Abstract
The elevated expression and receptor binding of gastrin-releasing peptide (GRP) in various types of cancer, especially in malignant melanoma of the skin, suggest that GRP might be a putative target for immunotherapy in neoplastic diseases. We have therefore constructed a novel DNA vaccine coding for six tandem repeats of a fragment of GRP from amino acids 18 to 27 (GRP6) flanked by helper T-cell epitopes for increased immunogenicity, including HSP65, a tetanus toxoid fragment from amino acids 830 to 844 (T), pan-HLA-DR-binding epitope (PADRE) (P), and two repeats of a mycobacterial HSP70 fragment from amino acids 407 to 426 (M). The anti-GRP DNA vaccine (pCR3.1-VS-HSP65-TP-GRP6-M2) was constructed on a backbone of a pCR3.1 plasmid vector with eight 5′-GACGTT-3′ CpG motifs and the VEGF183 signal peptide (VS). Intramuscular (IM) injections of anti-GRP vaccine in mice stimulated the production of high titers of specific antibodies against GRP and suppressed the growth of subcutaneous tumors of B16-F10 melanoma cells. Parallel results were obtained in vitro, showing inhibition of B16-F10 cell proliferation by GRP antisera. IM injections of the DNA vaccine also significantly attenuated tumor-induced angiogenesis associated with intradermal tumors of B16-F10 cells. In addition, lung invasion of intravenously injected cells was highly diminished, suggesting potent antimetastatic activity of the DNA vaccine. These findings support the highly immunogenic and potent antitumorigenic activity of specific anti-GRP antibodies elicited by the anti-GRP DNA vaccine.
In recent years, gastrin-releasing peptide (GRP) has been shown to be a potent mitogen for a variety of tumors (23). GRP plays an important role in human cancers exerting autocrine, paracrine, or endocrine growth factor effects (34). The GRP receptor (GRPR) is expressed aberrantly in various cancer cells (23), and GRP binding appears to activate multiple cellular signaling pathways, resulting in cellular proliferation and tumor formation (2, 8). Moreover, bombesin-like peptide (BLP) family members are involved in many steps of tumor progression, including angiogenesis (9, 14, 20) and distant metastasis (19, 22), resulting in increased aggressiveness and poorer prognosis of tumors.
Various GRPR antagonists, anti-GRP antibodies, and cytotoxic immunocomplexes have exhibited impressive antitumoral activity both in vitro and in vivo against human and murine tumors (3). DNA vaccines targeting GRP represent another promising approach. However, a key issue in developing subunit DNA vaccines is their relatively weak immunogenicity. The effectiveness of subunit vaccines could be increased by delivering them with adjuvants. Previous studies have demonstrated that the mycobacterial 65-kDa heat shock protein (HSP65) exhibits strong immunogenicity and contains strong T-cell epitopes presented by major histocompatibility complex class II molecules; accordingly, it has been used as a helper T-cell epitope for delivering B-cell epitopes in vivo (24). The low immunogenicity of self-peptides can also be overcome by immunization with immunogens containing multiple copies of the self-peptides in linear alignment (36). In addition, unmethylated bacterial DNA oligonucleotides (CpG motifs) (16) and synthetic peptides representing helper T-lymphocyte epitopes, such as those encoded by a tetanus toxoid fragment from amino acids 830 to 844 (tetanus toxoid 830-844) (18), pan-HLA-DR-binding epitope (PADRE) (1), or mycobacterial HSP70 fragment 407 to 426 (HSP70407-426) (32), can be incorporated as immunoadjuvants in vaccines to augment immunogenicity and promote biostable antibody response.
In this study, we constructed anti-GRP DNA vaccines incorporating various immunoadjuvants and tested whether they could induce strong humoral responses in immunized mice. The efficacy of the anti-GRP DNA vaccines against tumor-associated angiogenesis or distant metastases was evaluated with various tumor models utilizing the well-characterized mouse melanoma B16-F10 cell line.
MATERIALS AND METHODS
Animals.
For all experiments, 5-week-old male C57BL/6 mice, purchased from the Chinese Academy of Medical Sciences and housed under pathogen-free conditions, were used.
Tumor cell lines.
The B16-F10 cell line was obtained from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences, Beijing, China. Tumor cells were cultured in growth medium containing Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mmol/liter glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C under a humidified atmosphere of 95% air-5% CO2.
Construction of DNA vaccines.
