Abstract
Apical membrane antigen 1 (AMA1) and the 42-kDa merozoite surface protein 1 (MSP142) are leading malaria vaccine candidates. Several preclinical and clinical trials have been conducted, and an in vitro parasite growth inhibition assay has been used to evaluate the biological activities of the resulting antibodies. In a U.S. phase 1 trial with AMA1-C1/Alhydrogel plus CPG 7909, the vaccination elicited anti-AMA1 immunoglobulin G (IgG) which showed up to 96% inhibition. However, antibodies induced by MSP142-C1/Alhydrogel plus CPG 7909 vaccine showed less than 32% inhibition in vitro. To determine whether anti-MSP142 IgG had less growth-inhibitory activity than anti-AMA1 IgG in vitro, the amounts of IgG that produced 50% inhibition of parasite growth (Ab50) were compared for rabbit and human antibodies. The Ab50s of rabbit and human anti-MSP142 IgGs were significantly higher (0.21 and 0.62 mg/ml, respectively) than those of anti-AMA1 IgGs (0.07 and 0.10 mg/ml, respectively) against 3D7 parasites. Ab50 data against FVO parasites also demonstrated significant differences. We further investigated the Ab50s of mouse and monkey anti-AMA1 IgGs and showed that there were significant differences between the species (mouse, 0.28 mg/ml, and monkey, 0.14 mg/ml, against 3D7 parasites). Although it is unknown whether growth-inhibitory activity in vitro reflects protective immunity in vivo, this study showed that the Ab50 varies with both antigen and species. Our data provide a benchmark for antibody levels for future AMA1- or MSP142-based vaccine development efforts in preclinical and clinical trials.
The scourge of malaria remains a global health problem, and 2.4 billion people live in areas at risk of infection with Plasmodium falciparum, the most virulent species of malaria in humans (10). An effective vaccine directed to blood-stage malaria parasites, which are responsible for the pathology associated with this disease, is of importance to control or eliminate the disease (8, 32). Apical membrane antigen 1 (AMA1) and the 42-kDa merozoite surface protein 1 fragment (MSP142) are leading vaccine candidates, and various evidence from epidemiologic and animal studies supports these proteins as potential vaccine targets (8, 33). Both proteins are expressed on the surface of merozoites (the invasive form of the blood-stage parasite) and are thought to have an important role in the invasion process; MSP1 is responsible for initial attachment and AMA1 for reorientation (9, 18, 25).
In addition to the range of preclinical studies in animals, multiple phase 1 clinical trials have been conducted with either AMA1 or MSP142 (6, 7, 15, 16, 22, 23, 24, 26, 28, 30, 31, 33). However, the lack of a validated animal model or an in vitro correlate of protection makes it difficult to assess the efficacy of a vaccine candidate in terms of clinical protection. As of today, performing a large phase 2 trial is the only way to measure efficacy of a blood-stage vaccine; a trial with over 1,000 children is required to obtain precise estimates of efficacy against mild or uncomplicated clinical malaria disease (21). An in vitro parasite growth inhibition assay (GIA; also referred to as the invasion inhibition assay) is one of the few assays that can measure the biological activity of antibodies against parasites (or infected erythrocytes). Therefore, the GIA has been used in preclinical and phase 1 studies as one of the immunological measurements. A recent study showed that in Kenyan children and adults, time to infection after drug treatment was significantly associated with the growth-inhibitory activity measured by an in vitro GIA (5). Another study showed that individuals with high-level MSP119-specific invasion-inhibitory antibody had a 66% reduction in the risk of blood-stage infection during a 12-week follow-up after drug treatment (13).
In preclinical studies and also several clinical trials in malaria-naive populations, we have shown that when the anti-AMA1 or -MSP142 antibody units (as measured by enzyme-linked immunosorbent assay [ELISA]) of total immunoglobulin G (IgG) were plotted against the percent inhibition (as measured by GIA), there was a strong correlation between antibody units and percent inhibition, with the relationship following a symmetrical sigmoid curve (16, 20, 22, 29). In a U.S. phase 1 trial, AMA1-C1 vaccine (a mixture of FVO and 3D7 allelic forms of AMA1 formulated with Alhydrogel plus CPG 7909) elicited anti-AMA1 IgGs. The IgGs showed up to 96% inhibition of growth against P. falciparum 3D7 parasites (22). However, antibodies induced by MSP142-C1 vaccine (a mixture of FVO and 3D7 allelic forms of MSP142 formulated with Alhydrogel plus CPG 7909) in a U.S. trial showed less than 32% inhibition in vitro (L. Martin et al., 56th Annu. Meet. Am. Soc. Trop. Med. Hyg., abstr. 213, p. 62, 2007). Therefore, in this study, we attempted to test the hypothesis that anti-MSP142 antibody has less biological activity than anti-AMA1 antibody in the GIA.
