Abstract
A double-blind, randomized, controlled phase I study to assess the safety, immunogenicity, and antibody persistence of a new group A conjugate vaccine (PsA-TT) in volunteers aged 18 to 35 years was previously performed. Subjects received one dose of either the PsA-TT conjugate vaccine, meningococcal A/C polysaccharide vaccine (PsA/C), or tetanus toxoid vaccine. The conjugate vaccine was shown to be safe and immunogenic as demonstrated by a standardized group A-specific immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) and by a serum bactericidal antibody (SBA) assay using rabbit complement (rSBA). This report details further analysis of the sera using four additional immunologic assays to investigate the relationship between the different immunoassays. The immunoassays used were an SBA assay that used human complement (hSBA), a group A-specific IgG multiplexed bead assay, and two opsonophagocytic antibody (OPA) assays which used two different methodologies. For each vaccine group, geometric mean concentrations or geometric mean titers were determined for all assays before and 4, 24, and 48 weeks after vaccination. Pearson's correlation coefficients were used to assess the relationship between the six assays using data from all available visits. An excellent correlation was observed between the group A-specific IgG concentrations obtained by ELISA and those obtained by the multiplexed bead assay. hSBA and rSBA titers correlated moderately, although proportions of subjects with putatively protective titers and those demonstrating a ≥4-fold rise were similar. The two OPA methods correlated weakly and achieved only a low correlation with the other immunoassays. The correlation between hSBA and group A-specific IgG was higher for the PsA-TT group than for the PsA/C group.
Within the African meningitis belt, unpredictable epidemics of meningococcal disease continue to occur every 5 to 15 years. To prevent these epidemics, a novel serogroup A conjugate meningococcal vaccine was developed. The Meningitis Vaccine Project, a partnership between the World Health Organization (WHO) and PATH (Seattle, WA) with core funding from the Bill and Melinda Gates Foundation, was created in 2001 with the goal of eliminating meningococcal epidemics in sub-Saharan Africa through the development, testing and licensure, and widespread use of serogroup A meningococcal conjugate vaccines (11) (http://www.meningvax.org).
A group A meningococcal conjugate vaccine using tetanus toxoid as a carrier protein (PsA-TT) was developed at the Serum Institute of India Ltd., using a new licensed conjugation technique from the Center for Biologics Evaluation and Research/Food and Drug Administration (CBER/FDA, Bethesda, MD) (15). The results from a double-blind, randomized, controlled phase I study to assess safety, immunogenicity, and antibody persistence in healthy volunteers aged 18 to 35 years have been reported elsewhere (13). Subjects received either the PsA-TT vaccine, meningococcal A/C polysaccharide vaccine (PsA/C), or the tetanus toxoid vaccine. Blood samples were taken on the day of immunization and 4, 24, and 48 weeks later. Assessment by standardized enzyme-linked immunosorbent assay (ELISA) for group A-specific immunoglobulin G (IgG) and serum bactericidal antibody (SBA) assay using rabbit complement (rSBA) showed the vaccine to be immunogenic and able to elicit persistent functional antibody titers (13). Sera from this study were analyzed by additional immunologic assays, data from which have been used to investigate the relationship between different group A immunologic assays. Previously, knowledge of the relationship between group A immunoassays has been limited, with the majority of studies comparing only two assays; therefore, the goal of this study was to investigate the relationship between six different group A immunoassays, knowledge of which may aid our understanding of the immune response to this and other vaccines.
MATERIALS AND METHODS
Study group.
Full details of the study group were reported by Kshirsagar et al. (13); in brief, healthy adult volunteers aged 18 to 35 years were recruited from three sites within India and were randomized in a double-blind fashion to receive intramuscularly in the deltoid region one dose of either the PsA-TT conjugate vaccine (n = 24), the meningococcal polysaccharide vaccine A/C (Sanofi Pasteur, Lyon, France) (n = 25), or the tetanus toxoid adsorbed vaccine (SIIL, Pune, India) (n = 25). Blood samples were taken for immunogenicity studies on the day of immunization and 4, 24, and 48 weeks later.
Immunologic assays.
Samples from the subjects who received tetanus toxoid vaccine were not analyzed in the additional four immunoassays.
Testing laboratories.
The immunogenicity of the vaccine was determined by the rSBA assay and multiplex bead assay to determine group A-specific IgG (Health Protection Agency, Manchester, United Kingdom), group A-specific IgG ELISA and opsonophagocytic antibody (OPA) assay (Centers for Disease Control and Prevention, Atlanta, GA), OPA (Norwegian Institute of Public Health [NIPH], Oslo, Norway), and SBA assay using human complement (hSBA) (Center for Biologics Evaluation and Research, Bethesda, MD).
SBA assays.
SBA assays were performed against the group A target strain, CDC F8238 (phenotype A:4,21:P1.20,9, L11), as previously described by Maslanka et al. (21). The complement source used in the rSBA was active serum from 3- to 4-week-old rabbits (Pel Freez Biologicals, Wisconsin). Pooled active sera from healthy adult blood donors were used as a complement source in the hSBA. Individual sera were identified that had no intrinsic bactericidal or bacteriostatic activity against F8238, had rSBA titers of ≤8, and had total immunoglobulin levels of <30 μg/ml against group A polysaccharide. Pooled complement was tested to ensure complement activity, lack of intrinsic killing of the target strain, and accurate hSBA titer determination of control sera prior to use in the study assays. For both rSBA and hSBA, titers were expressed as the reciprocal serum dilutions yielding ≥50% killing after 60 min for rSBA and 90 min for hSBA. The lower limit of detection for both rSBA and hSBA was a titer of 4. Threshold analyses were performed using the titer of ≥8 for both rSBA and hSBA. Titers of <4 were assigned a value of 2 for geometric mean titer (GMT) analysis. hSBA assay was performed only on samples collected prior to vaccination and 4 and 48 weeks following vaccination.
An rSBA titer of ≥8 was defined here as putatively protective (2). Due to the lower limit of detection of the hSBA assay being 4, an hSBA titer of ≥8, rather than ≥4 as determined by Goldschneider et al. (7), was defined here as putatively protective so as to not overestimate protection.