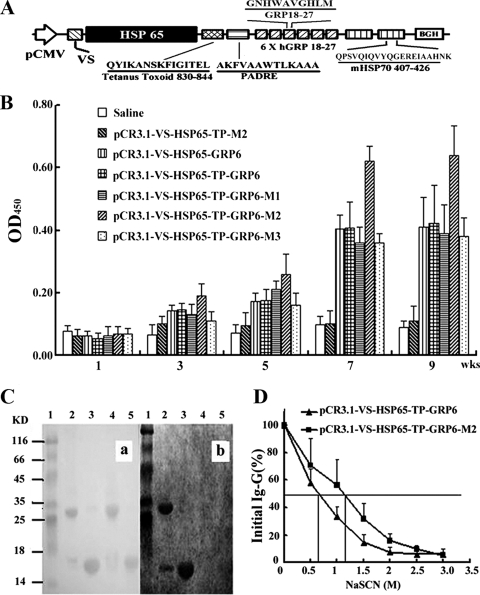
Plasmid pCR3.1 (Invitrogen Corp., CA) containing eight 5′-GACGTT-3′ CpG motifs was used as the backbone for the construction of the DNA vaccines. The DNA fragment encoding HSP65 was PCR amplified from Mycobacterium bovis BCG DNA template as described previously (35). DNA fragments encoding the VEGF183 signal peptide (VS), tetanus toxoid 830-844, PADRE, a fragment of human GRP from amino acids 18 to 27 (GRP18-27), and mycobacterial HSP70407-426 were all chemically synthesized. The VS cDNA was inserted downstream of the cytomegalovirus promoter pCMV, followed sequentially by the genes coding for HSP65, tetanus toxoid 830-844 (T), and PADRE (P). Six tandem repeats of the human GRP18-27 gene (GRP6) were inserted downstream of the PADRE gene. Tandem repeats (from one to three) of the HSP70407-426 gene (M) were then placed downstream of GRP6. These resulted in five DNA vaccines, which were designated pCR3.1-VS-HSP65-GRP6, pCR3.1-VS-HSP65-TP-GRP6, pCR3.1-VS-HSP65-TP-GRP6-M1, pCR3.1-VS-HSP65-TP-GRP6-M2 (Fig. 1A), and pCR3.1-VS-HSP65-TP-GRP6-M3. Plasmid DNA lacking the GRP6 genes (pCR3.1-VS-HSP65-TP-M2) served as the vaccine control. All constructed DNA vaccines and the control were verified by DNA sequencing.
FIG. 1.
Characterization of GRP-specific IgG from immunized mice. (A) Schematic diagram of pCR3.1-VS-HSP65-TP-GRP6-M2. In this DNA vaccine, the VS cDNA (VS) was placed under the control of promoter pCMV (arrow), followed sequentially by the genes encoding for HSP65 (black box), tetanus toxoid 830-844 (T), PADRE (P), six tandem repeats of human GRP18-27 (6 X hGRP 18-27; GRP6), and two copies of mycobacterial HSP70407-426 (mHSP70 407-426; M2). (B) Mice immunized with pCR3.1-VS-HSP65-TP-GRP6-M2 produced the highest titers of anti-GRP antibody than any other groups, especially at 7 weeks (wks) after the initial immunization (P < 0.001). OD450, OD at 450 nm; KD, kilodaltons. (C) Specificity of anti-GRP antibodies was verified using immunoblot analysis. Proteins transferred onto nitrocellulose membranes were stained with Ponceau red (a) or incubated with sera from immunized mice (b). Lanes 1, protein markers; lanes 2, rhVEGF121-GRP18-27 without DTT; lanes 3, rhVEGF121-GRP18-27 with DTT; lanes 4, rhVEGF121 without DTT; lanes 5, rhVEGF121 with DTT. (D) Anti-GRP antibodies from sera of mice immunized with HSP70-containing plasmids had relative avidity significantly (P < 0.0001) higher than that for the group without, according to modified ELISA.
DNA immunization.
Plasmid DNA used for immunization was purified using a Qiagen plasmid mega kit (Qiagen, Germany) and suspended in sterile saline at a concentration of 1 μg/μl. A total of eight C57BL/6 male syngeneic mice (n = 8) were used for each experimental group (one group for each of the six plasmid DNAs, including the saline control group). Mice were anesthetized via intramuscular (IM) injections in the anterior thigh with 100 μl of 0.25% bupivacaine hydrochloride solution. Afterward, mice received IM injections of 100 μl of each of the plasmid DNA vaccines (1 μg/μl) or saline. Booster injections were given every other week (weeks 3, 5, and 7) using the same protocol.
Characterization of anti-GRP antibodies.
The amount of anti-GRP antibodies in the immune sera was determined by enzyme-linked immunosorbent assays (ELISA) as described by Yi et al. (36), using recombinant human VEGF121-GRP18-27 (rhVEGF121-GRP18-27) proteins (10 μg/well) as an immunogen. Assays for each sample were performed in duplicate. For determination of the anti-GRP antibody titers, sera collected at week 9 with different dilutions were tested by using the ELISA method. Preimmune serum was used as the negative control. Antibody titers was defined as the maximum serum dilution that manifested an ELISA optical density (OD) reading that was at least 5 standard deviations greater than the mean OD obtained for wells that contained preimmune serum. The specificity of anti-GRP antibodies was determined by immunoblotting that was similar to that described previously (35), with purified fusion proteins rhVEGF121-GRP18-27 and rhVEGF121 as immunogens. Reduction of rhVEGF121-GRP18-27 and rhVEGF121 was performed by incubation of the recombinant proteins with 10 mM dithiothreitol (DTT) at 50°C for 30 min.
Determination of relative avidity of antibodies.
The method for avidity assays was similar to that described by Pullen et al. (25). Briefly, ELISA plates were coated with 100 μl/well of recombinant rhVEGF121-GRP18-27 proteins (10 μg/well). Plates were blocked and then incubated with 100 μl of 1:100 diluted sera (pCR3.1-VS-HSP65-TP-GRP6-M2 and pCR3.1-VS-HSP65-TP-GRP6 immunization group, week 9) per well. After incubation, the wells were washed and incubated with 100 μl of serial dilutions of 0 to 3 M sodium thiocyanate (NaSCN; Sigma), a chaotropic compound that interferes with the antigen-antibody reaction, in phosphate-buffered saline (PBS). The wells were incubated for 15 min at room temperature before being washed. The remainder of the assay was done in a manner similar to that for the ELISA analysis.
