Abstract
The gamma interferon assay is used to identify Mycobacterium avium subsp. paratuberculosis-infected animals. It has been suggested that regulatory mechanisms could influence the sensitivity of the test when it is performed with cells from cattle and that the neutralization of interleukin-10 (IL-10) in vitro would increase the gamma interferon responses. To investigate the regulatory mechanisms affecting the gamma interferon assay with cells from goats, blood was collected from M. avium subsp. paratuberculosis-infected, M. avium subsp. paratuberculosis-exposed, and noninfected goats. Neutralization of IL-10 by a monoclonal antibody resulted in increased levels of gamma interferon production in M. avium subsp. paratuberculosis purified protein derivative (PPDj)-stimulated samples from both infected and exposed goats. However, the levels of gamma interferon release were also increased in unstimulated cells and in PPDj-stimulated cells from some noninfected animals following neutralization. Depletion of putative regulatory CD25high T cells had no clear effect on the number of gamma-interferon-producing cells. The IL-10-producing cells were identified to be mainly CD14+ major histocompatibility complex class II-positive monocytes in both PPDj-stimulated and control cultures and not regulatory T cells. However, possible regulatory CD4+ CD25+ T cells produced IL-10 in response to concanavalin A stimulation. The numbers of CD4+, CD8+, and CD8+ γδT-cell receptor-positive cells producing gamma interferon increased following IL-10 neutralization. These results provide insight into the source and the role of IL-10 in gamma interferon assays with cells from goats and suggest that IL-10 from monocytes can regulate both innate and adaptive gamma interferon production from several cell types. Although IL-10 neutralization increased the sensitivity of the gamma interferon assay, the specificity of the test could be compromised.
Mycobacterium avium subsp. paratuberculosis is the causative agent of paratuberculosis, a chronic intestinal disease in ruminants (12) that has a worldwide distribution and that is of substantial economic importance (5, 29). To control paratuberculosis, it is of great importance to be able to identity infected animals at an early stage. Several methods can be used to diagnose paratuberculosis, but all of these methods have limitations. Fecal culture, PCR, and serum antibody assays have relatively low sensitivities in the early stages of the disease (2, 44), while the gamma interferon (IFN-γ) test has the greatest potential to detect paratuberculosis in subclinically infected animals (7, 37). Norwegian goat kids experimentally infected with high doses of M. avium subsp. paratuberculosis gave increased IFN-γ responses from week 7 after the first bacterial exposure (40), and the results of an evaluation of the test for the diagnosis of paratuberculosis in Norwegian goats was promising (39). However, data from a Norwegian surveillance program have suggested that positive IFN-γ results are not detected earlier than antibodies in blood or bacteria in feces in naturally infected goats (K. R. Lybeck, unpublished data).
IFN-γ production is induced by cytokines like interleukin-12 (IL-12) and IL-18, while IL-4 and IL-10 reduce the level of IFN-γ expression (13, 30, 34). IL-10 can limit IFN-γ production in M. avium subsp. paratuberculosis- or Mycobacterium bovis-infected cattle, and it has been suggested that the neutralization of IL-10 could be a way of increasing the sensitivity of the IFN-γ assay since this leads to an antigen-specific increase in the level of IFN-γ production (10, 15). The cells producing IL-10 in those studies were not identified, but it was speculated that regulatory T cells (Tregs) were involved (15). Lately, the role of Tregs in suppressing immune responses has been a focus, and regulatory IL-10-producing CD4+ CD25+ cells from M. avium subsp. paratuberculosis-infected cattle have been reported (14).
In humans and mice, Tregs control the immune responses to infections, balancing protective immunity and immunopathology. However, during mycobacterial infections as well as infections caused by some other organisms, it seems that the suppression of the protective immune response can lead to pathogen persistence and chronic disease (21, 25, 26). Both thymus-derived natural CD4+ CD25high Foxp3 Tregs and adaptive CD4+ CD25+/− Foxp3+/− Tregs induced outside the central lymphoid organs exist (8, 35). It is generally believed that natural Tregs mediate suppression through contact-dependent mechanisms, while adaptive Tregs act via production of the anti-inflammatory cytokine IL-10 or transforming growth factor β (TGF-β) (8, 26). IL-10 is also produced by macrophages, dendritic cells, B cells, and various subsets of CD4+ and CD8+ T cells; and excessive or mistimed IL-10 production can inhibit protective immune responses to intracellular pathogens (13). TGF-β is produced by most immune cells and has the potential to suppress IFN-γ production (11).
On the basis of the knowledge of regulatory T cells in humans and mice and the findings from studies with cattle linking suboptimal IFN-γ production to regulatory mechanisms, we hypothesized that the low sensitivity of the IFN-γ test observed with cells from subclinically paratuberculosis-infected goats could be due to the effect of regulatory mechanisms. These mechanisms are poorly described in goats, and the aim of this study was to investigate the regulatory factors influencing the IFN-γ responses to paratuberculosis in vitro and ultimately identify possible ways to increase the sensitivity of the IFN-γ assay.
MATERIALS AND METHODS
Animals. (i) Infected and exposed goats.
All goats came from a goat herd consisting of about 160 Norwegian dairy goats in which paratuberculosis had been diagnosed on the basis of histopathology, the identification of acid-fast rods, and the detection of IS900 by PCR 1 year earlier. Twenty goats from the herd were examined.
Goats 1 to 14 were termed infected. Goats 1 to 4 had clinical signs consistent with paratuberculosis and positive IFN-γ results. The animals were 2.5 to 3.5 years old and skinny and had a poor coat condition. They were antibody positive; and goats 1, 2, and 4 were fecal culture positive. Goats 5 to 14 had positive results for IFN-γ. They were 1.5 to 4.5 years old and in better physical condition than goats 1 to 4, and all goats except goat 12 were fecal culture positive.
Goats 15 to 20 were termed exposed, but they had negative results for IFN-γ. They were 6 months old, were clinically healthy, and were antibody as well as fecal culture negative at the beginning of the study. At the termination of the study, goat 15 was IFN-γ positive and goat 16 was culture positive. It was, however, considered likely that all goats were infected due to massive M. avium subsp. paratuberculosis contamination in the housing facilities.