Group A-specific IgG.
Group A-specific IgG levels were determined using an ELISA as described by Carlone et al. (4), except that the reference serum CDC 1992 and monoclonal-PAN anti-human IgG Fc labeled with horseradish peroxidase (Hybridoma Reagent Laboratory, Baltimore, MD) were used. For the reference serum, we used CDC 1992 with the previously assigned group A-specific IgG concentration (10). The lower limit of quantitation for the ELISA was 0.4 μg/ml; concentrations below this were reported as 0.2 μg/ml.
Group A-specific IgG levels were also determined using the multiplex bead assay for quantitation of serum antibodies to Neisseria meningitidis groups A, C, Y, and W135 as previously described by Lal et al. (14). Briefly, meningococcal group A polysaccharide (National Institute of Biological Standards and Control, Potters Bar, Herts, United Kingdom) was attached to poly-l-lysine and then covalently linked to carboxylated microspheres by using a two-step carbodiimide reaction. Serum and microspheres were incubated for 20 min at room temperature on a plate shaker followed by washing and incubation with R-phycoerythrin-conjugated anti-human IgG. The plate was then read on a Bio-plex reader, and data were analyzed using Bio-plex Manager version 4.1.1. A standard curve of CDC 1992, using the previously assigned group A-specific IgG concentration (10), was generated by four-parameter logistic analysis and used to determine concentrations of unknown test sera in μg/ml. The lower limit of quantitation of the multiplex bead assay is 0.12 μg/ml; concentrations below this were reported as 0.06 μg/ml.
Opsonophagocytic assay (CDC).
The OPA assay at the CDC was performed as previously described by Martinez et al. (20). Briefly, serum dilutions were incubated with group A polysaccharide conjugated to 1-μm-diameter carboxylate-modified FluroSpheres (Molecular Probes, Eugene, OR), followed by the addition of baby rabbit complement and HL60 cells (CCL240; American Type Culture Collection, Manassas, VA). The HL60 cells had been chemically differentiated into monocytes by being cultured in medium supplemented with 1 mM n-butyric acid dissolved in Hanks buffered salt solution containing Ca2+ and Mg2+ (Invitrogen, Carlsbad, CA) and allowed to differentiate for 4 days. Samples were assayed with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). OPA data were analyzed with Statlia software (Brendan Scientific) using a four-parameter logistic curve fitting program. Following curve analysis, titers were reported as the reciprocal of the highest serum dilution yielding ≥50% of the maximum phagocytic uptake. Samples with a maximum phagocytic uptake of <20% were considered negative and were reported to have a titer of 4. Titers of <8 were assigned a value of 4 for GMT analysis.
Opsonophagocytic assay (NIPH).
Opsonophagocytic activity was measured as the respiratory burst by using human complement and human polymorphonuclear neutrophils (PMNs) and utilizing live meningococci, target strain F8238, as previously described (1). The samples were analyzed with a CyFlow ML (Partec GmbH, Munster, Germany) flow cytometer with gating on the PMN population, and the percentages of respiratory-burst-positive PMNs were recorded. The highest reciprocal serum dilution giving respiratory burst in ≥50% of the PMNs was recorded as the final titer. The lower limit of detection was 2; titers of <2 were assigned a value of 1 for GMT analysis.
Data analysis.
The rSBA assay, hSBA assay, the group A-specific IgG ELISA, the group A-specific IgG tetraplex assay, and the CDC and NIPH OPA assay results from each time point were log transformed, and geometric means were calculated with 95% confidence intervals (CIs).
In addition to calculation of GMTs, the proportion of subjects with a ≥4-fold rise in SBA titers was analyzed and SBA titer threshold analysis using various cutoffs was performed. The proportions of subjects with group A-specific IgG levels of ≥2 μg/ml (18) were calculated for both the IgG ELISA and the IgG tetraplex assay. The proportion of subjects with OPA titers of ≥8 at each time point and the proportion of subjects with a ≥4-fold rise in OPA titer were calculated. Fourfold rises were calculated from the lower limit of detection. Significant differences in proportions between vaccine groups were calculated using Fisher's exact test.
Differences in GMTs and geometric mean concentrations (GMCs) between vaccination groups were expressed as ratios with 95% CIs. GMTs and GMCs between time points were significantly different when the CIs did not include 1.0. The results of the statistical analysis of the rSBA assay and group A-specific IgG ELISA results were as described by Kshirsagar et al. (13). The relationship between the different immunologic assays was determined using the Pearson correlation coefficient by using available-case analysis where data with missing values were not included in the analyses. A Pearson correlation coefficient of >0.8 is regarded as a good linear relationship between two variables. A correlation between 0.45 and 0.79 has been described as moderate, and a correlation below 0.44 has been described as weak, throughout this paper.
RESULTS
SBA assay.
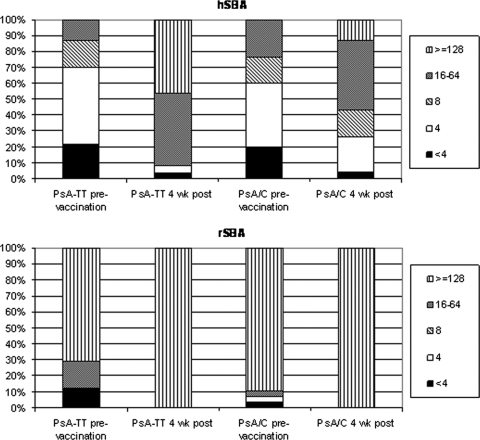
The rSBA and hSBA GMTs (with 95% CIs) are shown in Table 1, together with the proportions of subjects with a ≥4-fold rise in hSBA and rSBA titer from prevaccination to postvaccination. The proportions of subjects with SBA titers at various titer cutoffs are shown in Fig. 1.
TABLE 1.
