Abstract
The roles of the two ldh genes of Enterococcus faecalis were studied using knockout mutants. Deletion of ldh-1 causes a metabolic shift from homolactic fermentation to ethanol, formate, and acetoin production, with a high level of formate production even under aerobic conditions. Ldh-2 plays only a minor role in lactate production.
Carbohydrate metabolism in economically important lactic acid bacterial species, such as Lactococcus lactis, has been extensively studied. However, in lactic acid bacterial species such as Enterococcus faecalis, with less industrial value, research focus has so far been mostly on medical aspects. E. faecalis has two lactate dehydrogenase (ldh) genes (3, 17), with high similarity to ldhA and ldhB of Lactococcus lactis, respectively (2). In L. lactis, ldhA is responsible for all lactate production, while ldhB remains unexpressed. To understand the role of these genes in E. faecalis, and to gain more insight into energy metabolism, we made and characterized three ldh knockout mutants in E. faecalis V583, removing either ldh-1 (EF_0255), ldh-2 (EF_0641) or both.
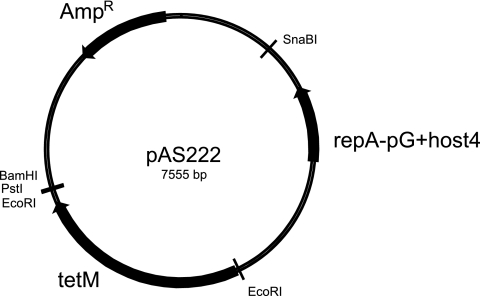
To achieve the construction of deletion mutants, we made a new shuttle vector based on the thermosensitive replicon of pG+host4 (12). Phusion DNA polymerase (Finnzyme, Espoo, Finland) was used for PCR in accordance with the manufacturer's recommendations. A part of pG+host4 was amplified with PCR using primers pgh41 and pgh42 (Table 1) and cloned in the HincII site of pBluescript SK+ to produce the plasmid pAS221. The tetM gene of Lactobacillus plantarum 5057 (4) was amplified by PCR using primers Dbd43f and Dbd44r (Table 1) and cloned in pCR2.1 (Invitrogen, United Kingdom), subsequently excised from the recombinant plasmid with EcoRI, and cloned in pAS221 cut with the same enzyme to yield pAS222 (Fig. 1). The various parts of the vector were verified by restriction enzyme analysis.
TABLE 1.
Primer sequences and their locations on the V583 chromosome
| Primer name | Sequence | Positions | Orientation |
|---|---|---|---|
| MJ1 | GAATACGTACTTGGCGGAAAATCAGCC | 230328-230346 | Forward |
| MJ2 | ATTACGTATGGCGCGTTCCTGTTGTC | 233258-233241 | Reverse |
| MJ3 | ACCAGGCGAAACTCGTTTAGACTTAGTTCATCGGTTGCACTAGCGCGTATC | 231989-231959, 231537-231518a | Reverse |
| MJ4 | ATGAACTAAGTCTAAACGAGTTTCGCCTGGTTTTTGAGGCGCACCAGCTG | 231959-232008 | Forward |
| MJ5 | TTGGTACGTATAGGTTAAGGTGG | 595643-595665 | Forward |
| MJ6 | TCATACGTACAATACTTCCTTCTC | 598586-598553 | Reverse |
| MJ7 | CTGCCAAGATGCCGATATCGTCGTGATTACTGAGTACTGCACGCATTG | 597301-597272, 596798-596781a | Reverse |
| MJ8 | GTAATCACGACGATATCGGCATCTTGGCAGTCTTGATAGTCGCCAGCCCG | 597272-597321 | Forward |
| Dbd43fb | GTCTAGATTTGAATGGAGGAAAATCACATGA | NAc | Forward |
| Dbd44rb | GGCATGCTGTCCGAACAGCGTTCGGATT | NA | Reverse |
| Pgh41 | AACCCTCTTTAATTTGGTTATATGA | NA | Forward |
| Pgh42 | CACGCATAAAATCCCCTTTCA | NA | Reverse |
Deletion primers spanning the gap.
Primers for amplification of the TetM gene, which is not located on the E. faecalis chromosome.
NA, not applicable.
FIG. 1.
Plasmid map of pAS222. Resistance genes and origins of replication are indicated by arrows. Restriction sites used in cloning are marked.
