Abstract
Summary: Primary antibody deficiencies are the most common primary immunodeficiency diseases. They are a heterogeneous group of disorders with various degrees of dysfunctional antibody production resulting from a disruption of B-cell differentiation at different stages. While there has been tremendous recent progress in the understanding of some of these disorders, the etiology remains unknown for the majority of patients. As there is a large spectrum of underlying defects, the age at presentation varies widely, and the clinical manifestations range from an almost complete absence of B cells and serum immunoglobulins to selectively impaired antibody responses to specific antigens with normal total serum immunoglobulin concentrations. However, all of these disorders share an increased susceptibility to infections, affecting predominantly the respiratory tract. A delay of appropriate treatment for some diseases can result in serious complications related to infections, while timely diagnosis and adequate therapy can significantly decrease morbidity and increase life expectancy and quality of life.
INTRODUCTION
Recurrent infection is the predominant presenting complaint for primary antibody deficiency (PAD) disorders. Thus, patients are often referred to infectious disease specialists prior to being seen by a clinical immunologist. Infectious disease consultants also play an important role in the management of infectious complications after immunodeficiency has been diagnosed. The infectious disease specialists’ familiarity with PADs and principles of their diagnosis and management is therefore paramount for early disease recognition and optimum interdisciplinary care.
The majority of the antibody deficiencies that have been defined at the molecular level arise from defects that are intrinsic to B-cell development and function. This is not surprising given that plasma cells derived from B cells are the only cells in the body that secrete immunoglobulin. However, some disorders that are manifested mainly by impaired antibody production may also arise due to defects in T cells or other cells that cooperate with or provide “help” to B cells for plasma cell development (14). Furthermore, the majority of patients with antibody deficiency have syndromes defined by clinical and laboratory criteria and whose molecular basis remains unknown.
The true incidence and prevalence of immunodeficiency are unknown, as no large prospective studies have been performed. The incidences and prevalences of all forms of primary immunodeficiency estimated from reports of surveys or registries in over 40 countries range from 1:10,000 to 1:2,000 individuals (113). However, this is suspected to be an underestimate since it is likely that many cases of immunodeficiency are undiagnosed. A recent random telephone survey in the United States determined a prevalence of all immunodeficiencies of approximately 1:2,000 to 1:800 individuals (18). Antibody deficiencies account for 50 to 60% of all immunodeficiencies (113).
In this review, we begin with a discussion of B-cell development with reference to specific biochemical pathways, whose derangement leads to clinical immunodeficiency. We will discuss in some detail the infections that are characteristic of this group of diseases, as well as the clinical, laboratory, and molecular elements defining each diagnosis. We conclude with a brief discussion of principles of management.
B-LYMPHOCYTE DEVELOPMENT
B lymphocytes develop in the bone marrow and spleen through a series of stages from a pluripotent precursor cell to a mature B cell. This process does not require B-cell contact with antigen. After antigen stimulation in concert with other signals, a B cell will become either an antibody-producing effector cell (plasma cell) or a memory cell. Genetic lesions affecting B-cell development and function result in the clinical manifestations of antibody deficiency.
Antigen-Independent B-Cell Development
The formation of mature B cells is independent of antigen contact and takes place predominantly in the primary lymphoid organs, the fetal liver during gestation, and, subsequently, the bone marrow throughout the remainder of life. A key event in the development of a diverse repertoire of antibody specificities is the functional rearrangement of the immunoglobulin heavy-chain (IgH) and immunoglobulin light-chain (IgL) segments of the B-cell receptor (BCR) genes (5, 94).
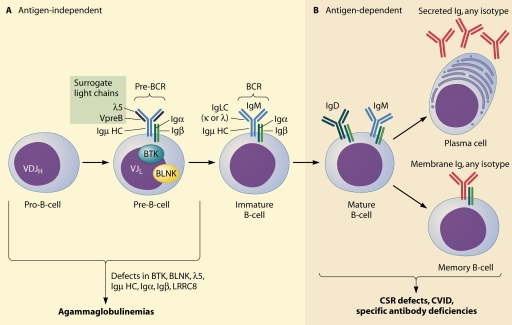
B-cell receptor gene rearrangement and early B-cell development.
Receptor gene rearrangement follows a sequential order, starting with recombination of the variable (V), diversity (D), and joining (J) (VDJ) gene segments of the IgH locus, followed by V and J rearrangement of the IgL locus (Fig. 1A). The recombinase-activating gene 1 (RAG1) and RAG2 enzymes play essential roles in this complex process as well as in the rearrangement of T-cell receptor gene segments. A mutation of RAG1 or RAG2 leads to severe combined (B- and T-cell) immunodeficiency (19, 73, 125).
FIG. 1.
Overview of B-cell development and defects causing antibody deficiency. (A) Early B-cell development progresses independently of antigen contact with the sequential VDJ rearrangement of the IgM heavy chain (Igμ HC) followed by the Ig light chain (Ig LC). The pre-BCR is assembled from the rearranged IgM heavy chain, the components of the surrogate light chain (VpreB and λ5), and the signaling molecules Igα and Igβ. After successful recombination of the Ig LC, the BCR is formed. Defects in components of the pre-BCR or in signaling molecules downstream of the receptor (BTK and BLNK) lead to an early developmental block, resulting in agammaglobulinemia. (B) Defects in later stages of antigen-dependent B-cell differentiation result in diseases with different degrees of disrupted antibody production. VDJH, Igμ HC recombination of the V (VH), D, and J (JH) gene segments; VJL, Ig LC recombination of VL and JL segments. The exact point at which mutations in LRRC8 lead to a block in B-cell development has not yet been determined.
Productive immunoglobulin M (IgM) (μ) heavy-chain recombination leads to progression from the pro-B-cell stage to the pre-B-cell stage, which is characterized by the expression of a pre-BCR (Fig. 1A). In order to assemble a receptor prior to having rearranged its light-chain locus, the μ heavy chain forms an immunoglobulin-like receptor with the heterodimer of the proteins λ5 and VpreB, which have strong homology to the constant regions of Ig light chains and function as surrogate light chains. The pre-BCR is formed by the association of the μ heavy chain, the surrogate light-chain heterodimer, and the signal-transducing proteins Igα and Igβ (67, 71, 118).
The pre-BCR is a critical checkpoint for the regulation of B-cell development, since the expression of a pre-BCR and signaling through it are prerequisites for expansion and further differentiation. Signaling is likely mediated by ligand-independent oligomerization of the pre-BCR. The cytoplasmic kinase Bruton's tyrosine kinase (BTK), which is encoded on the X chromosome, plays a pivotal role in signal transduction downstream of the pre-BCR and, later, of the mature BCR. BTK is expressed in B cells at all stages of development except the plasma cell stage and is also expressed in myeloid cells and platelets.
Mutation of the BTK gene in humans leads to a failure of early B-cell maturation, causing X-linked agammaglobulinemia (XLA). Mutations in components of the pre-BCR and intracellular signaling machinery account for most of the remainder of the agammaglobulinemia subtypes identified in humans to date. These include mutations in the genes for the μ heavy chain, λ5, Igα, Igβ, and B-cell linker protein (BLNK), an adaptor protein downstream of Btk in the B-cell signaling cascade (8, 29). Another form of agammaglobulinemia arises from the disrupted function of the protein leucine-rich repeat containing 8 (LRRC8). Its role in B-cell development is unknown (124).
Following heavy-chain gene rearrangement, the B-cell precursor proceeds with rearrangement of the V and J segments of its κ or λ light-chain locus to produce a light-chain protein, which associates with the previously synthesized IgM heavy chain. The immature, IgM-expressing B cell leaves the bone marrow to complete its maturation in the spleen, becoming a mature, or naïve, B cell expressing IgM and IgD (Fig. 1B). Subsequent B-cell development to form memory B cells or plasma cells takes place in peripheral lymphoid organs upon exposure to antigen (73, 86).
Antigen-Dependent B-Cell Development
Driven by contact with antigen and T cells, the B cell undergoes a further diversification of its antibody repertoire in the peripheral lymphoid organs, mainly through the events of class switch recombination (CSR) and somatic hypermutation (SHM), which are responsible for the acquisition of different immunoglobulin isotypes and increased antigen specificity, respectively. The lymph node germinal centers are the primary sites for CSR, SHM, B-cell memory, and plasma cell generation.
CSR and SHM.
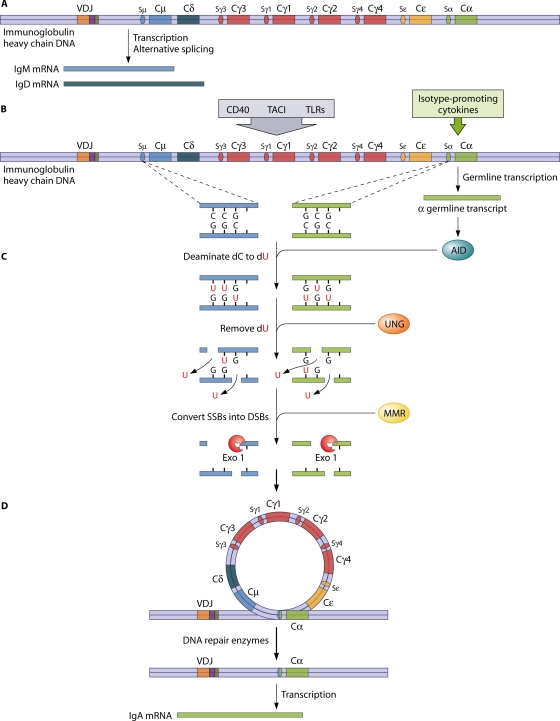
The immunoglobulin class or isotype is determined by its heavy-chain constant region (CH). IgM and IgD expressions occur through alternative splicing of a single mRNA encompassing the Cμ and Cδ genes (Fig. 2). The mechanism of class switching to the other isotypes (IgG, IgA, and IgE) is the recombination of the previously rearranged VDJ assembly with downstream CH regions by the intrachromosomal deletion of intervening segments, which are looped out as circular DNA. The recombination occurs between short GC-rich DNA sequences called switch (S) regions located upstream of each of the CH genes (other than Cδ), resulting in the substitution of Cμ or Cδ with either one of the subclasses of Cγ or Cα, or Cɛ, to produce the IgG and IgA subclasses, or IgE.
FIG. 2.
Molecular mechanisms of immunoglobulin CSR in B cells. As an example, isotype switching to IgA is shown (the human heavy-chain locus is depicted in a simplified way, not distinguishing the two IgA subclass constant regions). (A) After the productive rearrangement of heavy- and light-chain variable-region gene segments, nonswitched B cells produce IgM or IgD isotypes by alternative splicing. (B) Several B-cell activation signals, including the engagement of CD40, TACI, and TLRs, in combination with isotype-promoting cytokines lead to germ line transcription and the expression of AID. (C) In the S regions upstream of the heavy-chain genes, AID deaminates dC to dU, which is then removed by UNG. The resulting abasic sites are cleaved, creating SSBs that spontaneously form DSBs if they are close enough to each other. MMR is a second repair pathway that is thought to convert SSBs that are not near each other on opposite strands into DSBs through the recruitment of exonuclease 1 (Exo 1) and other proteins. (D) The intervening DNA is circularized and deleted. The two broken switch junctions are joined by the nonhomologous end-joining DNA repair machinery, and IgA is expressed.
In contrast, SHM targets the V regions of the immunoglobulins, introducing point mutations in the V genes of light- and heavy-chain loci at high frequency, permitting the selection of cells producing higher-affinity antibody required for neutralizing the infectivity and pathogenicity of some microbes and their toxins. Despite the distinct results of these two central processes of antigen-dependent B-cell maturation, they share several characteristics including activation signals and enzyme machinery for DNA cleavage and repair.
The transcription of non-protein-encoding, so-called “germ line” RNA transcripts is initiated in response to cytokines and specific activation signals, most importantly derived from CD40/CD40 ligand (CD40L) interactions but also from interactions of transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI [TNFRSF13B]) with its ligands and activation of Toll-like receptor (TLR)-mediated pathways (Fig. 2). The germ line transcripts form stable RNA-DNA hybrids with the template DNA strand, leaving both DNA strands accessible to the DNA-editing enzyme activation-induced cytidine deaminase (AID) (93).
AID deaminates dC to dU nucleotides, initiating a subsequent base excision repair process. The dU residues are removed after deglycosylation by uracil-N-glycosylase (UNG), leaving abasic sites. These are cleaved by ApeI endonuclease, resulting in nicked DNA strands. If single-strand DNA breaks (SSBs) are sufficiently near, double-strand breaks (DSBs) form due to the high density of GC base pairs in the switch regions. It has been shown that mismatch repair (MMR) proteins, which play a role primarily in meiosis, are also involved in CSR downstream of AID and UNG, with the likely function of creating DSBs, if the SSBs on opposite strands are not close enough to each other (128). The nicked ends are joined by a complex enzyme machinery that operates to repair DSBs caused by any means (enzymatic, chemical [e.g., by DNA-modifying chemotherapeutic drugs], or physical [ionizing radiation]). The process is called nonhomologous end joining; the same mechanism repairs the DNA breaks arising in VDJ recombination described above.
Genomic mutations in humans leading to an absence of the tumor necrosis factor (TNF) receptor family member CD40 (also called TNF receptor superfamily member 5 [TNFRSF5]) or its receptor CD40L (TNF superfamily member 5 [TNFSF5]) (also called CD154) or mutations of AID or UNG result in disorders characterized by defective CSR and SHM. These are collectively designated CSR defects, or hyper-IgM syndromes, due to the predominant production of IgM isotype antibodies as a consequence of a limited or absent capability of isotype switching (27, 93, 128).
Mutations in the gene encoding the MMR protein Pms2 were recently described for three patients with impaired isotype switching and a high rate of malignancies (101). Polymorphisms in the MMR protein MutS homolog 5 (Msh5) have been reported to be associated with IgA deficiency and common variable immunodeficiency (CVID) (126).
T-cell-dependent antibody responses.
The interaction of CD40 with its ligand CD40L is a central element in T-cell-dependent antibody responses. CD40, a transmembrane glycoprotein cell surface receptor, is constitutively expressed on the surface of B cells, dendritic cells, and macrophages. Its ligand, CD40L, is transiently expressed on the surface of T-helper cells after activation by antigen-presenting and costimulatory molecule-expressing dendritic cells. The engagement of CD40 on the B-cell surface leads to the transcription of target genes that result in B-cell clonal expansion, germinal-center formation, isotype switching, SHM, and the generation of long-lived memory cells and plasma cells. The formation of germinal centers depends largely on the presence of T-helper cells and CD40/CD40L interactions; hence, they form only during T-cell-dependent antibody responses to protein antigens (109).
