Abstract
An open mixed culture was enriched with glycogen-accumulating organisms (GAOs) by using a sequencing batch reactor and treating an agroindustrial waste (sugar cane molasses) under cyclic anaerobic-aerobic conditions. Over a 1-year operating period, the culture exhibited a very stable GAO phenotype with an average polyhydroxyalkanoate (PHA) content of 17% total suspended solids. However, the GAO microbial community evolved over the course of operation to a culture exhibiting unusual characteristics in producing PHAs comprised of short-chain-length monomers, namely, 3-hydroxybutyrate, 3-hydroxy-2-methylbutyrate, 3-hydroxyvalerate, and 3-hydroxy-2-methylvalerate, and also, up to 31 mol% of the medium-chain-length (MCL) monomer 3-hydroxyhexanoate (3HHx). Microbial community analysis by fluorescence in situ hybridization revealed a concurrent long-term drift in the GAO community balance, from mainly “Candidatus Competibacter phosphatis” to mainly Defluviicoccus vanus-related organisms. The production of 3HHx was confirmed by 13C nuclear magnetic resonance (NMR) and appeared to be related to the increased presence of D. vanus-related GAOs. These results suggest a broadened spectrum of material, chemical, and mechanical properties that can be achieved for biopolymers produced by open mixed cultures from fermented waste. The increased spectrum of polymer properties brings a wider scope of potential applications.
Polyhydroxyalkanoates (PHAs) are biopolymers synthesized by bacteria as intracellular reserves of carbon, energy, and reducing power, with physical properties that make them an attractive source of biobased thermoplastics. These polymers are completely biodegradable and biocompatible and show promise for a broad range of engineering applications, from commodity to high-value-added specialty niche products (27).
The most common PHAs are homopolymers of 3-hydroxybutyrate [P(3HB)] and copolymers with, for example, 3-hydroxyvalerate (3HV) [P(3HB-co-3HV)]. The homopolymer P(3HB) is highly crystalline with low impact strength. The copolymer P(3HB-co-3HV) exhibits contrasting mechanical properties, such as lower crystallinity and an increased impact strength and flexibility due to the incorporation of 3HV units. The copolymers with P(3HB) thus exhibit properties that are strongly dependent on the type and content of additional monomer elements. The incorporation of monomers other than 3HV may further extend the scope for PHA applications by tailoring the polymer's mechanical properties (8, 17).
The industrial production of PHAs is currently conducted using bacterial cultures of pure or recombinant strains that require sterile production conditions and strict process control. This state of the art has a high energy demand and, consequently, high production costs relative to those of, for example, polypropylene. Furthermore, well-defined and expensive substrates, such as glucose or propionic acid, are used as feedstock, contributing even further to the relatively high costs of production (10). As a result and notwithstanding the potential environmental benefits of a biodegradable thermoplastic, PHA production economics represent a considerable obstacle to widespread commercial use of these polymers.
In the search for more cost-effective PHA production, one promising cost-saving strategy is to use open mixed microbial cultures constrained by selection pressures of the process operating environment (17). With the right operating conditions, it is possible to select for a microbial culture with high PHA storage ability. The application of open mixed cultures avoids costs in sterilization and simplifies the process control, given that it is designed based on the ecological role of species survival with PHAs. Additionally, agroindustrial wastes and by-products, such as olive oil mill effluents (18), sugar cane molasses (1), or paper mill wastewater (7), may be used as the carbon source (42), rather than refined organic substrates.
To date, the research on PHA production by mixed cultures has been conducted mostly with aerobic dynamic feeding (ADF) systems, also known as “feast and famine” enrichment, where the substrate is provided intermittently and microbial growth occurs under non-steady-state conditions (1, 7, 19, 32, 42). An alternative approach to ADF is an anaerobic-aerobic selection strategy, where electron donor and acceptor are separated (i.e., the carbon source is provided intermittently and under anaerobic conditions). For a bioreactor inoculated with activated sludge, such anaerobic-aerobic selection in the absence of excess phosphorus concentrations promotes enrichment of a specific group of bacteria, glycogen-accumulating organisms (GAOs) (14, 38). GAOs have been thoroughly investigated in the context of enhanced biological phosphate removal (38).
The GAO phenotype occurs when an external source of soluble carbon (volatile fatty acids [VFAs]) is taken up anaerobically and stored as PHAs at the expense of intracellular glycogen. Glycogen is hydrolyzed in order to provide energy (ATP), reducing power (NADH), and PHA precursors. In the subsequent aerobic phase, PHAs are used as a source of carbon, energy, and reducing power for cell growth, maintenance, and glycogen replenishment (36).
This GAO phenotype has been observed for a number of bacterial organisms: “Candidatus Competibacter phosphatis,” belonging to the Gammaproteobacteria phylum (11); Sphingomonadales-related organisms, belonging to the Alphaproteobacteria phylum (5); and cluster 1 (48) and cluster 2 (35) Defluviicoccus vanus-related organisms, also belonging to the Alphaproteobacteria phylum. The latter organisms belonging to the Alphaproteobacteria phylum typically appear in arrangements of four or more cells and are generally referred to as tetrad-forming organisms (47). To date, no pure cultures of these organisms have been isolated. They have all been identified in situ with techniques such as fluorescence in situ hybridization (FISH).
In ADF mixed-culture systems fed with complex substrates, different bacterial groups may possibly develop, with different substrate affinities and metabolisms. The net result can be the production of different homopolymers rather than one copolymer. Although GAO mixed cultures may include several bacterial strains, all will have the same type of metabolism, involving glycogen turnover. GAOs use glycogen for polymer production in conjunction with the external substrates provided, making it more feasible to control for a true copolymer production (14). However, utilization of GAOs for PHA production has mainly been studied using synthetic substrates (13, 14). Only one previous study with a complex fermented waste, a paper mill wastewater, was reported (6).
In the present investigation, enrichment of GAOs using fermented sugar cane molasses, a carbon source that has been studied previously for ADF enrichment (1), was studied. The enrichment of the GAO culture for PHA production was accomplished at laboratory scale with an anaerobic-aerobic sequencing batch reactor (SBR). The laboratory system was operated over a 1-year period, and the evolution of both phenotypic and genotypic characteristics of the microbial community was systematically evaluated.
MATERIALS AND METHODS
SBR set-up and operation.
A laboratory SBR operated under alternating anaerobic-aerobic conditions was used for enrichment of the GAO culture on fermented sugar cane molasses. The SBR had a working volume of 1,000 ml and was maintained at 30°C. Data logging and pump control were managed by an in-house program, BioCTR, created under LabView (National Instruments) with Data Acquisition Control (NI USB-6009; National Instruments). Each 8-h SBR cycle consisted of feeding (8.25 min with 250 ml of influent), anaerobic phase (3 h), aerobic phase (3.7 h) with excess sludge withdrawal under well-mixed conditions (33 ml), settling (1 h), and effluent withdrawal (10 min with 227 ml of effluent). The hydraulic retention time and sludge retention time (SRT) were 1.33 days and 10 days, respectively. Stirring was kept at 500 rpm. Argon or air was supplied through a ceramic diffuser at a flow rate of 1 liter/min to generate conditions for the anaerobic and aerobic phases, respectively. pH was maintained with a set point of pH 7.7 by controlled dosing of 0.2 M H2SO4. Redox potential was also continuously monitored.
