Abstract
This longitudinal study evaluated the response to periodontal treatment by subjects infected with either JP2 (n = 25) or non-JP2 (n = 25) Aggregatibacter (Actinobacillus) actinomycetemcomitans. Participants were treated during the first 4 months by receiving (i) scaling and root planing, (ii) systemic antibiotic therapy, and (iii) periodontal surgery. Probing depth (PD), clinical attachment level (CAL), and gingival and plaque indices (GI and PI, respectively) were monitored at baseline and at 12 months, along with DNA-PCR-based subgingival detection of JP2 or non-JP2 A. actinomycetemcomitans. At baseline, PD, CAL, and GI scores were statistically higher in the JP2 strain-positive group than the non-JP2-strain-positive group. At 12 months, PD, CAL, and GI scores had decreased significantly for both groups, but the reduction rates of PD and CAL were higher in the non-JP2-strain-positive group. Among JP2-strain-positive patients in the baseline, patients who remained JP2 strain positive at 12 months showed significantly higher GIs than did the patients who had lost the detectable JP2 clone. Patients who remained JP2 strain positive at 12 months appeared to be more resistant to mechanical-chemical therapy than did those who were still non-JP2 strain positive, while the elimination of JP2 A. actinomycetemcomitans remarkably diminished gingival inflammation. Early identification and elimination of the JP2 clone of A. actinomycetemcomitans will enable practitioners to effectively predict the outcome of treatments applied to periodontal patients.
Compared to gingivitis, or disease-free periodontium, an increased incidence of Aggregatibacter (Actinobacillus) actinomycetemcomitans has been reported in the lesions of localized aggressive periodontitis (LAP) or severe chronic periodontitis (9, 13, 16, 41, 44, 45). It is also true that while polymicrobial infection is implicated in the pathogenesis of chronic adult periodontitis, oral colonization by A. actinomycetemcomitans is more directly associated with the pathogenesis of LAP. Such pathogenic outcomes related to A. actinomycetemcomitans infection seem to be partially derived from leukotoxin (LTX) produced by A. actinomycetemcomitans. LTX is a potent virulence factor, which is encoded in an operon consisting of four genes (5) and typically causes cytotoxicity in neutrophils (26) and macrophages (37). Furthermore, in vitro experiments revealed that a specific 530-bp sequence in the LTX promoter region, rather than the LTX operon, impacts the transcription regulation of LTX (5), whereas there are several factors that regulate the expression of LTX from A. actinomycetemcomitans (25). It is also true that there may be highly leukotoxic A. actinomycetemcomitans strains other than just the type with the 530-bp deletion (24). Nonetheless, the deletion of this 530-bp promoter region, which is found in some pathogenic strains of A. actinomycetemcomitans, also called JP2 strains, has critical ramifications. For example, faster expression of the LTX gene with the deletion has been observed, leading to the production of a larger amount of LTX than that in strains where this promoter region remains intact (5). Among genetic differences found in A. actinomycetemcomitans, the 530-bp deletion in the promoter region of the LTX gene operon increases LTX activity by the JP2 clone, the so-called highly leukotoxic A. actinomycetemcomitans clone. On the other hand, in some pathogenic strains of A. actinomycetemcomitans, termed minimally leukotoxic, the 530-bp deletion does not seem to occur (22); consequently, less LTX activity is observed. As a result, non-JP2 clones (e.g., A. actinomycetemcomitans strains 652 and γ4) appear to be associated with a lesser severity of periodontal disease (11, 30). In contrast, subjects with severe forms of periodontal disease have tended to harbor deletion-positive A. actinomycetemcomitans clones (7, 8, 17, 18, 19, 35). Haubek et al. (19) found a higher rate of progression of periodontal disease among JP2-strain-positive patients, while Wu et al. (43) reported that detection of the A. actinomycetemcomitans LTX gene (lktA), but not the gene for the fimbria-associated protein (fap) of A. actinomycetemcomitans, in the subgingival sulcus correlates with the severity of chronic periodontal disease in China. Furthermore, Haubek et al. (23) reported that the JP2 clone of A. actinomycetemcomitans is likely to be an important etiological agent in initiation of periodontal attachment loss in children and adolescents. These lines of evidence support the hypothesis that A. actinomycetemcomitans and the genetic variant of A. actinomycetemcomitans with the 530-bp deletion (JP2 strain) are tightly associated with the onset and progression of periodontitis.
