Abstract
Beta-hemolytic group C and G streptococci cause a considerable invasive disease burden and sometimes cause disease outbreaks. Little is known about the critical epidemiologic parameter of genetic relatedness between isolates. We determined the emm types of 334 Streptococcus dysgalactiae subsp. equisimilis isolates, and attempted emm typing of 5 Streptococcus canis isolates from a recent population-based surveillance for invasive isolates. Thirty-four emm types were observed, including one from S. canis. We formulated multilocus sequence typing (MLST) primers with six of the seven loci corresponding to the Streptococcus pyogenes MLST scheme. We performed MLST with 65 of the 334 surveillance isolates (61 S. dysgalactiae subsp. equisimilis isolates, 4 S. canis isolates) to represent each emm type identified, including 2 to 3 isolates for each of the 25 redundantly represented emm types. Forty-one MLST sequence types (STs) were observed. Isolates within 16 redundantly represented S. dysgalactiae subsp. equisimilis emm types shared identical or nearly identical STs, demonstrating concordance between the emm type and genetic relatedness. However, seven STs were each represented by two to four different emm types, and 7 of the 10 S. dysgalactiae subsp. equisimilis eBURST groups represented up to six different emm types. Thus, S. dysgalactiae subsp. equisimilis isolates were similar to S. pyogenes isolates, in that strains of the same emm type were often highly related, but they differed from S. pyogenes, in that S. dysgalactiae subsp. equisimilis strains with identical or closely similar STs often exhibited multiple unrelated emm types. The phylogenetic relationships between S. dysgalactiae subsp. equisimilis and S. pyogenes alleles revealed a history of interspecies recombination, with either species often serving as genetic donors. The four S. canis isolates shared highly homologous alleles but were unrelated clones without evidence of past recombination with S. dysgalactiae subsp. equisimilis or S. pyogenes.
Streptococcus pyogenes, Streptococcus dysgalactiae subsp. equisimilis, and Streptococcus canis are beta-hemolytic pyogenic species that are highly genetically related on the basis of 16S rRNA sequence comparisons, with S. canis being the closest relative to S. pyogenes (39, 16). In recent years, S. dysgalactiae subsp. equisimilis has increasingly been reported as a cause of invasive disease (11, 24, 36), yet the epidemiology and population genetics of this species is poorly understood. S. canis is a commensal and opportunistic pathogen of dogs and other animals (13, 16). S. canis rarely causes disease in humans (7, 39); however, its incidence in human disease is unknown.
As with S. pyogenes, S. dysgalactiae subsp. equisimilis isolates are almost always emm typeable (8, 11, 24, 39), with over 50 known emm types (emm types are downloadable from ftp://ftp.cdc.gov/pub/infectious_diseases/biotech/tsemm/). During 1998, we documented the occurrence of emm type stG1389 from an invasive S. canis infection in a dog (unpublished data), and we also recently noticed the same sequence in the GenBank database (GenBank accession number EU195120). To our knowledge, this is the only emm type documented from S. canis. Several other virulence determinants are known to be shared between S. dysgalactiae subsp. equisimilis and S. pyogenes (9, 10, 12, 19, 25). S. pyogenes exotoxin genes have been detected within S. dysgalactiae subsp. equisimilis and S. canis, and lysogenic transfers of prophages carrying superantigen genes between S. pyogenes and S. dysgalactiae subsp. equisimilis have been documented (20, 22, 31, 33, 38).
M-protein gene (emm) typing has been useful as a simple genetic tool for identifying and resolving Streptococcus pyogenes strains for epidemiologic studies (18, 27, 29, 35). This is consistent with the observation that within given regions, group A streptococcal (GAS) emm types are often restricted to only one or two defined clonal complexes, and often, these common clones are predominant in diverse locations (15) (available at www.mlst.net). In direct contrast, a previous report described a poor concordance between the emm type and genetic relatedness in S. dysgalactiae subsp. equisimilis (23). That report additionally showed evidence of the lateral movement of housekeeping genes between the two species, with the majority of gene transfer events involving S. pyogenes donors and S. dysgalactiae subsp. equisimilis recipients.
We recently carried out population-based surveillance for invasive disease caused by beta-hemolytic streptococci of groups other than A and B, and the results from 2 years of surveillance were reported (7). The majority of case isolates (about 80%) were S. dysgalactiae subsp. equisimilis. The only other emm-typeable species identified by active surveillance was S. canis, of which only one of five S. canis isolates was emm typeable. We found that the burden of invasive disease caused by S. dysgalactiae subsp. equisimilis is comparable to that caused by invasive GAS (29) and affects similar adult populations (7). In the work presented here, we describe newly discovered relationships between multilocus sequence types and emm types among an expanded collection of invasive S. dysgalactiae subsp. equisimilis and S. canis isolates recovered in the United States from 2002 to 2005.
MATERIALS AND METHODS
Isolates.
Surveillance for invasive streptococcal disease caused by beta-hemolytic streptococci of groups other than A and B was conducted as part of CDC's Active Bacterial Core surveillance from 2002 to 2005 in the eight-county metropolitan Atlanta, GA, area (population, 4,464,200 in 2003) and in 2003 and 2004 in the three-county San Francisco, CA, Bay Area (population, 3,213,848 in 2003). Isolates were recovered from a normally sterile site (e.g., blood, cerebrospinal fluid, or bone) from cases that included only residents of the two surveillance areas. The isolates were initially identified as non-group A and non-group B streptococci at local hospital laboratories by using standard, commercially available latex agglutination kits. All available isolates were sent to CDC for verification and further characterization. The results of emm typing for 212 S. dysgalactiae subsp. equisimilis isolates and 5 S. canis isolates were reported previously (7). An additional 122 S. dysgalactiae subsp. equisimilis isolates were collected through continued surveillance through 2005. The combined group of 344 invasive S. dysgalactiae subsp. equisimilis and S. canis isolates were further evaluated by multilocus sequence typing (MLST).
Species identification.
Conventional biochemical tests were used for the identification of the beta-hemolytic streptococcal species, as described previously (7) and at http://www.cdc.gov/ncidod/biotech/strep/strep-doc/index.htm.
emm typing.
Crude template preparation and emm typing were performed as described at http://www.cdc.gov/ncidod/biotech/strep/protocols.htm. For S. pyogenes, the M-protein gene (emm) types were determined exactly as described at http://www.cdc.gov/ncidod/biotech/strep/protocol_emm-type.htm. All emm type-specific sequences described here are downloadable from the CDC database (ftp://ftp.cdc.gov/pub/infectious_diseases/biotech/tsemm/).
MLST.
The 65 isolates were chosen from among 334 invasive S. dysgalactiae subsp. equisimilis and S. canis isolates to represent all different emm types and, when possible, to represent multiple independent isolates that shared the same emm type. When possible, we selected isolates that shared the same emm type and that were collected in different years and from different surveillance areas. Included among these 65 isolates were 4 of the 5 S. canis isolates recovered, 1 of which was emm typeable.