The inoculum was a GAO culture that had been previously enriched with fermented dairy industry wastewater as the substrate under identical SBR operating conditions except at an SRT of 7 days (AnoxKaldnes, Sweden). The inoculum for the SBR operated with the dairy wastewater came from a municipal wastewater treatment plant (Källby in Lund, Sweden).
Molasses fermentation and preparation of culture medium.
Acidogenic fermentation of the sugar cane molasses has been described in detail elsewhere (1). Briefly, fermentation was conducted in a continuously stirred tank reactor (CSTR) operated with 10-h hydraulic retention time at pH 6 and an influent C:N:P molar ratio of 100:3:1. The CSTR effluent comprised a fatty acid mixture (126 C-mmol [mmol of C]/liter) of acetate, propionate, butyrate, valerate, and lactate with a nominal lactate:acetate:propionate:butyrate:valerate ratio of 2:72:7:17:3 on a mol basis. This fatty acid mixture made up 75% of the dissolved organic carbon (DOC) of the CSTR effluent. The CSTR effluent was filtered through an ultrafiltration hollow-fiber membrane module (5 × 105 molecular-weight cutoff).
The filtered fermented molasses was then diluted with a mineral medium. This mineral medium, adapted from Serafim et al. (44), contained, per liter, 720 mg of MgSO4·7H2O, 84 mg of CaCl2·2H2O, 687.6 mg of NH4Cl, 24 mg of EDTA, 20 mg of N-allylthiourea (to inhibit nitrification), and 1.2 ml of a trace element solution (1,500 mg of FeCl3·6H2O, 150 mg of H3BO3, 30 mg of CuSO4·5H2O, 30 mg of KI, 120 mg of MnCl2·4H2O, 60 mg of Na2MoO4·2H2O, 120 mg of ZnSO4·7H2O, and 150 mg of CoCl2·6H2O per liter of solution). A solution of phosphate (165.6 mg of K2HPO4 and 81 mg of KH2PO4) was prepared separately. The mineral solution was supplemented with the phosphate solution after both had been autoclaved.
A 50:50 (vol/vol) mixture of the filtered fermented molasses and the phosphate-supplemented mineral medium generated the SBR feed (C:N:P molar ratio of 100:10:1). With three cycles per day, the organic loading rate (OLR) was 47.4 C-mmol/liter/day and the average VFA concentration measured at the beginning of each cycle was 15.8 C-mmol/liter. The SBR feed was maintained in an anaerobic condition with continuous stirring and purging with argon.
SBR sampling and sample preservation.
The SBR performance was evaluated by means of routinely performed cycle studies over the course of the 1-year monitoring period. In these cycle studies, the reactor contents were grab sampled (5 ml) at selected time intervals over the course of the 8-h SBR cycle in order to assess the metabolic activity and the fate of carbon and nitrogen. Each grab sample was centrifuged (13,000 rpm for 2 min), after which the supernatant was decanted and frozen pending analysis. The biomass pellet was also stored frozen pending lyophilization for subsequent quantification of glycogen and PHA contents. As part of the cycle study, the amounts of total suspended solids (TSS) and volatile suspended solids (VSS) were determined from sampling at the beginning and the end of the cycle.
Analytical procedures.
Concentrations of VFAs (acetate, propionate, butyrate, and valerate) and lactate were determined by high-performance liquid chromatography as described by Albuquerque et al. (1). Ammonia levels were monitored by using an ammonia gas-sensing combination electrode ThermoOrion 9512. DOC quantification was derived from total carbon and inorganic carbon sample quantification done using a Shimadzu TOC automatic analyzer (TOC-V CSH). For this organic analysis, thawed aqueous samples were diluted 10 times with MilliQ water.
TSS and VSS were determined according to standard methods for solids analysis (4), with minor modifications. Three-milliliter aliquots of mixed liquor were vacuum filtered onto tare-weighed glass microfiber filters (1.2-μm Whatman GF/C). All filters were pretreated at 550°C for 12 h and stored in a desiccator. The filter with harvested biomass was dried in the microwave (15 min at 30 W) and weighed for TSS determination (40).
Biomass glycogen was analyzed as glucose after acidic hydrolysis, which is common practice for GAO enriched cultures (13, 21, 29, 39, 49). Lyophilized biomass samples were weighed and combined with a 1-ml aliquot of 0.6 M HCl. The acid-biomass solutions were then purged for 10 min with argon and digested for 2 h at 100°C in sealed glass tubes. After cooling, the samples were centrifuged (13,000 rpm for 2 min) and the supernatant was filtered (0.2-μm Whatman) before injection. The glycogen concentration, as glucose, was then assessed by high-performance liquid chromatography using a Merck-Hitachi chromatograph (Bio-Rad Aminex 300-mm by 1.8-mm HPX-87H column with a guard column and a refractive index detector). A 0.01 N H2SO4 solution was used as the eluent at a flow rate of 0.6 ml/min at 50°C. Calibration was done with glucose standards in the range of 25 to 1,000 mg/liter.
The PHA content was assessed by acidic methanolysis followed by individual methyl ester quantification using gas chromatography as described by Lemos et al. (28).
SBR biomass samples were collected regularly, at the end of the feeding period, for microscopic observation and staining. An aliquot of each sample was fixed for later FISH analysis, as described by Amann (3). The 16S DNA probes employed are listed in Table 1. The specific probes used were all Cy3-labeled and used in combination with fluorescein isothiocyanate-labeled EUBmix. Intracellular PHA granules were shown by Nile blue staining (7). An Olympus BX51 microscope was used for the observations of the biomass in phase-contrast, bright-field (Nile blue staining), and epifluorescence (after Nile blue staining and FISH) microscopy. The abundance of hybridized cells was semiquantitatively estimated. Images were collected and analyzed with an F-View Soft Image System video camera and AnalySIS software.
TABLE 1.
16S rRNA gene probes used for FISH (helper probes and/or competitors according to the references)
| Probe or mixture | Probe composition | Target | Reference(s) |
|---|---|---|---|
| EUBmix | EUB338 + EUB338-II + EUB338-III | Bacteria domain | 2,16 |
| GAOmix | GB_G2 + GAOQ431 + GAOQ989 | “Candidatus Competibacter phosphatis” | 26,11 |
| TFOmix | TFO_DF218 + TFO_DF618 | Defluviicoccus vanus-related organisms, cluster 1 | 48 |
| DFmix | DF988 + DF1020 | Defluviicoccus vanus-related organisms, cluster 2 | 35 |
| PAOmix | PAO462 + PAO651 + PAO846 | “Candidatus Accumulibacter phosphatis” | 12 |
| ALF969 | ALF969 | Alphaproteobacteria | 39 |
| BET42a | BET42a | Betaproteobacteria | 33 |
| GAM42a | GAM42a | Gammaproteobacteria | 33 |
| AMAR839 | AMAR839 | Amaricoccus organisms (except A. tamworthensis) | 34 |
Natural-abundance 13C nuclear magnetic resonance (NMR) analysis was performed on the extracted PHAs. Fifty milliliters of chloroform was added to the biomass, and the flask was sealed and stirred for 3 days at 37°C. The chloroform mixture was filtered through a Whatman GF/F membrane (0.7 μm), and the chloroform extract was then dried under a steady argon flow. The recovered dried extract was then resuspended in deuterated chloroform. 13C-NMR spectra of the chloroform extracts were recorded at 125.76 MHz in a Bruker Avance II-400 spectrometer, using a QNP 1H/13C probe (5-mm diameter). The NMR acquisition conditions were 25 kHz spectral width, 60 s repetition delay, 5.5 ms pulse width corresponding to a flip angle of 90°, 128 K data size, and 28°C probe temperature. Proton broadband decoupling was applied during the acquisition time only (2.08 s). Chemical shifts were referenced with respect to the resonance of deuterated chloroform at 77.5 ppm. Benzoic acid was used as the internal standard (128.29 ppm). The polymer carbon resonances were assumed based on values in the literature (24, 25).
