Abstract
Four Bacillus thuringiensis δ-endotoxins, Cry3A, Cry4Aa, Cry11Aa, and Cyt1Aa, were found to exhibit low to moderate toxicity on the pea aphid, Acyrthosiphon pisum, in terms both of mortality and growth rate. Cry1Ab was essentially nontoxic except at high rates. To demonstrate these effects, we had to use exhaustive buffer-based controls.
Many species of aphids are important sucking-insect pests that feed on plant vascular fluids. Their feeding mechanism makes these insects excellent vectors for many plant pathogens, especially viruses, yet less amenable to standard, nonsystemic chemical control by insecticides. Minor effects on the survival and fecundity of aphids reared on Bacillus thuringiensis (Bt) crops have been noted in some studies but not in others (1, 3, 6). However, the sensitivity of aphids to Bt toxins, or the lack thereof, has not been previously tested through artificial-diet bioassays with exhaustive buffer-based controls.
Bt δ-endotoxins Cyt1A, Cry4A/Cry4B, and Cry11, obtained from three recombinant strains of B. thuringiensis subsp. israelensis, as well as Cry1Ab and Cry3A, obtained from recombinant Escherichia coli, were purified by ultracentrifugation in a discontinuous sucrose gradient as described previously (9). Cry proteins were solubilized in solubilization buffer (50 mM Na2CO3, 100 mM NaCl, pH 10) with dithiothreitol (10 mM) added before use. Cyt1A was first solubilized on 10 mM Na2CO3 (pH 11) buffer and then neutralized at pH 7.5 to 8 with 10 μl HCl (1 N). Both solubilized and trypsin-digested samples (1:30 over toxin weight) were used at different concentrations (32, 125, and 500 μg/ml; trypsin-activated toxin concentrations were calculated on the basis of the preactivation concentrations of the protoxins) to supplement the AP3 aphid synthetic diet (7) used to feed Acyrthosiphon pisum (LL01 green clone). Ampicillin (100 μg/ml), an ineffective antibiotic for A. pisum or its obligate symbiont Buchnera, was added to the medium to avoid bacterial growth. For each concentration, 30 nymphs (10 nymphs/box and three repetitions) were bioassayed at 20°C and under a 16:8 (light-dark) photoperiod. Survival time was calculated from aphid deposition on the test diet (day 0). Mortality was surveyed daily, and body weights of survivors were noted at day 7. ST50 (median survival time after challenge) was calculated by using an actuarial survival analysis (Statview) with censoring values of survivors at the end of the experiments. The approximate concentrations resulting in a 50% decrease in mean body weight (IC50) and killing of 50% of the insects tested (LC50) were calculated at the end of the experiments from the growth reduction and mortality data, respectively, derived with the three doses by using Statview and the censoring values of survivors.
All of the Cry δ-endotoxins tested were lethal to A. pisum and retarded the growth of survivors (Fig. 1 and 2). Mortalities ranged from only 25% (Cry1Ab) to 100% (Cry4 and Cry11) after 3 to 6 days of exposure to 500 μg/ml of solubilized protein (Fig. 1). When significant mortalities were achieved (Cry3A, Cry4, and Cry11), trypsin activation enhanced toxicity. Activation of Cry4 at the intermediate concentration tested (125 μg/ml) resulted in a twofold increase in mortality (Fig. 1D). ST50s were calculated for both solubilized protoxins and activated Cry3A, Cry4, and Cry11. The ST50s (at 500 μg/ml) ranged from 1.8 ± 0.14 days for solubilized Cry4 and Cry11 to 3.7 ± 1.2 days for trypsin-activated Cry3A (Table 1). Control aphids fed buffer all survived for >8 days. The LC50 of Cry1Ab was not calculated, since mortality associated with Cry1Ab reached a plateau at 500 μg/ml. The LC50 of Cry4 was estimated to be 70 to 100 μg/ml (data not shown).
FIG. 1.
Mortality assays over the nymphal life stage of the pea aphid, A. pisum, upon ingestion of artificial diets containing purified Bt toxins after either solubilization (open symbols) or solubilization and trypsin activation (solid symbols). The toxins used were Cry1Ab (circles), Cry3A (squares), a mixture of Cry4A and Cry4B (diamonds), and Cry11A (triangles). The soluble-toxin doses used were low at 32 μg/ml (blue), intermediate at 125 μg/ml (violet), and high at 500 μg/ml (red). Assays were carried out with 30 initial neonate insects in three batches of 10 individuals.