Cell proliferation assay.
The effects of the GRP1-27 full peptide or sera from immunized mice on the proliferation of B16-F10 cells were measured using a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (7). Briefly, B16-F10 cells plated on 96-well plates at 1 × 104 cells per well were incubated with serial dilutions of the GRP1-27 peptide (Sigma) or 1:3 and 1:10 diluted sera for 48 h followed by incubation with 20 μl of MTT for 4 h. At the end of the incubation, absorbances were read at 570 nm. All assays were performed in triplicate.
In vivo tumor studies.
Mice were immunized four times every 2 weeks followed by subcutaneous injections of 1 × 106 B16-F10 cells into the left flank. After 14 days, animals were sacrificed, and solid tumors were excised and weighed.
In vivo angiogenesis assay.
To investigate the effects of anti-GRP DNA vaccines on tumor angiogenesis in vivo, an intradermal tumor model using B16-F10 cells was used. Tumor-associated angiogenesis was quantified by vessel counting methods described previously (17). Briefly, mice immunized four times every 2 weeks were injected intradermally with 1.0 × 106 B16-F10 cells in 50 μl of PBS at two sites in the anterior abdominal wall. When the tumors reached ∼4 mm in diameter, the flap of the abdominal wall skin containing the injected cells was removed. Sections were examined by light microscopy at low magnification (×10), and the total number of blood vessels (major vessels and branch points) was determined within a 1-cm2 area around each implant site.
In vivo assay for tumor metastasis.
Mice immunized four times every 2 weeks were injected intravenously with 1.0 × 105 B16-F10 cells into the tail veins. After 21 days, mice were sacrificed, and each body organ was examined grossly for the presence of metastatic tumor nodules.
Statistical analysis.
Data were collected and subjected to statistical analyses using Student's t test. Differences were considered significant at a P value of <0.05.
RESULTS
DNA vaccines elicited production of specific antibodies against GRP.
To investigate whether DNA vaccines containing six tandem repeats of GRP18-27 could evoke a strong humoral immune response, we compared the levels of GRP-specific immunoglobulin G (IgG) in the sera collected from immunized mice by ELISA (Fig. 1B and Table 1) . Sera samples were collected 1 week, 3 weeks, 5 weeks, 7 weeks, and 9 weeks after the initial immunization. All GRP-based DNA vaccines, compared with the control vaccine (pCR3.1-VS-HSP65-TP-M2) or the saline control, greatly increased titers of specific anti-GRP antibodies 5 weeks postinoculation, and the antibody levels remained high for up to 9 weeks postinoculation. Among the GRP-based vaccines, the anti-GRP antibody titers in mice immunized with the plasmid containing two copies of the mycobacterial HSP70407-426 gene (M) were highest at 9 weeks postimmunization.
TABLE 1.
Titers of anti-GRP antibodies determined by ELISA
| Group | Antibody titer (log2)a |
|---|---|
| pCR3.1-VS-HSP65-GRP6 | 10.56 ± 0.81 |
| pCR3.1-VS-HSP65-TP-GRP6 | 10.68 ± 1.07 |
| pCR3.1-VS-HSP65-TP-GRP6-M1 | 10.37 ± 0.76 |
| pCR3.1-VS-HSP65-TP-GRP6-M2 | 14.84 ± 0.99b |
| pCR3.1-VS-HSP65-TP-GRP6-M3 | 10.49 ± 0.61 |
Sera for detection of antibody titers were collected at week 9 after the initial immunization. Values are means ± standard deviations; there were eight mice in each experimental group.
P < 0.01 by Student's t test.
The specificity of antibodies elicited in immunized mice was established with immunoblots using the rhVEGF121-GRP18-27 fusion protein as the antigen (Fig. 1C). Both the rhVEGF121-GRP18-27 fusion protein and rhVEGF121 are capable of forming homodimers through the disulfide bonds between monomers (11). Antibodies from mice immunized with pCR3.1-VS-HSP65-TP-GRP6-M2 reacted with the rhVEGF121-GRP18-27 fusion protein (lanes 2 and 3) but not with the rhVEGF121 proteins (lanes 4 and 5). Under nonreducing conditions, two reactive bands consistent with the sizes of the dimer and monomer of the rhVEGF121-GRP18-27 (lane 2) were observed; under reducing conditions, a single reactive band (lane 3) compatible with the size of the rhVEGF121-GRP18-27 monomer (lane 2) was observed. These findings suggest that the antibodies in the immune sera specifically recognized the GRP antigen.
To further investigate the relationship between the antibody titers and the biological effects of the GRP vaccines, avidity assays were performed with antisera from mice (n = 8, week 9) immunized with pCR3.1-VS-HSP65-TP-GRP6 or pCR3.1-VS-HSP65-TP-GRP6-M2. The relative avidities of antibodies were observed based on the concentrations of NaSCN required to depress the OD at 450 nm to half its initial value. The mean relative avidity of antibodies in the group vaccinated with pCR3.1-VS-HSP65-TP-GRP6-M2 was significantly higher than that from the group immunized with pCR3.1-VS-HSP65-TP-GRP6 (1.18 ± 0.15 versus 0.66 ± 0.14 M, P < 0.0001) (Fig. 1D).
The GRP1-27 peptide stimulated the proliferation of B16-F10 cells in vitro.