SBA titers of PsA-TT and PsA/C vaccination groups determined by using rabbit and human complement
| Vaccine group | GMT (95% CI; n)
|
No. of subjects with≥4-fold increase in antibody titer prevaccination to postvaccination/total no. of subjects (%) at time postvaccination
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rSBA
|
hSBA
|
rSBA
|
hSBA
|
|||||||
| Prevaccination | 4 wks postvaccination | 48 wks postvaccination | Prevaccination | 4 wks postvaccination | 48 wks postvaccination | 4 wks | 48 wks | 4 wks | 48 wks | |
| PsA-TT | 228 (84-617; 24) | 8,192 (5,221-12,885; 24)a | 4,211 (3,517-5,078; 24)b | 5 (3-7; 23) | 74 (39-142; 24)a,b | 35 (18-67; 24)b | 20/24 (83) | 20/24 (83) | 20/23 (87)c | 16/23 (70) |
| PsA/C | 422 (191-929; 25) | 5,257 (2,798-9,878; 25)a | 2,226 (1,552-3,191; 25)b | 7 (4-10; 25) | 19 (10-37; 23)a,b | 14 (7-25; 25)b | 18/25 (72) | 17/25 (68) | 11/23 (48)c | 8/25 (32) |
Significant rise prevaccination to 4 weeks postvaccination (P < 0.01).
Significant difference between vaccine groups (P < 0.05).
Significant difference in proportions (P = 0.011).
FIG. 1.
Percentages of subjects before and 4 weeks following vaccination with either PsA-TT or PsA/C at SBA titer thresholds of <4, 4, 8, 16 to 64, and ≥128.
SBA titers for both assays were seen to significantly increase from prevaccination to 4 weeks postvaccination (P < 0.01). Four weeks postvaccination, there was a significant difference (P < 0.05) between the hSBA GMTs of the PsA-TT and PsA/C vaccine groups which was not shown with the rSBA. A significant difference (P < 0.05) between the rSBA GMTs of the two vaccine groups was observed at 48 weeks following vaccination. A difference between the hSBA GMTs of the two vaccine groups was also observed 48 weeks following vaccination (P < 0.05).
Prevaccination, ≥88% of subjects in both vaccination groups had rSBA titers of ≥8, which rose to 100% 4 weeks following vaccination and remained at this level at 24 and 48 weeks after vaccination (Fig. 1 [24- and 48-week data not shown]). Using a threshold titer of ≥128, a large proportion of subjects (>70%) were at or above this threshold prevaccination, a proportion which increased to all subjects at 4 weeks following vaccination (Fig. 1). The proportion of subjects with a ≥4-fold rise in rSBA titer from before vaccination to 4 weeks following vaccination was 83% in the PsA-TT vaccine group, compared to 72% in the PsA/C vaccine group (Table 1).
The proportion of subjects with hSBA titers of ≥8 prevaccination was ≥30%, which increased to 92% in those who received PsA-TT and 74% in the PsA/C group, 4 weeks postvaccination (Fig. 1). Examination of the hSBA data at different SBA thresholds demonstrates that a higher percentage of subjects in the PsA-TT group than in the PsA/C group achieved a higher SBA titer at 4 weeks following vaccination (Fig. 1).
A greater proportion of subjects in the PsA-TT group than in the PsA/C group demonstrated a ≥4-fold rise in hSBA titer, 87% versus 48%, respectively (P = 0.011) (Table 1).
Group A-specific IgG.
The ELISA and the multiplex bead assay group A-specific IgG GMCs are shown in Table 2, together with the proportion of subjects with group A-specific IgG concentrations of ≥2.0 μg/ml.
TABLE 2.
Group A-specific IgG concentrations of PsA-TT and PsA/C vaccination groups
| Vaccine group | MenA IgG GMC (μg/ml) (95% CI; n) by assay and time
|
No. of subjects with MenA IgG level of ≥2 μg/ml/total no. of subjects (%)
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ELISA
|
Multiplex bead assay
|
ELISA
|
Multiplex bead assay
|
|||||||||||||
| Prevaccination | Wk postvaccination
|
Prevaccination | Wk postvaccination
|
Prevaccination | Wk postvaccination
|
Prevaccination | Wk postvaccination
|
|||||||||
| 4 | 24 | 48 | 4 | 24 | 48 | 4 | 24 | 48 | 4 | 24 | 48 | |||||
| PsA-TT | 4 (3-7; 24) | 110 (68-174; 24)a | 72 (43-123; 24) | 52 (30-93; 24) | 1.8 (1-3; 24) | 76 (47-123; 24)a | 50 (28-89; 24) | 32 (18-56; 24) | 18/24 (75) | 24/24 (100) | 24/24 (100) | 24/24 (100) | 13/24 (54) | 24/24 (100) | 24/24 (100) | 24/24 (100) |
| PsA/C | 5 (3-8; 25) | 44 (26-71; 25)a,b | 39 (22-66; 25) | 34 (21-54; 25) | 1.3 (0.81-2.2; 24) | 19 (10-34; 25)a | 19 (10-35; 24) | 15 (9-28; 25) | 21/25 (84) | 25/25 (100) | 24/25 (96) | 25/25 (100) | 10/24 (42)c | 23/25 (92) | 22/24 (92) | 23/25 (92) |
Significant difference between prevaccination and 4 weeks postvaccination (P < 0.005).
Significant difference between vaccine groups (P < 0.005).
Significant difference in percentages at prevaccination between vaccine groups (P < 0.005).
The group A-specific IgG GMC determined by both assays significantly increased 4 weeks after vaccination (P < 0.005) in both vaccine groups, and the PsA-TT GMC was significantly higher than the PsA/C GMC (P < 0.005 for both assays).
As described by Kshirsagar et al. (13), before vaccination a high percentage of subjects had group A-specific IgG concentrations of ≥2.0 μg/ml (as determined by ELISA). Concentrations increased in all subjects 4 weeks following conjugate or polysaccharide vaccination, but the proportions with group A-specific IgG concentrations of ≥2.0 μg/ml did not significantly change over 1 year. The proportion of subjects before vaccination with group A-specific IgG levels of ≥2.0 μg/ml as determined by the multiplex bead assay was lower (significantly lower in the PsA/C group, P < 0.005) than that determined by ELISA (Table 2) but increased to 100% in the PsA-TT group and 92% of subjects in the PsA/C group 4 weeks after vaccination and remained the same at 24 and 48 weeks following vaccination.