Goats 1 to 4 and 15 to 20 were moved to the animal premises at the National Veterinary Institute of Norway. The young goats and the adult goats were kept in separate rooms, both of which contained hygiene sluices. Blood was repeatedly collected from the vena jugularis over a period of 4 to 7 months. At the end of the study, the goats were euthanized with pentobarbital. Blood was collected only once from goats 5 to 14, which were kept in the original herd. The procedures and the animal management protocols used in the study were approved by the Norwegian Animal Research Authority, in accordance with the Animal Experimental and Scientific Purposes Act of 1986.
(ii) Noninfected goats.
Goats 21 to 37 were from a herd free of paratuberculosis located at the Animal Research Center at the Norwegian University of Life Sciences. The goats were 2 to 6 years old and tested negative for paratuberculosis by the IFN-γ test. Blood was collected from these goats on three separate occasions.
Antigens and antibodies.
Purified protein derivative from M. avium subsp. paratuberculosis (PPDj) was from the National Veterinary Institute of Norway. Staphylococcus aureus enterotoxin was obtained from Toxin Technology. Concanavalin A (ConA) was purchased from Sigma Aldrich. Anti-CD4 (clone 44.38) was from the Centre for Animal Biotechnology, Australia. Anti-CD8 (clone 38.65), anti-CD14 (clone TÜK4), anti-IFN-γ (clone CC302), and anti-IL-10 (clone CC320) were from AbD Serotec; anti-γδ T-cell receptor (anti-γδTCR; clone GB21A), anti-CD25 (clone LCTB2A), and anti-major histocompatibility complex class II (anti-MHCII; clone H42A) were from Veterinary Medical Research and Development (VMRD); anti-TGF-β (clone 1D11) was from R&D Systems; anti-Foxp3 (clone ebio7979) was from eBioscience; Alexa Fluor 633-conjugated anti-mouse immunoglobulin G2a (IgG2a) and IgG2b were from Invitrogen; and fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG2a and IgG3 and phycoerythrin (PE)-conjugated anti-mouse IgG1 were from Southern Biotech.
IFN-γ assay with neutralization of IL-10 or TGF-β.
Peripheral blood mononuclear cells (PBMCs) were isolated on a density gradient medium (Lymphoprep; Axis-Shield) by gradient centrifugation and washed once in phosphate-buffered saline (PBS) containing EDTA and twice in PBS without EDTA. The cells (2 × 106 cells/ml) were stimulated with PPDj (10 μg/ml) or no antigen in 24-well cell culture plates (Corning Incorporated) and incubated in complete medium (RPMI 1640 with l-glutamine and gentamicin; both from Sigma Aldrich) and 10% fetal calf serum for 24 h at 37°C in humidified air with 5% CO2. Anti-IL-10 antibodies were added, as indicated in Fig. 3A and in the legends to Fig. 3 and 5. The concentration of the anti-TGF-β monoclonal antibody (MAb) was 5 μg/ml, and the isotype control (CD18 clone H20A from VMRD) was used at 2.5 μg/ml. The supernatants were harvested and stored at −70°C until they were analyzed. IFN-γ production was assessed by a capture enzyme-linked immunosorbent assay (ELISA) for bovine IFN-γ, according to the manufacturer's instructions (Invitrogen). Recombinant bovine IFN-γ (AbD Serotec) was used as a standard.
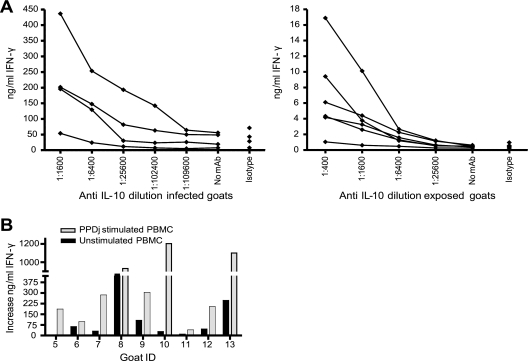
FIG. 3.
Effect of IL-10 neutralization on IFN-γ release. (A) Serial dilution of the IL-10 MAb in four infected goats (left panel) and six exposed goats (right panel); (B) increase in the amount of IFN-γ released following IL-10 neutralization (MAb concentration, 1:200) in unstimulated and PPDj-stimulated PBMCs from nine infected goats.
FIG. 5.
PBMCs from noninfected goats. (A) Increase in the amount of IFN-γ released following IL-10 neutralization (MAb concentration, 1:100) in unstimulated PBMCs and PPDj-stimulated PBMCs from 17 goats. (B) Phenotyping and intracellular IL-10 staining of PPDj-stimulated PBMCs from 10 goats. (C) Total number of IL-10-positive cells in unstimulated PBMCs and PPDj-stimulated PBMCs from 17 goats. (B and C) Uncorrected values are shown. Staining without the IL-10 MAb was used as a control, and the mean background was 0.1%.
Intracellular staining and flow cytometric analysis for IFN-γ-, IL-10-, and TGF-β-producing cells.
An adaptation of a flow cytometric method for intracellular IFN-γ staining was used (28). Cells were prepared and stimulated as described above with PPDj or no antigen in six-well cell culture plates (Corning Incorporated). S. aureus enterotoxin (0.5 μg/ml) and ConA (10 μg/ml) were used as positive controls. Incubation was for 18 h at 37°C in humidified air with 5% CO2. After 6 h, brefeldin A (Sigma Aldrich) was added. Cells were transferred into tubes, and PBS containing EDTA was used to remove adherent monocytes from the plastic wells (1). Cells (1 × 106) in 96-well trays (Becton Dickinson and Company) were stained for surface markers by the use of MAbs against CD4, CD8, γδTCR, CD25, CD14, and MHCII, followed by incubation with the appropriate Alexa Fluor 633- or FITC-conjugated secondary antibodies. Double staining for CD4 and CD25 plus CD8 and γδTCR was performed. Cytofix/Cytoperm solution (Becton Dickinson and Company) was used for fixation and permeabilization, and Perm/Wash solution (Becton Dickinson and Company) was used to wash the cells when intracellular staining was performed. The cells were first incubated with MAbs against IFN-γ, IL-10, or TGF-β and then with PE-conjugated secondary antibodies. For the TGF-β antibody, four different dilutions were tested; 1.25, 2.5, 5, and 12.5 μg/ml. Cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson and Company) equipped with Cell-Quest software. The results were calculated as the percentage of gated cells expressing PE plus FITC or Alexa Fluor 633. Alternatively, the percentage of PE-positive cells expressing FITC or Alexa Fluor 633 was determined. Positive fluorescence gates were set with reference to the negative controls, from which primary antibodies were omitted.