Determination of stoichiometry and kinetics.
The biomass PHA content was calculated as a percentage of TSS on a mass basis (% PHA = g PHA/g TSS × 100). Yields were calculated in C-mol/C-mol based on the amounts of substrates, storage products, and active biomass consumed and/or produced over the course of the anaerobic phase or the aerobic phase of an SBR cycle. Total VFA concentration was determined as the sum of the concentration of all the organic acids (in C-mmol/liter), and total PHA concentration was determined as the sum of all the monomers (in C-mmol/liter). Active biomass (X) was calculated by subtracting the storage products from the VSS as follows: X = VSS − PHA − glycogen (in g/liter).
It was assumed that all the ammonia consumed aerobically was used for growth and that it was the only possible source of nitrogen. Active biomass was assumed to be represented by the molecular formula C5H7NO2 (22).
For the anaerobic phase, stoichiometric coefficients for PHAs and glycogen were calculated on the basis of VFA or total carbon substrate (S) consumption in C-mol/C-mol, as follows: YPHA/VFA = ΔPHA/ΔVFA, YPHA/S = ΔPHA/(ΔVFA + ΔGlyc), and YGlyc/VFA = ΔGlyc/ΔVFA, where Y represents yield and Glyc is glycogen.
For the aerobic phase, yields of active biomass and glycogen were calculated on the basis of PHA consumption in C-mol/C-mol, as follows: YX/PHA = ΔX/ΔPHA and YGlyc/PHA = ΔGlyc/ΔPHA.
The observed yield of the aerobic phase was calculated as ΔY = YX/PHA + YGlyc/PHA. The respiratory yield was not taken into consideration in the material balances since the oxygen uptake rate was not measured. An average active biomass yield on VFA was also calculated by multiplying the active biomass concentration by the volume of sludge withdrawn at each cycle (33.3 ml) and dividing by the amount of VFAs consumed.
The total and individual specific VFA uptake rates (−qVFA, in C-mmol VFA/C-mmol X/h), the glycogen consumption rate (−qGlyc, in C-mmol Glyc/C-mmol X/h), and the total and individual specific PHA monomer production rates (q3HA, in C-mmol 3HA/C-mmol X/h) were determined from the cycle study time-series trends by linear regression and dividing the slope by the active biomass concentration. The anaerobic glycogen utilization rate for maintenance (mGlyc,An) was determined by performing linear regression of the time trend in glycogen consumption after complete VFA uptake and dividing the slope by the active biomass concentration.
RESULTS AND DISCUSSION
A mixed culture was enriched with GAOs in treating an agroindustrial waste, and the process was monitored for more than 1 year. From the available data, it was important to confirm the stability of the GAO-phenotypic behavior of the enrichment culture. More detailed PHA analysis was undertaken to positively identify a new monomer, 3-hydroxyhexanoate (3HHx). Observed trends in product formation were critically evaluated based on the measured yields and kinetics. Finally, the microbial community structure was characterized and the community dynamics were related to the observed changes in PHA composition.
Enrichment of a GAO culture.
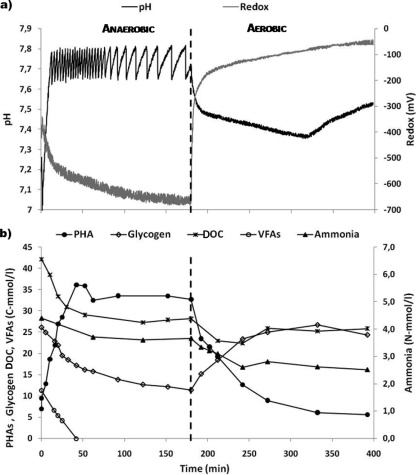
The performance of the reactor was evaluated by frequent cycle studies. The results for a typical SBR cycle time series are depicted in Fig. 1. A GAO culture was successfully enriched and maintained, as is evident from the stable phenotypic response. During the anaerobic phase of the cycle, there was consumption of the externally supplied VFAs, consumption of glycogen, and concomitant accumulation of PHAs. During the cycle studies, all VFAs were typically consumed within 1 h of anaerobic conditions. During the aerobic phase, PHAs were consumed, glycogen was produced, and ammonia was removed, indicating cellular growth. Typically, some ammonia was also removed during the anaerobic phase, although the level of ammonia removal was much higher during the aerobic phase. Dai et al. (15) and Zeng et al. (49) made similar observations, where minor removal of ammonia in the anaerobic phase by cultures enriched in GAOs was detected. Anaerobic nitrogen removal was probably due to adsorption of ammonia to biomass. Thus, ammonia was considered to be aerobically used for growth.
FIG. 1.
(a) Typical pH and redox profiles. (b) PHA, VFA, glycogen, DOC, and ammonia profiles during a cycle of the SBR. l, liter.
The DOC profile showed a decrease corresponding to VFA consumption during the anaerobic phase, remaining almost constant onwards, with a minor consumption of carbon during the aerobic phase (4 to 6% of DOC fed). The remaining DOC was not easily biodegradable either in anaerobic or aerobic conditions. Since no “Candidatus Accumulibacter phosphatis” organisms were detected by FISH (as shown below) and no turnover of phosphate related to polyphosphate accumulation was observed, organic carbon consumption was essentially due to GAO metabolism. SBR biomass concentration was, on average, 6 g of VSS/liter. The stoichiometric and kinetic parameters obtained from the cycle studies are presented in Tables 2 and 3, respectively.
TABLE 2.
Summary of the stoichiometric parametersa obtained for the different cycle studies performed
| Operation time (days) | Results for:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xi | La:Ac:Pr:Bu:Vab | 3HB:3H2MB:3HV: 3H2MV:3HHx | PHAmax (%) | VFAs | ΔNH4+ | Anaerobic phase
|
Aerobic phase
|
|||||
| YPHA/VFA | YPHA/(VFA+Glyc) | YGlyc/VFA | YGlyc/PHA | YX/PHA | ΔY | |||||||
| 81 | 3.6 | 0:44.8:33.1:14.2:7.8 | 25.3:0:74.2:0:0.5 | 14.9 | 11.3 | 0.23 | 2.28 | 0.94 | 1.43 | 0.48 | 0.21 | 0.69 |
| 137 | 5.1 | 9.1:66:8:14.3:2.6 | 38.4:0:59.9:0:1.7 | 17.4 | 20.9 | 1.59 | 2.11 | 0.87 | 1.43 | 0.45 | 0 | 0.45 |
| 198 | 3.6 | 2.8:72.7:5.9:15.6:2.9 | 31.8:10:46:7:5.3 | 15.8 | 15.8 | 0.01 | 2.50 | 0.99 | 1.53 | 0.71 | 0.09 | 0.81 |
| 266 | 3.7 | 1.3:75.6:7.1:13:3.1 | 26.7:6.1:39.5:4.6:23.1 | 13.2 | 11.6 | 0.24 | 2.53 | 0.94 | 1.70 | 0.74 | 0.02 | 0.77 |
| 304 | 4.2 | 0.9:70.3:5.9:21.4:1.5 | 29.5:6.7:30.2:4.8:28.9 | 20.0 | 18.5 | 0.77 | 2.78 | 0.98 | 1.84 | 0.42 | 0.14 | 0.56 |
| 365 | 4.8 | 1.4:65.5:8.4:23.1:1.5 | 25.5:5.6:33.4:4.7:30.8 | 20.8 | 18.6 | 1.31 | 2.89 | 0.90 | 2.21 | 0.50 | 0.03 | 0.53 |
Xi, initial specific biomass in g/liter; La:Ac:Pr:Bu:Va ratio in % mmol/mmol VFAs; 3HB:3H2MB:3HV:3H2MV:3HHx ratio in % mmol; PHAmax in mg PHA/mg TSS; VFAs in C-mmol/liter; ΔNH4+ in N-mmol/liter; YPHA/VFA and YPHA/(VFA+Glyc) in C-mol PHA/C-mol substrate; YGlyc/VFA in C-mol Glyc/C-mol VFA; YGlyc/PHA in C-mol Glyc/C-mol PHA; YX/PHA in C-mol X/C-mol PHA; aerobic ΔY in C-mol/C-mol.