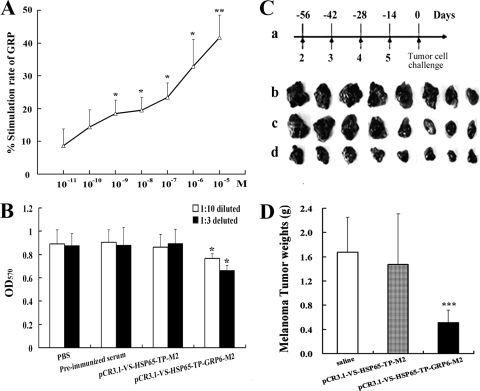
To determine the effect of GRP on malignant melanoma cells, B16-F10 cells plated on 96-well plates were incubated with the full GRP peptide (GRP1-27) in concentrations of 10−5 mol/liter to 10−11 mol/liter. The number of B16-F10 cells increased in a dose-dependent fashion in direct relation to the concentration of the full-length GRP1-27 peptide (Fig. 2A). Significant mitogenic effects, compared with those for the control, were observed initially starting at 10−8 mol/liter (stimulation rate, 19.5%; P < 0.05) and further increased at higher doses, with a stimulation rate of 41.7% at 10−5 mol/liter.
FIG. 2.
Effects of GRP and the anti-GRP vaccine on the proliferation of tumor cells. (A and B) GRP peptides stimulated the proliferation of cultured B16-F10 cells in a dose-dependent manner (*, P < 0.05; **, P < 0.01) (A), while GRP antisera suppressed their growth (*, P < 0.05), as seen with an MTT assay (B). OD570, OD at 570 nm. (C) Immunization scheme. Arrows indicate immunizations (a); the tumors were extracted from mice immunized with saline (b; control, n = 8), pCR3.1-VS-HSP65-TP-M2 (c; control, n = 8), or pCR3.1-VS-HSP65-TP-GRP6-M2 (d; n = 8) 14 days after the tumor cell challenge. (D) Tumor weights from mice immunized with pCR3.1-VS-HSP65-TP-GRP6-M2 were significantly lower than those from the saline group or the vaccine control group. ***, P < 0.001.
Sera from immunized mice inhibited the growth of B16-F10 cells in vitro.
To evaluate the inhibition effect of sera from immunized mice on B16-F10 cells in vitro, cultured cells were incubated with medium-diluted PBS, preimmunized serum, sera (n = 8) from mice immunized with pCR3.1-VS-HSP65-TP-M2, or sera (n = 8) from mice immunized with pCR3.1-VS-HSP65-TP-GRP6-M2. The growth of B16-F10 cells was significantly suppressed by GRP antisera in a dose-dependent manner (Fig. 2B).
The anti-GRP vaccine suppressed the growth of subcutaneous B16-F10 tumors.
B16-F10 cells injected subcutaneously formed large solid tumors in nonimmunized mice (saline) or in mice injected with a non-GRP-containing plasmid (pCR3.1-VS-HSP65-TP-M2). The tumor sizes decreased progressively in mice immunized with the anti-GRP vaccine (pCR3.1-VS-HSP65-TP-GRP6-M2) (Fig. 2C). The average weight of solid tumors in mice immunized with pCR3.1-VS-HSP65-TP-GRP6-M2 was significantly lower than that for mice in the saline group (0.517 ± 0.203 g versus 1.671 ± 0.579 g; P < 0.0001) or for mice injected with the control vaccine (0.517 ± 0.203 g versus 1.475 ± 0.835 g; P < 0.0001) (Fig. 2D).
The anti-GRP vaccine inhibited angiogenesis associated with intradermal B16-F10 tumors.
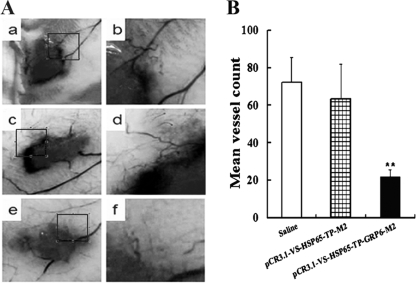
To assess the effect of the immune response on tumor-associated angiogenesis induced by anti-GRP DNA vaccine pCR3.1-VS-HSP65-TP-GRP6-M2, B16-F10 tumor cells were implanted intradermally at two sites in the abdominal region. It took approximately 7 days for the cells to form ∼4-mm intradermal tumors in the two control groups; however, the growth of intradermal tumors in pCR3.1-VS-HSP65-TP-GRP6-M2-immunized group was slightly delayed and required almost 11 days to form ∼4-mm tumors. As shown in Fig. 3A, tumor cells implanted intradermally were associated with significant angiogenesis. The total number of blood vessels around each implant site from pCR3.1-VS-HSP65-TP-GRP6-M2-immunized mice was significantly lower than that from the saline group (22 ± 4 versus 72 ± 14; P < 0.01) or from non-GRP-containing plasmid-immunized mice (22 ± 4 versus 63 ± 19; P < 0.01) (Fig. 3B). There were no significant differences between the two control groups ( P > 0.5).
FIG. 3.
