Abstract
Evaluation of the immune responses induced by childhood vaccines requires measurement of T-cell, as well as antibody, responses. However, cellular immune responses are often not analyzed because of technical hurdles and the volume of blood required. Therefore, a sensitive and specific assay for antigen-specific T cells that utilizes a small volume of blood would facilitate new vaccine evaluation. We developed a novel assay for quantifying virus-specific CD8+ T cells that combines the use of HLA-A2 immunoglobulin-based artificial antigen-presenting cells (aAPCs) for stimulation of antigen-specific CD8+ T cells in whole blood with quantitative real-time reverse transcription-PCR (qRT-PCR) to detect gamma interferon (IFN-γ) mRNA. This assay was optimized using a well-established cytomegalovirus (CMV) CD8+ T-cell system. The aAPC-qRT-PCR assay had comparable sensitivity to intracellular cytokine staining (ICS) in detecting CMV-specific CD8+ T cells with a detection limit of less than 0.004%. The assay was applied to the detection of low-frequency measles virus (MV)-specific CD8+ T cells by stimulating blood from five MV-immune HLA-A*0201 donors with four different MV-specific peptides (MV peptide aAPCs). Stimulation with three of the MV peptide aAPCs resulted in significant increases in IFN-γ mRNA ranging from 3.3- to 13.5-fold. Our results show that the aAPC-qRT-PCR assay is highly sensitive and specific and can be standardized for screening MV-specific CD8+ T cells in vaccine trials. The technology should be transferable to analysis of CD8+ T-cell responses to other antigens.
Vaccines are the most cost-effective method for decreasing deaths due to infectious diseases, and there are active efforts to develop new vaccines and improve old vaccines for a variety of infections. The live attenuated measles virus (MV) vaccine has eliminated measles in developed countries (2, 6, 18). Strategies such as mass immunization campaigns conducted by the World Health Organization, UNICEF, and their partners have led to an estimated 60% reduction in measles mortality worldwide relative to the global burden of mortality since 1999 (30). However, the disease still remains a leading vaccine-preventable cause of childhood mortality in some developing regions, such as parts of sub-Saharan Africa and Southeast Asia (9). This is in part due to the difficulty in protecting children less than 1 year of age with the current vaccine because of a combination of factors, including immaturity of the immune system and interference by transplacental antibodies (13, 24, 27). A novel vaccine that could be used early in infancy will help to close this window of susceptibility. Such a vaccine needs to be evaluated for its ability to induce both early innate and adaptive immune responses, as well as adequate humoral and cellular memory responses.
Although vaccine-induced protection from measles is most directly related to neutralizing antibody, T-cell responses contribute to protection and to the quality and durability of the antibody response (5, 20). However, cell-mediated immune (CMI) responses to MV have not been well characterized due to lack of tools for studying antigen-specific T-cell responses. Current technologies for analyzing immune cells, such as the enzyme-linked immunospot (ELISPOT) assay, intracellular cytokine staining (ICS), and major histocompatibility complex (MHC) tetramer staining provide opportunities to characterize antigen-specific T cells at the single-cell level. However, these methods usually involve isolation of peripheral blood mononuclear cells (PBMCs), which requires large blood volumes for sample preparation, a major hindrance, particularly in young children, for whom new measles vaccines are designed. Here we demonstrate a new assay to measure low-frequency MV-specific T-cell responses that may help define surrogate markers important in optimizing vaccination strategies for the induction of effective MV-specific T-cell responses.
The assay couples the standardized artificial-antigen-presenting-cell (aAPC) technique for stimulating CD8+ T cells in whole blood with quantitative real-time reverse transcription-PCR (qRT-PCR) for the detection of antigen-specific induction of gamma interferon (IFN-γ) mRNA. This assay overcomes many of the limitations associated with current assays because it uses a small sample volume, does not require prior processing of the sample, and is sensitive and reproducible. Furthermore, the assay could easily be adapted for monitoring low-frequency T-cell responses to other vaccines and infections.
MATERIALS AND METHODS
Healthy volunteers.
All donors provided written informed consent before enrolling. The HLA-A*0201 status of study subjects was determined by flow cytometry. A modified plaque reduction neutralization (PRN) assay was used to measure antibodies to MV. PBMCs were isolated from heparinized whole blood by Ficoll-Paque PLUS density gradient centrifugation (Amersham Pharmacia). The Institutional Review Board of Johns Hopkins Medical Institutions approved this investigation.
Peptides.
MV peptides MVppH30, MVppH516, MVppH576, and MVppC84 and cytomegalovirus (CMV) peptide CMVpp65 (Table 1) were prepared by the Johns Hopkins University Core Facility. The purity (>98%) of each peptide was confirmed by mass-spectral analysis and high-performance liquid chromatography.
TABLE 1.
Selected HLA-A*0201 epitopes for MV and CMV proteins
| Virus | Gene/peptide positiona | Amino acid sequence |
|---|---|---|
| MV | H30 | LMIDRPYVL |
| H516 | ILGQDLQYV | |
| H576 | KLWCRHFCV | |
| C84 | KLWESPQEI | |
| CMV | CMV65 | NLVPMVATV |
H, hemagglutinin protein of MV; C, nonstructural protein of MV.