To our knowledge, there is no study which has directly compared the amount of IgG that gives 50% inhibition of parasite growth (Ab50) between anti-AMA1 and anti-MSP142 antibodies in the GIA. Although there is an argument as to whether in vitro growth-inhibitory activity can be a surrogate marker for in vivo clinical protection for AMA1- and/or MSP1-based vaccines, the GIA is currently one of a few biological assays widely used to estimate the potential of blood-stage vaccines. In this study, the Ab50s for these two vaccine candidates were compared using both rabbit and human antibodies. In addition, for preclinical studies with AMA1- and/or MSP1-based vaccines in the future, we investigated the Ab50s of anti-AMA1 antibodies in two more species (mouse and monkey) to determine whether there are differences in biological activities of antibodies between species. This study showed that the Ab50s of anti-AMA1 IgGs were significantly lower than those of anti-MSP142 IgGs and that there were significant differences in Ab50s between species.
MATERIALS AND METHODS
Animal studies.
Mouse, rabbit, and monkey studies were done in compliance with National Institutes of Health guidelines and under the auspices of Animal Care and Use Committee-approved protocols. BALB/c mice, New Zealand White rabbits, and rhesus monkeys (Macaca mulatta) were immunized with either AMA1-C1 or MSP142-C1 formulated with several adjuvants (e.g., aluminum hydroxide [alum], Montanide ISA720, etc.).
Human trials.
Details of the three AMA1-C1 trials have been supplied elsewhere (22; www.clinicaltrials.gov, NCT00427167 and NCT00487916). In brief, malaria-naive adult volunteers were immunized with either 20 or 80 μg of AMA1-C1/Alhydrogel with or without CPG 7909 in the first trial. In the second study, malaria-naive adult volunteers were immunized with 80 μg of AMA1-C1/Alhydrogel with CPG 7909 using two different buffers. In the third trial, malaria-naive adult volunteers were immunized with 5, 20, or 80 μg of AMA1-C1/ISA720. Details of the MSP142-C1 trial have been provided elsewhere (www.clinicaltrials.gov, NCT00320658). In brief, malaria-naive adult volunteers were immunized with either 40 or 160 μg of MSP142-C1/Alhydrogel with or without CPG 7909. All trials were conducted under Investigational New Drug Applications reviewed by the U.S. Food and Drug Administration, and all were reviewed and approved by the Institutional Review Boards at the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and at the respective study sites. Written informed consent was obtained from all participants.
Total IgG purification and antigen-specific IgG purification.
Total IgGs were purified from individual or pooled serum/plasma by using protein G columns according to the manufacturer's instructions (Pierce, Inc., Rockford, IL). The eluted fractions were immediately neutralized with Tris buffer (pH 9.0), dialyzed against RPMI 1640, and concentrated with centrifugal filter devices to a concentration of 10 to 40 mg/ml. The AMA1-specific antibodies in the total IgG preparation were isolated by affinity adsorption to AMA1 protein immobilized on NHS-activated Sepharose 4 Fast Flow according to the manufacturer's instructions (GE Healthcare, Piscataway, NJ). Total IgGs (0.5 or 1 ml of each) were loaded onto the column and antigen-specific antibodies were eluted using elution buffer (0.1 M glycine, pH 2.7) and then immediately neutralized with Tris buffer (pH 9.0). AMA1-specific antibodies were dialyzed against RPMI 1640 and concentrated to 50 to 300 μl of final product. Anti-MSP142-specific IgGs in total IgGs were isolated in a similar manner using MSP142 affinity columns. The details of the AMA1 and MSP142 proteins used for the antigen-specific antibody purifications were described previously (14, 15). The antigens were cGMP-grade material and the purity of the proteins was ∼95% or more. For antigen-specific IgG purification, at least four different samples were prepared for each antigen and for each animal species. Total and antigen-specific IgGs were buffer exchanged and concentrated before ELISA and GIA. The recovery rate of the antigen-specific IgGs was ∼50%, regardless of antigen or species.
ELISA and GIA.
The standardized methodology for performing the ELISA and the GIA has been described previously (19). The ELISA unit value of a standard was assigned as the reciprocal of the dilution giving an optical density at 405 nm (OD405) of 1 in a standardized assay. The absorbance of individual test samples was converted into ELISA units using a standard curve generated by serially diluting the standard in the same plate. All GIAs were conducted with either protein G-purified total IgG or antigen-specific IgG. For the GIA, test IgG, synchronized P. falciparum parasites, and culture medium were applied to 96-well tissue culture plates and maintained for ∼40 h. Relative parasitemia levels were quantitated by biochemical determination of parasite lactate dehydrogenase. Percent inhibition of the immune IgG was calculated as follows: 100 − [(A650 of test IgG − A650 of normal red blood cells)/(A650 of infected red blood cells without any IgG − A650 of normal red blood cells) × 100]. Total IgGs were tested against both P. falciparum 3D7 and FVO parasites. In contrast, antigen-specific IgGs were tested against only homologous parasites (e.g., anti-AMA1-3D7 and MSP142-3D7-specific IgGs were tested against P. falciparum 3D7 parasites, etc.).