MLST was performed with seven primer sets based on the deduced closest matches of S. dysgalactiae subsp. equisimilis to the S. pyogenes MLST targets (gki, gtr, murI, mutS, recP, xpt, and yqiL), described at http://spyogenes.mlst.net/. These primer sequences are posted at http://www.cdc.gov/ncidod/biotech/strep/protocols.htm. To formulate these primers, we employed a nearly completed S. dysgalactiae subsp. equisimilis genome sequence provided by Awdhesh Kalia (381 contigs, approximately 2,000,000 bp, 300 to 20,000 reads each). Within this genome sequence, no target was highly similar (>93% identity) to yqiL sequences previously described from S. dysgalactiae subsp. equisimilis (23); however, we did identify a less similar putative acetyl coenzyme A acetyltransferase gene that we designated yqiZ and that shared about 65% identity to the closest matches from S. pyogenes. We were unable to amplify a yqiL-like gene; however, portions of yqiZ were variably amplified with primers described previously (23) or with the S. pyogenes yqiL-specific primers described at http://spyogenes.mlst.net/. Thus, we formulated optimal yqiZ-specific primers and substituted yqiZ into our scheme in place of yqiL. Our MLST scheme additionally differed from that described previously for S. dysgalactiae subsp. equisimilis (23) in that instead of generating 430-bp gtr sequences, we generated 450-base gtr sequences that correspond to the S. pyogenes MLST scheme. These seven primer sets also appear to provide a potentially reliable MLST scheme for S. canis. In summary, our MLST scheme directly corresponds to the existing system for S. pyogenes, except for the inclusion of yqiZ instead of yqiL.
MLST allele nomenclature.
Four-digit designations (Table 1) were assigned to alleles newly discovered during this study, with the exception that the sequences of S. canis mutS alleles 5426, 6318, and 8881 and S. dysgalactiae subsp. equisimilis mutS allele 8343 perfectly matched the sequences with GenBank accession numbers AJ413209, AJ413210, EF619610, and AJ413208, respectively. Three-digit designations correspond to alleles discovered during a previous study (23). Two-digit designations (xpt31 and recp70) correspond to alleles previously documented at the S. pyogenes MLST database (available at www.mlst.net). Five-digit designations (three digits separated by a hyphen from two digits) correspond to previously documented designations for S. dysgalactiae subsp. equisimilis alleles (23) that are also documented in the S. pyogenes MLST database (the first three digits and last two digits correspond to previous designations in S. dysgalactiae subsp. equisimilis and S. pyogenes, respectively; see, for example, mutS106-46 in Table 1).
TABLE 1.
emm type, allelic compositions, and demographics associated with 10 S. dysgalactiae subsp. equisimilis eBURST groups and 41 MLST types of S. dysgalactiae subsp. equisimilis and S. canis
| Genetic group | ST, group(s) | emm type(s) (no. of isolates, state, yr recovered)a | Allele
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| gkib,c | gtrb,c | murIb,c | mutSb | recPb,c | xptb,c | yqiZd | |||
| eBURST 1 | Se6 (G) | stG11 (1, GA, 2005) | 106 | 105 | 105 | 106-46e | 113-50e | 104 | 8337 |
| Se7 (G) | stG480 (1, CA, 2005) | 106 | 108 | 105 | 106-46e | 113-50e | 104 | 8343 | |
| Se8 (G) | stC839(1, GA, 2003) stG480 (1, GA, 2002) stG7860 (1, GA, 2004) stG11 (1, CA, 2003) | 106 | 108 | 105 | 106-46e | 113-50e | 104 | 8337 | |
| Se9 (G) | stC6979 (1, GA, 2004) | 106 | 108 | 105 | 106-46e | 113-50e | 104 | 6458 | |
| eBURST 2 | Se12 | stG643 (1, GA, 2002) | 107 | 6458 | 4127 | 105 | 6458 | 109 | 6458 |
| Se16 | stG3442 (1, CA, 2003) | 107 | 6458 | 105 | 105 | 6458 | 104 | 6458 | |
| Se17 | stG2078 (3, CA, GA, 04, 05), stG485 (1, GA, 2004) | 107 | 6458 | 105 | 105 | 6458 | 109 | 6458 | |
| Se36 | stG245 (1, CA, 2003) | 107 | 6458 | 105 | 105 | 6458 | 109 | 8343 | |
| eBURST 3 | Se18 | stG7882(1, GA, 2003) | 107 | 106 | 110 | 106-46e | 104 | 108 | 6458 |
| Se19 | stC6979 (1, CA, 2003) | 108-74e | 6742 | 110 | 106-46e | 104 | 108 | 6458 | |
| Se31 | stG6792 (1, CA, 2004) | 108-74e | 106 | 110 | 106-46e | 104 | 108 | 6458 | |
| Se4 | stG97 (2, GA, 2002, 2005), stC5344 (2, GA, 2005), stG6792 (1, GA, 2004), stG7882 (1, CA, 2005) | 105 | 106 | 110 | 106-46e | 104 | 108 | 6458 | |
| eBURST 4 | Se27 | stC6746 (1, GA, 2004) | 105 | 106 | 110 | 106-46e | 7917 | 6746 | 6746 |
| Se5 | stC6746 (1, CA, 2003) | 105 | 106 | 110 | 106-46e | 7505 | 6746 | 6746 | |
| eBURST 5 | Se20 | stG62647 (2, CA, GA, 2005) | 108-74e | 105 | 107 | 6532 | 105-2e | 109 | 6532 |
| Se33 | stG62647 (1, CA, 2003) | 108-74e | 6742 | 107 | 6532 | 105-2e | 109 | 6532 | |
| eBURST 6 | Se34 | stC1400 (2, GA, 2003, 2004), stG5063 (1, CA, 2005) | 108-74e | 7934 | 110 | 106-46e | 7934 | 31spe | 3499 |
| Se35 | stC7934 (1, GA, 2003) | 108-74e | 7934 | 110 | 106-46e | 7934 | 31spe | 6458 | |
| eBURST 7 | Se26 | stG840 (1, CA, 2003) | 108-74e | 106 | 105 | 106-46e | 102 | 112 | 6458 |
| Se30 | stG840 (1, GA, 2004) | 108-74e | 106 | 110 | 106-46e | 102 | 112 | 6458 | |
| eBURST 8 | Se15 | stG10 (2, CA, GA, 02, 2004), stG6 (1, CA, 2003), stG166B (1, GA, 2002), stG652 (1, GA, 2002) | 108-74e | 105 | 107 | 105 | 105-2e | 110 | 6458 |
| Se21 | stG245 (1, GA, 2002) | 108-74e | 6742 | 107 | 105 | 105-2e | 110 | 6458 | |
| eBURST 9 | Se10 | stC7505 (1, GA, 2005) | 9182 | 6458 | 7505 | 7505 | 7505 | 9182 | 9182 |
| Se14 | stC9431 (1, GA, 2004) | 9182 | 6458 | 7505 | 6461 | 7505 | 9182 | 9182 | |
| eBURST 10 | Se24 | stG6 (1, GA, 2005) | 108-74e | 106 | 105 | 8343 | 4012 | 103-2e | 8343 |
| Se25 | stG5420 (2, CA, GA, 2004, 2005), stG166B (1, CA, 2004) | 108-74e | 106 | 105 | 8343 | 102 | 103-2e | 8343 | |
| Single | Se3 | emm57 (2, CA, GA, 2005), stG653 (2, GA, 2002, 2005) | 103 | 105 | 110 | 106-46e | 101-83e | 107-71e | 6317 |
| Se1 | stL1929 (1, GA, 2003) | 9182 | 9182 | 9182 | 6461 | 7505 | 9182 | 9182 | |
| Se11 | stC7901 (1, CA, 2004) | 7901 | 105 | 110 | 106-46e | 106-4e | 108 | 9544 | |
| Se13 | stC9431 (1, CA, 2005) | 9182 | 6461 | 9182 | 6461 | 7505 | 9182 | 6461 | |
| Se2 | stG5063 (1, GA, 2004) | 103 | 6458 | 110 | 106-46e | 106-4e | 31spe | 6458 | |
| Se22 | stG643 (1, GA, 2004) | 108-74e | 105 | 107 | 6532 | 113-50e | 6532 | 6532 | |
| Se23 | stG2574 (1, CA, 2005) | 108-74e | 105 | 110 | 106-46e | 112 | 104 | 6317 | |
| Se28 | stC1400 (1, CA, 2004) | 108-74e | 105 | 110 | 105 | 70spe | 8983 | 6458 | |
| Se29 | stC74a (2, CA, 2003) | 108-74e | 106 | 110 | 105 | 102 | 104 | 8343 | |
| Se32 | stG652 (1, CA, 2004) | 108-74e | 106 | 110 | 106-46e | 107 | 112 | 9544 | |
| Se37 | stG485 (1, CA, 2005) | 108-74e | 106 | 105 | 106-46e | 107 | 104 | 8343 | |
| Sc1 | stG1389 (1, GA, 2002) | 4074 | 4074 | 4074 | 4074 | 8881 | Not done | 4074 | |
| Sc2 | Nontypeable (1, GA, 2002) | 6318 | 5426 | 6318 | 6318 | 6318 | 6318 | 6318 | |
| Sc3 | Nontypeable (1, CA, 2003) | 8881 | 8881 | 8881 | 8881 | 8881 | 8881 | 8881 | |
| Sc4 | Nontypeable (1, GA, 2005) | 5426 | 5426 | 5426 | 5426 | 5426 | 5426 | 5426 | |
Isolates of the 16 boldface emm types exhibit identical or highly similar multilocus STs (no more than one allelic difference), with the exception that one of the three stC1400 isolates was genetically divergent from the other two. emm types that are underlined are represented by only a single isolate.
