and how the disease may have been contracted. The absence of travel to areas where other Paragonimus species are endemic and the history of crayfish ingestion were helpful in establishing the identity of the causative agent of infection for each of the patients with North American paragonimiasis. The routine dietary history may not initially disclose the crayfish consumption. However, an in-depth dietary history, which in some instances was obtained after the diagnosis was made on morphological criteria in these cases, has revealed the consumption of locally caught crayfish. In four of the five cases, eggs with diagnostic features were present in respiratory secretions or tissue removed during surgery, whereas in the fifth case, the degenerate adults and eggs were seen in the pleural fluid. However, even if the morphological features of the eggs were not characteristic of P. kellicotti (e.g., if all of the eggs in a surgical specimen were distorted), the identity of the infecting Paragonimus species could have been deduced from the travel and dietary history.
The possibility of a Paragonimus species will be considered quite readily when helminth eggs are seen in respiratory specimens. However, when these are detected in the stool because the respiratory secretions containing the eggs have been swallowed, then the diagnostic possibilities are considerably greater. A number of helminthes produce operculate eggs that will appear in the stool specimens from infected individuals, and these need to be considered in the differential identification of a Paragonimus species. Other trematodes, such as Clonorchis sinensis, Opisthorchis species, Fasciola hepatica, and Fasciolopsis buski, as well as the cestode Diphyllobothrium latum, produce such operculate eggs. Size and microscopic morphological features are the means by which these eggs are successfully differentiated from the eggs of Paragonimus species, and these have been described in detail elsewhere (80, 144).
Immunodiagnostics
Antibody detection.
Serologic tests have emerged as important tools that aid clinicians in establishing the diagnosis of paragonimiasis. Many of these have been reviewed independently by Maleewong (102) and Blair et al. (19). These are particularly helpful when the clinical suspicion is high but the eggs cannot be demonstrated. As noted above, it is not uncommon that repeated sputum and stool examinations may be necessary to detect the eggs of Paragonimus. Uchiyama et al. studied 104 patients with paragonimiasis and found that although only 50% of patients had eggs in sputum or bronchoalveolar lavage specimens, almost all (102/104) had serologic evidence of disease (i.e., positive for IgG in serum and/or pleural effusion fluid) (182). Eggs may not be present in sputum or stool specimens from patients with predominantly pleura-based disease, unless there is also a parenchymal component of disease (165, 202, 208). Similarly, immunodiagnostics are particularly useful to assist in the diagnosis of patients with cerebral paragonimiasis, particularly early in the course of disease, as antibodies wane with chronicity (126).
The history of the development of immunodiagnostics for paragonimiasis is extensive and is only briefly reviewed here. A variety of serologic assays have been developed, which vary only slightly from one another with respect to sensitivity and specificity. Most of these are highly sensitive, often exceeding 95% sensitivity. Immunodiffusion assays, immunoelectrophoresis assays, and monoclonal antibody-based ELISAs that have been reported to have 100% sensitivity have been developed (132).
The improvement of the specificity of the immunodiagnostic assays has been the most challenging aspect of test development. Paragonimus immunoassays with specificities that are less than 100% usually have cross-reactivity with sera from patients with infections caused by certain other helminthes, such as Clonorchis. The degree of cross-reactivity depends on the particular antigen used in the assay for antibody capture. Several assays with 99 to 100% specificity have been developed after extensive refinements (see below); these include a monoclonal antibody-based IgG ELISA and an immunoblot assay, although surprisingly, one version of the immunoblot assay used only a crude antigen preparation (132). The development and incorporation of monoclonal antibodies into some of these assays have contributed to an increase in assay specificity (205).
The identification of an immunogenic protein from the crude protein extract of Paragonimus that elicits a highly specific antibody response is a complex and tedious task. Once successfully accomplished, however, this antigen holds great promise for use in a highly specific serologic assay. For example, Indrawati et al. evaluated the soluble crude antigen of P. heterotremus, an important cause of paragonimiasis in Thailand (67). The soluble crude antigen was separated into three fractions, F1, F2, and F3. These fractions were tested against sera from patients with the eggs of P. heterotremus in their respiratory secretions or feces, as well as three negative control groups. The negative control groups consisted of patients with other parasitic diseases, patients with culture-proven tuberculosis, and healthy disease-free individuals. The sera of Paragonimus-infected patients reacted best with the F1 fraction, which was found to contain what is believed to be an antigen that is highly specific for P. heterotremus. Subsequently, a 31.5-kDa protein was isolated from this preparation using isoelectric focusing and used to design ELISA and immunoblot assays. The sensitivity and specificity of this ELISA were shown to be 100% and 99%, respectively (67).
Maleewong has also described the challenges faced in the development of serologic assays (102). Crude parasite extracts as a source of antigen are difficult to obtain, since adult parasites must be harvested from experimentally infected animals. The reported sensitivity, specificity, and positive and negative predictive values of affinity-purified crude somatic antigen were 73.7%, 99.2%, 95.6%, and 94%, respectively. Another, although considerably more complex, method of obtaining a more-specific antigen has also been described (102, 103). These researchers constructed a monoclonal antibody library and then used Paragonimus-specific antibody-producing clones derived from this library to capture antigens from the crude extract preparation. These specific antigens were then incorporated into an ELISA that had a sensitivity, specificity, positive predictive value, and negative predictive value of 100% each.
The design of serologic tests with high sensitivity and specificity is possible but requires considerable effort and validation. Validation studies of serologic assays should include evaluation of sera from patients with paragonimiasis, from patients with other documented parasitic infections (particularly diseases caused by other trematodes), and from healthy individuals. Of the patients with paragonimiasis, it would be optimal to include the sera of patients infected with different Paragonimus species, but this is often difficult. In addition, negative controls for validation should also include patients with tuberculosis. As described above, patients with paragonimiasis are often misdiagnosed as having tuberculosis, so any potential nonspecific cross-reactivity between these groups that may occur would be important to detect.
It is important to know the performance parameters of the type of serologic test used, since some antibodies disappear within about a year after the successful treatment of disease, whereas others may persist for longer periods of time (105). Maleewong et al. have demonstrated that there is a significant decrease in the IgG antibody level following successful treatment with praziquantel with some assays, which makes it useful both for diagnosis and to monitor the response to therapy (102-106). Similarly, the complement fixation test becomes negative 6 to 12 months after appropriate therapy. ELISAs for the detection of IgG antibodies or total antibodies against Paragonimus, although often favored by laboratorians because they are easier to perform, may take longer to become positive following infection (4 to 24 months) and longer to normalize after a cure. Nakamura-Uchiyama et al. have found the detection and differentiation of IgM and IgG subclasses for antibodies specifically directed against Paragonimus to be useful for the differentiation of acute versus chronic disease (127, 128).
The most accessible immunodiagnostic assay in the United States is an excellent immunoblot assay that has been offered by the CDC since 1988 (168). This assay targets antibodies directed against an 8-kDa antigen of P. westermani, and it has a sensitivity of 96%. The specificity was found to be ≥99% when studied using a battery of sera from patients with paragonimiasis, as well as other parasitic diseases. The antibodies detected in the immunoblot assay, like those in the ELISAs, decline more slowly than those detected by the complement fixation test. Unfortunately, this test has been tested on only one patient with an infection caused by P. kellicotti and was negative (Patricia P. Wilkins, Parasitic Serology Section of the Centers for Disease Control and Prevention, personal communication). The immunoblot assay performed at the CDC is highly useful for patients with imported paragonimiasis, which in many instances may be due to P. westermani.
Although extensive work has been performed on the serodiagnosis of paragonimiasis, work specifically centered on P. kellicotti is extremely limited, particularly in humans (153, 154). Three of the five patients with North American paragonimiasis had serologic studies performed, but these were performed using three different serologic methods. In addition, there is uncertainty with regard to the location where these tests were performed. Two of the patients had active disease, whereas one had very remote disease. The two patients with active disease who were tested were reported to have positive serologic studies. The patient reported in the mid-1980s was tested using a complement fixation test, whereas the patient reported in 2002 was tested using an ELISA (49, 138). These serologic studies were both described as having been performed at the CDC, but this is unlikely, particularly for the latter case (i.e., the one tested by ELISA). The ELISA format has never been used at the CDC for Paragonimus serology (Patricia Wilkins, Parasitic Serology, CDC, personal communication). The Parasitic Serology Section at the CDC has tested the serum from only one patient with P. kellicottii, i.e., the patient with remote disease, and it was negative (100). This is not a particularly useful finding for determining the utility of the immunoblot assay for the detection of an active infection with P. kellicotti, since the antibody level in this patient may have naturally declined following the cessation of active infection. Practitioners with patients suspected of have paragonimiasis, either North American or otherwise, are encouraged to submit serum to the CDC for testing so that the scope of this assay, which is known to perform well for P. westermani, may be further defined. Information concerning these and other tests performed at the CDC is available at www.cdc.gov/ncidod/dpd/ or by calling (800) 232-4636 (CDC-INFO).