Statistical analysis.
For each antigen, in each species, a conversion factor (the concentration [in mg/ml] of IgG which gave 1 ELISA unit) was estimated using a least-squares model and log transformations.
The Ab50s in ELISA units and their standard errors were estimated for each antigen-species combination from a nonlinear mixed effects model using the nlme R package (version 3.1-89; J. Pinheiro, D. Bates, S. DebRoy, D. Sarkar, et al.). Specifically, to predict the growth inhibition from the jth animal in the ith study, say, Yij, we used a Hill model based on the associated ELISA units. For example, for Xij, Yij = [100(Xij/10bi)ai]/[1 + (Xij/10bi)ai] + ɛij, where ai is the Hill coefficient, bi is the log10(Ab50) for that study, and the ɛij term represents the independent error. The coefficients ai and bi reflect both a random study effect and the associated fixed antigen/species effect.
The fixed antigen/species Ab50 effects were converted to mg/ml by using the determined conversion factor. The log-transformed Ab50 effect (in mg/ml) for each antigen/species combination was assumed to be normally distributed with a mean equal to the sum of the fixed log10(Ab50) effect, in ELISA units, and the log-transformed conversion factor, with a variance equal to the sum of the two associated variances. Since several pairwise comparisons were done between species on the AMA1 data, we used Holm's adjusted P values for that family of tests.
RESULTS
Conversion factors.
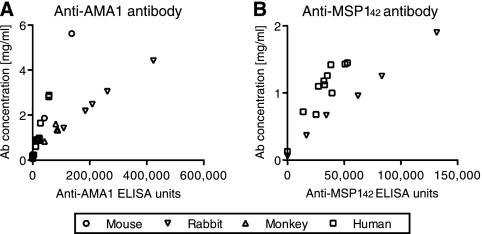
To compare the Ab50s between antigens and species, we first converted the arbitrary ELISA units to actual protein concentrations (i.e., mg/ml). We affinity purified AMA1-3D7- or MSP142-3D7-specific IgGs from more than four pooled samples for each antigen and for each species and then determined the ELISA units and protein concentration of each antigen-specific IgG. As shown in Fig. 1, there was a strong relationship between ELISA units and protein concentration of antigen-specific IgGs for each antigen in each species. Similarly, anti-AMA1/MSP142-FVO-specific IgGs also showed a strong relationship (data not shown). Based on these results, we calculated the conversion factor (the protein concentration of antibody equivalent to 1 ELISA unit) for each antigen in each species. The conversion factors were in the range of 9.8 × 10−6 to 62.7 × 10−6 mg/ml per 1 ELISA unit, depending on the antigen and species.
FIG. 1.
Correlation between ELISA units and protein concentration of AMA1-specific and MSP142-specific antibodies. Antigen-specific IgGs were purified from mouse, rabbit, monkey, and human samples and tested by ELISA following a standardized procedure. ELISA units (x axis) of the IgG are plotted against the protein concentration (y axis). (A) AMA1-3D7-specific IgGs were purified from mouse (from 4 samples), rabbit (6 samples), monkey (5 samples) and human (21 samples) specimens and the ELISA units were tested with AMA1-3D7-coated plates. (B) MSP142-3D7-specific IgGs were purified from rabbit (6 samples) and human (12 samples) specimens and tested with MSP142-3D7-coated ELISA plates.
Growth-inhibitory activity of total and antigen-specific IgGs.
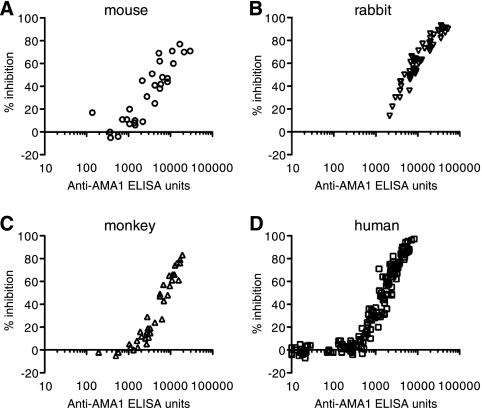
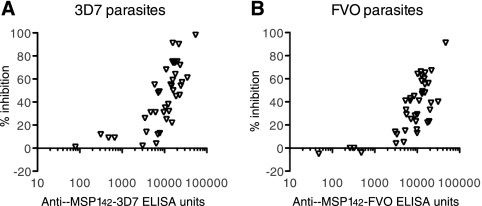
When the GIA was performed with individual anti-AMA1 total IgGs against 3D7 strains of P. falciparum, there was a strong correlation between ELISA units in the GIA well and growth-inhibitory activity for each species (Fig. 2). The results of the GIA against FVO parasites also showed a strong correlation (data not shown). Similarly, there was a strong correlation for rabbit anti-MSP142 total IgGs (Fig. 3).