Gene replacement was achieved by double-crossover homologous recombination, using pAS222 as cloning vector. Deletion of about one-third of each ldh gene was done by two-step PCR (7) using the outer primer pairs MJ1-MJ2 for ldh-1 and MJ5-MJ6 for ldh-2 (Table 1). The inner primer pairs carrying regions of homology for the fusion step were MJ3-MJ4 for Δldh-1 and MJ7-MJ8 for Δldh-2 (Table 1). The constructs were cloned into the SnabI site of pAS222 and propagated in Escherichia coli cells. To replace the genes on the chromosome withthe constructs, these were transformed into electrocompetent V583 cells, where electrocompetence was achieved as described by Holo and Nes (8), with 4% to 6% glycine in the growth medium. Selection for double-crossover events was done as described by Biswas et al. (1), and tetracycline-sensitive mutants could be detected after approximately 50 to 100 generations at 28°C. The double mutant was made by using the Δldh-2 construct in Δldh-1 cells. The mutants were verified by DNA sequencing. No enzymatic lactate dehydrogenase activity could be detected in the double mutant.
All strains were grown overnight (22 h) at 37°C in batch in filled, tightly capped tubes (anaerobic) or in Erlenmeyer flasks with vigorous shaking (aerobic) in a chemically defined medium with 61 mM glucose as the carbon source (16, 19), supplemented with 0.283 mM adenine, 0.666 mM asparagine, 1.37 mM glutamine, 0.181 mM guanine, 0.208 mM l-cystine, 0.196 mM uracil, and 23.6 mM sodium bicarbonate (14).
The growth rate of the mutants was similar to that of the wild-type strain, V583. This result is similar to observations regarding, for example, L. lactis (2), where little or no difference in growth rate between wild-type strains and ldh knockout mutants could be observed. The mutants did not show reduced growth yields compared to the wild type.
Metabolic end products in the medium were analyzed using high-performance liquid chromatography and headspace gas chromatography (13, 15) (Table 2). As shown in Table 2, the ldh-2 mutant showed hardly any differences in growth and metabolism in comparison to the wild type. The two mutants lacking ldh-1, on the other hand, produced much less lactate than the wild type but grew to higher cell densities and had higher final pHs (Table 2). Mutants lacking ldh-1 produced lactate levels about 25% relative to those of the wild type. This indicates that the ldh-2 gene is not completely silent, as has been reported for L. lactis (6), but contributes to the total lactate production in E. faecalis. Some residual lactate production was seen in the double ldh mutant. Small amounts of lactate can be formed in other pathways (5). The residual lactate was not due to a large increase in d-lactate production.
TABLE 2.
Metabolites in supernatants from batch-grown E. faecalis V583 and the ldh mutantsa
| Strain or genotype | Aeration | Final OD600 | Final pH | Metabolite concn (mM)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Citrate | Lactate | Formate | Acetate | Ethanol | Acetoin | ||||
| V583 | − | 1.7 | 4.5 | 25.5 | 2.2 | 60.1 | 6.5 | 16.5 | 3.2 | 0.3 |
| + | 2.6 | 4.4 | 4.9 | 1.8 | 56.3 | 3.9 | 45.4 | 0.2 | 7.2 | |
| Δldh-1 | − | 2.7 | 5.5 | 4.8 | 0.7 | 16 | 28.2 | 7.1 | 39.2 | 23.9 |
| + | 3.0 | 6.3 | 0.2 | 0.4 | 11.4 | 17.4 | 32 | 15.4 | 21.1 | |
| Δldh-2 | − | 1.7 | 4.6 | 25.6 | 2.2 | 59.9 | 6.5 | 16.6 | 3.3 | 0.5 |
| + | 2.7 | 4.4 | 4.3 | 1.8 | 55.0 | 2.5 | 44 | 0.3 | 8.7 | |
| Δldh-1 Δldh-2 | − | 2.8 | 5.9 | 4.0 | 0.2 | 5.3 | 27.3 | 8.5 | 41.7 | 21.1 |
| + | 2.7 | 6.3 | 0 | 0.2 | 3.5 | 20.8 | 33.1 | 11.4 | 24.6 | |
Values are averages of results from three separate experiments. The composition of the growth medium was as follows: glucose, 61 mM; citrate, 2.20 mM; lactate, 0.3 mM; formate, 1.4 mM; acetate, 15.7 mM; ethanol, 0.1 mM; and acetoin, 0.1 mM. OD600, optical density at 600 nm.
The wild-type V583 strain converted glucose almost exclusively to lactate under anaerobic conditions. With aeration, this strain made the same amounts of lactate as was observed during anaerobic growth but also produced considerable amounts of acetate, resulting in a lactate/acetate ratio of 2:1 (Table 2).
