Abstract
Anti-H5N1 antibody was determined by microneutralization, hemagglutination inhibition, and Western blotting assays in serial blood samples collected from eight Thai patients, including four fatal cases and four survivors. The antibody was detected as early as 5 days and, typically, with an increase in titer in paired blood at about 15 days after disease onset. The anti-H5 antibody response was long-lasting, for almost 5 years in cases which can be followed that far. In addition, cross-neutralizing activity to related clade 1 viruses was observed.
During the first outbreak of human highly pathogenic avian influenza (HPAI) virus H5N1 disease in Hong Kong in 1997, the microneutralization (microNT) assay was used to detect acute antibody responses in virologically confirmed cases, but the durability of the response was not assessed (4). The microNT assay was more sensitive than the traditional hemagglutination inhibition (HI) assay, which used erythrocytes from avian species in detecting human antibodies induced by H5 viruses (7). The sensitivity and specificity of microNT could be improved when it was combined with a confirmatory Western blot (WB) assay (7).
Since late 2003, highly pathogenic avian influenza (HPAI) H5N1 viruses have caused unprecedented outbreaks in poultry in 61 countries (10). Human disease has accompanied the global spread of the virus, and as of 14 January 2009, 394 human H5N1 virus infections had been reported by the World Health Organization (WHO), with an overall fatality rate of over 60% (13). Despite these case numbers, information on the kinetics, and in particular the persistence of H5 virus-specific antibody in humans infected with H5N1 viruses, is lacking. This information could guide H5N1 vaccine studies and the optimal design of seroepidemiological investigations to better understand the extent of and risk factors associated with human H5N1 infection. In 2006, WHO included serodiagnosis as one among other criteria for an H5N1 confirmed case, i.e., (i) a fourfold or greater rise in neutralizing antibody titer in paired blood samples of which the acute blood has been collected within 7 days after symptom onset and the convalescent sample achieves a titer of ≥1:80, or (ii) presence of a neutralizing antibody titer of ≥1:80 in a single serum sample collected 14 or more days after symptom onset together with a positive result using a different serological assay, such as the horse erythrocyte HI assay or an H5-specific WB positive result (12).
Thailand reported the first human case of H5N1 disease on 23 January 2004. No human case has occurred after July 2006; in total, there have been 25 human cases with 17 deaths. The diagnosis of H5N1 infection in human cases in Thailand is primarily based on results of conventional and real-time reverse transcription-PCR (RT-PCR) and virus isolation methods. Serological tests have been introduced more recently. The present study reports the results of serological analyses conducted by using the microNT, HI, and WB assays to explore the kinetics and longevity of the antibody response to H5N1 virus infection in fatal cases and survivors.
MATERIALS AND METHODS
Ethical issues.
This study has been approved by two Ethical Committee for Human Research panels: one from the Faculty of Medicine Siriraj Hospital, Mahidol University, and the second one from the Ministry of Public Health, Thailand. Subjects or parents gave consent to participate in the follow-up blood collection.
Subjects.
Eight individuals, four fatal cases and four survivors, were included in this study. All of them were diagnosed with H5N1 virus infection by both RT-PCR and virus isolation methods. Nucleotide sequencing showed that all of the isolated viruses belonged to clade 1. The demographic data for these subjects are shown in Table 1. Serial blood samples from the four survivors were collected at approximately 6-month intervals. Serum or plasma samples were kept frozen at −20°C until tested.
TABLE 1.
Demographic data for H5N1 patients
| Subject no. | Age (yrs) | Gender | Estimated incubation period (days) | Disease outcome | Yr of infection |
|---|---|---|---|---|---|
| 1 | 6 | M | Death | 2004 | |
| 2 | 2 | M | 5 | Survival | 2004 |
| 3 | 29 | M | 3 | Survival | 2004 |
| 4 | 32 | F | Survival | 2004 | |
| 5 | 48 | M | 12 | Death | 2005 |
| 6 | 7 | M | 8 | Survival | 2005 |
| 7 | 5 | M | 10 | Death | 2005 |
| 8 | 59 | M | Death | 2006 |
Viruses.
