Abstract
Many bacteria are naturally capable of accumulating biopolyesters composed of 3-hydroxy fatty acids as intracellular inclusions, which serve as storage granules. Recently, these inclusions have been considered as nano-/microbeads with surface-attached proteins, which can be engineered to display various protein-based functions that are suitable for biotechnological and biomedical applications. In this study, the food-grade, generally-regarded-as-safe gram-positive organism Lactococcus lactis was engineered to recombinantly produce the biopolyester poly(3-hydroxybutyrate) and the respective intracellular inclusions. The codon-optimized polyhydroxybutyrate biosynthesis operon phaCAB from Cupriavidus necator was expressed using the nisin-controlled gene expression system. Recombinant L. lactis accumulated up to 6% (wt/wt) poly(3-hydroxybutyrate) of cellular dry weight. Poly(3-hydroxybutyrate) granules were isolated and analyzed with respect to bound proteins using biochemical methods and with respect to shape/size using transmission electron microscopy. The immunoglobulin G (IgG) binding ZZ domain of Staphylococcus aureus protein A was chosen as an exemplary functionality to be displayed at the granule surface by fusing it to the N terminus of the granule-associated poly(3-hydroxybutyrate) synthase. The presence of the fusion protein at the surface of isolated granules was confirmed by peptide fingerprinting using matrix-assisted laser desorption ionization-time of flight (mass spectrometry). The functionality of the ZZ domain-displaying granules was demonstrated by enzyme-linked immunosorbent assay and IgG affinity purification. In both assays, the ZZ beads from recombinant L. lactis performed at least equally to ZZ beads from Escherichia coli. Overall, in this study it was shown that recombinant L. lactis can be used to manufacture endotoxin-free poly(3-hydroxybutyrate) beads with surface functionalities that are suitable for biomedical applications.
Polyhydroxyalkanoates (PHAs), a group of biopolyesters, are naturally produced by a large number of bacteria as energy and carbon storage material when they are exposed to imbalanced nutrient availability. The isolated biopolyesters have been considered as alternative renewable plastics for technical and medical applications (7, 41-43). The biosynthesis of one of the most common biopolyesters, poly[(R)-3-hydroxybutyrate] (PHB), comprises three enzyme-catalyzed reactions. Two molecules of acetyl-coenzyme A (CoA) are condensed to acetoacetyl-CoA by β-ketothiolase (PhaA). Acetoacetyl-CoA then is reduced to (R)-3-hydroxybutyryl-CoA by the NADPH-dependent acetoacetyl-CoA reductase (PhaB), and (R)-3-hydroxybutyryl-CoA is polymerized into PHB by PHA synthase (PhaC) with the concomitant release of CoA (42). The functional expression of these three genes in various organisms established recombinant biopolyester production (31). The PHA synthase is the key enzyme that catalyzes the formation of high-molecular-weight polyester molecules while mediating the assembly of insoluble inclusions inside the bacterial cell (1, 2, 44). These inclusions, which generally are regarded as spherical, are composed of a biopolyester core and a protein/phospholipid shell and have been considered shell-core micro-/nanobeads that are suitable for technical and medical applications (16, 43). Experimental evidence had been provided that the PHA synthase remains covalently attached at the surface of the biopolyester granules, and that this enzyme can be engineered to display various functionalities, such as binding domains or enzymes suitable for applications in affinity purification or in vitro catalysis (17, 22, 38, 39). These functionalized polyester beads have been produced so far only by recombinant gram-negative bacteria such as Escherichia coli or Pseudomonas aeruginosa. However, gram-negative bacteria contain lipopolysaccharide (LPS) endotoxins, which are known to be pyrogenic for the human body and are copurified with products derived from gram-negative hosts. Tedious purification steps are required to remove endotoxins to levels acceptable to the U.S. Food and Drug Administration (FDA). Methods commonly used to depyrogenate the plastic PHA (such as oxidizing agents or supercritical fluids) (54) are considered too harsh for the surface functionalities of PHA beads.
With the aim of establishing an alternative source of PHB beads that are free of LPS contamination, Lactococcus lactis was investigated in this study as a host to manufacture engineered biopolyester beads. L. lactis is a gram-positive homofermentative bacterium that traditionally has been used for the production of a variety of fermented milk products (50). Recently, L. lactis has attracted interest as a safe food-grade host for the production of heterologous proteins for medical applications (37, 47), and respective gene expression systems have been developed to use L. lactis as a cell factory. One of the best-known inducible systems for recombinant protein production in L. lactis is the nisin-controlled gene expression system (NICE) (10, 26-28, 35). Nisin is an antimicrobial peptide (lantibiotic) that acts as an inducer for the nisA promoter via NisK and NisR. The nisA promoter was shown to exhibit a linear inducer-response relationship with nisin as the inducer (26, 29). In this study, the NICE system in L. lactis was used for the recombinant production of tailor-made endotoxin-free biopolyester beads that are suitable for medical applications. The aims of this study were (i) to establish the Cupriavidus necator PHB biosynthesis pathway in L. lactis and (ii) to use engineered L. lactis to produce PHB beads that display a functional protein that is suitable for applications in diagnostics and affinity purification. Due to the different G+C contents of C. necator and L. lactis, problems with efficient transcription caused by codon usage bias were expected. The other main challenge was whether the metabolism of L. lactis would support PHB biosynthesis. While recombinant PHB production previously has been achieved for another gram-positive organism, Corynebacterium glutamicum (24, 25), this is the first report of recombinant PHB production and the generation of surface-functionalized PHB granules in a food-grade homofermentative lactic acid bacterium.
MATERIALS AND METHODS
Strains and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. E. coli strains were cultivated in Luria-Bertani medium at 37°C with aeration. L. lactis strains were cultivated in M17 medium (Merck) and 0.5% (wt/vol) glucose (0.5% GM17) at 30°C without aeration unless stated otherwise. If required, ampicillin was used at a concentration of 75 μg/ml for E. coli, and chloramphenicol was added to medium at 5 μg/ml for E. coli and 10 μg/ml for L. lactis.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Characteristics | Reference or source |
|---|---|---|
| E. coli | ||
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqlacZΔM15 Tn 10 (Tetr)] | Stratagene |
| L. lactis | ||
| MG1363 | NCDO 712 derivative, plasmid- and phage-free strain | 13 |
| NZ9000 | MG1363 derivative, pepN::nisRK | 28 |
| Plasmid | ||
| pUC57 | Cloning vector, ColE1 origin, Ampr | Fermentas |
| pUC57-phaC | Codon-optimized phaC in EcoRV site of pUC57 | This study |
| pUC57-phaA | Codon-optimized phaA in EcoRV site of pUC57 | This study |
| pUC57-phaB | Codon-optimized phaB in EcoRV site of pUC57 | This study |
| pUC57-phaAB | Codon-optimized phaAB in pUC57 | This study |
| pUC57-ZZ | Codon-optimized gene for ZZ domain in EcoRV site of pUC57 | This study |
| pNZ8148 | Cmr, pSH71 origin, PnisA | 35 |
| pNZ-AB | pNZ8148 derivative, PnisA-phaAB | This study |
| pNZ-CAB | pNZ8148 derivative, PnisA-phaCAB | This study |
| pNZ-ZZB | pNZ8148 derivative, PnisA-ZZ-phaB | This study |
| pNZ-ZZCAB | pNZ8148 derivative, PnisA-ZZphaC-phaAB | This study |
Isolation, analysis, and manipulation of DNA.