FIG. 2.
Biological activities of anti-AMA1 total IgGs against P. falciparum 3D7 parasites as judged by the in vitro GIA. Growth-inhibitory activities of mouse (A), rabbit (B), monkey (C), or human (D) anti-AMA1 total IgGs are shown. Anti-AMA1-3D7 ELISA units in the GIA well (x axis) are plotted against the percent inhibition (y axis) of P. falciparum 3D7 parasites.
FIG. 3.
Biological activities of rabbit anti-MSP142 total IgGs against P. falciparum 3D7 and FVO parasites. Growth-inhibitory activities of rabbit anti-MSP142 total IgGs against 3D7 (A) or FVO (B) parasites are shown. Anti-MSP142 ELISA units in the GIA well (x axis) are plotted against the percent inhibition (y axis).
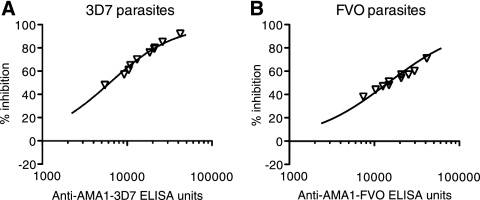
When rabbit anti-AMA1-specific IgG data (Fig. 4, individual points) were superimposed onto the best-fit curve of the data from rabbit anti-AMA1 total IgG data (Fig. 4), both total and antigen-specific IgGs showed similar growth-inhibitory activities when the ELISA units were normalized.
FIG. 4.
Biological activities of rabbit anti-AMA1-specific IgGs against P. falciparum 3D7 and FVO parasites. Growth-inhibitory activities of affinity-purified rabbit anti-AMA1-specific IgGs against 3D7 (A) or FVO (B) parasites are shown. Anti-AMA1 ELISA units in the GIA well (x axis) are plotted against the percent inhibition (y axis). The line represents the best fit of the data from rabbit anti-AMA1 total IgGs, not from anti-AMA1-specific IgGs.
Ab50 comparison.
Based on the best-fitting curve generated from GIA with anti-AMA1 and MSP142 total IgGs for both 3D7 and FVO, we calculated Ab50s in ELISA units for each species for each antigen. In the case of human MSP142, we calculated the Ab50 using both total and antigen-specific IgGs data, because the concentration of the total IgGs in the GIA well did not show more than 32% inhibition. Subsequently, the Ab50 in ELISA units was transformed into an Ab50 in mg/ml by using a conversion factor for each antigen in each species.
When we compared anti-MSP142 IgGs and anti-AMA1 IgGs (Table 1), the Ab50s of anti-MSP142 in both rabbits and humans were significantly higher than the Ab50s of anti-AMA1 IgGs (P < 0.001 for both species for both parasite strains); thus, a higher level of antibody was needed for MSP142 to achieve the same level of growth-inhibitory activity compared to AMA1.
TABLE 1.
Ab50 for growth-inhibitory activity of each P. falciparum antigen and species
| Antigen and species | Ab50 (mg/ml)a
|
|
|---|---|---|
| 3D7 parasites | FVO parasites | |
| AMA1 | ||
| Mouse | 0.28 (0.19-0.42) | 0.71 (0.41-1.25) |
| Rabbit | 0.07 (0.05-0.10) | 0.13 (0.10-0.18) |
| Monkey | 0.14 (0.09-0.20) | 0.27 (0.17-0.42) |
| Human | 0.10 (0.07-0.14) | 0.18 (0.13-0.24) |
| MSP142 | ||
| Rabbit | 0.21 (0.14-0.33) | 0.40 (0.25-0.64) |
| Human | 0.62 (0.39-1.00) | 1.11 (0.61-2.04) |
Data are means with 95% confidence intervals shown in parentheses.
When the Ab50s for 3D7 parasites of anti-AMA1 IgGs in different species were compared, there were significant differences between species: mouse > rabbit (P < 0.001); mouse > monkey (P = 0.036); mouse > human (P < 0.0001); monkey > rabbit (P = 0.037). The Ab50s of anti-AMA1 IgGs to FVO parasites showed a similar pattern: mouse > rabbit (P < 0.001); mouse > monkey (P = 0.029); mouse > human (P < 0.0001); monkey > rabbit (P = 0.044). In the case of anti-MSP142 IgGs, human IgGs required significantly more antibodies than rabbit IgGs (P = 0.001 for 3D7 and P = 0.009 for FVO parasites).