Two human H5N1 isolates belonging to genotype Z, clade 1, were used for serodiagnosis in this study. A/Thailand/1(KAN-1)/04 (KAN-1), the first human virus isolated in the country, was isolated in January 2004, and A/Thailand/676(NYK)/05 (NYK) was isolated in December 2005 during the third wave of the epidemic. The latter virus contains two mutational changes: A134V in the receptor binding site and R325K in the cleavage site of the hemagglutinin (HA) molecule (1, 6). Complete genomic sequences of the two virus isolates used in this study are available through GenBank. KAN-1 preferentially binds sialic acid α2,3-linked galactose (SAα2,3Gal), while NYK preferentially binds sialic acid α2,6-linked galactose (SAα2,6Gal) (1). The viruses were propagated in MDCK cell monolayers without serum supplement. Experiments related to infectious viruses were conducted in biosafety level 3 facilities.
Microneutralization assay.
An enzyme-linked immunosorbent assay (ELISA)-based microNT assay was conducted for detection of NT antibody. The test protocol followed what is described in the WHO manual, with a small modification (11). The test serum was heat inactivated at 56°C for 30 min and then twofold diluted with maintenance medium starting from a dilution of 1:5 to 1:2,560. The assay was performed by mixing 60 μl of the diluted serum with 60 μl of the virus suspension at a concentration of 200 50% tissue culture infective doses (TCID50) and incubated at 37°C for 2 hours. Then, 100 μl of the mixture was transferred onto an MDCK cell monolayer and further incubated at 37°C for 18 to 20 h. In order to verify the amount of virus inoculum, virus back-titrations at doses of 0.1, 1, 10, and 100 TCID50 were included in every assay plate, together with the positive control serum and cell culture control. The test reaction was run in duplicate. Viral nucleoprotein produced in the infected MDCK cells was detected by indirect ELISA using mouse monoclonal antibody to influenza A virus nucleoprotein (Chemicon International, Inc., CA) as the primary antibody and goat anti-mouse immunoglobulin (Ig) conjugated with horseradish peroxidase (Southern Biotechnology Associates, Inc. Birmingham, AL) as the second antibody. The tetramethylbenzidine (TMB) peroxidase substrate system (Kirkegaard & Perry, Gaithersburg, MD) was used as the chromogenic substrate. The color product was read for the optical density (OD) at dual wavelengths of 450 and 630 nm. The corrected OD value of the test serum was obtained after subtracting the cell control OD from the original OD value. The corrected OD value of the virus control at a working concentration of 100 TCID50 was also obtained in the same manner. A positive microNT result was obtained when the test serum yielded a ≥50% reduction in the corrected OD value compared with that of the virus control. The antibody titer was defined as the reciprocal value of the highest serum dilution that gave ≥50% neutralization of 100 TCID50 of the test virus. It should be noted that the antibody titer value as determined by our definition was twofold lower than that defined by WHO and CDC, such that our titer of 1:40 is equivalent to 80 as defined by WHO and CDC. In our assay protocol, the volume of the test virus in the reaction well was not taken into account in the calculation for the NT antibody titer; meanwhile, this volume is included in the WHO/CDC protocol (I. Stephenson, WHO manual on influenza microneutralization assay: CDC Influenza Training Course at Faculty of Medicine Siriraj Hospital, Mahidol University, November 2004). In addition, we found no significant difference in the NT titers, as the CDC reference serum samples were assayed in an MDCK cell monolayer or cell suspension such that under two types of cell conditions, a test serum showed the same antibody titer or the variation was within twofold of the difference.
Hemagglutination inhibition test.