General cloning procedures were performed as described previously (48). Plasmid isolation from L. lactis was performed in the same way as that from E. coli, except that THMS buffer (30 mM Tris-HCl [pH 8.0], 3 mM MgCl2, and 25% [wt/vol] sucrose) containing 2 mg/ml lysozyme was used as the lysis buffer. The transformation of E. coli was performed using the rubidium chloride method as described elsewhere (19). An incubation of 48 h was required to detect colonies of transformants after the introduction of pNZ8148-based plasmids. The transformation of L. lactis was performed by electroporation as described previously, with the modifications outlined below (20, 53). To make electrocompetent L. lactis cells, a 100-ml culture of 0.5% GM17 supplemented with 0.2 M sucrose and 1% (wt/vol) glycine was inoculated with 5% (vol/vol) of an overnight culture of L. lactis in 0.5% GM17, and then it was cultivated at 30°C with aeration until the optical density at 600 nm (OD600) reached 0.4 to 0.7. Cells were harvested by centrifugation (4,000 rpm, 4°C, 20 min) and washed with ice-cold 0.5 M sucrose-10% (vol/vol) glycerol. Cells were kept on ice for 15 min in 50 ml of 0.5 M sucrose-10% (vol/vol) glycerol-50 mM EDTA, harvested by centrifugation, and washed again. The pellet finally was resuspended in 1 ml 0.5 M sucrose-10% (vol/vol) glycerol (1/100 volume) and divided into aliquots of 40 μl.
One microliter of purified plasmid solution or 9 μl ligation mixture (each corresponding to about 1 μg of DNA) was mixed with 40 μl competent cells. After incubation on ice for 30 min, electroporation was performed using a 2-mm cuvette with a 2-kV pulse (with a pulse time of about 5.0 to 5.5 ms) (MicroPulser; Bio-Rad Laboratories). After being pulsed, 1 ml of recovery medium (M17 supplemented with 20 mM MgCl2 and 2 mM CaCl2) was added to the cells immediately. After incubation for 10 min on ice, cells were allowed to recover at 30°C for 1.5 to 2 h and subsequently plated onto M17 agar containing 0.5% (wt/vol) glucose and 10 μg/ml chloramphenicol. Colonies were detected after 24 to 36 h.
Construction of plasmids enabling biopolyester bead production by L. lactis.
The genes phaC, phaA, and phaB from C. necator were synthesized by GeneScript Corporation (Piscataway, NJ), who adapted them to the codon usage bias of L. lactis, i.e., avoiding rarely used codons. The construction of pNZ-AB was conducted as a two-step ligation as described below. pUC57-phaB was digested with BamHI and HindIII, and the resulting phaB fragment was inserted into pUC57-phaA downstream of phaA using the same restriction sites. The resulting plasmid, pUC57-phaAB, was digested with SphI and HindIII to release the phaAB-comprising DNA fragment, which subsequently was ligated into SphI-HindIII-hydrolyzed pNZ8148, generating pNZ-AB. pNZ-CAB, with the entire phaCAB operon under nisA promoter control, was obtained by the ligation of the NcoI-SphI DNA fragment comprising phaC from pUC57-phaC into NcoI-SphI-hydrolyzed pNZ-AB.
The DNA region encoding the immunoglobulin G (IgG) binding ZZ domain derived from Staphylococcus aureus protein A also was synthesized by GeneScript Corporation (Piscataway, NJ), who adapted it to the codon usage bias of L. lactis. A DNA fragment of pUC57-ZZ containing part of the nisA promoter (PnisA) and the ZZ region was obtained by BstBI and BamHI hydrolysis and inserted into the corresponding sites of pNZ-AB, yielding pNZ-ZZB. To introduce the phaC- and phaA-containing fragment of pNZ-CAB into pNZ-ZZB, both plasmids were hydrolyzed with NheI and BamHI, and the phaCA fragment of pNZ-CAB was inserted into pNZ-ZZB, resulting in pNZ-ZZCAB.
The DNA sequences of all constructed plasmids were confirmed by DNA sequencing according to the chain termination method using an ABI3730 Genetic Analyzer (Applied Biosystems Inc., CA).
Establishment of the PHB biosynthesis pathway in L. lactis.
To assess whether recombinant L. lactis was capable of PHB production, the respective cells were analyzed by gas chromatography/mass spectrometry (GC/MS). Briefly, cells were cultivated in 50 ml M17 broth supplemented with 1% (wt/vol) glucose (1% GM17), galactose (GalM17), or lactose (LacM17) for 24 h. For aerobic growth conditions, cultivations were performed in 250-ml flasks with agitation. For anaerobic growth conditions, cells were grown in sealed 50-ml bottles without agitation. Where necessary, l-arginine was added at a concentration of 0.3% (wt/vol), or hemin was added at 10 μg/ml (stock solution of 0.5 mg/ml in 0.05 N NaOH). Induction was obtained by adding nisin to a final concentration between 2.5 and 40 ng/ml (stock solution of 250 μg/ml in 0.05% acetic acid) when the OD600 reached 0.5. After cultivation, cells were harvested, washed once with an equal volume of 50 mM potassium phosphate buffer (pH 7.4), and then freeze-dried at −80°C. The PHB contents of the lyophilized samples were determined by GC/MS after the conversion of the PHB into 3-hydroxymethylesters by acid-catalyzed methanolysis (4).
Determination of acetate and lactate in culture supernatants.
The concentrations of acetate and lactate were measured in culture supernatants from different growth conditions. Cells were cultivated for 24 h as described in the previous section and centrifuged, and the supernatants were analyzed. Acetate was determined using the Megazyme Acetic Acid kit (acetate kinase format) (Megazyme International Ireland, Bray, Co. Wicklow, Ireland), and lactate was measured with the Lactate Assay kit from Eton Bioscience (San Diego, CA) by following the manufacturers' instructions.
