Abstract
The uncharacterized gene previously proposed as a mannose-6-phosphate isomerase from Bacillus subtilis was cloned and expressed in Escherichia coli. The maximal activity of the recombinant enzyme was observed at pH 7.5 and 40°C in the presence of 0.5 mM Co2+. The isomerization activity was specific for aldose substrates possessing hydroxyl groups oriented in the same direction at the C-2 and C-3 positions, such as the d and l forms of ribose, lyxose, talose, mannose, and allose. The enzyme exhibited the highest activity for l-ribulose among all pentoses and hexoses. Thus, l-ribose, as a potential starting material for many l-nucleoside-based pharmaceutical compounds, was produced at 213 g/liter from 300-g/liter l-ribulose by mannose-6-phosphate isomerase at 40°C for 3 h, with a conversion yield of 71% and a volumetric productivity of 71 g liter−1 h−1.
l-Ribose is a potential starting material for the synthesis of many l-nucleoside-based pharmaceutical compounds, and it is not abundant in nature (5, 19). l-Ribose has been produced mainly by chemical synthesis from l-arabinose, l-xylose, d-glucose, d-galactose, d-ribose, or d-mannono-1,4-lactone (2, 17, 23). Biological l-ribose manufacture has been investigated using ribitol or l-ribulose. Recently, l-ribose was produced from ribitol by a recombinant Escherichia coli containing an NAD-dependent mannitol-1-dehydrogenase (MDH) with a 55% conversion yield when 100 g/liter ribitol was used in a 72-h fermentation (18). However, the volumetric productivity of l-ribose in the fermentation is 28-fold lower than that of the chemical method synthesized from l-arabinose (8). l-Ribulose has been biochemically converted from l-ribose using an l-ribose isomerase from an Acinetobacter sp. (9), an l-arabinose isomerase mutant from Escherichia coli (4), a d-xylose isomerase mutant from Actinoplanes missouriensis (14), and a d-lyxose isomerase from Cohnella laeviribosi (3), indicating that l-ribose can be produced from l-ribulose by these enzymes. However, the enzymatic production of l-ribulose is slow, and the enzymatic production of l-ribose from l-ribulose has been not reported.
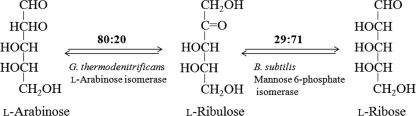
Sugar phosphate isomerases, such as ribose-5-phosphate isomerase, glucose-6-phosphate isomerase, and galactose-6-phosphate isomerase, work as general aldose-ketose isomerases and are useful tools for producing rare sugars, because they convert the substrate sugar phosphates and the substrate sugars without phosphate to have a similar configuration (11, 12, 21, 22). l-Ribose isomerase from an Acinetobacter sp. (9) and d-lyxose isomerase from C. laeviribosi (3) had activity with l-ribose, d-lyxose, and d-mannose. Thus, we can apply mannose-6-phosphate (EC 5.3.1.8) isomerase to the production of l-ribose, because there are no sugar phosphate isomerases relating to l-ribose and d-lyxose. The production of the expensive sugar l-ribose (bulk price, $1,000/kg) from the rare sugar l-ribulose by mannose-6-phosphate isomerase may prove to be a valuable industrial process, because we have produced l-ribulose from the cheap sugar l-arabinose (bulk price, $50/kg) using the l-arabinose isomerase from Geobacillus thermodenitrificans (20) (Fig. 1).
FIG. 1.
Schematic representation for the production of l-ribulose from l-arabinose by G. thermodenitrificans l-arabinose isomerase and the production of l-ribose from l-ribulose by B. subtilis mannose-6-phosphate isomerase.
In this study, the gene encoding mannose-6-phosphate isomerase from Bacillus subtilis was cloned and expressed in E. coli. The substrate specificity of the recombinant enzyme for various aldoses and ketoses was investigated, and l-ribulose exhibited the highest activity among all pentoses and hexoses. Therefore, mannose-6-phosphate isomerase was applied to the production of l-ribose from l-ribulose.
MATERIALS AND METHODS
Microorganisms, plasmid, medium, and culture conditions.
B. subtilis ATCC 23857, E. coli ER2566, and plasmid pET-28a(+) (Novagen, Darmstadt, Germany) were used as the sources of genomic DNA of mannose-6-phosphate isomerase, as host cells, and as the expression vector, respectively. The recombinant E. coli cells for the expression of the enzyme were cultivated in 500 ml of Luria-Bertani medium in a 2,000-ml flask containing 20 μg/ml of kanamycin at 37°C with shaking at 250 rpm. When the optical density of bacteria reached 0.6 at 600 nm, isopropyl-β-d-thiogalactopyranoside was added to a final concentration of 0.1 mM to induce mannose-6-phosphate isomerase expression, and the culture was incubated with shaking at 150 rpm at 16°C for 16 h.
Gene cloning.