Responses to periodontal treatment of patients infected with A. actinomycetemcomitans have been investigated (6, 10, 39). Some previously published studies have even demonstrated that A. actinomycetemcomitans is a pathogen which is able to invade periodontal tissues but evade mechanical-chemical therapies (27, 32, 33). It is well established in the literature that A. actinomycetemcomitans-associated periodontitis requires periodontal therapy composed of a three-step strategy: (i) subgingival plaque control by mechanical debridement, (ii) administration of systemic antibiotics, and, as required, (iii) gingival flap surgery (14). Especially, the combination of metronidazole plus amoxicillin (amoxicilline) has been shown to improve the clinical and microbial parameters of patients infected with A. actinomycetemcomitans since it was first reported by van Winkelhoff et al. (42). A recent study by Akincibay et al. (1) also demonstrated that mechanical debridement accompanied by systemic administration of amoxicillin plus metronidazole leads to improvement of clinical parameters as well as suppression of A. actinomycetemcomitans.
Although the pattern of periodontal colonization by either the JP2 or the non-JP2 clones of A. actinomycetemcomitans seemed to remain unchanged during a 2-year longitudinal study, patients infected with JP2 A. actinomycetemcomitans demonstrated prominently elevated periodontal breakdown, as evaluated by clinical attachment level (CAL), compared to those infected with the A. actinomycetemcomitans strain not having the deletion genotype (19). It is particularly noteworthy that such episodes of periodontal breakdown occurred in spite of intensive periodontal therapy and maintenance (20). Overall, the effectiveness of periodontal therapy for patients infected with either the JP2 or non-JP2 A. actinomycetemcomitans clones remains unclear.
It has been shown that the A. actinomycetemcomitans JP2 clone, which produces a large amount of LTX in vitro (5), is associated with the magnitude of tissue damage. We hypothesized that this result would most likely affect the clinical therapeutic response of patients infected with the JP2 clone to a greater degree than those infected with the non-JP2 A. actinomycetemcomitans clones. Specifically, patients infected by JP2 clones should respond less efficiently to the type of three-step clinical treatment described above than should subjects infected with non-JP2 clones. To confirm this theory, we compared the responses to mechanical-chemical periodontal therapy administered to patients infected with either a JP2 or a non-JP2 A. actinomycetemcomitans strain and monitored the colonization by each strain present in the subgingival samples isolated at pre- and posttreatment.
MATERIALS AND METHODS
Study population.
Following a verbal and written explanation of the study, all participants signed a consent form previously approved by the Institutional Committee on Research Involving Human Subjects at the University of Taubaté, SP, Brazil (390/07).
The sample size per group was calculated (Student's t test for independent samples) to provide 90% power (α = 0.05) to detect any possible between-group differences regarding the measured clinical parameters. We included five additional subjects per group to account for the estimated dropout generally observed in long-term studies.
Preliminary screening of patients in our clinic showed 150 patients who were eligible due to the presence of JP2 or non-JP2 A. actinomycetemcomitans clones in periodontal pockets. However, for this study, we recruited only lifelong nonsmokers with at least four periodontal sites in different teeth presenting both a probing depth (PD) of ≥4 mm and a CAL of ≥3 mm (29). Age and gender between the groups having JP2 and non-JP2 A. actinomycetemcomitans clones were matched as closely as possible. Additional exclusion criteria included the following conditions: (i) any systemic condition requiring antibiotic coverage for periodontal examination, (ii) allergic reaction to metronidazole and/or amoxicillin, (iii) diabetes or other immunocompromising conditions, and (iv) pregnancy or breast-feeding. Subjects with orthodontic and prosthetic devices, or iatrogenic restorations, were also excluded, as were subjects with ongoing periodontal treatment (including professional supragingival plaque control, scaling, and root planing, as well as periodontal flap surgery) 24 months before the beginning of the study or subjects who had taken systemic or local antibiotics within 6 months prior to the clinical examination. Subjects who had used mouth rinse routinely in the previous 6 months were also excluded. In accordance with periodontal surgical requirements, 37 subjects were also excluded after clinical reevaluation because they did not show any indication (active residual pockets of ≥6 mm; furcation lesions) for periodontal surgery. Only subjects who required surgery in at least two quadrants participated in this study (for details, see “Flap surgery” below). Therefore, out of the total 150 eligible subjects treated between 2003 and 2005 at the University of Taubaté, 50 patients (JP2-strain-positive subjects, n = 25; non-JP2-strain-positive subjects, n = 25) were selected for the present investigation. The clinical demographic data are shown in Table 1 .