FIG. 2.
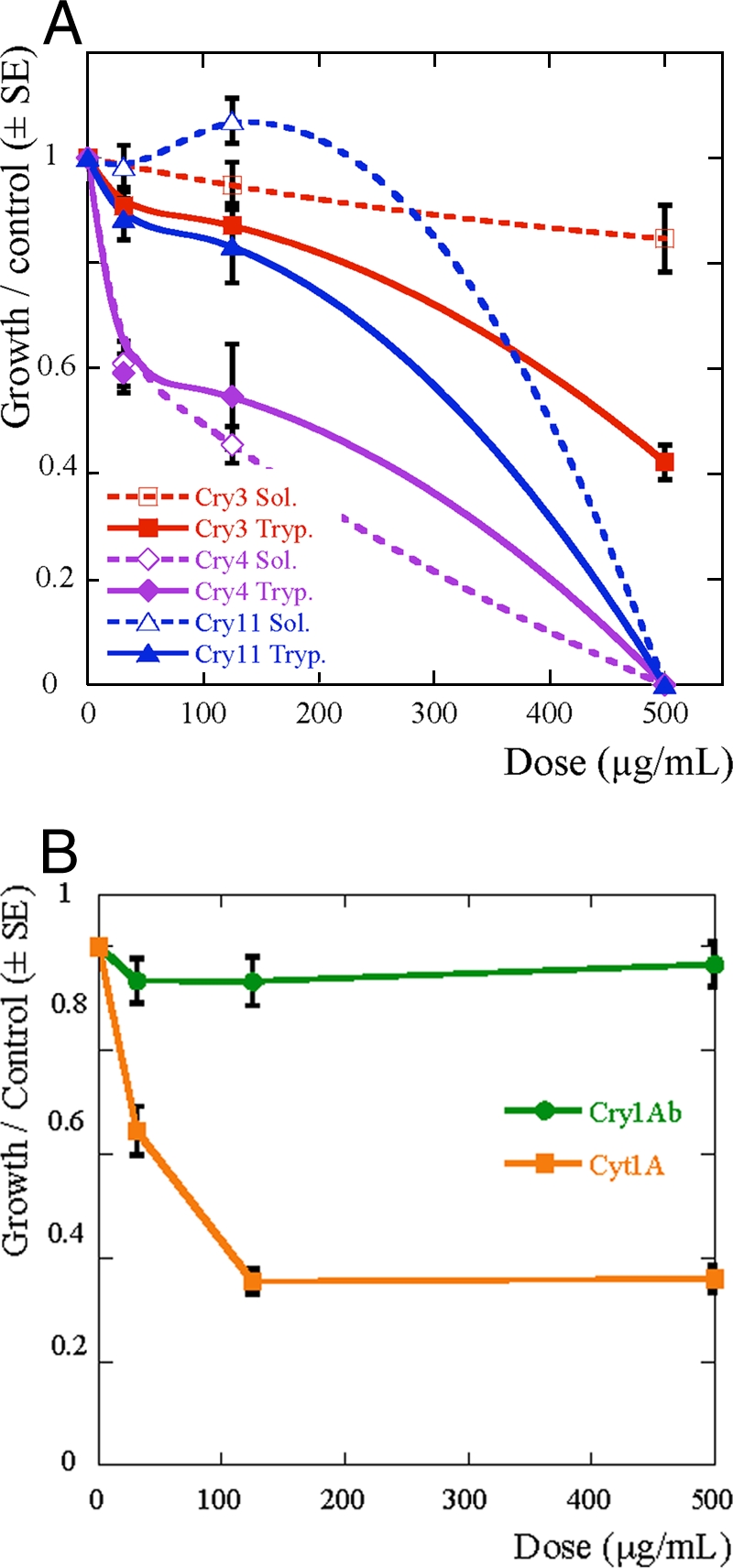
Growth inhibition assays with purified Bt toxins Cry3A, Cry4, and Cry 11 (A) and Cry1Ab and Cyt1A (B) on the pea aphid, A. pisum. Toxins were added to the diet either after solubilization (open symbols) or after solubilization and trypsin activation (solid symbols). Error bars show the standard errors (SE) of individual weights at day 7 of experiments, standardized by the control group mean weight (toxin dose, 0; initial number, 30). Color coding of toxins: Cry3A, red squares; Cry4A and Cry4B mixture, violet diamonds; Cry11A, blue triangles; Cry1Ab, green circles; Cyt1A, yellow squares. In the experiment with Cry1Ab (B), the toxin was purified by high-performance liquid chromatography and activated toxin was provided as a salt-free lyophilisate by W. Moar (Auburn University, Auburn, AL).