Three-digit designations correspond exactly to alleles discovered during the previous study (23), with the exception that our gtr sequences were 450 bases in length (as in http://spyogenes.mlst.net/) but shared identity in their overlap with the 430-base entries described previously (23). Four-digit designations correspond to alleles discovered during this study.
Boldface entries correspond to cluster II GGS/GCS-like alleles, according to previously described phylogenic analysis and the analysis performed during this study. Nonboldface entries correspond to cluster I GAS-like alleles. S. canis alleles belong to neither category and are italicized.
yqiZ is specific for the MLST scheme presented in this study, since we were unable to amplify the yqiL homolog of S. pyogenes that was described in the previous study (23).
Designations indicate alleles additionally found within S. pyogenes according to our previous data and according to data recorded at http://spyogenes.mlst.net/. In designations with hyphens (e.g., gki108-74), the first three-digit number refers to the designation described for S. dysgalactiae subsp. equisimilis in the previous study (23), while the number after the hyphen refers to the designation given for the same allele in S. pyogenes at http://spyogenes.mlst.net/. recP70sp and xpt31sp were not previously identified from S. dysgalactiae subsp. equisimilis and correspond to alleles found in the S. pyogenes MLST database.
eBURST analysis.
eBURST (version 3) analysis was performed with the program provided at http://spyogenes.mlst.net/eburst/with the MLST data generated from the study isolates. An eBURST group consists of a set of seven-locus allelic profiles in which each profile is related to at least one other profile in the set by sharing six of seven alleles.
Phylogenetic analysis.
Wisconsin Package programs (Wisconsin Package, version 10.3; Accelrys Inc., San Diego, CA) were used for all DNA sequence manipulations. Individual alleles (405 to 498 bases in length) and six-allele concatenated sequences totaling 2,700 bases (yqiL was not used since we could not amplify this gene from S. dysgalactiae subsp. equisimilis) from each different allelic combination found during this study were aligned precisely by use of the PileUp program. We used the Distances program to generate a matrix of the pairwise evolutionary distance between the aligned sequences. For analysis of individual loci, all known S. pyogenes MLST alleles and the alleles from S. dysgalactiae subsp. equisimilis and S. canis found in this study were included. For analysis of concatenated six-allele profiles, all study STs and 21 STs selected from the S. pyogenes MLST database were employed. These S. pyogenes STs were inclusive of certain major S. pyogenes clones found during U.S. surveillance and also of highly diverse STs from S. pyogenes that contained S. dysgalactiae subsp. equisimilis-like alleles. Tree construction was done by the neighbor-joining approach by use of the Jukes-Cantor model. One thousand bootstrap replicates were performed with the same data by using the Paupsearch program to give the frequencies of occurrence of bipartitions.
Nucleotide sequence accession numbers.
GenBank accession numbers EU768803 to EU768808 were assigned to the seven newly discovered gki alleles; GenBank accession numbers EU768892, EU938013 to EU938015, EU981290, EU999155, EU999156, FJ238468, and FJ414797-FJ515799 were assigned to the gtr alleles; GenBank accession numbers EU999157, FJ151276 to FJ151278, FJ156731, FJ128469, and FJ238470 were assigned to the murI alleles; GenBank accession numbers FJ238471 to FJ238474 were assigned to the new mutS alleles; GenBank accession numbers FJ238475 to FJ238481 and EU283342 were assigned to the recP alleles; GenBank accession numbers FJ238482 to FJ238488 were assigned to the xpt alleles; and GenBank accession numbers FJ348682, FJ263615, FJ263616, and FJ238646 to FJ268656 were assigned to the yqiZ alleles. The sequences of S. canis mutS alleles 5426, 6318, and 8881 and S. dysgalactiae subsp. equisimilis mutS allele 8343 perfectly matched the sequences with GenBank accession numbers AJ413209, AJ413210, EF619610, and AJ413208, respectively.
RESULTS
emm type diversity.
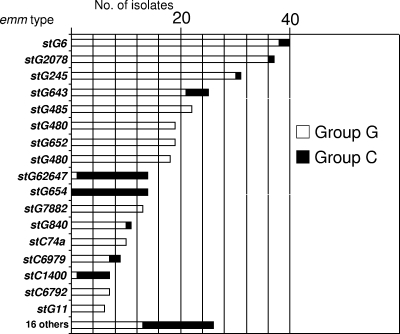
Thirty-three emm types were observed among the 334 invasive S. dysgalactiae subsp. equisimilis isolates, with five types each accounting for 20 to 40 isolates (Fig. 1). These S. dysgalactiae subsp. equisimilis isolates consisted of 275 group G streptococcal (GGS) strains, 58 group C streptococcal (GCS) strains, and 1 group L streptococcal (GLS) strain. Nine emm types represented both GGS and GCS strains. The type found from the single GLS strain, stL1929, has previously been shown to be associated only with GLS strains isolated as long as 28 years ago (unpublished observations; see http://www.cdc.gov/ncidod/biotech/strep/types_emm103-124.htm and ftp://ftp.cdc.gov/pub/infectious_diseases/biotech/tsemm/ for limited information regarding individual emm types). All five S. canis isolates were GGS, with only one of the isolates yielding an emm amplicon. This S. canis emm type was included in the CDC emm database in 1999 and corresponded to st1389 from an S. canis isolate recovered from a dog. The sequence of type stG1389 shares 99% identity in its 402-base overlap with the sequence with GenBank accession number EU195120 (from an S. canis isolate from the throat of a dog), deposited in the GenBank database in December 2007. None of the 34 emm types (including stG1389 from S. canis) were obvious derivatives of each other, and all shared less than 87% identity within the 50 codons encoding the predicted N terminus of the M protein, with most types sharing less than 50% identity. One type, emm57, is documented from reference strains of M serotype 57 S. pyogenes (4, 40) and was found in two GCS S. dysgalactiae subsp. equisimilis isolates recovered in California and Georgia in 2005 (Table 1; emm57 corresponds to MLST type Se3, which was not included within an eBURST group).
FIG. 1.
emm type distribution observed among 334 S. dysgalactiae subsp. equisimilis isolates recovered from population-based surveillance of invasive streptococcal disease in areas of California and Georgia from 2002 to 2005.
MLST results.