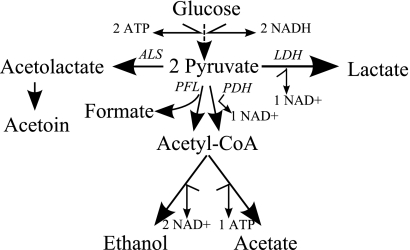
The metabolic profile of mutants lacking ldh-1 showed large differences from the wild type. Without aeration, the metabolic end products from Δldh-1 and Δldh-1 Δldh-2 were mostly formate, ethanol, and acetoin (Table 2). With aeration, Δldh-1 and Δldh-1 Δldh-2 produced acetate but also considerable amounts of ethanol and formate, indicating high activity levels for both pyruvate formate lyase (Pfl) and pyruvate dehydrogenase (Fig. 1). The data imply that these two enzymes made fairly equal contributions to metabolism of pyruvate under aerobic conditions. Like Pfl from other sources, the E. faecalis enzyme has been reported to be readily inactivated by oxygen (11, 22). However, citrate synthase-deficient E. coli can produce formate by using Pfl under aerobic conditions (10).
The ldh-1 mutants detoxified excess pyruvate by converting it to acetoin (Fig. 2), which can play a useful role in maintaining intracellular pH by affecting the proton motive force (9, 23). We observed no butanediol production (Table 2). The E. faecalis mutants produced the same amounts of acetoin during anaerobic and aerobic conditions, in contrast to L. lactis, where acetoin production increases in the presence of oxygen, and butanediol production can be observed under certain conditions during anaerobic growth (2, 18).
FIG. 2.
Glucose metabolism in Enterococcus faecalis. LDH, lactate dehydrogenase; ALS, acetolactate synthase; PFL, pyruvate formate lyase; PDH, pyruvate dehydrogenase.
In the Δldh-1 cultures, acetate and citrate were consumed under anaerobic conditions, indicating that E. faecalis can use these compounds as external electron acceptors. We measured citrate and glucose utilization in wild-type V583 cells and in Δldh-1 Δldh-2 at 1-h intervals during growth by using K-CITR and K-GLUHKR kits from Megazyme (Bray, Ireland). Unlike the wild type, the mutant showed concomitant consumption of citrate and glucose (data not shown). Citrate utilization is regulated by catabolite repression in E. faecalis (20), so apparently, one effect of Δldh-1 is to relieve, at least in part, catabolite repression.
In conclusion, we have shown that although Ldh-2 contributes to lactate production, Ldh-1 plays the major role in energy metabolism in E. faecalis. We show that Ldh-2 alone is not able to handle the large amounts of pyruvate formed in glycolysis. This leads to production of mixed acids, as is also observed in ldh knockouts in other lactic acid bacterial species. Further metabolic studies of the ldh mutants will provide valuable insight and maybe keys to regulation of pathogenicity and virulence (21).
Acknowledgments
This work was supported by the SysMO project, funded by the Norwegian Research Council.
Thanks to Kari Olsen for analyzing metabolic samples.
Footnotes
Published ahead of print on 22 May 2009.
REFERENCES
- 1.Biswas, I., A. Gruss, S. D. Ehrlich, and E. Maguin. 1993. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J. Bacteriol. 175:3628-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bongers, R. S., M. H. Hoefnagel, M. J. Starrenburg, M. A. Siemerink, J. G. Arends, J. Hugenholtz, and M. Kleerebezem. 2003. IS981-mediated adaptive evolution recovers lactate production by ldhB transcription activation in a lactate dehydrogenase-deficient strain of Lactococcus lactis. J. Bacteriol. 185:4499-4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourgogne, A., D. A. Garsin, X. Qin, K. V. Singh, J. Sillanpaa, S. Yerrapragada, Y. Ding, S. Dugan-Rocha, C. Buhay, H. Shen, G. Chen, G. Williams, D. Muzny, A. Maadani, K. A. Fox, J. Gioia, L. Chen, Y. Shang, C. A. Arias, S. R. Nallapareddy, M. Zhao, V. P. Prakash, S. Chowdhury, H. Jiang, R. A. Gibbs, B. E. Murray, S. K. Highlander, and G. M. Weinstock. 2008. Large scale variation in Enterococcus faecalis illustrated by the genome analysis of strain OG1RF. Genome Biol. 9:R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danielsen, M. 2002. Characterization of the tetracycline resistance plasmid pMD5057 from Lactobacillus plantarum 5057 reveals a composite structure. Plasmid 48:98-103. [DOI] [PubMed] [Google Scholar]
- 5.Ferain, T., A. N. Schanck, and J. Delcour. 1996. 13C nuclear magnetic resonance analysis of glucose and citrate end products in an ldhL-ldhD double-knockout strain of Lactobacillus plantarum. J. Bacteriol. 178:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaspar, P., A. R. Neves, C. A. Shearman, M. J. Gasson, A. M. Baptista, D. L. Turner, C. M. Soares, and H. Santos. 2007. The lactate dehydrogenases encoded by the ldh and ldhB genes in Lactococcus lactis exhibit distinct regulation and catalytic properties—comparative modeling to probe the molecular basis. FEBS J. 274:5924-5936. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi, R. 1990. Recombinant PCR, p. 177-183. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA.