Isolation of PHB beads from recombinant L. lactis.
L. lactis strains were cultivated for 24 h and harvested by centrifugation (6,000 rpm, 4°C, 20 min). After the pellet was resuspended in a 1/50 volume of 50 mM potassium phosphate buffer (pH 7.4) containing 3 mM MgCl2 and 25% (wt/vol) sucrose, cells were incubated with 10 mg/ml lysozyme at 37°C for 2 h. The supernatant was removed by centrifugation at 8,000 × g for 30 min, and the sediment was resuspended in the same volume of 50 mM potassium phosphate buffer. Cells were further disrupted by sonication (30 s, five times, 30 W), and the cell extract was removed by centrifugation at 6,000 × g for 20 min. The sediment was subjected to glycerol gradient ultracentrifugation. After 2.5 h of ultracentrifugation at 100,000 × g, beads could be isolated from the boundary of 44 and 88% (vol/vol) glycerol.
TEM analysis.
To confirm biopolyester bead formation inside recombinant L. lactis as well as to assess the shape and size of the biopolyester beads produced by L. lactis, transmission electron microscopy (TEM) analysis was performed. After 24 h of cultivation, 2 ml of cell culture was harvested and washed twice with 50 mM potassium phosphate buffer (pH 7.4), and sediments were prepared for electron microscopy analysis as previously described (17).
Analysis of proteins attached to PHB beads.
To assess the biopolyester bead protein profile, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described previously (30, 48). Dominant bands were cut out and identified by tryptic peptide fingerprint analysis using matrix-assisted laser desorption ionization-time of flight (mass spectrometry) [MALDI-TOF(MS)] as described previously (22).
Assessment of functional display of the IgG binding ZZ domain at the surface of engineered biopolyester beads from recombinant L. lactis.
Enzyme-linked immunosorbent assay (ELISA) analysis was performed as described previously (5), but with slight modifications. Briefly, biopolyester beads were attached to wells of an ELISA plate (starting from a protein amount of 5 μg per well). After being blocked with 3% (wt/vol) bovine serum albumin for 1 h, each well was incubated with horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG (Jackson ImmunoResearch Laboratories, PA) for 1 h. After being washed, 100 μl of an o-phenylenediamine solution (Abbott Diagnostics, IL) was added to each well, and after 15 min, the reaction was stopped by adding 100 μl of 1 N H2SO4. The amount of substrate conversion was measured at a wavelength of 490 nm using a microtiter plate reader.
Use of ZZ domain-displaying beads from recombinant L. lactis for purification of IgG from human serum.
IgG purification was performed as described previously (5). Briefly, beads were washed in a series of buffers (TBS [50 mM Tris-HCl, 150 mM NaCl, pH 7.4]; 50 mM glycine, pH 1.9; TBS; 50 mM Tris-HCl, 500 mM NaCl, pH 7.4; and 10 mM Tris-HCl, 37.5 mM NaCl, pH 7.4) and resuspended in TBS. Beads (corresponding to 500 μg of total bead protein) then were mixed with an excess of human serum and incubated at room temperature for 30 min. Following incubation, the beads were pelleted and washed with the following buffers: once with TBS, twice with 50 mM Tris-HCl, 500 mM NaCl, pH 7.4, and twice with 10 mM Tris-HCl, 37.5 mM NaCl, pH 7.4. Proteins bound to the beads then were eluted with 50 mM glycine, pH 2.7, and subsequently analyzed by SDS-PAGE.
RESULTS
Establishment of PHB biosynthesis in L. lactis.
The bioinformatic analysis of the L. lactis genome sequence did not reveal genes homologous to the PHA biosynthesis genes. Thus, to produce PHB granules in L. lactis cells, plasmid pNZ-CAB was constructed that harbors the codon-optimized PHB biosynthesis operon from C. necator under PnisA control. Plasmid pNZ-AB, harboring only the two PHB biosynthesis genes phaA and phaB, was constructed and used as a negative control in addition to the empty vector pNZ8148. These plasmids were transformed into L. lactis, and PHB production in recombinant L. lactis NZ9000 strains was assessed by GC/MS analysis using cells cultivated in 1% GM17 supplemented with 0.3% (wt/vol) l-arginine for 24 h (Table 2). The strain NZ9000 harboring pNZ8148 or pNZ-AB did not accumulate any PHB. However, strain NZ9000 harboring pNZ-CAB accumulated the biopolyester PHB, contributing to 4 to 6% (wt/wt) of cellular dry weight (CDW) when the inducer nisin was added at a concentration of ≥2.5 ng/ml (Table 2). Nisin concentrations greater than 2.5 ng/ml only slightly enhanced PHB accumulation. When more than 10 ng/ml nisin was added to the medium, the biomass production was slightly impaired. The final pH of the culture supernatant was independent of the nisin concentration (Table 2). When cells were cultivated for 48 h instead of 24 h, no increase in biomass or PHB production was observed (data not shown). Time course experiments indicated that the PHB content remained unchanged after cells reached the stationary growth phase (see Fig. S2 in the supplemental material).
TABLE 2.
Growth and PHB biosynthesis of L. lactis NZ9000 harboring various plasmidsa
| Plasmid | Description | Nisinb (ng/ml) | CDW (g/liter) | PHB contentc (%, wt/wt) | pHd |
|---|---|---|---|---|---|
| pNZ8148 | PnisA | 10 | 1.16 | 0 | 4.97 |
| pNZ-AB | PnisA-phaAB | 10 | 1.60 | 0 | 5.40 |
| pNZ-CAB | PnisA-phaCAB | 0 | 1.92 | 0 | 5.49 |
| 2.5 | 1.75 | 5.09 | 5.56 | ||
| 10 | 1.63 | 4.44 | 5.48 | ||
| 20 | 1.59 | 5.79 | 5.56 | ||
| 40 | 1.56 | 6.06 | 5.50 |
Experiments were conducted in triplicate, and the mean values are shown. The standard deviation was <15%. Cells were cultivated in 50 ml M17 medium containing 1% (wt/vol) glucose and 0.3% (wt/vol) arginine with aeration for 24 h at 30°C.
Nisin induction was performed when an OD600 of about 0.5 was reached.
PHB content was determined by GC/MS.
pH in culture supernatant.
Effect of the carbon source and oxygen availability on PHB and biomass production.
