Abstract
Halogenases have been shown to play a significant role in biosynthesis and introducing the bioactivity of many halogenated secondary metabolites. In this study, 54 reduced flavin adenine dinucleotide (FADH2)-dependent halogenase gene-positive strains were identified after the PCR screening of a large collection of 228 reference strains encompassing all major families and genera of filamentous actinomycetes. The wide distribution of this gene was observed to extend to some rare lineages with higher occurrences and large sequence diversity. Subsequent phylogenetic analyses revealed that strains containing highly homologous halogenases tended to produce halometabolites with similar structures, and halogenase genes are likely to propagate by horizontal gene transfer as well as vertical inheritance within actinomycetes. Higher percentages of halogenase gene-positive strains than those of halogenase gene-negative ones contained polyketide synthase genes and/or nonribosomal peptide synthetase genes or displayed antimicrobial activities in the tests applied, indicating their genetic and physiological potentials for producing secondary metabolites. The robustness of this halogenase gene screening strategy for the discovery of particular biosynthetic gene clusters in rare actinomycetes besides streptomycetes was further supported by genome-walking analysis. The described distribution and phylogenetic implications of the FADH2-dependent halogenase gene present a guide for strain selection in the search for novel organohalogen compounds from actinomycetes.
It is well known that actinomycetes, notably filamentous actinomycetes, have a remarkable capacity to produce bioactive molecules for drug development (4, 6). However, novel technologies are demanded for the discovery of new bioactive secondary metabolites from these microbes to meet the urgent medical need for drug candidates (5, 9, 31).
Genome mining recently has been used to search for new drug leads (7, 20, 42, 51). Based on the hypothesis that secondary metabolites with similar structures are biosynthesized by gene clusters that harbor certain homologous genes, such homologous genes could serve as suitable markers for distinct natural-product gene clusters (26, 51). A wide range of structurally diverse bioactive compounds are synthesized by polyketide synthase (PKS) and nonribosomal peptide synthetase (NRPS) systems in actinomycetes, therefore much attention has been given to revealing a previously unrecognized biosynthetic potential of actinomycetes through the genome mining of these genes (2, 3, 22). However, the broad distribution of PKS and NRPS genes and their high numbers even in a single actinomycete complicate their use (2, 3). To rationally exploit the genetic potential of actinomycetes, more and more special genes, such as tailoring enzyme genes, are being utilized for this sequence-guided genetic screening strategy (20, 38).
Tailoring enzymes, which are responsible for the introduction and generation of diversity and bioactivity in several structural classes during or after NRPS, PKS, or NRPS/PKS assembly lines, usually include acyltransferases, aminotransferases, cyclases, glycosyltransferases, halogenases, ketoreductases, methyltransferases, and oxygenases (36, 45). Halogenation, an important feature for the bioactivity of a large number of distinct natural products (16, 18, 30), frequently is introduced by one type of halogenase, called reduced flavin adenine dinucleotide (FADH2)-dependent (or flavin-dependent) halogenase (10, 12, 35). More than 4,000 halometabolites have been discovered (15), including commercially important antibiotics such as chloramphenicol, vancomycin, and teicoplanin (43).
Previous investigations of FADH2-dependent halogenase genes were focused largely on related gene clusters in the genera Amycolatopsis (33, 44, 53) and Streptomyces (8, 10, 21, 27, 32, 34, 47-49) and also on those in the genera Actinoplanes (25), Actinosynnema (50), Micromonospora (1), and Nonomuraea (39); however, none of these studies has led to the rest of the major families and genera of actinomycetes. In addition, there is evidence that FADH2-dependent halogenase genes of streptomycetes usually exist in halometabolite biosynthetic gene clusters (20), but we lack knowledge of such genes and clusters in other actinomycetes.
In the present study, we show that the distribution of the FADH2-dependent halogenase gene in filamentous actinomycetes does indeed correlate with the potential for halometabolite production based on other genetic or physiological factors. We also showed that genome walking near the halogenase gene locus could be employed to identify closely linked gene clusters that likely encode pathways for organohalogen compound production in actinomycetes other than streptomycetes.
MATERIALS AND METHODS
Strains and culture conditions.
A total of 228 reference strains proportionally encompassing 40 genera and 10 families in 7 suborders of Actinomycetales were chosen randomly (see Tables S1 and S2 in the supplemental material). The majority were type strains. Most strains were obtained from the China General Microbiological Culture Collection Center (CGMCC), and others were from the Agricultural Research Service Culture Collection (NRRL), the German Collection of Microorganisms and Cell Cultures (DSMZ), the Japan Collection of Microorganisms (JCM), and the NITE-Biological Resource Center (NBRC). Most strains were grown on GYM medium (DSMZ medium 65) and the rest, which could not readily grow on GYM medium, were cultivated on appropriate media, such as oatmeal agar (DSMZ medium 609), yeast-starch agar (DSMZ medium 1027), and Bennett's agar (DSMZ medium 548). The agars and corresponding liquid media were used to cultivate strains for antimicrobial assay and DNA extraction, respectively.
DNA extraction and PCR-based screening for putative halogenase genes.
Genomic DNAs were prepared as described by Hopwood et al. (19). The PCR amplification of FADH2-dependent halogenase genes was performed using the degenerate primer pair Halo-B4-FW/Halo-B7-RV and the procedure of Hornung et al. (20). Amplification products were examined by 1.0% agarose gel electrophoresis and stained with ethidium bromide. Positive fragments with the expected size of approximately 550 bp were purified and cloned into the pMD-18T vector (TakaRa). Two positive clones of each fragment were sequenced directly by using an Applied Biosystems DNA sequencer (model 3730XL). An additional two positive clones were sequenced if the initial two gave different sequences. The sequencing results were used to search the GenBank database with the BLASTN and BLASTP (NCBI) programs to determine the putative functions of the genes. Sequences that did not show identity to halogenase genes in the database were defined as false positive.