Generation of aAPCs.
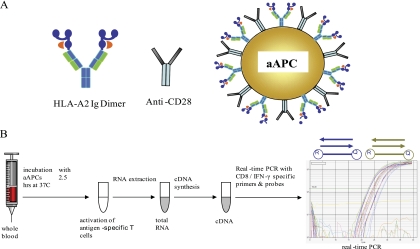
aAPCs were generated by coupling HLA immunoglobulin (Ig) and anti-CD28 onto magnetic beads (Dynal, Lake Success, NY) (Fig. 1A), as previously reported (23). Briefly, beads were incubated with a 1:1 mixture of HLA-A2 Ig and anti-CD28 monoclonal antibody in 0.1 M borate buffer for 24 h at 4°C on a rotator and then washed twice with bead wash buffer. Peptides were loaded onto HLA Ig-coated aAPCs at a 10-μg/ml final peptide concentration and then washed twice with phosphate-buffered saline (PBS) and adjusted to 107 beads/ml. Peptide-containing aAPC beads (peptide aAPCs) were stored up to 6 months in the peptide solution at 4°C.
FIG. 1.
(A) Schematic of aAPCs, followed by peptide loading as described in Materials and Methods. aAPCs were made by immobilizing HLA Ig and anti-CD28 onto magnetic beads. (B) Schematic representation of aAPC-qRT-PCR based assay for detection of CD8+ T-cell reactivity. One hundred microliters of whole blood was added to a vial containing 1 × 106 aAPCs in a total volume of 400 μl PBS. After incubation at 37°C for 2.5 h with rotation, RNA was isolated and subsequently reverse transcribed. qRT-PCR was performed to amplify CD8 and IFN-γ cDNA using primers and TaqMan probes specific for CD8 and IFN-γ, respectively. The circled R and Q represent the reporter and quencher dyes that are at the beginning and end of the TaqMan probes.
Whole-blood stimulation, RNA isolation, and cDNA synthesis.
One hundred microliters of heparinized whole blood was mixed with 400 μl PBS containing106 peptide aAPCs and incubated at 37°C for 2.5 h while rotating. After incubation, total RNA was extracted using the QIAamp RNA blood minikit (Qiagen GmbH, Hilden, Germany) according to the manufacturers' protocol. For cDNA synthesis total RNA was reverse transcribed using the iScript synthesis kit (Bio-Rad Laboratories, Hercules, CA) following the manufacturer's manual (Fig. 1B).
PRN assay.
MV-specific neutralizing antibodies were measured by the ability of serially diluted plasma to reduce plaque formation by the Chicago-1 strain of MV on Vero cells (i.e., PRN), as previously described (4, 19). The international standard serum 66/202 was included in all assays, and data were normalized to that standard to calculate international units of neutralizing antibody per milliliter.
qRT-PCR.
qRT-PCR was performed using primers and TaqMan probes specific for CD8 and IFN-γ (11). The primer and TaqMan probe sequences were as follows: IFN-γ forward primer, AGCTCTGCATCGTTTTGGGTT; IFN-γ reverse primer, GTTCCATTATCCGCTACATCTGAA; IFN-γ probe, 6-carboxyfluorescein (FAM)-TCTTGGCTGTTACTGCCAGGACCCA-Iowa Black fluorescence quencher (IBFQ); CD8 forward primer, CCCTGAGCAACTCCATCATGT; CD8 reverse primer, GTGGGCTTCGCTGGCA; and CD8 probe, HEX-TCAGCCACTTCGTGCCGGTCTTC-IBFQ. PCR was conducted with the iQ5 real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA). PCRs were performed in triplicate in a final volume of 25 μl using the TaqMan Universal PCR master mix (Roche, Branchburg, NJ). PCR mixtures contained 400 nM primers, 200 nM TaqMan probe, and 10% of the cDNA as template. The thermal cycler parameters were 95°C for 10 min, followed by 40 cycles involving denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min, with single fluorescence detection at the end of each cycle. The n-fold increase in IFN-γ mRNA copy number normalized to CD8 mRNA copy number and relative to unstimulated cells was calculated as follows: n-fold increase in IFN-γ mRNA = 2[ΔCT stimulated (IFN-γ- CD8)] − [ΔCT unstimulated (IFN-γ - CD8)], where CT is the threshold cycle.
For each real-time PCR experiment, background IFN-γ mRNA copy number was determined for the individual donors by stimulating the samples with unloaded aAPCs. A threshold value was calculated by adding 3 standard deviations to the mean background IFN-γ mRNA copy number. Based on the assumption that the acquired data follow a Gaussian distribution, this represents a confidence interval greater than 99%. Therefore, IFN-γ mRNA copy numbers higher than the threshold value were considered positive.
HLA-A*0201 MHC multimer staining.