Since it has been reported that L. lactis switches its metabolism between homolactic fermentation and mixed-acid fermentation depending on the carbon source as well as the availability of oxygen (14, 21, 51), strain NZ9000(pNZ-CAB) was grown under various cultivation conditions to evaluate the effect of these factors (Table 3). When the strain was cultivated in M17 medium containing galactose or glucose as the carbon source, the final OD600 and CDW were higher compared to those with lactose as the carbon source. Strain NZ9000(pNZ-CAB) accumulated about 4 to 6 and 1% (wt/wt) PHB of CDW when cultivated with glucose or galactose under aerobic and anaerobic conditions, respectively (Table 3). No PHB was detectable when lactose was used as the carbon source.
TABLE 3.
Effect of aeration, carbon source, and additives on PHB accumulation in L. lactis NZ9000 harboring pNZ-CABa
| Carbon source | Additiveb | Aerationc | CDW (g/liter) | PHB contentd (%, wt/wt) | pHe |
|---|---|---|---|---|---|
| Glucose | None | + | 1.23 | 4.10 | 4.64 |
| None | − | 1.26 | 0.98 | 4.46 | |
| Glucose | l-Arginine | + | 1.63 | 4.44 | 5.48 |
| l-Arginine | − | 1.44 | 0.92 | 5.17 | |
| Galactose | l-Arginine | + | 1.62 | 4.51 | 5.64 |
| l-Arginine | − | 1.70 | 0.99 | 5.21 | |
| Lactose | l-Arginine | + | 0.60 | 0 | 7.78 |
| l-Arginine | − | 0.55 | 0 | 8.59 | |
| Glucose | Hemin | + | 2.19 | 1.79 | 5.18 |
Experiments were conducted in triplicate, and the mean values are shown. The standard deviation was <15%. Cells were cultivated in 50 ml M17 supplemented with 1% of the carbon source shown in the table at 30°C for 24 h.
l-Arginine at 0.3% (wt/vol) or 10 μg/ml hemin was added to the growth medium (M17 with 1% of the carbon source) where applicable.
+, cultivated in a 250-ml flask with agitation; −, cultivated in a sealed 50-ml bottle without agitation.
PHB content was determined by GC/MS.
pH in culture supernatant.
Effect of l-arginine and hemin on PHB and biomass production by L. lactis.
It is known that L. lactis produces large amounts of lactate under conditions favoring homolactic fermentation, which eventually inhibits its growth by lowering the pH. In previous studies, it was assumed that growth would be improved by supplementation with l-arginine or hemin (8, 12, 14, 15, 34, 46). To evaluate the effect of these supplements on PHB and biomass production, cultivation was performed in the absence of these supplements or in the presence of either l-arginine or hemin. The presence of arginine only slightly increased biomass formation and PHB production, whereas the addition of hemin caused an increase in biomass formation but a decrease in PHB production (Table 3).
Lactate and acetate formation by recombinant L. lactis under various cultivation conditions.
It was further investigated how various cultivation parameters, e.g., the nisin concentration, the carbon source, or aeration/no aeration, affect lactate and acetate formation by recombinant L. lactis. Of particular interest was the correlation between PHB production and acid fermentation under various cultivation conditions. The presence or absence of nisin as well as various nisin concentrations did not significantly affect lactate formation, which was correlated with the finding that the absence or presence of PHB did not play a major role in lactate formation (Table 4). Only lactose led to strong lactate production. Lactate formation was not significantly affected by oxygen availability. The analysis of acetate formation showed similar results, except that anaerobic conditions strongly reduced acetate formation (Table 4). The same trends were observed for lactate and acetate formation when cells were grown using either galactose or lactose as the carbon source independently of supplementation with l-arginine (data not shown). More detailed results are given in Fig. S1 in the supplemental material.
TABLE 4.
Effect of growth condition modifications on lactate and acetate productiona
| Parameter changed or modificationa | Change in production ofc:
|
|
|---|---|---|
| Lactate | Acetate | |
| PHB accumulationb | — | — |
| Increasing nisin concn | — | — |
| Lack of aeration | — | ↓↓ |
| Carbon source | ||
| Glucose without arginine | ↓ | — |
| Glucose plus hemin | ↓ | — |
| Galactose | (↓) | (↑) |
| Lactose | ↑↑ | ↑ |
| Time (0 to 10 h) | ↓ | ↑ |
That is, changed compared to standard growth conditions: M17 medium plus 1% (wt/vol) glucose and 0.3% (wt/vol) arginine, aerobic cultivation for 24 h, and induction with 10 ng/ml nisin.
Lactococcus lactis NZ9000 harboring plasmid pNZ-CAB (PHB production) instead of plasmid pNZ-AB (no PHB production).
—, not affected; ↓ or ↑, decreased or increased, respectively; ↓↓ or ↑↑, strongly decreased or increased, respectively; (↓) or (↑), very slight decrease or increase, respectively.
Time course experiments monitoring acetate and lactate formation, respectively, by recombinant L. lactis showed that lactate formation decreased by about fourfold, whereas the acetate formation increased by about fivefold with increasing CDW and increasing PHB content (Table 4; also see Fig. S2 in the supplemental material). The pH of the growth medium decreased over time (from greater than 7 to 5), the decrease being inversely correlated with the increase in CDW and PHB (data not shown).
Formation of PHB granules and their analysis.
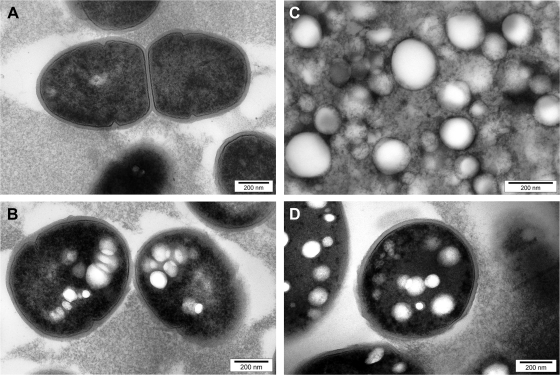
To confirm that the accumulated PHB is deposited as spherical inclusions inside recombinant L. lactis, cells were subjected to TEM analysis. Only cells harboring all three PHB biosynthesis genes and, thus, capable of PHB biosynthesis showed the formation of intracellular granules (Fig. 1A and B). The diameter of PHB granules accumulated in cells was about 150 nm. PHB granules were isolated and subjected to GC/MS (data not shown) and TEM analysis (Fig. 1C). Granule-associated proteins were identified by SDS-PAGE analysis combined with tryptic peptide fingerprinting using MALDI-TOF(MS). A dominant protein with an apparent molecular mass of about 60 kDa was identified as PHA synthase (PhaC). A further six prominent proteins were identified. SDS-PAGE and MALDI-TOF(MS) results are shown in Fig. S3 in the supplemental material.