TABLE 1.
Time course of mechanical and chemical treatments as well as clinical examinations employed in this study
| Experimental time | Examination(s) and therapeutic procedure(s) |
|---|---|
| Baseline (day 0) | Pretreatment clinical and microbial examinations |
| 1 mo | |
| Wk 1 | Oral hygiene instruction (1 time/ |
| Wk 2 | wk, 4 times/mo) |
| Wk 3 | |
| Wk 4 | |
| 2 mo | |
| Wk 1 | Scaling and root planning (1 time/wk); systemic antibiotics (metronidazole plus amoxicillin) |
| Wk 2 | Scaling and root planning (1 |
| Wk 3 | quadrant/wk, 4 times/mo) |
| Wk 4 | |
| 3 mo | |
| Wk 1 | Supragingival scaling plus dental |
| Wk 2 | prophylaxis (1 quadrant/wk, 4 |
| Wk 3 | times/mo) |
| Wk 4 | |
| 4 mo | |
| Wk 1 | Clinical reevaluation; supragingival plaque control (mechanical therapy plus 0.12% chlorhexidine mouth rinse) |
| Wk 2 | Periodontal surgery (first quadrant with diseased lesion); supragingival plaque control (mechanical therapy plus 0.12% chlorhexidine mouth rinse) |
| Wk 3 | Supragingival plaque control (mechanical therapy; 1 time/wk) |
| Wk 4 | Periodontal surgery (second quadrant with diseased lesion); supragingival plaque control (mechanical therapy plus 0.12% chlorhexidine mouth rinse) |
| End of active periodontal therapy | |
| 7 mo | Supragingival scaling plus dental prophylaxis (1 quadrant/wk, 4 times/mo) |
| 10 mo | Supragingival scaling plus dental prophylaxis (1 quadrant/wk, 4 times/mo) |
| 12 mo | Posttherapy clinical and microbial reevaluation |
Clinical measurements.
A complete periodontal examination, excluding third molars, was conducted to register PD, CAL, gingival index (GI) (28), and plaque index (PI) (40) in six periodontal sites per tooth by one trained and calibrated examiner using a manual periodontal probe (PCPUNC 15; Hu-Friedy, Chicago, IL) at baseline and 8 months after periodontal therapy. To avoid any contamination that might result from bleeding on probing, baseline measurements were carried out at least 7 days in advance of bacterial sampling (day 0).
The calibration protocol for the examiner followed methods similar to those previously described (2). Data analysis was performed to determine reliability using standard error of measurement (SEM) for the continuous variables, such as PD and CAL, and kappa statistics (κ) for the categorical variables. Theoretically, calibration of an examiner is obtained when the SEM or κ values reach more than 0.8 or between 0.8 and 0.95, respectively. In the present study, the examiner was considered calibrated because she showed an SEM of 0.89 and a κ of 0.85. Reproducibility of the intraexaminer measurements was recalculated at 6 months, and both SEMs and κ values remained within reliable ranges during the entire study period.
Protocol of periodontal therapy.
As shown in Table 1, the timeline and procedures which were used in this study followed the procedures developed by others, with some modification (1, 4). The details of all clinical procedures employed in this study are described as follows.
Initial treatment.
After the clinical baseline measurement (day −7 or earlier) and bacterial sampling (day 0; described below in detail), each subject received oral hygiene instructions and a standard kit for mechanical supragingival plaque control. The kit contained fluoride dentifrice, a regular toothbrush, interdental toothbrushes, and dental floss. Within 4 weeks, scaling and root planing were conducted under local anesthesia with Gracey and McCall curettes and Hirschfield periodontal files (Hu-Friedy, Chicago, IL). The time required for each quadrant was approximately 2.5 h, and quadrants I to IV were treated clockwise, requiring a total of four clinical visits.