Intradermal testing is another type of immunodiagnostic method that may be used to identify patients who have or have had paragonimiasis. Although this assay is highly sensitive (80 to 90%), its utility as a diagnostic tool is limited because patients remain positive for years to decades following a cure (19). For this reason, it has been used most successfully as an epidemiologic survey tool. It has, however, been used in conjunction with a serologic assay to diagnose active infection (165, 208). In this type of application, the intradermal skin test is applied to the population under consideration and the more specific serologic assay is applied to those individuals with a positive skin test. This is necessary because of the high false-positive rate of the intradermal skin test with respect to diagnosing active disease. Cheng et al. screened 9,197 people in Fujian Province in China using this approach; 650 (7%) had positive reactions using intradermal skin testing, whereas only 60 (0.7%) had a positive reaction with a more definitive serologic assay (17, 37).
Antigen detection.
The antigen detection assay is essentially the reverse of a serologic assay. Rather than using a specific antigen to assay for the presence of a specific antibody, an antibody is used for the detection of a particular antigen. Monoclonal antibodies directed against Paragonimus-specific epitopes are used alone or in conjunction with polyclonal antibodies to capture the Paragonimus-specific antigens. This application has been used to detect the presence of antigens from a Paragonimus species that are shed into the blood, respiratory secretions, or stool. Maleewong described an assay that when tested using the stools of experimentally infected cats demonstrated a sensitivity of 73.7% and a specificity of 100% (102). Similarly, Zhang et al. developed monoclonal antibodies to metacercarial and adult worm stage-specific antigens and then developed an antigen detection test for use with serum (207). They described positive reactions in all patients with parasitologically confirmed infections and positive reactions in many patients suspected to be infected. No false-positive results were reported with the sera of patients with infections caused by other helminthes, those with tuberculosis, or healthy donors. In the future, antigen detection assays may prove to be useful tests for the diagnosis of active paragonimiasis.
Molecular Testing
Molecular analyses.
Very sophisticated molecular analyses have been and are being undertaken to more fully understand the taxonomic relationships of Paragonimus species (184). It is these types of analyses that seek to understand the relationships between P. skrjabini and P. miyazakii, for example, which will resolve many of the taxonomic uncertainties that have occurred based on morphology alone (18). Unfortunately, it will likely be many years until enough studies are performed for us to fully understand the diversity and relationships of all Paragonimus species that may cause human disease. Numerous samples will be needed to clearly define intraspecies variation and which target genes should be used to define a species.
Such studies, however, are broadening the knowledge regarding the geographic distribution of these organisms. One advantage of the use of molecular methods to study the distribution of these organisms is that the investigator is not reliant on infected humans or mammals for specimens. The geographic distribution may be studied using intermediate forms of the parasite obtained from first and second intermediate hosts in the environment. Tandon et al., for example, used this approach to molecularly characterize metacercariae from northeastern India. In this study, they determined that the species present in this area was P. westermani. Once the species designation was fully established using molecular methods (i.e., once any doubt regarding species designation was removed), then the researchers were able to more fully characterize the microscopic morphology of this stage of this species (178). This is an example of how molecular methods may actually strengthen, rather than replace, traditional morphology-based parasitologic methods. A similar approach was used to study the relative prevalences of five Paragonimus species that occur in Thailand (177). Several other groups have used these and similar methods to characterize the Paragonimus species in their geographic regions (142, 163). Only limited molecular studies, however, have been undertaken in the Western Hemisphere. These have examined the DNA from P. mexicanus isolates from Guatemala and Ecuador, but analyses of P. kellicotti have yet to be performed (73).
Molecular methods of classification will continue to generate a more thorough understanding the genus Paragonimus. Furthermore, such studies will allow for the characterization of the distribution of these parasites. The refinement of such procedures often results in the development of diagnostic assays that may be used in human and animal medicine alike. Anecdotal reports regarding the use of a variety of molecular techniques will continue to be made, but the thorough evaluation of these assays will require well-controlled clinical trials, which may be difficult to perform given the declining incidence of this disease in many locales.
Molecular diagnostics.
Species-specific PCR assays have been constructed for the detection of some Paragonimus species (175). Likely more useful are broad-range PCR assays that target conserved regions within the Paragonimus genome. A variety of postamplification methods have been used to characterize the products of broad-range Paragonimus genus-generic assays. Traditional DNA sequencing has been used to determine the identity of the Paragonimus species under investigation through comparison with a genetic library of homologous DNA sequences from well-characterized species (46, 73, 74, 147). Commonly used genetic targets for such analyses include the internal transcribed spacer (ITS) regions of ribosomal genetic complexes, particularly ITS2, and the mitochondrial cytochrome c oxidase gene (46, 73, 147).
One of the earliest uses of molecular methods for the detection of Paragonimus used a DNA probe, which was reported to be both highly sensitive and specific (102). This probe reportedly could detect as few as five eggs or two metacercariae. Although this has been described and its feasibility demonstrated, it has not been developed into a commonly used or commercially available assay. Whether a DNA probe or nucleic acid amplification technology will prove useful for the routine detection of Paragonimus DNA in infected patients remains to be seen.
The feasibility of using PCR-based assays as diagnostic tools is under consideration, and preliminary work is being performed in animal models. For example, the detection of P. heterotremus using PCR has been explored in a cat model, with excellent results (69). Intapan et al. reported that this method could detect as few as five eggs per 0.6 g of feces and, apart from cross-reactivity with certain other Paragonimus species, was highly specific. The application of the broad-range PCR primer sets described above, which encompass the genus Paragonimus, has been used to assist in parsing difficult parasitologic differential diagnoses. For example, Schuster et al. were confronted with the difficulty of differentiating Achillurbainia congolensis from extrapulmonary infection caused by P. uterobilateralis. They used the combination of morphology and the fact the nucleic acid extracts from the eggs failed to amplify with Paragonimus-specific primers to secure the appropriate identification (152). Chang et al. have taken this approach a step further and used broad-range PCR and DNA sequencing to identify the infecting parasite in a human with paragonimiasis (34). Paragonimus species have also been differentiated using PCR followed by endonuclease digestion and fragment analysis, where the restriction patterns generated correlate with the species designations (175, 176). Other methods, such as randomly amplified polymorphic DNA markers, have also been used to differentiate Paragonimus species (68). Molecular diagnostic assays of various types hold great promise as potential human diagnostic assays, but how these will actually be employed and how they will compare with the traditional diagnostic methods remain to be seen.
TREATMENT
The therapeutic agent of choice for pulmonary paragonimiasis is praziquantel (75 mg/kg/day given in three doses for 2 days for both children and adults) (114, 181). Studies have demonstrated a 71 to 75% cure rate after 1 day, an 86 to 100% cure rate after 2 days, and a complete (100%) cure rate after 3 days with this dosage (132). The side effects of praziquantel are minimal but include dizziness, headache, and gastrointestinal disturbance. Praziquantel has been used successfully in the treatment of four of the five patients with North American paragonimiasis (30, 49, 141). Two patients, however, also required surgical intervention with decortication, and one of these patients, who particularly had severe disease, was given a second course of praziquantel. The fifth patient with North American paragonimiasis, who had remote disease, died from an overwhelming bacterial infection from an unrelated cause prior to being considered for antiparasitic therapy. In retrospect, however, praziquantel was likely not indicated for this patient, since the infection was believed to be old and inactive.
Bithionol was the drug most commonly used prior to praziquantel but was quickly replaced. Early studies investigating bithionol as an antiparasitic agent were performed in a rat model using P. kellicotti (172, 191). Bithionol is highly effective, with a 91 to 100% cure rate. In contrast to praziquantel, however, it requires extended treatment, and many patients develop gastrointestinal side effects (70%) or a rash. The adult and pediatric doses for bithionol are the same at 30 to 50 mg/kg on alternate days for 10 to 15 doses (114). Similarly, niclofan was an antiparasitic agent used in the past that demonstrated a high cure rate (95%) following a single dose. Unfortunately, neurotoxicity and hepatotoxicity limited the use of this drug as a first-line agent (132).
More recently, triclabendazole has been used successfully to treat paragonimiasis in animals. Preliminary investigations in humans have begun, with promising results (84, 132, 146). High cure rates have been reported for patients with paragonimiasis in Ecuador who were given only a single dose of triclabendazole at 10 mg/kg. One of the potential advantages of this therapeutic agent is that this agent has activity for patients with fascioliasis, which is in contrast to the case for praziquantel (17, 84). In addition, Calvopina et al. suggest that triclabendazole is better tolerated than praziquantel (29). The World Health Organization's recommended dosing and schedule of administration for this drug is 10 mg/kg twice a day (17, 197). Although additional studies are needed, triclabendazole may prove to be an effective alternative to prazequantel for patients with paragonimiasis.
PREVENTION AND CONTROL
The control of paragonimiasis in animals is impractical, given the widespread distribution of this genus of parasite in a variety of carnivorous and omnivorous animal hosts. The control of the snail and crustacean intermediate hosts is also impractical and may have untoward ecological consequences. However, control of human paragonimiasis is possible to a large extent through education and changes in customs and food preparation practices. The success to date in substantially reducing infections in countries that traditionally have had high incidences of infection has been directly related to educational efforts. Interestingly, some areas that are experiencing an emergence of disease have been linked with newly defined behaviors, such as the consumption of raw meat from wild boar (17).