HLA-A*0201 peptide-phycoerythrin (PE) tetrameric complexes for MVppH30, MVppH516, and MVppC84 and a control proprietary peptide were obtained from Beckman Coulter Immunomics Operation. Blood samples were stained with MHC tetramers at 2 μg/ml, followed by fluorescein isothiocyanate (FITC)-labeled anti-CD8. After washing, stained cells were fixed with 0.5% paraformaldehyde and analyzed. MVppH576 peptide was loaded into HLA-A2 Ig dimers (MVppH576 dimer) synthesized in our lab because a tetrameric complex for this peptide was not available during these studies. Freshly collected blood was stained with MVppH576 dimer and stained with PE-conjugated goat anti-mouse Ig (Caltag Laboratories) followed by FITC-conjugated anti-CD8. Unloaded HLA-A2 Ig dimer served as the negative control. Data were acquired on a FACSCalibur flow cytometer and analyzed using FlowJo software. The frequency of multimer-positive CD8+ T cells was determined by gating on CD3+ cells.
In vitro stimulation and intracellular cytokine expression of T lymphocytes.
To measure antigen-specific CD8+ T cells, a 2× cocktail containing Golgiplug and 2 μg/ml CD28-CD49d costimulatory reagent (BD Biosciences, San Jose, CA), with either peptide-loaded aAPCs (for antigen-specific stimulation) or unloaded aAPCs (nonspecific stimulation), was prepared. One hundred microliters of cocktail was added per well in a 96-well plate (Costar, Corning, NY). One hundred microliters of whole blood was added to each well and incubated at 37°C in 5% CO2 for 6 h.
At the end of the culture period, cells were transferred into fluorescence-activated cell sorter staining tubes (BD Biosciences, Bedford, MA); washed and incubated with human AB serum (United States Biologic, Swampscott, MA) to prevent nonspecific Fc receptor binding; and then labeled with anti-CD3, anti-CD4, and anti-CD8 monoclonal antibodies (Pharmingen, San Diego, CA) for 20 min. Following red-blood-cell lysis and surface staining, cells were incubated in permeabilization buffer (PBS [pH 7.4], 0.1% saponin, 1% bovine serum albumin, 0.1% sodium azide) for 20 min at room temperature, then stained with APC-labeled antibody to human IFN-γ and a relevant isotype control (Pharmingen, San Diego, CA) for 30 min. Intracellular cytokine expression was assessed by flow cytometry.
RESULTS
Optimization of the aAPC-qRT-PCR assay.
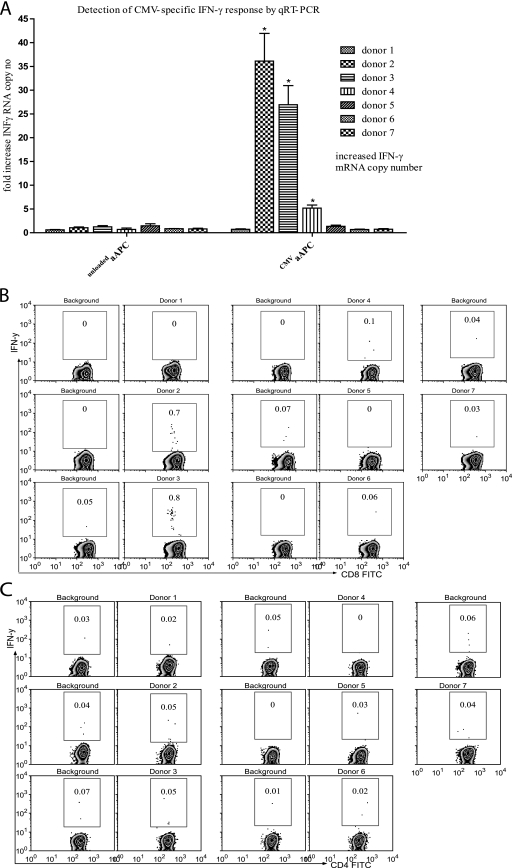
To evaluate the aAPC-qRT-PCR assay for the detection of antigen-specific CD8+ T cells, we used a well-established CMV-specific CD8+ T-cell system (17, 23). The HLA-A*0201 status and frequencies of CMV tetramer-positive cells of seven healthy donors were determined by flow cytometry (Table 2). The aAPC-qRT-PCR assay was performed in parallel with the IFN-γ ICS assay for detection of antigen-specific T cells. Two donors with 36-fold and 27-fold increases in IFN-γ mRNA by aAPC-qRT-PCR assay (Fig. 2A) also had high frequencies (2.97% and 2.69%) of CMV tetramer-positive cells (Table 2), and CMV peptide induced IFN-γ expression of 0.70% and 0.80% by ICS assay (Fig. 2B). Donor 4, with a fivefold increase in IFN-γ mRNA level by aAPC-qRT-PCR assay, had a low percentage (0.24%) of CMV tetramer-positive cells (Table 2) and low level (0.10%) of IFN-γ ICS (Fig. 2B). All donors who tested negative by the aAPC-qRT-PCR assay were also IFN-γ ICS and CMV tetramer negative (Table 2). To ensure that the IFN-γ detected by the aAPC-qRT-PCR assay was mainly from CD8+ T cells, all stimulated whole-blood samples for IFN-γ ICS analysis were surface stained with human anti-CD4 and anti-CD8 monoclonal antibodies. CD4+ T cells were IFN-γ negative for all donors by ICS (Fig. 2C).