Ac, acetate; Pr, propionate; Bu, butyrate; Va, valerate; La, lactate.
TABLE 3.
Summary of the kinetic parameters (C-mol/C-mol X/h)a obtained for the anaerobic phase of the different cycle studies performed
| Operation time (days) | −qVFA | −qAc | −qBu | −qPr | −qVa | −qGlyc | mGlyc,An | q3HB | q3HV | q3HHx | q3H2MB | q3H2MV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 81 | 0.102 (5, 0.003) | 0.036 (8, 0.001) | 0.024 (6, 0.001) | 0.042 (5, 0.002) | 0.024 (3, 0.001) | 0.096 (5, 0.007) | 0.010 (4, 0.001) | 0.042 (7, 0.002) | ND | 0.001 (5, <0.0001) | ND | ND |
| 137 | 0.108 (7, 0.003) | 0.060 (9, 0.002) | 0.024 (8, 0.001) | 0.042 (4, 0.007) | ND | 0.168 (4, 0.008) | 0.016 (3, 0.0003) | 0.078 (5, 0.004) | 0.120 (6, 0.005) | 0.012 (5, 0.001) | ND | ND |
| 198 | 0.114 (7, 0.006) | 0.060 (8, 0.002) | 0.036 (5, 0.002) | ND | ND | 0.150 (5, 0.007) | 0.013 (3, 0.0002) | 0.090 (5, 0.006) | 0.126 (4, 0.004) | 0.036 (5, 0.004) | 0.006 (5, 0.001) | ND |
| 266 | ND | 0.096 (3, 0.004) | ND | ND | ND | 0.102 (6, 0.014) | 0.013 (3, 0.002) | 0.072 (10, 0.006) | 0.102 (4, 0.004) | 0.090 (4, 0.005) | ND | 0.012 (4, 0.001) |
| 304 | 0.102 (6, 0.004) | 0.048 (9, 0.002) | 0.042 (6, 0.002) | ND | ND | 0.138 (3, 0.002) | 0.010 (4, 0.0004) | 0.060 (9, 0.001) | 0.066 (9, 0.002) | 0.072 (7, 0.002) | 0.012 (8, 0.0004) | 0.006 (6, 0.0005) |
| 365 | 0.174 (4, 0.004) | 0.084 (6, 0.004) | 0.066 (4, 0.005) | ND | ND | 0.144 (5, 0.009) | ND | 0.066 (6, 0.004) | 0.120 (5, 0.006) | 0.036 (6, 0.002) | 0.018 (5, 0.001) | 0.018 (7, 0.001) |
The number of observations followed by the standard deviation of the parameter is in parentheses. ND, not determined.
Some fluctuation was observed in the organic acid composition of the CSTR effluent used as feed for the SBR. However, with one exception (around day 81), these fluctuations could be considered minor (Table 2) and therefore typical for such open mixed-culture acidogenic fermentation. The fermented molasses fed to the SBR during the first cycle study (day 81) was distinctly different in VFA proportions from what was typically observed. Usually, the most-dominant VFA was acetate, followed by butyrate. However, in the cycle study for day 81, propionate was the second-most-dominant VFA. This disturbance was due to a pH control problem for the CSTR, confirming the sensitivity of molasses fermentation to operating conditions (1). Yet, this VFA shift was short-lived and, therefore, should not have affected the GAO culture enrichment.
The composition of PHAs during cycle studies was sensitive to the VFA composition (Table 2). The relatively high proportion of propionate for the cycle study at day 81 led to a higher synthesis of 3HV. At day 137, the feed was of the typical VFA composition, with more butyrate than propionate, and 3HV synthesis was reduced as proposed by Lemos et al. (28). Nevertheless, the fluctuations in VFA composition did not disrupt selection for a GAO-phenotypic biomass. As is evident from the very similar total specific VFA uptake rates at day 81 and day 137, the mixed culture had a robust ability to utilize a feed with variable VFA composition. Evidence for process robustness, such as the capability to handle short-term variations in influent composition, is of practical importance to industrial-scale operation.
Evolution in the composition of the PHAs produced.
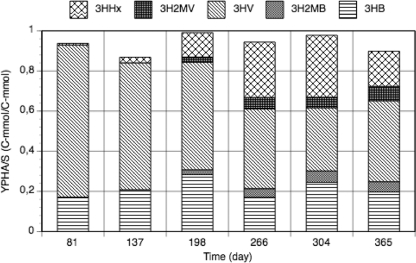
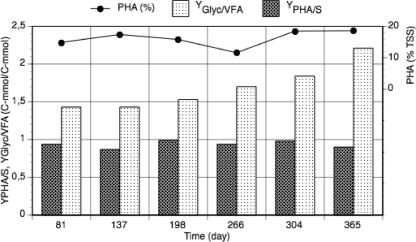
The proportions of PHA monomers between cycle studies revealed an unusual shift in the polymer composition (Table 2). Figure 2 shows this evolution in the PHA monomers. At first, the polymer was constituted mainly of 3HB and 3HV monomers. By day 198, the culture was also producing small amounts of 3-hydroxy-2-methylbutyrate (3H2MB) and 3-hydroxy-2-methylvalerate (3H2MV). These four types of monomers have previously been observed to be produced by GAOs (43). Interestingly, the culture also produced the medium-chain length (MCL) monomer 3HHx in increasing proportions over time, up to 30.8 mol% toward the end of the study.
FIG. 2.
Polymer yield on substrate and evolution of the PHA monomer proportions over reactor operation time.
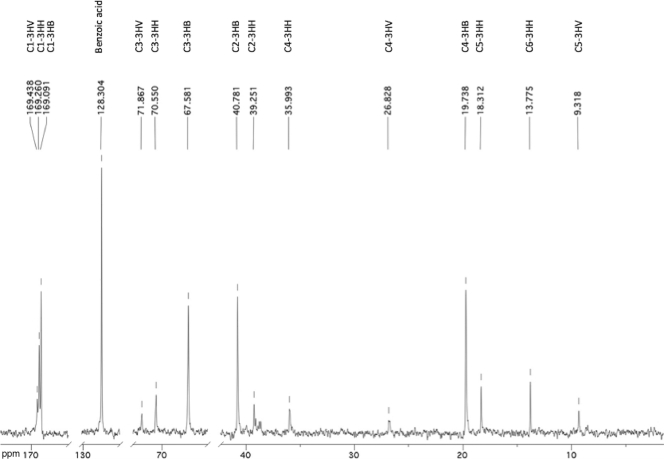
The presence of 3HHx was confirmed with a natural-abundance 13C-NMR analysis of polymer extracted from lyophilized biomass collected at the maximal storage (Fig. 3). All six peaks in this NMR spectrum that could not be assigned to 3HB and 3HV matched the different 3HHx carbon resonances obtained from the literature (24, 25).