Effects of the anti-GRP vaccine on the growth and angiogenesis of intradermal tumors. (A) Light microscope picture of B16-F10 tumor cells implanted intradermally in the anterior abdominal wall and the development of new blood vessels. Tumor-associated angiogenesis in mice injected with saline (a and b) and the pCR3.1-VS-HSP65-TP-M2 control vaccine (c and d) appeared to be significantly greater than in mice immunized with the pCR3.1-VS-HSP65-TP-GRP6-M2 anti-GRP vaccine (e and f). Representative images were taken with an objective that was ×10 (a, c, and e) or higher (b, d, and f). (B) The total number of blood vessels was determined within the precise 1-cm2 area around each implant site. **, P < 0.01.
The anti-GRP vaccine inhibited lung metastasis of intravenous B16-F10 tumors.
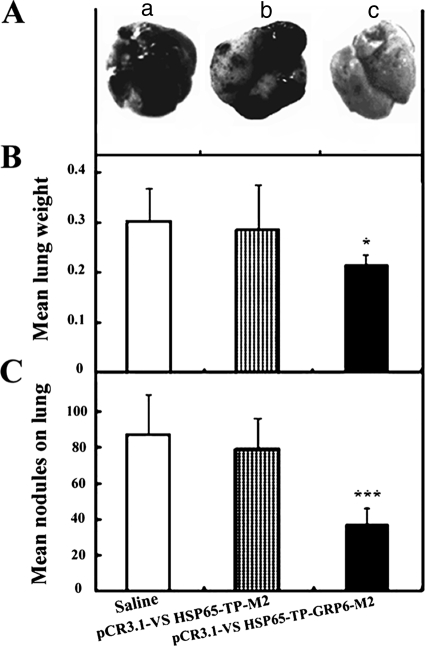
To further test the efficacy of the anti-GRP vaccine, the extent of lung metastasis by intravenously administered tumor cells in the tail vein of immunized mice was evaluated. Metastatic tumor nodules were often detected in the lungs 21 days after injection of tumor cells (Fig. 4A). The average weight of lungs of mice immunized with pCR3.1-VS-HSP65-TP-GRP6-M2 was significantly lower than that of the saline group (0.215 ± 0.020 g versus 0.301 ± 0.068 g; P < 0.05) or the pCR3.1-VS-HSP65-TP-M2 control group (0.215 ± 0.020 g versus 0.289 ± 0.087 g; P < 0.05) (Fig. 4B), which indicates that fewer metastases were formed in the lungs of the anti-GRP DNA vaccine-immunized group. In addition, the average number of metastatic nodules in mice immunized with pCR3.1-VS-HSP65-TP-GRP6-M2 was significantly less than that in mice that received saline (37.2 ± 9.4 versus 88.0 ± 22.6; P < 0.001) or in mice in the pCR3.1-VS-HSP65-TP-M2 control group (37.2 ± 9.4 versus 79.3.0 ± 16.8; P < 0.001) (Fig. 4C). There were no significant differences between results for the two control groups (P > 0.5).
FIG. 4.
Effects of the anti-GRP vaccine on lung metastasis of intravenous B16-F10 cells. (A) Representative lungs from mice (n = 8 for each group) immunized with saline (a) or with the pCR3.1-VS-HSP65-TP-M2 control vaccine (b) showed numerous darkly pigmented metastatic melanoma cells compared with mice immunized with the pCR3.1-VS-HSP65-TP-GRP6-M2 anti-GRP vaccine (c). (B and C) Lungs of mice (n = 8 for each group) immunized with the pCR3.1-VS-HSP65-TP-GRP6-M2 anti-GRP vaccine were significantly (P < 0.05) lighter and contained fewer tumor nodules than lungs from the saline group or from the pCR3.1-VS-HSP65-TP-M2 control vaccine group. Values are means ± standard deviations. *, P < 0.05; ***, P < 0.001.
DISCUSSION
The main obstacle in developing subunit cancer vaccines targeting self-proteins is the low immunogenicity of their epitopes. Our previous study has established that the increased immunogenicity as well as antitumor effects of immunogens containing tandemly repeated B- or T-cell epitopes is associated with the increased copy number of the target sequence (35). These results could be due to the enhanced recognition of the tandemly repeated self-epitope by Th1 cells, resulting in efficient inhibition of Th1 cells and a consequent increase in recruitment of Th2 cells (10). In this study, human GRP18-27 was chosen as the target epitope (21). The GRP epitope was tandemly repeated six times in order to enhance the humoral immune response. To further enhance the immunogenicity of the DNA vaccine, HSP65, tetanus toxoid 830-844, PADRE, and mycobacterial HSP70407-426 were used as fusion partners. Our results showed that the DNA vaccine pCR3.1-VS-HSP65-TP-GRP6-M2, containing two tandem repeats of the mHSP70407-426 epitope (M2), generated the highest titers of anti-GRP antibodies among the DNA vaccines.
HSP70407-426, located in the 70-kDa microbial protein HSP70, has been identified as a major epitope stimulating tumor necrosis factor alpha, interleukin-12, and CCL-5 in monocytes and dendritic cells, comparable to those stimulated by native HSP70 (32). Interestingly, the plasmid coding for two copies of HSP70407-426 showed a stronger immune-stimulatory potential than the one with three copies of HSP70407-426. It could be speculated that the reasons for this phenomenon are as follows. (i) Although mer-epitope effects can induce stronger immunogenicity of the epitope, as the number of epitope copies increases, the immunogenicity of the epitope becomes stronger, and then this effect could reach a crucial point at which the number is still increasing while the immunogenicity decreases (based on unpublished data from our lab). Thus, it is not the immunogen with more copies of the epitopes but rather the moderate one which can induce the strongest immune responses. (ii) The HSP70407-426 epitope in these plasmids is the T-helper epitope, and the leading role should be played by GRP epitope. Too many repeated copies of HSP70407-426 will decrease the immune responses to GRP. The exact mechanism underlying this phenomenon is now under detailed investigation in our lab.