Phylogenetic analysis.
Halogenase amino acid sequences of the positive strains and related known halometabolite-producing actinomycetes were aligned, using the ClustalW algorithm in MEGA 4.0 (41), with closely related flavin-dependent monooxygenase sequences (11, 54) retrieved by BLASTP searches from public databases and trimmed manually at the same position before being used for further analysis. The resulting alignment containing 197 amino acid residues (with gaps) was used to infer a neighbor-joining tree (37) and bootstrap values. The Poisson correction model (29) was chosen as a substitution model for the tree construction with pairwise deletion.
A 16S rRNA gene phylogenetic tree based on 1,390 nucleotides (with gaps) also was constructed using the same software described above. Pairwise distances between the nucleotide sequences were calculated using the K2P model (23).
PCR detection of PKS I, PKS II, and NRPS genes.
To further assess the genetic potential of the halogenase gene-positive strains for producing halometabolites, the specific primer pairs K1F/M6R, KSAF/KSAR, and A3F/A7R were used to detect the existence of PKS I, PKS II, and NRPS genes, respectively (2, 3, 28). The 50-μl PCR mixture contained 5 μl 10× PCR buffer [100 mM KCl, 80 mM (NH4)2SO4, 100 mM Tris-HCl (pH 9.0), 0.5% NP-40], 6 μl Mg2+ (25 mM), 1 μl each primer (20 μM), 2 μl deoxynucleoside triphosphates (10 mM), 1 μl Taq DNA polymerase (5 U/μl; Bio-Med), 2 μl genomic DNA, and 5 μl (for PKS I and PKS II) or 2.5 μl (for NRPS) dimethylsulfoxide (DMSO). The PCR was performed in an MJ Research cycler PTC-200 under the following cycling conditions: (i) 5 min at 95°C; (ii) 30 cycles of 1 min at 95°C, 1.5 min at 56°C, and 2 min at 72°C for K1F/M6R, or 30 cycles of 45 s at 95°C, 45 s at 63°C and 1 min at 72°C for KSAF/KSAR, or 30 cycles of 45 s at 95°C, 1 min at 57°C, and 1 min at 72°C for A3F/A7R; and (iii) 10 min at 72°C.
All of the amplification products were examined by 1.0% agarose gel electrophoresis, and bands of 1,200 to 1,400 bp, 600 to 800 bp, and 700 to 800 bp were classified as products of PKS I, PKS II, and NRPS genes, respectively.
Antimicrobial assays.
Antimicrobial activities of the actinomycetes were tested against those of Escherichia coli DSM 1103, Staphylococcus aureus subsp. aureus DSM 1104, Bacillus subtilis subsp. spizizenii DSM 347, Candida albicans CGMCC 2.538, Fusarium oxysporum CGMCC 3.2830, and Trichoderma viride CGMCC 3.2196 using the agar diffusion assay. Agar plugs of the reference strains cultivated on appropriate media (see Tables S1 and S2 in the supplemental material) were cut from agar plates that were incubated at 28°C for 14 to 21 days and were transferred to test plates containing individual indicator strains, followed by incubation for 12 to 48 h.
Genome walking.
The genetic information of halogenase gene-positive strains was extended by genome walking using the self-formed adaptor PCR method described by Wang et al. (46). Five rare actinomycetes were randomly selected for this purpose, namely, Actinocorallia aurantiaca DSM 43924T, Amycolatopsis orientalis IFO 12806T, Catenulispora yoronensis NBRC 103397T, Catenuloplanes nepalensis JCM 9536T, and Planobispora longispora CGMCC 4.1206T (JCM 3092T). The resulting PCR products were cloned into the pMD-18T vector (TakaRa) and then sequenced. Putative genes encoded by the flanking sequences of the corresponding halogenase genes were identified by searching the sequences against the GenBank database with the BLASTN and BLASTP programs.
Nucleotide sequence accession numbers.
The gene sequences of 16S rRNA and putative halogenases obtained in this study were deposited in the GenBank database under the accession numbers listed in Fig. 1 and 2 (also see Table S1 in the supplemental material). Also, the genomic sequence fragments (1.2 to 3.9 kb) obtained for the five positive strains by genome walking were deposited in the GenBank database under accession numbers FJ532346 to FJ532350.
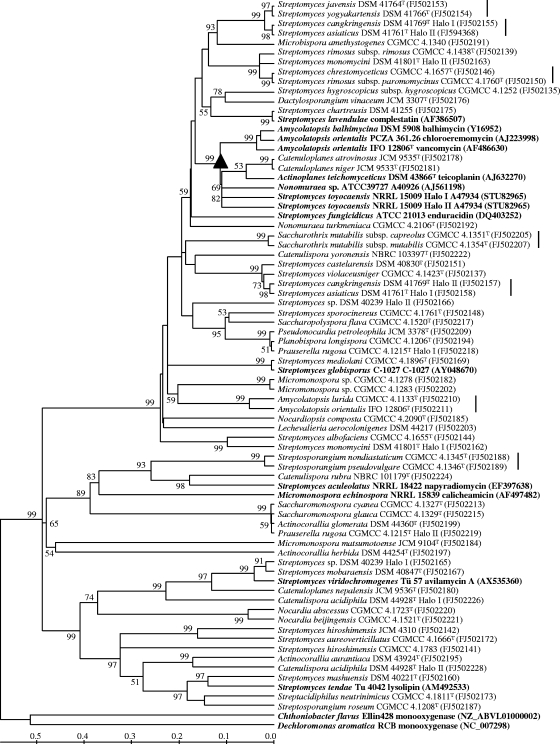
FIG. 1.