The gene (945 bp) encoding mannose-6-phosphate isomerase was obtained using the genomic DNA isolated from B. subtilis ATCC 23857. The sequence of the primers used for gene cloning was based on the DNA sequence of the mannose-6-phosphate isomerase from B. subtilis HB002 (GenBank accession number AF324506). Forward (5-TTTCATATGACGCATCCTTTATT) and reverse (5-TTTCTCGAGTTAAGGATGAGATATCA) primers were designed to introduce the underlined NdeI and XhoI restriction sites. The amplified DNA fragment obtained by PCR was cloned into the pGEM-T Easy vector (Promega, Madison, WI). The NdeI-XhoI fragment from T vector containing the gene encoding mannose-6-phosphate isomerase was subcloned into the same sites of pET-28a(+) and transformed into E. coli ER2566.
Purification of mannose-6-phosphate isomerase.
The washed recombinant cells were resuspended in 50 mM phosphate buffer containing 300 mM NaCl, 10 mM imidazole, and 0.1 mM phenylmethylsulfonyl fluoride as a protease inhibitor. The resuspended cells were disrupted by ultrasonication (Fisher Scientific, Pittsburgh, PA). The cell debris was removed by centrifugation at 13,000 × g for 20 min at 4°C, and the supernatant was filtered through a 0.45-μm filter. The filtrate was applied to a HisTrap HP chromatography column (Amersham Biosciences, Uppsala, Sweden) equilibrated with the phosphate buffer. The column was washed extensively with the same buffer, and the bound protein was eluted with a linear gradient from 10 to 250 mM imidazole at a flow rate of 1 ml/min. The active fractions were collected and dialyzed against 50 mM N-(2-hyroxyethyl)piperazine-N′-(3-propanesulfonic acid) (EPPS) buffer (pH 7.5). After dialysis, the resulting solution was used as the purified enzyme. The purification step using the column was carried out in a cold room at 4°C with a fast protein liquid chromatography system (Bio-Rad Laboratories, Hercules, CA).
Molecular mass determination.
The subunit molecular mass of mannose-6-phosphate isomerase was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. To determine the molecular mass of the native enzyme, purified enzyme was applied to a Sephacryl S-300 HR 16/60 gel filtration chromatograph (Amersham Biosciences) and eluted with 50 mM Tris-HCl (pH 7.5) buffer containing 150 mM NaCl at a flow rate of 1 ml/min. The column was calibrated with aldolase (158 kDa), albumin (67 kDa), chymotrypsinogen A (25 kDa), and RNase (13.7 kDa) as reference proteins (Amersham Biosciences).
Enzyme assay.
Mannose-6-phosphate isomerase was used without EDTA treatment. Unless otherwise stated, the reaction was performed at 40°C in 50 mM EPPS buffer (pH 7.5) containing 10 mM l-ribose, 1.5 U/ml of enzyme, and 0.5 mM Co2+ for 20 min. One unit of mannose-6-phosphate isomerase activity was defined as the amount of enzyme required to produce 1 nmol of l-ribulose per min at 40°C and pH 7.5. The specific activity was defined as the increased amount of aldose or ketose as a product per the enzyme amount per the reaction time.
Effects of pH and temperature on enzyme activity.
To examine the effects of pH and temperature on the activity of mannose-6-phosphate isomerase, the pH was varied between 6.5 and 8.5 using 50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer (pH 6.5 to 7.5) and 50 mM EPPS buffer (pH 7.5 to 8.5), and the temperature was varied from 20 to 50°C. The experimental enzyme deactivation data were fitted to a first-order curve, and the half-life of the enzyme was calculated using SigmaPlot 10.0 software (Systat Software, San Jose, CA). The influence of temperature on enzyme stability was monitored at temperatures from 25 to 50°C at pH 7.5.
Analytical methods.
The metal ion-treated enzyme of 12 mg/ml was prepared by adding 0.5 mM Co2+ or Zn2+ to the purified enzyme, followed by overnight dialysis at 4°C against distilled water. The concentrations of Co2+ and Zn2+ for the enzymes were analyzed by inductively coupled plasma mass spectrometry (Elan 6100; Perkin Elmer, Norwalk, CT). The concentrations of monosaccharides were determined by a Bio-LC system (Dionex ICS-3000, Sunnylvale, CA) with an electrochemical detector using a CarboPac PA I column. The column was eluted at 30°C with 200 mM sodium hydroxide at a flow rate of 1 ml/min. The concentrations of mannose-6-phosphate were determined by the same system. The column was eluted at 30°C with an Na-acetate gradient of 75 mM NaOH and 75 mM NaOH-500 mM Na-acetate. The gradient was increased to 100 mM between 0 to 35 min, to 150 mM between 35 and 38 min, to 350 mM between 38 and 65 min, and then to 500 mM for 75 min (7). The flow rate was 1 ml/min.
RESULTS AND DISCUSSION
Amino acid sequence alignment of B. subtilis mannose-6-phosphate isomerase.