- 8.Holo, H., and I. F. Nes. 1989. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl. Environ. Microbiol. 55:3119-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hugenholtz, J., L. Perdon, and T. Abee. 1993. Growth and energy generation by Lactococcus lactis subsp. lactis biovar diacetylactis during citrate metabolism. Appl. Environ. Microbiol. 59:4216-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee, J., A. Goel, M. M. Ataai, and M. M. Domach. 1994. Flux adaptations of citrate synthase-deficient Escherichia coli. Ann. N. Y. Acad. Sci. 745:35-50. [DOI] [PubMed] [Google Scholar]
- 11.Lindmark, D. G., P. Paolella, and N. P. Wood. 1969. The pyruvate formate-lyase system of Streptococcus faecalis. I. Purification and properties of the formate-pyruvate exchange enzyme. J. Biol. Chem. 244:3605-3612. [PubMed] [Google Scholar]
- 12.Maguin, E., H. Prevost, S. D. Ehrlich, and A. Gruss. 1996. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J. Bacteriol. 178:931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsili, R. T. 1981. Monitoring bacterial metabolites in cultured buttermilk by high performance liquid chromatography and headspace gas chromatography. J. Chromatogr. Sci. 19:451. [DOI] [PubMed] [Google Scholar]
- 14.Mickelson, M. N. 1964. Chemically defined medium for growth of Streptococcus pyogenes. J. Bacteriol. 88:158-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narvhus, J. A., K. Thorvaldsen, and R. K. Abrahamsen. 1990. Quantitative determination of volatile compounds produced by Lactococcus spp. using direct automatic headspace gas chromatography. Brief communications and abstracts of posters, vol. II, p. 522. XXII Int. Dairy Congr., Montreal, Canada. [Google Scholar]
- 16.Otto, R., A. S. Sonnenberg, H. Veldkamp, and W. N. Konings. 1980. Generation of an electrochemical proton gradient in Streptococcus cremoris by lactate efflux. Proc. Natl. Acad. Sci. USA 77:5502-5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulsen, I. T., L. Banerjei, G. S. Myers, K. E. Nelson, R. Seshadri, T. D. Read, D. E. Fouts, J. A. Eisen, S. R. Gill, J. F. Heidelberg, H. Tettelin, R. J. Dodson, L. Umayam, L. Brinkac, M. Beanan, S. Daugherty, R. T. DeBoy, S. Durkin, J. Kolonay, R. Madupu, W. Nelson, J. Vamathevan, B. Tran, J. Upton, T. Hansen, J. Shetty, H. Khouri, T. Utterback, D. Radune, K. A. Ketchum, B. A. Dougherty, and C. M. Fraser. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071-2074. [DOI] [PubMed] [Google Scholar]
- 18.Platteeuw, C., J. Hugenholtz, M. Starrenburg, I. van Alen-Boerrigter, and W. M. de Vos. 1995. Metabolic engineering of Lactococcus lactis: influence of the overproduction of alpha-acetolactate synthase in strains deficient in lactate dehydrogenase as a function of culture conditions. Appl. Environ. Microbiol. 61:3967-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poolman, B., and W. N. Konings. 1988. Relation of growth of Streptococcus lactis and Streptococcus cremoris to amino acid transport. J. Bacteriol. 170:700-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rea, M. C., and T. M. Cogan. 2003. Catabolite repression in Enterococcus faecalis. Syst. Appl. Microbiol. 26:159-164. [DOI] [PubMed] [Google Scholar]
- 21.Richardson, A. R., S. J. Libby, and F. C. Fang. 2008. A nitric oxide-inducible lactate dehydrogenase enables Staphylococcus aureus to resist innate immunity. Science 319:1672-1676. [DOI] [PubMed] [Google Scholar]
- 22.Snoep, J. L., M. J. Teixeira de Mattos, P. W. Postma, and O. M. Neijssel. 1990. Involvement of pyruvate dehydrogenase in product formation in pyruvate-limited anaerobic chemostat cultures of Enterococcus faecalis NCTC 775. Arch. Microbiol. 154:50-55. [DOI] [PubMed] [Google Scholar]
- 23.Tsau, J. L., A. A. Guffanti, and T. J. Montville. 1992. Conversion of pyruvate to acetoin helps to maintain pH homeostasis in Lactobacillus plantarum. Appl. Environ. Microbiol. 58:891-894. [DOI] [PMC free article] [PubMed] [Google Scholar]