The two H5N1 isolates and horse and goose erythrocytes were used for antibody detection by an HI test. The protocol was as described previously (8, 11). A test serum was mixed with receptor-destroying enzyme (Denka Seiken, Japan) to obtain a dilution of 1:4, followed by incubation for 16 h at 37°C, heat inactivation at 56°C for 30 min, and adsorption with packed red blood cells (RBC) for 60 min at 4°C. The treated serum was serially diluted twofold to yield the starting dilutions of 1:20 to 1:2,560 and then added with 4 HA units of the test virus. One HA unit is defined as the highest virus dilution that yields complete hemagglutination. A reaction well containing 25 μl of the diluted serum and 25 μl of the virus suspension was incubated at room temperature for 30 min before adding 50 μl of the erythrocyte suspension and further incubated for 1 hour at 4°C. Either a 0.5% goose red blood cell (GRBC) or 1% horse red blood cell (HRBC) suspension was employed in our HI assay. Hemagglutination patterns of the test wells were examined. The HI antibody titer was defined as the reciprocal of the highest serum dilution that gave complete inhibition of hemagglutination.
Western blot assay.
Based on WHO criteria, a human serum sample that possesses an NT antibody titer of ≥80 (or ≥40 by our assay) should be further confirmed for its specificity to H5 HA in a WB assay. The serum sample at the dilution of 1:100 was tested against baculovirus recombinant H5 HA antigen (Protein Sciences Corporation, CT). According to the product brochure, the HA1 and HA2 subunits possess molecular masses of 45 and 25 kDa, respectively. Goat antiserum against purified HA of A/Vietnam/1203/04(H5N1), kindly provided by Robert G. Webster and Richard Webby, St. Jude Children's Research Hospital, and a commercial monoclonal antibody to H5 HA (US Biological, Swampscott, MA) was used as the reference antibody control. The nitrocellulose membrane blotted with antigens was blocked with 5% skim milk in Tris-buffer saline plus 0.1% Tween 20 (TBS-T). The test sera were incubated with the blotted membrane overnight at 4°C before washing three times with TBS-T, followed by the secondary antibody, horseradish peroxidase enzyme conjugated with either rabbit anti-goat Ig (Dako Cytomation, Denmark), goat anti-mouse IgG (Santa Cruz Biotechnology Inc., Santa Cruz, CA), or goat anti-human IgG (Zymed Laboratories Inc., San Francisco, CA) for 2 hours at room temperature. Diaminobenzidine (Sigma-Aldrich, St. Louis, MO) mixed with NiCl2 and H2O2 was used as the chromogenic substrate.
RESULTS AND DISCUSSION
A fourfold rise in antibody titer either by microNT or HI assay was noted in paired blood specimens, typically when the second specimen was collected 15 or more days after onset of disease (patient nos. 1, 4, and 6). The highest microNT and HI titers of 1:1,280 were detected in sera collected at 15 days (patient no. 6) or 21 days (patient nos. 1 and 4) after onset of disease (Table 2). In one surviving subject from whom repeated serial blood specimens were collected (patient no. 6), the early peak antibody level was followed by a marked decrease in titer about 5 months later, after which the antibody titer remained stable for at least another year. Similarly, all four survivors (patient nos. 2, 3, 4, and 6) also demonstrated microNT titers of ≥1:40 that persisted in all serial serum samples collected at over 3 or almost 5 years after disease onset, the longest periods that we could follow for an individual subject. Our titer of 1:40 was a critical value for indicating previous H5N1 infection, because it was not found in the general Thai population. When establishing the microNT assay, we had investigated more than 200 serum samples from healthy subjects. The results showed that 70% of the subjects had NT antibody to H1N1 virus as tested against A/New Caledonia/20/99 (H1N1) but none had antibody to KAN-1 virus (data not shown). We previously reported the result of an ELISA-based microNT assay in 901 residents of four villages with H5N1 human cases and found that 888 (98.6%) had no NT antibody titer to KAN-1 virus as screened at the serum dilution of 1:5. Among 13 positive cases, 11 had an NT titer of 5 and the other 2 had titers of 10 and 20 (2).