TABLE 2.
Detection of CMV-specific CD8+ T-cell reactivity by tetramer, ICS, and aAPC-qRT-PCRa
| Donor | HLA-A*0201 status | % of CD8+ cells
|
Fold increase in IFN-γ copies (aAPC-qRT-PCR)b | ||
|---|---|---|---|---|---|
| PBMCs | CMV tetramer positive | IFN-γ (ICS) | |||
| 1 | Negative | 6.06 | 0.00 | 0.00 | 1 |
| 2 | Positive | 4.76 | 2.97 | 0.70 | 36* |
| 3 | Positive | 8.76 | 2.69 | 0.75 | 27* |
| 4 | Positive | 6.47 | 0.24 | 0.10 | 5* |
| 5 | Positive | 10.25 | 0.01 | 0.00 | 1 |
| 6 | Positive | 8.58 | 0.06 | 0.06 | 1 |
| 7 | Positive | 8.26 | 0.03 | 0.00 | 1 |
Blood samples from seven healthy donors were analyzed. Frequencies of CMV-specific CD8+ T cells were measured by tetramer and IFN-γ ICS. All values for tetramer and ICS are background subtracted. Increases (fold) in IFN-γ mRNA expression were measured by aAPC-qRT-PCR assay and were computed using the formula described in Materials and Methods.
Asterisks mark all values considered positive by the aAPC-qRT-PCR assay.
FIG. 2.
Evaluation of the aAPC-qRT-PCR-based assay using the CMV-specific CD8+ T-cell system. Seven donors were tested for CMV-specific CD8+ T-cell responses using the aAPC-qRT-PCR assay. (A) One hundred microliters of whole blood per stimulation condition was analyzed with the aAPC-qRT-PCR-based assay for activation and detection of CMV-specific T-cell responses. Asterisks mark all values considered positive. For details, see Materials and Methods. (B) Four-color ICS was performed on whole-blood samples after 6 h of stimulation with CMV aAPCs. The frequencies of CMV-specific CD8+ T cells were assessed by gating on CD3, CD8, and IFN-γ triple-positive cells. (C) The CMV specificity for stimulating CD8+T cells in whole blood was tested by assessing the frequency of IFN-γ-producing CD4+T cells. CD3, CD4, and IFN-γ triple-positive cells are gated.
Determination of the detection limit of the aAPC-qRT-PCR assay.
We used donor 4, who had the lowest detectable level of IFN-γ mRNA by aAPC-qRT-PCR assay, to estimate the detection limit of the assay. Based on an estimated leukocyte count of 106 leukocytes in 100 μl of blood and the acquired T-cell data for donor 4 (Table 2), we estimated the number of CMV-specific IFN-γ-producing cells to be 0.004%, which represents 40 CMV-specific CD8+ T cells in 100 μl of blood.
Analysis of MV-specific CD8+ T cells using the aAPC-qRT-PCR-based assay.
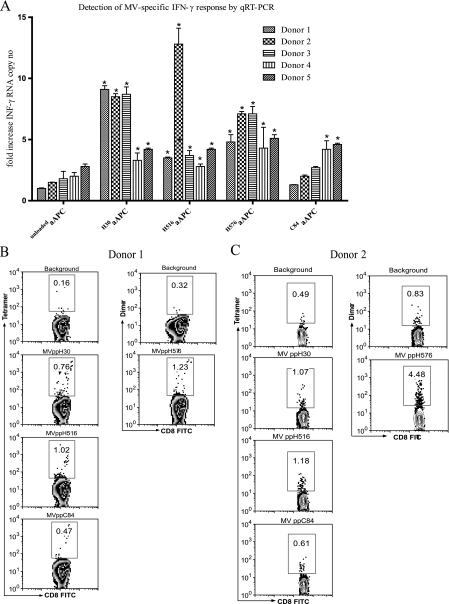
MV infection or immunization generally confers lifelong immunity. Consequently, MV-specific CD8+ T cells can be detected in individuals several decades after immunization. However, the frequencies of these cells are generally much lower than those of CMV-specific CD8+ T cells (15, 25). We evaluated the aAPC-qRT-PCR assay for the detection of low-frequency MV-specific memory CD8+ T cells. Four MV peptides were chosen for these experiments based on previous studies (Table 1) (25). Five healthy HLA-A*0201 donors with detectable MV-neutralizing antibodies were studied. All donors had a remote history of measles immunization, except for donor 2, who received measles-mumps-rubella (MMR) vaccine less than 2 years prior to sampling and had a higher PRN titer (PRNT) (Table 3). Blood samples obtained from all donors were stimulated with MV peptides (Table 1) loaded onto HLA-Ig aAPCs, followed by qRT-PCR. Means for three repeat experiments are shown (Fig. 3A). In addition, the frequencies of MV peptide-specific memory CD8+ T cells were determined by staining whole blood with multimers loaded with each of the four different MV peptides. Representative multimer staining results for donor 1 and 2 are shown in Fig. 3B and C. Donors 1 and 3 with a remote history of measles immunization showed a similar pattern of IFN-γ mRNA expression in response to MV-specific stimulation conditions. Both donors had elevated responses to two hemagglutinin (H) protein-derived peptides, MVppH30 and MVppH576, ranging from 5.1- to 9.8-fold increases in IFN-γ mRNA levels, while stimulation with MVppC84 was negative for both donors (Table 3). In contrast, donor 2, who received MMR booster vaccine less than 2 years prior to sampling, had relatively higher (9.1-, 13.5-, and 7.5-fold) increases in IFN-γ mRNA levels following stimulation with all three H protein-derived peptides (Table 3). Donors 4 and 5 had low but positive responses to all four peptides tested. Overall, although no single peptide stimulation dominated the responses, MVppH30 and MVppH576 generally induced stronger responses in most of the donors. PRNT, multimer staining, and aAPC-qRT-PCR results for the measles studies are summarized in Table 3.