DISCUSSION
This study shows that more anti-MSP142 antibodies than anti-AMA1 antibodies were required to attain comparable levels of parasite growth-inhibitory activity in both rabbit and human. This is consistent with the lower growth-inhibitory activity observed in the MSP142 clinical trials compared to the AMA1 clinical trials. In addition, this study also showed that different species had different Ab50 values even when they were immunized with the same antigen. Since this study provides the Ab50 in mg/ml for various species, investigators can use these numbers to compare their vaccines on the same scale for future AMA1- or MSP142-based vaccine development efforts in preclinical and clinical trials.
The ELISA units of samples were calculated based on the ELISA units of a reference standard tested in the same plate, and we used different secondary antibodies to detect the primary anti-AMA1 or anti-MSP142 antibodies. Therefore, 1 ELISA unit in one species does not equate to the same amount of antibody in other species. Even when we used the same secondary antibody from the same company at the same dilution, we sometimes observed different signal strengths (i.e., different OD values), depending on the lot number of the product (unpublished results). Therefore, to compare Ab50s in different species and/or for different antigens, we needed to convert the arbitrary OD-based ELISA units to actual protein concentrations (i.e., in mg/ml). To do that, we affinity purified antigen-specific IgGs and calculated the conversion factor (the protein concentration of antibody equivalent to 1 ELISA unit) for each animal species for each antigen. Actually, we observed an almost-sixfold difference in conversion factors. However, there is a concern whether the affinity purification procedure may change the quality of antibody (e.g., destroy the function or select only lower-affinity antibody, as high-affinity antibody is thought to be hard to elute from a column). However, as we previously reported when we used human IgGs (19), both rabbit total IgGs and antigen-specific IgGs showed similar growth-inhibitory activities when the ELISA units were normalized (Fig. 4). In addition, we have also shown (19) that AMA1-specific IgGs purified from Malian sera by the same method used in this study showed similar levels of growth-inhibitory activities as AMA1-specific IgGs isolated from Papua New Guinea samples by Hodder and his colleagues (11). These data suggest that the affinity purification procedure used in this study did not change the quality of antibody as judged by GIA. Therefore, we believe that it is acceptable to calculate Ab50s of total IgGs for each antigen and species in ELISA units and then convert the Ab50 (in mg/ml) by using conversion factors, which are calculated from antigen-specific IgGs.
To the best of our knowledge, this is the first report to compare directly the functional activities (Ab50s) of anti-AMA1 and anti-MSP142 antibodies. In both rabbits and humans, more anti-MSP142 antibodies were required to reach 50% inhibition than anti-AMA1 antibodies. Although we do not know the mechanism responsible for this difference, one hypothesis is that more anti-MSP142 antibodies are required to block the growth, as MSP142 protein is the most abundant protein on the surface of the merozoite while AMA1 protein is presented only in the merozoite's apical pole (4, 25). This study also shows different Ab50s not only between antigens but also between species immunized. For each species, several adjuvants, protein doses, and bleed time points were tested. However, as shown in Fig. 2, all of the data points basically followed a single dose-response curve in each species. Therefore, we did not consider the difference between the species to be due to vaccine formulation. Further research is needed to uncover the bases for the differences between antigens and species.
Although this study shows that more anti-MSP142 antibody is required to reach the same level of inhibition in the GIA, it does not necessarily mean that AMA1 is a better vaccine candidate than MSP142. First, it is still unresolved whether any in vitro assay correlates with clinical protection. Second, other mechanisms of immune protection have been reported in the case of MSP119, a small fragment of MSP142. A recent study using transgenic rodent malaria and mice also transgenic for human Fc receptors showed that Fc receptor-mediated protection is important (17). The GIA does not measure the Fc receptor-mediated immune response and so would fail to detect this possible mechanism of protection. Although there is another in vitro functional assay that is considered to be able to measure the Fc receptor-mediated response, called the antibody-dependent cellular inhibitory (2), no antibody-dependent cellular inhibitory activity has been reported for MSP142 at this stage.
It has been shown that transfusion of IgG from malaria-immune individuals reduces parasitemia in malaria patients (3, 27), providing evidence that antibodies are important for clinical protection against blood-stage malaria in humans. As noted above, recent studies suggested that there are some correlations between the total (5) or MSP119-specific (13) growth-inhibitory activity and clinical protection under certain conditions. However, while it is unknown whether growth-inhibitory activity in vitro can be a marker of clinical protection induced by a blood-stage vaccine, the GIA is one of only a few assays to measure functional activity of anti-AMA1/MSP1 antibodies against parasites at this time. Indeed, to demonstrate biologic activity of induced antibodies, the GIA has been used not only for preclinical development but also for clinical phase 1 trials of AMA1- and/or MSP1-based vaccines (6, 12, 15, 16, 22, 23, 24, 26).