OPA assay.
The GMTs for both OPA methods are shown in Table 3. For the OPA assay performed at the CDC, the GMTs of both vaccine groups increased following vaccination (P < 0.001 for both groups) but declined to prevaccination levels from 4 weeks to 24 weeks (P < 0.001 for both groups) with a further decline to below baseline levels at 48 weeks after vaccination. At 48 weeks following vaccination, the GMT of the PsA/C group was ∼6-fold lower than that determined prevaccination (P < 0.01). Interestingly, the control group prevaccination and week 48 GMTs also differed by ∼6-fold (data not shown). This was not observed in the PsA-TT vaccine group, where the prevaccination and week 48 GMTs differed by less than twofold. As well, the prevaccination GMT for the PsA-TT vaccine group and the week 48 GMT for the PsA/C group were not statistically different. For some reason, the prevaccination PsA/C GMT was higher than the PsA-TT GMT. The week 48 GMTs of the two vaccine groups were not significantly different.
TABLE 3.
Immune responses to PsA-TT and PsA/C by OPA assay
| Vaccine group | OPA GMT (95% CI; n) by assay and time
|
No. of subjects with≥4-fold increase in titer between prevaccination and postvaccination/total no. of subjects (%) at time postvaccination
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CDC OPA
|
NIPH OPA
|
CDC OPA
|
NIPH OPA
|
|||||||||||
| Prevaccination | Wk postvaccination
|
Prevaccination | Wk postvaccination
|
4 wks | 24 wks | 48 wks | 4 wks | 24 wks | 48 wks | |||||
| 4 | 24 | 48 | 4 | 24 | 48 | |||||||||
| PsA-TT | 443 (232-845; 24) | 2,656 (1,713-4,118; 24)a | 813 (571-1,156; 24)b | 271 (163-452; 24)c,d | 11 (6-20; 24) | 50 (31-78; 24)a,f | 11 (6-20; 24)b | 10 (6-18; 24)d,f | 15/24 (63) | 9/24 (38) | 6/24 (25) | 12/24 (50) | 5/24 (21) | 4/24 (17) |
| PsA/C | 820 (526-1,277; 25) | 2,165 (1,268-3,695; 25)a | 556 (350-883; 25)b | 147 (81-268; 25)c,d,e | 10 (6-15; 25) | 22 (13-37; 25)a,f | 6 (4-9; 24)b | 5 (3-8; 25)d,e,f | 13/25 (52) | 2/25 (8) | 1/25 (4) | 8/25 (32) | 2/24 (8) | 1/25 (4) |
Significant rise between prevaccination and 4 weeks postvaccination (P < 0.001).
Significant decline from 4 weeks postvaccination to 24 weeks postvaccination (P < 0.001).
Significant decline from 24 weeks postvaccination to 48 weeks postvaccination (P < 0.001).
Significant decline from 4 weeks postvaccination to 48 weeks postvaccination (P < 0.001).
Significant decline between prevaccination and 48 weeks postvaccination (P < 0.01).
Significant difference between the two vaccine groups (P = 0.02).
For the CDC OPA assay, similar proportions of subjects in the two vaccine groups demonstrated a fourfold rise from prevaccination to 4 weeks postvaccination (Table 3). A greater proportion of subjects in the PsA-TT group than in the PsA/C group maintained a fourfold rise at 24 and 48 weeks following vaccination. All subjects, in both vaccine groups, at all time points had OPA titers of ≥8.
The OPA GMT for the assay performed at NIPH increased 4 weeks following vaccination for both vaccine groups (P < 0.001), with a decline to prevaccination levels at 24 weeks (P < 0.001 for both groups), and remained at this level 48 weeks following vaccination. At 48 weeks following vaccination, the GMT of the PsA/C group was significantly lower than that determined prevaccination (P < 0.01). There was a significant difference between the NIPH OPA GMTs of the two vaccine groups 4 weeks after vaccination (P = 0.02). A higher proportion of subjects in the PsA-TT group than in the PsA/C group demonstrated a fourfold rise in OPA titer from prevaccination to 4 weeks postvaccination (Table 3). High proportions of subjects, 71% and 72%, had OPA titers of ≥8 prior to vaccination, which increased to 96% and 80% in the PsA-TT and PsA/C vaccine groups, respectively (data not shown).
Comparison and correlation of results between immunologic assays.
Correlations between immunoassays were calculated for each visit individually and all visits combined. The Pearson correlation coefficients per visit compared to those for all visits combined did not fluctuate greatly for the majority. Correlations between immunoassays were calculated for all visits combined, and the Pearson linear correlation coefficients between assays are given in Table 4.
TABLE 4.
Pearson correlation coefficients for the PsA-TT and PsA/C vaccine groups (all visits combined)
| Assay | Correlation coefficient (n) by assaya:
|
|||||
|---|---|---|---|---|---|---|
| ELISA | Multiplex | hSBA | OPA (CDC) | OPA (NIPH) | rSBA | |
| ELISA | 0.89 (96) | 0.77 (71) | 0.25 (96) | 0.10 (96) | 0.54 (96) | |
| Multiplex | 0.90 (98) | 0.73 (71) | 0.29 (96) | 0.19 (96) | 0.56 (96) | |
| hSBA | 0.45 (73) | 0.46 (72) | 0.23 (71) | 0.32 (71) | 0.50 (71) | |
| OPA (CDC) | 0.09 (100) | 0.08 (98) | 0.19 (73) | 0.36 (96) | 0.37 (96) | |
| OPA (NIPH) | −0.07 (100) | −0.05 (98) | 0.25 (73) | 0.21 (100) | 0.37 (96) | |
| rSBA | 0.55 (100) | 0.53 (98) | 0.34 (73) | 0.32 (100) | 0.11 (100) | |
Values for the PsA-TT group are indicated by boldface; those for the PsA/C group are in lightface.