Administration of antibiotics.
Systemic antibiotic therapy was conducted in combination with mechanical debridement in the second month. All participants were prescribed 21 tablets of metronidazole at 250 mg plus amoxicillin at 500 mg (one tablet every 8 h for 1 week).
Supplemental treatment.
After 3 months, all patients received supragingival scaling and dental prophylaxis. Root planing, flap surgery, or antibiotic administration was not used for this supplemental treatment.
Flap surgery.
In the 4th month, after reevaluation of the basic periodontal clinical measurements, all 50 subjects were treated with the modified Widman flap introduced by Ramfjörd and Nissle (38). More specifically, periodontal sites that still presented bleeding on probing and a residual pocket depth of at least 6 mm received periodontal flap surgery under local anesthesia without the procedure of osseous recontouring. After each periodontal surgery, patients were asked to use chlorhexidine mouthwash (0.12%) twice a day for 1 week.
Compliance.
Patients were encouraged to comply with the study protocol. Compliance and desirable and undesirable side effects were evaluated by use of a questionnaire. Participants were reminded daily by telephone to take the prescribed antibiotics. Additional phone calls to monitor any adverse effects of the therapy were made every 15 days up to 12 months.
Sampling of subgingival bacterial plaque.
To determine the colonization pattern of A. actinomycetemcomitans strains, a pooled subgingival sample was collected at pretreatment (baseline, day 0) and 12 months from baseline examination. The total 12-month study involved an initial 4-month period of treatment followed by 8 months of posttherapy relaxation (Table 1). The site that was bleeding and also the deepest, otherwise either the site that was deepest or the site that was bleeding, in each quadrant was selected at the baseline measurement (day −7 or earlier). A total of four sites were isolated with sterile cotton rolls, and the supragingival plaque was removed with sterile cotton pellets. The subgingival sample was collected using a sterile paper point (Tanari 30; Tanariman Industrial Ltd., Manacapuru, Brazil) inserted into a gingival sulcus (periodontal pocket) for 60 s. The sterile paper points for each patient (n = 4) were placed in the same microtube filled with cold reduced Ringer's solution (1 ml/tube) (Oxoid, Basingstoke, Hampshire, United Kingdom). The samples were immediately stored at −80°C until the following analyses were performed.
Identification of A. actinomycetemcomitans substrains expressing deletion (JP2) or nondeletion (non-JP2) genotypes by DNA-PCR analysis.
The microtubes containing the paper points were centrifuged for 3 min (8.064 × g), and the supernatant was removed. From the bacterial cell pellet, genomic DNA was extracted using a commercial DNA purification kit (InstaGene; Bio-Rad Laboratories, Hercules, CA), according to the manufacturer's instructions.
To detect the specific 530-bp sequence that determines the JP2 or non-JP2 promoter region, the primers used for DNA amplification were designed in accordance with the work of Orrù et al. (35) and were synthesized by a commercial service (Invitrogen, Carlsbad, CA). Specifically, the pair of primer sequences (forward, 5′-CATTCTCGGCGAAAAAACTA-3′, and reverse, 5′-CCCATAACCAAGCCACATAC-3′) circumscribe a fragment of 530 bp present in the A. actinomycetemcomitans non-JP2 strain 652 but absent in the JP2 strain. The expected sizes of PCR products obtained from the JP2 and non-JP2 clone types were 195 bp and 696 bp, respectively.
PCR amplification was performed in a thermal cycler (Perkin-Elmer, Wellesley, MA) using a standard protocol as follows: initial denaturing at 95°C for 5 min, followed by a total of 35 cycles of denaturation at 95°C for 30 s; annealing at 50°C for 15 s, and extension at 72°C for 1 min. The final extension was carried out at 72°C for 5 min. The PCR products were analyzed by electrophoresis (5 V/cm2) in a 1.5% agarose gel, stained with ethidium bromide (0.5 μg/ml), and photographed under UV light. PCR fragments were compared with both positive- and negative-control DNAs isolated from A. actinomycetemcomitans 652 and JP2 strains, respectively.
Statistical analysis.