Yoon et al. used a different method for determination of a conversion factor (34), and Bergmann-Leitner et al. have shown that the total IgG purification method affects the growth-inhibitory activity (1). In addition, as we showed previously with an AMA1 vaccine in rabbits (20), the AMA1 combination vaccine (FVO plus 3D7) induced more cross-reactive antibodies than a single-allele vaccine, and the growth-inhibitory activity of the cross-reactive antibody was slightly weaker than that of strain-specific antibody when antibody titers were normalized. Therefore, the actual Ab50 values might be slightly different when one uses a different method of purification or evaluates the GIA with purified IgGs from individuals immunized with a single-allele antigen. However, since we used the same method for all of the samples in this study and differences between cross-reactive and strain-specific antibodies in the GIA were not large, it is likely that the relative differences in the Ab50s between the AMA1 and MSP142 vaccines will hold even with other methods and/or a single-allele vaccine. Therefore, future preclinical and clinical trials with AMA1/MSP142-based vaccines need to take into account the relative differences in Ab50s shown in this study in evaluating the potential of a vaccine by using the GIA.
Acknowledgments
We are very grateful to all volunteers who participated in the clinical trials. We also appreciate John Treanor, Anna Durbin, Joanne Margason, Ev Tierney, and Allan Saul for contributions to the human trials. We are also very grateful to Lynn Lambert and her team for meticulous execution of animal immunization studies.
The studies were supported by the intramural program of the National Institute of Allergy and Infectious Diseases, NIH, and the GIA Reference Center was supported by the PATH/Malaria Vaccine Initiative.
Footnotes
Published ahead of print on 13 May 2009.
REFERENCES
- 1.Bergmann-Leitner, E. S., R. M. Mease, E. H. Duncan, F. Khan, J. Waitumbi, and E. Angov. 2008. Evaluation of immunoglobulin purification methods and their impact on quality and yield of antigen-specific antibodies. Malar. J. 7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouharoun-Tayoun, H., P. Attanath, A. Sabchareon, T. Chongsuphajaisiddhi, and P. Druilhe. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 1721633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen, S., I. A. Mcgregor, and S. Carrington. 1961. Gamma-globulin and acquired immunity to human malaria. Nature 192733-737. [DOI] [PubMed] [Google Scholar]
- 4.Cowman, A. F., and B. S. Crabb. 2006. Invasion of red blood cells by malaria parasites. Cell 124755-766. [DOI] [PubMed] [Google Scholar]
- 5.Dent, A. E., E. S. Bergmann-Leitner, D. W. Wilson, D. J. Tisch, R. Kimmel, J. Vulule, P. O. Sumba, J. G. Beeson, E. Angov, A. M. Moormann, and J. W. Kazura. 2008. Antibody-mediated growth inhibition of Plasmodium falciparum: relationship to age and protection from parasitemia in Kenyan children and adults. PLoS ONE 3e3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dicko, A., D. J. Diemert, I. Sagara, M. Sogoba, M. B. Niambele, M. H. Assadou, O. Guindo, B. Kamate, M. Baby, M. Sissoko, E. M. Malkin, M. P. Fay, M. A. Thera, K. Miura, A. Dolo, D. A. Diallo, G. E. Mullen, C. A. Long, A. Saul, O. Doumbo, and L. H. Miller. 2007. Impact of a Plasmodium falciparum AMA1 vaccine on antibody responses in adult Malians. PLoS ONE 2e1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dicko, A., I. Sagara, R. D. Ellis, K. Miura, O. Guindo, B. Kamate, M. Sogoba, M. B. Niambele, M. Sissoko, M. Baby, A. Dolo, G. E. Mullen, M. P. Fay, M. Pierce, D. A. Diallo, A. Saul, L. H. Miller, and O. K. Doumbo. 2008. Phase 1 study of a combination AMA1 blood stage malaria vaccine in Malian children. PLoS ONE 3e1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girard, M. P., Z. H. Reed, M. Friede, and M. P. Kieny. 2007. A review of human vaccine research and development: malaria. Vaccine 251567-1580. [DOI] [PubMed] [Google Scholar]