The thorough cooking of the crustacean intermediate host (i.e., crab or crayfish) effectively kills this parasite. The emergence of paragonimiasis in Japan through the consumption of raw boar indicates the importance of also thoroughly cooking any potential paratenic host (17). Infections with P. kellicotti may be avoided by not consuming crayfish or by thoroughly cooking the crayfish prior to consumption. Each of the five patients with North American paragonimiasis had a history of eating crayfish. Two reportedly ate them raw, whereas one reported eating crayfish that were undercooked. The culinary preparation methods used by the two other patients were not reported. Similarly, imported crabs should also be thoroughly cooked prior to consumption. Crab meat should never be eaten raw. Crabs pickled in alcohol (i.e., drunken crabs) also pose a danger, since this method of preservation is insufficient to kill the parasite. Poor food preparatory practices may also lead to paragonimiasis, as well as other food-borne illnesses. Whenever fresh crustaceans are cut or processed with a food processor, the utensils and cutlery boards that have been used should be thoroughly cleaned prior to using these to prepare any other foods, particularly those foods that will be consumed without cooking (e.g., salads) (80, 166).
The future prevention and control of paragonimiasis will employ previously used highly effective educational methods. In addition, information should be provided to target populations who are contracting paragonimiasis in unconventional ways (e.g., Japanese hunters contracting paragonimiasis through the consumption of raw or undercooked meat from wild boars). Although vaccination is conceivably possible, it will not likely occur in the near future, given the more pressing need for vaccinations for more prevalent, medically severe, and economically impactful infections (17).
CONCLUSION
Paragonimiasis is a zoonotic infection caused by a highly evolved parasite with a complex life cycle that includes at least three hosts. Two intermediate hosts, a snail and a crustacean, and a mammalian definitive host are necessary to complete the life cycle of this parasite. Human and animal infections result from the consumption of raw or undercooked crustaceans (i.e., the second intermediate host) or through consumption of raw or undercooked meat from a paratenic host. Paragonimus is the only human parasite where the adults reside in the human lung. The genus Paragonimus is highly successful and is endemic in Asia, Africa, and the Americas. Paragonimus kellicotti is the species endemic to North America. Physicians in countries without endemic disease may encounter patients with paragonimiasis who have immigrated with the disease or who contracted the disease while traveling or through ingestion of imported contaminated food. Many infections are subclinical and cause only mild disease. Sometimes, however, severe infections occur and may be fatal. Two of the five patients with P. kellicotti infections that have been reported over the past three decades were associated with relatively severe disease. These patients required surgery, with one requiring a second course of praziquantel. The most recently reported patient had a remote P. kellicotti infection, but the residual pleural effusion caused by the Paragonimus infection became infected by bacteria and directly contributed to his demise.
The diagnosis of paragonimiasis should be considered when the appropriate clinical signs and symptoms, radiologic findings, and food history are supportive. Cough and hemoptysis are the most common presenting symptoms in patients with paragonimiasis. Diagnosis may be achieved through the demonstration of the characteristic operculate eggs in respiratory secretions, stool, or tissue biopsy specimens. Immunodiagnostics are excellent tools for assisting with the diagnosis of imported paragonimiasis, particularly for patients who do not have demonstrable eggs in clinical specimens. The utility of immunodiagnostics for detecting infections caused by P. kellicotti remains to be determined. Praziquantel is an effective treatment, but surgery may be necessary for patients with complicated pleural disease or cerebral paragonimiasis. Control of paragonimiasis in animals is impractical because of wild animal reservoirs, but in humans infections may be averted through the avoidance of certain foods and by thorough cooking. Crayfish in North America may harbor P. kellicotti and therefore should be thoroughly cooked prior to consumption.
Biography
 Gary W. Procop (MD, MS) is Chairman of the Department of Clinical Pathology at the Cleveland Clinic and Medical Director of the Molecular Microbiology, Mycology, and Parasitology laboratories. He completed anatomic and clinical pathology training at Duke University Medical Center and a clinical microbiology fellowship at the Mayo Clinic. He is a diplomat of the American Board of Pathology in Anatomic and Clinical Pathology, and Medical Microbiology. He is a Fellow of the American Academy of Microbiology, the College of American Pathologists, the American Society for Clinical Pathology, the Infectious Diseases Society of America, and the Royal Society of Tropical Medicine and Hygiene. He has given more than 375 scientific presentations, and has 124 published manuscripts, 25 chapters, and 1 book to his credit. He is a Trustee of the American Board of Pathology, and Chair of the Microbiology Test Development Committee for the Board. He is also a member of the Board of the American College of Microbiology. He holds the Vice Chair position on both the Public Affairs Committee and the Microbiology Resource Committee for the College of American Pathologists. His primary interests are the practical applications of molecular diagnostic methods for the diagnosis and treatment of infections, infectious disease pathology, mycology, and parasitology. His main hobby is sailing, and he holds certifications in basic keelboat sailing, basic and intermediate coastal cruising, and coastal navigation.
Gary W. Procop (MD, MS) is Chairman of the Department of Clinical Pathology at the Cleveland Clinic and Medical Director of the Molecular Microbiology, Mycology, and Parasitology laboratories. He completed anatomic and clinical pathology training at Duke University Medical Center and a clinical microbiology fellowship at the Mayo Clinic. He is a diplomat of the American Board of Pathology in Anatomic and Clinical Pathology, and Medical Microbiology. He is a Fellow of the American Academy of Microbiology, the College of American Pathologists, the American Society for Clinical Pathology, the Infectious Diseases Society of America, and the Royal Society of Tropical Medicine and Hygiene. He has given more than 375 scientific presentations, and has 124 published manuscripts, 25 chapters, and 1 book to his credit. He is a Trustee of the American Board of Pathology, and Chair of the Microbiology Test Development Committee for the Board. He is also a member of the Board of the American College of Microbiology. He holds the Vice Chair position on both the Public Affairs Committee and the Microbiology Resource Committee for the College of American Pathologists. His primary interests are the practical applications of molecular diagnostic methods for the diagnosis and treatment of infections, infectious disease pathology, mycology, and parasitology. His main hobby is sailing, and he holds certifications in basic keelboat sailing, basic and intermediate coastal cruising, and coastal navigation.
REFERENCES
- 1.Abend, L. 1910. Ueber Haemoptysis Parasitaria. Dtsch. Arch. Klin. Med. 100:501-511. [Google Scholar]
- 2.Ameel, D. 1934. Paragonimus, its life history and distribution in North America and its taxonomy. Am. J. Hyg. 19:270-317. [Google Scholar]
- 3.Reference deleted.
- 4.Ameel, D. J., W. W. Cort, and A. van der Woude. 1951. Development of the mother sporocyst and rediae of Paragonimus kellicotti Ward, 1908. J. Parasitol. 37:395-404. [PubMed] [Google Scholar]
- 5.Amunarriz, M. 1991. Intermediate hosts of Paragonimus in the eastern Amazonic region of Ecuador. Trop. Med. Parasitol. 42:164-166. [PubMed] [Google Scholar]
- 6.Anantaphruti, M. T. 2001. Parasitic contaminants in food. Southeast Asian J. Trop. Med. Public Health 32(Suppl. 2):218-228. [PubMed] [Google Scholar]
- 7.Ando, R. 1921. The first intermediate host of Paragonimus westermani. Trop. Dis. Bull. 17:51. [Google Scholar]
- 8.Arce, J. 1915. La Paragonimiasis en el Peru. Cron. Med. 32:249-254. [Google Scholar]
- 9.Ashitani, J., K. Kumamoto, and S. Matsukura. 2000. Paragonimiasis westermani with multifocal lesions in lungs and skin. Intern. Med. 39:433-436. [DOI] [PubMed] [Google Scholar]
- 10.Asor, J. E., E. S. Ibanga, and F. O. I. Arene. 2003. Paragonimus uterobilateralis: peak period of egg output in sputum of infected subjects in Cross River Basin, Nigeria. Mary Slessor J. Med. 3:24-27. [Google Scholar]
- 11.Bae, W. T., M. K. Jung, and H. K. Kang. 1988. Computed tomographic fidings of cerebral paragonimiasis. J. Korean Radiol. Soc. 24:692-701. [Google Scholar]
- 12.Baelz, E. 1883. Uber eine neue Parasiten des Menshen. Berlin Klic. Wscher. 20:234-236. [Google Scholar]
- 13.Beaver, P., E. Malek, and M. Little. 1964. Development of Spirometra and Paragonimus eggs in Harada-Mori cultures. J. Parasitol. 50:664-666. [PubMed] [Google Scholar]
- 14.Bech-Nielsen, S., R. W. Fulton, H. U. Cox, J. D. Hoskins, J. B. Malone, Jr., and R. K. McGrath. 1980. Feline respiratory tract disease in Louisiana. Am. J. Vet. Res. 41:1293-1298. [PubMed] [Google Scholar]
- 15.Beckett, J. V., and V. Gallicchio. 1966. Occurrence of the lung fluke, Paragonimus kellicotti Ward, 1908, in Ohio mink. J. Parasitol. 52:511. [Google Scholar]
- 16.Bemrick, W. J., and J. C. Schlotthauer. 1971. Paragonimus kellicotti (Ward, 1908) in a Minnesota skunk (Melphitis mephitis). J. Wildl. Dis. 7:36. [DOI] [PubMed] [Google Scholar]
- 17.Blair, D., T. Agatsuma, and W. Wang. 2008. Paragonimiasis, p. 117-150. In K. D. Murrell and B. Fried (ed.), Food-borne parasitic zoonoses, vol. 11. Fish and plant-borne parasites. Springer, New York, NY. [Google Scholar]
- 18.Blair, D., Z. Chang, M. Chen, A. Cui, B. Wu, T. Agatsuma, M. Iwagami, D. Corlis, C. Fu, and X. Zhan. 2005. Paragonimus skrjabini Chen, 1959 (Digenea: Paragonimidae) and related species in eastern Asia: a combined molecular and morphological approach to identification and taxonomy. Syst. Parasitol. 60:1-21. [DOI] [PubMed] [Google Scholar]
- 19.Blair, D., Z. B. Xu, and T. Agatsuma. 1999. Paragonimiasis and the genus Paragonimus. Adv. Parasitol. 42:113-222. [DOI] [PubMed] [Google Scholar]
- 20.Boe, D. M., and M. I. Schwarz. 2007. A 31-year-old man with chronic cough and hemoptysis. Chest 132:721-726. [DOI] [PubMed] [Google Scholar]
- 21.Braun, M. 1901. Zur Kenntniss der Trematoden der Saugethiere. Zool. Jahrb. Abt. Syst. 14:311-348. [Google Scholar]
- 22.Braun, M. G. C. C. 1899. Ueber Clinostomun Leidy. Zool. Anz. 22:489-493. [Google Scholar]
- 23.Brown, R. W., R. J. Clarke, I. Denham, and P. W. Trembath. 1983. Pulmonary paragonimiasis in an immigrant from Laos. Med. J. Aust. 2:668-669. [DOI] [PubMed] [Google Scholar]
- 24.Bunnag, B., and T. Harinasuta. 1984. Opisthorchis, clonorchiasis and paragonimiasis, p. 461-470. In A. A. F. Mahmoud and K. S. Warren (ed.), Tropical and geographic medicine. McGraw-Hill, New York, NY.