Neighbor-joining tree based on halogenase amino acid sequences of 54 reference strains and known halometabolite-producing strains (in boldface). Two closely related flavin-dependent monooxygenase sequences are used as outgroups (in boldface). GenBank accession numbers are given in parentheses. The clade of vancomycin group antibiotics is marked with a triangle. Strains containing multiple halogenase genes are differentiated by the terms Halo I and Halo II. Taxonomically closely related strains sharing highly homologous halogenases are marked with vertical lines to the right of the strain names. Percentage bootstrap values based on 1,000 resampled datasets are shown at the nodes; only values above 50% are given. The scale bar indicates the amino acid substitution rate.
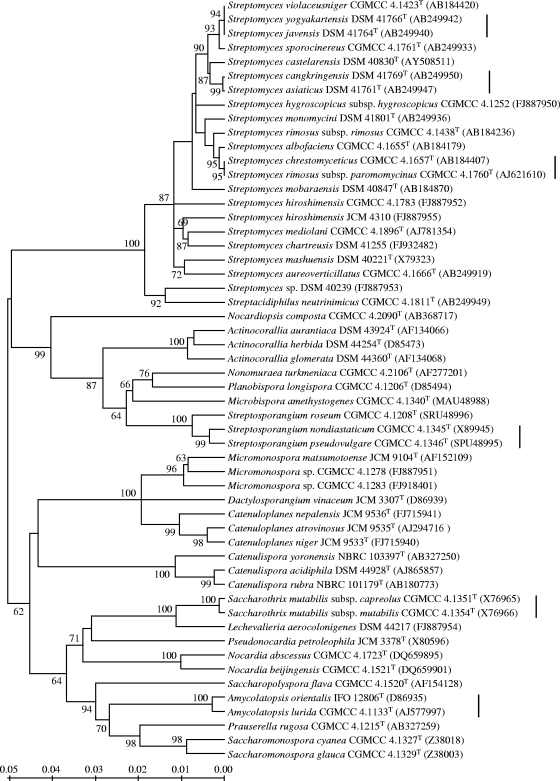
FIG. 2.
Neighbor-joining tree based on 16S rRNA gene sequences of the halogenase gene-positive reference strains, showing their taxonomic relationships. GenBank accession numbers are given in parentheses. Taxonomically closely related strains sharing highly homologous halogenases are marked with vertical lines to the right of the strain names. Percentage bootstrap values based on 1,000 resampled datasets are shown at the nodes; only values above 50% are given. The scale bar indicates the nucleotide substitution rate.
RESULTS
Detection and distribution of putative halogenase genes.
Fifty-six PCR fragments were obtained from 228 actinomycete reference strains using the FADH2-dependent halogenase gene-specific primer set Halo-B4-FW/Halo-B7-RV. Fifty-four (23.7%) halogenase gene-positive strains belonging to 20 genera and 9 families in 6 suborders of Actinomycetales were identified after BLAST analyses of the sequences of PCR products (see Table S1 in the supplemental material), among which at least 6 were found to possess multiple halogenase loci. Two (3.6%) PCR products were revealed to be false positive, with sequence identities to other genes.
Comparatively high occurrences of FADH2-dependent halogenase genes were observed in the families Catenulisporaceae (75.0%), Streptosporangiaceae (46.2%), and Thermomonosporaceae (30.0%) (Table 1). Moreover, extremely high occurrences of the halogenase genes were obtained in the genera Actinocorallia, Catenulispora, and Streptosporangium of these families, where all three Actinocorallia strains and three out of four strains of Catenulispora and Streptosporangium each in this test were positive. The halogenase genes also were frequently identified in the families Actinosynnemataceae (30.0%), Nocardiopsaceae (25.0%), and Pseudonocardiaceae (26.9%), with high incidences in the genera Amycolatopsis, Lechevalieria, Saccharomonospora, and Saccharothrix but less frequency among strains of the genus Pseudonocardia (Table 1). Amycolatopsis orientalis IFO12806T, the producer of vancomycin, was detected to have another copy of the halogenase gene that shared only 61% amino acid sequence identity with that previously reported (GenBank accession number AF486630).
TABLE 1.
Number of strains detected and positive strains in each family and genus
| Family and genus | No. of strains detected | No. of positive strains |
|---|---|---|
| Actinosynnemataceae | 10 | 3 |
| Actinosynnema | 1 | 0 |
| Lechevalieria | 2 | 1 |
| Lentzea | 2 | 0 |
| Saccharothrix | 5 | 2 |
| Catenulisporaceae | 4 | 3 |
| Catenulispora | 4 | 3 |
| Glycomycetaceae | 2 | 0 |
| Glycomyces | 2 | 0 |
| Micromonosporaceae | 48 | 7 |
| Actinocatenispora | 1 | 0 |
| Actinoplanes | 9 | 0 |
| Asanoa | 3 | 0 |
| Catellatospora | 5 | 0 |
| Catelliglobosispora | 1 | 0 |
| Catenuloplanes | 7 | 3 |
| Couchioplanes | 2 | 0 |
| Dactylosporangium | 5 | 1 |
| Longispora | 1 | 0 |
| Micromonospora | 9 | 3 |
| Pilimelia | 1 | 0 |
| Spirilliplanes | 1 | 0 |
| Verrucosispora | 1 | 0 |
| Virgisporangium | 2 | 0 |
| Nocardiaceae | 11 | 2 |
| Nocardia | 11 | 2 |
| Nocardiopsaceae | 4 | 1 |
| Nocardiopsis | 4 | 1 |
| Pseudonocardiaceae | 26 | 7 |
| Actinoalloteichus | 2 | 0 |
| Allokutzneria | 1 | 0 |
| Amycolatopsis | 6 | 2 |
| Kutzneria | 1 | 0 |
| Prauserella | 1 | 1 |
| Pseudonocardia | 8 | 1 |
| Saccharomonospora | 3 | 2 |
| Saccharopolyspora | 4 | 1 |
| Streptomycetaceae | 100 | 22 |
| Kitasatospora | 2 | 0 |
| Streptacidiphilus | 3 | 1 |
| Streptomyces | 95 | 21 |
| Streptosporangiaceae | 13 | 6 |
| Microbispora | 3 | 1 |
| Nonomuraea | 4 | 1 |
| Planobispora | 1 | 1 |
| Planomonospora | 1 | 0 |
| Streptosporangium | 4 | 3 |
| Thermomonosporaceae | 10 | 3 |
| Actinocorallia | 3 | 3 |
| Actinomadura | 7 | 0 |
Relatively lower occurrences of FADH2-dependent halogenase genes were observed in the families Streptomycetaceae (22.0%), Micromonosporaceae (14.6%), and Nocardiaceae (18.2%) (Table 1). Twenty-one out of 95 (22.1%) strains of Streptomyces displayed the genetic potential to produce FADH2-dependent halogenases. In the family Micromonosporaceae (excluding the genus Salinispora), the halogenase genes were identified only in the genera Catenuloplanes, Dactylosporangium, and Micromonospora.