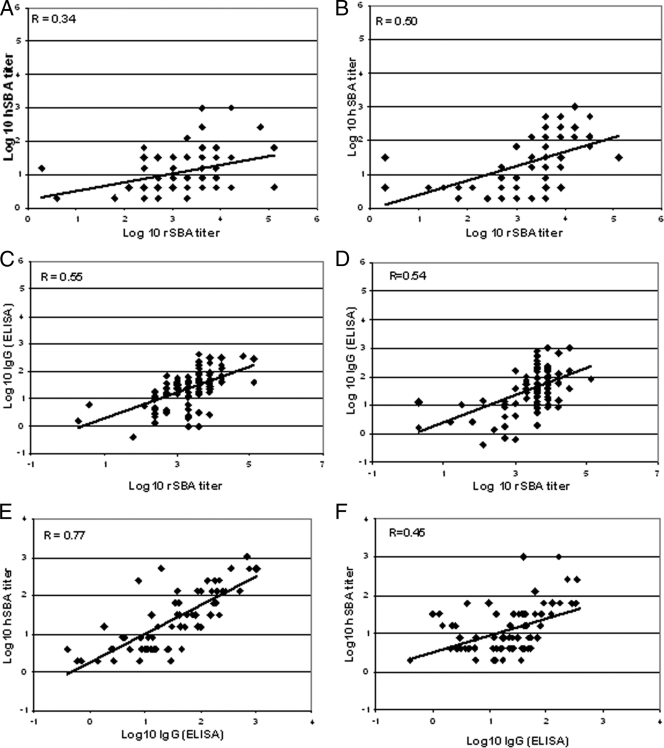
For all visits combined, only a moderate correlation between rSBA and hSBA titers was observed in the PsA-TT group (r = 0.50), and a weaker correlation was observed in the PsA/C group (r = 0.34). SBA titers generated using the two different complement sources differ in magnitude, as can be seen in Fig. 2. The proportion of subjects in the PsA-TT group with SBA titers of ≥8 at 4 weeks postvaccination determined in the hSBA or rSBA assay showed an association with 92% and 100% of subjects in the hSBA and rSBA assays, respectively. Similarly, in the PsA/C group, 74% and 100% of subjects exhibited titers of ≥8 in the hSBA and rSBA assays, respectively. The agreement between the two SBA assays regarding subjects who did and did not demonstrate a ≥4-fold rise in SBA titer prior to and 4 weeks following vaccination was evaluated. In the PsA-TT group, 70% of subjects demonstrated a ≥4-fold rise in titer in both SBA assays and 17% and 13% of subjects demonstrated only a ≥4-fold rise in titer in hSBA or rSBA assays, respectively. In the PsA/C group, 43% of subjects demonstrated a ≥4-fold rise in titer and 22% did not by both assays (overall agreement of 65%), and 4% and 30% of subjects demonstrated only a ≥4-fold rise SBA titer in the hSBA or rSBA assays, respectively.
FIG. 2.
Scatter plots showing correlation data for rSBA, hSBA, and IgG (ELISA) along with the Pearson correlation coefficient, r. (A, C, and E) PsA-TT; (B, D, and F) PsA/C; (A and B) rSBA versus hSBA; (C and D) rSBA versus IgG (ELISA); (E and F) IgG (ELISA) versus hSBA.
A good linear relationship was observed between the group A-specific IgG level measured by ELISA and that measured by the multiplex bead assay for both the PsA-TT and PsA/C vaccine groups (0.89 and 0.90, respectively). Although the results of the two assays were correlated, they were significantly different, with the IgG concentrations determined by the multiplex bead assay being consistently lower. The proportions of subjects with group A-specific IgG concentrations of ≥2 μg/ml determined by the two assays differed prevaccination (significantly lower in the PsA/C group [P < 0.005]) but were similar at all other time points (Table 2).
The results of the two OPA methods correlated weakly in both vaccine groups (r = 0.21 and r = 0.36 for PsA/C and PsA-TT groups, respectively, for all visits combined). Despite a weak correlation between the titers obtained by the two assays, an association was observed between the two assays specifically in the percentages of subjects who did and did not demonstrate a ≥4-fold rise in titers as determined by the two assays. In the PsA-TT vaccination group, 62.5% showed either a ≥4-fold rise in titer (37.5%) or a <4-fold rise (25%) by both assays while in the PsA/C group a total of 64% showed either a ≥4-fold rise in titer (24%) or a <4-fold rise (40%) by both assays.
The correlation observed between group A-specific IgG (measured by either method) and hSBA for the PsA-TT group was higher than that for the PsA/C group and higher than the correlation between the two SBA assays for both immunization groups (Table 4).
Moderate correlations between group A-specific IgG (measured by either method) and rSBA titers were observed for both vaccine groups (r = 0.53 to 0.56). Scatter plots of these four correlations are shown in Fig. 2. From the plots of hSBA versus IgG (measured by ELISA), a slight difference in the distribution of the hSBA data can be seen between the two groups, which probably accounts for the higher PsA-TT Pearson correlation coefficient.
Both OPA methods demonstrated only low correlations with group A-specific IgG concentrations measured by either ELISA or the multiplex bead assay for both vaccine groups (r < 0.29). Low correlations (for all visits combined) were also observed between the results obtained by either of the OPA methods and those determined by either SBA methodology (r = 0.11 to 0.37). Analysis of these data at each visit showed there to be variation in the correlation between CDC OPA and rSBA. Moderate correlations were observed between the CDC OPA and rSBA in both vaccine groups prior to vaccination (r = 0.48), and this correlation improved in the PsA-TT group 4 weeks postvaccination (r = 0.74), but this improvement was not seen in the PsA/C group. At 24 and 48 weeks postvaccination, the correlations between the CDC OPA and rSBA were similar in the two vaccination groups (r < 0.27).