Inducible costimulator (ICOS) is a costimulatory molecule upregulated on activated T effector cells. The interaction of ICOS with its ligand ICOSL (also known as B7RP-1, B7h, and B7-H2) on B cells in secondary lymphoid organs is thought to play a critical role in T-B-cell cooperation, which is required for generating T-cell-dependent antibody responses (81). A homozygous deletion in the ICOS gene was reported to be the first genetic defect causing CVID in humans (<1% of all cases). Germinal centers do not form in these patients, and numbers of switched memory B cells are markedly reduced (51).
T-cell-independent antigen responses.
While T-cell-dependent antibody responses take several days to form, T-cell-independent or thymus-independent (TI) antibody responses are characterized by a quick production of generally lower-affinity antibodies with limited isotype switching. TI antigens are nonprotein antigens such as polysaccharides, glycolipids, and nucleic acids. Therefore, they cannot be presented to T cells in the context of major histocompatibility complex molecules (136). TI antigens can elicit antibody responses through a variety of pathways, such as cross-linking of BCRs on the B-cell surface through repetitive antigenic epitopes, as is the case for bacterial capsular polysaccharides, or the activation of B cells through the engagement of TLR pathways, as has been shown for hypomethylated CpG-containing DNAs from bacteria and viruses through TLR9 (58, 136). In response to TLR activation, dendritic cells and B cells also upregulate and release the TNF superfamily factors B-cell-activating factor of the TNF family (BAFF) (TNFSF13B) and a proliferation-inducing ligand (APRIL) (TNFSF13) (see below) (76). These factors can mediate CD40/CD40L-independent isotype switching upon engagement with their common receptor, TACI, on B cells.
TACI receptor-ligand interactions.
The TNF family receptor TACI and its ligands BAFF and APRIL were recently found to play an essential role in mediating T-cell-independent antigen responses. TACI, BAFF, and APRIL are part of a complex system of TNF family receptors and ligands, which are involved in B-cell homeostasis, activation, and isotype switching. TACI is expressed mainly on B cells and is upregulated upon B-cell activation. APRIL and BAFF also bind to B-cell maturation antigen (BCMA) (TNFRSF17), which is not involved in class switching but promotes the survival of long-lived plasma cells. Additionally, BAFF binds to its own receptor, BAFF-R, driving B-cell development and survival (76). BAFF- and BAFF-R-deficient mice have a severe defect in B-cell development, with an almost complete absence of mature B cells. TACI-deficient mice and APRIL-deficient mice have an impaired ability to mount antibody responses to polysaccharide antigens and have decreased isotype switching to IgA (76, 80).
In humans, the engagement of TACI with its ligands BAFF and APRIL induces B-cell class switching to IgA, IgG, and IgE in the presence of isotype-specific cytokines (25). TACI can be upregulated by the engagement of innate receptors such as TLR9 (68). As further evidence of the synergy between TACI and TLRs in B-cell activation, a recent study by Xu et al. revealed that viral double-stranded RNA can induce CD40-independent isotype switching to IgA and IgG by engaging TLR3 in upper respiratory mucosal B cells, augmented by BAFF, which was found to be produced abundantly by surrounding mucosal dendritic cells (144).
TACI gene mutations have been identified in a subgroup of patients with CVID (see below) (24).
MZ and B1 B cells in response to polysaccharides.
Specialized subsets of B cells, marginal zone (MZ) B cells and B1 B cells, are mediators of the early T-cell-independent humoral immune response. MZ B cells reside in the spleen at the border of the white and red pulps in the periarteriolar lymphoid sheath, where they encounter and react to blood-borne pathogens as one of the first lines of host defense. Their response to polysaccharides makes them particularly important for the defense against potentially life-threatening but widely prevalent encapsulated bacteria. Polysaccharides can activate the complement system by the alternative pathway, generating complement fragments including C3d, which then, in conjunction with antigen, can further enhance B-cell activation (79). The complement receptor for C3d (CD21 [also called complement receptor 2]) is highly expressed on MZ B cells.
B1 B cells are a subset of B cells that are enriched in the peritoneum and pleural cavities and also have innate-like function with a restricted antibody repertoire specific to common pathogen structures, such as bacterial cell wall polysaccharides. They are thought to produce so-called “natural antibodies” to bacteria in naïve hosts and are capable of generating rapid responses to pathogens introduced to mucosal surfaces (57). TACI is upregulated on MZ and B 1 B cells and likely plays a role in the activation of these cell populations (80). Together, MZ and B1 B cells are thought to bridge the gap between innate and highly specific adaptive immune responses (57, 79).
The absence of these B-cell populations in asplenic patients and the immaturity of the splenic MZ in young children have been suggested to contribute to the decreased polysaccharide antibody responses and the resulting high susceptibility to encapsulated bacteria in these patient groups (137).
The B-cell surface protein CD19 cooperates in a cell surface complex with CD21 in lowering the BCR signaling threshold after the binding of immune complexes containing complement. Furthermore, CD19 appears to influence the differentiation of the B1 B-cell subset. CD19-deficient mice have decreased numbers of natural antibody-producing B1 B cells and are susceptible to infections with Streptococcus pneumoniae (52). CD19 deficiency has been recognized to cause CVID in a small number of patients (135).
B-cell memory.
Memory is a classical phenomenon in humoral immunity, characterized by accelerated antibody responses upon rechallenge with antigen in conjunction with increased antibody affinity for the antigen. It is mediated by memory B cells, which are generated in germinal centers in response to T-cell-dependent antigens. This population of B cells can be distinguished by expressing the surface marker CD27 (TNFRSF7), which interacts with its ligand CD70 (TNFSF7), which is expressed on T cells. Memory B cells can rapidly differentiate into antibody-secreting plasma cells and are poised to interact with T cells as a result of an efficient presentation of antigen in the context of major histocompatibility complex class II, a high level of expression of adhesion molecules on their surface, and the capacity for a rapid upregulation of the costimulatory molecules B7-1 and B7-2 (also called CD80 and CD86, respectively). Memory B cells circulate in a resting state and are capable of producing high-affinity, isotype-switched antibodies upon reexposure to antigen (112).
The continued presence of protective antibodies of a given specificity even after antigen has been eliminated is thought to be derived from long-lived plasma cells, which reside in survival niches in the bone marrow. If depleted, they can be replenished by the pool of memory B cells, suggesting that antigen contact or activation signals such as cytokines or TLR signals may chronically drive memory B cells to differentiate into long-lived antibody-secreting plasma cells (131).
Patients with CSR defects including CD40L, CD40, and AID deficiencies have decreased numbers of or absent class-switched memory B cells (93). Markedly decreased numbers of switched memory B cells are also found for a subgroup of patients with CVID, which is characterized by a higher rate of disease-associated complications (122, 138, 140).
ANTIBODY DEFICIENCY STATES
The differential diagnosis of hypogammaglobulinemia encompasses a variety of pathological conditions. Causes not related to primary immunodeficiency include nephrotic syndrome, protein-losing enteropathy, malabsorption, hematologic malignancies, chemotherapy, solid-organ transplantation, infectious diseases (e.g., human immunodeficiency virus and congenital rubella), and adverse effects of specific therapeutic drugs (95, 96). Hypogammaglobulinemia can be caused by prolonged courses of systemic corticosteroids, although specific antibody responses are not affected in most of these patients (72). Rituximab, a monoclonal antibody targeting the B-cell marker CD20, is now often used for the treatment of antibody-mediated autoimmune diseases and can cause long-lasting B-cell depletion and hypogammaglobulinemia. It is usually well tolerated, although rare cases of infectious complications characteristic of severe antibody deficiency have been reported (108). Other drugs that are known to cause hypogammaglobulinemia are anticonvulsants, antimalarial drugs, gold salts, sulfasalazine, d-penicillamine, and fenclofenac (95). Not all forms of secondary hypogammaglobulinemia are thought to confer an increased risk of infections, but this has not been well studied for the majority of conditions (97).
Hypogammaglobulinemia and impaired antibody responses are the hallmark of PAD disorders yet can also be due to combined immunodeficiency and can be part of the presentation of a number of specific immunodeficiencies that affect primarily the cellular immune compartment. The focus of this review is on PADs and the infectious complications characteristically associated with these disorders (Table 1).
TABLE 1.
Antibody deficiency disorders
| Disease | Laboratory feature(s) | Clinical feature(s) | Molecular defect(s) |
|---|---|---|---|
| Agammaglobulinemia (X linked and autosomal recessive) | Absent IgM, IgG, and IgA; B cells <2% of lymphocytes; absent specific antibody responses | Recurrent and severe bacterial infections, enteroviral infections, absent lymphoid tissue, some autoimmunity and malignancy | X linked (BTK), autosomal recessive (μ heavy chain, λ5, Igα, Igβ, BLNK, LRRC8), unknown in 5-10% of cases |
| CSR defects or hyper-IgM syndromes | Low IgG and IgA levels and normal-to-increased IgM levels, normal B-cell numbers, impaired specific antibody responses, decreased T-cell responses in CD40L/CD40 deficiency | Recurrent and severe bacterial infections, opportunistic infections and liver disease in CD40L/CD40 deficiency, lymphoid hyperplasia in AID/UNG deficiency, autoimmunity and malignancy | Intrinsic B-cell defects (AID, UNG), combined immunodeficiencies (CD40L, CD40) |
| CVID | Low IgG and variably low IgA and IgM levels, normal or decreased B-cell numbers, impaired specific antibody responses, variably decreased T-cell responses | Recurrent and severe bacterial infections, autoimmunity and malignancy, lymphoproliferative and granulomatous disease, gastrointestinal disorders | TACI, ICOS, CD19; unknown in 90% of cases |
| Selective IgA deficiency | IgA levels of <7 mg/dl and normal IgM and IgG levels, normal B-cell numbers, normal specific antibody responses in most patients | Usually asymptomatic; some association with atopy, autoimmunity, and gastrointestinal disorders | Unknown |
| IgG subclass deficiency | Low levels of one or more IgG subclasses; normal total IgG, IgM, and IgA levels; normal B-cell numbers; impaired specific antibody responses in some patients | Usually asymptomatic, some association with atopy and autoimmunity | Unknown |
| Specific antibody deficiency | Normal IgG, IgM, and IgA levels; impaired specific antibody responses (polysaccharides); normal B-cell numbers | Recurrent, predominantly respiratory tract bacterial infections | Unknown |
| THI | Low IgG levels and variably low IgA and rarely low IgM levels, normal specific antibody responses in most patients, normal B-cell numbers | Recurrent, predominantly respiratory tract bacterial infections | Unknown |
PRIMARY ANTIBODY DEFICIENCIES
Agammaglobulinemias
Agammaglobulinemias are rare antibody deficiencies caused by defects in early B-cell development and characterized by low numbers of or absent B cells, marked hypogammaglobulinemia, and increased susceptibility to infections.
XLA.
XLA accounts for ∼85% of known cases of agammaglobulinemia and is caused by a deficiency of the tyrosine kinase BTK (29). BTK mutations have been identified throughout the five functional domains of the gene, which is located in the middle portion of the X chromosome at Xq22. It is usually a fully penetrant, X-linked recessive disorder. However, milder atypical phenotypes have been described. Some mutations that permit the production of a small amount of functioning BTK protein lead to milder disease, but a completely consistent genotype-phenotype correlation has not been found (29).
The incidence of XLA in the United States has been estimated to be at least 1/190,000 male births. The age of onset of symptoms for most patients is between 3 months and 3 years, with over 50% of patients becoming symptomatic by 1 year of age and more than 90% of patients becoming symptomatic by 5 years of age (141). The transfer of maternal IgG through the placenta during gestation provides protection to affected infants for the first few months of life and can obscure early diagnoses.
T-cell numbers and function are normal for patients with XLA. Neutropenia, often severe, is found in 15 to 25% of patients at the time of diagnosis. Neutropenia appears to be a result of the patient's high bacterial burden related to associated infections, since it has been observed only in the presence of infections and resolves with antimicrobial and immunoglobulin therapy (39). Characteristic physical examination findings of XLA are paucity or absence of lymphatic tissue such as tonsils or palpable lymph nodes.
Noninfectious complications have been described for XLA, including a higher incidence of juvenile rheumatoid arthritis, aseptic polyarthritis, sensorineural hearing loss, and colorectal cancer (12, 20, 75). Autoimmune diseases such as Crohn's disease and type I diabetes mellitus have been described for a few patients with XLA, albeit much less frequently than for CVID (15). The occurrence of a dermatomyositis-like syndrome, marked by peripheral edema, erythematous rash, and evidence of inflammation of the skin and muscle on biopsy specimens, has been associated with disseminated enteroviral disease in patients with XLA (84) (see below).
At the time of diagnosis, most, but not all, patients have low serum immunoglobulin levels, with IgG levels of <200 mg/dl, IgA levels of <15 mg/dl, and IgM levels of <40 mg/dl (141). Almost all patients with classic XLA have markedly decreased B-cell numbers, with <2% of CD19- or CD20-positive lymphocytes in the blood. B-cell lymphopenia with normal T-cell numbers can be considered diagnostic in the context of a positive family history or if the mother is an identified carrier for the disease. Maternal carrier status can be established by an evaluation of maternal B cells for a pattern of nonrandom X-chromosome inactivation. If there is no family history of XLA, it can be helpful to measure BTK protein expression by Western blotting or by flow cytometric analysis of the patient's monocytes or platelets (47). Flow cytometric analysis for cytoplasmic BTK expression can also detect carrier females, revealing BTK-positive and BTK-negative populations (46). In cases of atypical XLA, in which a poorly functional BTK protein is expressed, genetic analysis of the BTK gene might be necessary to distinguish it from other forms of severe antibody deficiency such as CVID (70).
ARA.
In an estimated 10% of cases, agammaglobulinemia is not due to mutations in BTK; rather, it is inherited as an autosomal trait and is therefore classified as autosomal-recessive agammaglobulinemia (ARA). Underlying molecular defects are heterogeneous, with several key components of early B-cell development found to be affected in different individuals (Fig. 1). A specific defect has not been identified for approximately 5% of patients.
Clinical and laboratory features of ARA are essentially identical to those of XLA. ARA is suspected for females with agammaglobulinemia, for families with an autosomal pattern of inheritance, if consanguinity is found to be present, or if a BTK mutation has been excluded for an affected male (14, 29).
Immunodeficiency with Low IgG and Normal or High IgM Levels (Class Switch Recombination Defects)
The term hyper-IgM has been used to describe a group of disorders of CSR with characteristically elevated IgM levels and low levels of switched isotypes (IgG, IgA, and IgE). In the past decade, the molecular defects for the majority of these disorders have been identified, and their characterization has been instrumental in elucidating some of the central mechanisms of antibody isotype switching and SHM (Fig. 2).
X-linked hyper-IgM is caused by mutations of the gene encoding CD40L (TNFSF5). A very rare autosomal-recessive form with an identical phenotype is due to mutations in the gene encoding CD40 (TNFRSF5). Defective CD40L or CD40 expression results in a combined humoral and cellular immunodeficiency with absent or abortive germinal center formation as well as impaired interactions of T cells with dendritic cells. Patients usually present in infancy with severe and/or recurrent bacterial respiratory and gastrointestinal infections as well as opportunistic infections such as Pneumocystis jirovecii pneumonia, disseminated fungal infections, disseminated cytomegalovirus (CMV) and herpes simplex virus infections, and cholangitis due to Cryptosporidium parvum. Other clinical features are neutropenia, chronic anemia associated with erythrovirus (parvovirus B19) infections, and an increased incidence of gastrointestinal tumors (77, 93, 142).