Phylogenetic analyses.
The 60 putative FADH2-dependent halogenases of the reference strains shared amino acid sequence identities ranging from 44 to 100% with halogenases in the public databases (see Table S1 in the supplemental material), and 34 of them showed less than 70% identity with their counterparts. Phylogenetic analysis based on amino acid sequences showed that the putative halogenases were distinct from homologous flavin-dependent monooxygenases while also presenting great diversity, especially in Streptomyces and Actinocorallia, with identities ranged from 38.1% (corresponding to 122 amino acid differences out of 197 residues) to 99.5% (1 amino acid difference out of 197) (Fig. 1). Strains of different families and genera are mixed with each other in the halogenase phylogeny, with Streptomyces strains interspersed among the whole tree and some taxonomically distant strains located closely together (Fig. 1). Large sequence differences (65 to 113 amino acid differences out of 197) also were found between copies of halogenase genes within the same strains. The 16S rRNA gene phylogeny of the 54 halogenase gene-positive strains was shown in Fig. 2, with every family and genus clearly circumscribed as expected.
Genetic and physiological potentials of reference strains.
PKS I, PKS II, and NRPS amplicons were obtained in 79.6, 70.4, and 57.4%, respectively, of the 54 halogenase gene-positive strains. A total of 52 (96.3%) strains were found to possess at least one of the PKS and NRPS systems (see Table S1 in the supplemental material). In addition, 32 (59.3%) out of the 54 positive strains showed antimicrobial activities against at least one of the indicator strains tested (see Table S1 in the supplemental material). In the 21 halogenase gene-positive streptomycetes, 20 (95.2%) were found to possess PKS/NRPS genes, and 20 (95.2%) showed antimicrobial activities. Microbispora amethystogenes CGMCC 4.1340T was the only strain found to harbor the halogenase gene but without the appearance of either PKS/NRPS genes or antimicrobial activity.
To compare the biosynthetic potential between the halogenase gene-positive and -negative strains, 61 halogenase gene-negative strains across almost all genera that contained halogenase-positive strains were proportionally and randomly selected to run parallel tests. PKS I, PKS II, and NRPS loci were found in 65.6, 45.9, and 34.4% of them, respectively, and 52 (85.2%) strains contained at least one of the PKS and NRPS systems (see Table S2 in the supplemental material). Twenty-eight (45.9%) out of the 61 negative strains showed antimicrobial activities against at least one of the indicator strains tested (see Table S2 in the supplemental material). In the 24 halogenase gene-negative streptomycetes, 22 (91.7%) were found to possess PKS/NRPS genes, and 15 (62.5%) showed antimicrobial activities. Seven halogenase gene-negative strains were found to have neither PKS/NRPS genes nor antimicrobial activity.
Genome walking.
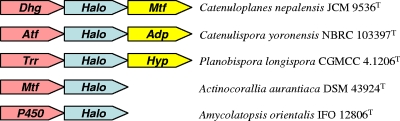
Sequence comparisons revealed that genes adjacent to the halogenase genes of the five randomly selected rare actinomycetes (Fig. 3) had significant identities to genes that are components of distinct biosynthetic gene clusters, although the flanking genes were not all obtained for the five strains. Sequences flanking the halogenase gene of Catenuloplanes nepalensis JCM 9536T matched pyruvate dehydrogenase and methyltransferase genes that are functional in avilamycin A biosynthesis; similarly, the counterparts of aminotransferase and amino acid adenylation domain-containing protein genes flanking the halogenase gene of Catenulispora yoronensis NBRC 103397T play roles in viomycin and sporolide biosyntheses, respectively. The second halogenase gene locus in the vancomycin producer Amycolatopsis orientalis IFO 12806T was found next to a cytochrome P450 monooxygenase gene that is active in the biosynthesis of vancomycin as well. Although a putative transcriptional regulator gene and a hypothetical protein gene are adjacent to the halogenase gene of Planobispora longispora CGMCC 4.1206T, their counterparts lie in the daptomycin and salinomycin biosynthetic gene clusters, respectively.
FIG. 3.
Genetic organization of parts of the putative halometabolite biosynthetic gene clusters in five reference strains. Abbreviations within each gene symbol indicate the proposed function based on sequence analysis. Adp, amino acid adenylation domain-containing protein; Atf, aminotransferase; Dhg, dehydrogenase; Halo, halogenase; Hyp, hypothetical protein; Mtf, methyltransferase; P450, cytochrome P450 monooxygenase; Trr, transcriptional regulator.
DISCUSSION
Filamentous actinomycetes have a rich history as the biological source of numerous secondary metabolites that have found utility as drugs and biologically active natural products (6). The biosynthetic gene clusters encoding the pathways for these metabolites also have become exciting sources of enzymatic modules for introducing structural diversity into customized small molecules through combinatorial biosynthesis (9). However, in employing genome mining and biological screening, the high rate of the rediscovery of known compounds has revealed that careful strain selection is critical to the efficient discovery of new gene clusters using these methods (2, 40). In general, greater phylogenetic diversity within a group of actinomycetes predicts greater structural diversity in the corresponding natural products the group can produce.