- 9.Goel, V. K., X. Li, H. Chen, S. C. Liu, A. H. Chishti, and S. S. Oh. 2003. Band 3 is a host receptor binding merozoite surface protein 1 during the Plasmodium falciparum invasion of erythrocytes. Proc. Natl. Acad. Sci. USA 1005164-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerra, C. A., P. W. Gikandi, A. J. Tatem, A. M. Noor, D. L. Smith, S. I. Hay, and R. W. Snow. 2008. The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS Med. 5e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodder, A. N., P. E. Crewther, and R. F. Anders. 2001. Specificity of the protective antibody response to apical membrane antigen 1. Infect. Immun. 693286-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu, J., Z. Chen, J. Gu, M. Wan, Q. Shen, M. P. Kieny, J. He, Z. Li, Q. Zhang, Z. H. Reed, Y. Zhu, W. Li, Y. Cao, L. Qu, Z. Cao, Q. Wang, H. Liu, X. Pan, X. Huang, D. Zhang, X. Xue, and W. Pan. 2008. Safety and immunogenicity of a malaria vaccine, Plasmodium falciparum AMA-1/MSP-1 chimeric protein formulated in montanide ISA 720 in healthy adults. PLoS ONE 3e1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.John, C. C., R. A. O'Donnell, P. O. Sumba, A. M. Moormann, T. F. de Koning-Ward, C. L. King, J. W. Kazura, and B. S. Crabb. 2004. Evidence that invasion-inhibitory antibodies specific for the 19-kDa fragment of merozoite surface protein-1 (MSP-119) can play a protective role against blood-stage Plasmodium falciparum infection in individuals in a malaria endemic area of Africa. J. Immunol. 173666-672. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy, M. C., J. Wang, Y. Zhang, A. P. Miles, F. Chitsaz, A. Saul, C. A. Long, L. H. Miller, and A. W. Stowers. 2002. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect. Immun. 706948-6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malkin, E., C. A. Long, A. W. Stowers, L. Zou, S. Singh, N. J. Macdonald, D. L. Narum, A. P. Miles, A. C. Orcutt, O. Muratova, S. E. Moretz, H. Zhou, A. Diouf, M. Fay, E. Tierney, P. Leese, S. Mahanty, L. H. Miller, A. Saul, and L. B. Martin. 2007. Phase 1 study of two merozoite surface protein 1 (MSP142) vaccines for Plasmodium falciparum malaria. PLoS Clin. Trials 2e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malkin, E. M., D. J. Diemert, J. H. McArthur, J. R. Perreault, A. P. Miles, B. K. Giersing, G. E. Mullen, A. Orcutt, O. Muratova, M. Awkal, H. Zhou, J. Wang, A. Stowers, C. A. Long, S. Mahanty, L. H. Miller, A. Saul, and A. P. Durbin. 2005. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect. Immun. 733677-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIntosh, R. S., J. Shi, R. M. Jennings, J. C. Chappel, T. F. de Koning-Ward, T. Smith, J. Green, M. van Egmond, J. H. Leusen, M. Lazarou, J. van de Winkel, T. S. Jones, B. S. Crabb, A. A. Holder, and R. J. Pleass. 2007. The importance of human FcγRI in mediating protection to malaria. PLoS Pathog. 3e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell, G. H., A. W. Thomas, G. Margos, A. R. Dluzewski, and L. H. Bannister. 2004. Apical membrane antigen 1, a major malaria vaccine candidate, mediates the close attachment of invasive merozoites to host red blood cells. Infect. Immun. 72154-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miura, K., H. Zhou, S. E. Moretz, A. Diouf, M. A. Thera, A. Dolo, O. Doumbo, E. Malkin, D. Diemert, L. H. Miller, G. E. Mullen, and C. A. Long. 2008. Comparison of biological activity of human anti-apical membrane antigen-1 antibodies induced by natural infection and vaccination. J. Immunol. 1818776-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miura, K., H. Zhou, O. V. Muratova, A. C. Orcutt, B. Giersing, L. H. Miller, and C. A. Long. 2007. In immunization with Plasmodium falciparum apical membrane antigen 1, the specificity of antibodies depends on the species immunized. Infect. Immun. 755827-5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moorthy, V. S., Z. Reed, and P. G. Smith. 2008. MALVAC 2008. Measures of efficacy of malaria vaccines in phase 2b and phase 3 trials: scientific, regulatory and public health perspectives. Vaccine 27624-628. [DOI] [PubMed] [Google Scholar]
- 22.Mullen, G. E., R. D. Ellis, K. Miura, E. Malkin, C. Nolan, M. Hay, M. P. Fay, A. Saul, D. Zhu, K. Rausch, S. Moretz, H. Zhou, C. A. Long, L. H. Miller, and J. Treanor. 2008. Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria. PLoS ONE 3e2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ockenhouse, C. F., E. Angov, K. E. Kester, C. Diggs, L. Soisson, J. F. Cummings, A. V. Stewart, D. R. Palmer, B. Mahajan, U. Krzych, N. Tornieporth, M. Delchambre, M. Vanhandenhove, O. Ofori-Anyinam, J. Cohen, J. A. Lyon, and D. G. Heppner. 2006. Phase I safety and immunogenicity trial of FMP1/AS02A, a Plasmodium falciparum MSP-1 asexual blood stage vaccine. Vaccine 243009-3017. [DOI] [PubMed] [Google Scholar]