TABLE 3.
Detection of MV-specific immune responses by PRNT, MHC tetramer, and aAPC-qRT-PCRa
| Donor peptide | PRNT of mlU | % of multimer-positive CD8+ T cells | Fold increase in IFN-γ mRNA copy no.b |
|---|---|---|---|
| Donor 1 | 1,778.9 | ||
| MVppH30 | 0.6 | 9.8* | |
| MVppH516 | 0.86 | 4.5* | |
| MVppH576 | 0.93 | 5.1* | |
| MVppC84 | 0.31 | 2.0 | |
| Donor 2 | 3,302.9 | ||
| MVppH30 | 0.58 | 9.1* | |
| MVppH516 | 0.69 | 13.5* | |
| MVppH576 | 3.65 | 7.5* | |
| MVppC84 | 0.12 | 2.5 | |
| Donor 3 | 983.0 | ||
| MVppH30 | 0.04 | 8.7* | |
| MVppH516 | 0.14 | 3.7* | |
| MVppH576 | 3.31 | 7.1* | |
| MVppC84 | 0.13 | 2.7 | |
| Donor 4 | |||
| MVppH30 | 2,980.7 | 0.03 | 3.3* |
| MVppH516 | 0.04 | 2.8* | |
| MVppH576 | 1.03 | 4.3* | |
| MVppC84 | 0.14 | 4.2* | |
| Donor 5 | 2,632.2 | ||
| MVppH30 | 0.01 | 4.2* | |
| MVppH516 | 0.00 | 4.2* | |
| MVppH576 | 0.08 | 5.1* | |
| MVppC84 | 0.01 | 4.2* |
Blood samples from 5 HLA-A*0201 healthy donors were analyzed for measles virus-neutralizing antibody by PRNT. Frequencies of MV-specific CD8+T cells were measured by tetramer staining. Values for tetramer staining are background subtracted. Increases (fold) in IFN-γ mRNA expression were measured by aAPC-qRT-PCR assay and were computed using the formula described in Materials and Methods.
Asterisks mark all values considered positive by the aAPC-qRT-PCR assay.
FIG. 3.
Analysis of measles-reactive cells. Five HLA-A2-positive donors were analyzed for MV peptide-specific memory CD8+ T cells using the aAPC-qRT-PCR method. Blood samples were collected and tested using the aAPC-qRT-PCR assay for MV peptide reactivity as indicated. (A) Mean levels of IFN-γ mRNA expression for the three repeat experiments are reported. Asterisks mark all values considered positive; for details, see Materials and Methods. The frequency of MV peptide-specific CD8+ T cells was also assessed by staining whole blood with multimers loaded with the MV peptides listed in Table 1. Representative multimer staining results for donor 1 (B) and donor 2 (C) are reported.
DISCUSSION
We developed a new aAPC-qRT-PCR assay for the detection of antigen-specific memory T cells in whole blood by combining a standardized antigen-specific aAPC stimulation method and a high-sensitivity qRT-PCR assay for amplification and quantification of IFN-γ gene expression. It is at least as sensitive as current standard assays such as ICS and tetramer analysis but requires much smaller blood volumes. A feature that allows for easy application of this assay in field studies is that sample tubes containing the aAPCs can be set up in advance, and 50 to 100 μl of blood is added when samples are collected and incubated for 2 to 3 h. Once RNA is isolated, the sample can be stored or shipped for PCR analysis. Therefore, this assay is potentially field-friendly and suitable for studies involving young children.