Depletion of CD25+ T cells.
PBMCs (up to 1 × 108 cells) were diluted in 400 μl PBS with EDTA, and the mixture was incubated with 6.25 μg/ml anti-CD25 antibodies in siliconated blood tubes on a shaker for 20 min at 4°C. The cells were washed with PBS containing EDTA and incubated as described above with 100 μl MACS anti-mouse IgG MicroBeads (Miltenyi Biotec). LD columns (Miltenyi Biotec) were used to remove CD25-positive cells. The efficiency of the procedure was controlled with staining for CD25 and flow cytometric analysis. The same numbers of depleted and nondepleted cells were incubated with PPDj or no antigen. Incubation, staining, and flow cytometric analysis for IFN-γ were performed as described above.
Intracellular staining and flow cytometric analysis for detection of Foxp3-positive cells.
PBMCs were isolated and stained for surface markers (CD4 and CD25) as described above. Intracellular staining for Foxp3 was performed according to the manufacturer's instructions, with modifications, by use of the recommended fixation and permeabilization working solution and permeabilization buffer from eBioscience. The time of incubation with the PE-conjugated MAb against Foxp3 (ebio7979 at 0.5 μg/100 μl) was adjusted to 3 h. The isotype control was PE mouse IgG1(κ) at 0.125 μg/100 μl (eBioscience). Human PBMCs were used as positive controls. Cells were analyzed as described above for intracellular IFN-γ staining. The positive fluorescence gates were set with reference to staining with isotype controls.
Statistical analysis.
Statistical significance was determined by the Wilcoxon matched-pairs signed-rank test (within a group) and the two-tailed Mann-Whitney U test (between groups). P values of less than 0.05 were regarded as significant. Only a change in the concentration (ng/ml) of IFN-γ (concentration for PPDj-stimulated wells − concentration for unstimulated wells) of ≥1 was considered a biological difference, and smaller differences were considered equal for the purposes of statistical analysis.
RESULTS
Identification of cell types producing IFN-γ in M. avium subsp. paratuberculosis-infected goats.
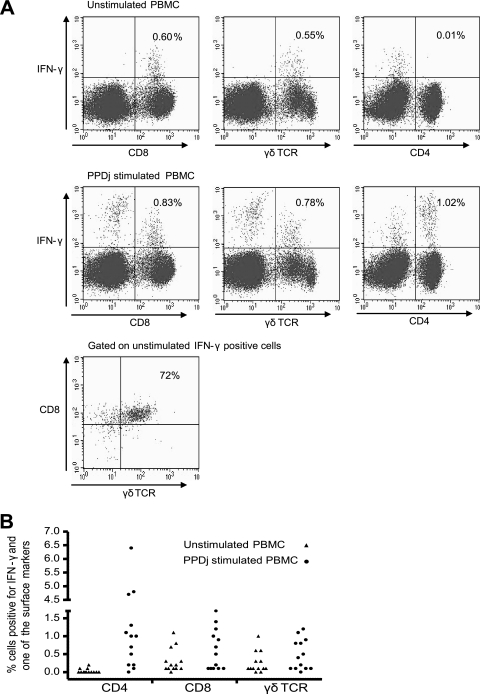
To study the regulatory immune mechanisms that affect IFN-γ production in vitro, we initially identified the IFN-γ-producing cells in blood from goats naturally infected with M. avium subsp. paratuberculosis by intracellular staining and flow cytometry. The numbers of CD4+, CD8+, and γδTCR-positive (γδTCR+) cells producing IFN-γ increased significantly in PPDj-stimulated cultures compared to the numbers of the control cells producing IFN-γ. However, some IFN-γ-producing cells were also detected in unstimulated control samples, and these were mainly CD8+ or CD8+ γδTCR+ (P < 0.05) (Fig. 1A and B). On average, 76% (range, 30 to 92%) of IFN-γ-producing CD8+ cells in unstimuated samples expressed the γδTCR receptor, and similar results were seen following PPDj stimulation (Fig. 1A). In general, few CD8− γδTCR+ cells produced IFN-γ, with average levels of production of 3% in unstimulated cultures and 10% in PPDj-stimulated cultures. Only small amounts of IFN-γ were seen in exposed goats, and there was no difference between PPDj-stimulated and control cells (data not shown).
FIG. 1.
(A) Flow cytometric dot plots showing the phenotypes and IFN-γ expression of unstimulated PBMCs (upper panels) and PPDj-stimulated PBMCs (middle panels) from one representative infected goat. (Lower panel) IFN-γ-positive, unstimulated cells are shown in a CD8-versus-γδTCR plot. (B) Identification of IFN-γ-producing cells in 13 infected goats. The experiments were repeated two to five times with cells from four animals. Uncorrected values are shown. Staining without the IFN-γ MAb was used as a control, and the mean background was 0.04%.
Attempts to identify Foxp3+ cells and effect of CD25 depletion on PPDj-induced IFN-γ production.
Regulatory T cells have been shown to suppress Th1 responses, and we wanted to see how these cells could affect in vitro IFN-γ production in blood from M. avium subsp. paratuberculosis-infected animals. We first attempted to identify CD4+ CD25 high Foxp3-positive (Foxp3+) regulatory T cells by intracellular staining using a human anti-Foxp3 antibody. Human PBMCs were used as a control. Foxp3 has not been described in goats, but the protein sequence is similar for a range of species, with the degree of identity of the Foxp3 sequence from humans (GenBank accession number EU855812) and cattle (GenBank accession number NM_001045933) being 90%. The staining for the transcription factor in goats was not convincing. Incubation of PBMCs with MAb ebio7979 for 3 h occasionally showed a small population of Foxp3+ cells (up to 0.20 to 0.40% of lymphocytes). The results were, however, not consistent in repeated experiments (data not shown).