For the seven targets, the number of different alleles encountered among the 61 S. dysgalactiae subsp. equisimilis isolates ranged from as few as 6 (murI and mutS) to as many as 14 (recP) (Table 1). Sixty-two distinct alleles were found, 27 of which were identical to previously discovered alleles within a set of 34 S. dysgalactiae subsp. equisimilis isolates (23).
Our results differed markedly from those of a previous study in which each S. dysgalactiae subsp. equisimilis isolate corresponded to 1 of 34 distinct allelic profiles (23). In our study of 61 S. dysgalactiae subsp. equisimilis isolates, we found 37 allelic profiles, with 9 profiles each accounting for multiple (two to five) isolates. Also in contrast to the findings of the previous study (23), in which only a single pair of isolates shared the same eBURST group (by virtue of sharing six of seven identical alleles), our S. dysgalactiae subsp. equisimilis isolate set revealed 10 eBURST groups that represented 46 of the 61 isolates, with up to four different allelic profiles and 6 different isolates being represented in individual eBURST groups (Table 1). In addition, three individual STs that were not within eBURST groups accounted for three pairs of isolates.
We successfully applied the MLST protocol used for S. dysgalactiae subsp. equisimilis to our small set of four S. canis isolates, except for the failure to amplify xpt from one S. canis isolate. There were no related allelic profiles among the four S. canis isolates (Table 1), with only one instance of a shared allele between two isolates (recP8881) occurring.
Concordance between emm type and genetic relatedness in S. dysgalactiae subsp. equisimilis.
Twenty-five emm types found among S. dysgalactiae subsp. equisimilis isolates were represented in this MLST study by two to three independent isolates (Table 1). For 16 of these 25 emm types, concordance was observed between the emm type and genetic relatedness, in that the isolates shared the same eBURST group; and for 10 of these 16 concordant sets, isolates with the same emm type shared the same ST. Fifteen of these 16 concordant sets displayed close genetic relatedness (allelic identity or a single divergent allele) among all members of an emm type included in the study (two isolates each of types emm57, stC5344, stC6746, stC74a, stG10, stG11, stG480, stG5420, stG653, stG6792, stG7882, stG840, and stG97 and three isolates each of stG2078 and stG62647), with stC1400 representing two isolates with an identical allelic profile and a third isolate with a divergent allelic profile. In addition to the 16 observed examples of concordance between emm type and genetic relatedness, we found that the ST-Se37 (stG485) detected in this study shared allelic identity (excluding yqiZ) with a previously determined genotype (designated ST21) from a GCS isolate of the same emm type that was recovered prior to 2001 (23).
High degree of genetic relatedness between S. dysgalactiae subsp. equisimilis isolates with different emm types.
The 10 small eBURST groups, consisting of two to four STs each, consisted solely of highly related isolates (Table 1). In only 3 of the 10 eBURST groups were double-locus variants observed (data not shown), and in all other eBURST groups, all isolates were single-locus variants of one another. As mentioned above, in 15 of the 16 examples of observed concordance between the emm type and genetic relatedness, all isolates within a given emm type were encompassed within a single eBURST group or ST.
It was striking that only 2 of the 10 eBURST groups were represented by a single emm type. Seven individual STs were represented by two to four distinct emm types, and 8 of 10 eBURST groups represented two to five different emm types. In contrast to S. pyogenes, in which single- and double-locus variants usually share the same emm types, four of these S. dysgalactiae subsp. equisimilis eBURST groups displayed five different emm types.
Genotypes shared between GGS and GCS isolates.
For five different emm types, both GGS and GCS isolates were chosen for genetic analysis. Unrelated genotypes (only zero to three identical alleles) were observed between GGS and GCS isolates within three of these five emm types (stC1400, stG5063, and stG643), while GGS and GCS isolates within types st5344 and st62647 shared six to seven identical alleles.
Evidence of housekeeping gene transfer between S. dysgalactiae subsp. equisimilis and S. pyogenes.
Since S. pyogenes, S. canis, and S. dysgalactiae subsp. equisimilis are close relatives, we wished to use the MLST scheme described previously (15, 23) and a more recent collection of invasive S. dysgalactiae subsp. equisimilis and S. canis isolates (7) to assess potential housekeeping gene flow between the three species.
Within gki, mutS, recP, and xpt there were a total of 10 alleles found in our S. dysgalactiae subsp. equisimilis data set that have also been documented within S. pyogenes (Table 1). These included, gki108-74, mutS106-46, recP101-83, recP105-2, recP106-4, recP113-50, recP70sp, xpt103-2, xpt107-71, and xpt31sp. Fifty S. dysgalactiae subsp. equisimilis isolates contained one to three of these alleles that had previously been documented in S. pyogenes.
gtr and murI data.
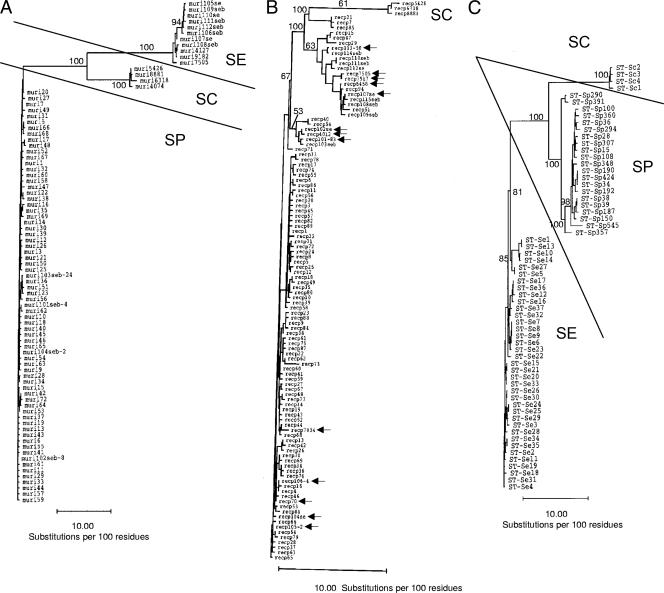
Unlike the previous study (23), our study set S. dysgalactiae subsp. equisimilis gtr and murI alleles showed no examples of allelic identity or similarity between the two species. As reported previously (23), the S. pyogenes gtr and murI alleles display very little variation. The 8 S. dysgalactiae subsp. equisimilis gtr alleles shared ≥97.8% sequence identity but only ≤82% identity with 78 of 79 known S. pyogenes gtr alleles. The gtr60 allele in the S. pyogenes MLST database differed by only a single base from the S. dysgalactiae subsp. equisimilis allele gtr106 (99.8% sequence identity); however, there is no existing documentation of an S. pyogenes strain with this allele. The 6 S. dysgalactiae subsp. equisimilis murI alleles shared ≥98.4% sequence identity but ≤77% identity with the 70 known S. pyogenes murI alleles. These alleles were closely similar to 5 S. dysgalactiae subsp. equisimilis murI alleles from a previous study (23), and these 11 alleles clustered together in a species-specific cluster in the phylogram shown in Fig. 2A.
FIG. 2.
Phylograms of murI (A); recP (B); and concatenated gki, gtr, murI, mutS, recP, and xpt (C) MLST data. We employed the neighbor-joining approach, using the Jukes-Cantor model. One thousand bootstrap replicates were performed on the same data by using the Paupsearch program to give the indicated frequencies of occurrence of bipartitions. seb, alleles exclusively found in a previous study of S. dysgalactiae subsp. equisimilis isolates. All known S. pyogenes and S. dysgalactiae subsp. equisimilis murI and recP alleles (as of June 2008) were also included in the analysis. Alleles with hyphens have been documented in both S. pyogenes and S. dysgalactiae subsp. equisimilis (B and C). Arrows show the S. dysgalactiae subsp. equisimilis recP alleles found during this study (B). In panel C, murI alleles are distinctly clustered in the indicated species-specific clusters (S. dysgalactiae subsp. equisimilis, S. canis, and S. pyogenes), while recP alleles from S. dysgalactiae subsp. equisimilis and S. pyogenes are not as distinctly separated. For the phylogram in panel C, the analysis included all STs from S. dysgalactiae subsp. equisimilis and S. canis found in this study and the S. pyogenes STs included in Table 2. For ST-Sc1 in panel C, the xpt6318 allele from another S. canis strain was employed since we were unable to amplify xpt from this strain. SP, S. pyogenes; SE, S. dysgalactiae subsp. equisimilis; SC, S. canis.
gki data.