- 25.Burton, K., R. Yogev, N. London, K. Boyer, and S. T. Shulman. 1982. Pulmonary paragonimiasis in Laotian refugee children. Pediatrics 70:246-248. [PubMed] [Google Scholar]
- 26.Burzynski, J., and N. W. Schluger. 2008. The epidemiology of tuberculosis in the United States. Semin. Respir. Crit. Care Med. 29:492-498. [DOI] [PubMed] [Google Scholar]
- 27.Cabrera, B. D. 1984. Paragonimiasis in the Philippines: current status. Arzneimittelforschung 34:1188-1192. [PubMed] [Google Scholar]
- 28.Cabrera, B. D. 1977. Studies on Paragonimus and paragonimiasis in the Philippines. II. Prevalence of pulmonary paragonimiasis in wild rats Rattus norvigicus in Jaro, Leyte, Philippines. Int. J. Zoonoses 4:49-55. [PubMed] [Google Scholar]
- 29.Calvopina, M., R. H. Guderian, W. Paredes, M. Chico, and P. J. Cooper. 1998. Treatment of human pulmonary paragonimiasis with triclabendazole: clinical tolerance and drug efficacy. Trans. R. Soc. Trop. Med. Hyg. 92:566-569. [DOI] [PubMed] [Google Scholar]
- 30.Castilla, E. A., R. Jessen, D. N. Sheck, and G. W. Procop. 2003. Cavitary mass lesion and recurrent pneumothoraces due to Paragonimus kellicotti infection: North American paragonimiasis. Am. J. Surg. Pathol. 27:1157-1160. [DOI] [PubMed] [Google Scholar]
- 31.Cha, S. H., K. H. Chang, S. Y. Cho, M. H. Han, Y. Kong, D. C. Suh, C. G. Choi, H. K. Kang, and M. S. Kim. 1994. Cerebral paragonimiasis in early active stage: CT and MR features. Am. J. Roentgenol. 162:141-145. [DOI] [PubMed] [Google Scholar]
- 32.Chang, H. T., C. W. Wang, and C. F. Yu. 1958. Paragonimiasis: a clinical study of 200 adult cases. Chin. Med. J. 77:3-9. [PubMed] [Google Scholar]
- 33.Chang, K. H., S. Y. Cho, J. R. Hesselink, M. H. Han, and M. C. Han. 1991. Parasitic diseases of central nervous system. Neuroimaging Clin. N. Am. 1:159-178. [Google Scholar]
- 34.Chang, Z. S., B. Wu, D. Blair, Y. N. Zhang, L. Hu, S. H. Chen, M. G. Chen, Z. Feng, and G. M. Davis. 2000. Gene sequencing for identification of Paragonimus eggs from a human case. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 18:213-215. [PubMed] [Google Scholar]
- 35.Chen, H. 1965. Paragonimus, Pagumogonimus, and a Paragonimus-like trematode in man. Chin. Med. J. 84:781-791. [PubMed] [Google Scholar]
- 36.Chen, M. G., Z. S. Chang, X. Y. Shao, M. D. Liu, D. Blair, S. H. Chen, Y. N. Zhang, J. L. Hong, G. B. Shen, and Z. Feng. 2001. Paragonimiasis in Yongjia County, Zhejiang Province, China: clinical, parasitological and karyotypic studies on Paragonimus westermani. Southeast Asian J. Trop. Med. Public Health 32:760-769. [PubMed] [Google Scholar]
- 37.Cheng, Y. Z., L. S. Xu, B. J. Chen, L. S. Li, R. Y. Zhang, C. X. Lin, J. X. Lin, Y. S. Li, Y. R. Le, Y. Y. Fang, K. Q. Lin, and G. B. Zheng. 2005. Survey on the current status of important human parasitic infections in Fujian Province. Chin. J. Parasitol. Parasit. Dis. 23:283-287. [PubMed] [Google Scholar]
- 38.Cho, S. Y., Y. Kong, and S. Y. Kang. 1997. Epidemiology of paragonimiasis in Korea. Southeast Asian J. Trop. Med. Public Health 28:32-36. [PubMed] [Google Scholar]
- 39.Choi, D. W. 1990. Paragonimus and paragonimiasis in Korea. Kisaengchunghak Chapchi 28(Suppl.):79-102. [DOI] [PubMed] [Google Scholar]
- 40.Choi, W. Y. 1984. Paragonimus westermani: pathogenesis and clinical features of infection. Arzneimittelforschung 34:1184-1185. [PubMed] [Google Scholar]
- 41.Reference deleted.
- 42.Chung, Y. B., H. J. Yang, S. Y. Kang, Y. Kong, and S. Y. Cho. 1997. Activities of different cysteine proteases of Paragonimus westermani in cleaving human IgG. Korean J. Parasitol. 35:139-142. [DOI] [PubMed] [Google Scholar]
- 43.Clara, M. K. 1937. Zur Histobiologie des Bronchalepithels. Z. Mikrosk.-Anat. Forsch. 41:321-347. [Google Scholar]
- 44.Cole, R. A., and W. L. Shoop. 1987. Helminths of the raccoon (Procyon lotor) in western Kentucky. J. Parasitol. 73:762-768. [PubMed] [Google Scholar]
- 45.Corvetto, A. 1921. Un caso de paragonimiasis pulmonar, observado en el Peru. Med. Ibera. 15:359-362. [Google Scholar]
- 46.Cui, A. L., Z. S. Chang, M. G. Chen, D. Blair, S. H. Chen, Y. N. Zhang, and Z. Feng. 2003. Taxonomic status in DNA sequences of five species of genus Paragonimus. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 21:27-30. [PubMed] [Google Scholar]
- 47.Davis, G. M., C. E. Chen, Z. B. Kang, and Y. Y. Liu. 1994. Snail hosts of Paragonimus in Asia and the Americas. Biomed. Environ. Sci. 7:369-382. [PubMed] [Google Scholar]
- 48.De, N. V., K. D. Murrell, L. D. Cong, P. D. Cam, L. V. Chau, N. D. Toan, and A. Dalsgaard. 2003. The food-borne trematode zoonoses of Vietnam. Southeast Asian J. Trop. Med. Public Health 34(Suppl. 1):12-34. [PubMed] [Google Scholar]
- 49.DeFrain, M., and R. Hooker. 2002. North American paragonimiasis: case report of a severe clinical infection. Chest 121:1368-1372. [DOI] [PubMed] [Google Scholar]
- 50.de Leon, W. U., and J. N. C. Piad. 2005. Paragonimiasis in the Philappines, vol. I. FAP Journal, Japan.
- 51.Doanh, P. N., N. T. Le, and D. T. The. 2005. Paragonimus and paragonimiasis in Vietnam, vol. I. FAP Journal, Japan. [DOI] [PMC free article] [PubMed]
- 52.Faust, E. C., P. F. Russell, and R. C. Jung. 1970. Craig and Fausts' clinical parasitology, 8th ed. Lea & Febiger, Philadelphia, PA.
- 53.Fehlisen, F., and C. M. Cooper. 1910. Paragonimiasis or parasitic hemoptysis. JAMA 54:679-699. [Google Scholar]
- 54.Feldman, W., and H. Essex. 1929. Distomatose pulmonaire chez le chat. Ann. Parastiol. Hum. Comp. 7:204-208. [Google Scholar]
- 55.Fogel, S. P., and P. T. Chandrasoma. 1994. Paragonimiasis in a cystic breast mass: case report and implications for examination of aspirated cyst fluids. Diagn. Cytopathol. 10:229-231. [DOI] [PubMed] [Google Scholar]
- 56.Galatius-Jensen, F., and I. K. Uhm. 1965. Radiological aspects of cerebral paragonimiasis. Br. J. Radiol. 38:494-502. [DOI] [PubMed] [Google Scholar]
- 57.Grove, D. I. 1990. A history of helminthology. CAB International, Wallingford, England.