Despite various correlation coefficients between the SBA and group A-specific IgG, similar proportions of subjects are regarded as protected (hSBA titer of ≥8, rSBA titer of ≥8, and MenA-specific IgG titer of ≥2 μg/ml) by the different assays. Four weeks postvaccination, for the PsA-TT group, 92% of subjects were regarded as protected by all four assays; the remaining two subjects had hSBA titers of <8. For the PsA/C group, all subjects were regarded as protected by rSBA and ELISA and 68% of subjects were regarded as protected by all four assays. For the PsA/C group, 92% of subjects were regarded as protected by rSBA, ELISA, and multiplex bead assay; of the remaining 8% (two subjects), one had no hSBA titer determined and one was regarded as protected by the other three assays.
DISCUSSION
This is the first study in which multiple immunoassays have been compared to evaluate the serum antibody responses induced by either a group A conjugate vaccine or a bivalent meningococcal A/C polysaccharide vaccine.
Two serologic assays, the SBA assay and the group-specific ELISA, have standardized protocols, and the results from multicenter collaborative studies have shown that different laboratories can obtain comparable results (3, 6, 21).
The SBA assay measures the ability of serum antibody to kill meningococci in the presence of complement. Many factors can influence the results of measurement of meningococcal bactericidal activity; these include the choice of the bacterial test strain, conditions for growth of the bacteria, and the source of the exogenous complement. Classic studies in the 1960s demonstrated a correlation between SBA activity and protection against meningococcal disease; however, these studies used normal human serum, which lacked intrinsic bactericidal activity, as the complement source (7, 8). Following international workshops on the development of standardized laboratory protocols, subsequent multicenter collaborative studies used baby rabbit complement (21) that is commercially available (Pel-Freez). Until now, no meningococcal serogroup A hSBA interlaboratory studies have been conducted. In this study, significantly different antibody titers were observed when SBA titers were generated using baby rabbit serum than when SBA titers were generated using human serum as the complement source, as has been noted in previous studies (21, 22). The modest correlation between hSBA and rSBA observed in this study agrees with the correlation noted by Norheim et al. (22). Interestingly, a significant difference between the two vaccine groups was found at 4 weeks postvaccination by the hSBA assay and by both SBA assays at 48 weeks. Despite the difference in the magnitude of the SBA titers obtained with human and rabbit complement, a high proportion of responders were observed in the PsA-TT group by either assay when these data were analyzed by titers of ≥8 and by a ≥4-fold titer rise. Goldschneider et al. (7) demonstrated an hSBA titer of ≥4 to correlate with protection from meningococcal disease, but due to the limit of detection of the hSBA assay used in this study being a titer of 4, a more conservative level of ≥8 was used to analyze the hSBA data in order not to overestimate protection. Despite standardization of MenA rSBA assays (21), serologic correlates of protection have not been established for MenA; however, by extrapolation from protective MenC rSBA titers (2, 3), SBA titers of ≥8 have been used to indicate protection. With the use of a threshold titer of ≥8 for both SBA assays, at 4 weeks postvaccination all subjects in both groups had rSBA titers of ≥8 while 92% and 74% of subjects in the PsA-TT and PsA/C vaccine groups, respectively, had hSBA titers of ≥8. With the use of a higher discriminatory titer of ≥128 for rSBA, at 4 weeks postvaccination all subjects in both groups had rSBA titers above this level. The proportion of subjects demonstrating a ≥4-fold rise in rSBA titers at 4 weeks postvaccination was slightly higher in the PsA-TT vaccine group than in the PsA/C vaccine group (83% versus 72%, respectively), as were these proportions for hSBA titers, which were 87% and 48%, respectively. Among subjects demonstrating a ≥4-fold rise in titer from baseline to 4 weeks postvaccination, there was moderate agreement between rSBA and hSBA (>65%).
Antibody-dependent complement-mediated bactericidal killing is considered a surrogate of protection in meningococcal disease. However, the only correlate of protection for group A is that which was derived from the Finnish efficacy trials of a meningococcal group A polysaccharide vaccine (16, 18). For this correlation, a group A-specific Ig concentration of 2 μg/ml, as determined by radioimmunoassay, was derived from the mean level in unimmunized adults (18) who were assumed to be protected, and this level corresponded to that achieved by the majority of individuals in the age groups in which the vaccine was shown to be effective (23). In our study, group A-specific IgG was measured by two methods, the standardized ELISA (4) and a multiplex bead assay (14). A good correlation was observed between the group A-specific IgG concentrations obtained by these two methods, as was previously reported by Lal et al. (14), although the IgG results from the multiplex bead assay were consistently lower. This is probably due to differences in reaction kinetics, with the ELISA detecting both high- and low-avidity antibodies and the multiplex bead assay detecting high-avidity antibody and hence overall a lower MenA-specific IgG concentration.
A moderate correlation between rSBA and group A-specific IgG was observed in this study, which agrees with correlations previously described (17). Results of an earlier study that showed improved correlation when a modified ELISA that selected for high-avidity antibodies was used (12) suggest that this moderate correlation between rSBA and group A-specific IgG is due to the extent to which antibody binding assays measure both functional and nonfunctional IgG, with the latter usually being of low avidity. Bactericidal activity is determined not only by the quantity but also by the quality of antibody, e.g., the isotype, subclass, and avidity. Despite various correlation coefficients between the SBA and group A-specific IgG, similar proportions of subjects are regarded as protected (hSBA titer of ≥8, rSBA titer of ≥8, and MenA-specific IgG titer of ≥2 μg/ml) by the different assays. Four weeks postvaccination, there was an agreement between 92% of subjects being regarded as protected by the four different assays for the PsA-TT group whereas there was only an agreement between the four assays for 68% of subjects in the PsA/C group. Norheim et al. (22) observed a correlation between hSBA titers and group A-specific IgG concentrations in Ethiopian case sera similar to that which was observed in this study for the PsA/C group. Interestingly, the Pearson correlation coefficient between the hSBA and group A-specific IgG (either method) was higher for the PsA-TT group than for the PsA/C group, indicating the higher quality of antibody induced by the PsA-TT vaccine.