FIG. 3.
Natural-abundance 13C-NMR spectra of the polymers extracted from cells collected from the SBR. The peaks were identified by comparing them to results in the literature (24, 25).
The production of 3HHx by the mixed culture was not expected given that it is an MCL monomer. Until now, the synthesis of MCL monomers has only been reported for pure-culture PHA production processes. Typically, production of MCL monomers occurs from structurally related substrates, such as long-chain fatty acids (9). However, synthesis of MCL monomers has also been observed from structurally nonrelated substrates, such as sugars (23, 25, 37) and soy molasses (46). Synthesis of MCL monomers from nonrelated substrates can occur via de novo fatty acid biosynthesis coupled to PHA biosynthesis (41). Copolymerization of short-chain-length (SCL) monomers, such as 3HB, and MCL monomers both from related (10) and nonrelated (25, 37) substrates has been reported. The monomer 3HHx was not observed for a GAO-enriched system using paper mill effluent as feedstock, but this difference may be attributed to a difference in balance among the range of possible organisms presenting the GAO phenotype (6).
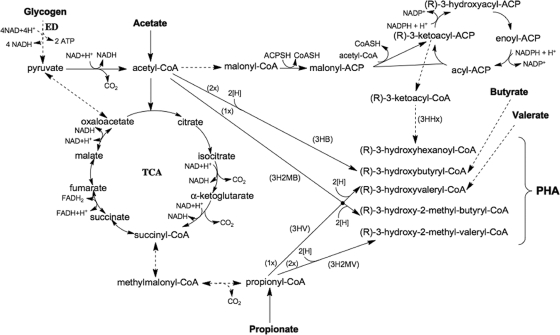
Probable metabolic pathways for the PHA production observed during this study are shown in Fig. 4. In the case of this GAO mixed culture, 3HHx must have been produced from VFA and/or glycogen since no consumption of residual DOC was detected during the anaerobic phase. It is likely that the synthesis of 3HHx monomers occurred via the de novo fatty acid biosynthesis pathway, possibly from intermediates of the glycolytic pathway, such as pyruvate or acetyl-coenzyme A. The use of these intermediates could explain the observed reciprocal decrease in the synthesis of 3HV with the increase of 3HHx synthesis, given that glycogen is the main source of precursors for 3HV synthesis by GAOs under anaerobic conditions with acetate as the predominant substrate (29). The redox balance can also play a role in this metabolism shift. The production of 3HHx through de novo fatty acid biosynthesis leads to a higher consumption of NADH, and less pyruvate needs to be drifted to propionyl-coenzyme A for NADH consumption.
FIG. 4.
Proposed metabolic pathways for synthesis of the different monomers observed in the PHAs produced by GAOs in this study. ED, Entner-Doudoroff pathway; TCA, tricarboxylic acid cycle; CoA, coenzyme A; CoASH, reduced form of CoA; ACP, acyl carrier protein; ACPSH, reduced form of ACP; FADH2, flavin adenine dinucleotide (reduced form).
The synthesis of SCL-MCL PHAs is motivating from the point of view of the potential broadening in the spectrum of biopolymers that may be produced from fermented waste substrates and mixed cultures. MCL copolymers have been shown to exhibit material properties, with respect to lower crystallinity, lower tensile strength, and higher elongation to break, that are distinct from those of PHAs consisting only of SCL monomers (20).
Stoichiometry and kinetics.
Stoichiometric and kinetic parameters derived from the cycle studies are summarized in Tables 2 and 3 and in Fig. 5.
FIG. 5.
Evolution of the PHAs produced per C-mol of total carbon consumed during the anaerobic phase, glycogen consumed per C-mol of VFA consumed during the anaerobic phase, and PHA content at the end of the anaerobic phase of each cycle study.
With respect to glycogen values, it is assumed that changes in biomass glucose content are in close correspondence to glycogen levels. In principle, glucose from other biomass sources could have contributed to the measured changes, but such contributions were expected to be very low compared to the glycogen dynamics from GAOs. The measured glucose levels in the enriched GAO culture were up to 23% of TSS, whereas typical “background” levels of total polysaccharides in microbial biomass are around 5% of dry weight (31). Therefore, it has been established as common practice to use glucose measurements for glycogen levels in GAO-enriched cultures (13, 21, 29, 39, 49) because short-term changes in biomass glucose are dominated by changes in the glycogen storage pool.
The amount of glycogen consumed with respect to VFA consumption during the anaerobic phase increased over the reactor operation period from 1.43 C-mol/C-mol at day 81 to 2.21 C-mol/C-mol at day 365. As a consequence, the amount of PHAs accumulated on VFA also increased from 2.28 C-mol/C-mol to 2.89 C-mol/C-mol. The specific glycogen consumption rate for anaerobic maintenance was constant over the period of operation (Table 3). Therefore, the increase in glycogen utilization for the anaerobic metabolism of PHA synthesis of the culture is likely related to the synthesis of 3HHx. According to available metabolic models for GAOs, the PHA yield on VFA is 1.85 C-mol/C-mol with acetate as the sole carbon source (49) and 1.50 C-mol/C-mol with propionate (39). Generally, PHA yields decrease with increased VFA chain length due to higher efficiency in activation per C-mol. As the activation energy is 1 mol ATP per mol VFA, less glycogen needs to be converted into PHAs per C-mol of VFA to provide energy for propionate than for acetate metabolism (21, 39). For the same reason, yields for butyrate and valerate could be expected to be lower than for propionate (Table 2). The PHA yields observed with the fermented-molasses VFA mixture were higher than the models would predict. Since GAOs convert glycogen to PHAs for maintenance purposes (21), these yields are probably affected by the maintenance requirements. The average specific glycogen consumption rate for maintenance at 30°C (0.013 C-mol/C-mol/h) was around three times higher than has been reported for GAOs at 20°C (0.0041 C-mol/C-mol/h) (21). For these organisms, Lopez-Vasquez et al. (30) showed that the consumption rate is positively affected by temperature.
Over the reactor operation time, the rates of accumulation of 3HHx, 3H2MB, and 3H2MV increased (Table 3). The highest accumulation rate was always obtained for 3HV, indicating that this is a preferable monomer to be synthesized in anaerobic conditions.
The observed total specific VFA uptake rates at pH 7.7 and 30°C were 0.10 to 0.17 C-mol/C-mol X/h. These rates are approximately half of what has previously been observed at pH 7 and a temperature range of 20°C to 35°C with acetate as the sole carbon source (21, 30) but similar to what has been observed for acetate at pH 8 and 20°C (21). The butyrate consumption rate increased over the operational period, probably reflecting a higher affinity for this substrate of D. vanus-related organisms, which became enriched during the period studied (see below).
