Abstract
Current serological diagnosis of Trypanosoma evansi infection in camels is based on the native variable antigen type RoTat 1.2. The goal of this study was to develop a novel serological diagnostic test based on a nonvariable protein and freed from the use of rats or mice for its production. An enzyme-linked immunosorbent assay using a recombinant extracellular domain of invariant surface glycoprotein 75 (ELISA/rISG75) was developed and tested on a collection of 184 camel sera. The results were compared to those obtained from three established antibody detection tests based on variable surface glycoprotein RoTat 1.2: an ELISA for T. evansi (ELISA/T. evansi), a card agglutination test for trypanosomiasis (CATT/T. evansi), and an immune trypanolysis (TL) assay. The ELISA/rISG75 and the ELISA/T. evansi showed a sensitivity of 94.6% (95% confidence interval [CI], 87.8 to 98.2%, at 19% positivity cutoff value) and 98.9% (95% CI, 94.1 to 99.8, at 12% positivity cutoff value), respectively. The ELISA/rISG75 had 100% specificity (CI, 95.9 to 100%), while the ELISA/T. evansi showed 98.9% specificity (CI, 95.9 to 100%). The ELISA/rISG75 demonstrated an almost perfect agreement with the TL assay, the CATT/T. evansi, and the ELISA/T. evansi, with kappa scores of at least 0.94. The ELISA/rISG75, having a performance comparable to that of the gold standard (the TL assay) and being independent of antigenic variation, may become a new reference test for surra in camels. It opens avenues for the diagnosis of T. evansi infections in other hosts as well as for the development of a pan-Trypanozoon test for detection of Trypanosoma brucei brucei, T. b. gambiense, T. b. rhodesiense, T. evansi, and T. equiperdum.
Trypanosoma evansi is the causative agent of surra in domestic animals such as camels, equines, cattle, buffaloes, small ruminants, and dogs. Wild animals such as capybaras can act as reservoir hosts. Although T. evansi is noninfective for healthy humans, a case of human infection by T. evansi in India in 2004 has been reported; however, this infection was due to a genetic mutation in the host's APOL1 gene (20). Camels and horses are very sensitive to T. evansi, and death can occur within 3 months without treatment. Cattle and other ruminants infected by T. evansi suffer from immunosuppression, resulting in increased susceptibility to other diseases or vaccination failure (5, 17). T. evansi is mechanically transmitted by bloodsucking flies, such as Tabanidae and Stomoxys species. The disease occurs in Africa, Asia, and South and Central America and causes important economic losses (16).
Control of surra still relies mainly on the observation of clinical signs and subsequent treatment of sick animals, which is inefficient and results in high morbidity and mortality of undiagnosed animals that in the meantime act as reservoirs. Definitive diagnosis of T. evansi infection is achieved by microscopic demonstration of the parasite, which method, however, suffers from limited sensitivity. The most sensitive parasite detection test is the mini-anion-exchange centrifugation method, but it is seldom used (4, 9). Indirect diagnosis is possible through detection of specific antibodies in the mammalian hosts. All currently available antibody detection tests, including an immune trypanolysis (TL) assay (21), an enzyme-linked immunosorbent assay for T. evansi (ELISA/T. evansi) (22), a card agglutination test for trypanosomiasis for T. evansi (CATT/T. evansi) (1), and a latex agglutination test for T. evansi (24), are based on the native variant surface glycoprotein (VSG) of the predominant variable antigen type (VAT) RoTat 1.2 of T. evansi. Production of these tests requires the mass culture of T. evansi homogeneously expressing RoTat 1.2 VSG in rats or mice. In addition, the results of all these serological tests may remain negative in animals infected with T. evansi type B in Kenya, as this type has been reported not to express the RoTat 1.2 VAT (13-15).
On the cell surface of a bloodstream form trypanosome, among the VSGs and nonvariable surface proteins, there are an estimated 5 × 104 invariant surface glycoprotein (ISG75) molecules (28). The ISG75 gene family is present and transcribed in the bloodstream form of all species and subspecies of the Trypanozoon subgenus, including Trypanosoma brucei brucei, T. b. gambiense, T. b. rhodesiense, T. evansi, and T. equiperdum. This multicopy gene family consists of two main groups that share at least 75% similarity among their cDNA and genomic DNA sequences (19). Within each group, there is at least 92% similarity among the cDNA sequences. A putative ISG75, regardless of its belonging to group I or group II, has a conserved topology: a large N-terminal extracellular domain, a single α-helix transmembrane domain, and a small cytoplasmic domain at the C terminus (19, 27). Since ISG75 is not subject to antigenic variation, its corresponding antibodies circulate during the course of infections in mice (29), and its extracellular domain can be produced in Escherichia coli by a standardized protocol (18), it is considered a highly relevant antigen for diagnosis of Trypanozoon infections.