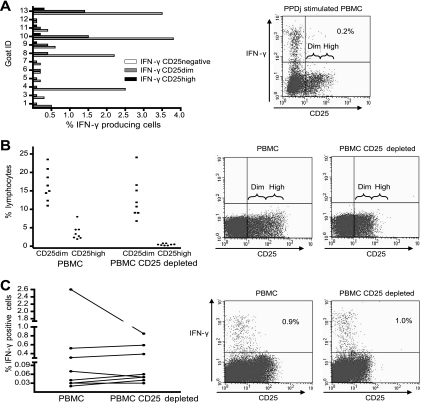
We thus decided to see how the removal of CD25high cells would affect IFN-γ production. CD25 is expressed on Tregs, but in addition CD25 can be an activation marker on T cells; we therefore had to establish if the IFN-γ-producing cells were CD25+. In general, the majority of IFN-γ-producing cells did not express CD25. IFN-γ-producing CD25+ cells were detected, but mostly in the CD25dim population (Fig. 2A). The proportion of IFN-γ-producing cells expressing CD25dim varied from 4 to 50%, with a mean of 27%. On average, only 5% (range, 0 to 13%) of IFN-γ-producing cells expressed high levels of CD25, making it possible to remove regulatory T cells and not activated IFN-γ-producing cells. Using magnetic beads and an anti-CD25 MAb, we reduced the proportion of CD25high cells by an average of 88% and the proportion of CD25dim cells by an average of 26% (Fig. 2B). CD25 depletion did not significantly alter the number of IFN-γ-producing cells and generally resulted in only slightly higher or unaffected numbers of IFN-γ producing cells in both infected and exposed goats (P > 0.05) (Fig. 2C). Similar results were seen when the level of IFN-γ production was measured by ELISA (data not shown).
FIG. 2.
(A) IFN-γ production related to CD25 expression in PPDj-stimulated PBMCs from infected goats. (Left panel) Results for 12 goats; (right panel) dot plot for one representative animal. (B) Efficiency of CD25 depletion; (C) effect of CD25 depletion on the number of IFN-γ-producing cells after PPDj stimulation. (B and C) Depletion was performed with cultures of cells from three infected goats and five exposed goats (left panels); (right panels) dot plots for one representative animal.
Effect of IL-10 and TGF-β neutralization on IFN-γ release in goats from a M. avium subsp. paratuberculosis-infected herd.
Next, we wanted to investigate how immunosuppressive cytokines could influence IFN-γ release. An MAb against IL-10 or TGF-β was added to PPDj-stimulated and unstimulated PBMCs from infected and exposed goats. After incubation, the amount of IFN-γ was measured by ELISA. IL-10 neutralization led to a dose-responsive increase in the level of PPDj-induced IFN-γ release (Fig. 3A). The absolute increase in the level of IFN-γ production following IL-10 neutralization was high in infected goats and was significantly higher than that in exposed goats. However, increased levels of IFN-γ production were seen in all animals: 13 infected goats and 6 exposed goats (P < 0.05). An increased level of IFN-γ production was also detected in control cells (Fig. 3B) (P < 0.05), but it was always lower than that in PPDj-stimulated cells for all goats except goat 17 (data not shown). An increase in the level of PPDj-induced IFN-γ production could also be demonstrated by flow cytometry in most infected goats. No effect of TGF-β neutralization was observed (data not shown).
Identification of IL-10-producing cells in infected and exposed goats.
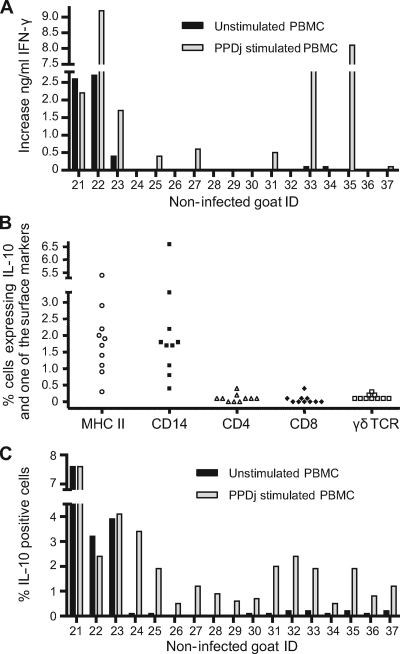
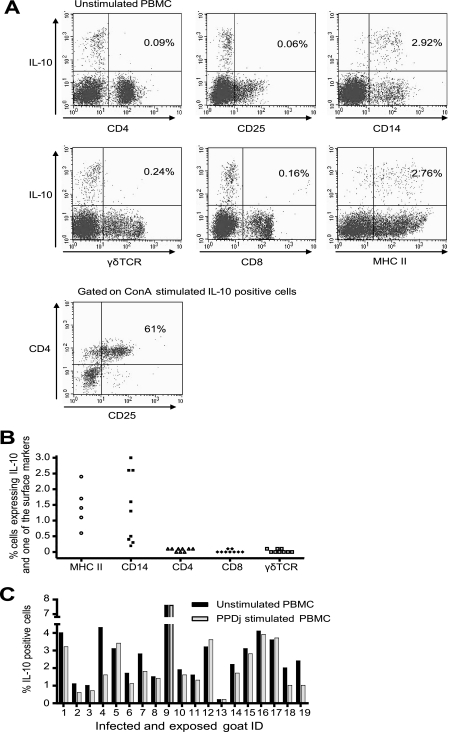
The strong effect of IL-10 neutralization on IFN-γ production together with the minimal effect of CD25 depletion made us investigate which cell type produced IL-10. The cell type producing most of the IL-10 in both PPDj-stimulated and control wells was CD14+ and MHCII positive (MHCII+). Significantly more IL-10-producing cells expressed CD14 than CD4, CD8, or γδTCR (P < 0.05) (Fig. 4A and B). Some IL-10-producing CD25+ cells were seen in individual goats; however, only 2% (range, 0 to 12%) of the IL-10-producing cells expressed high levels of CD25. On average, approximately 2 to 3% of the PBMCs produced IL-10, and in the majority of animals, the level of IL-10 production by unstimulated cells was slightly higher than that by PPDj-stimulated cells (Fig. 4C) (P < 0.05). There was no significant difference in the number of IL-10-producing cells between infected and exposed goats (data not shown).