- 24.Polhemus, M. E., A. J. Magill, J. F. Cummings, K. E. Kester, C. F. Ockenhouse, D. E. Lanar, S. Dutta, A. Barbosa, L. Soisson, C. L. Diggs, S. A. Robinson, J. D. Haynes, V. A. Stewart, L. A. Ware, C. Brando, U. Krzych, R. A. Bowden, J. D. Cohen, M. C. Dubois, O. Ofori-Anyinam, E. De-Kock, W. R. Ballou, and D. G. Heppner, Jr. 2007. Phase I dose escalation safety and immunogenicity trial of Plasmodium falciparum apical membrane protein (AMA-1) FMP2.1, adjuvanted with AS02A, in malaria-naive adults at the Walter Reed Army Institute of Research. Vaccine 254203-4212. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez, L. E., H. Curtidor, M. Urquiza, G. Cifuentes, C. Reyes, and M. E. Patarroyo. 2008. Intimate molecular interactions of P. falciparum merozoite proteins involved in invasion of red blood cells and their implications for vaccine design. Chem. Rev. 1083656-3705. [DOI] [PubMed] [Google Scholar]
- 26.Roestenberg, M., E. Remarque, E. de Jonge, R. Hermsen, H. Blythman, O. Leroy, E. Imoukhuede, S. Jepsen, O. Ofori-Anyinam, B. Faber, C. H. Kocken, M. Arnold, V. Walraven, K. Teelen, W. Roeffen, Q. de Mast, W. R. Ballou, J. Cohen, M. C. Dubois, S. Ascarateil, A. van der Ven, A. Thomas, and R. Sauerwein. 2008. Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Alhydrogel, Montanide ISA 720 or AS02. PLoS ONE 3e3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabchareon, A., T. Burnouf, D. Ouattara, P. Attanath, H. Bouharoun-Tayoun, P. Chantavanich, C. Foucault, T. Chongsuphajaisiddhi, and P. Druilhe. 1991. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 45297-308. [DOI] [PubMed] [Google Scholar]
- 28.Saul, A., G. Lawrence, A. Allworth, S. Elliott, K. Anderson, C. Rzepczyk, L. B. Martin, D. Taylor, D. P. Eisen, D. O. Irving, D. Pye, P. E. Crewther, A. N. Hodder, V. J. Murphy, and R. F. Anders. 2005. A human phase 1 vaccine clinical trial of the Plasmodium falciparum malaria vaccine candidate apical membrane antigen 1 in Montanide ISA720 adjuvant. Vaccine 233076-3083. [DOI] [PubMed] [Google Scholar]
- 29.Singh, S., K. Miura, H. Zhou, O. Muratova, B. Keegan, A. Miles, L. B. Martin, A. J. Saul, L. H. Miller, and C. A. Long. 2006. Immunity to recombinant Plasmodium falciparum merozoite surface protein 1 (MSP1): protection in Aotus nancymai monkeys strongly correlates with anti-MSP1 antibody titer and in vitro parasite-inhibitory activity. Infect. Immun. 744573-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoute, J. A., J. Gombe, M. R. Withers, J. Siangla, D. McKinney, M. Onyango, J. F. Cummings, J. Milman, K. Tucker, L. Soisson, V. A. Stewart, J. A. Lyon, E. Angov, A. Leach, J. Cohen, K. E. Kester, C. F. Ockenhouse, C. A. Holland, C. L. Diggs, J. Wittes, and D. G. Heppner, Jr. 2007. Phase 1 randomized double-blind safety and immunogenicity trial of Plasmodium falciparum malaria merozoite surface protein FMP1 vaccine, adjuvanted with AS02A, in adults in western Kenya. Vaccine 25176-184. [DOI] [PubMed] [Google Scholar]
- 31.Thera, M. A., O. K. Doumbo, D. Coulibaly, D. A. Diallo, I. Sagara, A. Dicko, D. J. Diemert, D. G. Heppner, Jr., V. A. Stewart, E. Angov, L. Soisson, A. Leach, K. Tucker, K. E. Lyke, and C. V. Plowe. 2006. Safety and allele-specific immunogenicity of a malaria vaccine in Malian adults: results of a phase I randomized trial. PLoS Clin. Trials 1e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vekemans, J., and W. R. Ballou. 2008. Plasmodium falciparum malaria vaccines in development. Expert Rev. Vaccines 7223-240. [DOI] [PubMed] [Google Scholar]
- 33.Withers, M. R., D. McKinney, B. R. Ogutu, J. N. Waitumbi, J. B. Milman, O. J. Apollo, O. G. Allen, K. Tucker, L. A. Soisson, C. Diggs, A. Leach, J. Wittes, F. Dubovsky, V. A. Stewart, S. A. Remich, J. Cohen, W. R. Ballou, C. A. Holland, J. A. Lyon, E. Angov, J. A. Stoute, S. K. Martin, and D. G. Heppner, Jr. 2006. Safety and reactogenicity of an MSP-1 malaria vaccine candidate: a randomized phase Ib dose-escalation trial in Kenyan children. PLoS Clin. Trials 1e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon, I. K., E. Angov, D. Larson, D. G. Heppner, J. F. Cummings, and V. A. Stewart. 2005. Characterization of a human reference standard for antibody to Plasmodium falciparum merozoite surface protein 142. Am. J. Trop. Med. Hyg. 72714-718. [PubMed] [Google Scholar]