In the present study, the wide distribution of the FADH2-dependent halogenase gene in actinomycetes was observed when examining a large collection of 228 randomly selected reference actinomycetes. Twenty out of 40 genera were found to contain halogenase gene-positive strains, where more than 10 genera exhibited higher incidences of halogenase genes than the halometabolite primary producer Streptomyces, suggesting that more organohalogen compounds can be discovered from strains of these genera. Moreover, the relatively low sequence identities (<70%) of more than half (57.6%) of the putative halogenases with those in public databases imply that these halogenases are different from the known ones and therefore contribute to novel halogenated compounds. Interestingly, halogenases in the genera Actinocorallia, Catenulispora, Saccharomonospora, Saccharothrix, and Streptosporangium, where none of the strains have been reported to be producers of halometabolites to our knowledge, were found not only with higher occurrences but also with generally lower sequence identities (45 to 72%) to known halogenases than those in the intensively exploited genus Streptomyces, offering an unprecedented and promising source of strains for novel halometabolites. However, due to the small number of species known for some rare actinomycetes, the high occurrences of the halogenase gene in these lineages were based on a low number of strains tested and may be subjected to a decrease when more species are discovered and more strains of rare actinomycetes are included for evaluation.
Three major findings could be derived from phylogenetic analyses. The first is that strains containing highly homologous halogenases are likely to produce halometabolites with similar structures. It is particularly interesting that strains producing vancomycin group antibiotics (see the structures in Fig. S1 in the supplemental material) contain highly homologous halogenases and that therefore are clustered into a stable clade in the phylogenetic tree (Fig. 1). Although more evidence is necessary to determine whether similar correlations could be extended to other clades, a reasonable deduction can be drawn that Catenuloplanes atrovinosus JCM 9535T and Catenuloplanes niger JCM 9533T, which fall within the vancomycin group antibiotic clade and share halogenases with high sequence identities, should be producers of vancomycin-type antibiotics as well. The presence of NRPS genes for the biosynthesis of glycopeptide antibiotics in these strains also supports such an inference. Likewise, based on the facts that the halogenase of Catenuloplanes nepalensis JCM 9536T is phylogenetically close to the halogenase of Streptomyces viridochromogenes, which produces avilamycin A, and that the genes upstream and downstream of the halogenase gene in Catenuloplanes nepalensis share high identities with their first BLAST hits, which are located in the avilamycin A biosynthetic gene cluster in Streptomyces viridochromogenes, it is indicated that Catenuloplanes nepalensis is able to produce avilamycin A group antibiotics.
Second, halogenase genes likely display some widespread horizontal gene transfer (HGT) within actinomycetes. This conclusion is supported by the apparent incongruity between halogenase and 16S rRNA gene phylogenies (Fig. 1 and 2) and by the unduly high level of halogenase similarity between some distantly related actinomycete taxa. For instance, Prauserella rugosa CGMCC 4.1215T, Planobispora longispora CGMCC 4.1206T, and Pseudonocardia petroleophila JCM 3378T, which belong to distinct genera and are well separated in the 16S rRNA gene phylogeny, fall within a closely related subclade in the halogenase phylogeny, sharing nearly identical halogenase sequences; Prauserella rugosa CGMCC 4.1215T has another halogenase and forms another closely related subclade with Actinocorallia glomerata DSM 44360T and Saccharomonospora glauca CGMCC 4.1329T. These data strongly suggest that halogenase genes have transferred horizontally between these distant species, and Prauserella rugosa might acquire the genes by HGT from two different most recent ancestors. Furthermore, HGT events also are detected by the observation of genes, with restricted distributions, present in some taxa but absent from closely related taxa (14). Because only two of four strains belonging to the species Streptomyces hiroshimensis harbor the FADH2-dependent halogenase gene locus, it is possible that some strains of the species have not acquired the gene yet or lost it during its evolutionary history. The same trend is seen with Streptomyces mashuensis, where we observed one of two strains positive for a halogenase gene (see Tables S1 and S2 in the supplemental material), suggesting the recent acquisition of the gene by HGT. It is also possible that the negative strains of these species harbor halogenases that are evolutionarily distant from those in positive strains and thus cannot be amplified under present PCR conditions. If this is the case, however, the atypical gene sequences in strains of the same species are still an index of HGT (24).
Third, halogenase genes seem to be transferred vertically as well. Although horizontal gene transfer and the inter- and intraspecies diversity of halogenases are evident in our study, some taxonomically closely related strains also carry genes for highly homologous halogenases, as exemplified by the six pairs of strains marked with vertical lines in Fig. 1 and 2. Significantly, Streptomyces rimosus subsp. paromomycinus CGMCC 4.1760T is closer to Streptomyces chrestomyceticus CGMCC 4.1657T than to Streptomyces rimosus subsp. rimosus CGMCC 4.1438T in the 16S rRNA gene phylogeny, and this relationship is recovered exactly in the halogenase phylogeny; the strain pair Streptomyces asiaticus DSM 41761T and Streptomyces cangkringensis DSM 41769T each has multiple halogenases, nevertheless these organisms consistently cluster together in respective halogenase subclades, as they do in the 16S rRNA gene phylogeny (Fig. 1). All of these data provide evidence that halogenase genes have transferred vertically into these strains. This is not surprising, because the evolutionary history of biosynthetic gene clusters is complicated, and the genetic elements often propagate by both horizontal transfer and vertical inheritance (13).
It is worth noting that when we used PKS/NRPS genes and antimicrobial activities to serve as auxiliary, but not necessary, indicators for possible halometabolite production, we found a higher percentage of strains with genetic or physiological potentials (96.3 and 59.3%, respectively) for secondary metabolite biosynthesis in halogenase gene-positive actinomycetes than in halogenase gene-negative ones (85.2 and 45.9%, respectively). However, further tests would be needed to evaluate the bioactivity, considering that the actinomycetes may produce bioactive compounds other than antimicrobial agents or have secondary metabolite gene clusters that were not expressed under our experimental circumstances.