TABLE 1.
ST50s of pea aphids feeding on solubilized Cry toxins and solubilized Cry toxins activated with trypsin
| Toxin | Mean ST50a ± SE (days) at dose of:
|
||
|---|---|---|---|
| 32 mg/ml | 125 mg/ml | 500 mg/ml | |
| Cry1Ab | |||
| Solubilized | NL | >8 | >8 |
| Trypsinized | NL | >8 | >8 |
| Cry3A | |||
| Solubilized | NL | >8 | >8 |
| Trypsinized | NL | >8 | 3.7 ± 1.2 |
| Cry4A | |||
| Solubilized | NL | >8 | 1.8 ± 0.14 |
| Trypsinized | >8 | 1.8 ± 0.15 | 1.9 ± 0.17 |
| Cry11A | |||
| Solubilized | NL | >8 | 1.8 ± 0.14 |
| Trypsinized | NL | >8 | 2.5 ± 0.10 |
NL, nonlethal; >8, survival for >8 days.
Aphids that survived ingestion of the Cry and Cyt proteins in the bioassays had markedly reduced growth rates compared to those of the control group (Fig. 2). Growth inhibition by each Cry protein correlated with mortality. Cry4 inhibited growth the most (Fig. 2A), whereas Cry1Ab inhibited growth the least (Fig. 2B). The IC50 of Cry4 was calculated to be 135 μg/ml. The growth of aphids surviving Cyt1A ingestion was strongly inhibited, with an average weight at the end of the assay, for doses of 125 μg/ml or higher, corresponding to less than 40% of that of the control group (Fig. 2B). This decrease in aphid weight associated with the ingestion of Cyt1A is in contrast to the low mortality (about 10%) produced by the same dose of this protein. Most of the surviving insects did not reach adulthood as a result of feeding on Cyt1A, whereas control insects completed their nymphal development by the end of the bioassay.
Cofeeding experiments with a mixture of toxins (Cry and Cyt1A) currently under way suggest that there is no identifiable synergy between Cry and Cyt toxins in this model, at least in the concentration range of 32 to 500 μg/ml (A.-M. Grenier et al., unpublished data).
In two previous studies (10, 11), sensitivity of another aphid, Macrosiphum euphorbiae, to suspensions of Cry2, Cry3A, and Cry4 crystals was reported but no sensitivity to solubilized endotoxins was found. This may be explained by the lack of complete solubilization of the Bt crystals (10) and by the fact that control groups were fed a water-based artificial diet instead of a diet containing the buffer used to solubilize the crystals. Our bioassays, performed with buffer-based controls, show that A. pisum is indeed sensitive to Bt δ-endotoxins, although to a low degree. In fact, the IC50s and LC50s we calculated are very high compared to those of highly susceptible targets of B. thuringiensis (http://www.glfc.forestry.ca/bacillus/) but similar to those of organisms with low sensitivity, such as nematodes. For example, in feeding bioassays in which growth inhibition was measured against Caenorhabditis elegans fed E. coli/Cry strains, IC50s ranged from 16 μg/ml for Cry14A to as high as 230 μg/ml for Cry6A (12). The low activity of Bt endotoxins against aphids suggests that these proteins have not evolved to kill aphids. In fact, the ecological niches of B. thuringiensis and these insects are very different and it is unlikely that aphids, feeding on a virtually germfree environment such as plant phloem, come in contact with bacteria living either in other susceptible insects or on the plant surface. It might be hypothesized that the sensitivity of pea aphids to these Bt endotoxins is a consequence of similarities among midgut microvillar proteins and lipids, especially the surface molecules that compose the sugar residues known to serve as the initial binding sites for Bt toxins (4), rather than a result of direct selection for aphid sensitivity.