Descriptive analysis was performed for all variables. Quantitative variables were described throughout mean values, standard deviations, and minimum and maximum values. Qualitative variables were described throughout absolute and relative frequencies.
Initially, the intragroup prevalence data at the two time points (baseline preexamination and posttherapy examination) were compared by applying the Wilcoxon test, while the intergroup analysis was done using the Mann-Whitney test. In addition, comparisons were made between groups (JP2 and non-JP2) for independent variables (GI, PI, PD, and CAL) at baseline and 12 months by applying analysis of variance (ANOVA), the Wilcoxon test, and Student's t test when appropriate. Moreover, at 12 months, evaluation of inter- and intragroup statistical analysis was done by using the Mann-Whitney test.
All tests were performed using statistical software (SPSS for Windows Release 12.0; SPSS Inc., Chicago, IL), and the differences were considered significant when the P value was less than 0.05.
RESULTS
The study population consisted of 50 periodontally diseased patients who had no previous history of smoking. These patients were subclassed into groups infected with either JP2 or non-JP2 clones of A. actinomycetemcomitans (n = 25/group), while age and gender in each group were matched as closely as possible (Table 2).
TABLE 2.
Distribution of the study population according to A. actinomycetemcomitans LTX profile, age, gender, and previous periodontal treatment
| Characteristic | Value for group infected with A. actinomycetemcomitans clone type:
|
|
|---|---|---|
| JP2 (n = 25) | Non-JP2 (n = 25) | |
| Age (yrs) | ||
| Range | 16-39 | 15-38 |
| Mean ± SD | 24 ± 6.56 | 24.4 ± 6.44 |
| Gender (no. of patients) | ||
| Female | 18 | 18 |
| Male | 7 | 7 |
| Previous periodontal treatment (no. of patients) | ||
| Within 24 mo | 5 | 6 |
| Never | 20 | 19 |
| Periodontal diagnosis (no. of patients) | ||
| Advanced generalized chronic | 4 | 23 |
| Generalized aggressive | 21 | 2 |
In response to clinical treatment (measured at 12-month examination), the prevalence of detectable levels of A. actinomycetemcomitans decreased among patients in both groups compared to the prevalence measured at baseline preexamination (Table 3). There was no statistically significant difference between the prevalence of JP2 and non-JP2 strains found at the 12-month examination (Mann-Whitney test, P = 0.3320). It should be noted that patients remaining JP2 strain positive appeared to suffer local gingival inflammation, as determined by persistently elevated GI scores (see Fig. 2). In the context of the present study, this finding is significant because identification and elimination of JP2 are the key to effectively treating periodontitis in patients infected with A. actinomycetemcomitans. Very intriguingly, no shift of the colonizing strain from JP2 to non-JP2, or vice versa, between pre- and posttreatment examinations was observed in any patients examined, indicating that the colonizations by A. actinomycetemcomitans strains were exclusive of each other (Table 3).
TABLE 3.
Shift of prevalence of A. actinomycetemcomitans strain(s) recovered from the periodontal crevice
| Groupa | Clone type(s) | No. (%) of patients positive for strain(s) of A. actinomycetemcomitans at 12 mo |
|---|---|---|
| JP2 strain infected | JP2 | 14 (56)b,c |
| Non-JP2 | 0 (0) | |
| Both JP2 and non-JP2 | 0 (0) | |
| Not detectable | 11 (44) | |
| Non-JP2 strain | JP2 | 0 (0) |
| infected | Non-JP2 | 18 (72)b,c |
| Both JP2 and non-JP2 | 0 (0) | |
| Not detectable | 7 (28) |
At 0 months, both groups had 25 patients (100%) positive for A. actinomycetemcomitans.
Intragroup reduction from 0 months to 12 months is significant as analyzed by the Wilcoxon test (JP2-strain-infected group, P = 0.003; non-JP2-strain-infected group, P = 0.0180).
Prevalence of non-JP2 strains at 12 months is not significantly different from that of JP2 strains at 12 months by the Mann-Whitney test (P = 0.3320).