In general, the kinetic parameters, together with the evolution in proportions of PHA monomers, clearly indicate that there was a slow and continuous change of metabolism. Such drift can be indicative of a possible change in the community toward a higher specialization in PHA accumulation from fermented molasses.
The cycle study PHA storage yields (YPHA/S) show a high efficiency for carbon (VFA plus glycogen) conversion to PHAs (Table 2). At the end of the anaerobic phase, the amount of PHAs stored in the biomass was between 14.9% and 20.8% (g PHA/g TSS) with an average organic load of 15.8 C-mmol VFAs/liter for each cycle (OLR, 47.4 C-mmol/liter/day, and SRT, 10 days) and an average VSS of 6 g/liter. A mixed culture selected under ADF conditions that was also fed with fermented sugar cane molasses reached a maximum PHA content of 10% (g PHA/g TSS) with an average organic load of 30 C-mmol VFAs/liter for each cycle (OLR, 60 C-mmol/liter/day, and SRT, 10 days) and an average VSS of 4.4 g/liter (1). The production of PHAs by GAOs using acetate as the sole carbon source was recently studied by Dai et al. (14). The SBR used for culture selection had an average TSS of 2.5 g/liter, and the organic load at the beginning of each cycle was nominally 6 C-mmol/liter acetate (OLR, 25 C-mmol/liter/day, and SRT, 7 days). These operating conditions resulted in an average PHA content of 14% of dry cell weight. All the above-reported PHA content values are not the maximum capacities for PHAs of each one of the processes but represent only the maximum levels obtained in the enrichment reactors.
The yield of active biomass over VFA consumed in the SBR for the cycle studies was, on average, 0.39 C-mol/C-mol (Table 2). This value is close to the 0.37 C-mol/C-mol that has been suggested by a metabolic model for GAOs utilizing acetate as the sole carbon source (49). The active biomass yield calculated in the same way from the data presented by Albuquerque et al. (1) for the ADF culture enriched with fermented molasses was 0.32 C-mol/C-mol (assuming that the active biomass concentration was equal to the VSS concentration at the end of the cycle).
Usually, maximum PHA storage is obtained under conditions different from those used for selection of a PHA-producing mixed culture. Most of the time, PHAs are accumulated to significant levels in the wasted biomass in batch-fed reactors (45). Evaluation of the overall PHA productivity and economy in polymer recovery requires that the maximum potential biomass PHA content be established. Maximum content can be estimated from batch-fed accumulation experiments. However, optimum accumulation may depend on the structure of the enrichment community and its stability and capacity for PHA storage. In addition, environmental conditions may influence the accumulation in order to reach this maximum PHA capacity. Optimization of PHA accumulation for the GAO enrichment mixed culture is part of ongoing investigations.
Microbial community characterization.
The observed long-term evolution in PHA monomer composition could have been caused by a metabolic adaptation by the culture, a drift in microbial community composition, or both. In order to clarify this aspect of the process dynamics, the culture was examined using morphological characterization and FISH designed for organisms known to have the GAO phenotype.
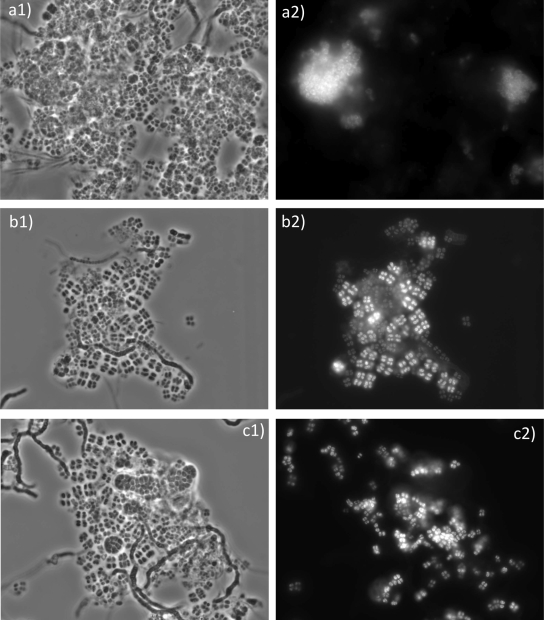
The SBR bacterial community was mainly composed of four different morphological types: bacteria organized in groups of four (tetrads), coccus-shaped bacteria, thin filamentous bacteria, and filamentous bacteria resembling a bead string (Fig. 6). The use of Nile blue staining in fresh samples taken from the SBR near the end of the anaerobic phase, at maximum PHA level, revealed the presence of PHA granules inside the different bacteria types, except for the thin filaments.
FIG. 6.
Morphologies of bacteria present in the GAO mixed culture. (a1, b1, and c1) Phase-contrast images. (a2) Overlay of results for EUBmix with those for the specific probe GAOmix. (b2) Overlay of results for EUBmix with those for the specific probe TFOmix. (c2) Overlay of results for EUBmix with those for the specific probe DFmix. Magnification, ×1,000. Results with for EUBmix are in green, and those for specific probes in red.
Cells were collected periodically for FISH analysis. From the several observations, two samples of the SBR bacterial community were characterized in more detail: one taken at day 98, in the early phase of operation, and one from the end of operation. Observations were performed with epifluorescence microscopy using EUBmix in fluorescein isothiocyanate together with specific probes with Cy3 labeling.
At day 98, organisms binding to the GAOmix probes, hence “Candidatus Competibacter phosphatis,” were the most abundant. In accordance with previous observations, these bacteria exhibited the coccus morphology (11). In addition, two types of TFOs binding to TFOmix and DFmix, hence belonging to cluster 1 (48) and cluster 2 (35) of organisms related to Defluviicoccus vanus, were detected. These organisms were considerably less abundant than “Ca. Competibacter.” All of these bacteria are GAOs and are known to occur frequently in anaerobic-aerobic activated sludge systems at laboratory scale with synthetic substrates, as well as in full-scale municipal wastewater treatment plants (38).
Characterization of the sample taken at the end of operation revealed a clear shift in the microbial community. At this time, there was a large predominance of the tetrad-forming-organism morphology, and these organisms hybridized with the TFOmix and DFmix probes, besides the general group-specific probe ALF969 that targets Alphaproteobacteria. In total, the cells binding to TFOmix and DFmix now corresponded to no less than 70% of all organisms present. Both groups are represented in Fig. 6b and c, where it is clear that, although both groups of organisms are arranged in tetrads, the ones that hybridized with TFOmix are larger, allowing for a distinction by morphology.
Large, coccus-shaped organisms hybridizing with GAOmix were still present in the biomass for the last sample (Fig. 6a) but at a lower abundance than on day 98. These cells also bound the group-specific probe for Gammaproteobacteria, GAM42a. Some small, rod-shaped bacteria hybridized with the BET42a probe that targets Betaproteobacteria. These bacteria were only detected in small numbers. No hybridization was observed with PAOmix, for “Candidatus Accumulibacter phosphatis,” or with AMAR839, confirming the absence of polyphosphate-accumulating organisms and Amaricoccus sp.
Hence, according to the FISH analysis, there was a clear drift in microbial community composition with time. Although the culture was highly enriched in GAOs at both sampling occasions, the community composition with respect to different types of GAOs was significantly changed. A very low production of 3HHx at the beginning of the reactor operational period occurred in the presence of both types of Defluviicoccus vanus-related GAOs. Hence, it appears that production of 3HHx is related to the abundance of these organisms, given that their enrichment occurred alongside an increase in the production of 3HHx. Even though the community shift is not conclusively linked with levels of 3HHx, the time scales involved make metabolic adaption a less likely explanation.