- 58.Hahn, S. T., S. H. Park, C. Y. Kim, and K. S. Shinn. 1996. Adrenal paragonimiasis simulating adrenal tumor—a case report. J. Korean Med. Sci. 11:275-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harinasuta, T., S. Pungpak, and J. S. Keystone. 1993. Trematode infections. Opisthorchiasis, clonorchiasis, fascioliasis, and paragonimiasis. Infect. Dis. Clin. N. Am. 7:699-716. [PubMed] [Google Scholar]
- 60.Hayashi, E. 1978. A study on paragonimiasis, p. 1627-1680. In I. Tashiro (ed.), Medical History of Miyazaki Prefecture. Miyazaki Prefecture Doctoral Association, Miyazaki, Japan.
- 61.Heinert, J. 1949. Paragonimiasis pulmonar en el Ecuador. Rev. Med. Cordoba 37:289-309. [PubMed] [Google Scholar]
- 62.Heinert, J. F. 1947. Paragonimiasis pulmonar o distomatosis en el Ecuador. Rev. Kuba Med. Trop. 3:101-106. [Google Scholar]
- 63.Higashi, K., H. Aoki, K. Tatebayashi, H. Morioka, and Y. Sakata. 1971. Cerebral paragonimiasis. J. Neurosurg. 34:515-527. [DOI] [PubMed] [Google Scholar]
- 64.Ikeda, T. 2003. Involvement of cysteineproteinases in excystment of Paragonimus ohirai metacercariae induced sodium cholate and A23187. J. Helminthol. 77:21-26. [DOI] [PubMed] [Google Scholar]
- 65.Im, J. G., Y. Kong, Y. M. Shin, S. O. Yang, J. G. Song, M. C. Han, C. W. Kim, S. Y. Cho, and E. K. Ham. 1993. Pulmonary paragonimiasis: clinical and experimental studies. Radiographics 13:575-586. [DOI] [PubMed] [Google Scholar]
- 66.Im, J. G., H. Y. Whang, W. S. Kim, M. C. Han, Y. S. Shim, and S. Y. Cho. 1992. Pleuropulmonary paragonimiasis: radiologic findings in 71 patients. Am. J. Roentgenol. 159:39-43. [DOI] [PubMed] [Google Scholar]
- 67.Indrawati, I., W. Chaicumpa, P. Setasuban, and Y. Ruangkunaporn. 1991. Studies on immunodiagnosis of human paragonimiasis and specific antigen of Paragonimus heterotremus. Int. J. Parasitol. 21:395-401. [DOI] [PubMed] [Google Scholar]
- 68.Intapan, P. M., T. Kosuwan, C. Wongkham, and W. Maleewong. 2004. Genomic characterization of lung flukes, Paragonimus heterotremus, P. siamensis, P. harinasutai, P. westermani and P. bangkokensis by RAPD markers. Vet. Parasitol. 124:55-64. [DOI] [PubMed] [Google Scholar]
- 69.Intapan, P. M., C. Wongkham, K. J. Imtawil, W. Pumidonming, T. K. Prasongdee, M. Miwa, and W. Maleewong. 2005. Detection of Paragonimus heterotremus eggs in experimentally infected cats by a polymerase chain reaction-based method. J. Parasitol. 91:195-198. [DOI] [PubMed] [Google Scholar]
- 70.Ito, J., M. Yokogawa, R. Lamothe-Argumedo, and H. Hata. 1985. Studies on the cercaria of Paragonimus mexicanus in Aroapyrgus alleei from Colima, Mexico, with special reference to its morphology. Jpn. J. Parasitol. 34:71-77. [Google Scholar]
- 71.Ito, J., K. Yoshimura, and Y. Hishinuma. 1969. Comparative studies on Paragonimus sadoensis Miyazaki, Kawashima, Hamajima et Otsuru, 1968 and P. ohirai Miyazaki, 1939. I. Morphology of the rediae and cercariae, with special reference to the excretory systems. Jpn. J. Parasitol. 18:530-538. [Google Scholar]
- 72.Iwagami, M., L. Y. Ho, K. Su, P. F. Lai, M. Fukushima, M. Nakano, D. Blair, K. Kawashima, and T. Agatsuma. 2000. Molecular phylogeographic studies on Paragonimus westermani in Asia. J. Helminthol. 74:315-322. [DOI] [PubMed] [Google Scholar]
- 73.Iwagami, M., C. Monroy, M. A. Rosas, M. R. Pinto, A. G. Guevara, J. C. Vieira, Y. Agatsuma, and T. Agatsuma. 2003. A molecular phylogeographic study based on DNA sequences from individual metacercariae of Paragonimus mexicanus from Guatemala and Ecuador. J. Helminthol. 77:33-38. [DOI] [PubMed] [Google Scholar]
- 74.Iwagami, M., R. P. Rajapakse, W. Paranagama, and T. Agatsuma. 2003. Identities of two Paragonimus species from Sri Lanka inferred from molecular sequences. J. Helminthol. 77:239-245. [DOI] [PubMed] [Google Scholar]
- 75.Jiang, X., Z. Fan, X. Wang, and Y. Du. 2000. Clinical analysis of 258 cases with Pagumogonimus skrjabini in west of Hubei Province. Endemic Dis. Bull. 15:88-90. [Google Scholar]
- 76.Johnson, J. R., A. Falk, C. Iber, and S. Davies. 1982. Paragonimiasis in the United States. A report of nine cases in Hmong immigrants. Chest 82:168-171. [DOI] [PubMed] [Google Scholar]
- 77.Johnson, R. J., and J. R. Johnson. 1983. Paragonimiasis in Indochinese refugees. Roentgenographic findings with clinical correlations. Am. Rev. Respir. Dis. 128:534-538. [DOI] [PubMed] [Google Scholar]
- 78.Johnson, R. J., E. C. Jong, S. B. Dunning, W. L. Carberry, and B. H. Minshew. 1985. Paragonimiasis: diagnosis and the use of praziquantel in treatment. Rev. Infect. Dis. 7:200-206. [DOI] [PubMed] [Google Scholar]
- 79.Kadota, T., R. Ishikura, Y. Tabuchi, N. Nakao, H. Fujikawa, K. Kaba, E. Tani, and T. Miura. 1989. MR imaging of chronic cerebral paragonimiasis. Am. J. Neuroradiol. 10:S21-S22. [PMC free article] [PubMed] [Google Scholar]
- 80.Kagawa, F. T. 1997. Pulmonary paragonimiasis. Semin. Respir. Infect. 12:149-158. [PubMed] [Google Scholar]
- 81.Kan, H., T. Ogata, A. Taniyama, M. Migita, I. Matsuda, and Y. Nawa. 1995. Extraordinarily high eosinophilia and elevated serum interleukin-5 level observed in a patient infected with Paragonimus westermani. Pediatrics 96:351-354. [PubMed] [Google Scholar]
- 82.Kanazawa, T., H. Hata, S. Kojima, and M. Yokogawa. 1987. Paragonimus westermani: a comparative study on the migration route of the diploid and triploid types in the final hosts. Parasitol. Res. 73:140-145. [DOI] [PubMed] [Google Scholar]
- 83.Karrer, H. 1956. Electron microscope study of bronchiolar epithelium of normal mouse lung. Exp. Cell Res. 10:237-241. [DOI] [PubMed] [Google Scholar]
- 84.Keiser, J., D. Engels, G. Buscher, and J. Utzinger. 2005. Triclabendazole for the treatment of fascioliasis and paragonimiasis. Expert Opin. Investig. Drugs 14:1513-1526. [DOI] [PubMed] [Google Scholar]
- 85.Keiser, J., and J. Utzinger. 2005. Emerging foodborne trematodiasis. Emerg. Infect. Dis. 11:1507-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kellicott, D. S. 1894. Certain entozoa of the dog and sheep. Trans. Ohio State Med. Soc., p. 122-130.
- 87.Kerbert, C. 1878. Zur Trematoden-Kenntnis. Zoologischer. Anzeiger. 1:529-578. [Google Scholar]
- 88.Kim, D. C. 1984. Paragonimus westermani: life cycle, intermediate hosts, transmission to man and geographical distribution in Korea. Arzneimittelforschung 34:1180-1183. [PubMed] [Google Scholar]
- 89.Kim, J. S. 1970. A comparison of sensitivity on stool and sputum examination for diagnosis of paragonimiasis. Korean J. Parasitol. 8:22-24. [DOI] [PubMed] [Google Scholar]
- 90.Kim, S. K., and A. E. Walker. 1961. Cerebral paragonimiasis. Acta Psychiatr. Scand. 153:(Suppl.)70-76. [PubMed] [Google Scholar]
- 91.Kirkpatrick, C. E., and E. A. Shelly. 1985. Paragonimiasis in a dog: treatment with praziquantel. J. Am. Vet. Med. Assoc. 187:75-76. [PubMed] [Google Scholar]
- 92.Kisch, B. 1958. Electron microscopy of the lungs. III. Endothelial cells of the smallest air surfaces. Exp. Med. Surg. 16:1-16. [PubMed] [Google Scholar]
- 93.Kusner, D. J., and C. H. King. 1993. Cerebral paragonimiasis. Semin. Neurol. 13:201-208. [DOI] [PubMed] [Google Scholar]
- 94.Lara, A. 1913. Hemoptisis endemica de los paises tropicales. Rev. Med. Yucatan 9:1-5. [Google Scholar]
- 95.Leon, L. A. 1955. Diagnostico de la paragonimiasis. Gac. Med. 10:903-910. [Google Scholar]
- 96.Li, H. Z., F. W. Xie, and S. C. Sun. 1992. CT findings in “fresh” cerebral paragonimiasis. No Shinkei Geka 20:91-97. [PubMed] [Google Scholar]
- 97.Lu, C. H. 1957. Clinical observations of 195 cases of Paragonimus in children. Chin. J. Pediatr. 8:143-146. (In Chinese; English abstract.) [Google Scholar]
- 98.Lumsden, R. D., and F. Sogandares-Bernal. 1968. Electron microscopy of histopathology in pulmonary paragonimiasis, abstr. 45. Abstr. 43rd Annu. Meet. Am. Soc. Parasitol.