A search using the active-site motif (LSVQVHPDD) of mannose-6-phosphate isomerase from B. subtilis ATCC 23857 was performed, and the enzyme exhibited 59, 57, 54, 55, 56, 25.5, and 27.5% amino acid sequence similarities to proposed mannose-6-phosphate isomerases from B. amyloliquefaciens, B. halodurans, Geobacillus kaustophilus, Paenibacillus sp. strain JDR-2, Listeria monocytogenes, Thermotoga maritima, and Thermus thermophilus, respectively.
Previously cloned l-ribose-converting enzymes, such as the l-ribose isomerase from Acinetobacter sp. (9), l-arabinose isomerase mutant from E. coli (4), d-xylose isomerase mutants from A. missouriensis (14), and d-lyxose isomerase from C. laeviribosi (3) exhibit no extensive homology with mannose-6-phosphate isomerase, which is genetically different from other l-ribose-converting enzymes.
Molecular mass determination.
The mannose-6-phosphate isomerase was purified with a final purification of 27-fold, a yield of 33%, and a specific activity of 22.5 U/mg. The subunit molecular mass of the purified mannose-6-phosphate isomerase by sodium dodecyl sulfate-polyacrylamide gel electrophoresis was approximately 36.5 kDa (data not shown), which was consistent with the calculated value of 36,444 Da based on the 315 amino acid residues and 6 histidine residues. The molecular masses of other proposed mannose-6-phosphate isomerases from B. halodurans, Candida albicans, Salmonella enterica, Homo sapiens, and Thermus thermophilus were 35,521, 48,867, 42,591, 46,656, and 28,102 Da, respectively. The molecular mass of the native enzyme was estimated using a Sephacryl S-300 HR 16/60 gel filtration chromatograph to be 36.6 kDa as a monomer (data not shown).
Effects of metal ions, pH, and temperature.
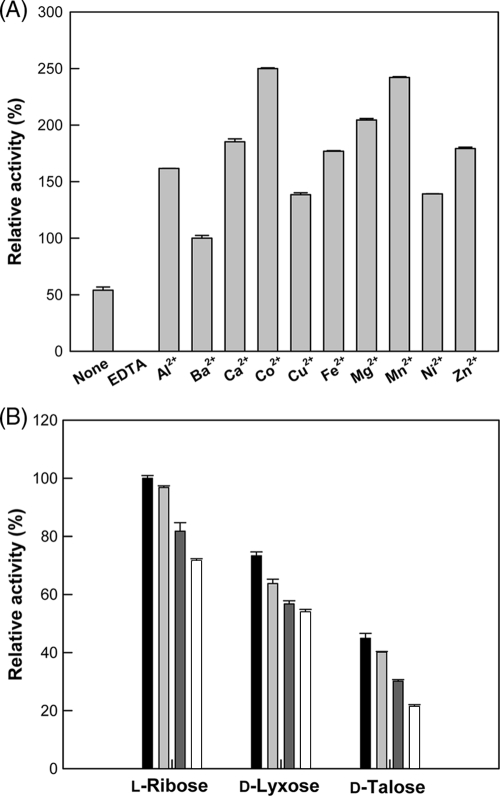
Typically, phosphate sugar isomerases are metal independent (11, 12), but mannose-6-phosphate isomerases are metalloenzymes that require a divalent ion metal cofactor, like a zinc ion, for activity and catalysis (6, 13, 16). The effect of metal ions on enzyme activity was investigated. The purified enzyme showed 54% activity relative to that of Ba2+, whereas the enzyme after the removal of metal ions by treatment with EDTA displayed no activity (Fig. 2A). Among the metal ions tested, Co2+ was the most effective metal ion for l-ribose isomerization by B. subtilis mannose-6-phosphate isomerase, and its optimal concentration was 0.5 mM (data not shown). The affinity of Co2+, Mn2+, Mg2+, or Zn2+ for a specific sugar, such as l-ribose, d-lyxose, or d-talose, was investigated (Fig. 2B). The results showed that the order of isomerization activity for the metal ions tested was the same regardless of the kind of sugar, and that the isomerization activity was highest in the presence of Co2+ among the sugars tested. Thus, all subsequent experiments were performed in the presence of 0.5 mM Co2+ as a cofactor.
FIG. 2.
(A) Effect of metal ions on the activity of B. subtilis mannose-6-phosphate isomerase. The reactions were performed in 50 mM EPPS buffer (pH 7.5) containing 10 mM l-ribose, 1.5 U/ml of enzyme, and 0.5 mM of each metal ion at 40°C for 20 min. (B) Effect of metal ions on activity of B. subtilis mannose-6-phosphate isomerase with different substrates. The reactions were performed in 50 mM EPPS buffer (pH 7.5) containing 10 mM sugar such as l-ribose, d-lyxose, or d-talose, 1.5 U/ml of enzyme, and 0.5 mM metal ion such as Co2+ (▪), Mn2+ (), Mg2+ (), or Zn2+ (□) at 40°C for 20 min. Data represent the means from three experiments, and error bars represent standard deviations. Data represent the means from three separate experiments.