FIG. 1.
TEM analysis of L. lactis NZ9000 accumulating PHB and isolated PHB granules. (A) Cells of L. lactis NZ9000(pNZ-AB) not showing any PHB accumulation (negative control); (B) cells of L. lactis NZ9000(pNZ-CAB) filled with small PHB granules (diameter, 100 to 200 nm); (C) PHB granules isolated from L. lactis NZ9000(pNZ-CAB), demonstrating the retention of size and shape during the isolation process; (D) cells of L. lactis NZ9000(pNZ-ZZCAB).
Production of functionalized PHB beads by recombinant L. lactis.
To assess whether recombinant L. lactis harboring the respective hybrid gene encoding a PHA synthase fusion protein can be used to manufacture PHB beads displaying a desired functionality for, e.g., medical applications, the IgG antibody binding ZZ domain of protein A from S. aureus was chosen as the protein function to be displayed and fused to the N terminus of the PHA synthase as previously described (5) but adapted to the codon usage bias of L. lactis. The production of functionalized PHB granules was monitored by GC/MS (data not shown) and TEM (Fig. 1D). The respective ZZ domain-displaying PHB beads (ZZ beads) were isolated from L. lactis NZ9000(pNZ-ZZCAB) and subjected to TEM (data not shown) and SDS-PAGE analysis (see Fig. S3 in the supplemental material). A very dominant protein with an apparent molecular mass of 66 kDa was identified by tryptic peptide fingerprinting using MALDI-TOF(MS) as the ZZ domain PhaC fusion protein (ZZ-PhaC).
Assessment of the functionality of ZZ beads from recombinant L. lactis by ELISA and IgG purification.
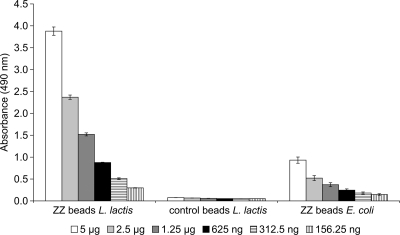
The specific IgG binding of the ZZ beads isolated from L. lactis was assessed by ELISA to demonstrate the functional display of the ZZ domain at the bead surface. A higher absorbance (representing IgG-conjugated HRP activity) was measured for the ZZ beads isolated from recombinant L. lactis than for ZZ beads isolated from recombinant E. coli, indicating that the L. lactis ZZ beads are functional (Fig. 2). PHB beads displaying only wild-type PHB synthase did not show any significant binding of IgG.
FIG. 2.
ELISA demonstrating specific binding of IgG to ZZ domain-displaying beads isolated from L. lactis; ZZ beads L. lactis indicates beads isolated from L. lactis NZ9000(pNZ-ZZCAB) that produce ZZ-PhaC; control beads L. lactis indicates granules isolated from L. lactis NZ9000(pNZ-CAB) that produce PhaC only; and ZZ beads E. coli indicates granules isolated from E. coli producing ZZ-PhaC. Isolated PHB granules were bound to ELISA plates, and HRP-conjugated rabbit anti-mouse IgG was used to detect the functional display of the ZZ domain. o-Phenylenediamine was used as the substrate for HRP. L. lactis-derived wild-type granules not displaying the ZZ domain and ZZ domain-displaying granules from E. coli were used as negative and positive controls, respectively. The increasingly darker gray shading of the columns indicates decreasing amounts of granule protein used in the assays (5 μg, 2.5 μg, 1.25 μg, 625 ng, 312.5 ng, and 156.25 ng). Measurements were performed in triplicate, and the mean values and standard deviations are indicated.
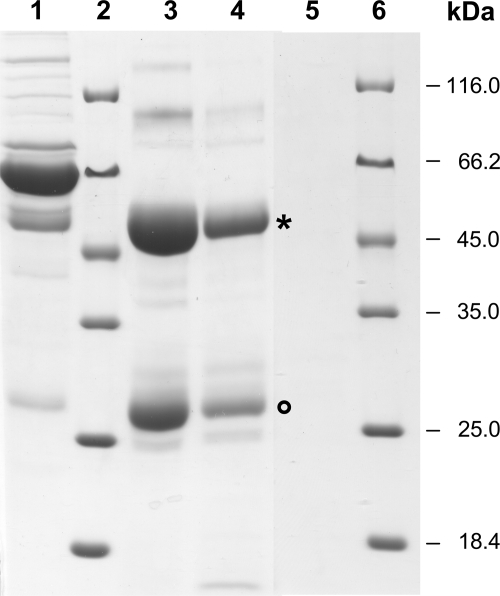
Furthermore, ZZ beads from recombinant L. lactis were applied for the purification of IgG antibodies from human serum. SDS-PAGE analysis of proteins eluted from the beads showed that two proteins corresponding in size to the antibody heavy and light chains were strongly enriched in the elution fractions compared to their levels in the original serum (Fig. 3). Analogously to ZZ beads from recombinant E. coli, the Igs eluted from L. lactis ZZ beads at pH 2.7 and with a similar degree of purity. However, more IgG was eluted from the L. lactis ZZ beads than from the E. coli ZZ beads. No Igs were eluted from PHB beads not displaying the ZZ protein (Fig. 3).
FIG. 3.
SDS-PAGE analysis of human serum proteins bound in vitro to ZZ domain-displaying beads isolated from L. lactis or E. coli and released after elution, demonstrating IgG purification. Lane 1, human serum; lanes 2 and 6, molecular size standard; lane 3, proteins eluted at pH 2.7 from L. lactis ZZ beads; lane 4, proteins eluted at pH 2.7 from E. coli ZZ beads; and lane 5, proteins eluted at pH 2.7 from L. lactis control beads not displaying the ZZ domain. Beads were washed as described in Materials and Methods, incubated with human serum for 30 min, and washed again, and bound proteins were eluted with glycine, pH 2.7. The heavy (asterisk) and light chains (circle) of the strongly enriched Igs are indicated.
DISCUSSION
In previous studies, it was demonstrated that the PHA synthase can be engineered to display various protein functions at the PHA granule surface when produced by gram-negative bacteria (5, 43). These granules were applicable as beads in affinity purification, diagnostics, and biocatalysis. However, for medical applications of these biopolyester beads, it would be favorable to establish production in a safe host such as the food-grade and generally-regarded-as-safe microorganism L. lactis. As a gram-positive bacterium, L. lactis does not produce LPS (49, 52), which often represent a problem regarding the medical applications of products derived from gram-negative bacteria (33, 40).