- 99.Lumsden, R. D., and F. Sogandares-Bernal. 1970. Ultrastructural manifestations of pulmonary paragonimiasis. J. Parasitol. 56:1095-1109. [PubMed] [Google Scholar]
- 100.Madariaga, M. G., T. Ruma, and J. H. Theis. 2007. Autochthonous human paragonimiasis in North America. Wild. Environ. Med. 18:203-205. [DOI] [PubMed] [Google Scholar]
- 101.Mahajan, R. 2005. Paragonimiasis: an emerging public health problem in India. Indian J. Med. Res. 121:716-718. [PubMed] [Google Scholar]
- 102.Maleewong, W. 1997. Recent advances in diagnosis of paragonimiasis. Southeast Asian J. Trop. Med. Public Health 28(Suppl. 1):134-138. [PubMed] [Google Scholar]
- 103.Maleewong, W., S. Pariyanonda, C. Wongkham, P. Intapan, W. Daenseegaew, and N. Morakote. 1990. Comparison of adult somatic and excretory-secretory antigens in enzyme-linked immunosorbent assay for serodiagnosis of human infection with Paragonimus heterotremus. Trans. R. Soc. Trop. Med. Hyg. 84:840-841. [DOI] [PubMed] [Google Scholar]
- 104.Maleewong, W., C. Wongkham, P. Intapan, S. Pariyanonda, and N. Morakote. 1992. Excretory-secretory antigenic components of Paragonimus heterotremus recognized by infected human sera. J. Clin. Microbiol. 30:2077-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maleewong, W., C. Wongkham, S. Pariyanonda, and P. Intapan. 1992. Analysis of antibody levels before and afer praziquantel treatment in human paragonimiasis heterotremus. Asian Pac. J. Allergy Immunol. 10:69-72. [PubMed] [Google Scholar]
- 106.Maleewong, W., C. Wongkham, S. Pariyanonda, P. Intapan, V. Pipitgool, W. Daenseegaew, and N. Morakote. 1991. Antigenic components of Paragonimus heterotremus recognized by infected human serum. Parasite Immunol. 13:89-93. [DOI] [PubMed] [Google Scholar]
- 107.Malek, E. 1980. Trematodes, 6th ed. Saunders, Philadelphia, PA.
- 108.Malek, E. A. 1980. Paragonimiasis, vol. II. CRC Press, Boca Raton, FL.
- 109.Malvy, D., K. Ezzedine, M. Receveur, T. Pistone, P. Mercié, and M. Longy-Boursier. 2006. Extra-pulmonary paragonimiasis with unusual arthritis and cutaneous features among a tourist returning from Gabon. Clin. Infect. Dis. 4:340-342. [DOI] [PubMed] [Google Scholar]
- 110.Manson, P. 1881. Distoma ringeri. China Imperial Maritime Customs. Medical Reports for the half year ended 30th September 1880. Medical Times Gazette 20:8-9. [Google Scholar]
- 111.Mariano, E. G., S. R. Borja, and M. J. Vruno. 1986. A human infection with Paragonimus kellicotti (lung fluke) in the United States. Am. J. Clin. Pathol. 86:685-687. [DOI] [PubMed] [Google Scholar]
- 112.Marty, A. M., and R. C. Neafie. 2000. Paragonimiasis, p. 49-67. In W. M. Myers, R. C. Neafie, A. M. Marty, and D. J. Wear (ed.), Pathology of infectious diseases, vol. I. Helminthiases. Armed Forces Institute of Pathology, Washington, DC. [Google Scholar]
- 113.Maruyama, H., S. Noda, and Y. Nawa. 1996. Emerging problems of parasitic disease in southern Kyushu, Japan. Jpn. J. Parasitol. 45:192-200. [Google Scholar]
- 114.Medical Letter, Inc. April 2002. Drugs for parasitic infections. Med. Lett. 4. Medical Letter, Inc., New Rochelle, NY.
- 115.Meehan, A. M., A. Virk, K. Swanson, and E. M. Poeschla. 2002. Severe pleuropulmonary paragonimiasis 8 years after emigration from a region of endemicity. Clin. Infect. Dis. 35:87-90. [DOI] [PubMed] [Google Scholar]
- 116.Min, D. Y., Y. A. Lee, J. S. Ryu, M. H. Ahn, Y. B. Chung, S. Sim, and M. H. Shin. 2004. Caspase-3-mediated apoptosis of human eosinophils by the tissue-invading helminth Paragonimus westermani. Int. Arch. Allergy Immunol. 133:357-364. [DOI] [PubMed] [Google Scholar]
- 117.Miyazaki, I. 1974. Lung flukes in the world—morphology and life history. International Medical Foundation of Japan, Toyko, Japan.
- 118.Miyazaki, I., and S. Habe. 1976. A newly recognized mode of human infection with the lung fluke Paragonimus westermani. J. Parasitol. 62:646-648. [PubMed] [Google Scholar]
- 119.Miyazaki, I., and Y. Ishii. 1968. Comparative study of the Mexican lung flukes with Paragonimus kellicotti Ward, 1908. J. Parasitol. 54:845-846. [PubMed] [Google Scholar]
- 120.Miyazaki, I., and Y. Ishii. 1968. Studies on the Mexican lung flukes, with special reference to a description of Paragonimus mexicanus sp.n. Jpn. J. Parasitol. 17:445-453. [Google Scholar]
- 121.Miyazaki, I., K. Terasaki, and S. Habe. 1981. Comparison of single worm infection to cats between Paragonimus westermani and P. pulmonalis. Med. Bull. Fukuoka Univ 8:405-416. [Google Scholar]
- 122.Miyazaki, I., K. Terasaki, and K. Iwata. 1978. Natural infection of muscle of wild boars in Japan by immature Paragonimus westermani (Kerbert 1878). J. Parasitol. 64:559-560. [PubMed] [Google Scholar]
- 123.Moore, T., and T. Nutman. 1998. Eosinophila in the returning traveler. Infect. Dis. Clin. N. Am. 12:503-521. [DOI] [PubMed] [Google Scholar]
- 124.Morris, M. W., and F. R. Davey. 2001. Basic examination of blood. W. B. Saunders, Phildelphia, PA.
- 125.Mukerjee, C. M., S. E. Simpson, R. J. Bell, and J. C. Walker. 1992. Pleuropulmonary paragonimiasis in a Laotian immigrant to Australia. Chest 101:849-851. [DOI] [PubMed] [Google Scholar]
- 126.Nakamura-Uchiyama, F., H. Mukae, and Y. Nawa. 2002. Paragonimiasis: a Japanese perspective. Clin. Chest Med. 23:409-420. [DOI] [PubMed] [Google Scholar]
- 127.Nakamura-Uchiyama, F., D. N. Onah, and Y. Nawa. 2001. Clinical features and parasite-specific IgM/IgG antibodies of paragonimiasis patients recently found in Japan. Southeast Asian J. Trop. Med. Public Health 32(Suppl. 2):55-58. [PubMed] [Google Scholar]
- 128.Nakamura-Uchiyama, F., D. N. Onah, and Y. Nawa. 2001. Clinical features of paragonimiasis cases recently found in Japan: parasite-specific immunoglobulin M and G antibody classes. Clin. Infect. Dis. 32:e151-e153. [DOI] [PubMed] [Google Scholar]
- 129.Nana, A., and S. Bovornkitti. 1991. Pleuropulmonary paragonimiasis. Semin. Respir. Med. 12:46-54. [Google Scholar]
- 130.Narain, K., K. R. Devi, and J. Mahanta. 2005. Development of enzyme-linked immunosorbent assay for serodiagnosis of human paragonimiasis. Indian J. Med. Res. 121:739-746. [PubMed] [Google Scholar]
- 131.Nawa, Y. 1998. Histopathological and immunological diagnosis for parasitic zoonoses, p. 39-52. In E. Ishikura, M. Aikawa, and H. Itakura (ed.), Host response to international parasitic zoonoses. Springer, Tokyo, Japan.