Inductively coupled plasma mass spectrometry analysis demonstrated that the concentration of Zn2+ (9,280 parts per billion [ppb]) in the purified enzyme (12 mg/ml) was 232-fold higher than that of Co2+ (40 ppb). The metal ions were not removed by dialysis, suggesting that they were tightly bound to the enzyme. After 0.5 mM Zn2+ or Co2+ was added to the enzyme and followed by dialysis, the concentration of Zn2+ or Co2+ in the metal ion-treated enzyme (12 mg/ml) was increased to 51,440 or 17,870 ppb, respectively. The increased concentration of Zn2+ (42,160 ppb) in the Zn2+-treated enzyme was 2.4-fold higher than that of Co2+ (17,830 ppb) in the Co2+-treated enzyme. These data indicated that Zn2+ is an effective metal ion for mannose-6-phosphate isomerase, and that Co2+ also can be bound to the enzyme. Although B. subtilis mannose-6-phosphate isomerase typically contained a zinc ion, the enzyme had the highest activity in the presence of Co2+. Similar results for the highest activity in the presence of Co2+ also were reported for the mannose-6-phosphate isomerases from Xanthomonas campestris and P. aeruginosa (10, 15).
The effects of pH and temperature on enzyme activity were investigated, and maximum activity was observed at pH 7.5 and 40°C. Mannose-6-phosphate isomerase displayed first-order kinetics for thermal inactivation, and the half-lives of the enzyme at 25, 30, 35, 40, 45, and 50°C were 461, 325, 236, 111, 56, and 10 h, respectively. Since the thermal stability of this enzyme is not particularly good, the use of mannose-6-phosphate isomerases from thermophilic organisms such as G. kaustophilus, Thermotoga maritima, and Thermus thermophilus may be more effective for industrial applications.
Substrate specificity of B. subtilis mannose-6-phosphate isomerase.
The specific activity of B. subtilis mannose-6-phosphate isomerase was investigated with all pentoses and hexoses. Among the aldose substrates, the specific activity was the highest for l-ribose, followed by d-lyxose, d-talose, d-mannose, l-allose, d-ribose, l-talose, d-allose, l-lyxose, and l-mannose (Table 1). Among the ketose substrates, the highest specific activity was observed with l-ribulose, followed by d-xylulose, d-ribulose, l-xylulose, d-tagatose, d-fructose, l-psicose, l-fructose, d-psicose, and l-tagatose. These results suggested that mannose-6-phosphate isomerase was a potential l-ribose producer.
TABLE 1.
Specific activity and equilibrium ratio of B. subtilis mannose 6-phosphate isomerase for monosaccharidesa
| Substrate | Product | Sp actb (nmol min−1 mg−1) | Equilibrium ratioc (aldose:ketose, %) |
|---|---|---|---|
| Aldose | |||
| l-Ribose | l-Ribulose | 22.5 ± 0.1 | 71:29 |
| d-Lyxose | d-Xylulose | 16.5 ± 1.5 | 60:40 |
| d-Talose | d-Tagatose | 10.1 ± 0.4 | 9:91 |
| d-Mannose | d-Fructose | 5.5 ± 0.51 | 76:24 |
| l-Allose | l-Psicose | 4.7 ± 0.20 | 76:24 |
| d-Ribose | d-Ribulose | 1.1 ± 0.04 | 85:15 |
| l-Talose | l-Tagatose | 0.47 ± 0.09 | 10:90 |
| d-Allose | d-Psicose | 0.21 ± 0.00 | 82:18 |
| l-Lyxose | l-Xylulose | 0.16 ± 0.00 | 75:25 |
| l-Mannose | l-Fructose | 0.09 ± 0.00 | 83:17 |
| Ketose | |||
| l-Ribulose | l-Ribose | 91.8 ± 3.2 | 29:71 |
| d-Xylulose | d-Lyxose | 65.9 ± 0.9 | 40:60 |
| d-Ribulose | d-Ribose | 8.4 ± 0.28 | 15:85 |
| l-Xylulose | l-Lyxose | 4.3 ± 0.20 | 25:75 |
| d-Tagatose | d-Talose | 2.4 ± 0.02 | 91:9 |
| d-Fructose | d-Mannose | 2.1 ± 0.02 | 24:76 |
| l-Psicose | l-Allose | 2.0 ± 0.06 | 24:76 |
| l-Fructose | l-Mannose | 0.53 ± 0.00 | 17:83 |
| d-Psicose | d-Allose | 0.09 ± 0.00 | 18:82 |
| l-Tagatose | l-Talose | 0.08 ± 0.00 | 90:10 |
Data represent the means and standard deviations from three separate experiments.