TABLE 2.
Kinetics and longevity of antibody response to H5N1 virus infection
| Subject no. | Blood sample No. | Specimen collection (time after disease onset) | Antibody titer to:
|
|||||
|---|---|---|---|---|---|---|---|---|
| A/Thailand/1(KAN-1)/04a as determined by:
|
A/Thailand/676(NYK)/05b as determined by:
|
|||||||
| NT | HI with:
|
NT | HI with:
|
|||||
| GRBC | HRBC | GRBC | HRBC | |||||
| 1 | 1 | 10 days | <5 | 20 | 20 | 20 | 20 | |
| 2 | 21 days | 1,280 | 640 | 1,280 | 1,280 | 640 | ||
| 2 | 1 | 2 yrs, 3 mos | 80 | 80 | 160 | 160 | 160 | 80 |
| 2 | 2 yrs, 9 mos | 160 | 80 | 160 | 160 | 160 | 160 | |
| 3 | 3 yrs, 3 mos | 80 | 80 | 80 | 160 | 160 | 160 | |
| 4 | 3 yrs, 11 mos | 80 | 80 | 160 | 160 | |||
| 5 | 4 yrs, 5 mos | 40 | 40 | 160 | 160 | |||
| 6 | 4 yrs, 11 mos | 40 | 40 | 160 | 160 | |||
| 3 | 1 | 2 yrs, 2 mos | 160 | 80 | 160 | 80 | 160 | 80 |
| 2 | 2 yrs, 8 mos | 160 | 80 | 160 | 160 | 160 | 160 | |
| 3 | 3 yrs, 2 mos | 160 | 80 | 160 | 160 | 160 | 160 | |
| 4 | 3 yrs, 10 mos | 160 | 80 | 160 | 160 | |||
| 5 | 4 yrs, 3 mos | 80 | 80 | 160 | 160 | |||
| 6 | 4 yrs, 10 mos | 80 | 80 | 160 | 160 | |||
| 4 | 1 | 10 days | <5 | 20 | 20 | |||
| 2 | 12 days | 5 | 20 | 20 | 20 | 20 | ||
| 3 | 21 days | 1,280 | ||||||
| 4 | 1 yrs, 6 mos | 160 | 80 | 80 | 320 | 160 | 160 | |
| 5 | 2 yrs | 160 | 80 | 160 | 320 | 160 | 160 | |
| 6 | 2 yrs, 6 mos | 160 | 80 | 160 | 160 | 80 | 160 | |
| 7 | 3 yrs, 3 mos | 80 | 80 | 160 | 160 | |||
| 8 | 3 yrs, 8 mos | 80 | 80 | 160 | 160 | |||
| 9 | 4 yrs, 2 mos | 80 | 80 | 160 | 160 | |||
| 5 | 1 | 5 days | 320 | 80 | 320 | 160 | 640 | 640 |
| 6 | 1 | 4 days | 5 | <20 | <20 | <10 | 20 | <20 |
| 2 | 15 days | 1,280 | 320 | 640 | 2,560 | 1,280 | 1,280 | |
| 3 | 20 days | 640 | 160 | 320 | 640 | 640 | ||
| 4 | 5 mos | 80 | 80 | 160 | 80 | 160 | 160 | |
| 5 | 11 mos | 80 | 80 | 160 | 80 | 160 | 160 | |
| 6 | 1 yrs, 5 mos | 40 | 80 | 80 | 80 | 160 | 160 | |
| 7 | 2 yrs, 2 mos | 40 | 40 | 80 | 80 | |||
| 8 | 2 yrs, 7 mos | 40 | 40 | 80 | 80 | |||
| 9 | 3 yrs, 1 mos | 40 | 40 | 80 | 80 | |||
| 7 | 1 | 9 days | <5 | <20 | <20 | <5 | 20 | 20 |
| 8 | 1 | 24 days | 5 | <20 | <20 | <20 | <20 | |
| 2 | 27 days | 5 | <20 | <20 | <20 | <20 | ||
Autologous virus of patient no. 1.