To well evaluate this halogenase gene-screening strategy, five strains belonging to different rare genera were chosen randomly for chromosome walking, of which three strains did not produce any bioactivity in our tests. The adjacent genes we identified here are common in secondary metabolite clusters. Although some of them, such as methyltransferase and the cytochrome P450 monooxygenase genes, also occur elsewhere in actinomycete genomes, the functions of their counterparts, as verified by BLAST searches, convincingly indicated that the flanking genes should be located in corresponding biosynthetic gene clusters. The results testified that this strategy can be applied efficiently to hunting for halometabolite gene clusters in rare actinomycetes without giving much consideration to their bioactive status.
There are two subgroups of FADH2-dependent halogenases that are acknowledged to play a significant role in halometabolite biosynthesis; however, only the subgroup with specificity for phenol and pyrrole derivatives was involved in this study, due to its extremely high incidence in halometabolite synthesis in actinomycetes (43). The other subgroup, which acts upon indole or tryptophan derivatives, also could be used for such a screening procedure to discover more and different classes of halogenated metabolites (17, 52).
The large sequence diversity and novelty of the putative FADH2-dependent halogenases identified in this study illustrate how little we know about halogenases and corresponding halometabolites. Fortunately, the detection and distribution analysis presented here sets the stage for more extensive future work to characterize halogenase-positive actinomycetes for the production of halogenated metabolites with activity in a larger panel of bioassays. Finally, the combined phylogenetic analysis of halogenases together with strain phylogeny performed here likely will be useful in predicting the existence of structurally novel halometabolites.
Supplementary Material
Acknowledgments
We are grateful to Ning Liu, Xiaoying Rong, Danheng Qiu, and Yuhua Piao (Institute of Microbiology, CAS) for their assistance in antimicrobial tests and DNA preparation and to Michael Goodfellow (Newcastle University, United Kingdom) and John J. Mekalanos (Harvard Medical School) for their valuable comments and suggestions on the manuscript. We also thank the international culture collections CGMCC, DSMZ, JCM, NBRC, and NRRL for providing reference strains.
This publication was supported by the Specialized Research Fund for State Key Laboratories of China and by the National Hi-Tech Research and Development Program of China (grant no. 2007AA09Z420).
Footnotes
Published ahead of print on 15 May 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Ahlert, J., E. Shepard, N. Lomovskaya, E. Zazopoulos, A. Staffa, B. O. Bachmann, K. Huang, L. Fonstein, A. Czisny, R. E. Whitwam, C. M. Farnet, and J. S. Thorson. 2002. The calicheamicin gene cluster and its iterative type I enediyne PKS. Science 297:1173-1176. [DOI] [PubMed] [Google Scholar]
- 2.Ayuso, A., D. Clark, I. Gonzalez, O. Salazar, A. Anderson, and O. Genilloud. 2005. A novel actinomycete strain de-replication approach based on the diversity of polyketide synthase and nonribosomal peptide synthetase biosynthetic pathways. Appl. Microbiol. Biotechnol. 67:795-806. [DOI] [PubMed] [Google Scholar]
- 3.Ayuso-Sacido, A., and O. Genilloud. 2005. New PCR primers for the screening of NRPS and PKS-I systems in actinomycetes: detection and distribution of these biosynthetic gene sequences in major taxonomic groups. Microb. Ecol. 49:10-24. [DOI] [PubMed] [Google Scholar]
- 4.Baltz, R. H. 2007. Antimicrobials from actinomycetes: back to the future. Microbe 2:125-131. [Google Scholar]
- 5.Baltz, R. H. 2008. Renaissance in antibacterial discovery from actinomycetes. Curr. Opin. Pharmacol. 8:557-563. [DOI] [PubMed] [Google Scholar]
- 6.Berdy, J. 2005. Bioactive microbial metabolites. J. Antibiot. (Tokyo) 58:1-26. [DOI] [PubMed] [Google Scholar]
- 7.Challis, G. L. 2008. Mining microbial genomes for new natural products and biosynthetic pathways. Microbiology 154:1555-1569. [DOI] [PubMed] [Google Scholar]
- 8.Chiu, H. T., B. K. Hubbard, A. N. Shah, J. Eide, R. A. Fredenburg, C. T. Walsh, and C. Khosla. 2001. Molecular cloning and sequence analysis of the complestatin biosynthetic gene cluster. Proc. Natl. Acad. Sci. USA 98:8548-8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clardy, J., M. A. Fischbach, and C. T. Walsh. 2006. New antibiotics from bacterial natural products. Nat. Biotechnol. 24:1541-1550. [DOI] [PubMed] [Google Scholar]
- 10.Dairi, T., T. Nakano, K. Aisaka, R. Katsumata, and M. Hasegawa. 1995. Cloning and nucleotide sequence of the gene responsible for chlorination of tetracycline. Biosci. Biotechnol. Biochem. 59:1099-1106. [DOI] [PubMed] [Google Scholar]
- 11.Dong, C., S. Flecks, S. Unversucht, C. Haupt, K. H. van Pee, and J. H. Naismith. 2005. Tryptophan 7-halogenase (PrnA) structure suggests a mechanism for regioselective chlorination. Science 309:2216-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorrestein, P. C., E. Yeh, S. Garneau-Tsodikova, N. L. Kelleher, and C. T. Walsh. 2005. Dichlorination of a pyrrolyl-S-carrier protein by FADH2-dependent halogenase PltA during pyoluteorin biosynthesis. Proc. Natl. Acad. Sci. USA 102:13843-13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischbach, M. A., C. T. Walsh, and J. Clardy. 2008. The evolution of gene collectives: how natural selection drives chemical innovation. Proc. Natl. Acad. Sci. USA 105:4601-4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gogarten, J. P., W. F. Doolittle, and J. G. Lawrence. 2002. Prokaryotic evolution in light of gene transfer. Mol. Biol. Evol. 19:2226-2238. [DOI] [PubMed] [Google Scholar]
- 15.Gribble, G. W. 2004. Natural organohalogens: a new frontier for medicinal agents. J. Chem. Edu. 81:1441-1449. [Google Scholar]
- 16.Groll, M., R. Huber, and B. C. M. Potts. 2006. Crystal structures of salinosporamide A (NPI-0052) and B (NPI-0047) in complex with the 20S proteasome reveal important consequences of beta-lactone ring opening and a mechanism for irreversible binding. J. Am. Chem. 28:5136-5141. [DOI] [PubMed] [Google Scholar]
- 17.Hammer, P. E., D. S. Hill, S. T. Lam, K. H. Van Pee, and J. M. Ligon. 1997. Four genes from Pseudomonas fluorescens that encode the biosynthesis of pyrrolnitrin. Appl. Environ. Microbiol. 63:2147-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris, C. M., R. Kannan, H. Kopecka, and T. M. Harris. 1985. The role of the chlorine substituents in the antibiotic vancomycin: preparation and characterization of mono- and didechlorovancomycin. J. Am. Chem. Soc. 107:6652-6658. [Google Scholar]
- 19.Hopwood, D. A., M. J. Bibb, K. F. Chater, T. Kieser, C. J. Bruton, H. M. Kieser, D. J. Lydiate, C. P. Smith, and J. M. Ward. 1985. Genetic manipulation of Streptomyces: a laboratory manual. John Innes Foundation, Norwich, CT.