The reactions were performed in 50 mM EPPS buffer (pH 7.5) containing 10 mM sugar and 0.5 mM Co2+ at 40°C for 20 min by adjusting the enzyme amount (1.0 to 10 U/ml).
The reactions were performed in 50 mM EPPS buffer (pH 7.5) containing 1 mM sugars, 120 U/ml of enzyme, and 0.5 mM Co2+ at 35°C for 72 to 144 h.
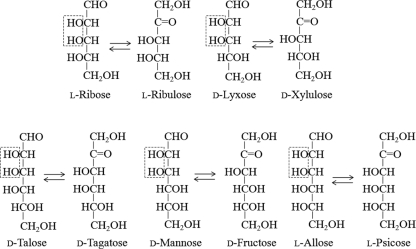
The aldose substrates with hydroxyl groups oriented in the same direction at C-2 and C-3 were converted reversibly by the enzyme to the corresponding ketoses. The enzyme preferred aldose substrates with the C-2 and C-3 hydroxyl groups in the left-hand configuration (Fischer projections), such as l-ribose, d-lyxose, d-talose, d-mannose, and l-allose, compared to those with right-hand hydroxyl groups, such as d-ribose, l-talose, d-allose, l-lyxose, and l-mannose (Fig. 3). The specific activity of l-ribose isomerase from an Acinetobacter sp. followed the order l-ribose > d-lyxose > d-mannose, whereas the specific activity of d-lyxose isomerase from C. laeviribosi followed the order d-lyxose > d-mannose > l-ribose (3, 9). No activity was found from the two enzymes for d-talose, l-allose, d-ribose, l-talose, d-allose, l-lyxose, and l-mannose, demonstrating the different substrate specificities of the B. subtilis mannose-6-phosphate isomerase.
FIG. 3.
Schematic representation of aldose-ketose isomerization reactions catalyzed by B. subtilis mannose-6-phosphate isomerase. The boxed structure indicates the hydroxyl configurations at C-2 and C-3 of the sugars.
The orders of turnover numbers (kcat) and catalytic efficiencies (kcat/Km) for B. subtilis mannose-6-phosphate isomerase with aldoses were the same as the order of specific activity (Table 2). l-Ribose displayed the highest kcat/Km among the aldoses tested. The Michaelis-Menten constant (Km) for l-ribulose (849 mM) was about 1.2-fold lower than that for l-ribose. The kcat (3,694 s−1) using l-ribulose was about 2.1-fold higher than that using l-ribose. As a result, the kcat/Km (43.5 mM−1 s−1) for l-ribulose was 2.5-fold higher than that (17.6 mM−1 s−1) for l-ribose. This enzyme displayed kcat/Km values for l-ribose that were significantly higher (88 × 105- and 3.4 × 105-fold) than those of the d-lyxose isomerase from C. laeviribosi and the d-xylose isomerase mutant from A. missouriensis, respectively (3, 14). Thus, B. subtilis mannose-6-phosphate isomerase has the highest kcat/Km for l-ribose among the l-ribose-converting enzymes reported to date, suggesting that the enzyme is an l-ribose producer.
TABLE 2.
Kinetic parameters of B. subtilis mannose 6-phosphate isomerase for aldosesa
| Substrate | Km (mM) | kcat (s−1) | kcat/Km (mM−1 s−1) |
|---|---|---|---|
| l-Ribose | 998 ± 44 | 17,595 ± 670 | 17.6 ± 1.0 |
| d-Lyxose | 433 ± 3 | 7,089 ± 78 | 16.4 ± 0.1 |
| d-Talose | 469 ± 31 | 3,969 ± 150 | 6.8 ± 0.5 |
| d-Mannose | 946 ± 7 | 3,748 ± 240 | 3.5 ± 0.2 |
| l-Allose | 312 ± 14 | 920 ± 41 | 2.9 ± 0.2 |
| d-Ribose | 110 ± 1 | 72 ± 1 | 0.6 ± 0.01 |
Data represent the means and standard deviations from three separate experiments. The reactions were performed in 50 mM EPPS buffer (pH 7.5) containing various amounts of sugar (50 to 1,500 mM) and 0.5 mM Co2+ at 40°C for 20 min.
The Km, kcat, and kcat/Km of the enzyme for mannose-6-phosphate were 29 mM, 22,730 s−1, and 770 mM−1 s−1, respectively, and were 34-fold lower, 1.3-fold greater, and 44-fold greater than those for l-ribose, indicating that the enzyme is a true mannose-6-phosphate isomerase, and that mannose-6-phosphate is an authentic substrate for the enzyme.
Equilibrium ratio.