ELISPOT, ICS, and MHC tetramer techniques are the three main assays that have been employed to characterize cellular immune response to MV infection and measles vaccination. Ovsyannikova et al. (26) used PBMCs from seronegative and highly seropositive adults to evaluate the longevity of MV-specific CMI responses following vaccination by measuring frequencies of memory T cells using the ELISPOT assay. Another group used IFN-γ ICS to examine the profile of MV vaccine-induced antigen-specific T cells over time following vaccination (22). We recently reported three new MV HLA-A*0201 CD8+ T-cell epitopes and demonstrated their use in evaluating CD8+ T-cell responses to MV infection and measles vaccination using HLA-A*0201 tetramers and HLA Ig dimers (25). While current techniques have allowed for the analysis of MV-specific CD8+ T cells in research laboratories, they are not well suited for analysis of MV-specific CD8+ T cells of children, especially in resource-poor settings. ELISPOT has the advantage of being highly sensitive with a detection limit in PBMCs of 10/106 (1, 28), but requires a large blood volume to obtain sufficient numbers of cells and takes 2 to 3 days to perform. ICS has the advantage of identifying the active cell type and multiparameter capability, but also requires large blood volume and on-site availability of sophisticated instruments because samples must be analyzed shortly after stimulation. Tetramer staining has the advantage of speed and sensitivity, but positive tetramer staining does not always reflect cell function (16). This may in part explain the inconsistencies between multimer staining and aAPC-qRT-PCR assay results for measles-reactive cells. For example, donor 2 showed high levels of MVppH576-specific cells by Ig dimer staining but had a lower aAPC-qRT-PCR result (Fig. 3A and C).
We first used the well-established CMV system to optimize the assay because of its high-affinity CMVpp65 peptide antigen and also because many of our donors had relatively high precursor frequencies that could be detected by CMV tetramer as well as ICS without prior in vitro manipulation. These studies allowed us to determine optimum blood sample volume, incubation period, and RNA isolation conditions (data not shown). After establishing the assay conditions, we compared the aAPC-qRT-PCR assay performance to that of ICS. The aAPC-qRT-PCR assay was able to detect low frequencies of CMV-specific cells. For example, we were able to detect approximately 40 CMV-specific T cells in 106 PBMCs (0.004%) in the donor with the weakest CMV-specific cytotoxic T-lymphocyte reactivity. This implies that our assay has equivalent sensitivity to the standard ICS assay, for which the lower limit of detection has been reported to range between 0.01% and 0.001% antigen-specific T cells (3, 12, 14, 31). However, to identify 0.001% antigen-specific cells by ICS would require acquisition of several hundred thousand events and a much larger sample volume for analysis than is needed for our aAPC-qRT-PCR assay.
Specificity is another important attribute of a good assay. The aAPC-qRT-PCR assay did not give false-positive results. All samples that were negative by the standard assays (ICS and MHC tetramer staining) were also negative by the aAPC-qRT-PCR assay. Although whole blood was used, the IFN-γ detected by the assay, as expected, mainly came from CD8+ T cells, because aAPCs express class I MHC molecules and stimulate CD8+ T cells. CD4+ T cells were negative for intracellular IFN-γ in all seven donors stimulated with CMV aAPCs.
Once the conditions were optimized, we next used the aAPC-qRT-PCR assay to detect MV-specific memory CD8+ T cells. Unlike for CMV and influenza viruses, no single immunodominant peptide has been identified for MV-specific CD8+ T cells. Therefore, four MV-derived peptides were selected for stimulation: three from the H protein and one from the C protein (7, 8, 10, 21, 25, 29). aAPCs loaded with peptides derived from the H protein induced IFN-γ secretion in MV-specific T cells in all of the donors, consistent with H protein as a primary target of the MV-specific HLA-A*0201-restricted CD8+ T-cell response during measles and after vaccination (25). The MVppC84 derived from the C gene of MV, which codes for a nonstructural protein, did not induce detectable CD8+ T-cell IFN-γ production in three out of the five donors tested. Interestingly, stimulation with MV peptides H30 and H576 induced stronger responses in three of the five donors studied. While additional tests for these peptides in patients with natural MV infection and individuals receiving measles vaccination are needed to substantiate these findings, it appears that peptides H30 and H576 are immunodominant HLA-A*0201-restricted MV peptides.
The versatility of the aAPC-qRT-PCR assay was shown by the ability to successfully detect antigen-specific CD8+ T cells in both the CMV system with one immunodominant epitope and in the generally low-frequency immunodemocratic MV-specific CD8+ T-cell system. However, the new aAPC-qRT-PCR assay as described has some limitations, such as the requirements for isolation of RNA and the subsequent generation of cDNA, which impose a level of complexity that may not be available in field sites. In addition, its application is limited to knowledge of epitopes and HLA-A2 haplotype. Nevertheless, it represents a starting platform that is already advantageous over other methods such as multimer staining, ICS, and ELISPOT assay and has the potential for further improvements. For example, in its current form, it can only be used to identify one cytokine at a time, an issue we are currently addressing by multiplexing the real-time-PCR protocol to allow for the simultaneous detection of multiple cytokines.