Here, the PHB biosynthesis pathway was established in recombinant L. lactis. The main conclusion is that PHB production in recombinant L. lactis is possible, but that it is more of a challenge to push the metabolism toward higher PHB yields. Furthermore, the key biosynthesis enzyme, the PHA synthase, and a fusion protein variant of the PHA synthase were found to be overproduced at the PHB granule surface when using the NICE system.
GC/MS analysis showed that recombinant L. lactis harboring the phaCAB genes on plasmid pNZ8148 could accumulate PHB to up to 6% (wt/wt) of its CDW when 40 ng/ml nisin was used for induction (Table 2). However, at this nisin concentration, the CDW was decreased by about 15% (wt/wt) compared to the maximum CDW obtained in the absence of inducer. The growth inhibitory effect of nisin generally was observed at concentrations above 10 ng/ml, which is in the range of previously observed inhibitory levels (9, 26). Interestingly, the CDW of L. lactis NZ9000 harboring the empty vector pNZ8148 was lower than that of the strain carrying either pNZ-AB or pNZ-CAB (Table 2). This decreased growth most likely was due to the lower pH of the NZ9000(pNZ8148) culture, which in turn could be related to the fact that in strains NZ9000(pNZ-CAB) and NZ9000(pNZ-AB) PhaA affects acetyl-CoA availability and, thus, acid formation.
Under anaerobic conditions, the final pH was slightly lower than that under aerobic conditions (Table 3). Due to the switch from mixed-acid fermentation to homolactic fermentation, under anaerobic conditions one would expect an increase in lactate production and a decrease in acetate production. Aerobic conditions should be favorable for PHB production, because the carbon flux from pyruvate to acetate via acetyl-CoA, a PHB precursor, is promoted during mixed-acid fermentation. While acetate levels and PHB production during growth on glucose were indeed higher under aerobic and lower under anaerobic conditions, no significant difference in lactate production depending on the presence or absence of oxygen was observed (Tables 3 and 4). Thus, the lower pH in culture under anaerobic conditions might be due mainly to formate production.
PHB production with galactose as a carbon source was comparable to that with glucose under both aerobic and anaerobic conditions (Table 3). This was contrary to the theoretical expectation that cultivation on galactose would increase the supply of acetyl-CoA by switching the flux from lactate production to acetyl-CoA in mixed-acid fermentation. However, it was previously reported that galactose also can cause the induction of the nisA promoter, to a much smaller extent than nisin but causing a significant decrease of nisin-mediated induction if the two components are present simultaneously (6). The respective effectors, NisR for nisin and an unknown compound for galactose, were found to act on overlapping sites of the nisA promoter. This effect of galactose on nisin induction was observed only in strains that metabolize galactose via the Leloir pathway, which is the case in L. lactis MG1363 (6, 11, 18). With lactose as a carbon source, cells did not grow well and did not accumulate any PHB (Table 3). A probable reason is an impaired lactose uptake ability of L. lactis MG1363-derived strains that lack a plasmid involved in lactose utilization (13). This is supported by the observation that the growth of NZ9000(pNZ-CAB) was no better in M17 medium with lactose than in M17 medium alone (data not shown).
The supplementation of the medium with l-arginine or hemin improved the CDW of L. lactis but did not increase PHB accumulation (Table 3). Two moles of ammonium produced from 1 mol of l-arginine via the arginine deiminase pathway previously was shown to improve the growth of L. lactis by neutralizing the lactate-mediated acidic pH of the medium (8, 34). Hemin has been demonstrated to enable the respiratory growth of L. lactis in the presence of oxygen (12). It was found to increase the final growth yield and improve long-term survival, probably by helping to maintain a higher pH (12, 14, 46). Hemin also was reported to have a suppressive effect on lactate formation, which was thought to be due to the presence of alternative electron sinks for NAD regeneration and/or lactate consumption (36). Improved growth with both l-arginine and hemin did not result in increased PHB production (Table 3), thus carbon must have been converted to biomass and not PHB precursors.
L. lactis accumulated only 6% (wt/wt) PHB of CDW compared to 60 to 80% (wt/wt) for recombinant E. coli and C. necator, as well as 50% (wt/wt) reported for the gram-positive recombinant C. glutamicum (25, 32). It is expected that the PHB yield for L. lactis can be improved, but this most likely will require more fundamental measures than modifications of medium composition, growth, and induction conditions. In order to determine the most effective means to increase PHB production, it might be advisable to monitor metabolic fluxes. The use of an Ldh (lactate dehydrogenase) mutant strain might help to redistribute the pyruvate pool/flux. Another possibility is modifying the strength of the expression of the individual pha genes. In the case of C. glutamicum, a significant increase in recombinant PHB accumulation was obtained by gene dosage (25).
PHB granules isolated from L. lactis NZ9000 harboring pNZ-CAB and pNZ-ZZCAB were small (diameter, 100 to 200 nm) and uniform in size (Fig. 1) compared to the average sizes reported in the literature for other organisms (100 to 500 nm or even larger) (43). Even after the longer cultivation of L. lactis (48 h instead of 24 h), granule size did not significantly increase (data not shown). Thus, the small granule size might reflect the small cell size of L. lactis or the relatively meager PHB accumulation. With regard to therapeutic applications of nanoparticles, and when, for example, considering drug delivery, size has been shown to be one of the major factors for successful delivery apart from chemical properties. The efficiency and speed of cellular uptake and even the mechanism of endocytic internalization (and thus the fate of the drug) can be size dependent (23, 45).
The protein profile of proteins associated with granules isolated from L. lactis was different from that obtained for E. coli, but interestingly one of the more prominent proteins in both organisms was elongation factor Tu (see Fig. S3 in the supplemental material) (3). PhaC or ZZ-PhaC was the dominant protein at the surface of the respective granules from recombinant L. lactis. Another protein found at the granule surface was β-ketothiolase (PhaA), but like the identified L. lactis proteins, this is most likely an isolation artifact and not physiologically relevant.
ZZ domain-displaying PHB beads isolated from L. lactis were functional in both ELISA and IgG purification (Fig. 2 and 3). However, the L. lactis ZZ beads showed a greater IgG binding capacity in ELISA and a slightly increased IgG purification capacity than those of E. coli ZZ beads. This could be due to a higher density of ZZ-PhaC fusion protein at the bead surface as well as the significantly smaller size of the L. lactis-derived beads. The superior performance of the L. lactis ZZ domain-displaying granules and the advantage of this safe, LPS-free production organism suggested that L. lactis can be conceived as an alternative host for the manufacture of functionalized biopolyester beads. This is particularly relevant for the production of functionalized biopolyester beads designed for medical application where a food-grade production host such as L. lactis and even the food-grade inducer nisin might provide an important advantage. In addition, the NICE system has a strong inducible promoter that is suitable for the efficient production of heterologous proteins, which is required for the functionality of biopolyester beads displaying the PHA synthase fusion proteins at high density (35, 47).