Autologous virus of patient no. 7.
There were two patients (nos. 5 and 7) who died before convalescent blood samples were collected. No anti-H5 antibody was detected in patient no.7, whose acute blood was collected 9 days after onset of disease; even the NT assay was performed against NYK, which was the autologous virus. On the other hand, patient no. 5, who had a single serum sample collected 5 days after onset of disease, remarkably exhibited a serum antibody titer of about 1:320 by either microNT or HI assay. Finally, patient no. 8 had no detectable anti-H5 antibody in two blood samples collected at 24 and 27 days after onset of symptoms, beyond the time when a response was typically seen in other patients. Patient no. 8 was negative for anti-human immunodeficiency virus and anti-hepatitis C virus antibodies and was naturally immune to hepatitis B virus infection (positive anti-HBs, positive anti-HBc, and negative HBs antigen). However, he was an alcohol abuser, an underlying condition that might lead to the inability to develop high antibody response. He received standard oseltamivir treatment beginning 14 days after onset of symptoms but died 13 days later. We further tested these two blood samples against autologous virus (A/Thailand/1(NBL)/06 (H5N1), but still no NT antibody titer was detected (data not shown). Katz et al. (4) previously reported an adult H5N1 patient in whom no H5-specific antibody response was detected, despite the collection of appropriately timed paired blood specimens. The diagnosis of H5N1 virus infection in this case was similarly based on positive RT-PCR and virus isolation. However, in this case, the inability to mount an H5 antibody response was attributed to the immunocompromised status of the patient due to systemic lupus erythematosus.
Interestingly, we found that sera obtained from patients infected in 2004 could cross-neutralize the two virus isolates to comparable antibody titers. Similarly, sera collected in 2005 cross-neutralized the earlier H5N1 strain, despite the two viruses sharing 96% amino acid identity in the HA sequence (GenBank accession nos. AAS65615 and ABC72655) and 90% identity in NA sequence (GenBank accession nos. AAS65616 and ABC72646) and the difference in receptor site binding preference of SAα2,3Gal or SAα2,6Gal (1, 6). These results suggest that the two H5N1 viruses examined were antigenically closely related with respect to the human antibody response.
This study used infectious viruses as the test antigens in the HI assay. The protocol as described previously employed either 1% horse or 0.5% goose erythrocytes (5, 8). Even though horse erythrocytes have been recommended by previous investigators and WHO (8, 11) for detection of antibody to AI virus by HI assay, we did not see a marked difference in the antibody titers obtained when either kind of erythrocytes was used (Table 2). Our finding provides an alternative choice of erythrocytes that can be accessed more easily for Asian countries. We previously reported this finding in a smaller number of blood samples (5).
The result from the WB assay in 20 serum samples from healthy subjects was clearly negative, including the serum sample with anti-H5N1 NT antibody titers of 10 and 20 (data not shown). On the other hand, sera from all four survivors, including when using goat anti-H5 HA, reacted with both HA1 and HA2 domains of the H5 HA, while the monoclonal antibody reacted with the HA1 domain only. The WB assay confirmed the persistence of H5N1 antibody in serial serum samples collected at all time points in all survivors. Examples of serum specimens from patients 4 and 6 are shown in Fig. 1.
FIG. 1.