The growth of B16-F10 cells has been shown to be regulated by both GRP and monoclonal antibodies in vitro. Our data demonstrate that the anti-GRP DNA vaccine was highly efficacious against B16-F10 tumors in vivo. High titers of GRP-specific antibodies could neutralize elevated levels of the GRP self-peptide made by tumor cells, thereby blocking activation of GRPR in malignant melanoma cells. Very recently and in analogy, Garcia et al. (6) reported the humoral immune response of patients enrolled in a randomized phase II clinical trial, in which an epidermal growth factor (EGF) vaccination blocked the binding of EGF to its receptor (EGFR), slowing down tumor cell proliferation, which correlated with survival in vaccinated non-small-cell lung cancer patients. The strategy of exploiting anti-EGF autoantibodies to block the EGF/EGFR autocrine loop reduces the growth rate of EGF-dependent tumors, which can also support the function of anti-GRP autoantibodies in blocking the GRP/GRPR autocrine loop of GRP-dependent tumors.
Studies have demonstrated that GRP could contribute to the increased metastatic potential of cancers through the stimulation of proangiogenic gene expression. GRP has been reported to stimulate GPRR-induced NF-κB activation and upregulation of interleukin-8 gene expression (20) as well as the expression of various angiogenic markers, including PECAM-1 and VEGF, and to increase phosphorylated Akt levels (14). To our knowledge, our study is the first to demonstrate that immune responses induced by the pCR3.1-VS-HSP65-TP-GRP6-M2 anti-GRP vaccine significantly reduced tumor-associated angiogenesis and vascularization of solid tumors.
The metastatic spread of cancer cells to different organs represents the major cause of death in cancer patients. BLPs have been shown to stimulate invasion and/or migration of many tumor cell lines (3, 23). BLPs mediate tumor cell invasion and metastasis by stimulating the expression and activation of several integrins, PP125FAK tyrosine phosphorylation, and MMP-9 (4, 5, 19, 28, 33). Bombesin significantly increased the incidence of peritoneal metastasis from gastric cancers and intestinal adenocarcinomas (30, 31). Very recently, Qiao et al. have demonstrated that GRPR silencing suppressed the metastatic potential of neuroblastoma (26). In the current study, we showed for the first time that anti-GRP vaccine-activated GRP immune deprivation suppressed the lung metastasis of intravenously injected tumor cells. The inhibition of tumor invasion to the lungs could be attributed to the following: (i) inhibition of the proliferation of B16-F10 cells invading the lungs and suppression of tumor-associated angiogenesis (29); (ii) downregulation of PKC (27), resulting in antimetastatic effects (37); (iii) upregulation of wild-type p53 (13), leading to the induction of apoptosis of B16-F10, which has very low levels of endogenous p53 (12); or (iv) antigenic epitopes in degenerating B16-F10 cells engulfed by immune-activated antigen-presenting cells could be presented to cytotoxic T lymphocytes, leading to a potent immune response against the remaining tumor cells (15).
In conclusion, we have demonstrated for the first time that immune responses which are elicited by a novel anti-GRP DNA vaccine suppress the proliferation and growth of melanoma tumors in mice. The antiangiogenesis and antimetastastic activities of this DNA vaccine suggest a novel approach against various cancers, especially malignant melanoma.
Acknowledgments
This work was supported by the China National Natural Science Fund Committee (grants no. 30500458, 30701023, 30672464, 30572272, and 30772570) and the Natural Science Foundation of Jiangsu Province (grant no. BK2007170).
Footnotes
Published ahead of print on 20 May 2009.