FIG. 4.
(A) Identification of IL-10-producing cells. (Upper and middle panels) Phenotype and IL-10 expression in unstimulated PBMCs from one representative infected goat; (lower panel) IL-10-positive ConA-stimulated cells in a CD4-versus-CD25 plot. (B) Phenotyping and intracellular IL-10 staining of PPDj-stimulated PBMCs from four infected goats and five exposed goats. MHCII staining was performed only with cells from two infected goats and three exposed goats. All experiments were repeated one to four times with cells from two infected and three exposed goats. CD14 staining was performed with cells from an additional 10 infected goats. (C) Total number of IL-10-positive cells in unstimulated and PPDj-stimulated PBMCs from 14 infected goats and 5 exposed goats. The experiments were repeated one to four times with cells from two infected and three exposed goats. (B and C) Uncorrected values are shown. Staining without the IL-10 MAb was used as a control, and the mean background was 0.15%.
In prior studies, ConA has been regarded as a generator of suppressor T cells (23, 33). We thus wanted to see if ConA could induce IL-10 from CD4+ CD25+ cells, which might indicate Tregs. After ConA stimulation, 19% (range, 7 to 35%) of IL-10-producing cells expressed high levels of CD25. Furthermore, 37% (range, 18 to 54%) of IL-10-producing cells were CD4+ CD25+, whereas only 2% (range, 0 to 3%) of unstimulated and PPDj-stimulated cells were CD4+ CD25+ (P < 0.05) (Fig. 4A). Cells were also stained for the expression of TGF-β, but no cells or a negligible number of cells producing this cytokine could be detected (data not shown).
Neutralization of IL-10 and identification of IL-10-producing cells in healthy goats.
Next, we wanted to see if the increased amount of IFN-γ released following IL-10 neutralization was specific for M. avium subsp. paratuberculosis-infected goats and if it could be used to increase the sensitivity of the IFN-γ assay. We examined 17 healthy goats from an M. avium subsp. paratuberculosis-free herd, and an increase in the amount of IFN-γ released was detected in less than half of the goats after stimulation with PPDj (P < 0.05) (Fig. 5A). The absolute increase was, however, less than the increases in both infected and exposed goats (P < 0.05). Increased levels of IFN-γ production in unstimulated PBMCs were seen in only three goats (Fig. 5A). The main cell type producing IL-10 in healthy goats was, as for infected goats, CD14+ MHCII+ cells (Fig. 5B). In contrast to the findings for infected and exposed goats, IL-10 production was seen by cells stimulated with PPDj and not in unstimulated cells in healthy goats (P < 0.05), with the exception of three goats (Fig. 5C). There was no significant difference in the number of IL-10-producing cells between healthy and infected or exposed goats on PPDj stimulation.
Identification of cells with increased levels of IFN-γ production after IL-10 neutralization.
Finally, we wanted to see which cell types caused the increased level of IFN-γ production on IL-10 neutralization. Phenotyping, intracellular staining for IFN-γ, and flow cytometric analysis were performed after incubation of PBMCs with or without the anti-IL-10 MAb. Despite substantial variations between animals, increased numbers of CD4+, CD8+, and γδTCR+ T cells producing IFN-γ were seen following IL-10 neutralization in both PPDj-stimulated and unstimulated PBMCs. The increase was, however, significant only for CD8+ and γδTCR+ T cells on PPDj stimulation (Table 1). The IFN-γ-producing γδTCR+ cells were, as described earlier, largely cells that also expressed CD8.
TABLE 1.
IFN-γ expression in lymphocytes incubated with or without the anti-IL-10 MAb and the difference caused by IL-10 neutralizationa
| Incubation condition | Mean no. of lymphocytes ± SEM expressing:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IFN-γ and CD4
|
IFN-γ and CD8
|
IFN-γ and γδTCR
|
IFN-γ
|
|||||
| Unstimulated PBMCs | PPDj-stimulated PBMCs | Unstimulated PBMCs | PPDj-stimulated PBMCs | Unstimulated PBMCs | PPDj-stimulated PBMCs | Unstimulated PBMCs | PPDj-stimulated PBMCs | |
| No MAb | 0.08 ± 0.07 | 1.99 ± 1.04 | 0.41 ± 0.28 | 0.72 ± 0.32 | 0.15 ± 0.07 | 0.37 ± 0.14 | 0.52 ± 0.36 | 3.11 ± 1.60 |
| Anti-IL-10 | 0.36 ± 0.19 | 2.65 ± 1.22 | 0.87 ± 0.42 | 1.42 ± 0.44 | 0.34 ± 0.23 | 0.61 ± 0.20 | 1.41 ± 0.56 | 4.73 ± 1.77 |
| Difference | 0.28 ± 0.13 | 0.66 ± 0.32 | 0.46 ± 0.32 | 0.70 ± 0.19 | 0.20 ± 0.22 | 0.25 ± 0.09 | 0.89 ± 0.38 | 1.61 ± 0.44 |
The experiments were preformed with cells from eight infected goats, were repeated twice with two goats, and were performed on three separate occasions. Staining without the IFN-γ MAb was used as a control, and the results for the control were subtracted from the number of IFN-γ-producing cells.