These data demonstrate that this assay can be used to identify antigen-specific (CMV and MV) memory CD8+ T-cell responses. Further studies will be required to evaluate the ability of the assay to characterize acute immune responses to measles vaccination and the kinetics of MV-specific CD8+ T-cell responses over time. In summary, we have developed a novel assay that can detect as few as 40 antigen-specific CD8+ T cells in 100 μl of whole blood. Special features of this assay include utilization of small sample volume and no requirement for presample processing or sophisticated instruments on site. Because it can use as little as 100 μl of blood, it is especially suited for studying antigen-specific T-cell responses in young children.
Acknowledgments
We thank William Moss and Fidel Zavala for helpful discussions. We also thank Joan Glick Bieler for critical reading of the manuscript and Brandyn Lau for valuable assistance with the PRNT assay.
This work was funded by the Bill and Melinda Gates Foundation and the Johns Hopkins Malaria Research Institute.
Footnotes
Published ahead of print on 3 June 2009.
REFERENCES
- 1.Asai, T., W. J. Storkus, and T. L. Whiteside. 2000. Evaluation of the modified ELISPOT assay for gamma interferon production in cancer patients receiving antitumor vaccines. Clin. Diagn. Lab. Immunol. 7145-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canada Communicable Disease Report. 2002. Global measles mortality reduction and regional elimination, 2000-2001. Can. Commun. Dis. Rep. 2881-98. [PubMed] [Google Scholar]
- 3.Clay, T. M., A. C. Hobeika, P. J. Mosca, H. K. Lyerly, and M. A. Morse. 2001. Assays for monitoring cellular immune responses to active immunotherapy of cancer. Clin. Cancer Res. 71127-1135. [PubMed] [Google Scholar]
- 4.Cohen, B. J., S. Audet, N. Andrews, and J. Beeler. 2007. Plaque reduction neutralization test for measles antibodies: description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine 2659-66. [DOI] [PubMed] [Google Scholar]
- 5.Coovadia, H. M., A. Wesley, and P. Brain. 1978. Immunological events in acute measles influencing outcome. Arch. Dis. Child. 53861-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutts, F. T., A. Henao-Restrepo, and J. M. Olive. 1999. Measles elimination: progress and challenges. Vaccine 17(Suppl. 3)S47-S52. [DOI] [PubMed] [Google Scholar]
- 7.Day, E. K., A. J. Carmichael, I. J. M. ten Berge, E. C. P. Waller, J. G. P. Sissons, and M. R. Wills. 2007. Rapid CD8+ T cell repertoire focusing and selection of high-affinity clones into memory following primary infection with a persistent human virus: human cytomegalovirus. J. Immunol. 1793203-3213. [DOI] [PubMed] [Google Scholar]
- 8.Dunbar, P. R., G. S. Ogg, J. Chen, N. Rust, P. van der Bruggen, and V. Cerundolo. 1998. Direct isolation, phenotyping and cloning of low-frequency antigen-specific cytotoxic T lymphocytes from peripheral blood. Curr. Biol. 8413-416. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari, M. J., R. F. Grais, N. Bharti, A. J. K. Conlan, O. N. Bjornstad, L. J. Wolfson, P. J. Guerin, A. Djibo, and B. T. Grenfell. 2008. The dynamics of measles in sub-Saharan Africa. Nature 451679-684. [DOI] [PubMed] [Google Scholar]
- 10.Herberts, C. A., K. J. Stittelaar, E. van der Heeft, J. van Gaans-van den Brink, M. C. M. Poelen, P. J. M. Roholl, L. J. W. van Alphen, C. J. M. Melief, A. P. J. M. de Jong, and C. A. C. M. van Els. 2001. A measles virus glycoprotein-derived human CTL epitope is abundantly presented via the proteasomal-dependent MHC class I processing pathway. J. Gen. Virol. 822131-2142. [DOI] [PubMed] [Google Scholar]
- 11.Kammula, U. S., K.-H. Lee, A. I. Riker, E. Wang, G. A. Ohnmacht, S. A. Rosenberg, and F. M. Marincola. 1999. Functional analysis of antigen-specific T lymphocytes by serial measurement of gene expression in peripheral blood mononuclear cells and tumor specimens. J. Immunol. 1636867-6875. [PubMed] [Google Scholar]
- 12.Karanikas, V., J. Lodding, V. C. Maino, and I. F. McKenzie. 2000. Flow cytometric measurement of intracellular cytokines detects immune responses in MUC1 immunotherapy. Clin. Cancer Res. 6829-837. [PubMed] [Google Scholar]
- 13.Leuridan, E., and P. Van Damme. 2007. Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine 256296-6304. [DOI] [PubMed] [Google Scholar]
- 14.Maecker, H. T., S. Auffermann-Gretzinger, L. E. Nomura, A. Liso, D. K. Czerwinski, and R. Levy. 2001. Detection of CD4 T-cell responses to a tumor vaccine by cytokine flow cytometry. Clin. Cancer Res. 7902s-908s. [PubMed] [Google Scholar]