Two forms of hyper-IgM with autosomal-recessive inheritance are caused by deficiencies of AID or UNG, both resulting in profoundly defective CSR. Patients present with recurrent or severe infections of the type classically associated with profound antibody deficiency. Lymphoid hyperplasia is present in about two-thirds of patients due to massive germinal center enlargement, which often manifests as prominent cervical lymphadenopathy and tonsillar hypertrophy. Patients have an increased incidence of autoimmune disease such as autoimmune hemolytic anemia and autoimmune thrombocytopenia (62, 105).
Mutations in the genes encoding the I-κB kinase γ chain (also called NF-κB essential modulator [NEMO]) or I-κB kinase α chain result in a distinct phenotype characterized by variable manifestations of ectodermal dysplasia (conical or absent teeth, sparse hair, frontal bossing, and decreased eccrine sweat glands) and susceptibility to mycobacterial infections in addition to antibody deficiency with often high serum IgM or IgA levels (63, 99). These disorders are sometimes classified as hyper-IgM syndromes. However, these defects include significant elements of T-cell and NK cell dysfunction and are best classified among the combined (cellular and humoral) immunodeficiencies.
Patients with a CD40L or CD40 deficiency have markedly reduced IgG levels (IgG levels of <250 mg/dl), often low IgA levels, and normal to elevated IgM levels (142). Among those patients with AID and UNG deficiency, IgG and IgA levels are generally profoundly decreased (IgG levels of <200 mg/dl and IgA levels of <20 mg/dl), and IgM levels are normal to increased (100 to 3,700 mg/dl) (105). Specific IgG responses are poor for all subtypes of CSR defects. Lymphocyte subset composition is normal. T-cell proliferative responses are normal except for patients with CD40L and CD40 deficiencies, who have impaired responses to recall antigens. CD40 and CD40L expression can be evaluated by flow cytometric analysis. Gene testing for mutations in CD40, CD40L, AID, UNG, and NEMO is available (14).
Common Variable Immunodeficiency
CVID is a heterogeneous disorder of B-cell differentiation and maturation with dysfunctional antibody production. Patients have markedly reduced serum concentrations of IgG, impaired specific antibody responses, and recurrent infections. With an estimated prevalence of 1 in 25,000 to 1 in 75,000 individuals, it is the most common of the more severe primary immunodeficiencies that come to medical attention (41, 54). A familial pattern of inheritance has been demonstrated for approximately 10 to 20% of patients with CVID (54). The variability of its phenotype and the incomplete penetrance of some of its associated molecular defects suggest that the etiology of CVID is multifactorial, with a combination of defects of B-cell differentiation resulting in antibody deficiency.
While the etiology remains unknown for the majority of patients, gene defects have been revealed for approximately 10 to 15% of CVID patients in recent years. Thus far, mutations or polymorphisms in four genes, two of which appear to affect only very few patients, have been described. Mutations in the gene encoding TACI have been identified for 8 to 10% of patients with CVID (24, 120). Two TACI sequence variants, Cys104Arg and Ala181Glu, have shown a significant association with CVID even in a heterozygous state. However, these TACI mutations are also present in some healthy individuals; hence, it has been proposed that additional genetic and/or environmental factors are needed to develop disease (76, 119).
Several single nucleotide polymorphisms in the gene encoding Msh5, an MMR protein with likely involvement in CSR, have recently been reported to occur more frequently in patients with CVID and IgA deficiency in a combined analysis of U.S. and Swedish cohorts (126). Again, these polymorphisms are also found in healthy individuals, and other potentially disease-modifying factors are yet to be determined.
The other two recently identified monogenetic defects are very rare causes for CVID but may be exemplary of how defects affecting different stages of antigen-dependent B-cell maturation can result in primary antibody production failure. Nine individuals with CVID due to ICOS deficiency have been reported. They all carry an identical large homozygous deletion in the ICOS gene and likely descend from a common ancestor (121). Four patients from two unrelated families who developed CVID due to homozygous mutations in the gene encoding the B-cell surface protein CD19 have been described. Their phenotype was characterized by normal total peripheral B-cell numbers but severely reduced numbers of memory B cells (135).
The age of presentation of CVID varies widely. According to a recent large European registry study of 334 patients, the most common age at the onset of symptoms was in the third decade, with a mean of 26.3 years and a median of 24 years. The mean age at diagnosis was 35.3 years, with a median of 33 years (26). This indicates that many patients suffer from their disease for many years before their immunodeficiency is recognized.
Patients often come to medical attention due to acute or chronic bacterial and, less frequently, viral infections. Noninfectious complications are also frequent, encompassing autoimmune diseases, lymphoproliferative disease, granulomatous disease, a spectrum of gastrointestinal disorders, as well as malignancies. Significant morbidity and mortality arise from chronic lung disease as a consequence of recurrent and chronic infections as well as granulomatous and lymphoid interstitial pneumonitis (32). Granulomatous disease is present in 5 to 10% of patients with CVID. Granulomas are of the noncaseating type and resemble those found in sarcoidosis. In many cases, patients are misdiagnosed with sarcoidosis before their antibody deficiency is detected and the diagnosis of CVID is made (40, 85). Although granulomas most commonly affect the lungs, they are also found in almost any other organ including skin, liver, spleen, bone marrow, and the gastrointestinal tract of patients (85). Hypertrophy of lymphoid tissue is common in CVID. Splenomegaly has been reported for 26% of patients according to one study (111).
The overall prevalence of autoimmune disease is estimated to be 20 to 30% and includes a wide spectrum of disorders. The most frequently encountered are idiopathic thrombocytopenic purpura, autoimmune hemolytic anemia, rheumatoid arthritis, and pernicious anemia (32, 64, 111). Both hematologic malignancies and solid tumors have an increased prevalence in patients with CVID. Particularly common are non-Hodgkin B-cell lymphoma and gastric cancer, with reported prevalences of approximately 2 to 8% and 1 to 2%, respectively (14, 69, 87). Studies have found no increased risk of malignancies among relatives of patients with CVID, suggesting that the immunodeficiency itself predisposes one to cancer (87).
For patients with CVID, IgG levels are reduced by greater than 2 standard deviations (SDs) below the mean for age. An associated reduction in either the IgA or IgM level has been included as part of the diagnostic criteria by some authors. Also essential for establishing the diagnosis is evidence of impaired specific antibody responses to infection or vaccine challenge. Peripheral B-cell numbers can be either normal or reduced. T-cell numbers and function are also reduced in some patients.
CVID remains predominantly a diagnosis of exclusion. Other specific immunodeficiency diagnoses have to be ruled out by careful evaluation of clinical features and laboratory phenotypes. In some cases, molecular testing may be required to distinguish other diseases with similar phenotypes, including CSR defects, agammaglobulinemia, and X-linked lymphoproliferative syndrome (caused by mutations in SH2D1A) (37). ICOS expression on T cells can be assessed by flow cytometric analysis; however, it is not routinely done due to the extraordinarily rare occurrence of this mutation. It may be considered for individuals who can trace their ancestry to the Black Forest region of Germany. The expression of CD19 is routinely assessed as part of the lymphocyte subset evaluation. In a rare individual with other clinical and laboratory features of CVID in whom no B cells are detected after staining for CD19, the presence of measurable numbers of B cells with analysis of another marker, such as CD20, may suggest the defect. TACI gene sequence analysis is commercially available.
The number of isotype-switched memory B cells in the peripheral blood has been found to be one of several useful parameters to distinguish distinct phenotypes of CVID. Reduced numbers of switched memory B cells (<2% of total B cells) have been shown to correlate with disease-associated complications such as splenomegaly and granulomatous disease (140). One study reported a significant correlation with autoimmune disease if the cutoff level for switched memory B cells was lowered to <0.55% of total B cells (122). A low percentage of naïve CD4 T cells also correlates with overall disease severity and splenomegaly (50).
IgA Deficiency
Selective IgA deficiency is a common immunological variant with a prevalence of 1:400 to 1:600 in the healthy U.S. population. Affected individuals are asymptomatic in up to 90% of cases (28). There is familial clustering of IgA deficiency with CVID, with 20 to 25% of individuals having a positive family history of either IgA deficiency or CVID. Some IgA-deficient patients progress over time to CVID. The pattern of inheritance of selective IgA deficiency is unclear (54). The underlying defect has also not been determined for the majority of patients. Patients with associated chromosomal abnormalities, particularly on chromosome 18, have been described. Acquired IgA deficiency has been associated with a relatively large number of medications (see above) (14).
Despite its association with a benign clinical course in most patients, a subgroup of IgA-deficient patients develops recurrent sinopulmonary and gastrointestinal infections typical of antibody deficiency, yet invasive infections such as meningitis or sepsis generally do not occur. IgA-deficient individuals are at an increased risk of developing autoimmune disease, particularly systemic lupus erythematosus and rheumatoid arthritis, and gastrointestinal disease such as inflammatory bowel disease and celiac disease (2). A higher prevalence of asthma and allergies has also been reported.
Selective IgA deficiency is defined as a serum IgA level below 7 mg/dl in the presence of normal serum IgG and IgM levels for a patient older than 4 years of age after other causes for hypogammaglobulinemia have been excluded. Young children can have a physiological delay in IgA production that they will outgrow and therefore are not considered deficient. The clinical presentation together with an evaluation of specific antibody responses should determine further management (14).
IgG Subclass Deficiency
IgG subclass deficiency is defined as an abnormally low level of one or more IgG subclasses (IgG1, IgG2, IgG3, or IgG4) with normal levels of total IgG and normal levels of the other immunoglobulin isotypes. It is sometimes associated with IgA deficiency (14).
The diagnosis of IgG subclass deficiency is controversial, since low levels of one or more IgG subclasses can be found in 2 to 20% of healthy individuals. Diagnosis is further complicated by the variation of IgG subclass levels with age and by different methods used in individual laboratories for determining the serum levels (17). The sequence of appearance of the different subclasses throughout childhood reflects the order of the respective heavy-chain constant-region genes on chromosome 14 (Fig. 2). Levels of IgG3 and IgG1 rise quickly during infancy, followed by delayed increases in IgG2 and IgG4 levels, with adult levels often not being reached before puberty. Since IgG2 is the isotype that is primarily responsible for responses against polysaccharides, it was postulated that a deficiency of this subclass in children would predispose patients to infections with encapsulated bacteria. However, some studies identified children with low IgG2 levels in the healthy population without evidence of increased susceptibility to infections (127). Another study revealed a resolution of IgG2 deficiency in all children observed over a time period of 6 years (6). The presence of symptoms seems to correlate rather with an impairment of specific antibody responses to vaccines or to natural exposure to pathogens (21). If patients are symptomatic, they present with recurrent sinopulmonary bacterial infections. Association with atopy and autoimmune disease has been reported, similarly to IgA deficiencies (14).
IgG subclass deficiency can be established if one or more IgG subclasses are 2 SDs below the age-adjusted norm, with normal total serum IgG levels. Only if associated infections are present is further immunological workup warranted, including evaluation of specific antigen responses to protein and polysaccharide antigens (21).
Specific Antibody Deficiency
Specific antibody deficiency (SAD) is characterized by impaired IgG responses to polysaccharides in the presence of normal serum concentrations of IgG, IgM, and IgA. It is therefore also called selective antibody deficiency with normal immunoglobulin. Patients have poor responses to polysaccharide antigens such as pneumococcal polysaccharide and Haemophilus influenzae type b capsular polysaccharide. The underlying molecular defect is not known. The prevalence of SAD is also unknown but is estimated to be high, with a few studies reporting 5 to 10% of children who are evaluated for recurrent infections being affected (61). Patients present with infections characteristic of PAD, with a predominance of recurrent upper and lower respiratory tract bacterial infections.
A diagnosis of SAD requires a demonstration of poor responses to polysaccharide vaccines in the context of normal serum immunoglobulin concentrations. It may be difficult to establish the diagnosis with confidence for young children below the age of 2 years, as they have less consistent responses to polysaccharide vaccine challenge. Abnormal responses to protein vaccines or evidence of further abnormal laboratory findings can be indicative of a more extensive defect and should prompt evaluation for other immunodeficiencies.
Transient Hypogammaglobulinemia of Infancy
Transplacentally acquired maternal antibodies protect the infant against pathogens until his or her own antibody production has reached sufficient levels. The hiatus between the loss of maternal antibodies and the onset of a robust antibody synthesis represents a physiological period of hypogammaglobulinemia, usually lasting from 3 months to about 6 months of age. Prolongation and accentuation of this phase with decreased levels of IgG and, in some cases, also IgA and IgM production until early childhood are considered to account for cases of transient hypogammaglobulinemia of infancy (THI). The term is somewhat misleading given that hypogammaglobulinemia persists until childhood in most cases and may not be transient in other cases but rather represents an early presentation of a more profound antibody deficiency such as CVID (33, 83). The delay in antibody production is sometimes associated with recurrent infections. Infectious manifestations include mostly upper respiratory tract infections and, less commonly, pneumonia. Rarely, invasive infections such as sepsis or meningitis have been reported (14).
Laboratory evaluation reveals serum IgG levels 2 SDs below the mean for age-matched controls. Serum levels of IgA and, less frequently, IgM can also be decreased. Evaluation should include specific antibody responses to vaccines and also flow cytometric quantitation of lymphocyte subsets to rule out more substantial defects. Specific antibody responses are most often normal in patients with transient hypogammaglobulinemia. Patients should be monitored over time until levels have normalized. The disease is self-limited by definition; however, medical intervention is indicated for some patients, and the diagnosis has to be revised for others (91).
Unspecified Antibody Deficiencies
Patients with primary hypogammaglobulinemia and intact cellular immunity who do not fulfill diagnostic criteria of any of the PADs are said to have unspecified hypogammaglobulinemia. Some of these individuals are predisposed to developing infections and have to be managed according to their clinical presentation, sometimes requiring intervention with antimicrobials and/or gammaglobulin (14).
INFECTIONS AND PRIMARY ANTIBODY DEFICIENCIES
Despite the heterogeneity in the clinical spectrum of diseases and age of presentation, the high prevalence of chronic or recurrent infections is shared by all clinically significant PADs. Pyogenic encapsulated bacteria, predominantly Streptococcus pneumoniae and Haemophilus influenzae, are overrepresented as the most commonly isolated pathogens for patients with PAD, which is reflective of the important role of antibodies in the opsonization of encapsulated bacteria. However, for subgroups of patients, commonly encountered infectious organisms also include Staphylococcus aureus, Pseudomonas spp., Mycoplasma spp., Enterobacteriaceae, Campylobacter, Giardia, and enteroviruses. In rare cases of CVID and XLA, severe infections with opportunistic pathogens such as Pneumocystis jirovecii and other fungi can occur (32, 74, 90, 103, 141).