DISCUSSION
Regulatory cells and cytokines may have an impact on the in vitro production of IFN-γ in blood from M. avium subsp. paratuberculosis-infected goats. We found that IL-10 neutralization had a profound effect on IFN-γ secretion and that IL-10 was mainly produced by monocytes and not CD4+ CD25high, putative Treg cells. The strongest effect of neutralization was seen in PPDj-stimulated samples from clinically ill animals; however, increased levels of IFN-γ production were also detected in unstimulated control samples and in PPDj-stimulated cultures of cells from some healthy animals. This is probably because IFN-γ is produced in both an innate and adaptive fashion and because IL-10 can influence both of these pathways.
In the present study, neutralization of IL-10 resulted in enhanced IFN-γ release in infected and exposed goats, confirming the expected suppressive effect of IL-10 on Th1 immune responses. This is in accordance with the findings of other studies with cattle with paratuberculosis (10) and M. bovis infection (15). It was speculated that Tregs might be the source of IL-10, but this was not confirmed (15). We found that the main IL-10-producing cell type in both infected and healthy goats was CD14+ MHCII+ monocytes. This is in agreement with the findings of a recent study in which CD14+ monocytes were found to act as regulatory IL-10-producing cells in healthy cattle (17). IL-10 expression has also been seen in monocytes and macrophages incubated with M. avium subsp. paratuberculosis bacteria (36, 42, 43). One report of cattle infected with M. avium subsp. paratuberculosis suggested that M. avium subsp. paratuberculosis-responsive IL-10-producing cells were CD4+ and/or CD25+ (14). In our hands, possible Treg cells producing IL-10 were mainly detected after ConA stimulation and not after specific stimulation with M. avium subsp. paratuberculosis antigens, although additional characterization is necessary to definitively determine that these are actually Tregs. Nevertheless, our results suggest that if Tregs are present in the PBMCs, their role as IL-10-producing cells is limited compared to the role of monocytes. Furthermore, the effect of CD25+ cell depletion was variable and generally did not result in a convincing increase in the level of IFN-γ expression. Our results suggest that CD25+ cells are not the most important cells limiting IFN-γ production in an overnight in vitro assay with PBMCs from goats. However, some adaptive Tregs are CD25 negative. It is therefore possible that CD25-negative Tregs could have suppressed IFN-γ production through mechanisms other than IL-10 production.
The level of IL-10 production by unstimulated PBMCs from infected animals was high, but IL-10 production was almost absent in unstimulated cells from most of the healthy goats. It thus seemed that M. avium subsp. paratuberculosis infection can result in a high level of constitutive IL-10 production by monocytes. Previously, the levels of IL-10 production by unstimulated cells from clinically infected animals were found to be higher than those by unstimulated cells from healthy cattle (22). PPDj stimulation had opposite effects on IL-10 production by cells from infected and healthy animals. The number of IL-10-expressing cells increased after PPDj stimulation of cells from healthy goats, while a slight decrease was detected for most infected animals. Others have noted the upregulation of IL-10 expression in cultures of cells from healthy cattle when they were stimulated with M. avium subsp. paratuberculosis or M. avium subsp. paratuberculosis antigen (10, 22, 36). Such stimulation also resulted in higher levels of IL-10 production in M. avium subsp. paratuberculosis-infected cattle than in noninfected cattle (10, 14, 22, 43). This finding is in contrast to our results. However, we measured the number of IL-10-producing cells, while previous studies measured the quantity of IL-10 produced. Individual cells can produce variable amounts of cytokines, and the flow cytometric method does not give a direct quantitative measurement of the amount of cytokine produced per cell. Furthermore, the kinetics of IL-10 production seem to be particularly relevant. The maximum expression of IL-10 in infected animals was usually detected after a few hours of stimulation, while less IL-10 was detected at later time points (10, 14, 43). We measured IL-10 after 18 h, and one could suggest that the IFN-γ induced by PPDj in infected animals could downregulate IL-10 production at this time point. In line with this, IFN-γ has been described to inhibit IL-10 production from human monocytes, macrophages, and dendritic cells (19, 24).
As expected, CD4+ cells produced large amounts of IFN-γ in M. avium subsp. paratuberculosis-infected animals after PPDj stimulation. CD8+ and CD8+ γδT cells also contributed to IFN-γ production on PPDj stimulation and were the main IFN-γ producers in unstimulated samples. IFN-γ production from CD8+ cells has previously been seen in both unstimulated samples (38) and antigen-stimulated samples from M. avium subsp. paratuberculosis-infected or vaccinated ruminants (3, 16, 38), while studies with contradictory results regarding the ability of ruminant γδT cells to produce IFN-γ in response to mycobacterial antigens exist (3, 16, 32). IFN-γ expression by CD8+ γδT cells has, to our knowledge, not previously been related to mycobacterial infections in ruminants. In rats, however, IFN-γ production has been attributed to these cells in other pathological settings (20), and the cell type has been described in cattle (18, 31).
In our studies, IL-10 seemed to suppress IFN-γ production by several subsets of lymphocytes. The mechanism for IL-10-mediated regulation of IFN-γ secretion was not explored further. IL-10 can limit IFN-γ production by acting directly on T cells (13), at least in humans. Alternatively, IL-10 can regulate T cells indirectly by acting on monocytes and macrophages, inhibiting the expression of MHCII and costimulatory molecules and the production of proinflammatory cytokines, such as IL-12 and IL-18 (13). An inhibitory effect of IL-10 on MHCII expression (41) and an increase in the levels of IL-12 and IL-18 following IL-10 neutralization have also been described in cattle (14, 41). This could potentially promote both innate and adaptive IFN-γ production. The IFN-γ production from CD4+ cells in PPDj-stimulated cultures in the present study was likely an adaptive response, while CD8 cells seemed to produce IFN-γ in both an adaptive and an innate fashion. This is supported by the findings of studies with mice that showed innate IFN-γ production from CD8+ cells (6). γδT cells also seem to be a source of early IFN-γ in both humans and cattle, bridging the innate and the adaptive immune responses (4, 9). Furthermore, innate IFN-γ production from NK cells cannot be excluded (27). The broad actions of IL-10 can thus provide possible explanations for the increased levels of IFN-γ production by both PPDj-stimulated and control cells, as well as for the enhanced IFN-γ production in samples from healthy goats.