The gki108-74 allele shared a much higher degree of similarity with other S. dysgalactiae subsp. equisimilis alleles (≥96%) than nearly all other S. pyogenes alleles (≤92% identity to 98/101 S. pyogenes alleles), indicating that the potential interspecies transfer of this allele was from an S. dysgalactiae subsp. equisimilis donor to S. pyogenes; however, there is no past documentation of the association of the gki74 allele with any specific GAS allelic profile or strain. Two additional S. dysgalactiae subsp. equisimilis-like gki alleles are evident in the S. pyogenes database, and these share 95 to 98.2% identity with the S. dysgalactiae subsp. equisimilis gki alleles in Table 1. These include gki79 and gki75, found in GAS strains recovered in Australia and Nepal, respectively (28, 34).
mutS data.
The S. dysgalactiae subsp. equisimilis mutS alleles shared ≥98.3% identity and 86 to 92% identity with the 56 S. pyogenes-specific mutS alleles (available at www.mlst.net). The mutS106-46 allele, found in both S. pyogenes and in this study, shared ≥98.8% identity with the other five S. dysgalactiae subsp. equisimilis mutS alleles encountered in this study, suggesting the possible transfer of this allele from S. dysgalactiae subsp. equisimilis into S. pyogenes. With the exception of the mutS62 allele, all S. dysgalactiae subsp. equisimilis, S. pyogenes, and S. canis mutS alleles were each resolved by phylogenetic analysis into separate, well-supported, species-specific clusters (data not shown), as shown for the data for murI (Fig. 2A). The mutS62 allele, found in two separate instances from S. pyogenes isolates causing invasive disease in rhesus monkeys (17), shared only 95.1% and 92% identity with the closest-matching alleles of S. pyogenes and S. dysgalactiae subsp. equisimilis, respectively.
recP and xpt data.
Unlike gki, gtr, murI, and mutS, for which phylogenetic analysis primarily resolved the alleles from S. pyogenes and S. dysgalactiae subsp. equisimilis into two simple clusters, the recP and xpt allele clusters were heterogeneous (the recP phylogram is shown in Fig. 2B).
Of the 14 S. dysgalactiae subsp. equisimilis recP alleles found in this study, 5 (alleles 70, 101-83, 105-2, 106-4, 113-50) have previously been observed in reported S. pyogenes isolates, although only recP alleles 70, 2, 4, and 50 are associated at mlst.net with complete STs that correspond to verified S. pyogenes strains with typical S. pyogenes emm types. By using a tree based upon the Distances program (Fig. 2B), recP113-50 and five other S. dysgalactiae subsp. equisimilis recP alleles from this study (alleles 112se, 7505, 7917, 107se, and 6458) clustered together within a distinct branch with six S. dysgalactiae subsp. equisimilis alleles from a previous study (alleles 114seb, 110seb, 111seb, 112seb, 115seb, and 108seb) that were described as cluster II alleles characteristic of S. dysgalactiae subsp. equisimilis (21). Additionally, the S. pyogenes recP alleles (recP alleles 15, 47, 29, 94, and 51) clustered within this main group of S. dysgalactiae subsp. equisimilis-like recP alleles (Fig. 2B). The remaining eight recP S. dysgalactiae subsp. equisimilis alleles found during this study (alleles 70, 4012, 7934, 101-83, 102se, 104se,105-2, and 106-4) clustered together with the majority of known S. pyogenes alleles and are categorized as cluster I GAS-like alleles by use of the criteria from the previous study (23).
The xpt and recP data sets from S. dysgalactiae subsp. equisimilis shared similarities, in that several alleles were previously documented in S. pyogenes, revealing that both S. dysgalactiae subsp. equisimilis and S. pyogenes could serve as recombinational donors or recipients of these housekeeping alleles. Three xpt alleles found in this study (alleles 103-2, 107-71, and 31sp) have previously been documented in S. pyogenes. xpt allele 31sp clustered with five additional closely similar S. dysgalactiae subsp. equisimilis alleles from this study (alleles 6746, 110se, 6532, 112se, and 108se) and four more S. dysgalactiae subsp. equisimilis cluster II S. dysgalactiae subsp. equisimilis-like alleles from the previous study (23) (alleles 114seb, 115seb, 113seb, and 111seb). Included within cluster II were S. pyogenes xpt alleles 73, 51, 29, and 62. xpt alleles 103-2 and 107-71 were typical of cluster I and were closely similar to various S. pyogenes alleles. Additional S. dysgalactiae subsp. equisimilis cluster I alleles found in this study were xpt104se and xpt898304, which were in the same major branch as four previously documented S. dysgalactiae subsp. equisimilis alleles (data not shown).
yqiZ data.
As described in Materials and Methods, we were unable to amplify sequences from our S. dysgalactiae subsp. equisimilis isolates that corresponded to the yqiL MLST alleles described earlier (23), and a closely similar target was also not apparent in a nearly completed S. dysgalactiae subsp. equisimilis genome sequence (Awdhesh Kalia, personal communication). Among our S. dysgalactiae subsp. equisimilis isolate set, 10 different alleles of yqiZ were observed, and these shared ≥97.2% identity and only 65 to 85% identity to various S. dysgalactiae subsp. equisimilis yqiL alleles described previously (23).
S. canis MLST data.
There were no examples of the detection of alleles in the four S. canis isolates that were also found in the two other species. Within the seven MLST targets, all the S. canis alleles shared at least 97.5% identity (for six alleles, ≥98.5% identity) and shared no more than 79 to 94.2% identity within individual MLST targets to the closest S. pyogenes or S. dysgalactiae subsp. equisimilis alleles.
Phylogenetic analysis.
We used the Distances program to generate a matrix of the pairwise evolutionary distance between aligned sequences. We found that the dendrograms generated from these data clustered sequences in a manner concordant with the previous, more detailed phylogenetic analysis (23). Using our dendrograms to construct trees for allelic data generated for combined S. dysgalactiae subsp. equisimilis and S. pyogenes data, we assigned different alleles as GAS-like or GGS/GCS-like according to how the alleles clustered with typical S. pyogenes alleles represented within the entire S. pyogenes MLST database and with the S. dysgalactiae subsp. equisimilis alleles generated in the previous study (23). The trees for murI and recP are shown in Fig. 2A and B, respectively. Equivalent trees for the other five MLST alleles are available upon request.
Resolution of the three species by use of MLST data.
For the construction of phyograms from concatenated MLST data, we selected the most diverse existing S. pyogenes allelic profiles as well as allelic profiles from some of the most common global clones, including the prevalent M1, M3, M75, M12, and M4 strains. Four typical S. pyogenes allelic profiles (from predominant types emm1, emm3, and emm12 and the rarer type emm100 clone) were characterized by the use of highly conserved MLST alleles (data not shown). We also included diverse allelic profiles containing known S. dysgalactiae subsp. equisimilis-like alleles and other obviously divergent alleles.