Patients with a CSR defect due to a CD40L, CD40, or NEMO deficiency commonly present in early childhood with severe opportunistic infections such as Pneumocystis jirovecii pneumonia and gastrointestinal Cryptosporidium infections (93). This is a result of an additional profound impairment of cellular immunity associated with these disorders, which are therefore better classified as combined immunodeficiencies.
Even though at the time of diagnosis of PAD, the presence of infections is often established at one anatomical site, infections tend to occur at multiple sites over time. A review of 96 XLA patients showed that more than two-thirds of patients with upper respiratory tract infections at the time of diagnosis also developed infections of the lower respiratory tract and/or gastrointestinal tract (74).
Respiratory Infections
Almost all patients with PAD suffer from upper and lower respiratory tract bacterial infections, predominantly otitis media, sinusitis, and pneumonia. This is true for both pediatric and adult patients, although otitis media is less common in the adult patient population. If nasal cultures are obtained, they grow Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis for the majority of patients, often with evidence of more than one pathogen present. Concomitant infections with viruses, predominantly rhinovirus and enteroviruses, have been reported (22, 65). Sinusitis is chronic in most patients and often refractory to conventional antimicrobial therapy. Surgical intervention alone often provides only temporary relief (22, 103, 106).
Pneumonia is a common infectious manifestation of PAD. Studies of CVID revealed that at least two-thirds of patients had one or more episodes of pneumonia prior to diagnosis (32, 59). Patients are prone to pneumonia-associated complications; one study reported that more than half of CVID patients who developed pneumonia required hospitalization (23). In the majority of reported cases, acute pneumonia was treated empirically without isolation of a causative pathogen. If isolated, common pathogens are Streptococcus pneumoniae, Haemophilus influenzae type b, Haemophilus parainfluenzae, Mycoplasma spp., Pseudomonas spp., and Staphylococcus spp. (141). Evidence for an increased susceptibility to mycoplasma infections in PAD patients is the increased incidence of pneumonia caused by Ureaplasma urealyticum, which is rarely associated with nonurogenital infections in immunocompetent hosts (117). In rare cases of pneumonia in patients with XLA or CVID, other organisms have been isolated, including Pneumocystis jirovecii, Mycobacterium hominis, Mycobacterium avium, and adenovirus (15, 32, 114, 141). A case of measles pneumonia, which developed after exposure to wild-type virus, has been reported for a patient with XLA who had absent B cells (141).
Chronic lung disease is a well-recognized complication of PAD and one of the primary causes of morbidity and early mortality for patients with CVID and XLA (32, 66). Bronchiectasis is present in a large number of patients with CVID at the time of diagnosis, even younger patients (111). Some studies revealed that bronchiectasis correlated with a late diagnosis of PAD, suggesting that it develops as a consequence of chronic pulmonary infections prior to the initiation of aggressive therapy (103). However, chronic pulmonary complications, particularly in CVID, also stem from dysregulated inflammation and lymphoproliferation or autoimmune disease. These are manifested as granulomatous lung disease and interstitial lymphoid pneumonitis. Progression of chronic pulmonary changes remains a significant problem for patients even after the institution of immunoglobulin replacement therapy (66, 103, 106, 111).
Gastrointestinal and Hepatic Infections
Patients with PAD frequently suffer from gastrointestinal complications, with chronic or recurrent diarrhea being the most common clinical presentation sometimes accompanied by malabsorption and weight loss. In CVID, the differential diagnosis for gastrointestinal complaints is broad, with a high prevalence of noninfectious complications including nodular lymphoid hyperplasia, inflammatory bowel disease, atrophic gastritis, sprue-like illness with villous atrophy, and intestinal lymphangiectasia (32, 143). The reported incidence of infectious diarrhea for patients with CVID and XLA varies from 5% to approximately 30% (143). Giardia lamblia is the most frequently identified pathogen in the stool of symptomatic patients, and a resolution of symptoms upon antiprotozoal therapy has been reported (78). In mice, the elimination of Giardia from the gut has been shown to correlate with the onset of secretion of Giardia-specific IgA into the intestinal mucosa. A lack thereof might provide a potential explanation for the increased susceptibility of humans with PAD to giardiasis. Studies of humans have not consistently confirmed this observation, but data are scant (43).
Other pathogens identified in patients with infectious colitis include Rotavirus, Campylobacter, Enterovirus, Salmonella spp., and Clostridium difficile (141). Rare cases of Cryptosporidium and CMV infections of the small bowel have been reported for patients with CVID. Evidence of latent CMV infection has been found for the majority of patients with CVID, yet an increased risk of developing clinical CMV disease has not been clearly established for PAD patients (34). The occurrence of Cryptosporidium parvum infections is usually indicative of a T-cell defect in addition to antibody deficiency, as seen in patients with CD40L deficiency. Cryptosporidium infections of these patients are associated with the development of sclerosing cholangitis and severe chronic liver disease (115).
Cases of hepatitis C virus infection have been reported, most of which could be traced back to contaminated intravenous (i.v.) immunoglobulin or other blood preparations more than 15 years ago. Improved methods of donor screening and manufacturing of gammaglobulin have eliminated this particular risk (110). There have been no further cases of any disease transmission by purified immunoglobulin since that time.
Systemic Bacterial Infections
Agammaglobulinemic patients and, to a lesser extent, patients with CVID have an increased risk of developing blood-borne bacterial infections, particularly prior to the diagnosis and institution of adequate therapy with gammaglobulin. These are thought to arise from localized infections with secondary dissemination of bacteria through the bloodstream. About 6 to 10% of patients with XLA have been reported to develop sepsis, in most cases before a diagnosis of primary immunodeficiency is established, compared to approximately 1% of CVID patients. For both patient groups, the most commonly isolated bacteria in cases of sepsis are Pseudomonas spp., Streptococcus pneumoniae, and Haemophilus influenzae (32, 103, 141). Bacterial meningitis is caused predominantly by Streptococcus pneumoniae and Haemophilus influenzae type b and has been reported to occur in 3 to 4% of XLA patients and in 1 to 2% of CVID patients (10, 32, 64, 103, 141). Other reported causative bacteria were Pseudomonas spp., Neisseria meningitidis, Staphylococcus aureus, Escherichia coli, and Listeria monocytogenes (32, 59). Septic arthritis and osteomyelitis have been reported for 7% and 3% of XLA patients, respectively, and rarely for patients with CVID (32, 141).
Chronic Infectious Arthritis
Mycoplasma and Ureaplasma infections have been described to be a cause of chronic, often severe, erosive arthritis in patients with severe antibody deficiency due to either XLA or CVID (44, 45). Infections presented mostly as large-joint monoarthritis involving the knee, shoulder, elbow, or hip joints and, in a few cases, as symmetrical polyarthritis. Ureaplasma urealyticum, Mycoplasma pneumoniae, Mycoplasma hominis, Mycoplasma felis, Mycoplasma orale, and Mycoplasma salivarium have been isolated from synovial fluid of affected patients (16, 44, 45, 100). Antibody-deficient patients have been shown to have an increased rate of colonization of respiratory tract and urogenital tract mucosa with Mycoplasma and Ureaplasma organisms, which is likely a result of lacking protective antibodies at mucosal sites (45, 117). Why there is predilection for joint involvement remains unknown. Treatment consists of adequate immunoglobulin replacement combined with tetracyclines. The outcome is variable, with most patients responding to treatment but with some patients suffering from therapy-refractory disease with tetracycline-resistant organisms. The occasional resemblance to rheumatoid arthritis presents a diagnostic challenge given that seronegative noninfectious arthritis is also prevalent in the CVID population (44).
Enteroviral Infections
Although cell-mediated immunity plays a dominant role in host defense against most viruses, defense against enteroviruses appears to also depend on antibody-mediated mechanisms. This is evidenced by the increased susceptibility of severely antibody-deficient patients with XLA or, in rarer cases, CVID to severe, often fatal infections with enteroviruses including echoviruses, polioviruses, and coxsackieviruses (53, 84).
A few patients with XLA were reported to have developed vaccine-related poliomyelitis after vaccination with live attenuated poliovirus, which is associated with a high rate of mortality. Those who survived had severe neurological sequelae such as permanent paralysis (53). A case of wild-type poliovirus infection of a patient with XLA has also been described (123).
Chronic enteroviral meningoencephalitis of agammaglobulinemia is a recognized complication of patients suffering from severe antibody deficiency. It clinically presents either with slowly progressive neurological symptoms such as ataxia, paresthesias, loss of cognitive skills, developmental regression, and sensorineural hearing loss or, in other cases, acutely with fever, headaches, seizures, and/or paralysis (53, 84). Infections often disseminate to involve multiple organs, causing hepatitis, arthritis, and a dermatomyositis-like syndrome with edema of the subcutaneous (s.c.) tissue, erythematous rash, and myositis (84). Enteroviruses have been isolated from the cerebrospinal fluid of patients or, less commonly, from other anatomical sites such as joints and muscle and have rarely been found in the stool. Echoviruses are the most commonly encountered species, with a predominance of echovirus 11. In rare cases, coxsackievirus A or B could be identified. The virus may not grow in culture in all cases; the detection of viral nucleic acid by PCR may be diagnostic (139). Cerebrospinal fluid pleocytosis and abnormal protein or glucose levels are inconsistently found. Treatment usually consists of high-dose i.v. immunoglobulin. Intrathecal immunoglobulin applications have also been tried in severe cases, with variable outcomes (53, 89, 107). Fortunately, it appears that the incidence of chronic enteroviral meningoencephalitis of agammaglobulinemia has decreased significantly since it has become standard practice to maintain higher serum IgG doses for patients with PAD.
Conjunctivitis
An increased occurrence of acute and/or recurrent bacterial conjunctivitis has been reported, with an incidence of 8 to 20% for agammaglobulinemic patients; the reported numbers for patients with CVID vary (103, 141). The causative infectious agent, if isolated, has been reported for only a few cases, with Haemophilus influenzae being the predominantly isolated organism. Conjunctivitis is sometimes associated with more serious corneal ulceration and keratitis (55, 103). A single case report described bilateral Chlamydia trachomatis conjunctivitis with corneal scarring in a boy with XLA (3).
Cutaneous Infections
While the skin is a less common site for infections in the overall population of patients with humoral immunodeficiency, cutaneous infectious complications such as cellulitis and s.c. abscesses do occur with increased frequency in patients with XLA, with a reported incidence of 18 to 27%, and have also been encountered at an increased rate in cohorts of patients with CVID (56, 103, 141). Recurrent subacute and chronic skin infections with Flexispira-like organisms and also with Helicobacter and Campylobacter have been reported for patients with XLA. The spectrum of presentation extends from atypical cellulitis lacking overt signs of inflammation to chronic ulcerations often accompanied by bacteremia and systemic illness. Organisms are difficult to culture, and treatment typically requires prolonged courses of i.v. antibiotics (30, 49, 134). Severe herpes zoster virus manifestations are a known skin complication of CVID patients (32, 64). Apart from infectious problems, patients with CVID often suffer from noninfectious cutaneous complications associated with autoimmune conditions.
GENERAL DIAGNOSTIC CONSIDERATIONS
Awareness of PAD remains limited among health care professionals, which is likely due to the presumed rarity of these diseases. A large retrospective study of CVID patients revealed a mean diagnostic delay of 7 years, with 20% of patients being diagnosed more than 15 years after the onset of symptoms (26).
Who Should Be Screened for PAD?
Patients presenting with infections that are characterized by either an increased frequency of occurrence, unusual severity, or lack of response to therapy should be evaluated for an underlying immunodeficiency. As described above, the majority of infections associated with PAD are caused by pathogens that are prevalent in the community, most commonly, but not exclusively, by encapsulated bacteria.
Children may also present with poor growth, failure to thrive, or recurrent fever of unknown origin in addition to recurrent infections of the respiratory or gastrointestinal tracts (143). Congenital absence of tonsillar tissue should raise the suspicion of agammaglobulinemia. The age of disease onset is very variable. More severe forms of PAD present during infancy or early childhood; the most severe defects present around the age of 6 months, when maternal antibodies have dissipated. However, some common forms of PAD often do not manifest until early adulthood to mid-adulthood (32, 111).
In a number of cases, the presenting symptoms are related to noninfectious complications such as autoimmune disease in CVID patients. As an example, a study of 21 patients with CVID and immune thrombocytopenic purpura revealed that CVID was diagnosed in only 19% of cases prior to the onset of immune thrombocytopenic purpura (88). Other clinical features associated with PAD can be clues for diagnosis, such as granulomatous disease, splenomegaly, unexplained chronic lung disease, unexplained chronic gastrointestinal disease, or inflammatory arthropathy (32, 64, 111). Patients with suspected antibody deficiency should be referred to a clinical immunologist.
Initial Evaluation
A detailed clinical history, including family history, is critical for the evaluation of a patient with suspected PAD. In addition to the physical examination, radiographic evaluation of target organs such as lungs or sinuses may be indicated. A systematic, stepwise approach to the laboratory evaluation of the humoral immune system is recommended, particularly since initial screening tests are easy to perform. A complete blood count is essential to determine the lymphocyte count and the presence of other possibly associated cytopenias. Initial laboratory testing includes quantitative serum immunoglobulin levels (IgM, IgG, and IgA) and evaluation of specific antibody responses to both protein and polysaccharide antigens (92). It is important to interpret serum immunoglobulin levels according to age of pediatric patients. IgG during early infancy is derived from placental transfer; therefore, serum levels do not reflect the infant's own level of production; immunoglobulin serum levels for some isotypes and isotype subclasses often do not reach adult levels until early adolescence.
Specific Antibody Responses
Antibodies against tetanus and diphtheria toxoids are indicative of functional T-cell-dependent antibody responses. The same is true for antibodies against the polyribose phosphate capsular antigen of Haemophilus influenzae type b and capsular polysaccharides of Streptococcus pneumoniae when elicited by vaccines in which these moieties are coupled to a protein carrier. Antibodies against S. pneumoniae produced after natural exposure or vaccination with the unconjugated 23-valent pneumococcal polysaccharide vaccine are indicative of T-cell-independent responses.