The enzyme reactions were performed in 50 mM EPPS buffer (pH 7.5) with 1 mM total sugars of aldose and/or ketose with five initial ratios, 0:100, 25:75, 50:50, 75:25, and 100:0, in the presence of 0.5 mM Co2+ using a high enzyme concentration (120 U ml−1) with stable temperature control (35°C) under extended reaction times (144 h). The extended reaction time was longer than the half-life of the enzyme at 40°C (111 h) but shorter than the half-life of the enzyme at 35°C (236 h). Thus, a reaction temperature of 35°C resulted in attaining the equilibrium ratio between the aldose and ketose under stable conditions. The reactions were performed with l-ribose, d-lyxose, and d-talose for 72 h, with d-mannose, l-allose, and d-ribose for 96 h, and with l-talose, d-allose, l-lyxose, and l-mannose for 144 h. Equilibrium ratios between sugars (except that between talose and tagatose) shifted toward the aldoses (Table 1). The equilibrium ratio between l-ribose and l-ribulose was 71:29, which was essentially the same as that obtained (70:30) by l-ribose isomerase from an Acinetobacter sp. (1).
Production of l-ribose.
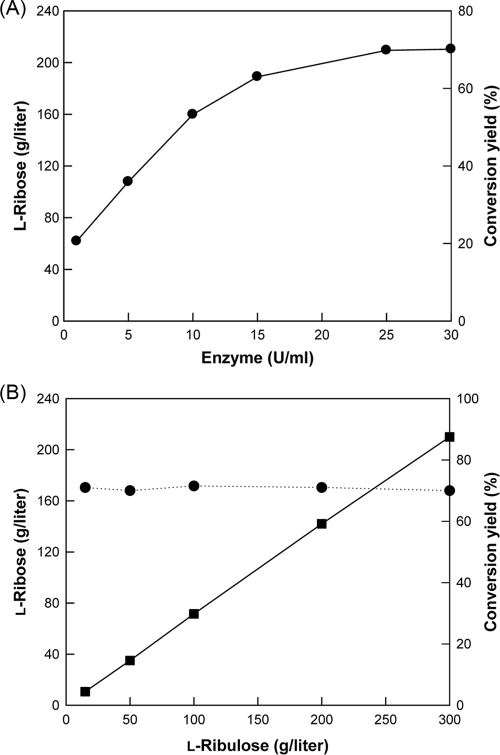
l-Ribose production was tested at enzyme concentrations ranging from 1 to 30 U/ml of enzyme with 300 g/liter l-ribulose as the substrate for 3 h (Fig. 4A). Below 25 U/ml of enzyme, l-ribose production (or conversion yield) increased with increasing amounts of enzyme, whereas it reached a plateau at concentrations above 25 U/ml of enzyme. Thus, the enzyme concentration for effective l-ribose production was determined to be 25 U/ml of enzyme. The production and conversion yield of l-ribose were assessed using various l-ribulose concentrations from 15 to 300 g/liter after 3 h (Fig. 4B). Increases in the l-ribulose concentration led to proportional increases in l-ribose production, while the yield of l-ribose from l-ribulose was almost constant at 70 to 71% regardless of the l-ribulose concentration.
FIG. 4.
(A) Effect of enzyme activity on the production and conversion yield of l-ribose by B. subtilis mannose-6-phosphate isomerase. The reactions were performed in 50 mM EPPS buffer (pH 7.5) containing 300 g/liter l-ribulose and 0.5 mM Co2+ at 40°C for 3 h. (B) Effect of substrate concentration on production (•) and conversion yield (▪) of l-ribose by B. subtilis mannose-6-phosphate isomerase. The reactions were performed in 50 mM EPPS buffer (pH 7.5) containing 25 U/ml of enzyme and 0.5 mM Co2+ at 40°C for 3 h.
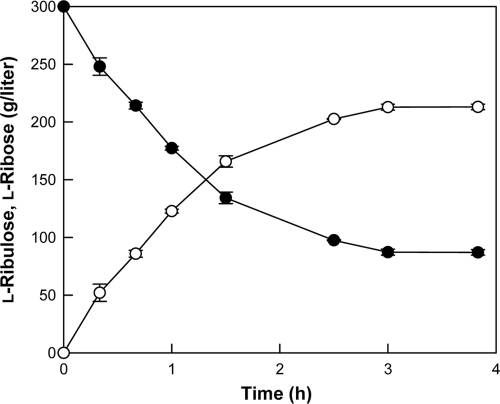
We performed time-course reactions of l-ribose production by B. subtilis mannose-6-phospate isomerase under the conditions of 40°C, pH 7.5, 300 g/liter l-ribulose, 0.5 mM Co2+, and 25 U/ml of enzyme (Fig. 5). As a result, l-ribose at 213 g/liter was obtained from 300 g/liter l-ribulose after 3 h, with a conversion yield of 71% and a volumetric productivity of 71 g liter−1 h−1. The chemical method using molybdic acid resulted in a 23% conversion yield from l-arabinose and 20 g liter−1 h−1 volumetric productivity (8). l-Ribose at 52 g/liter was produced from 100 g/liter ribitol by a recombinant E. coli containing an MDH for 72 h, corresponding to a volumetric productivity of 0.72 g liter−1 h−1 (18). This is the highest reported volumetric productivity and production in biological l-ribose manufacture. The volumetric productivity and production of l-ribose with B. subtilis mannose-6-phosphate were 98- and 4-fold higher, respectively, than the values attained previously with the recombinant E. coli. The enzymatic production of l-ribose described in the present study represents a remarkable improvement in terms of the volumetric productivity, product concentration, and ease of purification compared to those of fermentation and chemical methods.