In this study, an open mixed culture was enriched in GAOs with fermented molasses as the carbon source in a SBR for 1 year of continuous operation at laboratory scale. The phenotypic behavior of the culture suggested a very stable GAO enrichment. However, the composition of PHAs produced from the fermented molasses drifted in time during the reactor's operation from PHAs rich in 3HB and 3HV to PHAs also containing up to 31 mol% of the MCL monomer 3HHx alongside minor amounts of 3H2MB and 3H2MV. The production of 3HHx suggests that open mixed cultures can be versatile with respect to monomer synthesis, including both SCL and MCL monomers, with a fermented agroindustrial waste as substrate. In parallel with the observed evolution in PHA composition, FISH indicated a long-term shift in the community of GAO species from domination by “Candidatus Competibacter phosphatis” to domination by Defluviicoccus vanus-related organisms. This ecological drift is a possible cause for the alteration in PHA monomer composition.
Acknowledgments
We acknowledge financial support by European Integrated Project no. 026515-2, Bioproduction—Sustainable Microbial and Biocatalytic Production of Advanced Functional Materials, and by Fundação para a Ciência e a Tecnologia project PPCDT/AMB/56075/2004.
We also acknowledge Maria Albuquerque for the operation of the acidogenic reactor.
Footnotes
Published ahead of print on 22 May 2009.
REFERENCES
- 1.Albuquerque, M. G. E., M. Eiroa, C. Torres, B. R. Nunes, and M. A. M. Reis. 2007. Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses. J. Biotechnol. 130:411-421. [DOI] [PubMed] [Google Scholar]
- 2.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann, R. I. 1995. In situ identification of microorganisms by whole cell hybridization with rRNA-targeted nucleic acid probes, MMEM-3.3.6/1-MMEM-3.3.6/15. In A. Akkermans, J. van Elsas, and F. Bruijn (ed.), Molecular microbiology ecology manual. Kluwer Academic Publications, London, United Kingdom.
- 4.APHA. 1995. Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC.
- 5.Beer, M., Y. H. Kong, and R. J. Seviour. 2004. Are some putative glycogen-accumulating organisms (GAO) in anaerobic:aerobic activated sludge systems members of the α-Proteobacteria? Microbiology 150:2267-2275. [DOI] [PubMed] [Google Scholar]
- 6.Bengtsson, S., A. Werker, and T. Welander. 2008. Production of polyhydroxyalkanoates by glycogen-accumulating organisms treating a paper mill wastewater. Water Sci. Technol. 58:323-330. [DOI] [PubMed] [Google Scholar]
- 7.Bengtsson, S., A. Werker, M. Christensson, and T. Welander. 2008. Production of polyhydroxyalkanoates by activated sludge treating a paper mill wastewater. Bioresour. Technol. 99:509-516. [DOI] [PubMed] [Google Scholar]
- 8.Braunegg, G., G. Lefebvre, and K. F. Genser. 1998. Polyhydroxyalkanoates, biopolyesters from renewable resources: physiological and engineering aspects. J. Biotechnol. 65:127-161. [DOI] [PubMed] [Google Scholar]
- 9.Chen, G. Q., G. Zhang, S. J. Park, and S. Y. Lee. 2001. Industrial scale production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Appl. Microbiol. Biotechnol. 57:50-55. [DOI] [PubMed] [Google Scholar]
- 10.Choi, J., and S. Lee. 1997. Process analysis and economic evaluation for poly(3-hydroxybutyrate) production by fermentation. Bioprocess Eng. 17:335-342. [Google Scholar]
- 11.Crocetti, G. R., J. F. Banfield, J. Keller, P. L. Bond, and L. L. Blackall. 2002. Glycogen-accumulating organisms in laboratory-scale and full scale wastewater treatment process. Microbiology 148:3353-3364. [DOI] [PubMed] [Google Scholar]
- 12.Crocetti, G. R., P. Hugenholtz, P. L. Bond, A. Schuler, J. Keller, D. Jenkins, and L. L. Blackall. 2000. Identification of polyphosphate-accumulating organisms and design of 16S rRNA-directed probes for their detection and quantitation. Appl. Environ. Microbiol. 66:1175-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai, Y., L. Lambert, Z. Yuan, and J. Keller. 2008. Characterisation of polyhydroxyalkanoate copolymers with controllable four-monomer composition. J. Biotechnol. 134:137-145. [DOI] [PubMed] [Google Scholar]
- 14.Dai, Y., Z. Yuan, K. Jack, and J. Keller. 2007. Production of targeted poly(3-hydroxyalkanoates) copolymers by glycogen-accumulating organisms using acetate as sole carbon source. J. Biotechnol. 129:489-497. [DOI] [PubMed] [Google Scholar]
- 15.Dai, Y., Z. Yuan, X. Wang, A. Oehmen, and J. Keller. 2007. Anaerobic metabolism of Defluviicoccus vanus related glycogen-accumulating organisms (GAOs) with acetate and propionate as carbon sources. Water Res. 41:1885-1896. [DOI] [PubMed] [Google Scholar]
- 16.Daims, H., A. Brühl, R. Amann, K.-H. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB 338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 17.Dias, J. M. L., P. C. Lemos, L. S. Serafim, C. Oliveira, M. Eiroa, M. G. E. Albuquerque, A. M. Ramos, R. Oliveira, and M. A. M. Reis. 2006. Recent advances in polyhydroxyalkanoate production by mixed aerobic cultures: from the substrate to the final product. Macromol. Biosci. 6:885-906. [DOI] [PubMed] [Google Scholar]
- 18.Dionisi, D., G. Carucci, M. P. Papini, C. Riccardi, M. Majone, and F. Carrasco. 2005. Olive oil mill effluents as a feedstock for production of biodegradable polymers. Water Res. 39:2076-2084. [DOI] [PubMed] [Google Scholar]
- 19.Dionisi, D., M. Majone, V. Papa, and M. Beccari. 2004. Biodegradable polymers from organic acids by using activated sludge enriched by aerobic periodic feeding. Biotechnol. Bioeng. 85:569-579. [DOI] [PubMed] [Google Scholar]
- 20.Doi, Y., S. Kitamura, and H. Abe. 1995. Microbial synthesis and characterisation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 28:4822-4828. [Google Scholar]
- 21.Filipe, C., G. Daigger, and C. Grady. 2001. A metabolic model for acetate uptake under anaerobic conditions by glycogen-accumulating organisms: stoichiometry, kinetics, and the effect of pH. Biotechnol. Bioeng. 76:17-31. [DOI] [PubMed] [Google Scholar]
- 22.Henze, M., P. Harremoës, J. J. LaCour, and E. Arvin. 1995. Wastewater treatment: biological and chemical processes. Springer, Heidelberg, Germany.