The purpose of this study was to develop an antibody detection ELISA for T. evansi infection in camels by using recombinant ISG75 (rISG75). This ELISA/rISG75 was tested against a panel of 184 camel sera in parallel with the currently used serological diagnostic tests, including the TL assay, the CATT/T. evansi, and the ELISA/T. evansi.
MATERIALS AND METHODS
Production of rISG75.
An ISG75 cDNA derived from T. b. gambiense LiTat 1.3 (GenBank accession number DQ200239) (19) was expressed in the cytoplasmic space of E. coli strain Origami B (DE3). The pTbG-ISG75Strep-29-457-His construct covered the N-terminal 429 amino acids of the mature polypeptide to the region upstream of the transmembrane domain of the ISG75. This extracellular domain of ISG75 contained an N-terminal Strep-Tag II and a C-terminal His tag to facilitate protein purification (IBA). The ISG75Strep-29-457-His (or rISG75) was purified by tandem affinity chromatography using Strep-Tactin Poros 50 (IBA) and Ni-nitrilotriacetic acid (Qiagen), as described by Tran et al. (18).
Serum collection.
Samples were collected from dromedary camels between February 1995 and September 1995 in the Tahoua, Abalak, and Tchin-Tabaraden districts in Niger (24). Parasitological examination by wet blood film and mini-hematocrit centrifugation (25) and serological screening by the CATT/T. evansi at a 1:4 serum dilution were conducted in the field immediately after sampling. The sera were shipped to Belgium on dry ice and stored at −70°C. A total of 184 sera were used in this study, of which 93 were from camels that were positive by the TL assay, including 47 parasitological test-confirmed cases, and 91 from TL-negative control animals without clinical, parasitological, or serological evidence of infection.
ELISA/T. evansi.
The ELISA/T. evansi was performed by the method of Verloo et al. (22) but adapted for camels as described by Lejon et al. (10). The antigen in the ELISA/T. evansi was the native RoTat 1.2 VSG (0.2 μg/well) purified from bloodstream form T. evansi RoTat 1.2 cultured in rats.
CATT/T. evansi and TL assay.
The CATT/T. evansi and the TL assay were carried out as described by Verloo et al. (24) and Van Meirvenne et al. (21), respectively. The CATT/T. evansi, a direct agglutination test, uses formaldehyde-fixed, Coomassie blue-stained, freeze-dried parasites of T. evansi RoTat 1.2 (1). The TL test uses a suspension of live T. evansi RoTat 1.2 with guinea pig serum as a source of the complement that is incubated with the test serum. Lysis of the trypanosomes occurs when RoTat 1.2-specific antibodies are present in the test serum. The TL assay is considered the gold standard for the presence of specific antibodies against African trypanosomes (21, 23).
ELISA/rISG75.
Microplates (Maxisorp; Nunc) were coated overnight at 4°C with 100 μl/well of recombinant ISG75Strep-29-457-His at 2 μg/ml in phosphate-buffered saline (PBS) (0.01 M phosphate, 0.14 M NaCl [pH 7.4]). Antigen-negative control wells were left empty. Further manipulations were done at room temperature. Plates were blocked for 1 h with 300 μl/well of PBS-1% (wt/vol) casein. For testing, camel serum was diluted 1:400 in PBS-1% (wt/vol) casein. Portions (150 μl) of serum dilutions were added in duplicate to both antigen-containing and control wells for 1 h. By using an automated plate washer (Elx50 washer; BioTek Instruments), microplates were washed three times with 350 μl/well of PBS-Tween (0.05% [vol/vol] Tween 20). Protein A conjugated with horseradish peroxidase (Sigma) was diluted to 1:5,000 in PBS-Tween and incubated for 1 h (150 μl/well). After five washes with 350 μl/well of PBS-Tween, wells were incubated for 1 h with 150 μl ABTS [2,2′-azinobis(3-ethylbenzothiazoline)-6-sulfonic acid; Boehringer] substrate-chromogen solution. The latter was prepared from 50 mg ABTS dissolved in 100 ml of ABTS buffer (phosphate-citrate-sodium perborate solution [pH 4.6]; Boehringer). The plate was shaken for 10 s, and the optical densities (OD) were read at 415 nm (Multiskan RC version 6.0; Labsystems).