Supplementary Material
Acknowledgments
We thank NIZO Food Research (Ede, The Netherlands) for permission to use the strains and plasmids of the NICE system and Gillian Norris (IMBS, Massey University, New Zealand) for making these strains and plasmids available to us. Anika Jahns and Jane Atwood provided E. coli-derived ZZ beads. Furthermore, we thank Toshiaki Fukui (Department of Bioengineering, Graduate School of Bioscience and Biotechnology, Tokyo Institute of Technology, Japan) for his kind acceptance of this collaborative project.
J.M. was supported financially by the Integrated Doctoral Course, Tokyo Institute of Technology. In addition, we are grateful for research funding provided by Massey University.
Footnotes
Published ahead of print on 22 May 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Amara, A. A., and B. H. A. Rehm. 2003. Replacement of the catalytic nucleophile cysteine 296 by serine in class II polyhydroxyalkanoate synthase from Pseudomonas aeruginosa mediated synthesis of a new polyester: identification of catalytic residues. Biochem. J. 374:413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amara, A. A., A. Steinbüchel, and B. H. A. Rehm. 2002. In vivo evolution of the Aeromonas punctata polyhydroxyalkanoate (PHA) synthase: isolation and characterization of modified PHA synthases with enhanced activity. Appl. Microbiol. Biotechnol. 59:477-482. [DOI] [PubMed] [Google Scholar]
- 3.Bäckström, T. B., J. A. Brockelbank, and B. H. A. Rehm. 2007. Recombinant Escherichia coli produces tailor-made biopolyester granules for applications in fluorescence activated cell sorting: Functional display of the mouse interleukin-2 and myelin oligodendrocyte glycoprotein. BMC Biotechnol. 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandl, H., R. A. Gross, R. W. Lenz, and R. C. Fuller. 1988. Pseudomonas oleovorans as a source of poly(beta-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl. Environ. Microbiol. 54:1977-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brockelbank, J. A., V. Peters, and B. H. A. Rehm. 2006. Recombinant Escherichia coli strain produces a ZZ domain displaying biopolyester granules suitable for immunoglobulin G purification. Appl. Environ. Microbiol. 72:7394-7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandrapati, S., and D. J. O'Sullivan. 2002. Characterization of the promoter regions involved in galactose- and nisin-mediated induction of the nisA gene in Lactococcus lactis ATCC 11454. Mol. Microbiol. 46:467-477. [DOI] [PubMed] [Google Scholar]
- 7.Chen, G. Q., and Q. Wu. 2005. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 26:6565-6578. [DOI] [PubMed] [Google Scholar]
- 8.Crow, V. L., and T. D. Thomas. 1982. Arginine metabolism in lactic streptococci. J. Bacteriol. 150:1024-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Ruyter, P. G., O. P. Kuipers, M. M. Beerthuyzen, I. van Alen-Boerrigter, and W. M. de Vos. 1996. Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J. Bacteriol. 178:3434-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Ruyter, P. G., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vos, W. M., and E. E. Vaughan. 1994. Genetics of lactose utilization in lactic acid bacteria. FEMS Microbiol. Rev. 15:217-237. [DOI] [PubMed] [Google Scholar]
- 12.Duwat, P., S. Sourice, B. Cesselin, G. Lamberet, K. Vido, P. Gaudu, Y. Le Loir, F. Violet, P. Loubiere, and A. Gruss. 2001. Respiration capacity of the fermenting bacterium Lactococcus lactis and its positive effects on growth and survival. J. Bacteriol. 183:4509-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaudu, P., G. Lamberet, S. Poncet, and A. Gruss. 2003. CcpA regulation of aerobic and respiration growth in Lactococcus lactis. Mol. Microbiol. 50:183-192. [DOI] [PubMed] [Google Scholar]
- 15.Gaudu, P., K. Vido, B. Cesselin, S. Kulakauskas, J. Tremblay, L. Rezaiki, G. Lamberet, S. Sourice, P. Duwat, and A. Gruss. 2002. Respiration capacity and consequences in Lactococcus lactis. Antonie van Leeuwenhoek 82:263-269. [PubMed] [Google Scholar]
- 16.Grage, K., A. C. Jahns, N. Parlane, R. Palanisamy, I. A. Rasiah, J. A. Atwood, and B. H. A. Rehm. 2009. Bacterial polyhydroxyalkanoate granules: biogenesis, structure, and potential use as nano-/micro-beads in biotechnological and biomedical applications. Biomacromolecules 10:660-669. [DOI] [PubMed] [Google Scholar]
- 17.Grage, K., and B. H. A. Rehm. 2008. In vivo production of scFv-displaying biopolymer beads using a self-assembly promoting fusion partner. Bioconj. Chem. 19:254-262. [DOI] [PubMed] [Google Scholar]
- 18.Grossiord, B. P., E. J. Luesink, E. E. Vaughan, A. Arnaud, and W. M. de Vos. 2003. Characterization, expression, and mutation of the Lactococcus lactis galPMKTE genes involved in galactose utilization via the Leloir pathway. J. Bacteriol. 185:870-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 20.Holo, H., and I. F. Nes. 1989. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl. Environ. Microbiol. 55:3119-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hugenholtz, J., M. Kleerebezem, M. Starrenburg, J. Delcour, W. de Vos, and P. Hols. 2000. Lactococcus lactis as a cell factory for high-level diacetyl production. Appl. Environ. Microbiol. 66:4112-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jahns, A. C., R. G. Haverkamp, and B. H. A. Rehm. 2008. Multifunctional inorganic-binding beads self-assembled inside engineered bacteria. Bioconj. Chem. 19:2072-2080. [DOI] [PubMed] [Google Scholar]
- 23.Jiang, W., B. Y. Kim, J. T. Rutka, and W. C. Chan. 2008. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 3:145-150. [DOI] [PubMed] [Google Scholar]
- 24.Jo, S. J., M. Maeda, T. Ooi, and S. Taguchi. 2006. Production system for biodegradable polyester polyhydroxybutyrate by Corynebacterium glutamicum. J. Biosci. Bioeng. 102:233-236. [DOI] [PubMed] [Google Scholar]