In conclusion, we have shown that the neutralization of IL-10, produced mainly by CD14+ monocytes, enhanced IFN-γ production in M. avium subsp. paratuberculosis-infected goats. Neutralization seemed to have the potential to increase the amount of IFN-γ released in subclinically infected, test-negative goats. However, since the amount of IFN-γ in unstimulated cells, as well as in some noninfected goats, increased following IL-10 neutralization, further work is needed to determine if manipulation of regulatory immune mechanisms is a way to increase the sensitivity of the IFN-γ assay for the detection of paratuberculosis.
Acknowledgments
We thank Knut Erik Witberg and Ina Killingrød-Greve for skilled animal handling. We also thank Inger Austrheim Heffernan for excellent technical assistance.
This work was supported by TINE Norwegian Dairies BA and the Norwegian Research Council (project 173247).
Footnotes
Published ahead of print on 6 May 2009.
REFERENCES
- 1.Ackerman, S. K., and S. D. Douglas. 1978. Purification of human monocytes on microexudate-coated surfaces. J. Immunol. 1201372-1374. [PubMed] [Google Scholar]
- 2.Bakker, D., P. T. Willemsen, and F. G. van Zijderveld. 2000. Paratuberculosis recognized as a problem at last: a review. Vet. Q. 22200-204. [DOI] [PubMed] [Google Scholar]
- 3.Bassey, E. O., and M. T. Collins. 1997. Study of T-lymphocyte subsets of healthy and Mycobacterium avium subsp. paratuberculosis-infected cattle. Infect. Immun. 654869-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beetz, S., D. Wesch, L. Marischen, S. Welte, H. H. Oberg, and D. Kabelitz. 2008. Innate immune functions of human gamma delta T cells. Immunobiology 213173-182. [DOI] [PubMed] [Google Scholar]
- 5.Benedictus, G., A. A. Dijkhuizen, and J. Stelwagen. 1987. Economic losses due to paratuberculosis in dairy cattle. Vet. Rec. 121142-146. [DOI] [PubMed] [Google Scholar]
- 6.Berg, R. E., and J. Forman. 2006. The role of CD8 T cells in innate immunity and in antigen non-specific protection. Curr. Opin. Immunol. 18338-343. [DOI] [PubMed] [Google Scholar]
- 7.Billman-Jacobe, H., M. Carrigan, F. Cockram, L. A. Corner, I. J. Gill, J. F. Hill, T. Jessep, A. R. Milner, and P. R. Wood. 1992. A comparison of the interferon gamma assay with the absorbed ELISA for the diagnosis of Johne's disease in cattle. Aust. Vet. J. 6925-28. [DOI] [PubMed] [Google Scholar]
- 8.Bluestone, J. A., and A. K. Abbas. 2003. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3253-257. [DOI] [PubMed] [Google Scholar]
- 9.Buza, J., T. Kiros, A. Zerihun, I. Abraham, and G. Ameni. 25 December 2008. Vaccination of calves with Mycobacteria bovis Bacilli Calmete Guerin (BCG) induced rapid increase in the proportion of peripheral blood gammadelta T cells. Vet. Immunol. Immunopathol. [Epub ahead of print.] [DOI] [PubMed]
- 10.Buza, J. J., H. Hikono, Y. Mori, R. Nagata, S. Hirayama, G. Aodon, A. M. Bari, Y. Shu, N. M. Tsuji, and E. Momotani. 2004. Neutralization of interleukin-10 significantly enhances gamma interferon expression in peripheral blood by stimulation with Johnin purified protein derivative and by infection with Mycobacterium avium subsp. paratuberculosis in experimentally infected cattle with paratuberculosis. Infect. Immun. 722425-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerwenka, A., and S. L. Swain. 1999. TGF-beta1: immunosuppressant and viability factor for T lymphocytes. Microbes Infect. 11291-1296. [DOI] [PubMed] [Google Scholar]
- 12.Chiodini, R. J., H. J. Van Kruiningen, and R. S. Merkal. 1984. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 74218-262. [PubMed] [Google Scholar]
- 13.Couper, K. N., D. G. Blount, and E. M. Riley. 2008. IL-10: the master regulator of immunity to infection. J. Immunol. 1805771-5777. [DOI] [PubMed] [Google Scholar]
- 14.de Almeida, D. E., C. J. Colvin, and P. M. Coussens. 2008. Antigen-specific regulatory T cells in bovine paratuberculosis. Vet. Immunol. Immunopathol. 125234-245. [DOI] [PubMed] [Google Scholar]
- 15.Denis, M., D. N. Wedlock, A. R. McCarthy, N. A. Parlane, P. J. Cockle, H. M. Vordermeier, R. G. Hewinson, and B. M. Buddle. 2007. Enhancement of the sensitivity of the whole-blood gamma interferon assay for diagnosis of Mycobacterium bovis infections in cattle. Clin. Vaccine Immunol. 141483-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasvold, H. J., M. Valheim, G. Berntsen, and A. K. Storset. 2002. In vitro responses to purified protein derivate of caprine T lymphocytes following vaccination with live strains of Mycobacterium avium subsp. paratuberculosis. Vet. Immunol. Immunopathol. 9079-89. [DOI] [PubMed] [Google Scholar]
- 17.Hoek, A., V. P. Rutten, J. Kool, G. J. Arkesteijn, R. J. Bouwstra, I. Van Rhijn, and A. P. Koets. 2008. Subpopulations of bovine WC1+ gammadelta T cells rather than CD4+ CD25high Foxp3+ T cells act as immune regulatory cells ex vivo. Vet. Res. 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hope, J. C., L. S. Kwong, P. Sopp, R. A. Collins, and C. J. Howard. 2000. Dendritic cells induce CD4+ and CD8+ T-cell responses to Mycobacterium bovis and M. avium antigens in Bacille Calmette Guerin vaccinated and nonvaccinated cattle. Scand. J. Immunol. 52285-291. [DOI] [PubMed] [Google Scholar]
- 19.Hu, X., S. D. Chakravarty, and L. B. Ivashkiv. 2008. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol. Rev. 22641-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isogai, S., A. Athiviraham, R. S. Fraser, R. Taha, Q. Hamid, and J. G. Martin. 2007. Interferon-gamma-dependent inhibition of late allergic airway responses and eosinophilia by CD8+ gammadelta T cells. Immunology 122230-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joosten, S. A., and T. H. Ottenhoff. 2008. Human CD4 and CD8 regulatory T cells in infectious diseases and vaccination. Hum. Immunol. 69760-770. [DOI] [PubMed] [Google Scholar]