FIG. 5.
Time course of l-ribose production (○) from 300 g/liter l-ribulose (•) by B. subtilis mannose-6-phospate isomerase. Data represent the means from three separate experiments. The reactions were performed in 50 mM EPPS buffer (pH 7.5) containing 300 g/liter l-ribulose, 25 U/ml of enzyme, and 0.5 mM Co2+ at 40°C.
The equilibrium ratio between l-arabinose and l-ribulose is reported to be 80:20 (20), and that between l-ribulose and l-ribose is 30:70 (1). The conversion yield of l-ribose from l-arabinose via a two-step bioconversion, obtained by making a simple calculation using these equilibrium ratios, was 14%. The theoretical conversion yield is expected to be higher than the simple calculated conversion yield due to the draw-through effect of the second reaction in the two-step bioconversion. The theoretical conversion yield is calculated by the continuous iteration of the two equilibriums between l-arabinose and l-ribulose and between l-ribulose and l-ribose until the ratios of l-arabinose, l-ribulose, and l-ribose are constant. As a result, the final equilibrium ratios of l-arabinose, l-ribulose, and l-ribose equal 54:14:32. Thus, the overall conversion efficiency of l-ribose from l-arabinose by the two-step bioconversion using l-arabinose isomerase and mannose-6-phospate isomerase was estimated to be 32%.
Since mannose-6-phosphate isomerase exhibited the highest activity for l-ribulose among all pentoses and hexoses, the unprecedented volumetric productivity and production of l-ribose (71 g liter−1 h−1 and 213 g/liter, respectively) using l-ribulose were obtained. The enzymatic production of l-ribose from l-ribulose in the present study was the first trial. In the future, we will attempt to produce l-ribose from l-arabinose via a two-enzyme system, in which l-ribulose will be produced first from l-arabinose by l-arabinose isomerase, and then l-ribose will be produced from l-ribulose by mannose-6-phosphate isomerase.
Acknowledgments
This study was supported by a grant (R0A-2007-000-20015-0) from the Korea Science and Engineering Foundation (KOSEF) through the National Research Laboratory. The program was funded by the Ministry of Education, Science, and Technology and by a grant (code 2007-0301034024) from BioGreen 21 Program, Rural Development Administration.
Footnotes
Published ahead of print on 15 May 2009.
REFERENCES
- 1.Ahmed, Z., T. Shimonishi, S. H. Bhuiyan, M. Utamura, G. Takada, and K. Izumori. 1999. Biochemical preparation of L-ribose and L-arabinose from ribitol: a new approach. J. Biosci. Bioeng. 88:444-448. [DOI] [PubMed] [Google Scholar]
- 2.Akagi, M., D. Omae, Y. Tamura, T. Ueda, T. Kumashiro, and H. Urata. 2002. A practical synthesis of L-ribose. Chem. Pharm. Bull. (Tokyo) 50:866-868. [DOI] [PubMed] [Google Scholar]
- 3.Cho, E. A., D. W. Lee, Y. H. Cha, S. J. Lee, H. C. Jung, J. G. Pan, and Y. R. Pyun. 2007. Characterization of a novel d-lyxose isomerase from Cohnella laeviribosi RI-39 sp. nov. J. Bacteriol. 189:1655-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Muynck, C., J. Van der Borght, M. De Mey, S. L. De Maeseneire, I. N. Van Bogaert, J. Beauprez, W. Soetaert, and E. Vandamme. 2007. Development of a selection system for the detection of L-ribose isomerase expressing mutants of Escherichia coli. Appl. Microbiol. Biotechnol. 76:1051-1057. [DOI] [PubMed] [Google Scholar]
- 5.Doong, S. L., C. H. Tsai, R. F. Schinazi, D. C. Liotta, and Y. C. Cheng. 1991. Inhibition of the replication of hepatitis B virus in vitro by 2′,3′-dideoxy-3′-thiacytidine and related analogues. Proc. Natl. Acad. Sci. USA 88:8495-8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gracy, R. W., and E. A. Noltmann. 1968. Studies on phosphomannose isomerase. 3. A mechanism for catalysis and for the role of zinc in the enzymatic and the nonenzymatic isomerization. J. Biol. Chem. 243:5410-5419. [PubMed] [Google Scholar]
- 7.Groussac, E., M. Ortiz, and J. Francois. 2000. Improved protocols for quantitative determination of metabolites from biological samples using high performance ionic-exchange chromatography with conductimetric and pulsed amperometric detection. Enzyme Microb. Technol. 26:715-723. [DOI] [PubMed] [Google Scholar]
- 8.Jumppanen, J., J. Nurmi, and O. Pastinen. October 2000. Process for the continuous production of high purity of L-ribose. U.S. patent 6,140,498.