- 23.Huijberts, G. N. M., G. Eggink, P. Dewaard, G. W. Huisman, and B. Witholt. 1992. Pseudomonas putida Kt2442 cultivated on glucose accumulates poly(3-hydroxyalkanoates) consisting of saturated and unsaturated monomers. Appl. Environ. Microbiol. 58:536-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huijberts, G. N. M., T. C. Rijk, P. Waard, and G. Eggink. 1994. 13C Nuclear magnetic resonance studies of Pseudomonas putida fatty acid metabolic routes involved in poly(3-hydroxyalkanoate) synthesis. J. Bacteriol. 176:1661-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato, M., H. J. Bao, C.-K. Kang, T. Fukui, and Y. Doi. 1996. Production of a novel copolyester of 3-hydroxybutyric acid and medium-chain-length 3-hydroxyalkanoic acids by Pseudomonas sp. 61-3 from sugars. Appl. Microbiol. Biotechnol. 45:363-370. [Google Scholar]
- 26.Kong, Y. H., S. L. Ong, W. J. Ng, and W. T. Liu. 2002. Diversity and distribution of a deeply branched novel proteobacterial group found in anaerobic-aerobic activated sludge processes. Environ. Microbiol. 4:753-757. [DOI] [PubMed] [Google Scholar]
- 27.Lee, S. Y. 1996. Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 49:1-14. [DOI] [PubMed] [Google Scholar]
- 28.Lemos, P. C., L. S. Serafim, and M. A. M. Reis. 2006. Synthesis of polyhydroxyalkanoates from different short-chain fatty acids by mixed cultures submitted to aerobic dynamic feeding. J. Biotechnol. 122:226-238. [DOI] [PubMed] [Google Scholar]
- 29.Lemos, P. C., Y. Dai, Z. Yuan, J. Keller, and M. A. M. Reis. 2007. Elucidation of metabolic pathways in glycogen-accumulating organisms with in vivo 13C nuclear magnetic resonance. Environ. Microbiol. 9:2694-2706. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Vazquez, C. M., Y.-I. Song, C. M. Hooijmans, D. Brdjanovic, M. S. Moussa, H. J. Gijzen, and M. M. C. van Loosdrecht. 2007. Short-term temperature effects on the anaerobic metabolism of glycogen-accumulating organisms. Biotechnol. Bioeng. 97:483-495. [DOI] [PubMed] [Google Scholar]
- 31.Madigan, M. T., and M. Martinko. 2005. Brock biology of microorganisms. Prentice-Hall, Upper Saddle River, NJ.
- 32.Majone, M., P. Masanisso, A. Carucci, K. Lindrea, and V. Tandoi. 1996. Influence of storage on kinetic selection to control aerobic filamentous bulking. Water Sci. Technol. 34:223-232. [Google Scholar]
- 33.Manz, W., R. Amann, W. Ludwig, M. Wagner, and K.-H. Scheifer. 1992. Phylogenetic oligonucleotide probes for the major subclasses of Proteobacteria: problems and solutions. Syst. Appl. Microbiol. 15:593-600. [Google Scholar]
- 34.Maszenan, A. M., R. J. Seviour, B. K. C. Patel, and J. Wanner. 2000. A fluorescently-labelled r-RNA targeted oligonucleotide probe for the in situ detection of G-bacteria of the genus Amaricoccus in activated sludge. J. Appl. Microbiol. 88:826-835. [DOI] [PubMed] [Google Scholar]
- 35.Meyer, R. L., A. M. Saunders, and L. L. Blackall. 2006. Putative glycogen-accumulating organisms belonging to the Alphaproteobacteria identified through rRNA-based stable isotope probing. Microbiology 152:419-429. [DOI] [PubMed] [Google Scholar]
- 36.Mino, T., W. T. Liu, F. Kurisu, and T. Matsuo. 1995. Modelling glycogen storage and denitrification capability of microorganisms in enhanced biological phosphate removal processes. Water Sci. Technol. 31:25-34. [Google Scholar]
- 37.Nomura, C. T., K. Taguchi, Z. Gan, K. Kuwabara, T. Tanaka, K. Takase, and Y. Doi. 2005. Expression of 3-ketoacyl-acyl carrier protein reductase (fabG) genes enhances production of polyhydroxyalkanoate copolymer from glucose in recombinant Escherichia coli JM109. Appl. Environ. Microbiol. 71(8):4297-4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oehmen, A., P. C. Lemos, G. Carvalho, Z. Yuan, J. Keller, L. L. Blackall, and M. A. M. Reis. 2007. Advances in enhanced biological phosphorus removal: from micro to macro scale. Water Res. 41:2271-2300. [DOI] [PubMed] [Google Scholar]
- 39.Oehmen, A., R. J. Zeng, M. A. Saunders, L. L. Blackall, J. Keller, and Z. Yuan. 2006. Anaerobic and aerobic metabolism of glycogen-accumulating organisms selected with propionate as the sole carbon source. Microbiology 152:2767-2778. [DOI] [PubMed] [Google Scholar]
- 40.Olsson, L., and J. Nielsen. 1997. On-line and in situ monitoring of biomass in submerged cultivations. Trends Biotechnol. 15:517-522. [Google Scholar]
- 41.Rehm, B. H. A., N. Kruger, and A. Steinbüchel. 1998. A new metabolic link between fatty acid de novo synthesis and polyhydroxyalkanoic acid synthesis: the phaG gene from Pseudomonas putida KT2440 encodes a 3-hydroxyacyl-acyl carrier protein coenzyme A transferase. J. Biol. Chem. 273:24044-24051. [DOI] [PubMed] [Google Scholar]
- 42.Reis, M. A. M., L. S. Serafim, P. C. Lemos, A. M. Ramos, F. R. Aguiar, and M. C. M. van Loosdrecht. 2003. Production of polyhydroxyalkanoates by mixed microbial cultures. Bioprocess Biosyst. Eng. 25:377-385. [DOI] [PubMed] [Google Scholar]
- 43.Satoh, H., T. Mino, and T. Matsuo. 1994. Deterioration of enhanced biological phosphorus removal by the domination of microorganisms without polyphosphate accumulation. Water Sci. Technol. 30:203-211. [Google Scholar]
- 44.Serafim, L. S., P. C. Lemos, R. Oliveira, and M. A. M. Reis. 2004. Optimization of polyhydroxybutyrate production submitted to aerobic dynamic feeding conditions. Biotechnol. Bioeng. 87:145-160. [DOI] [PubMed] [Google Scholar]
- 45.Serafim, L. S., P. C. Lemos, M. G. E. Albuquerque, and M. A. M. Reis. 2008. Strategies for PHA production by mixed cultures and renewable waste materials. Appl. Microb. Biotech. 81:615-628. [DOI] [PubMed] [Google Scholar]
- 46.Solaiman, D. K. Y., R. D. Ashby, A. T. Hotchkiss, Jr., and T. A. Foglia. 2006. Biosynthesis of medium-chain-length poly(hydroxyalkanoates) from soy molasses. Biotechnol. Lett. 28:157-162. [DOI] [PubMed] [Google Scholar]
- 47.Tsai, C. S., and W. T. Liu. 2002. Phylogenetic and physiological diversity of tetrad-forming organisms in deteriorated biological phosphorus removal systems. Water Sci. Technol. 46:179-184. [PubMed] [Google Scholar]
- 48.Wong, M.-T., F. M. Tan, W. J. Ng, and W. T. Liu. 2004. Identification and occurrence of tetrad-forming Alphaproteopbacteria in anaerobic-aerobic activated sludge processes. Microbiology 150:3741-3748. [DOI] [PubMed] [Google Scholar]
- 49.Zeng, R. J., M. C. M. van Loosdrecht, Z. Yuan, and J. Keller. 2003. Metabolic model for glycogen-accumulating organisms in anaerobic/aerobic activated sludge systems. Biotechnol. Bioeng. 81:92-105. [DOI] [PubMed] [Google Scholar]