Statistical analysis.
Corrected OD values for each test sample were obtained by subtracting the mean OD of the antigen-negative control wells from the mean OD of the corresponding antigen-containing wells. Subsequently, these values were recalculated to percent positivity (PP) using camel serum B5/28 as the reference serum (100 PP). This serum originated from a parasitological test-confirmed, TL-positive camel. Moreover, this serum gave the same corrected OD value in both the ELISA/T. evansi and the ELISA/rISG75.
The optimal combination of diagnostic sensitivity and specificity in the function of the cutoff value was determined by receiver operating characteristic (ROC) analysis (3, 26). ROC curves and the area under the curve (AUC) were generated using Stata Software version 9.2 (StataCorp LP). The concordance between the different diagnostic tests was determined using Cohen's kappa test (2).
RESULTS
Diagnostic performance of the ELISA/rISG75 versus the ELISA/T. evansi.
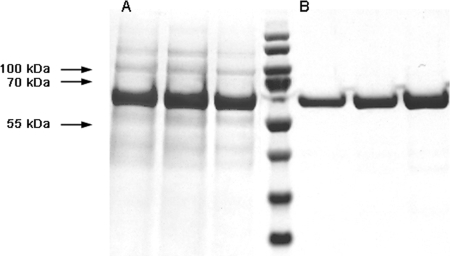
The recombinant extracellular domain of ISG75 (ISG75Strep-29-457-His) of 52 kDa was expressed in Escherichia coli. The cytoplasmic protein was purified using tandem affinity chromatography based on the N-terminal Strep-Tag and the C-terminal histidine tag (Fig. 1). The soluble pure protein was stored as a lyophilized powder that was subsequently resuspended in PBS upon performance of the ELISAs.
FIG. 1.
Coomassie blue-stained polyacrylamide gels containing the peak fractions of the first-step purification using Strep-Tactin Poros 50 (A, lanes 1 to 3) and second-step purification with Ni-NTA (B, lanes 5 to 7) of ISG75Strep-29-457-His. Lane 4, molecular mass markers.
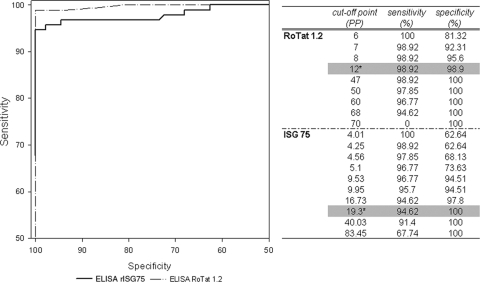
All 184 sera of the collection were tested in duplicate in the ELISA/T. evansi and the ELISA/rISG75. ROC analysis of the ELISA/rISG75 against the TL assay showed an optimum combination of sensitivity of 94.6% (95% CI, 87.8 to 98.2) and specificity of 100% (95% CI, 95.9 to 100) using a cutoff at 19 PP (Fig. 2). ROC analysis of the ELISA/T. evansi on these same samples showed an optimum combination of sensitivity of 98.9% (95% CI, 94.1 to 99.8) and specificity of 98.9% (CI, 95.9 to 100) at a 12 PP cutoff value (Fig. 2). Figure 2, right panel, shows combinations of sensitivity and specificity at different PP cutoff values of the ELISAs with the TL assay as the reference test. Furthermore, chi-square comparison of the AUCs showed no significant differences between results from the two ELISAs (P = 0.12).
FIG. 2.
ROC curves of the ELISA/rISG75 and the ELISA/T. evansi (ELISA/RoTat 1.2) for detection of T. evansi in camels, with the TL assay as a reference test. The right panel shows combinations of sensitivity and specificity of the ELISAs at different cutoff values. The optimal combinations of sensitivity and specificity are shaded in gray. The asterisks indicate the corresponding cutoff values.
Concordance analysis.