- 20.Hornung, A., M. Bertazzo, A. Dziarnowski, K. Schneider, K. Welzel, S. E. Wohlert, M. Holzenkampfer, G. J. Nicholson, A. Bechthold, R. D. Sussmuth, A. Vente, and S. Pelzer. 2007. A genomic screening approach to the structure-guided identification of drug candidates from natural sources. Chembiochem 8:757-766. [DOI] [PubMed] [Google Scholar]
- 21.Jia, X. Y., Z. H. Tian, L. Shao, X. D. Qu, Q. F. Zhao, J. Tang, G. L. Tang, and W. Liu. 2006. Genetic characterization of the chlorothricin gene cluster as a model for spirotetronate antibiotic biosynthesis. Chem. Biol. 13:575-585. [DOI] [PubMed] [Google Scholar]
- 22.Kim, T. K., A. K. Hewavitharana, P. N. Shaw, and J. A. Fuerst. 2006. Discovery of a new source of rifamycin antibiotics in marine sponge actinobacteria by phylogenetic prediction. Appl. Environ. Microbiol. 72:2118-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide-sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence, J. G., and H. Ochman. 2002. Reconciling the many faces of lateral gene transfer. Trends Microbiol. 10:1-4. [DOI] [PubMed] [Google Scholar]
- 25.Li, T. L., F. Huang, S. F. Haydock, T. Mironenko, P. F. Leadlay, and J. B. Spencer. 2004. Biosynthetic gene cluster of the glycopeptide antibiotic teicoplanin: characterization of two glycosyltransferases and the key acyltransferase. Chem. Biol. 11:107-119. [DOI] [PubMed] [Google Scholar]
- 26.Liu, W., J. Ahlert, Q. Gao, E. Wendt-Pienkowski, B. Shen, and J. S. Thorson. 2003. Rapid PCR amplification of minimal enediyne polyketide synthase cassettes leads to a predictive familial classification model. Proc. Natl. Acad. Sci. USA 100:11959-11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, W., S. D. Christenson, S. Standage, and B. Shen. 2002. Biosynthesis of the enediyne antitumor antibiotic C-1027. Science 297:1170-1173. [DOI] [PubMed] [Google Scholar]
- 28.Metsä-Ketelä, M., V. Salo, L. Halo, A. Hautala, J. Hakala, P. Mantsala, and K. Ylihonko. 1999. An efficient approach for screening minimal PKS genes from Streptomyces. FEMS Microbiol. Lett. 180:1-6. [DOI] [PubMed] [Google Scholar]
- 29.Nei, M., and S. Kumar. 2000. Molecular evolution and phylogenetics. Oxford University Press, New York, NY.
- 30.Neumann, C. S., D. G. Fujimori, and C. T. Walsh. 2008. Halogenation strategies in natural product biosynthesis. Chem. Biol. 15:99-109. [DOI] [PubMed] [Google Scholar]
- 31.Newman, D. J., and G. M. Cragg. 2007. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 70:461-477. [DOI] [PubMed] [Google Scholar]
- 32.Otsuka, M., K. Ichinose, I. Fujii, and Y. Ebizuka. 2004. Cloning, sequencing, and functional analysis of an iterative type I polyketide synthase gene cluster for biosynthesis of the antitumor chlorinated polyenone neocarzilin in “Streptomyces carzinostaticus.” Antimicrob. Agents Chemother. 48:3468-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelzer, S., R. Sussmuth, D. Heckmann, J. Recktenwald, P. Huber, G. Jung, and W. Wohlleben. 1999. Identification and analysis of the balhimycin biosynthetic gene cluster and its use for manipulating glycopeptide biosynthesis in Amycolatopsis mediterranei DSM 5908. Antimicrob. Agents Chemother. 43:1565-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pootoolal, J., M. G. Thomas, C. G. Marshall, J. M. Neu, B. K. Hubbard, C. T. Walsh, and G. D. Wright. 2002. Assembling the glycopeptide antibiotic scaffold: the biosynthesis of A47934 from Streptomyces toyocaensis NRRL 15009. Proc. Natl. Acad. Sci. USA 99:8962-8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puk, O., P. Huber, D. Bischoff, J. Recktenwald, G. Jung, R. D. Sussmuth, K. H. van Pee, W. Wohlleben, and S. Pelzer. 2002. Glycopeptide biosynthesis in Amycolatopsis mediterranei DSM 5908: function of a halogenase and a haloperoxidase/perhydrolase. Chem. Biol. 9:225-235. [DOI] [PubMed] [Google Scholar]
- 36.Rix, U., C. Fischer, L. L. Remsing, and J. Rohr. 2002. Modification of post-PKS tailoring steps through combinatorial biosynthesis. Nat. Prod. Rep. 19:542-580. [DOI] [PubMed] [Google Scholar]
- 37.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 38.Sigmund, J. M., D. C. Clark, F. A. Rainey, and A. S. Anderson. 2003. Detection of eubacterial 3-hydroxy-3-methylglutaryl coenzyme A reductases from natural populations of actinomycetes. Microb. Ecol. 46:106-112. [DOI] [PubMed] [Google Scholar]
- 39.Sosio, M., and S. Donadio. 2006. Understanding and manipulating glycopeptide pathways: the example of the dalbavancin precursor A40926. J. Ind. Microbiol. Biotechnol. 33:569-576. [DOI] [PubMed] [Google Scholar]
- 40.Strohl, W. R. 1997. Industrial antibiotics: today and the future, p. 1-47. In W. R. Strohl (ed.), Biotechnology of antibiotics, 2nd ed. Marcel Dekker, New York, NY.