REFERENCES
- 1.Alexander, J., M. F. del Guercio, A. Maewal, L. Qiao, J. Fikes, R. W. Chesnut, J. Paulson, D. R. Bundle, S. DeFrees, and A. Sette. 2000. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J. Immunol. 1641625-1633. [DOI] [PubMed] [Google Scholar]
- 2.Aprikian, A. G., L. Tremblay, K. Han, and S. Chevalier. 1997. Bombesin stimulates the motility of human prostate-carcinoma cells through tyrosine phosphorylation of focal adhesion kinase and of integrin-associated proteins. Int. J. Cancer 72498-504. [DOI] [PubMed] [Google Scholar]
- 3.Cornelio, D. B., R. Roesler, and G. Schwartsmann. 2007. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann. Oncol. 181457-1466. [DOI] [PubMed] [Google Scholar]
- 4.Festuccia, C., A. Angelucci, G. Gravina, E. Eleuterio, C. Vicentini, and M. Bologna. 2002. Bombesin-dependent pro-MMP-9 activation in prostatic cancer cells requires beta1 integrin engagement. Exp. Cell Res. 2801-11. [DOI] [PubMed] [Google Scholar]
- 5.Festuccia, C., F. Guerra, S. D'Ascenzo, D. Giunciuglio, A. Albini, and M. Bologna. 1998. In vitro regulation of pericellular proteolysis in prostatic tumor cells treated with bombesin. Int. J. Cancer 75418-431. [DOI] [PubMed] [Google Scholar]
- 6.Garcia, B., E. Neninger, A. de la Torre, I. Leonard, R. Martinez, C. Viada, G. Gonzalez, Z. Mazorra, A. Lage, and T. Crombet. 2008. Effective inhibition of the epidermal growth factor/epidermal growth factor receptor binding by anti-epidermal growth factor antibodies is related to better survival in advanced non-small-cell lung cancer patients treated with the epidermal growth factor cancer vaccine. Clin. Cancer Res. 14840-846. [DOI] [PubMed] [Google Scholar]
- 7.Guojun, W., G. Wei, O. Kedong, H. Yi, X. Yanfei, C. Qingmei, Z. Yankai, W. Jie, F. Hao, L. Taiming, L. Jingjing, and C. Rongyue. 2008. A novel vaccine targeting gastrin-releasing peptide: efficient inhibition of breast cancer growth in vivo. Endocr. Relat. Cancer 15149-159. [DOI] [PubMed] [Google Scholar]
- 8.Hellmich, M. R., K. L. Ives, V. Udupi, M. S. Soloff, G. H. Greeley, Jr., B. N. Christensen, and C. M. Townsend, Jr. 1999. Multiple protein kinase pathways are involved in gastrin-releasing peptide receptor-regulated secretion. J. Biol. Chem. 27423901-23909. [DOI] [PubMed] [Google Scholar]
- 9.Heuser, M., T. Schlott, A. V. Schally, E. Kahler, R. Schliephake, S. O. Laabs, and B. Hemmerlein. 2005. Expression of gastrin releasing peptide receptor in renal cell carcinomas: a potential function for the regulation of neoangiogenesis and microvascular perfusion. J. Urol. 1732154-2159. [DOI] [PubMed] [Google Scholar]
- 10.Jin, L., A. Zhu, Y. Wang, Q. Chen, Q. Xiong, J. Li, Y. Sun, T. Li, R. Cao, J. Wu, and J. Liu. 2008. A Th1-recognized peptide P277, when tandemly repeated, enhances a Th2 immune response toward effective vaccines against autoimmune diabetes in nonobese diabetic mice. J. Immunol. 18058-63. [DOI] [PubMed] [Google Scholar]
- 11.Jingjing, L., Y. Xue, N. Agarwal, and R. S. Roque. 1999. Human Muller cells express VEGF183, a novel spliced variant of vascular endothelial growth factor. Investig. Ophthalmol. Vis. Sci. 40752-759. [PubMed] [Google Scholar]
- 12.Kalechman, Y., G. Strassmann, M. Albeck, and B. Sredni. 1998. Up-regulation by ammonium trichloro(dioxoethylene-0,0′) tellurate (AS101) of Fas/Apo-1 expression on B16 melanoma cells: implications for the antitumor effects of AS101. J. Immunol. 1613536-3542. [PubMed] [Google Scholar]
- 13.Kanashiro, C. A., A. V. Schally, K. Groot, P. Armatis, A. L. Bernardino, and J. L. Varga. 2003. Inhibition of mutant p53 expression and growth of DMS-153 small cell lung carcinoma by antagonists of growth hormone-releasing hormone and bombesin. Proc. Natl. Acad. Sci. USA 10015836-15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang, J., T. A. Ishola, N. Baregamian, J. M. Mourot, P. G. Rychahou, B. M. Evers, and D. H. Chung. 2007. Bombesin induces angiogenesis and neuroblastoma growth. Cancer Lett. 253273-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kayaga, J., B. E. Souberbielle, N. Sheikh, W. J. Morrow, T. Scott-Taylor, R. Vile, H. Chong, and A. G. Dalgleish. 1999. Anti-tumour activity against B16-F10 melanoma with a GM-CSF secreting allogeneic tumour cell vaccine. Gene Ther. 61475-1481. [DOI] [PubMed] [Google Scholar]
- 16.Klinman, D. M., G. Yamshchikov, and Y. Ishigatsubo. 1997. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J. Immunol. 1583635-3639. [PubMed] [Google Scholar]
- 17.Kreisle, R. A., and W. B. Ershler. 1988. Investigation of tumor angiogenesis in an id mouse model: role of host-tumor interactions. J. Natl. Cancer Inst. 80849-854. [DOI] [PubMed] [Google Scholar]
- 18.Kumar, A., R. Arora, P. Kaur, V. S. Chauhan, and P. Sharma. 1992. “Universal” T helper cell determinants enhance immunogenicity of a Plasmodium falciparum merozoite surface antigen peptide. J. Immunol. 1481499-1505. [PubMed] [Google Scholar]