We employed concatenated MLST allele sequences from six of the seven targets (excluding yqiZ) of all 65 GGS/GCS isolates from this study and the corresponding sequences from documented S. pyogenes strains. Despite clear instances in which S. pyogenes-like and S. dysgalactiae subsp. equisimilis-like MLST alleles were shared between S. dysgalactiae subsp. equisimilis and S. pyogenes, phylograms of concatenated allelic data from all 37 S. dysgalactiae subsp. equisimilis STs and 21 S. pyogenes STs clearly resolved the two species (Fig. 2C).
DISCUSSION
A major purpose of this study was to examine the genetic diversity of a recent sample of population-based invasive S. dysgalactiae subsp. equisimilis isolates that were recovered in the United States and that represented all emm types recovered. We also wished to make limited observations from an analogous analysis of a very small sample of four S. canis isolates. The main contribution of this work regarding S. canis is simply a method for MLST that should prove useful for epidemiologic and other studies. While we were unable to amplify xpt from one of the four distinct S. canis strains, we doubt that this will be a serious impediment for large MLST projects. Even with S. pyogenes and pneumococci, we find rare individual loci that will not amplify with standard MLST primers.
Among the S. pyogenes isolates recovered from economically developed geographic regions and associated with either invasive disease or pharyngitis, emm types are often highly associated with specific clonal types (1, 2, 3, 6, 14, 21). In order to assess clonal relationships among S. dysgalactiae subsp. equisimilis isolates, our study included multiple members of 25 different emm types. It has been known for at least the past decade that certain S. pyogenes emm types are associated with multiple clonal types that can easily be resolved by phenotypic or genotypic testing (1, 4, 5, 34). For example, based upon a combination of phenotypic and genotypic data (T typing, antiopacity factor testing, macrorestriction profile analysis, sof gene sequence typing), classic M44-M61 and M27-M77 S. pyogenes reference strain pairs are obviously divergent clones that share the same emm sequence types (1, 2, 4). Ten S. dysgalactiae subsp. equisimilis emm types presented here (stC1400, stC6979, stC9431, stG166B, stG245, stG485, stG5063, stG6, stG643, stG652) each show associations with two different clonal types (Table 1; note that there was a concordant relationship between emm type and genetic relatedness in two of the three stC1400 isolates).
In the previous study, 34 distinct allelic profiles were observed among 34 S. dysgalactiae subsp. equisimilis isolates, with only a single pair of conserved but nonidentical allelic profiles being detected (23). In striking contrast, we found that more than 75% (46/61) of our invasive S. dysgalactiae subsp. equisimilis isolates were resolved into 10 different eBURST groups comprised of highly similar allelic profiles, with 9 of 37 allelic profiles being completely shared between multiple isolates. In addition, in the previous study (23) no genetic relatedness between isolates within all nine emm types that represented multiple isolates was observed, while we found a high degree of genetic relatedness between multiple isolates of the majority of emm types examined (16/25 [64%]). These marked differences may be due in part to the greater geographic and temporal diversities of the isolates described in the previous study (23) compared to the diversities of the isolates evaluated in the present study, all of which were recovered within a 3-year period in the United States. In addition, at least 6 of the 34 isolates from the previous study were not recovered from sterile sites. We do note, however, the identical allelic profile between an invasive stG485 isolate from our study (ST-Se37) that was recovered at least 8 years later than an invasive stG485 isolate described in the previous study (designated ST21). We received this stG485 strain, whose isolation date is unknown, in 1997 and supplied it for the study by Kalia et al. (23).
Similar to observations made with S. pyogenes (1, 5), in at least some cases the S. dysgalactiae subsp. equisimilis emm type is not predictive of genetic relatedness. For example, the two isolates of the most common emm type chosen, stG6 (Fig. 1), had divergent allelic profiles (Table 2), and in total, 9 of the 25 emm types represented by multiple isolates displayed no concordance between genetic relatedness and emm type. It is likely that additional tests could easily resolve strains that share an identical emm type distributed among two or more genetic backgrounds in the same manner that T-antigen profiles or sof sequence types currently resolve the analogous situation in GAS (1, 2, 3, 14).
TABLE 2.
Concatenated MLST types from documented S. pyogenes strains used for comparison with S. dysgalactiae subsp. equisimilis and S. canis MLST types from this study
| ST | Associated emm type | S. dysgalactiae subsp. equisimilis-like or other divergent allele | Reference or contributor of S. pyogenes allele |
|---|---|---|---|
| ST34 | emm49 | recP7 | 15 |
| ST38 | emm4 | recP15 | 15 |
| ST39 | emm4 | recP15 | 15 |
| ST100 | emm55 | xpt29 | 26, 34 |
| ST108 | st3765 | xpt31 | 26 |
| ST150 | emm75 | recp113-50a | 37 |
| ST187 | st213 | recP47 | 26 |
| ST190 | emm49 | recP21, mutS101-7a | 26 |
| ST192 | emm25 | xpt51 | 26 |
| ST290 | emm81 | gki79 | 26 |
| ST294 | st6030 | xpt51 | 34 |
| ST348 | emm57 | mutS106-46a | 34 |
| ST357 | emm75 | gki75 | 34 |
| ST360 | st1759 | xpt62 | 34 |
| ST391 | emm4 | recp15 | 32 |
| ST424 | emm49 | recP85, mutS101-7 | D. R. Martin |
| ST545 | st804 | recP94, xpt73 | 17 |
| ST15 | emm3 | None | 15 |
| ST28 | emm1 | None | 15 |
| ST36 | emm12 | None | 15 |
| ST307 | emm100 | None | 27 |
recP113-50, mutS101-7, and mutS106-46 correspond to S. pyogenes alleles recP50, mutS7, and mutS46, respectively.
A considerable fraction (7/37 [19%]) of the allelic profiles represented multiple emm types, with up to four emm types associated with individual allelic profiles. Furthermore, for the majority (8/10 [80%]) of eBURST groups, within which isolates generally varied from one another by only a single locus, multiple emm types were observed (up to six different types). It is unusual for S. pyogenes isolates that differ by one or two MLST loci to represent multiple emm types (28). It is even more rare for a single allelic profile to be associated with S. pyogenes isolates of divergent emm types, although within at least some of these instances, evidence of intragenic changes within the emm gene that result in a new emm type is apparent (28).
Our study shares basic similarities to a previous study of clonal relationships between S. dysgalactiae subsp. equisimilis invasive and noninvasive disease isolates collected in Portugal over a 6-year period (30), in which a majority of the clones deduced by pulsed-field gel electrophoresis included more than one emm type and the same emm type was sometimes found among isolates with diverse genetic backgrounds. This study also indicated a significant association in S. dysgalactiae subsp. equisimilis between certain clone-emm type combinations and invasive disease.
The results of our study also indicate that the emm type is likely to be a useful genetic marker for epidemiologic studies and other investigations involving S. dysgalactiae subsp. equisimilis, as it is useful for studies of S. pyogenes. It is entirely conceivable that the 16 instances in which all or nearly all S. dysgalactiae subsp. equisimilis isolates within a given emm type shared identical or closely similar allelic profiles are generally reflective of the clonality of these emm types at the national level. Obviously, a more thorough analysis of invasive S. dysgalactiae subsp. equisimilis isolates sharing the same emm type is necessary to verify such a projection. It also appears that additional parameters will be necessary to resolve major strains of S. dysgalactiae subsp. equisimilis, since multiple emm types are often superimposed upon the same allelic profile or set of highly related allelic profiles (Table 1). While it is likely that in S. dysgalactiae subsp. equisimilis certain emm types are largely predictive of clonal type in the same manner that certain emm types are predictive of clonal type in S. pyogenes, the situation appears to be more complex in S. dysgalactiae subsp. equisimilis. For example, while the common emm type stG2078 was associated with ST-Se17 among all three isolates of this emm type recovered from Georgia and California, an isolate carrying an unrelated emm type (stG485) also shared ST-Se17 (Table 2). In order to use S. dysgalactiae subsp. equisimilis emm types as a meaningful parameter in epidemiologic studies, it is important to elucidate and understand emm type correlates with clonal relatedness. From the findings of the work presented here, it appears that emm type deduction alone, while informative, is often insufficient for the resolution of S. dysgalactiae subsp. equisimilis invasive clones.