- 132.Nawa, Y. 2000. Re-emergence of paragonimiasis. Intern. Med. 39:353-354. [DOI] [PubMed] [Google Scholar]
- 133.Nawa, Y. 1991. Recent trends of paragonimiasis westermani in Miyazaki Prefecture, Japan. Southeast Asian J. Trop. Med. Public Health 22(Suppl.):342-344. [PubMed] [Google Scholar]
- 134.Null, M. M. 1910. Tochil, or endemic hemoptysis. Northwest Med. 2:364-366. [Google Scholar]
- 135.Oh, S. 1978. Paragonimiasis in the central nervous system, p. 243-266. In P. Vinken and G. Bruyn (ed.), Handbook of clinical neurology, vol. 35. North-Holland, Amsterdam, The Netherlands. [Google Scholar]
- 136.Oh, S. 1968. Roentgen findings in cerebral paragonimiasis. Radiology 90:292-299. [Google Scholar]
- 137.Otsuji, Y. 2003. Paragonimiasis. Prog. Med. Parasitol. Jpn. 8:183-200. [Google Scholar]
- 138.Pachucki, C., R. Levandowski, V. A. Brown, B. Sonnenkalb, and M. Vruno. 1984. American paragonimiasis treated with praziquantel. N. Engl. J. Med. 311:582-583. [DOI] [PubMed] [Google Scholar]
- 139.Pechman, R. D., Jr. 1980. Pulmonary paragonimiasis in dogs and cats: a review. J. Small Anim. Pract. 21:87-95. [DOI] [PubMed] [Google Scholar]
- 140.Presidente, J. J., and R. O. Ramsden. 1975. Paragonimus kellicotti infection in wild carnivores in southwestern Ontario. II. Histopathologic features. J. Wildl. Dis. 11:364-375. [DOI] [PubMed] [Google Scholar]
- 141.Procop, G. W., A. M. Marty, D. N. Scheck, D. R. Mease, and G. M. Maw. 2000. North American paragonimiasis. A case report. Acta Cytol. 44:75-80. [DOI] [PubMed] [Google Scholar]
- 142.Qian, B. Z., H. Sugiyama, J. Waikagul, and Z. H. Zhu. 2006. Sequence analysis of ITS2 and CO1 genes of Paragonimus harinasutai. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 24:119-121. [PubMed] [Google Scholar]
- 143.Ramsden, R. O., and P. J. Presidente. 1975. Paragonimus kellicotti infection in wild carnivores in southwestern Ontario. I. Prevalence and gross pathologic features. J. Wildl. Dis. 11:136-141. [DOI] [PubMed] [Google Scholar]
- 144.Rangdaeng, S., L. C. Alpert, A. Khiyami, K. Cottingham, and I. Ramzy. 1992. Pulmonary paragonimiasis. Report of a case with diagnosis by fine needle aspiration cytology. Acta Cytol. 36:31-36. [PubMed] [Google Scholar]
- 145.Rhee, C. H., C. K. Chung, H. W. Jung, S. Y. Cho, and H. J. Son. 1987. Cerebral paragonimiasis treted with praziquantel: case report. J. Korean Neurosurg Soc. 16:853-857. [Google Scholar]
- 146.Ripert, C., B. Couprie, R. Moyou, F. Gaillard, M. Appriou, and J. Tribouley-Duret. 1992. Therapeutic effect of triclabendazole in patients with paragonimiasis in Cameroon: a pilot study. Trans. R. Soc. Trop. Med. Hyg. 86:417. [DOI] [PubMed] [Google Scholar]
- 147.Ryu, J. S., U. W. Hwang, D. Y. Min, K. S. Shin, S. J. Nam, and O. R. Lee. 2000. Molecular identification of Paragonimus ohirai and P. westermani from Anhui Province, China. Parasite 7:305-309. [DOI] [PubMed] [Google Scholar]
- 148.Saborio, P., R. Lanzas, G. Arrieta, and A. Arguedas. 1995. Paragonimus mexicanus pericarditis: report of two cases and review of the literature. J. Trop. Med. Hyg. 98:316-318. [PubMed] [Google Scholar]
- 149.Sachs, R., E. J. Albiez, and J. Voelker. 1986. Prevalence of Paragonimus uterobilateralis infection in children in a Liberian village. Trans. R. Soc. Trop. Med. Hyg. 80:800-801. [DOI] [PubMed] [Google Scholar]
- 150.Sachs, R., and N. Cumberlidge. 1990. Distribution of metacercariae in freshwater crabs in relation to Paragonimus infection of children in Liberia, West Africa. Ann. Trop. Med. Parasitol. 84:277-280. [DOI] [PubMed] [Google Scholar]
- 151.Sadun, E. H., and A. A. Buck. 1960. Paragonimiasis in South Korea—immunodiagnostic, epidemiologic, clinical, roentgenologic and therapeutic studies Am. J. Trop. Med. Hyg. 9:562-599. [DOI] [PubMed] [Google Scholar]
- 152.Schuster, H., F. O. Agada, A. R. Anderson, R. S. Jackson, D. Blair, H. McGann, and G. Kelly. 2007. Otitis media and a neck lump—current diagnostic challenges for Paragonimus-like trematode infections. J. Infect. 54:e103-106. [DOI] [PubMed] [Google Scholar]
- 153.Seed, J., F. Sogandares-Bernal, and R. Mills. 1966. Studies on American paragonimiasis. II. Serological observations of infected cats. J. Parasitol. 52:358-362. [PubMed] [Google Scholar]
- 154.Seed, J. R., F. Sogandares-Bernal, and A. A. Cam. 1968. Studies on American paragonimiasis. VI. Antibody response in three cats infected with Paragonimus kellicotti. Tulane Stud. Zool. Bot. 15:70-74. [Google Scholar]
- 155.Sharma, O. P. 1989. The man who loved drunken crabs. A case of pulmonary paragonimiasis. Chest 95:670-672. [DOI] [PubMed] [Google Scholar]
- 156.Shibahara, T. 1991. The route of infection of Paragonimus westermani (diploid type) cercariae in the freshwater crab, Geothelphusa dehaani. J. Helminthol. 65:38-42. [Google Scholar]
- 157.Shih, Y. C., Y. H. Ch'En, and Y. C. Chang. 1958. Paragonimiasis of central nervous system. Observations on 76 cases. Chin. Med. J. 77:10-19. [PubMed] [Google Scholar]
- 158.Shim, Y.-S., S.-Y. Cho, and Y.-C. Han. 1991. Pulmonary paragonimiasis: a Korean perspective. Semin. Respir. Med. 12:35-45. [Google Scholar]
- 159.Shin, D. H., and C. Y. Joo. 1990. Prevalence of Paragonimus westermani in some Ulchin school children. Acta Paediatr. Jpn. 32:269-274. [DOI] [PubMed] [Google Scholar]
- 160.Shin, M. H., H. Kita, H. Y. Park, and J. Y. Seoh. 2001. Cysteine protease secreted by Paragonimus westermani attenuates effector functions of human eosinophils stimulated with immunoglobulin G. Infect. Immun. 69:1599-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shin, M. H., and D. Y. Min. 1999. Infection status of Paragonimus westermani metacercariae in crayfish (Cambaroides similis) collected from Bogildo (Islet), Wando-gun, Chollanam-do, Korea. Korean J. Parasitol. 37:55-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Singh, N. P., and R. Somvanshi. 1978. Paragonimus westermanni in tigers (Panthera tigris) in India. J. Wildl. Dis. 14:322-324. [DOI] [PubMed] [Google Scholar]
- 163.Singh, S. T., D. L. Singh, and H. Sugiyama. 2006. Possible discovery of Chinese lung fluke, Paragonimus skrjabini in Manipur, India. Southeast Asian J. Trop. Med. Public Health 37(Suppl. 3):53-56. [PubMed] [Google Scholar]
- 164.Singh, T. N., S. Kananbala, and K. S. Devi. 2005. Pleuropulmonary paragonimiasis mimicking pulmonary tuberculosis—a report of three cases. Indian J. Med. Microbiol. 23:131-134. [DOI] [PubMed] [Google Scholar]
- 165.Singh, T. N., H. R. Singh, S. Devi Kh, N. B. Singh, and Y. I. Singh. 2004. Pulmonary paragonimiasis. Indian J. Chest Dis. Allied Sci. 46:225-227. [PubMed] [Google Scholar]
- 166.Singh, T. S., S. Mutum, M. A. Razaque, Y. I. Singh, and E. Y. Singh. 1993. Paragonimiasis in Manipur. Indian J. Med. Res. 97:247-252. [PubMed] [Google Scholar]
- 167.Singh, T. S., S. S. Mutum, and M. A. Razaque. 1986. Pulmonary paragonimiasis: clinical features, diagnosis and treatment of 39 cases in Manipur. Trans. R. Soc. Trop. Med. Hyg. 80:967-971. [DOI] [PubMed] [Google Scholar]
- 168.Slemenda, S. B., S. E. Maddison, E. C. Jong, and D. D. Moore. 1988. Diagnosis of paragonimiasis by immunoblot. Am. J. Trop. Med. Hyg. 39:469-471. [DOI] [PubMed] [Google Scholar]
- 169.Snyder, D. E., A. N. Hamir, V. F. Netles, and C. E. Rupprecht. 1991. Lesions associated with pulmonary parasites in bobcats (Felis rufus) from Arkansas. J. Wildl. Dis. 27:170-174. [DOI] [PubMed] [Google Scholar]
- 170.Sogandares-Bernal, F. 1965. Studies on American paragonimiasis. I. Age immunity of the snail host. J. Parasitol. 51:958-960. [PubMed] [Google Scholar]
- 171.Sogandares-Bernal, F. 1966. Studies on American paragonimiasis. IV. Observations on the pairing of adult worms in laboratory infections of domestic cats. J. Parasitol. 52:701-703. [PubMed] [Google Scholar]
- 172.Sogandares-Bernal, F., and J. R. Seed. 1973. American paragonimiasis. Curr. Top. Comp. Pathobiol. 2:1-56. [DOI] [PubMed] [Google Scholar]
- 173.Stromberg, P. C., and J. P. Dubey. 1978. The life cycle of Paragonimus kellicotti in cats. J. Parasitol. 64:998-1002. [PubMed] [Google Scholar]
- 174.Sugiyama, H., Y. Morishima, Y. Kameoka, K. Arakawa, and M. Kawanaka. 2004. Paragonimus ohirai metacercariae in crabs collected along the Arakawa River in Tokyo, Japan. J. Vet. Med. Sci. 66:927-931. [DOI] [PubMed] [Google Scholar]
- 175.Sugiyama, H., Y. Morishima, Y. Kameoka, and M. Kawanaka. 2002. Polymerase chain reaction (PCR)-based molecular discrimination between Paragonimus westermani and P. miyazakii at the metacercarial stage. Mol. Cell Probes 16:231-236. [DOI] [PubMed] [Google Scholar]
- 176.Sugiyama, H., Y. Morishima, A. Rangsiruji, S. Binchai, P. Ketudat, Y. Kameoka, and M. Kawanaka. 2005. Molecular discrimination between individual metacercariae of Paragonimus heterotremus and P. westermani occurring in Thailand. Southeast Asian J. Trop. Med. Public Health 36(Suppl. 4):102-106. [PubMed] [Google Scholar]
- 177.Sugiyama, H., Y. Morishima, A. Rangsiruji, S. Binchai, P. Ketudat, and M. Kawanaka. 2006. Application of multiplex PCR for species discrimination using individual metacercariae of Paragonimus occurring in Thailand. Southeast Asian J. Trop. Med. Public Health 37(Suppl. 3):48-52. [PubMed] [Google Scholar]
- 178.Tandon, V., P. K. Prasad, A. Chatterjee, and P. T. Bhutia. 2007. Surface fine topography and PCR-based determination of metacercaria of Paragonimus sp. from edible crabs in Arunachal Pradesh, Northeast India. Parasitol. Res. 102:21-28. [DOI] [PubMed] [Google Scholar]
- 179.Taylor, C. R., and H. A. Swett. 1982. Pulmonary paragonimiasis in Laotian refugees. Radiology 143:411-412. [DOI] [PubMed] [Google Scholar]
- 180.Terasaki, K., S. Habe, L. Y. Ho, H. Jian, T. Agatsuma, Shibahara, T., H. Sugiyama, and K. Kawashima. 1995. Tetraploids of the lung fluke Paragonimus westermani found in China. Parasitol. Res. 81:627-630. [DOI] [PubMed] [Google Scholar]
- 181.Turner, J. A. 1998. Trematodes, 4th ed., vol. 2. W. B. Saunders, Philadelphia, PA.