Cohen's kappa was calculated to measure the concordance between the ELISA/rISG75 and the three other antibody detection tests, the TL assay, the CATT/T. evansi, and the ELISA/T. evansi. All 91 TL assay-negative and 91 CATT/T. evansi-negative samples were also negative by the ELISA/rISG75. One ELISA/T.evansi-negative sample scored positive by the ELISA/rISG75. Five camel sera that scored positive by the TL assay, the CATT/T. evansi, and the ELISA/T.evansi were negative by the ELISA/rISG75 (Table 1). Overall, kappa scores for the ELISA/rISG75 versus the TL assay and versus the CATT/T. evansi were both 0.95; the kappa score for the ELISA/rISG75 versus the ELISA/T. evansi was 0.94. These scores indicate an almost perfect agreement between the compared tests (8).
TABLE 1.
Comparison of ELISA/rISG75 scores with scores from three other testsa
| ELISA/rISG75 result | No. of serum samples with indicated result by:
|
|||||
|---|---|---|---|---|---|---|
| TL assay
|
CATT/T. evansi
|
ELISA/T. evansi
|
||||
| Positive | Negative | Positive | Negative | Positive | Negative | |
| Positive | 88 | 0 | 88 | 0 | 87 | 1 |
| Negative | 5 | 91 | 5 | 91 | 5 | 91 |
n = 184.
DISCUSSION
RoTat 1.2 is a predominant VSG expressed by most strains of T. evansi, and therefore it is being used as the major antigen in several serological diagnostic tests (1, 23). The CATT/T. evansi, a direct agglutination test, is the most widely applied test and has a proven record of reliability for different host species, such as buffaloes and camels (6, 7). An indirect latex agglutination test for T. evansi also has been used successfully with several host species but is less widely used (22). Both tests can be conducted under field conditions and do not need host species-specific conjugates. The TL assay, the reference test for the presence of VAT-specific antibodies, and the ELISA/T. evansi are laboratory-bound tests, the latter of which can be adapted to different host species by changing the host-specific conjugate. All these tests make use of native VSGs, implicating mass culture of bloodstream form trypanosomes in laboratory rodents. Recombinant RoTat 1.2 VSG has been expressed in Spodoptera frugiperda and incorporated in an ELISA and an indirect latex agglutination test, the latter showing a sensitivity of 89.3% and a specificity of 99.1% when used with 29 camel sera (10). Still, the occurrence of T. evansi type B, hitherto isolated only in Kenya, that does not possess the RoTat 1.2 gene may generate false-negative results in the RoTat 1.2-based tests.
ISG75, a nonvariable protein on the surface of the bloodstream form of all species of the Trypanozoon subgenus and heterologously expressed in E. coli, may overcome the above-mentioned drawbacks. Indeed, its production is independent of in vivo culture of trypanosomes in rodents. An antibody test using rISG75 as an antigen is expected to be reactive with sera from animals infected with T. evansi type B strains not expressing RoTat 1.2. Indeed, sera from mice infected with T. evansi type B (KETRI 2479) were positive by the ELISA/rISG75 using anti-mouse antibody conjugated with peroxidase (data not shown). Furthermore, ELISA/rISG75 tests on a small serum sample set of T. b. brucei-infected goats, T. evansi RoTat 1.2-infected horses, and T. equiperdum-infected rabbits showed that rISG75 is panreactive within the Trypanozoon subgenus. While the protein A-horseradish peroxidase conjugate used in the ELISA/rISG75 protocol may also be used with horse sera, a simple replacement of the host-specific conjugate allows adaptation of the tests to other host species. Therefore, the same rISG75 antigen may be incorporated in diagnostic tests for other trypanosome infections in other hosts, such as T. brucei (nagana in animals, sleeping sickness in humans) or T. equiperdum (dourine in equines).
Our study shows that an ELISA/rISG75, used with a large collection of well-documented sera from Niger, is highly specific (100%) and sensitive (94.6%) for T. evansi infection in camels. Furthermore, although the ELISA/rISG75 targets other antibodies, the results obtained by using the ELISA/rISG75 are highly concordant with those obtained with the RoTat 1.2-based assays (the TL assay, ELISA/T. evansi, and CATT/T. evansi). The performance of this test is even comparable to that of the TL assay, the gold standard for serological diagnosis, as proven by the AUC analysis. Thus, we here deliver the proof of principle of a diagnostic test for surra in camels based on a recombinant invariant protein derived from T. b. gambiense. The same antigen may be incorporated into other test formats, such as lateral flow or agglutination, and bears potential for the development of a pan-Trypanozoon test applicable to other host species (bovine, small ruminants, horses, and humans).