Specific antibody titers must be interpreted in the context of the patient's age and immunization history. There are no well-validated criteria to define age-specific normal vaccine responsiveness to pneumococcal polysaccharides. It is current practice to evaluate postimmunization serotype-specific IgG antibody concentrations with respect to the increase over the preimmunization level for a given serotype and the percentage of serotypes to which a patient responds. A postimmunization serum antibody concentration of >1.0 to 1.3 μg/ml (note that this may not be the correct value for all methods of measurement) or at least fourfold over baseline is considered to be an adequate response (14). Polysaccharide responses cannot be tested reliably for children below the age of 2 years; some may respond, but many healthy children will have low responses. Between the ages of 2 and 5 years, children should respond to at least half of the vaccinated pneumococcal serotypes, and individuals older than 5 years should respond to 70% of serotypes. If titers are low, patients might receive a booster immunization with a subsequent determination of responses 3 to 6 weeks later (14). For some patients, serum isohemagglutinins (IgG and IgM antibodies against ABO blood group antigens, which occur naturally in response to polysaccharide antigens of the gut flora) are measured, but these are generally considered to be less reliable than the assessment of vaccine antibody responses for evaluating humoral immunodeficiency. It has to be noted that nonresponsiveness to some vaccines can also be found in healthy individuals with otherwise intact humoral immunity. For example, the nonconversion rate following a full series of immunizations with the hepatitis B virus vaccine is about 5 to 15% for healthy individuals and has been linked to the presence of specific HLA haplotypes (4). Primary nonresponsiveness to the hepatitis B virus vaccine is diagnosed for a healthy immunocompetent individual who fails to seroconvert following two full series of hepatitis B virus immunizations (82). For these patients, a more global PAD can be ruled out by the demonstration of otherwise normal vaccination responses.
Further Laboratory Evaluation
The specific diagnostic criteria for the different forms of PAD are discussed in their respective sections. If immunoglobulin serum levels and antibody responses to specific antigens are normal for patients with susceptibility to encapsulated bacteria, alternative diagnoses such as anatomical defects, ciliary dyskinesia, complement deficiencies, or other rare defects of innate immune mechanisms must be considered.
Patients with significant hypogammaglobulinemia should have their lymphocyte subset numbers and distributions determined (14, 92). Flow cytometric analysis is now widely available to enumerate T-cell, B-cell, and NK cell subsets and will identify patients with agammaglobulinemia. Patients with CVID might benefit from a further characterization of their specific phenotype. Several classification schemes for CVID have been proposed on the basis of B-cell and T-cell immunophenotyping (50, 102, 138, 140). Clinical or laboratory features that are suggestive of defects of cellular immunity or of a combined defect, including T-cell lymphopenia, opportunistic infections, or failure of young children to thrive, might warrant an assessment of T-cell function by testing of lymphocyte proliferative responses to recall antigens and mitogens.
Analysis of specific protein expression by Western blotting or flow cytometry is required for the diagnosis of some PADs. Gene sequencing is commercially available for many of the known gene defects and, depending on the presenting clinical and laboratory features, may be indicated to confirm or rule out specific diagnoses. Whenever possible, molecular diagnosis is desirable for accurate genetic counseling.
MANAGEMENT
As infections and infection-related complications constitute the predominant clinical problems that arise as a consequence of antibody deficiency, management focuses largely on the prevention and treatment of infections as well as monitoring for early detection of associated complications. Antibody replacement therapy and antimicrobial therapy thus play central roles in the care of patients with PAD. Surveillance for and treatment of noninfectious complications such as autoimmunity and malignancies are also critical elements in the management of primary disorders of humoral immunity; these were reviewed elsewhere previously (31). Periodic follow-up of patients with antibody deficiency is recommended to detect complications that can occur despite adequate treatment with gammaglobulin and/or antimicrobials.
Immunoglobulin Replacement Therapy
Several studies have demonstrated the efficacy of gammaglobulin replacement therapy for decreasing the incidence of upper and lower respiratory tract infections and reducing the use of antimicrobials and the rate of hospital admissions for patients with PAD (23, 106, 129). It is generally accepted that patients with significantly diminished levels of specific antibody production require replacement therapy with immunoglobulin (98). Replacement therapy is the mainstay of treatment for PADs at the severe end of the spectrum, including agammaglobulinemia, CVID, and CSR defects (or hyper-IgM syndromes), which are all characterized by different degrees of hypogammaglobulinemia, impaired specific antibody production, and susceptibility to recurrent and/or severe infections. Early diagnosis and institution of immunoglobulin therapy for these patients have been demonstrated to be crucial not only for the prevention of acute infections such as pneumonia or meningitis but also for decreasing the morbidity and mortality associated with chronic complications such as chronic lung disease or chronic enteroviral infections (36, 64, 103, 105, 116, 142).
The decision to treat patients with less pronounced defects of antibody production with immunoglobulin is based upon the patient's clinical and laboratory presentation and response to conventional antimicrobial therapy. IgG replacement therapy is generally recommended under these conditions if failure to mount specific antibody responses has been documented and if there is evidence of recurrent or severe infections that are inadequately controlled with antimicrobial therapy. Significant infectious morbidity usually has to be present, such as recurrent pneumonias that predispose a patient to bronchiectasis or recurrent episodes of documented bacterial sinusitis (14, 98).
Patients with milder antibody deficiencies (particularly children, who require treatment with immunoglobulin) may be reevaluated at some time after initiation of therapy if they are doing well. Replacement therapy is preferably discontinued in spring or summer, when the incidence of respiratory infections is low; antibody responses can be tested 4 to 6 months later. In some cases, clinical course and vaccine challenge responses improve over time; in others, therapy has to be reinstituted (14, 98).
While the majority of symptomatic patients with THI can be managed with antibiotic prophylaxis, those who fail or do not tolerate antimicrobial treatment may benefit from short-term gammaglobulin replacement but should be reevaluated periodically (14). IgG subclass deficiency and selective IgA deficiency per se are not indications for replacement therapy, but it might be warranted in cases in which poor specific antibody production can be demonstrated in the context of otherwise insufficiently controlled recurrent infections and inadequate responses to antibiotics.
Patients with agammaglobulinemia or severe hypogammaglobulinemia (<200 mg/dl) might require a loading dose (e.g., 1 dose of 1 g/kg body weight or divided into separate doses). For maintenance therapy of PAD, a starting dose of 400 to 600 mg/kg/month is generally recommended. This is administered i.v. every 3 to 4 weeks or s.c. once weekly or every other week. The mean physiological elimination half-life for IgG is approximately 23 days, with a wide variability, ranging from about 20 to 60 days, observed among individuals receiving i.v. or s.c. replacement therapy (13). Generally, doses and intervals are adjusted to keep serum trough levels at least above 500 mg/dl. However, the replacement regimen has to be individualized for each patient by also taking into account the specific underlying condition and the clinical response to treatment. Some studies have shown that higher-than-standard doses are beneficial for selected patients (38, 116). Maintenance of increased IgG trough levels (>800 mg/dl) might be indicated for patients with chronic lung disease or and/or chronic refractory sinusitis. Both conditions present a challenge in the treatment of PAD, since progression of disease even despite adequate therapy has been reported (66, 106). Whether or not sustaining higher IgG trough levels can consistently prevent the occurrence or progression of these complications remains to be determined.
Specific infectious conditions may require a temporary institution of even higher doses of immunoglobulin therapy, e.g., treatment of XLA patients suffering from chronic enteroviral meningoencephalitis, where maintaining IgG trough levels of >1,000 mg/dl has shown some success (84, 89). For patients with SAD who have quantitatively normal total serum IgG levels, obtaining trough levels is not helpful; therefore, treatment has to be titrated according to the clinical response.
s.c. administration of IgG has been shown to be safe and at least as efficacious as i.v. infusion of IgG in treating PAD and is gaining popularity as an alternative to i.v. administration of IgG (42, 48). Its main advantages are fewer systemic adverse effects, smaller fluctuations in IgG serum concentrations, and improved quality of life for patients due mostly to the convenience of self-administration at home (7, 11). It should be considered in particular for patients with reactions to i.v. administration of IgG or difficult venous access. Local side effects (erythema, swelling, and tenderness) occur commonly with s.c. administration of IgG. These are mostly mild and resolve within 24 h. Only rarely do they cause significant discomfort. The total amount of IgG administered per month is generally the same for both i.v. administration of IgG and s.c. administration of IgG. The more frequent s.c. dosing of IgG is necessitated by the limited amount of medication that can be accommodated s.c. (7, 11).
Prophylactic Antibiotics
Many patients with a milder phenotype of PAD, including patients with THI, selective IgA deficiency, or IgG subclass deficiency, who present with recurrent upper respiratory infections can be managed successfully with antibacterial chemoprophylaxis only (9, 60). It might be particularly helpful for patients with mild transient deficiency. Thus, prophylaxis with antibiotics is considered to be the initial mode of therapy for most patients with THI and is often required only during the winter months for a few years (14).
Many experts recommend antibiotic prophylaxis in addition to immunoglobulin replacement for patients with CVID or XLA who have chronic infectious complications that are difficult to control, such as chronic sinusitis or bronchiectasis. The presumed lack of transport of infused IgG to mucosal surface sites and the lack of IgM and IgA in some patients have been postulated to contribute to the persistence of infections in spite of replacement with gammaglobulin (103). There are no published controlled trials examining the potential benefits of additional antibiotic prophylaxis for patients who are treated with gamma globulin. However, the effectiveness in reducing pulmonary complications was demonstrated in a retrospective analysis when treatment was used in conjunction with intramuscular immunoglobulin prior to the availability of the currently used i.v. or s.c. preparations (130).
Standard prophylactic antibiotic regimens for PAD are derived largely from data from studies of immunocompetent patients with recurrent upper respiratory infections (35, 104). Prophylaxis is usually initiated with amoxicillin (amoxicilline), trimethoprim-sulfamethoxazole, or azithromycin. If not effective, other agents such as amoxicillin-clavulanate or clarithromycin (or others) may be used. Some practitioners use full therapeutic doses, and others use half-doses. Some rotate preventive antibiotics every 1 to 6 months, and others maintain therapy with a single agent. There are no published data on the effectiveness of any specific regimen for this patient population.
Treatment of Infections
General principles that guide the management of infections in immunocompetent individuals, such as the early recognition of specific symptoms, precise definition of the focus of infection, as well as identification of the causative organism with the help of imaging, cultures, and/or biopsy specimen data, if necessary, also apply to PADs. Antimicrobial treatment should be based on susceptibilities, if possible. Some patients need longer courses of antibiotics than immunocompetent individuals, and vigilance has to be exercised in making sure that infections are completely eradicated at the end of a treatment course. Bronchiectasis is highly prevalent in patients with CVID and XLA and is often characterized by insidious onset and silent progression (66). Infections in the context of bronchiectasis usually require intensified treatment, often in a multidisciplinary setting involving adequate pulmonary airway clearing, culture-guided aggressive antimicrobial therapy, anti-inflammatory therapy, and monitoring of disease progression by pulmonary function testing and, at periodic intervals, by computerized tomography (or high-resolution computerized tomography) (14, 66, 133). Finally, the increased prevalence of certain infections in PAD (such as Giardia enteritis, arthritis due to Mycoplasma or Ureaplasma, or cutaneous infections with Flexispira-like organisms) has to be taken into consideration for the choice of microbiological identification techniques and antimicrobial selection. As serology is unreliable for detecting infections of patients with PAD, culture- or molecular-based assays often have to be used. For many infectious organisms such as Mycoplasma or Ureaplasma species, conventional PCR or real-time PCR is available, providing equivalent or superior sensitivity for the detection of acute infection compared to serology and culture (1, 132).
CONCLUSION
Recent discoveries of some of the molecular defects that cause PAD have shed much light on central pathways of B-cell biology, such as the molecular mechanisms of CSR and the regulation of terminal B-cell differentiation. Our rapidly growing understanding of the underlying pathophysiology of these diseases will likely have many implications for diagnosis and management in the near future.
Significant advances in the preparation of immunoglobulin and in its administration have made treatment safe and highly effective. Timely diagnosis and early onset of replacement therapy with immunoglobulin have been shown to significantly decrease the morbidity and mortality associated with diseases such as XLA and CVID. The recognition and accurate diagnosis of the milder forms of antibody deficiency are also of great importance. Most of these patients will benefit from early and aggressive antibiotic therapy. Some patients require prevention with antibiotic prophylaxis or immunoglobulin replacement, which can break the cycle of recurring infection, often resulting in a greatly improved quality of life.
Biography
 Ari J. Fried obtained his medical degree from the Christian-Albrechts-University of Kiel, Germany, in 2001. After medical school, he conducted research as a fellow in the field of transplantation immunology at the University of Iowa and completed a residency in Pediatrics at the University of Iowa Hospitals and Clinics. In 2007, he started a fellowship in Allergy/Immunology at Children's Hospital Boston. In his second year of fellowship, he joined the basic science laboratory of Raif S. Geha, where he currently studies the pathogenesis of common variable immunodeficiency with a focus on mutations in the TACI gene. His research and clinical interests lie in the study of the molecular defects underlying primary immunodeficiencies (PIDs) and in the treatment of patients with PID.
Ari J. Fried obtained his medical degree from the Christian-Albrechts-University of Kiel, Germany, in 2001. After medical school, he conducted research as a fellow in the field of transplantation immunology at the University of Iowa and completed a residency in Pediatrics at the University of Iowa Hospitals and Clinics. In 2007, he started a fellowship in Allergy/Immunology at Children's Hospital Boston. In his second year of fellowship, he joined the basic science laboratory of Raif S. Geha, where he currently studies the pathogenesis of common variable immunodeficiency with a focus on mutations in the TACI gene. His research and clinical interests lie in the study of the molecular defects underlying primary immunodeficiencies (PIDs) and in the treatment of patients with PID.
 Francisco A. Bonilla received his M.D. degree from the Mount Sinai Medical School and his Ph.D. from the City University of New York, both in New York, NY. He completed a residency in Pediatrics at the Floating Hospital for Infants & Children of the Tufts University New England Medical Center and a fellowship in Allergy/Immunology at Children's Hospital Boston. He is currently the Director of the Clinical Immunology Program at Children's Hospital Boston and Assistant Professor of Pediatrics at Harvard Medical School. He is the Secretary of the Basic and Clinical Immunology Interest Section, a former Chair and current member of the Primary Immunodeficiency Diseases Committee, and a Fellow of the American Academy of Allergy, Asthma & Immunology.
Francisco A. Bonilla received his M.D. degree from the Mount Sinai Medical School and his Ph.D. from the City University of New York, both in New York, NY. He completed a residency in Pediatrics at the Floating Hospital for Infants & Children of the Tufts University New England Medical Center and a fellowship in Allergy/Immunology at Children's Hospital Boston. He is currently the Director of the Clinical Immunology Program at Children's Hospital Boston and Assistant Professor of Pediatrics at Harvard Medical School. He is the Secretary of the Basic and Clinical Immunology Interest Section, a former Chair and current member of the Primary Immunodeficiency Diseases Committee, and a Fellow of the American Academy of Allergy, Asthma & Immunology.