Besides the obvious assessment of correlations of S. dysgalactiae subsp. equisimilis emm types with specific clones, we wished to assess if we could deduce a history of housekeeping gene transfer between S. dysgalactiae subsp. equisimilis and S. pyogenes, and if so, we wondered if we could deduce an obvious net directionality in transfer events between the two species. Previous work suggested a net directionality of housekeeping gene transfer from S. pyogenes donors to S. dysgalactiae subsp. equisimilis recipients, resulting in highly mosaic S. dysgalactiae subsp. equisimilis genomes. This conclusion was based primarily upon the observations that at the time known S. pyogenes housekeeping gene alleles displayed little diversity and a heterogeneous collection of S. dysgalactiae subsp. equisimilis isolates exhibited a great deal of genetic diversity because they contained one to four S. pyogenes-like housekeeping gene sequences. The great contrast between our observations and those from the previous study (23) in the degree of mosaicism observed within S. dysgalactiae subsp. equisimilis due to the presence of S. pyogenes-like MLST alleles is quite apparent when the MLST data are compared. Whereas 10 of 34 isolates in the previous study revealed S. pyogenes-like alleles for three to four MLST targets, no isolates in our data set contained more than two S. pyogenes-like alleles. S. pyogenes-like alleles were completely absent within gki, gtr, murI, and mutS loci from our 61-isolate data set, while 9 of 34 isolates from the previous study contained one to two S. pyogenes-like alleles within these four targets. Typical alleles of these four targets are much less related between the two species, while the S. dysgalactiae subsp. equisimilis and S. pyogenes recP and xpt alleles are much more closely related, as evidenced by the relatively smaller number of substitutions per 100 residues seen between recP allelic clusters most characteristic of each species compared to the markedly greater separation in S. pyogenes and S. dysgalactiae subsp. equisimilis murI alleles (Fig. 2A and B). In fact, when the five isolates from the previous study with an S. pyogenes-like allele at either gki, gtr, murI, or mutS were included within the same phylogenetic analysis with concatenated alleles shown in Fig. 2C, the five isolates were poorly resolved from other S. dysgalactiae subsp. equisimilis strains and formed a separate branch (data not shown). The four isolates from the previous study with two S. pyogenes-like alleles within these four loci actually showed greater shared relatedness to S. pyogenes than to S. dysgalactiae subsp. equisimilis and formed a subgroup connected with the main S. pyogenes branch shown (data not shown). At present, it appears that assessments of housekeeping gene transfer directionality between S. pyogenes and S. dysgalactiae subsp. equisimilis are heavily dependent upon the sources of the isolate sets. The majority of MLST results that have been documented for S. pyogenes have been based on collections from developed countries. More recent limited studies from more diverse S. pyogenes isolate sets from less developed countries have revealed a disproportionate number of S. dysgalactiae subsp. equisimilis-like alleles (26, 34). We have also found an S. pyogenes strain (represented by ST545 in Fig. 2C) that was recovered on two different occasions from monkeys with invasive infections (17) (data not shown) and that contained two different S. dysgalactiae subsp. equisimilis-like housekeeping alleles (Table 2). It is possible that the cumulative MLST databases are not sufficiently reflective of the global diversity of either of these species to make generalizations regarding the net directionality of gene flow between them.
Acknowledgments
We sincerely thank Awdhesh Kalia for sharing unpublished sequence data from his S. dysgalactiae subsp. equisimilis genome project. We are grateful to the California and Georgia Active Bacterial Core surveillance participants in this study.
Footnotes
Published ahead of print on 22 April 2009.
REFERENCES
- 1.Beall, B., G. Gherardi, M. Lovgren, R. R. Facklam, B. A. Forwick, and G. J. Tyrrell. 2000. emm and sof gene sequence variation in relation to serological typing of opacity-factor-positive group A streptococci. Microbiology 1461195-1209. [DOI] [PubMed] [Google Scholar]
- 2.Beall, B., R. R. Facklam, J. A. Elliott, A. R. Franklin, T. Hoenes, D. Jackson, L. Laclaire, T. Thompson, and R. Viswanathan. 1998. Streptococcal emm types associated with T-agglutination types and the use of conserved emm gene restriction fragment patterns for subtyping group A streptococci. J. Med. Microbiol. 47893-898. [DOI] [PubMed] [Google Scholar]
- 3.Beall, B., R. Facklam, T. Hoenes, and B. Schwartz. 1997. Survey of emm gene sequences and T-antigen types from systemic Streptococcus pyogenes infection isolates collected in San Francisco, California; Atlanta, Georgia; and Connecticut in 1994 and 1995. J. Clin. Microbiol. 351231-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beall, B., R. Facklam, and T. Thompson. 1996. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J. Clin. Microbiol. 34953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessen, D. E., K. F. McGregor, and A. M. Whatmore. 2008. Relationships between emm and multilocus sequence types within a global collection of Streptococcus pyogenes. BMC Microbiol. 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bessen, D. E., J. R. Carapetis, B. Beall, R. Katz, M. Hibble, B. J. Currie, T. Collingridge, M. W. Izzo, D. A. Scaramuzzino, and K. S. Sriprakash. 2000. Contrasting molecular epidemiology of group A streptococci causing tropical and nontropical infections of the skin and throat. J. Infect. Dis. 1821109-1116. [DOI] [PubMed] [Google Scholar]
- 7.Broyles, L. N., C. Van Beneden, B. Beall, R. Facklam, P. L. Shewmaker, P. Malpiedi, P. Daily, A. Reingold, and M. M. Farley. 2009. Population-based study of invasive disease caused by beta-hemolytic streptococci of groups other than A and B. Clin. Infect. Dis. 48706-712. [DOI] [PubMed] [Google Scholar]
- 8.Campo, R. E., D. R. Schultz, and A. L. Bisno. 1995. M proteins of group G streptococci: mechanisms of resistance to phagocytosis. J. Infect. Dis. 171601-606. [DOI] [PubMed] [Google Scholar]
- 9.Chhatwal, G. S., and S. R. Talay. 2000. Pathogenicity factors in C and G streptococci, p. 177-183. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, DC.