- 19.Lacoste, J., A. G. Aprikian, and S. Chevalier. 2005. Focal adhesion kinase is required for bombesin-induced prostate cancer cell motility. Mol. Cell. Endocrinol. 23551-61. [DOI] [PubMed] [Google Scholar]
- 20.Levine, L., J. A. Lucci III, B. Pazdrak, J. Z. Cheng, Y. S. Guo, C. M. Townsend, Jr., and M. R. Hellmich. 2003. Bombesin stimulates nuclear factor kappa B activation and expression of proangiogenic factors in prostate cancer cells. Cancer Res. 633495-3502. [PubMed] [Google Scholar]
- 21.Papac, D. I., J. Hoyes, and K. B. Tomer. 1994. Epitope mapping of the gastrin-releasing peptide/anti-bombesin monoclonal antibody complex by proteolysis followed by matrix-assisted laser desorption ionization mass spectrometry. Protein Sci. 31485-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel, O., C. Dumesny, A. S. Giraud, G. S. Baldwin, and A. Shulkes. 2004. Stimulation of proliferation and migration of a colorectal cancer cell line by amidated and glycine-extended gastrin-releasing peptide via the same receptor. Biochem. Pharmacol. 682129-2142. [DOI] [PubMed] [Google Scholar]
- 23.Patel, O., A. Shulkes, and G. S. Baldwin. 2006. Gastrin-releasing peptide and cancer. Biochim. Biophys. Acta 176623-41. [DOI] [PubMed] [Google Scholar]
- 24.Perraut, R., A. R. Lussow, S. Gavoille, O. Garraud, H. Matile, C. Tougne, J. van Embden, R. van der Zee, P.-H. Lambert, J. Gysin, and G. del Giudice. 1993. Successful primate immunization with peptides conjugated to purified protein derivative or mycobacterial heat shock proteins in the absence of adjuvants. Clin. Exp. Immunol. 93382-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pullen, G. R., M. G. Fitzgerald, and C. S. Hosking. 1986. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 8683-87. [DOI] [PubMed] [Google Scholar]
- 26.Qiao, J., J. Kang, T. A. Ishola, P. G. Rychahou, B. M. Evers, and D. H. Chung. 2008. Gastrin-releasing peptide receptor silencing suppresses the tumorigenesis and metastatic potential of neuroblastoma. Proc. Natl. Acad. Sci. USA 10512891-12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozengurt, E. 1990. Bombesin stimulation of mitogenesis: specific receptors, signal transduction, and early events. Am. Rev. Respir. Dis. 142S11-S15. [DOI] [PubMed] [Google Scholar]
- 28.Salazar, E. P., and E. Rozengurt. 1999. Bombesin and platelet-derived growth factor induce association of endogenous focal adhesion kinase with Src in intact Swiss 3T3 cells. J. Biol. Chem. 27428371-28378. [DOI] [PubMed] [Google Scholar]
- 29.Saurin, J. C., M. Fallavier, B. Sordat, J. C. Gevrey, J. A. Chayvialle, and J. Abello. 2002. Bombesin stimulates invasion and migration of Isreco1 colon carcinoma cells in a Rho-dependent manner. Cancer Res. 624829-4835. [PubMed] [Google Scholar]
- 30.Tatsuta, A., H. Iishi, M. Baba, H. Yano, K. Murata, M. Mukai, and H. Akedo. 2000. Suppression by apigenin of peritoneal metastasis of intestinal adenocarcinomas induced by azoxymethane in Wistar rats. Clin. Exp. Metastasis 18657-662. [DOI] [PubMed] [Google Scholar]
- 31.Tatsuta, M., H. Iishi, M. Baba, H. Narahara, N. Uedo, H. Yano, R. Yamamoto, M. Mukai, and H. Akedo. 2001. Induction by bombesin of peritoneal metastasis of gastric cancers induced by N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats. Gastric Cancer 414-19. [DOI] [PubMed] [Google Scholar]
- 32.Wang, Y., T. Whittall, E. McGowan, J. Younson, C. Kelly, L. A. Bergmeier, M. Singh, and T. Lehner. 2005. Identification of stimulating and inhibitory epitopes within the heat shock protein 70 molecule that modulate cytokine production and maturation of dendritic cells. J. Immunol. 1743306-3316. [DOI] [PubMed] [Google Scholar]
- 33.Wood, M., K. Fudge, J. L. Mohler, A. R. Frost, F. Garcia, M. Wang, and M. E. Stearns. 1997. In situ hybridization studies of metalloproteinases 2 and 9 and TIMP-1 and TIMP-2 expression in human prostate cancer. Clin. Exp. Metastasis 15246-258. [DOI] [PubMed] [Google Scholar]
- 34.Xiao, D., X. Qu, and H. C. Weber. 2002. GRP receptor-mediated immediate early gene expression and transcription factor Elk-1 activation in prostate cancer cells. Regul. Pept. 109141-148. [DOI] [PubMed] [Google Scholar]
- 35.Yankai, Z., Y. Rong, H. Yi, L. Wentao, C. Rongyue, Y. Ming, L. Taiming, L. Jingjing, and W. Jie. 2006. Ten tandem repeats of beta-hCG 109-118 enhance immunogenicity and anti-tumor effects of beta-hCG C-terminal peptide carried by mycobacterial heat-shock protein HSP65. Biochem. Biophys. Res. Commun. 3451365-1371. [DOI] [PubMed] [Google Scholar]
- 36.Yi, H., Y. Rong, Z. Yankai, L. Wentao, Z. Hongxia, W. Jie, C. Rongyue, L. Taiming, and L. Jingjing. 2006. Improved efficacy of DNA vaccination against breast cancer by boosting with the repeat beta-hCG C-terminal peptide carried by mycobacterial heat-shock protein HSP65. Vaccine 242575-2584. [DOI] [PubMed] [Google Scholar]
- 37.Yoshikawa, N., K. Nakamura, Y. Yamaguchi, S. Kagota, K. Shinozuka, and M. Kunitomo. 2003. Effect of PKC412, a selective inhibitor of protein kinase C, on lung metastasis in mice injected with B16 melanoma cells. Life Sci. 721377-1387. [DOI] [PubMed] [Google Scholar]