REFERENCES
- 1.Abele-Horn, M., C. Wolff, P. Dressel, A. Zimmermann, W. Vahlensieck, F. Pfaff, and G. Ruckdeschel. 1996. Polymerase chain reaction versus culture for detection of Ureaplasma urealyticum and Mycoplasma hominis in the urogenital tract of adults and the respiratory tract of newborns. Eur. J. Clin. Microbiol. Infect. Dis. 15:595-598. [DOI] [PubMed] [Google Scholar]
- 2.Alaswad, B., and P. Brosnan. 2000. The association of celiac disease, diabetes mellitus type 1, hypothyroidism, chronic liver disease, and selective IgA deficiency. Clin. Pediatr. (Philadelphia) 39:229-231. [DOI] [PubMed] [Google Scholar]
- 3.al Ghonaium, A., J. B. Ziegler, and D. Tridgell. 1996. Bilateral chronic conjunctivitis and corneal scarring in a boy with X-linked hypogammaglobulinaemia. J. Paediatr. Child. Health 32:463-465. [DOI] [PubMed] [Google Scholar]
- 4.Alper, C. A. 1995. The human immune response to hepatitis B surface antigen. Exp. Clin. Immunogenet. 12:171-181. [DOI] [PubMed] [Google Scholar]
- 5.Alt, F. W., T. K. Blackwell, and G. D. Yancopoulos. 1987. Development of the primary antibody repertoire. Science 238:1079-1087. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson, A. R., and C. M. Roifman. 2007. Low serum immunoglobulin G2 levels in infancy can be transient. Pediatrics 120:e543-e547. [DOI] [PubMed] [Google Scholar]
- 7.Ballow, M. 2008. Immunoglobulin therapy: methods of delivery. J. Allergy Clin. Immunol. 122:1038-1039. [DOI] [PubMed] [Google Scholar]
- 8.Bankovich, A. J., S. Raunser, Z. S. Juo, T. Walz, M. M. Davis, and K. C. Garcia. 2007. Structural insight into pre-B cell receptor function. Science 316:291-294. [DOI] [PubMed] [Google Scholar]
- 9.Barlan, I. B., R. S. Geha, and L. C. Schneider. 1993. Therapy for patients with recurrent infections and low serum IgG3 levels. J. Allergy Clin. Immunol. 92:353-355. [DOI] [PubMed] [Google Scholar]
- 10.Basile, N., S. Danielian, M. Oleastro, S. Rosenzweig, E. Prieto, J. Rossi, A. Roy, and M. Zelazko. 2009. Clinical and molecular analysis of 49 patients with X-linked agammaglobulinemia from a single center in Argentina. J. Clin. Immunol. 29:123-129. [DOI] [PubMed] [Google Scholar]
- 11.Berger, M. 2008. Subcutaneous administration of IgG. Immunol. Allergy Clin. N. Am. 28:779-802. [DOI] [PubMed] [Google Scholar]
- 12.Berlucchi, M., A. Soresina, L. O. Redaelli De Zinis, L. Valetti, R. Valotti, V. Lougaris, A. Meini, D. Salsi, P. Nicolai, and A. Plebani. 2008. Sensorineural hearing loss in primary antibody deficiency disorders. J. Pediatr. 153:293-296. [DOI] [PubMed] [Google Scholar]
- 13.Bonilla, F. A. 2008. Pharmacokinetics of immunoglobulin administered via intravenous or subcutaneous routes. Immunol. Allergy Clin. N. Am. 28:803-819. [DOI] [PubMed] [Google Scholar]
- 14.Bonilla, F. A., I. L. Bernstein, D. A. Khan, Z. K. Ballas, J. Chinen, M. M. Frank, L. J. Kobrynski, A. I. Levinson, B. Mazer, R. P. Nelson, Jr., J. S. Orange, J. M. Routes, W. T. Shearer, and R. U. Sorensen. 2005. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann. Allergy Asthma Immunol. 94:S1-S63. [DOI] [PubMed] [Google Scholar]
- 15.Bonilla, F. A., and R. S. Geha. 2004. Immunodeficiencies caused by B cell defects, p. 403-416. In T. Honjo, F. W. Alt, and M. S. Neuberger (ed.), Molecular biology of B cells. Elsevier Academic Press, San Diego, CA.
- 16.Bonilla, H. F., C. E. Chenoweth, J. G. Tully, L. K. Blythe, J. A. Robertson, V. M. Ognenovski, and C. A. Kauffman. 1997. Mycoplasma felis septic arthritis in a patient with hypogammaglobulinemia. Clin. Infect. Dis. 24:222-225. [DOI] [PubMed] [Google Scholar]
- 17.Bossuyt, X., G. Marien, I. Meyts, M. Proesmans, and K. De Boeck. 2005. Determination of IgG subclasses: a need for standardization. J. Allergy Clin. Immunol. 115:872-874. [DOI] [PubMed] [Google Scholar]
- 18.Boyle, J. M., and R. H. Buckley. 2007. Population prevalence of diagnosed primary immunodeficiency diseases in the United States. J. Clin. Immunol. 27:497-502. [DOI] [PubMed] [Google Scholar]
- 19.Brack, C., M. Hirama, R. Lenhard-Schuller, and S. Tonegawa. 1978. A complete immunoglobulin gene is created by somatic recombination. Cell 15:1-14. [DOI] [PubMed] [Google Scholar]
- 20.Brosens, L. A., K. M. Tytgat, F. H. Morsink, R. J. Sinke, I. J. Ten Berge, F. M. Giardiello, G. J. Offerhaus, and J. J. Keller. 2008. Multiple colorectal neoplasms in X-linked agammaglobulinemia. Clin. Gastroenterol. Hepatol. 6:115-119. [DOI] [PubMed] [Google Scholar]
- 21.Buckley, R. H. 2002. Immunoglobulin G subclass deficiency: fact or fancy? Curr. Allergy Asthma Rep. 2:356-360. [DOI] [PubMed] [Google Scholar]
- 22.Buehring, I., B. Friedrich, J. Schaaf, H. Schmidt, P. Ahrens, and S. Zielen. 1997. Chronic sinusitis refractory to standard management in patients with humoral immunodeficiencies. Clin. Exp. Immunol. 109:468-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Busse, P. J., S. Razvi, and C. Cunningham-Rundles. 2002. Efficacy of intravenous immunoglobulin in the prevention of pneumonia in patients with common variable immunodeficiency. J. Allergy Clin. Immunol. 109:1001-1004. [DOI] [PubMed] [Google Scholar]
- 24.Castigli, E., S. A. Wilson, L. Garibyan, R. Rachid, F. Bonilla, L. Schneider, and R. S. Geha. 2005. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat. Genet. 37:829-834. [DOI] [PubMed] [Google Scholar]
- 25.Castigli, E., S. A. Wilson, S. Scott, F. Dedeoglu, S. Xu, K. P. Lam, R. J. Bram, H. Jabara, and R. S. Geha. 2005. TACI and BAFF-R mediate isotype switching in B cells. J. Exp. Med. 201:35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapel, H., M. Lucas, M. Lee, J. Bjorkander, D. Webster, B. Grimbacher, C. Fieschi, V. Thon, M. R. Abedi, and L. Hammarstrom. 2008. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood 112:277-286. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhuri, J., and F. W. Alt. 2004. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat. Rev. Immunol. 4:541-552. [DOI] [PubMed] [Google Scholar]
- 28.Clark, J. A., P. A. Callicoat, N. A. Brenner, C. A. Bradley, and D. M. Smith, Jr. 1983. Selective IgA deficiency in blood donors. Am. J. Clin. Pathol. 80:210-213. [DOI] [PubMed] [Google Scholar]
- 29.Conley, M. E., A. Broides, V. Hernandez-Trujillo, V. Howard, H. Kanegane, T. Miyawaki, and S. A. Shurtleff. 2005. Genetic analysis of patients with defects in early B-cell development. Immunol. Rev. 203:216-234. [DOI] [PubMed] [Google Scholar]
- 30.Cuccherini, B., K. Chua, V. Gill, S. Weir, B. Wray, D. Stewart, D. Nelson, I. Fuss, and W. Strober. 2000. Bacteremia and skin/bone infections in two patients with X-linked agammaglobulinemia caused by an unusual organism related to Flexispira/Helicobacter species. Clin. Immunol. 97:121-129. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham-Rundles, C. 2008. Autoimmune manifestations in common variable immunodeficiency. J. Clin. Immunol. 28(Suppl. 1):S42-S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunningham-Rundles, C., and C. Bodian. 1999. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin. Immunol. 92:34-48. [DOI] [PubMed] [Google Scholar]
- 33.Dalal, I., B. Reid, E. Nisbet-Brown, and C. M. Roifman. 1998. The outcome of patients with hypogammaglobulinemia in infancy and early childhood. J. Pediatr. 133:144-146. [DOI] [PubMed] [Google Scholar]
- 34.Daniels, J. A., H. M. Lederman, A. Maitra, and E. A. Montgomery. 2007. Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): a clinicopathologic study and review. Am. J. Surg. Pathol. 31:1800-1812. [DOI] [PubMed] [Google Scholar]
- 35.De Diego, J. I., M. P. Prim, C. Alfonso, N. Sastre, I. Rabanal, and J. Gavilan. 2001. Comparison of amoxicillin and azithromycin in the prevention of recurrent acute otitis media. Int. J. Pediatr. Otorhinolaryngol. 58:47-51. [DOI] [PubMed] [Google Scholar]
- 36.de Gracia, J., M. Vendrell, A. Alvarez, E. Pallisa, M. J. Rodrigo, D. de la Rosa, F. Mata, J. Andreu, and F. Morell. 2004. Immunoglobulin therapy to control lung damage in patients with common variable immunodeficiency. Int. Immunopharmacol. 4:745-753. [DOI] [PubMed] [Google Scholar]
- 37.Eastwood, D., K. C. Gilmour, K. Nistala, C. Meaney, H. Chapel, Z. Sherrell, A. D. Webster, E. G. Davies, A. Jones, and H. B. Gaspar. 2004. Prevalence of SAP gene defects in male patients diagnosed with common variable immunodeficiency. Clin. Exp. Immunol. 137:584-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eijkhout, H. W., J. W. van Der Meer, C. G. Kallenberg, R. S. Weening, J. T. van Dissel, L. A. Sanders, P. F. Strengers, H. Nienhuis, and P. T. Schellekens. 2001. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. A randomized, double-blind, multicenter crossover trial. Ann. Intern. Med. 135:165-174. [DOI] [PubMed] [Google Scholar]
- 39.Farrar, J. E., J. Rohrer, and M. E. Conley. 1996. Neutropenia in X-linked agammaglobulinemia. Clin. Immunol. Immunopathol. 81:271-276. [DOI] [PubMed] [Google Scholar]
- 40.Fasano, M. B., K. E. Sullivan, S. B. Sarpong, R. A. Wood, S. M. Jones, C. J. Johns, H. M. Lederman, M. J. Bykowsky, J. M. Greene, and J. A. Winkelstein. 1996. Sarcoidosis and common variable immunodeficiency. Report of 8 cases and review of the literature. Medicine (Baltimore) 75:251-261. [DOI] [PubMed] [Google Scholar]
- 41.Fasth, A. 1982. Primary immunodeficiency disorders in Sweden: cases among children, 1974-1979. J. Clin. Immunol. 2:86-92. [DOI] [PubMed] [Google Scholar]
- 42.Fasth, A., and J. Nystrom. 2007. Safety and efficacy of subcutaneous human immunoglobulin in children with primary immunodeficiency. Acta Paediatr. 96:1474-1478. [DOI] [PubMed] [Google Scholar]
- 43.Faubert, G. 2000. Immune response to Giardia duodenalis. Clin. Microbiol. Rev. 13:35-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franz, A., A. D. Webster, P. M. Furr, and D. Taylor-Robinson. 1997. Mycoplasmal arthritis in patients with primary immunoglobulin deficiency: clinical features and outcome in 18 patients. Br. J. Rheumatol. 36:661-668. [DOI] [PubMed] [Google Scholar]
- 45.Furr, P. M., D. Taylor-Robinson, and A. D. Webster. 1994. Mycoplasmas and ureaplasmas in patients with hypogammaglobulinaemia and their role in arthritis: microbiological observations over twenty years. Ann. Rheum. Dis. 53:183-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Futatani, T., T. Miyawaki, S. Tsukada, S. Hashimoto, T. Kunikata, S. Arai, M. Kurimoto, Y. Niida, H. Matsuoka, Y. Sakiyama, T. Iwata, S. Tsuchiya, O. Tatsuzawa, K. Yoshizaki, and T. Kishimoto. 1998. Deficient expression of Bruton's tyrosine kinase in monocytes from X-linked agammaglobulinemia as evaluated by a flow cytometric analysis and its clinical application to carrier detection. Blood 91:595-602. [PubMed] [Google Scholar]
- 47.Futatani, T., C. Watanabe, Y. Baba, S. Tsukada, and H. D. Ochs. 2001. Bruton's tyrosine kinase is present in normal platelets and its absence identifies patients with X-linked agammaglobulinaemia and carrier females. Br. J. Haematol. 114:141-149. [DOI] [PubMed] [Google Scholar]
- 48.Gardulf, A., U. Nicolay, O. Asensio, E. Bernatowska, A. Bock, B. C. Carvalho, C. Granert, S. Haag, D. Hernandez, P. Kiessling, J. Kus, J. Pons, T. Niehues, S. Schmidt, I. Schulze, and M. Borte. 2006. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies—a prospective, multi-national study. J. Clin. Immunol. 26:177-185. [DOI] [PubMed] [Google Scholar]