- 10.Cleary, P. P., J. Peterson, C. Chen, and C. Nelson. 1991. Virulent human strains of group G streptococci express a C5a peptidase enzyme similar to that produced by group A streptococci. Infect. Immun. 592305-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen-Poradosu, R., J. Jaffe, D. Lavi, S. Grisariu-Greenzaid, R. Nir-Paz, L. Valinsky, M. Dan-Goor, C. Block, B. Beall, and A. E. Moses. 2004. Group G streptococcal bacteremia in Jerusalem. Emerg. Infect. Dis. 101455-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies, M. R., D. J. McMillan, R. G. Beiko, V. Barroso, R. Geffers, K. S. Sriprakas, and G. S. Chhatwal. 2007. Virulence profiling of Streptococcus dysgalactiae subspecies equisimilis isolated from infected humans reveals 2 distinct genetic lineages that do not segregate with their phenotypes or propensity to cause diseases. Clin. Infect. Dis. 441442-1454. [DOI] [PubMed] [Google Scholar]
- 13.Devriese, L. A., J. Hommez, R. Kilpper-Bälz, and K.-H. Schleifer. 1986. Streptococcus canis sp. nov.: a species of group G streptococci from animals. Int. J. Syst. Bacteriol. 36422-425. [Google Scholar]
- 14.Dicuonzo, G., G. Gherardi, G. Lorino, S. Angeletti, M. De Cesaris, E. Fiscarelli, D. E. Bessen, and B. Beall. 2001. Group A streptococcal genotypes from pediatric throat isolates in Rome, Italy. J. Clin. Microbiol. 391687-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enright, M. C., B. G. Spratt, A. Kalia, J. H. Cross, and D. E. Bessen. 2001. Multilocus sequence typing of Streptococcus pyogenes and the relationships between emm type and clone. Infect. Immun. 692416-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Facklam, R. 2002. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin. Microbiol. Rev. 15613-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia, A., K. Paul, B. Beall, and H. McClure. 2006. Toxic shock due to Streptococcus pyogenes in a rhesus monkey (Macaca mulatta). J. Am. Assoc. Lab. Anim. Sci. 4579-82. [PubMed] [Google Scholar]
- 18.Gooskens, J., A. J. Neeling, R. J. Willems, J. W. Wout, and E. J. Kuijper. 2005. Streptococcal toxic shock syndrome by an iMLS resistant M type 77 Streptococcus pyogenes in The Netherlands. Scand. J. Infect. Dis. 3785-89. [DOI] [PubMed] [Google Scholar]
- 19.Humar, D., V. Datta, D. J. Bast, B. Beall, J. C. De Azavedo, and V. Nizet. 2002. Streptolysin S and necrotising infections produced by group G streptococcus. Lancet 359124-129. [DOI] [PubMed] [Google Scholar]
- 20.Igwe, E. I., P. L. Shewmaker, R. R. Facklam, M. M. Farley, C. van Beneden, and B. Beall. 2003. Identification of superantigen genes speM, ssa, and smeZ in invasive strains of beta-hemolytic group C and G streptococci recovered from humans. FEMS Microbiol. Lett. 229259-264. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, D. R., E. L. Kaplan, A. VanGheem, R. R. Facklam, and B. Beall. 2006. Characterization of group A streptococci (Streptococcus pyogenes): correlation of M-protein and emm-gene type with T-protein agglutination pattern and serum opacity factor. J. Med. Microbiol. 55157-164. [DOI] [PubMed] [Google Scholar]
- 22.Kalia, A., and D. E. Bessen. 2003. Presence of streptococcal pyrogenic exotoxin A and C genes in human isolates of group G streptococci. FEMS Microbiol. Lett. 219291-295. [DOI] [PubMed] [Google Scholar]
- 23.Kalia, A., M. C. Enright, B. G. Spratt, and D. E. Bessen. 2001. Directional gene movement from human-pathogenic to commensal-like streptococci. Infect. Immun. 694858-4869. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Lopardo, H. A., P. Vidal, M. Sparo, P. Jeric, D. Centron, R. R. Facklam, H. Paganini, N. G. Pagniez, M. Lovgren, and B. Beall. 2005. Six-month multicenter study on invasive infections due to Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis in Argentina. J. Clin. Microbiol. 43802-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malke, H. 2000. Genetics and pathogenicity factors of group C and G streptococci, p. 163-176. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, DC.
- 26.McGregor, K. F., N. Bilek, A. Bennett, A. Kalia, B. Beall, J. R. Carapetis, B. J. Currie, K. S. Sriprakash, B. G. Spratt, and D. E. Bessen. 2004. Group A streptococci from a remote community have novel multilocus genotypes but share emm types and housekeeping alleles with isolates from worldwide sources. J. Infect. Dis. 189717-723. [DOI] [PubMed] [Google Scholar]
- 27.McGregor, K. F., and B. G. Spratt. 2005. Identity and prevalence of multilocus sequence typing-defined clones of group A streptococci within a hospital setting. J. Clin. Microbiol. 431963-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGregor, K. F., B. G. Spratt, A. Kalia, A. Bennett, N. Bilek, B. Beall, and D. E. Bessen. 2004. Multilocus sequence typing of Streptococcus pyogenes representing most known emm types and distinctions among subpopulation genetic structures. J. Bacteriol. 1864285-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Loughlin, R. E., A. Roberson, P. R. Cieslak, R. Lynfield, K. Gershman, A. Craig, B. A. Albanese, M. M. Farley, N. L. Barrett, N. L. Spina, B. Beall, L. H. Harrison, A. Reingold, C. Van Beneden, and Active Bacterial Core Surveillance Team. 2007. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin. Infect. Dis. 45853-862. [DOI] [PubMed] [Google Scholar]
- 30.Pinho, M. D., J. Melo-Cristino, and M. Ramirez. 2006. Clonal relationships between invasive and noninvasive Lancefield group C and G streptococci and emm-specific differences in invasiveness. J. Clin. Microbiol. 44841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proft, T., P. D. Webb, V. Handley, and J. D. Fraser. 2003. Two novel superantigens found in both group A and group C Streptococcus. Infect. Immun. 711361-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson, D. A., J. A. Sutcliffe, W. Tewodros, A. Manoharan, and D. E. Bessen. 2006. Evolution and global dissemination of macrolide-resistant group A streptococci. Antimicrob. Agents Chemother. 502903-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachse, S., P. Seidel, D. Gerlach, E. Günther, J. Rödel, E. Straube, and K. H. Schmidt. 2002. Superantigen-like gene(s) in human pathogenic Streptococcus dysgalactiae, subsp equisimilis: genomic localisation of the gene encoding streptococcal pyrogenic exotoxin G (speG(dys)). FEMS Immunol. Med. Microbiol. 34159-167. [DOI] [PubMed] [Google Scholar]
- 34.Sakota, V., A. M. Fry, T. M. Lietman, R. R. Facklam, Z. Li, and B. Beall. 2006. Genetically diverse group A streptococci from children in far-western Nepal share high genetic relatedness with isolates from other countries. J. Clin. Microbiol. 442160-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shulman, S. T., R. R. Tanz, W. Kabat, K. Kabat, E. Cederlund, D. Patel, Z. Li, V. Sakota, J. B. Dale, B. Beall, and U.S. Streptococcal Pharyngitis Surveillance Group. 2004. Group A streptococcal pharyngitis serotype surveillance in North America, 2000-2002. Clin. Infect. Dis. 39325-332. [DOI] [PubMed] [Google Scholar]
- 36.Sylvetsky, N., D. Raveh, Y. Schlesinger, B. Rudensky, and A. M. Yinnon. 2002. Bacteremia due to beta-hemolytic Streptococcus group G: increasing incidence and clinical characteristics of patients. Am. J. Med. 112622-626. [DOI] [PubMed] [Google Scholar]
- 37.Szczypa, K., E. Sadowy, R. Izdebsk, and W. Hryniewicz. 2004. A rapid increase in macrolide resistance in Streptococcus pyogenes isolated in Poland during 1996-2002. J. Antimicrob. Chemother. 54828-831. [DOI] [PubMed] [Google Scholar]
- 38.Vojtek, I., Z. A. Pirzada, B. Henriques-Normark, M. Mastny, R. P. Janapatla, and E. Charpentier. 2008. Lysogenic transfer of group A Streptococcus superantigen gene among streptococci. J. Infect. Dis. 197225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whatmore, A. M., K. H. Engler, G. Gudmundsdottir, and A. Efstratiou. 2001. Identification of isolates of Streptococcus canis infecting humans. J. Clin. Microbiol. 394196-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whatmore, A. M., V. Kapur, D. J. Sullivan, J. M. Musser, and M. A. Kehoe. 1994. Non-congruent relationships between variation in emm gene sequences and the population genetic structure of group A streptococci. Mol. Microbiol. 14619-631. [DOI] [PubMed] [Google Scholar]