Demonstration of killing of meningococci by opsonophagocytosis (24, 25), coupled with the establishment of the OPA as the surrogate of protection for Streptococcus pneumoniae (19), has led to the development of an OPA against meningococci (1, 20). Immunization with meningococcal polysaccharide vaccines elicits complement-dependent serum bactericidal and opsonizing antibodies, with both mechanisms operating simultaneously to clear meningococci (7, 8, 9, 26). In this study there was little correlation between the two OPA assays, possibly due to methodologic differences. The CDC OPA assay measures internalization and uses antigen-coated particles and rabbit complement while the NIPH OPA assay measures respiratory burst and employs live meningococci and human complement. In addition, different effector cells are used in these OPA assays. Poor correlations were observed between either the CDC or the NIPH OPA assay and all other assays, although the reasons for this discrepancy are unclear. This is in contrast to measurement of levels of OPA against group B meningococci, which usually demonstrate a good correlation with SBA and IgG levels (1, 5). Also, OPA levels were found to rapidly decline at 1 year following vaccination, which was not the case with the rSBA. The decline at week 48 to levels below prevaccination levels in the CDC OPA assay is puzzling, as this was not observed for any other assays and the week 24 and 48 samples were tested at the same time, so that it appears that the unexpected inexplicability of the result is not likely to be due to the testing method. These data call into question the relevance of the OPA assays as they currently exist in measuring the immune response to meningococci.
In summary, the best linear relationship was observed between group A-specific IgG levels as determined by ELISA and by the multiplex bead assay. Only a moderate correlation was observed between the hSBA and rSBA; however, the assays were similar regarding the proportions of subjects that demonstrated a fourfold rise in titer from baseline to 4 weeks postvaccination. Only a weak correlation was found between the two OPAs examined. Both OPAs were shown to correlate weakly with each other and showed little or no correlation with SBA or IgG concentration. Other than the two antibody binding assays (ELISA and multiplex bead assay), none of the serogroup A immunoassays showed sufficiently high correlation to consider one assay able to predict or represent another assay. Without an accompanying study of vaccine efficacy, the question of how each assay relates to protection cannot be definitively determined. However, this study may be useful in examining postimmunization efficacy data in relation to serogroup A immunoassays. We recommend that for future group A vaccine trials, a rational approach is to determine functional bactericidal activity as a primary endpoint, while IgG can provide additional supporting evidence of immunogenicity. This study suggests that when rSBA is used as a primary serologic assay for all sera, hSBA performed on a subset provides additional supportive data and additional information may be provided by measuring IgG-specific antibody. World Health Organization requirements have been established for meningococcal polysaccharide vaccines using rabbit complement, but currently no criteria have been set for data generated using human complement (27). The difference in titers obtained when rabbit complement is used and when human complement is used in the SBA assay may be investigated further by the addition of human factor H to rabbit complement.
Acknowledgments
We are thankful to the study participants and the study principal investigators (Nilima Kshirsagar, Naidu Mur, and Urmila Thatte) and their teams involved at the three study centers in India (Seth G. S. Medical College and KEM Hospital Parel, Mumbai; The Nizam's Institute of Medical Sciences, Punjagutta, Hyderabad; and Topiwala National Medical College and BYl, Mumbai).
M. P. Preziosi is a staff member of the World Health Organization. M. P. Preziosi alone is responsible for the views expressed in this publication, and they do not necessarily represent the decisions, policy, or views of the World Health Organization.
The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any agency determination or policy.
Footnotes
Published ahead of print on 27 May 2009.
REFERENCES
- 1.Aase, A., L. M. Næss, R. H. Sandin, T. K. Herstad, F. Oftung, J. Holst, I. L. Haugen, E. A. Høiby, and T. E. Michaelsen. 2003. Comparison of functional immune responses in humans after intranasal and intramuscular immunisations with outer membrane vesicle vaccines against group B meningococcal disease. Vaccine 212042-2051. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, N., R. Borrow, and E. Miller. 2003. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin. Diagn. Lab. Immunol. 10780-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrow, R., N. Andrews, D. Goldblatt, and E. Miller. 2001. Serological basis for use of meningococcal group C conjugate vaccines in the United Kingdom: a reevaluation of correlates of protection. Infect. Immun. 691568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlone, G. M., C. E. Frasch, G. R. Siber, S. Quataert, L. L. Gheesling, S. H. Turner, B. D. Plikaytis, L. O. Helsel, W. E. DeWitt, W. F. Bibb, B. Swaminathan, G. Arakere, C. Thompson, D. Phipps, D. Madore, and C. Broome. 1992. Multicenter comparison of levels of antibody to the Neisseria meningitidis group A capsular polysaccharide measured by using an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 30154-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Findlow, J., S. Taylor, A. Aase, R. Horton, R. Heyderman, J. Southern, N. Andrews, R. Barchha, E. Harrison, A. Lowe, E. Boxer, C. Heaton, P. Balmer, E. Kaczmarski, P. Oster, A. Gorringe, R. Borrow, and E. Miller. 2006. Comparison and correlation of Neisseria meningitidis group B immunologic assay results and human antibody responses following three doses of the Norwegian meningococcal outer membrane vesicle vaccine MenBvac. Infect. Immun. 