- 25.Jo, S. J., K. Matsumoto, C. R. Leong, T. Ooi, and S. Taguchi. 2007. Improvement of poly(3-hydroxybutyrate) [P(3HB)] production in Corynebacterium glutamicum by codon optimization, point mutation and gene dosage of P(3HB) biosynthetic genes. J. Biosci. Bioeng. 104:457-463. [DOI] [PubMed] [Google Scholar]
- 26.Kuipers, O. P., M. M. Beerthuyzen, P. G. de Ruyter, E. J. Luesink, and W. M. de Vos. 1995. Autoregulation of nisin biosynthesis in Lactococcus lactis by signal transduction. J. Biol. Chem. 270:27299-27304. [DOI] [PubMed] [Google Scholar]
- 27.Kuipers, O. P., M. M. Beerthuyzen, R. J. Siezen, and W. M. De Vos. 1993. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for development of immunity. Eur. J. Biochem. 216:281-291. [DOI] [PubMed] [Google Scholar]
- 28.Kuipers, O. P., P. de Ruyter, M. Kleerebezem, and W. M. de Vos. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15-21. [Google Scholar]
- 29.Kuipers, O. P., P. G. de Ruyter, M. Kleerebezem, and W. M. de Vos. 1997. Controlled overproduction of proteins by lactic acid bacteria. Trends Biotechnol. 15:135-140. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 31.Lee, S. Y., K. M. Lee, H. N. Chan, and A. Steinbüchel. 1994. Comparison of recombinant Escherichia coli strains for synthesis and accumulation of poly-(3-hydroxybutyric acid) and morphological changes. Biotechnol. Bioeng. 44:1337-1347. [DOI] [PubMed] [Google Scholar]
- 32.Madison, L. L., and G. W. Huisman. 1999. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. 63:21-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magalhães, P. O., A. M. Lopes, P. G. Mazzola, C. Rangel-Yagui, T. C. Penna, and A. Pessoa, Jr. 2007. Methods of endotoxin removal from biological preparations: a review. J. Pharm. Pharm. Sci. 10:388-404. [PubMed] [Google Scholar]
- 34.Marquis, R. E., G. R. Bender, D. R. Murray, and A. Wong. 1987. Arginine deiminase system and bacterial adaptation to acid environments. Appl. Environ. Microbiol. 53:198-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mierau, I., and M. Kleerebezem. 2005. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl. Microbiol. Biotechnol. 68:705-717. [DOI] [PubMed] [Google Scholar]
- 36.Nagayasu, M., A. K. Wardani, K. Nagahisa, H. Shimizu, and S. Shioya. 2007. Analysis of hemin effect on lactate reduction in Lactococcus lactis. J. Biosci. Bioeng. 103:529-534. [DOI] [PubMed] [Google Scholar]
- 37.Nouaille, S., L. A. Ribeiro, A. Miyoshi, D. Pontes, Y. Le Loir, S. C. Oliveira, P. Langella, and V. Azevedo. 2003. Heterologous protein production and delivery systems for Lactococcus lactis. Genet. Mol. Res. 2:102-111. [PubMed] [Google Scholar]
- 38.Peters, V., and B. H. A. Rehm. 2006. In vivo enzyme immobilization by use of engineered polyhydroxyalkanoate synthase. Appl. Environ. Microbiol. 72:1777-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters, V., and B. H. A. Rehm. 2008. Protein engineering of streptavidin for in vivo assembly of streptavidin beads. J. Biotechnol. 134:266-274. [DOI] [PubMed] [Google Scholar]
- 40.Petsch, D., and F. B. Anspach. 2000. Endotoxin removal from protein solutions. J. Biotechnol. 76:97-119. [DOI] [PubMed] [Google Scholar]
- 41.Rehm, B. H. A. 2003. Polyester synthases: natural catalysts for plastics. Biochem. J. 376:15-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rehm, B. H. A. 2006. Genetics and biochemistry of polyhydroxyalkanoate granule self-assembly: the key role of polyester synthases. Biotechnol. Lett. 28:207-213. [DOI] [PubMed] [Google Scholar]
- 43.Rehm, B. H. A. 2007. Biogenesis of microbial polyhydroxyalkanoate granules: a platform technology for the production of tailor-made bioparticles. Curr. Issues Mol. Biol. 9:41-62. [PubMed] [Google Scholar]
- 44.Rehm, B. H. A., R. V. Antonio, P. Spiekermann, A. A. Amara, and A. Steinbüchel. 2002. Molecular characterization of the poly(3-hydroxybutyrate) (PHB) synthase from Ralstonia eutropha: in vitro evolution, site-specific mutagenesis and development of a PHB synthase protein model. Biochim. Biophys. Acta 1594:178-190. [DOI] [PubMed] [Google Scholar]
- 45.Rejman, J., V. Oberle, I. S. Zuhorn, and D. Hoekstra. 2004. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 377:159-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rezaïki, L., B. Cesselin, Y. Yamamoto, K. Vido, E. van West, P. Gaudu, and A. Gruss. 2004. Respiration metabolism reduces oxidative and acid stress to improve long-term survival of Lactococcus lactis. Mol. Microbiol. 53:1331-1342. [DOI] [PubMed] [Google Scholar]
- 47.Rupa, P., V. Monedero, and B. N. Wilkie. 2008. Expression of bioactive porcine interferon-gamma by recombinant Lactococcus lactis. Vet. Microbiol. 129:197-202. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Plainview, NY.
- 49.Sutcliffe, I. C., and N. Shaw. 1991. Atypical lipoteichoic acids of gram-positive bacteria. J. Bacteriol. 173:7065-7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teuber, M. 1995. The genus Lactococcus, p. 173-234. In B. J. B. Wood and W. H. Holzapfel (ed.), The genera of lactic acid bacteria. Blackie Academic & Professional, London, United Kingdom.
- 51.Thomas, T. D., K. W. Turner, and V. L. Crow. 1980. Galactose fermentation by Streptococcus lactis and Streptococcus cremoris: pathways, products, and regulation. J. Bacteriol. 144:672-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valappil, S. P., A. R. Boccaccini, C. Bucke, and I. Roy. 2007. Polyhydroxyalkanoates in gram-positive bacteria: insights from the genera Bacillus and Streptomyces. Antonie van Leeuwenhoek 91:1-17. [DOI] [PubMed] [Google Scholar]
- 53.Wells, J. M., P. W. Wilson, and R. W. Le Page. 1993. Improved cloning vectors and transformation procedure for Lactococcus lactis. J. Appl. Bacteriol. 74:629-636. [DOI] [PubMed] [Google Scholar]
- 54.Williams, S. F., D. P. Martin, D. M. Horowitz, and O. P. Peoples. 1999. PHA applications: Addressing the price performance issue I. Tissue engineering. Int. J. Biol. Macromol. 25:111-121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.