- 49.Gerrard, J., D. Alfredson, and I. Smith. 2001. Recurrent bacteremia and multifocal lower limb cellulitis due to Helicobacter-like organisms in a patient with X-linked hypogammaglobulinemia. Clin. Infect. Dis. 33:E116-E118. [DOI] [PubMed] [Google Scholar]
- 50.Giovannetti, A., M. Pierdominici, F. Mazzetta, M. Marziali, C. Renzi, A. M. Mileo, M. De Felice, B. Mora, A. Esposito, R. Carello, A. Pizzuti, M. G. Paggi, R. Paganelli, W. Malorni, and F. Aiuti. 2007. Unravelling the complexity of T cell abnormalities in common variable immunodeficiency. J. Immunol. 178:3932-3943. [DOI] [PubMed] [Google Scholar]
- 51.Grimbacher, B., A. Hutloff, M. Schlesier, E. Glocker, K. Warnatz, R. Drager, H. Eibel, B. Fischer, A. A. Schaffer, H. W. Mages, R. A. Kroczek, and H. H. Peter. 2003. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat. Immunol. 4:261-268. [DOI] [PubMed] [Google Scholar]
- 52.Haas, K. M., J. C. Poe, D. A. Steeber, and T. F. Tedder. 2005. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23:7-18. [DOI] [PubMed] [Google Scholar]
- 53.Halliday, E., J. Winkelstein, and A. D. Webster. 2003. Enteroviral infections in primary immunodeficiency (PID): a survey of morbidity and mortality. J. Infect. 46:1-8. [DOI] [PubMed] [Google Scholar]
- 54.Hammarstrom, L., I. Vorechovsky, and D. Webster. 2000. Selective IgA deficiency (SIgAD) and common variable immunodeficiency (CVID). Clin. Exp. Immunol. 120:225-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hansel, T. T., D. P. O'Neill, M. L. Yee, J. M. Gibson, and R. A. Thompson. 1990. Infective conjunctivitis and corneal scarring in three brothers with sex linked hypogammaglobulinaemia (Bruton's disease). Br. J. Ophthalmol. 74:118-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hausser, C., J. L. Virelizier, D. Buriot, and C. Griscelli. 1983. Common variable hypogammaglobulinemia in children. Clinical and immunologic observations in 30 patients. Am. J. Dis. Child. 137:833-837. [DOI] [PubMed] [Google Scholar]
- 57.Hayakawa, K., and R. R. Hardy. 2000. Development and function of B-1 cells. Curr. Opin. Immunol. 12:346-353. [DOI] [PubMed] [Google Scholar]
- 58.He, B., X. Qiao, and A. Cerutti. 2004. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J. Immunol. 173:4479-4491. [DOI] [PubMed] [Google Scholar]
- 59.Hermaszewski, R. A., and A. D. Webster. 1993. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Q. J. Med. 86:31-42. [PubMed] [Google Scholar]
- 60.Herrod, H. G. 1993. Management of the patient with IgG subclass deficiency and/or selective antibody deficiency. Ann. Allergy 70:3-8. [PubMed] [Google Scholar]
- 61.Hidalgo, H., C. Moore, L. E. Leiva, and R. U. Sorensen. 1996. Preimmunization and postimmunization pneumococcal antibody titers in children with recurrent infections. Ann. Allergy Asthma Immunol. 76:341-346. [DOI] [PubMed] [Google Scholar]
- 62.Imai, K., G. Slupphaug, W. I. Lee, P. Revy, S. Nonoyama, N. Catalan, L. Yel, M. Forveille, B. Kavli, H. E. Krokan, H. D. Ochs, A. Fischer, and A. Durandy. 2003. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat. Immunol. 4:1023-1028. [DOI] [PubMed] [Google Scholar]
- 63.Jain, A., C. A. Ma, S. Liu, M. Brown, J. Cohen, and W. Strober. 2001. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat. Immunol. 2:223-228. [DOI] [PubMed] [Google Scholar]
- 64.Kainulainen, L., J. Nikoskelainen, and O. Ruuskanen. 2001. Diagnostic findings in 95 Finnish patients with common variable immunodeficiency. J. Clin. Immunol. 21:145-149. [DOI] [PubMed] [Google Scholar]
- 65.Kainulainen, L., J. Suonpaa, J. Nikoskelainen, E. Svedstrom, T. Vuorinen, O. Meurman, and O. Ruuskanen. 2007. Bacteria and viruses in maxillary sinuses of patients with primary hypogammaglobulinemia. Arch. Otolaryngol. Head Neck Surg. 133:597-602. [DOI] [PubMed] [Google Scholar]
- 66.Kainulainen, L., M. Varpula, K. Liippo, E. Svedstrom, J. Nikoskelainen, and O. Ruuskanen. 1999. Pulmonary abnormalities in patients with primary hypogammaglobulinemia. J. Allergy Clin. Immunol. 104:1031-1036. [DOI] [PubMed] [Google Scholar]
- 67.Karasuyama, H., A. Kudo, and F. Melchers. 1990. The proteins encoded by the VpreB and lambda 5 pre-B cell-specific genes can associate with each other and with mu heavy chain. J. Exp. Med. 172:969-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katsenelson, N., S. Kanswal, M. Puig, H. Mostowski, D. Verthelyi, and M. Akkoyunlu. 2007. Synthetic CpG oligodeoxynucleotides augment BAFF- and APRIL-mediated immunoglobulin secretion. Eur. J. Immunol. 37:1785-1795. [DOI] [PubMed] [Google Scholar]
- 69.Kinlen, L. J., A. D. Webster, A. G. Bird, R. Haile, J. Peto, J. F. Soothill, and R. A. Thompson. 1985. Prospective study of cancer in patients with hypogammaglobulinaemia. Lancet i:263-266. [DOI] [PubMed] [Google Scholar]
- 70.Kornfeld, S. J., R. N. Haire, S. J. Strong, H. Tang, S. S. Sung, S. M. Fu, and G. W. Litman. 1996. A novel mutation (Cys145→stop) in Bruton's tyrosine kinase is associated with newly diagnosed X-linked agammaglobulinemia in a 51-year-old male. Mol. Med. 2:619-623. [PMC free article] [PubMed] [Google Scholar]
- 71.Kudo, A., and F. Melchers. 1987. A second gene, VpreB in the lambda 5 locus of the mouse, which appears to be selectively expressed in pre-B lymphocytes. EMBO J. 6:2267-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lack, G., H. D. Ochs, and E. W. Gelfand. 1996. Humoral immunity in steroid-dependent children with asthma and hypogammaglobulinemia. J. Pediatr. 129:898-903. [DOI] [PubMed] [Google Scholar]
- 73.LeBien, T. W., and T. F. Tedder. 2008. B lymphocytes: how they develop and function. Blood 112:1570-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lederman, H. M., and J. A. Winkelstein. 1985. X-linked agammaglobulinemia: an analysis of 96 patients. Medicine (Baltimore) 64:145-156. [PubMed] [Google Scholar]
- 75.Lee, A. H., A. I. Levinson, and H. R. Schumacher, Jr. 1993. Hypogammaglobulinemia and rheumatic disease. Semin. Arthritis Rheum. 22:252-264. [DOI] [PubMed] [Google Scholar]
- 76.Lee, J. J., E. Ozcan, I. Rauter, and R. S. Geha. 2008. Transmembrane activator and calcium-modulator and cyclophilin ligand interactor mutations in common variable immunodeficiency. Curr. Opin. Allergy Clin. Immunol. 8:520-526. [DOI] [PubMed] [Google Scholar]
- 77.Levy, J., T. Espanol-Boren, C. Thomas, A. Fischer, P. Tovo, P. Bordigoni, I. Resnick, A. Fasth, M. Baer, L. Gomez, E. A. Sanders, M. D. Tabone, D. Plantaz, A. Etzioni, V. Monafo, M. Abinun, L. Hammarstrom, T. Abrahamsen, A. Jones, A. Finn, T. Klemola, E. DeVries, O. Sanal, M. C. Peitsch, and L. D. Notarangelo. 1997. Clinical spectrum of X-linked hyper-IgM syndrome. J. Pediatr. 131:47-54. [DOI] [PubMed] [Google Scholar]
- 78.LoGalbo, P. R., H. A. Sampson, and R. H. Buckley. 1982. Symptomatic giardiasis in three patients with X-linked agammaglobulinemia. J. Pediatr. 101:78-80. [DOI] [PubMed] [Google Scholar]
- 79.Lopes-Carvalho, T., and J. F. Kearney. 2004. Development and selection of marginal zone B cells. Immunol. Rev. 197:192-205. [DOI] [PubMed] [Google Scholar]
- 80.Mackay, F., and P. Schneider. 2008. TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytok. Growth Factor Rev. 19:263-276. [DOI] [PubMed] [Google Scholar]
- 81.Mak, T. W., A. Shahinian, S. K. Yoshinaga, A. Wakeham, L. M. Boucher, M. Pintilie, G. Duncan, B. U. Gajewska, M. Gronski, U. Eriksson, B. Odermatt, A. Ho, D. Bouchard, J. S. Whorisky, M. Jordana, P. S. Ohashi, T. Pawson, F. Bladt, and A. Tafuri. 2003. Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell-dependent B cell responses. Nat. Immunol. 4:765-772. [DOI] [PubMed] [Google Scholar]
- 82.Mast, E. E., C. M. Weinbaum, A. E. Fiore, M. J. Alter, B. P. Bell, L. Finelli, L. E. Rodewald, J. M. Douglas, Jr., R. S. Janssen, and J. W. Ward. 2006. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP) part II: immunization of adults. MMWR Recommend. Rep. 55:1-33. [PubMed] [Google Scholar]
- 83.McGeady, S. J. 1987. Transient hypogammaglobulinemia of infancy: need to reconsider name and definition. J. Pediatr. 110:47-50. [DOI] [PubMed] [Google Scholar]
- 84.McKinney, R. E., Jr., S. L. Katz, and C. M. Wilfert. 1987. Chronic enteroviral meningoencephalitis in agammaglobulinemic patients. Rev. Infect. Dis. 9:334-356. [DOI] [PubMed] [Google Scholar]
- 85.Mechanic, L. J., S. Dikman, and C. Cunningham-Rundles. 1997. Granulomatous disease in common variable immunodeficiency. Ann. Intern. Med. 127:613-617. [DOI] [PubMed] [Google Scholar]
- 86.Melchers, F. 2005. The pre-B-cell receptor: selector of fitting immunoglobulin heavy chains for the B-cell repertoire. Nat. Rev. Immunol. 5:578-584. [DOI] [PubMed] [Google Scholar]
- 87.Mellemkjaer, L., L. Hammarstrom, V. Andersen, J. Yuen, C. Heilmann, T. Barington, J. Bjorkander, and J. H. Olsen. 2002. Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: a combined Danish and Swedish study. Clin. Exp. Immunol. 130:495-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Michel, M., V. Chanet, L. Galicier, M. Ruivard, Y. Levy, O. Hermine, E. Oksenhendler, A. Schaeffer, P. Bierling, and B. Godeau. 2004. Autoimmune thrombocytopenic purpura and common variable immunodeficiency: analysis of 21 cases and review of the literature. Medicine (Baltimore) 83:254-263. [DOI] [PubMed] [Google Scholar]
- 89.Misbah, S. A., G. P. Spickett, P. C. Ryba, J. M. Hockaday, J. S. Kroll, C. Sherwood, J. B. Kurtz, E. R. Moxon, and H. M. Chapel. 1992. Chronic enteroviral meningoencephalitis in agammaglobulinemia: case report and literature review. J. Clin. Immunol. 12:266-270. [DOI] [PubMed] [Google Scholar]
- 90.Moin, M., A. Aghamohammadi, A. Farhoudi, Z. Pourpak, N. Rezaei, M. Movahedi, M. Gharagozlou, B. M. Ghazi, A. Zahed, K. Abolmaali, M. Mahmoudi, L. Emami, and M. Bashashati. 2004. X-linked agammaglobulinemia: a survey of 33 Iranian patients. Immunol. Investig. 33:81-93. [DOI] [PubMed] [Google Scholar]
- 91.Moschese, V., S. Graziani, M. A. Avanzini, R. Carsetti, M. Marconi, M. La Rocca, L. Chini, C. Pignata, A. R. Soresina, R. Consolini, G. Bossi, A. Trizzino, S. Martino, F. Cardinale, P. Bertolini, G. L. Marseglia, M. Zecca, S. Di Cesare, I. Quinti, R. Rondelli, M. C. Pietrogrande, P. Rossi, and A. Plebani. 2008. A prospective study on children with initial diagnosis of transient hypogammaglobulinemia of infancy: results from the Italian Primary Immunodeficiency Network. Int. J. Immunopathol. Pharmacol. 21:343-352. [DOI] [PubMed] [Google Scholar]
- 92.Noroski, L. M., and W. T. Shearer. 1998. Screening for primary immunodeficiencies in the clinical immunology laboratory. Clin. Immunol. Immunopathol. 86:237-245. [DOI] [PubMed] [Google Scholar]
- 93.Notarangelo, L. D., G. Lanzi, S. Peron, and A. Durandy. 2006. Defects of class-switch recombination. J. Allergy Clin. Immunol. 117:855-864. [DOI] [PubMed] [Google Scholar]
- 94.Nunez, C., N. Nishimoto, G. L. Gartland, L. G. Billips, P. D. Burrows, H. Kubagawa, and M. D. Cooper. 1996. B cells are generated throughout life in humans. J. Immunol. 156:866-872. [PubMed] [Google Scholar]
- 95.Ochs, H. D., E. R. Stiehm, J. A. Winkelstein, C. Cunningham-Rundles, A. Durandy, H. M. Lederman, L. D. Notarangelo, D. J. Rawlings, and C. M. Roifman. 2004. Antibody deficiencies, p. 356-426. In E. R. Stiehm, H. D. Ochs, and J. A. Winkelstein (ed.), Immunologic disorders in infants and children, 5th ed. Saunders, Philadelphia, PA.