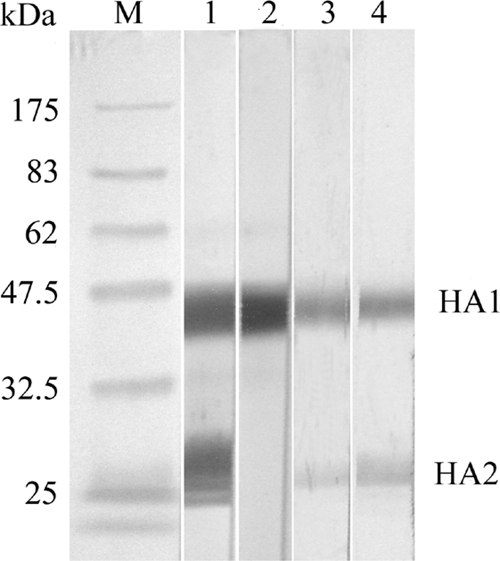
Detection of anti-H5 HA antibody by WB assay using H5 HA recombinant protein as the test antigen. Lane M, protein molecular mass markers; lane 1, goat antiserum to purified H5 HA; lane 2, mouse monoclonal antibody to H5 HA; lane 3, serum from patient no. 4 at 1 year 6 months after disease onset; lane 4, serum from patient no. 6 at 11 months after disease onset.
Our study was limited by the amount and number of blood specimens available for investigation for several reasons: diagnosis of H5N1 virus infection is generally performed on respiratory samples, not sera; criteria for serologic diagnosis have only been established more recently and are not useful for acute clinical care; based on early disease manifestations, clinicians were more likely to suspect other more common diseases, such as dengue, resulting in only small volumes of sera remaining after dengue serodiagnosis; most important is the high fatality rate of human H5N1 disease. Another limitation was the inability to obtain the exact time of exposure and infection of an individual. During a poultry die-off in a village, it was difficult to pinpoint the exact date of exposure, and some cases may have experienced multiple exposures. The incubation period for H5N1 virus infection in humans after exposure to infected poultry in many cases is 2 to 5 days and is generally 7 days or less. In clusters with probable human-to-human transmission, the incubation period appeared to be approximately 3 to 5 days but in one instance was estimated to be 8 to 9 days (3, 9, 14).
In summary, our study demonstrated high neutralizing antibody titers were achieved 2 to 3 weeks after disease onset in the majority of H5N1-infected symptomatic individuals tested. Furthermore, the anti-H5 neutralizing antibody response was long-lasting and cross-neutralizing for related clade 1 viruses. To what extent these features are implicated among asymptomatic individuals remains to be determined.
Acknowledgments
This work was supported by the National Center of Genetic Engineering and Biotechnology, the Thailand Research Fund for Senior Research Scholars, and the Department of Medical Sciences, Ministry of Public Health.
We thank Jacqueline Katz, U.S. CDC, for her valuable comments and for editing part of the manuscript.
Footnotes
Published ahead of print on 20 May 2009.
REFERENCES
- 1.Auewarakul, P., O. Suptawiwat, A. Kongchanagul, C. Sangma, Y. Suzuki, K. Ungchusak, S. Louisirirotchanakul, H. Lerdsamran, P. Pooruk, A. Thititanyanont, C. Pittayawanganon, C.-T. Gao, H. Hiramatsu, W. Jampangern, S. Chunsutthiwat, and P. Puthavathana. 2007. An avian influenza H5N1 virus that binds to a human-type receptor. J. Virol. 819950-9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dejpichai, R., Y. Laosiritaworn, P. Puthavathana, T. M. Uyeki, M. O'Reilly, N. Yampikulsakul, S. Phurahong, P. Pooruk, J. Prasertsopon, R. Kularb, K. Nateerom, N. Sawanpanyalert, and C. Jiraphongsa. 2009. Seroprevalence of antibodies to avian influenza virus A (H5N1) among residents of villages with human cases, Thailand, 2005. Emerg. Infect. Dis. 15756-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandun, I. N., H. Wibisono, E. R. Sedyaningsih, Yusharmen, W. Hadisoedarsuno, W. Purba, H. Santoso, C. Septiawati, E. Tresnaningsih, B. Heriyanto, D. Yuwono, S. Harun, S. Soeroso, S. Giriputra, P. J. Blair, A. Jeremijenko, H. Kosasih, S. D. Putnam, G. Samaan, M. Silitonga, K. H. Chan, L. L. M. Poon, W. Lim, A. Klimov, S. Lindstrom, Y. Guan, R. Donis, J. Katz, N. Cox, M. Peiris, and T. M. Uyeki. 2006. Three Indonesian clusters of H5N1 virus infection in 2005. N. Engl. J. Med. 3552186-2194. [DOI] [PubMed] [Google Scholar]