The possibility of cross-reactivity of rISG75 with Trypanosoma congolense and Trypanosoma vivax may be negligible for the following reasons. First, sera from goats experimentally infected with T. congolense and T. vivax showed minimal reactivities by the ELISA/rISG75 using a goat-specific conjugate (data not shown). Second, PCR detection of ISG75 in total DNA of T. congolense and T. vivax, using ISG75-1F and ISG75-2R primers from Tran et al. (19), was negative. Finally, in silico analysis of T. congolense and T. vivax genome projects reveals no significant sequence homology with ISG75.
In summary, 30 years after the pioneering ELISA/T. evansi was developed by Luckins et al. (11, 12), we have developed a highly specific and sensitive ELISA using a recombinant nonvariable antigen for diagnosis of surra in camels. The ELISA/rISG75 may be applicable for diagnosis of infections by other members of the Trypanozoon subgenus and in other host species.
Acknowledgments
We thank the individuals involved in the serum collection in Niger.
This study received financial support from the European Commission Sixth Framework Programme, INCO-DEV-PL003716. F.C. is a postdoctoral fellow of the Research Foundation Flanders (FWO).
Footnotes
Published ahead of print on 29 April 2009.
REFERENCES
- 1.Bajyana Songa, E., and R. Hamers. 1988. A card agglutination test (CATT) for veterinary use based on an early VAT RoTat 1/2 of Trypanosoma evansi. Ann. Soc. Belg. Méd. Trop. 68233-240. [PubMed] [Google Scholar]
- 2.Cohen, J. 1960. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 2037-46. [Google Scholar]
- 3.Greiner, M., D. Sohr, and P. Göbel. 1995. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J. Immunol. Methods 185123-132. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez, C., J. A. Corbera, F. Doreste, and P. Büscher. 2004. Use of the miniature anion exchange centrifugation technique to isolate Trypanosoma evansi from goats. Ann. N. Y. Acad. Sci. 1026149-151. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez, C., J. A. Corbera, M. Morales, and P. Büscher. 2006. Trypanosomosis in goats. Current status. Ann. N. Y. Acad. Sci. 1081300-310. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez, C., M. C. Juste, J. A. Corbera, E. Magnus, D. Verloo, and J. A. Montoya. 2000. Camel trypanosomosis in the Canary Islands: assessment of seroprevalence and infection rates using the card agglutination test (CATT/T. evansi) and parasite detection tests. Vet. Parasitol. 90155-159. [DOI] [PubMed] [Google Scholar]
- 7.Holland, W. G., N. G. Thanh, L. N. My, E. Magnus, D. Verloo, P. Büscher, B. Goddeeris, and J. Vercruysse. 2002. Evaluation of whole fresh blood and dried blood on filter paper discs in serological tests for Trypanosoma evansi in experimentally infected water buffaloes. Acta Trop. 81159-165. [DOI] [PubMed] [Google Scholar]
- 8.Landis, J. R., and G. G. Koch. 1977. The measurement of observer agreement for categorical data. Biometrics 33159-174. [PubMed] [Google Scholar]
- 9.Lanham, S. M., and D. G. Godfrey. 1970. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp. Parasitol. 28521-534. [DOI] [PubMed] [Google Scholar]
- 10.Lejon, V., F. Claes, D. Verloo, M. Maina, T. Urakawa, P. A. O. Majiwa, and P. Büscher. 2005. Recombinant RoTat 1.2 variable surface glycoprotein for diagnosis of Trypanosoma evansi in dromedary camels. Int. J. Parasitol. 35455-460. [DOI] [PubMed] [Google Scholar]
- 11.Luckins, A. G., A. R. Gray, and P. Rae. 1978. Comparison of the diagnostic value of serum immunoglobulin levels, an enzyme-linked immunosorbent assay and fluorescent antibody test in experimental infections with Trypanosoma evansi in rabbits. Ann. Trop. Med. Parasitol. 72429-441. [DOI] [PubMed] [Google Scholar]
- 12.Luckins, A. G., and D. Mehlitz. 1978. Evaluation of an indirect fluorescent antibody test, enzyme-linked immunosorbent assay and quantification of immunoglobulins in the diagnosis of bovine trypanosomiasis. Trop. Anim. Health Prod. 10149-159. [DOI] [PubMed] [Google Scholar]
- 13.Ngaira, J. M., B. Bett, S. M. Karanja, and E. N. M. Njagi. 2003. Evaluation of antigen and antibody rapid detection tests for Trypanosoma evansi infection in camels in Kenya. Vet. Parasitol. 114131-141. [DOI] [PubMed] [Google Scholar]
- 14.Ngaira, J. M., E. N. M. Njagi, J. J. N. Ngeranwa, and N. K. Olembo. 2004. PCR amplification of RoTat 1.2 VSG gene in Trypanosoma evansi isolates in Kenya. Vet. Parasitol. 12023-33. [DOI] [PubMed] [Google Scholar]
- 15.Ngaira, J. M., N. K. Olembo, E. N. M. Njagi, and J. Ngeranwa. 2005. The detection of non-RoTat 1.2 Trypanosoma evansi. Exp. Parasitol. 11030-38. [DOI] [PubMed] [Google Scholar]
- 16.Reid, S. A. 2002. Trypanosoma evansi control and containment in Australia. Trends Parasitol. 18219-224. [DOI] [PubMed] [Google Scholar]
- 17.Stephen, L. E. 1986. Trypanosomiasis. A veterinary perspective. Pergamon Press, Oxford, United Kingdom.