- 41.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA 4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 42.Tohyama, S., T. Eguchi, R. P. Dhakal, T. Akashi, M. Otsuka, and K. Kakinuma. 2004. Genome-inspired search for new antibiotics. Isolation and structure determination of new 28-membered polyketide macrolactones, halstoctacosanolides A and B, from Streptomyces halstedii HC34. Tetrahedron 60:3999-4005. [Google Scholar]
- 43.van Pee, K.-H., and S. Zehner. 2003. Enzymology and molecular genetics of biological halogenation, p. 171-199. In G. W. Gribble (ed.), Natural production of organohalogen compounds. vol. 3P. Springer, Berlin, Germany. [Google Scholar]
- 44.van Wageningen, A. M., P. N. Kirkpatrick, D. H. Williams, B. R. Harris, J. K. Kershaw, N. J. Lennard, M. Jones, S. J. Jones, and P. J. Solenberg. 1998. Sequencing and analysis of genes involved in the biosynthesis of a vancomycin group antibiotic. Chem. Biol. 5:155-162. [DOI] [PubMed] [Google Scholar]
- 45.Walsh, C. T., H. Chen, T. A. Keating, B. K. Hubbard, H. C. Losey, L. Luo, C. G. Marshall, D. A. Miller, and H. M. Patel. 2001. Tailoring enzymes that modify nonribosomal peptides during and after chain elongation on NRPS assembly lines. Curr. Opin. Chem. Biol. 5:525-534. [DOI] [PubMed] [Google Scholar]
- 46.Wang, S., J. He, Z. Cui, and S. Li. 2007. Self-formed adaptor PCR: a simple and efficient method for chromosome walking. Appl. Environ. Microbiol. 73:5048-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weitnauer, G., A. Muhlenweg, A. Trefzer, D. Hoffmeister, R. D. Sussmuth, G. Jung, K. Welzel, A. Vente, U. Girreser, and A. Bechthold. 2001. Biosynthesis of the orthosomycin antibiotic avilamycin A: deductions from the molecular analysis of the avi biosynthetic gene cluster of Streptomyces viridochromogenes Tü57 and production of new antibiotics. Chem. Biol. 8:569-581. [DOI] [PubMed] [Google Scholar]
- 48.Winter, J. M., M. C. Moffitt, E. Zazopoulos, J. B. McAlpine, P. C. Dorrestein, and B. S. Moore. 2007. Molecular basis for chloronium-mediated meroterpene cyclization: cloning, sequencing, and heterologous expression of the napyradiomycin biosynthetic gene cluster. J. Biol. Chem. 282:16362-16368. [DOI] [PubMed] [Google Scholar]
- 49.Yin, X., and T. M. Zabriskie. 2006. The enduracidin biosynthetic gene cluster from Streptomyces fungicidicus. Microbiology 152:2969-2983. [DOI] [PubMed] [Google Scholar]
- 50.Yu, T. W., L. Bai, D. Clade, D. Hoffmann, S. Toelzer, K. Q. Trinh, J. Xu, S. J. Moss, E. Leistner, and H. G. Floss. 2002. The biosynthetic gene cluster of the maytansinoid antitumor agent ansamitocin from Actinosynnema pretiosum. Proc. Natl. Acad. Sci. USA 99:7968-7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zazopoulos, E., K. Huang, A. Staffa, W. Liu, B. O. Bachmann, K. Nonaka, J. Ahlert, J. S. Thorson, B. Shen, and C. M. Farnet. 2003. A genomics-guided approach for discovering and expressing cryptic metabolic pathways. Nat. Biotechnol. 21:187-190. [DOI] [PubMed] [Google Scholar]
- 52.Zehner, S., A. Kotzsch, B. Bister, R. D. Sussmuth, C. Mendez, J. A. Salas, and K. H. van Pee. 2005. A regioselective tryptophan 5-halogenase is involved in pyrroindomycin biosynthesis in Streptomyces rugosporus LL-42D005. Chem. Biol. 12:445-452. [DOI] [PubMed] [Google Scholar]
- 53.Zerbe, K., O. Pylypenko, F. Vitali, W. Zhang, S. Rouset, M. Heck, J. W. Vrijbloed, D. Bischoff, B. Bister, R. D. Sussmuth, S. Pelzer, W. Wohlleben, J. A. Robinson, and I. Schlichting. 2002. Crystal structure of OxyB, a cytochrome P450 implicated in an oxidative phenol coupling reaction during vancomycin biosynthesis. J. Biol. Chem. 277:47476-47485. [DOI] [PubMed] [Google Scholar]
- 54.Ziegler, D. M. 2002. An overview of the mechanism, substrate specificities, and structure of FMOs. Drug Metab. Rev. 34:503-511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.