The low sensitivity of aphids to Bt toxins is not in contrast to recent reports on the lack of deleterious effects of genetically modified crops on aphid populations (5). A recent report confirms the presence of Cry1Ac in the phloem of transgenic oilseed rape and in aphids feeding on these plants (2). However, the concentration of Cry1Ac in phloem, being low, is compatible with the absence of deleterious effects of transgenic oilseed rape on aphids, as well as with previous studies reporting no detectable levels of Cry toxins in phloem translocated through sieves of commercial transgenic plants (8). Although low, the susceptibility of aphids to B. thuringiensis we report here could theoretically lead to the development of effective strategies for controlling these and other sucking insect pests with genetically modified crops expressing appropriate toxins. However, two conditions should concur. (i) Toxins must be present in the plant phloem to be accessible to these pests and vectors, and (ii) more effective toxins should be found, and thus screening programs with a range of natural and engineered toxins should be performed in order to determine their activity on sucking insects. Although a wide range of further studies are still needed to assess the potential of Bt crops for controlling aphids and other sucking insect pests, the substantial economic losses sucking insects cause to agriculture worldwide clearly merit exploration of the possibilities our results suggest.
Acknowledgments
Manuel Porcar has a Ramón y Cajal research contract from the Spanish Ministerio de Educación y Ciencia. This research was facilitated in part by a grant to B.F. from the U.S. National Institutes of Health (AI 145817).
Footnotes
Published ahead of print on 15 May 2009.
REFERENCES
- 1.Ashouri, A., D. Michaud, and C. Cloutier. 2001. Unexpected effects of different potato resistance factors to the Colorado potato beetle (Coleoptera: Chrysomelidae) on the potato aphid (Homoptera: Aphididae). Environ. Entomol. 30:524-532. [Google Scholar]
- 2.Burgio, G., A. Lanzoni, G. Accinelli, G. Dinelli, A. Bonetti, I. Marotti, and F. Ramilli. 2007. Evaluation of Bt-toxin uptake by the non-target herbivore, Myzus persicae (Hemiptera: Aphididae), feeding on transgenic oilseed rape. Bull. Entomol. Res. 97:211-215. [DOI] [PubMed] [Google Scholar]
- 3.Faria, C. A., F. L. Wäckers, J. Pritchard, D. A. Barrett, and T. C. J. Turlings. 2007. High susceptibility of Bt maize to aphids enhances the performance of parasitoids of Lepidopteran pests. PloS One 7:e600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffitts, J. S., S. M. Haslam, T. Yang, S. F. Garczynski, B. Mulloy, H. Morris, P. S. Cremer, A. Dell, M. J. Adang, and R. V. Aroian. 2005. Glycolipids as receptors for Bacillus thuringiensis crystal toxin. Science 307:922-925. [DOI] [PubMed] [Google Scholar]
- 5.Lawo, N. C., F. L. Wäckers, and J. Romeis. 2009. Indian Bt cotton varieties do not affect the performance of cotton aphids. PLoS One 4:e4804. doi: 10.1371/journal.pone.0004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellet, M. A., and A. S. Schoeman. 2007. Effect of Bt-cotton on chrysopids, ladybird beetles and their prey: aphids and whiteflies. Indian J. Exp. Biol. 45:554-562. [PubMed] [Google Scholar]
- 7.Rahbé, Y., and G. Febvay. 1993. Protein toxicity to aphids—an in vitro test on Acyrthosiphon pisum. Entomol. Exp. Appl. 67:149-160. [Google Scholar]
- 8.Raps, A., J. Kehr, P. Gugerli, W. J. Moar, F. Bigler, and A. Hilbeck. 2001. Immunological analysis of phloem sap of Bacillus thuringiensis corn and of the nontarget herbivore Rhopalosiphum padi (Homoptera: Aphididae) for the presence of Cry1Ab. Mol. Ecol. 10:525-533. [DOI] [PubMed] [Google Scholar]
- 9.Thomas, W. E., and D. J. Ellar. 1983. Bacillus thuringiensis var. israelensis crystal delta-endotoxin: effects on insect and mammalian cells in vitro and in vivo. J. Cell Sci. 60:181-197. [DOI] [PubMed] [Google Scholar]
- 10.Walters, F. S., and L. H. English. 1995. Toxicity of Bacillus thuringiensis δ-endotoxins toward the potato aphid in an artificial diet bioassay. Entomol. Exp. Appl. 77:211-216. [Google Scholar]
- 11.Walters, F. S., C. A. Kulesza, A. T. Phillips, and L. H. English. 1994. A stable oligomer of Bacillus thuringiensis delta-endotoxin, CryIIIA. Insect Biochem. Mol. Biol. 24:963-968. [Google Scholar]
- 12.Wei, J. Z., K. Hale, L. Carta, E. Platzer, C. Wong, S. C. Fang, and R. V. Aroian. 2003. Bacillus thuringiensis crystal proteins that target nematodes. Proc. Natl. Acad. Sci. USA 100:2760-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]