- 4.Katz, J. M., W. Lim, C. B. Bridges, T. Rowe, J. Hu-Primmer, X. Lu, R. A. Abernathy, M. Clarke, L. Conn, H. Kwong, M. Lee, G. Au, Y. Y. Ho, K. H. Mak, N. J. Cox, and K. Fukuda. 1999. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J. Infect. Dis. 1801763-1770. [DOI] [PubMed] [Google Scholar]
- 5.Louisirirotchanakul, S., H. Lerdsamran, W. Wiriyarat, K. Sangsiriwut, K. Chaichoun, T. Songserm, R. Kitphati, P. Sawanpanyalert, C. Komoltri, P. Auewarakul, and P. Puthavathana. 2007. Erythrocyte binding preference of avian influenza H5N1 viruses. J. Clin. Microbiol. 452284-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puthavathana, P., P. Auewarakul, P. C. Charoenying, K. Sangsiriwut, P. Pooruk, K. Boonnak, R. Khanyok, P. Thawachsupa, R. Kitphati, and P. Sawanpanyalert. 2005. Molecular characterization of the complete genome of human influenza H5N1 virus isolates from Thailand. J. Gen. Virol. 86423-433. [DOI] [PubMed] [Google Scholar]
- 7.Rowe, T., R. A. Abernathy, J. Hu-Primmer, W. W. Thompson, X. Lu, W. Lim, K. Fukuda, N. Cox, and J. M. Katz. 1999. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J. Clin. Microbiol. 37937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephenson, I., J. M. Wood, K. G. Nicholson, and M. C. Zambon. 2003. Sialic acid receptor specificity on erythrocytes affects detection of antibody to avian influenza hemagglutinin. J. Med. Virol. 70391-398. [DOI] [PubMed] [Google Scholar]
- 9.Ungchusak, K., P. Auewarakul, S. F. Dowell, R. Kitphati, W. Auwanit, P. Puthavathana, M. Uiprasertkul, K. Boonnak, C. Pittayawonganon, N. J. Cox, S. R. Zaki, P. Thawatsupha, M. Chittaganpitch, R. Khongtong, J. M. Simmerman, and S. Chunsutthiwat. 2005. Probable person-to-person transmission of avian influenza A (H5N1). N. Engl. J. Med. 352333-340. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. 2009. Countries report H5N1 avian influenza in domestic poultry/wildlife 2003-2009. http://www.oie.int/eng/info_ev/en_AI_factoids_2.htm. Accessed 15 January 2009.
- 11.World Health Organization. 2002. WHO manual on animal influenza diagnosis and surveillance. WHO/CDS/CSR/NCS/2002.5. http://www.who.int/csr/resources/publications/influenza/en/whocdscsrncs20025rev.pdf. Accessed 24 November 2006.
- 12.World Health Organization. 2006. WHO case definitions for human infections with influenza A (H5N1) virus. 29 August 2006. http://www.who.int/csr/disease/avian_influenza/guidelines/case_definition2006_08_29/en/print.html. Accessed 22 October 2006.
- 13.World Health Organization. 2009. Cumulative number of confirmed human cases of avian influenza reported to WHO 14 January 2009. http://www.who.int/csr/disease/avian_influenza/country/cases_table_2009_01_14/en/index.html. Accessed 15 January 2009.
- 14.Writing Committee of the Second WHO Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Viruses. 2008. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 358261-273. [DOI] [PubMed] [Google Scholar]