- 15.Maino, V. C., and L. J. Picker. 1998. Identification of functional subsets by flow cytometry: intracellular detection of cytokine expression. Cytometry 34207-215. [DOI] [PubMed] [Google Scholar]
- 16.Markovic, S. N., W. K. Nevala, C. B. Uhl, E. Celis, and D. J. McKean. 2006. A reproducible method for the enumeration of functional (cytokine producing) versus non-functional peptide-specific cytotoxic T lymphocytes in human peripheral blood. Clin. Exp. Immunol. 145438-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita, Y., M. Hosokawa, M. Ebisawa, T. Sugita, O. Miura, Y. Takaue, and Y. Heike. 2005. Evaluation of cytomegalovirus-specific cytotoxic T-lymphocytes in patients with the HLA-A*02 or HLA-A*24 phenotype undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 36803-811. [DOI] [PubMed] [Google Scholar]
- 18.Moss, W. J., and D. E. Griffin. 2006. Global measles elimination. Nat. Rev. Microbiol. 4900-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moss, W. J., S. Scott, N. Mugala, Z. Ndhlovu, J. A. Beeler, S. A. Audet, M. Ngala, S. Mwangala, C. Nkonga-Mwangilwa, J. J. Ryon, M. Monze, F. Kasolo, T. C. Quinn, S. Cousens, D. E. Griffin, and F. T. Cutts. 2007. Immunogenicity of standard-titer measles vaccine in HIV-1 infected and uninfected Zambian children: an observational study. J. Infect. Dis. 196347-355. [DOI] [PubMed] [Google Scholar]
- 20.Nair, N., H. Gans, L. Lew-Yasukawa, A. Long-Wagar, A. Arvin, and D. Griffin. 2007. Age-dependent differences in IgG isotype and avidity induced by measles vaccine received during the first year of life. J. Infect. Dis. 1961339-1345. [DOI] [PubMed] [Google Scholar]
- 21.Nanan, R., C. Carstens, and H. W. Kreth. 1995. Demonstration of virus-specific CD8+ memory T cells in measles-seropositive individuals by in vitro peptide stimulation. Clin. Exp. Immunol. 10240-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nanan, R., A. Rauch, E. Kampgen, S. Niewiesk, and H. W. Kreth. 2000. A novel sensitive approach for frequency analysis of measles virus-specific memory T-lymphocytes in healthy adults with a childhood history of natural measles. J. Gen. Virol. 811313-1319. [DOI] [PubMed] [Google Scholar]
- 23.Oelke, M., M. V. Maus, D. Didiano, C. H. June, A. Mackensen, and J. P. Schneck. 2003. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat. Med. 9619-624. [DOI] [PubMed] [Google Scholar]
- 24.Osterhaus, A., G. van Amerongen, and R. van Binnendijk. 1998. Vaccine strategies to overcome maternal antibody mediated inhibition of measles vaccine. Vaccine 161479-1481. [DOI] [PubMed] [Google Scholar]
- 25.Ota, M. O., Z. Ndhlovu, S. Oh, S. Piyasirisilp, J. A. Berzofsky, W. J. Moss, and D. E. Griffin. 2007. Hemagglutinin protein is a primary target of the measles virus-specific HLA-A2-restricted CD8+ T cell response during measles and after vaccination. J. Infect. Dis. 1951799-1807. [DOI] [PubMed] [Google Scholar]
- 26.Ovsyannikova, I. G., N. Dhiman, R. M. Jacobson, R. A. Vierkant, and G. A. Poland. 2003. Frequency of measles virus-specific CD4+ and CD8+ T cells in subjects seronegative or highly seropositive for measles vaccine. Clin. Diagn. Lab. Immunol. 10411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Premenko-Lanier, M., G. Hodge, P. Rota, A. Tamin, W. Bellini, and M. McChesney. 2006. Maternal antibody inhibits both cellular and humoral immunity in response to measles vaccination at birth. Virology 350429-432. [DOI] [PubMed] [Google Scholar]
- 28.Renkvist, N., C. Castelli, P. F. Robbins, and G. Parmiani. 2001. A listing of human tumor antigens recognized by T cells. Cancer Immunol. Immunother. 503-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Els, C. A. C. M., C. A. Herberts, E. van der Heeft, M. C. M. Poelen, J. A. M. van Gaans-van den Brink, A. van der Kooi, P. Hoogerhout, G. Jan ten Hove, H. D. Meiring, and A. P. J. M. de Jong. 2000. A single naturally processed measles virus peptide fully dominates the HLA-A*0201-associated peptide display and is mutated at its anchor position in persistent viral strains. Eur. J. Immunol. 301172-1181. [DOI] [PubMed] [Google Scholar]
- 30.Weekly Epidemiological Record. 2007. Progress in global measles control and mortality reduction, 2000-2006. Wkly. Epidemiol. Rec. 82418-424. [PubMed] [Google Scholar]
- 31.Whiteside, T. L., Y. Zhao, T. Tsukishiro, E. M. Elder, W. Gooding, and J. Baar. 2003. Enzyme-linked immunospot, cytokine flow cytometry, and tetramers in the detection of T-cell responses to a dendritic cell-based multipeptide vaccine in patients with melanoma. Clin. Cancer Res. 9641-649. [PubMed] [Google Scholar]