- 96.Onigbanjo, M. T., J. S. Orange, E. E. Perez, and K. E. Sullivan. 2007. Hypogammaglobulinemia in a pediatric tertiary care setting. Clin. Immunol. 125:52-59. [DOI] [PubMed] [Google Scholar]
- 97.Orange, J. S., R. S. Geha, and F. A. Bonilla. 2003. Acute chylothorax in children: selective retention of memory T cells and natural killer cells. J. Pediatr. 143:243-249. [DOI] [PubMed] [Google Scholar]
- 98.Orange, J. S., E. M. Hossny, C. R. Weiler, M. Ballow, M. Berger, F. A. Bonilla, R. Buckley, J. Chinen, Y. El-Gamal, B. D. Mazer, R. P. Nelson, Jr., D. D. Patel, E. Secord, R. U. Sorensen, R. L. Wasserman, and C. Cunningham-Rundles. 2006. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J. Allergy Clin. Immunol. 117:S525-S553. [DOI] [PubMed] [Google Scholar]
- 99.Orange, J. S., O. Levy, S. R. Brodeur, K. Krzewski, R. M. Roy, J. E. Niemela, T. A. Fleisher, F. A. Bonilla, and R. S. Geha. 2004. Human nuclear factor kappa B essential modulator mutation can result in immunodeficiency without ectodermal dysplasia. J. Allergy Clin. Immunol. 114:650-656. [DOI] [PubMed] [Google Scholar]
- 100.Paessler, M., A. Levinson, J. B. Patel, M. Schuster, M. Minda, and I. Nachamkin. 2002. Disseminated Mycoplasma orale infection in a patient with common variable immunodeficiency syndrome. Diagn. Microbiol. Infect. Dis. 44:201-204. [DOI] [PubMed] [Google Scholar]
- 101.Peron, S., A. Metin, P. Gardes, M. A. Alyanakian, E. Sheridan, C. P. Kratz, A. Fischer, and A. Durandy. 2008. Human PMS2 deficiency is associated with impaired immunoglobulin class switch recombination. J. Exp. Med. 205:2465-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Piqueras, B., C. Lavenu-Bombled, L. Galicier, F. Bergeron-van der Cruyssen, L. Mouthon, S. Chevret, P. Debre, C. Schmitt, and E. Oksenhendler. 2003. Common variable immunodeficiency patient classification based on impaired B cell memory differentiation correlates with clinical aspects. J. Clin. Immunol. 23:385-400. [DOI] [PubMed] [Google Scholar]
- 103.Plebani, A., A. Soresina, R. Rondelli, G. M. Amato, C. Azzari, F. Cardinale, G. Cazzola, R. Consolini, D. De Mattia, G. Dell'Erba, M. Duse, M. Fiorini, S. Martino, B. Martire, M. Masi, V. Monafo, V. Moschese, L. D. Notarangelo, P. Orlandi, P. Panei, A. Pession, M. C. Pietrogrande, C. Pignata, I. Quinti, V. Ragno, P. Rossi, A. Sciotto, and A. Stabile. 2002. Clinical, immunological, and molecular analysis in a large cohort of patients with X-linked agammaglobulinemia: an Italian multicenter study. Clin. Immunol. 104:221-230. [DOI] [PubMed] [Google Scholar]
- 104.Principi, N., P. Marchisio, E. Massironi, R. M. Grasso, and G. Filiberti. 1989. Prophylaxis of recurrent acute otitis media and middle-ear effusion. Comparison of amoxicillin with sulfamethoxazole and trimethoprim. Am. J. Dis. Child. 143:1414-1418. [DOI] [PubMed] [Google Scholar]
- 105.Quartier, P., J. Bustamante, O. Sanal, A. Plebani, M. Debre, A. Deville, J. Litzman, J. Levy, J. P. Fermand, P. Lane, G. Horneff, G. Aksu, I. Yalcin, G. Davies, I. Tezcan, F. Ersoy, N. Catalan, K. Imai, A. Fischer, and A. Durandy. 2004. Clinical, immunologic and genetic analysis of 29 patients with autosomal recessive hyper-IgM syndrome due to activation-induced cytidine deaminase deficiency. Clin. Immunol. 110:22-29. [DOI] [PubMed] [Google Scholar]
- 106.Quartier, P., M. Debre, J. De Blic, R. de Sauverzac, N. Sayegh, N. Jabado, E. Haddad, S. Blanche, J. L. Casanova, C. I. Smith, F. Le Deist, G. de Saint Basile, and A. Fischer. 1999. Early and prolonged intravenous immunoglobulin replacement therapy in childhood agammaglobulinemia: a retrospective survey of 31 patients. J. Pediatr. 134:589-596. [DOI] [PubMed] [Google Scholar]
- 107.Quartier, P., S. Foray, J. L. Casanova, I. Hau-Rainsard, S. Blanche, and A. Fischer. 2000. Enteroviral meningoencephalitis in X-linked agammaglobulinemia: intensive immunoglobulin therapy and sequential viral detection in cerebrospinal fluid by polymerase chain reaction. Pediatr. Infect. Dis. J. 19:1106-1108. [DOI] [PubMed] [Google Scholar]
- 108.Quartier, P., O. Tournilhac, C. Archimbaud, L. Lazaro, C. Chaleteix, P. Millet, H. Peigue-Lafeuille, S. Blanche, A. Fischer, J. L. Casanova, P. Travade, and M. Tardieu. 2003. Enteroviral meningoencephalitis after anti-CD20 (rituximab) treatment. Clin. Infect. Dis. 36:e47-e49. [DOI] [PubMed] [Google Scholar]
- 109.Quezada, S. A., L. Z. Jarvinen, E. F. Lind, and R. J. Noelle. 2004. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22:307-328. [DOI] [PubMed] [Google Scholar]
- 110.Quinti, I., M. Pierdominici, M. Marziali, A. Giovannetti, S. Donnanno, H. Chapel, J. Bjorkander, and F. Aiuti. 2002. European surveillance of immunoglobulin safety—results of initial survey of 1243 patients with primary immunodeficiencies in 16 countries. Clin. Immunol. 104:231-236. [DOI] [PubMed] [Google Scholar]
- 111.Quinti, I., A. Soresina, G. Spadaro, S. Martino, S. Donnanno, C. Agostini, P. Claudio, D. Franco, A. M. Pesce, F. Borghese, A. Guerra, R. Rondelli, and A. Plebani. 2007. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J. Clin. Immunol. 27:308-316. [DOI] [PubMed] [Google Scholar]
- 112.Rajewsky, K., and A. Radbruch. 2004. The cellular basis of B cell memory, p. 247-254. In T. Honjo, F. Alt, and M. Neuberger (ed.), Molecular biology of B cells. Elsevier Academic Press, San Diego, CA.
- 113.Rezaei, N., F. A. Bonilla, K. E. Sullivan, E. de Vries, and J. S. Orange. 2008. An introduction to primary immunodeficiency diseases, p. 1-38. In N. Rezaei, A. Aghamohammadi, and L. D. Notarangelo (ed.), Primary immunodeficiency diseases, 5th ed. Springer-Verlag, Berlin, Germany.
- 114.Roberts, D., A. E. Murray, B. C. Pratt, and R. E. Meigh. 1989. Mycoplasma hominis as a respiratory pathogen in X-linked hypogammaglobulinaemia. J. Infect. 18:175-177. [DOI] [PubMed] [Google Scholar]
- 115.Rodrigues, F., E. G. Davies, P. Harrison, J. McLauchlin, J. Karani, B. Portmann, A. Jones, P. Veys, G. Mieli-Vergani, and N. Hadzic. 2004. Liver disease in children with primary immunodeficiencies. J. Pediatr. 145:333-339. [DOI] [PubMed] [Google Scholar]
- 116.Roifman, C. M., H. Levison, and E. W. Gelfand. 1987. High-dose versus low-dose intravenous immunoglobulin in hypogammaglobulinaemia and chronic lung disease. Lancet i:1075-1077. [DOI] [PubMed] [Google Scholar]
- 117.Roifman, C. M., C. P. Rao, H. M. Lederman, S. Lavi, P. Quinn, and E. W. Gelfand. 1986. Increased susceptibility to Mycoplasma infection in patients with hypogammaglobulinemia. Am. J. Med. 80:590-594. [DOI] [PubMed] [Google Scholar]
- 118.Sakaguchi, N., and F. Melchers. 1986. Lambda 5, a new light-chain-related locus selectively expressed in pre-B lymphocytes. Nature 324:579-582. [DOI] [PubMed] [Google Scholar]
- 119.Salzer, U., C. Bacchelli, S. Buckridge, Q. Pan-Hammarstrom, S. Jennings, V. Lougaris, A. Bergbreiter, T. Hagena, J. Birmelin, A. Plebani, A. D. Webster, H. H. Peter, D. Suez, H. Chapel, A. McLean-Tooke, G. P. Spickett, S. Anover-Sombke, H. D. Ochs, S. Urschel, B. H. Belohradsky, S. Ugrinovic, D. S. Kumararatne, T. C. Lawrence, A. M. Holm, J. L. Franco, I. Schulze, P. Schneider, E. M. Gertz, A. A. Schaffer, L. Hammarstrom, A. J. Thrasher, H. B. Gaspar, and B. Grimbacher. 2009. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood 113:1967-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salzer, U., H. M. Chapel, A. D. Webster, Q. Pan-Hammarstrom, A. Schmitt-Graeff, M. Schlesier, H. H. Peter, J. K. Rockstroh, P. Schneider, A. A. Schaffer, L. Hammarstrom, and B. Grimbacher. 2005. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat. Genet. 37:820-828. [DOI] [PubMed] [Google Scholar]
- 121.Salzer, U., A. Maul-Pavicic, C. Cunningham-Rundles, S. Urschel, B. H. Belohradsky, J. Litzman, A. Holm, J. L. Franco, A. Plebani, L. Hammarstrom, A. Skrabl, W. Schwinger, and B. Grimbacher. 2004. ICOS deficiency in patients with common variable immunodeficiency. Clin. Immunol. 113:234-240. [DOI] [PubMed] [Google Scholar]
- 122.Sanchez-Ramon, S., L. Radigan, J. E. Yu, S. Bard, and C. Cunningham-Rundles. 2008. Memory B cells in common variable immunodeficiency: clinical associations and sex differences. Clin. Immunol. 128:314-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sarpong, S., H. S. Skolnick, H. D. Ochs, T. Futatani, and J. A. Winkelstein. 2002. Survival of wild polio by a patient with XLA. Ann. Allergy Asthma Immunol. 88:59-60. [DOI] [PubMed] [Google Scholar]
- 124.Sawada, A., Y. Takihara, J. Y. Kim, Y. Matsuda-Hashii, S. Tokimasa, H. Fujisaki, K. Kubota, H. Endo, T. Onodera, H. Ohta, K. Ozono, and J. Hara. 2003. A congenital mutation of the novel gene LRRC8 causes agammaglobulinemia in humans. J. Clin. Investig. 112:1707-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schwarz, K., G. H. Gauss, L. Ludwig, U. Pannicke, Z. Li, D. Lindner, W. Friedrich, R. A. Seger, T. E. Hansen-Hagge, S. Desiderio, M. R. Lieber, and C. R. Bartram. 1996. RAG mutations in human B cell-negative SCID. Science 274:97-99. [DOI] [PubMed] [Google Scholar]
- 126.Sekine, H., R. C. Ferreira, Q. Pan-Hammarstrom, R. R. Graham, B. Ziemba, S. S. de Vries, J. Liu, K. Hippen, T. Koeuth, W. Ortmann, A. Iwahori, M. K. Elliott, S. Offer, C. Skon, L. Du, J. Novitzke, A. T. Lee, N. Zhao, J. D. Tompkins, D. Altshuler, P. K. Gregersen, C. Cunningham-Rundles, R. S. Harris, C. Her, D. L. Nelson, L. Hammarstrom, G. S. Gilkeson, and T. W. Behrens. 2007. Role for Msh5 in the regulation of Ig class switch recombination. Proc. Natl. Acad. Sci. USA 104:7193-7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shackelford, P. G., D. M. Granoff, J. V. Madassery, M. G. Scott, and M. H. Nahm. 1990. Clinical and immunologic characteristics of healthy children with subnormal serum concentrations of IgG2. Pediatr. Res. 27:16-21. [DOI] [PubMed] [Google Scholar]
- 128.Stavnezer, J., J. E. Guikema, and C. E. Schrader. 2008. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 26:261-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stiehm, E. R. 1997. Human intravenous immunoglobulin in primary and secondary antibody deficiencies. Pediatr. Infect. Dis. J. 16:696-707. [DOI] [PubMed] [Google Scholar]
- 130.Sweinberg, S. K., R. A. Wodell, M. P. Grodofsky, J. M. Greene, and M. E. Conley. 1991. Retrospective analysis of the incidence of pulmonary disease in hypogammaglobulinemia. J. Allergy Clin. Immunol. 88:96-104. [DOI] [PubMed] [Google Scholar]
- 131.Tarlinton, D., A. Radbruch, F. Hiepe, and T. Dorner. 2008. Plasma cell differentiation and survival. Curr. Opin. Immunol. 20:162-169. [DOI] [PubMed] [Google Scholar]
- 132.Templeton, K. E., S. A. Scheltinga, A. W. Graffelman, J. M. Van Schie, J. W. Crielaard, P. Sillekens, P. J. Van Den Broek, H. Goossens, M. F. Beersma, and E. C. Claas. 2003. Comparison and evaluation of real-time PCR, real-time nucleic acid sequence-based amplification, conventional PCR, and serology for diagnosis of Mycoplasma pneumoniae. J. Clin. Microbiol. 41:4366-4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Thickett, K. M., D. S. Kumararatne, A. K. Banerjee, R. Dudley, and D. E. Stableforth. 2002. Common variable immune deficiency: respiratory manifestations, pulmonary function and high-resolution CT scan findings. Q. J. Med. 95:655-662. [DOI] [PubMed] [Google Scholar]
- 134.Tokuda, K., J. Nishi, H. Miyanohara, J. Sarantuya, M. Iwashita, A. Kamenosono, K. Hizukuri, N. Wakimoto, and M. Yoshinaga. 2004. Relapsing cellulitis associated with Campylobacter coli bacteremia in an agammaglobulinemic patient. Pediatr. Infect. Dis. J. 23:577-579. [DOI] [PubMed] [Google Scholar]
- 135.van Zelm, M. C., I. Reisli, M. van der Burg, D. Castano, C. J. van Noesel, M. J. van Tol, C. Woellner, B. Grimbacher, P. J. Patino, J. J. van Dongen, and J. L. Franco. 2006. An antibody-deficiency syndrome due to mutations in the CD19 gene. N. Engl. J. Med. 354:1901-1912. [DOI] [PubMed] [Google Scholar]
- 136.Vos, Q., A. Lees, Z. Q. Wu, C. M. Snapper, and J. J. Mond. 2000. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol. Rev. 176:154-170. [DOI] [PubMed] [Google Scholar]
- 137.Wardemann, H., T. Boehm, N. Dear, and R. Carsetti. 2002. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J. Exp. Med. 195:771-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Warnatz, K., A. Denz, R. Drager, M. Braun, C. Groth, G. Wolff-Vorbeck, H. Eibel, M. Schlesier, and H. H. Peter. 2002. Severe deficiency of switched memory B cells (CD27(+)IgM(−)IgD(−)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood 99:1544-1551. [DOI] [PubMed] [Google Scholar]
- 139.Webster, A. D., H. A. Rotbart, T. Warner, P. Rudge, and N. Hyman. 1993. Diagnosis of enterovirus brain disease in hypogammaglobulinemic patients by polymerase chain reaction. Clin. Infect. Dis. 17:657-661. [DOI] [PubMed] [Google Scholar]
- 140.Wehr, C., T. Kivioja, C. Schmitt, B. Ferry, T. Witte, E. Eren, M. Vlkova, M. Hernandez, D. Detkova, P. R. Bos, G. Poerksen, H. von Bernuth, U. Baumann, S. Goldacker, S. Gutenberger, M. Schlesier, F. Bergeron-van der Cruyssen, M. Le Garff, P. Debre, R. Jacobs, J. Jones, E. Bateman, J. Litzman, P. M. van Hagen, A. Plebani, R. E. Schmidt, V. Thon, I. Quinti, T. Espanol, A. D. Webster, H. Chapel, M. Vihinen, E. Oksenhendler, H. H. Peter, and K. Warnatz. 2008. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood 111:77-85. [DOI] [PubMed] [Google Scholar]
- 141.Winkelstein, J. A., M. C. Marino, H. M. Lederman, S. M. Jones, K. Sullivan, A. W. Burks, M. E. Conley, C. Cunningham-Rundles, and H. D. Ochs. 2006. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore) 85:193-202. [DOI] [PubMed] [Google Scholar]
- 142.Winkelstein, J. A., M. C. Marino, H. Ochs, R. Fuleihan, P. R. Scholl, R. Geha, E. R. Stiehm, and M. E. Conley. 2003. The X-linked hyper-IgM syndrome: clinical and immunologic features of 79 patients. Medicine (Baltimore) 82:373-384. [DOI] [PubMed] [Google Scholar]
- 143.Wood, P., S. Stanworth, J. Burton, A. Jones, D. G. Peckham, T. Green, C. Hyde, and H. Chapel. 2007. Recognition, clinical diagnosis and management of patients with primary antibody deficiencies: a systematic review. Clin. Exp. Immunol. 149:410-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu, W., P. A. Santini, A. J. Matthews, A. Chiu, A. Plebani, B. He, K. Chen, and A. Cerutti. 2008. Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J. Immunol. 181:276-287. [DOI] [PMC free article] [PubMed] [Google Scholar]