- 22.Khalifeh, M. S., and J. R. Stabel. 2004. Effects of gamma interferon, interleukin-10, and transforming growth factor beta on the survival of Mycobacterium avium subsp. paratuberculosis in monocyte-derived macrophages from naturally infected cattle. Infect. Immun. 721974-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lobo, P. I., and C. E. Spencer. 1979. Inhibition of humoral and cell-mediated immune responses in man by distinct suppressor cell systems. J. Clin. Investig. 631157-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyakh, L., G. Trinchieri, L. Provezza, G. Carra, and F. Gerosa. 2008. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol. Rev. 226112-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majlessi, L., R. Lo-Man, and C. Leclerc. 2008. Regulatory B and T cells in infections. Microbes Infect. 101030-1035. [DOI] [PubMed] [Google Scholar]
- 26.Mills, K. H. 2004. Regulatory T cells: friend or foe in immunity to infection? Nat. Rev. Immunol. 4841-855. [DOI] [PubMed] [Google Scholar]
- 27.Olsen, I., P. Boysen, S. Kulberg, J. C. Hope, G. Jungersen, and A. K. Storset. 2005. Bovine NK cells can produce gamma interferon in response to the secreted mycobacterial proteins ESAT-6 and MPP14 but not in response to MPB70. Infect. Immun. 735628-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsen, I., and A. K. Storset. 2001. Innate IFN-gamma production in cattle in response to MPP14, a secreted protein from Mycobacterium avium subsp. paratuberculosis. Scand. J. Immunol. 54306-313. [DOI] [PubMed] [Google Scholar]
- 29.Ott, S. L., S. J. Wells, and B. A. Wagner. 1999. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev. Vet. Med. 40179-192. [DOI] [PubMed] [Google Scholar]
- 30.Peleman, R., J. Wu, C. Fargeas, and G. Delespesse. 1989. Recombinant interleukin 4 suppresses the production of interferon gamma by human mononuclear cells. J. Exp. Med. 1701751-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platt, R., C. Coutu, T. Meinert, and J. A. Roth. 2008. Humoral and T cell-mediated immune responses to bivalent killed bovine viral diarrhea virus vaccine in beef cattle. Vet. Immunol. Immunopathol. 1228-15. [DOI] [PubMed] [Google Scholar]
- 32.Rhodes, S. G., R. G. Hewinson, and H. M. Vordermeier. 2001. Antigen recognition and immunomodulation by gamma delta T cells in bovine tuberculosis. J. Immunol. 1665604-5610. [DOI] [PubMed] [Google Scholar]
- 33.Rich, R. R., and C. W. Pierce. 1973. Biological expressions of lymphocyte activation. II. Generation of a population of thymus-derived suppressor lymphocytes. J. Exp. Med. 137649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoenborn, J. R., and C. B. Wilson. 2007. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 9641-101. [DOI] [PubMed] [Google Scholar]
- 35.Shevach, E. M. 2006. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity 25195-201. [DOI] [PubMed] [Google Scholar]
- 36.Souza, C. D., O. A. Evanson, and D. J. Weiss. 2006. Mitogen activated protein kinase p38 pathway is an important component of the anti-inflammatory response in Mycobacterium avium subsp. paratuberculosis-infected bovine monocytes. Microb. Pathog. 4159-66. [DOI] [PubMed] [Google Scholar]
- 37.Stabel, J. R. 1996. Production of gamma-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of subclinical paratuberculosis. J. Vet. Diagn. Investig. 8345-350. [DOI] [PubMed] [Google Scholar]
- 38.Stabel, J. R., K. Kimura, and S. Robbe-Austerman. 2007. Augmentation of secreted and intracellular gamma interferon following Johnin purified protein derivative sensitization of cows naturally infected with Mycobacterium avium subsp. paratuberculosis. J. Vet. Diagn. Investig. 1943-51. [DOI] [PubMed] [Google Scholar]
- 39.Storset, A. K., I. Berg, and B. Djonne. 2005. Evaluation of the gamma interferon test for diagnosis of paratuberculosis in goats. Vet. Immunol. Immunopathol. 10787-94. [DOI] [PubMed] [Google Scholar]
- 40.Storset, A. K., H. J. Hasvold, M. Valheim, H. Brun-Hansen, G. Berntsen, S. K. Whist, B. Djonne, C. M. Press, G. Holstad, and H. J. Larsen. 2001. Subclinical paratuberculosis in goats following experimental infection. An immunological and microbiological study. Vet. Immunol. Immunopathol. 80271-287. [DOI] [PubMed] [Google Scholar]
- 41.Weiss, D. J., O. A. Evanson, C. de Souza, and M. S. Abrahamsen. 2005. A critical role of interleukin-10 in the response of bovine macrophages to infection by Mycobacterium avium subsp. paratuberculosis. Am. J. Vet. Res. 66721-726. [DOI] [PubMed] [Google Scholar]
- 42.Weiss, D. J., O. A. Evanson, A. Moritz, M. Q. Deng, and M. S. Abrahamsen. 2002. Differential responses of bovine macrophages to Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium. Infect. Immun. 705556-5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss, D. J., O. A. Evanson, and C. D. Souza. 2005. Expression of interleukin-10 and suppressor of cytokine signaling-3 associated with susceptibility of cattle to infection with Mycobacterium avium subsp. paratuberculosis. Am. J. Vet. Res. 661114-1120. [DOI] [PubMed] [Google Scholar]
- 44.Whittington, R. J., and E. S. Sergeant. 2001. Progress towards understanding the spread, detection and control of Mycobacterium avium subsp. paratuberculosis in animal populations. Aust. Vet. J. 79267-278. [DOI] [PubMed] [Google Scholar]