- 9.Mizanur, R. M., G. Takata, and K. Izumori. 2001. Cloning and characterization of a novel gene encoding L-ribose isomerase from Acinetobacter sp. strain DL-28 in Escherichia coli. Biochim. Biophys. Acta 1521:141-145. [DOI] [PubMed] [Google Scholar]
- 10.Papoutsopoulou, S. V., and D. A. Kyriakidis. 1997. Phosphomannose isomerase of Xanthomonas campestris: a zinc activated enzyme. Mol. Cell. Biochem. 177:183-191. [DOI] [PubMed] [Google Scholar]
- 11.Park, C. S., S. J. Yeom, H. J. Kim, S. H. Lee, J. K. Lee, S. W. Kim, and D. K. Oh. 2007. Characterization of ribose-5-phosphate isomerase of Clostridium thermocellum producing d-allose from d-psicose. Biotechnol. Lett. 29:1387-1391. [DOI] [PubMed] [Google Scholar]
- 12.Park, H. Y., C. S. Park, H. J. Kim, and D. K. Oh. 2007. Substrate specificity of a galactose 6-phosphate isomerase from Lactococcus lactis that produces D-allose from D-psicose. J. Biotechnol. 132:88-95. [DOI] [PubMed] [Google Scholar]
- 13.Roux, C., J. H. Lee, C. J. Jeffery, and L. Salmon. 2004. Inhibition of type I and type II phosphomannose isomerases by the reaction intermediate analogue 5-phospho-D-arabinonohydroxamic acid supports a catalytic role for the metal cofactor. Biochemistry 43:2926-2934. [DOI] [PubMed] [Google Scholar]
- 14.Santa, H., J. Kammonen, O. Lehtonen, J. Karimaki, O. Pastinen, M. Leisola, and O. Turunen. 2005. Stochastic boundary molecular dynamics simulation of L-ribose in the active site of Actinoplanes missouriensis xylose isomerase and its Val135Asn mutant with improved reaction rate. Biochim. Biophys. Acta 1749:65-73. [DOI] [PubMed] [Google Scholar]
- 15.Shinabarger, D., A. Berry, T. B. May, R. Rothmel, A. Fialho, and A. M. Chakrabarty. 1991. Purification and characterization of phosphomannose isomerase-guanosine diphospho-D-mannose pyrophosphorylase. A bifunctional enzyme in the alginate biosynthetic pathway of Pseudomonas aeruginosa. J. Biol. Chem. 266:2080-2088. [PubMed] [Google Scholar]
- 16.Swan, M. K., T. Hansen, P. Schönheit, and C. Davies. 2004. Structural basis for phosphomannose isomerase activity in phosphoglucose isomerase from Pyrobaculum aerophilum: a subtle difference between distantly related enzymes. Biochemistry 43:14088-14095. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi, H., Y. Iwai, Y. Hitomi, and S. Ikegami. 2002. Novel synthesis of L-ribose from D-mannono-1,4-lactone. Org. Lett. 4:2401-2403. [DOI] [PubMed] [Google Scholar]
- 18.Woodyer, R. D., N. J. Wymer, F. M. Racine, S. N. Khan, and B. C. Saha. 2008. Efficient production of l-ribose with a recombinant Escherichia coli biocatalyst. Appl. Environ. Microbiol. 74:2967-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodyer, R. D., N. J. Wymer, F. M. Racine, S. N. Khan, and B. C. Saha. December 2003. Method of treating hepatitis delta virus infection. U.S. patent 6,670,342.
- 20.Yeom, S. J., J. H. Ji, R. Y. Yoon, and D. K. Oh. 2008. l-Ribulose production from l-arabinose by an l-arabinose isomerase mutant from Geobacillus thermodenitrificans. Biotechnol. Lett. 30:1789-1793. [DOI] [PubMed] [Google Scholar]
- 21.Yoon, R. Y., S. J. Yeom, H. J. Kim, and D. K. Oh. 2009. Novel substrates of a ribose-5-phosphate isomerase from Clostridium thermocellum. J. Biotechnol. 139:26-32. [DOI] [PubMed] [Google Scholar]
- 22.Yoon, R. Y., S. J. Yeom, C. S. Park, and D. K. Oh. 2009. Substrate specificity of a glucose-6-phosphate isomerase from Pyrococcus furiosus for monosaccharides. Appl. Microbiol. Biotechnol. 83:295-303. [DOI] [PubMed] [Google Scholar]
- 23.Yun, M., H. R. Moon, H. O. Kim, W. J. Choi, Y. C. Kim, C. S. Park, and L. S. Jeong. 2005. A highly efficient synthesis of unnatural L-sugars from D-ribose. Tetrahedron Lett. 46:5903-5905. [Google Scholar]