744557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gheesling, L. L., G. M. Carlone, L. B. Pais, P. F. Holder, S. E. Maslanka, B. D. Plikaytis, M. Achtman, P. Densen, C. E. Frasch, H. Käyhty, J. Mays, L. Nencioni, C. Peeters, D. Phipps, J. Poolman, E. Rosenqvist, G. Siber, B. Thiesen, J. Tai, C. Thompson, P. Vella, and J. Wenger. 1994. Multicenter comparison of Neisseria meningitidis group C anti-capsular polysaccharide antibody levels measured by a standardized enzyme-linked immunosorbent assay. J. Clin. Microbiol. 321475-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 1291307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 1291327-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotschlich, E., I. Goldschneider, and M. S. Artenstein. 1969. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J. Exp. Med. 129267-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holder, P. K., S. E. Maslanka, L. B. Pais, J. Dykes, B. D. Plikaytis, and G. M. Carlone. 1995. Assignment of Neisseria meningitidis group A and C class-specific anticapsular antibody concentrations to the new standard reference serum CDC1992. Clin. Diagn. Lab. Immunol. 2132-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jodar, L., F. M. LaForce, C. Ceccarini, T. Aguado, and D. M. Granoff. 2003. Meningococcal conjugate vaccine for Africa: a model for development of new vaccines for the poorest countries. Lancet 3611902-1904. [DOI] [PubMed] [Google Scholar]
- 12.Joseph, H., R. Ryall, M. Bybel, T. Papa, J. MacLennan, J. Buttery, and R. Borrow. 2003. Immunogenicity and immunological priming of the group A portion of a bivalent meningococcal A/C conjugate vaccine in 2-year-old children. J. Infect. Dis. 1871142-1146. [DOI] [PubMed] [Google Scholar]
- 13.Kshirsagar, N., N. Mur, U. Thatte, N. Gogtay, S. Viviani, M. P. Preziosi, C. Elie, H. Findlow, G. Carlone, R. Borrow, V. Parulekar, B. Plikaytis, P. Kulkarni, N. Imbault, and F. M. LaForce. 2007. Safety, immunogenicity, and antibody persistence of a new meningococcal group A conjugate vaccine in healthy Indian adults. Vaccine 25SA101-A107. [DOI] [PubMed] [Google Scholar]
- 14.Lal, G., P. Balmer, H. Joseph, M. Dawson, and R. Borrow. 2004. Development and evaluation of a tetraplex flow cytometric assay for quantitation of serum antibodies to Neisseria meningitidis groups A, C, Y, and W135. Clin. Diagn. Lab. Immunol. 11272-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, C. H., W. C. Kuo, S. Beri, S. Kapre, J. S. Joshi, N. Bouveret, F. M. LaForce, and C. E. Frasch. 2009. Preparation and characterisation of an immunogenic meningococcal group A conjugate vaccine for use in Africa. Vaccine 27726-732. [DOI] [PubMed] [Google Scholar]
- 16.Lepow, M. L., I. Goldschneider, R. Gold, M. Randolph, and E. C. Gotschlich. 1977. Persistence of antibody following immunization of children with groups A and C meningococcal polysaccharide vaccines. Pediatrics 60673-680. [PubMed] [Google Scholar]
- 17.Lieberman, J. M., S. S. Chiu, V. K. Wong, S. Partidge, S. J. Chang, C. Y. Chiu, L. L. Gheesling, G. M. Carlone, and J. I. Ward. 1996. Safety and immunogenicity of a groups A/C Neisseria meningitidis oligosaccharide-protein conjugate vaccine in young children. A randomized controlled trial. JAMA 2751499-1503. [PubMed] [Google Scholar]
- 18.Makela, H., H. Peltola, H. Kayhty, H. Jousimies, O. Pettay, E. Ruoslahti, A. Sivonen, and O. V. Renkonen. 1977. Polysaccharide vaccines of group A Neisseria meningitidis and Haemophilus influenzae type b: a field trial in Finland. J. Infect. Dis. 136S43-S50. [DOI] [PubMed] [Google Scholar]
- 19.Martinez, J., S. Romero-Steiner, T. Pilishvili, S. Barnard, J. Schinsky, D. Goldblatt, and G. M. Carlone. 1999. A flow cytometric opsonophagocytic assay for measurement of functional antibodies elicited after vaccination with the 23-valent pneumococcal polysaccharide vaccine. Clin. Diagn. Lab. Immunol. 6581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez, J., T. Pilishvili, S. Barnard, J. Caba, W. Spear, S. Romero-Steiner, and G. M. Carlone. 2002. Opsonophagocytosis of fluorescent polystyrene beads coupled to Neisseria meningitidis group A, C, Y, or W135 polysaccharide correlates with serum bactericidal activity. Clin. Diagn. Lab. Immunol. 9485-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maslanka, S. E., L. Gheesling, D. Libutti, K. Donaldson, H. Harakeh, J. Dykes, F. Arhin, S. Devi, C. E. Frasch, J. Huang, P. Kriz-Kuzemenska, R. Lemmon, M. Lorange, C. Peeters, S. Quataert, J. Tai, G. Carlone, and the Multilaboratory Study Group. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis group A and C serum bactericidal assays. Clin. Diagn. Lab. Immunol. 4156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norheim, G., A. Aseffa, M. A. Yassin, G. Mengistu, A. Kassu, D. Fikremariam, W. Tamire, Y. Merid, E. A. Hoiby, D. A. Caugant, E. Fritzsonn, T. Tangen, T. Alebel, D. Berhanu, M. Harboe, and E. Rosenqvist. 2007. Serum antibody responses in Ethiopian meningitis patients infected with Neisseria meningitidis serogroup A sequence type 7. Clin. Diagn. Lab. Immunol. 14451-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peltola, H., H. Makela, H. Kayhty, H. Jousimies, E. Herva, K. Hallstrom, A. Sivonen, O. V. Renkonen, O. Pettay, V. Karanko, P. Ahvonen, and S. Sarna. 1977. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N. Engl. J. Med. 297686-691. [DOI] [PubMed] [Google Scholar]
- 24.Roberts, R. 1970. Relationship between group A and group C meningococcal polysaccharides and serum opsonins in man. J. Exp. Med. 131499-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlesinger, M., R. Greenberg, J. Levy, H. Kayhty, and R. Levy. 1994. Killing of meningococci by neutrophils: effect of vaccination on patients with complement deficiency. J. Infect. Dis. 170449-453. [DOI] [PubMed] [Google Scholar]
- 26.Smith, L. F., and G. H. Lowell. 1980. Antibody-dependent cell-mediated antibacterial activity of human mononuclear cells. II. Immune specificity of antimeningococcal activity. J. Infect. Dis. 141748-751. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. 1976. Requirements for meningococcal polysaccharide vaccine. WHO Tech. Rep. Ser. 58850-75. [Google Scholar]