- 18.Tran, T., P. Büscher, G. Vandenbussche, L. Wyns, J. Messens, and H. De Greve. 2008. Heterologous expression, purification and characterisation of the extracellular domain of trypanosome invariant surface glycoprotein ISG75. J. Biotechnol. 135247-254. [DOI] [PubMed] [Google Scholar]
- 19.Tran, T., F. Claes, J.-C. Dujardin, and P. Büscher. 2006. The invariant surface glycoprotein ISG75 gene family consists of two main groups in the Trypanozoon subgenus. Parasitology 133613-621. [DOI] [PubMed] [Google Scholar]
- 20.Vanhollebeke, B., P. Truc, P. Poelvoorde, A. Pays, P. P. Joshi, R. Katti, J. Jannin, and E. Pays. 2006. Human Trypanosoma evansi infection linked to a lack of apolipoprotein L-1. N. Engl. J. Med. 3552752-2756. [DOI] [PubMed] [Google Scholar]
- 21.Van Meirvenne, N., E. Magnus, and P. Büscher. 1995. Evaluation of variant specific trypanolysis tests for serodiagnosis of human infections with Trypanosoma brucei gambiense. Acta Trop. 60189-199. [DOI] [PubMed] [Google Scholar]
- 22.Verloo, D., W. Holland, L. N. My, N. G. Thanh, P. T. Tam, B. Goddeeris, and J. Vercruysse. 2000. Comparison of serological tests for Trypanosoma evansi natural infections in water buffaloes from north Vietnam. Vet. Parasitol. 9287-96. [DOI] [PubMed] [Google Scholar]
- 23.Verloo, D., E. Magnus, and P. Büscher. 2001. General expression of RoTat 1.2 variable antigen type in Trypanosoma evansi isolates from different origin. Vet. Parasitol. 97183-189. [DOI] [PubMed] [Google Scholar]
- 24.Verloo, D., R. Tibayrenc, E. Magnus, P. Büscher, and N. Van Meirvenne. 1998. Performance of serological tests for Trypanosoma evansi infections in camels from Niger. J. Protozool. Res. 8190-193. [Google Scholar]
- 25.Woo, P. T. K. 1969. The haematocrit centrifuge for the detection of trypanosomes in blood. Can. J. Zool. 47921-923. [DOI] [PubMed] [Google Scholar]
- 26.Youden, W. J. 1950. An index for rating diagnostic tests. Cancer 332-35. [DOI] [PubMed] [Google Scholar]
- 27.Ziegelbauer, K., G. Multhaup, and P. Overath. 1992. Molecular characterization of two invariant surface glycoproteins specific for the bloodstream stage of Trypanosoma brucei. J. Biol. Chem. 26710797-10803. [PubMed] [Google Scholar]
- 28.Ziegelbauer, K., and P. Overath. 1992. Identification of invariant surface glycoproteins in the bloodstream stage of Trypanosoma brucei. J. Biol. Chem. 26710791-10796. [PubMed] [Google Scholar]
- 29.Ziegelbauer, K., and P. Overath. 1993. Organization of two invariant surface glycoproteins in the surface coat of Trypanosoma brucei. Infect. Immun. 614540-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]