- 182.Uchiyama, F., Y. Morimoto, and Y. Nawa. 1999. Re-emergence of paragonimiasis in Kyushu, Japan. Southeast Asian J. Trop. Med. Public Health 30:686-691. [PubMed] [Google Scholar]
- 183.Udaka, F., B. Okuda, M. Okada, T. Tsuji, and M. Kameyama. 1988. CT findings of cerebral paragonimiasis in the chronic state. Neuroradiology 30:31-34. [DOI] [PubMed] [Google Scholar]
- 184.van Herwerden, L., D. Blair, and T. Agatsuma. 1999. Intra- and interindividual variation in ITS1 of Paragonimus westermani (Trematoda: digenea) and related species: implications for phylogenetic studies. Mol. Phylogenet. Evol. 12:67-73. [DOI] [PubMed] [Google Scholar]
- 185.Vanijanonta, S., D. Bunnag, and T. Harinasuta. 1984. Paragonimus heterotremus and other Paragonimus spp. in Thailand: pathogenesis, clinic and treatment. Arzneimittelforschung 34:1186-1188. [PubMed] [Google Scholar]
- 186.Velez, I. D., J. Ortega, M. I. Hurtado, A. L. Salazar, S. M. Robledo, J. N. Jimenez, and L. E. Velasquez. 2000. Epidemiology of paragonimiasis in Colombia. Trans. R. Soc. Trop. Med. Hyg. 94:661-663. [DOI] [PubMed] [Google Scholar]
- 187.Vieira, J. C., H. D. Blankespoor, P. J. Cooper, and R. H. Guderian. 1992. Paragonimiasis in Ecuador: prevalence and geographical distribution of parasitisation of second intermediate hosts with Paragonimus mexicanus in Esmeraldas province. Trop. Med. Parasitol. 43:249-252. [PubMed] [Google Scholar]
- 188.Waikagul, J., R. Lazo, E. Cornejo, M. Lazo, M. Llaguno, J. Barrezueta, M. Llaguno, M. Botta, T. Yoonuan, and H. Akahane. 2003. Paragonimus infection in the Pedernales, Ecuador. Bull. Central Res. Inst. Fukuoka Univ. Ser. E 1:259-273. [Google Scholar]
- 189.Waikagul, J., S. Yaemput, and K. Visiassuk. 1986. The route of migration of Paragonimus siamensis Miyazaki & Wykoff, 1965 in the white rat. Southeast Asian J. Trop. Med. Public Health 17:587-590. [PubMed] [Google Scholar]
- 190.Waikagul, J., and T. Yoonuan. 2005. Paragonimus and paragonimiasis in Thailand, vol. I. FAP Journal, Japan.
- 191.Waitz, J. A., P. McClay, and P. E. Thompson. 1963. Activity of 2,2′-thiobis(4,6 dichorophenol (Bithionol) against Paragonimus kellicotti in rats. J. Parasitol. 49:16. [DOI] [PubMed] [Google Scholar]
- 192.Ward, H. 1894. On the presence of Distoma westermanni in the United States. Vet. Mag 1:355-357. [Google Scholar]
- 193.Ward, H. B. 1908. Data for determination of human entozoa. Trans. Am. Microsc. Soc. 28:177-201. [Google Scholar]
- 194.Ward, H. B. 1894. Ueber das Vorkommen von Distoma westermanii in den Vereinigten Staaten. Zentralbl. Bakteriol. Parasitenkd. Abt. 1 Orig. 15:362-364. [Google Scholar]
- 195.Ward, H. B., and E. F. Hirsch. 1915. The species of Paragonimus and their differentiation. Ann. Trop. Med. Parasitol. 9:109-162. [Google Scholar]
- 196.World Health Organization. 1995. Control of foodborne trematode infections. Report of a W. H. O. Study Group. WHO Tech. Rep. Ser. 849:1-157. [PubMed] [Google Scholar]
- 197.World Health Organization. 2004. Report on Joint WHO/FAO Workshop on Foodborne Trematode Infections in Asia. Report series no. RS/2002/GE/40(VTN). WHO, Geneva, Switzerland.
- 198.Xie, B. W., Y. S. Yang, Q. Z. Li, and L. Y. Tang. 1985. Rana boulengeri as the paratenic host of Pagumogonimus skrjabini. Chin. J. Parasitol. Parasit. Dis. 3:157. (In Chinese.) [Google Scholar]
- 199.Xu, Z. B. 1991. Studies on clinical manifestations, diagnosis and control of paragonimiasis in China. Southeast Asian J. Trop. Med. Public Health 22:345-348. [PubMed] [Google Scholar]
- 200.Yee, B., J. I. Hsu, C. B. Favour, and E. Lohne. 1992. Pulmonary paragonimiasis in Southeast Asians living in the central San Joaquin Valley. West. J. Med. 156:423-425. [PMC free article] [PubMed] [Google Scholar]
- 201.Yokogawa, M. 1969. Paragonimus and paragonimiasis. Adv. Parasitol. 7:375-387. [DOI] [PubMed] [Google Scholar]
- 202.Yokogawa, M. 1965. Paragonimus and paragonimiasis. Adv. Parasitol. 3:99-158. [DOI] [PubMed] [Google Scholar]
- 203.Yokogawa, M. 1964. Paragonimus and paragonimiasis, vol. 1. Sankyo Bijutsu Insatsu, Tokyo, Japan.
- 204.Yokogawa, M., S. Kojima, K. Araki, H. Tomioka, and S. Yoshida. 1976. Immunoglobulin E: raised levels in sera and pleural exudates of patients with paragonimiasis. Am. J. Trop. Med. Hyg. 25:581-586. [DOI] [PubMed] [Google Scholar]
- 205.Yong, T. S., J. H. Seo, and I. S. Yeo. 1993. Serodiagnosis of human paragonimiasis by ELISA-inhibition test using monoclonal antibodies. Korean J. Parasitol. 31:141-147. [DOI] [PubMed] [Google Scholar]
- 206.Yun, D. J. 1960. Paragonimiasis in children in Korea. J. Pediatr. 56:736-751. [DOI] [PubMed] [Google Scholar]
- 207.Zhang, Z., Y. Zhang, L. Liu, C. Dong, Z. Wu, and W. F. Piessens. 1996. Antigen detection assay to monitor the efficacy of praziquantel for treatment of Paragonimus westermani infections. Trans. R. Soc. Trop. Med. Hyg. 90:43. [DOI] [PubMed] [Google Scholar]
- 208.Zhong, H. L., L. Y. He, Z. B. Xu, and W. J. Cao. 1981. Recent progress in studies of Paragonimus and paragonimiasis control in China. Chin. Med. J. 94:483-494. [PubMed] [Google Scholar]