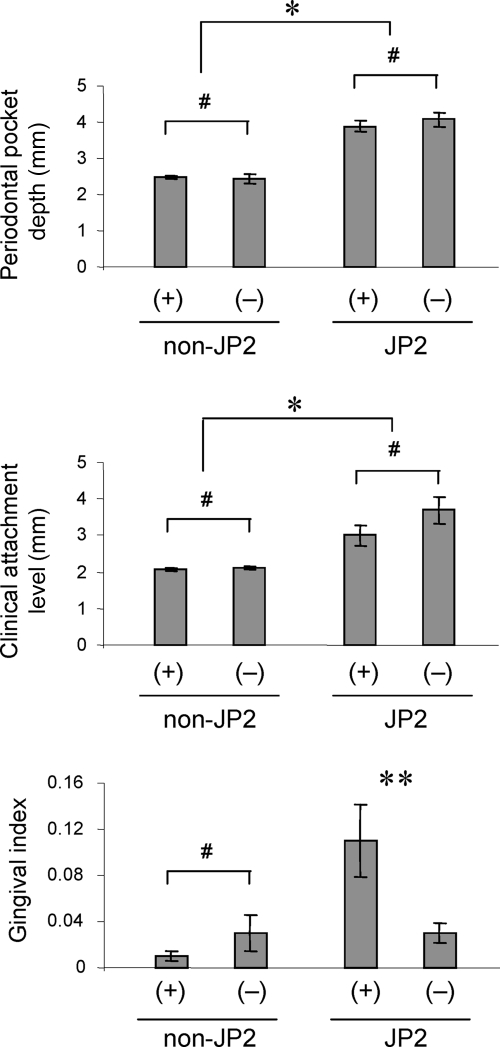
FIG. 2.
Intragroup analyses of clinical parameters at posttherapy examination. In the non-JP2-strain-infected group, clinical parameters, including PD, CAL, and GI, were compared between the subgroup of subjects who retained a detectable colonization by non-JP2 strains [non-JP2(+); n = 18] and the subgroup of subjects who lost detectable colonization by non-JP2 strains [non-JP2(−); n = 7], as measured at posttreatment examination (12 months). The same intragroup analyses for PD, CAL, and GI were carried out for the JP2-strain-infected group by comparison between JP2(+) subjects (n = 14) and JP2(−) subjects (n = 11) at posttreatment examination. The columns and bars indicate averages and standard errors, respectively. *, either the value for the JP2(+) subgroup or the value for the JP2(−) subgroup is significantly higher than that for any subgroup in the non-JP2-strain-infected group (Mann-Whitney test); **, significantly higher than non-JP2(+), non-JP2(−), or JP2(−) subgroups, by Mann-Whitney test; #, no statistical difference by ANOVA and Student's t tests.
According to the clinical readouts collected at baseline measurement, GI and PI, as well as PD and CAL, were statistically higher among the group of patients infected with the JP2 clone than the group infected with non-JP2 clones (Table 4). However, all four clinical indices measured at 12 months (i.e., 8 months posttherapy) showed significant reductions compared to the indices measured at baseline (Table 4). Nonetheless, at the 12-month examination, PD, CAL, and GI all remained significantly higher in the JP2-strain-infected group than in the non-JP2-strain-infected group, whereas PI did not show any significant difference between the JP2-strain-infected and non-JP2-strain-infected groups (Table 4). These results indicate that clinical parameters representing the results of past tissue damage and destruction (PD and CAL) as well as the parameter of currently occurring tissue damage (GI) remained higher in the JP2-strain-infected group than the non-JP2-strain-infected group, even though dental prophylaxis was equally applied to the two groups throughout the study.
TABLE 4.
Data from all participants regarding periodontal pocket depth, CAL of plaque, and GIs between day 0 and 12 months in relation to A. actinomycetemcomitans JP2 or non-JP2 strain profiles
| Clinical parameter and time (n = 25 for all times)a | Mean ± SD (range) for patient group infected with A. actinomycetemcomitans clone:
|
P | |
|---|---|---|---|
| Non-JP2 (n = 25) | JP2 (n = 25) | ||
| PD (mm) | |||
| Baseline | 3.64 ± 0.37 (2-6)b | 4.45 ± 0.82 (2-12)b,c | 0.0001 |
| 12 mo | 2.45 ± 0.26 (2-7) | 3.92 ± 0.64 (2-9)c | 0.00001 |
| CAL (mm) | |||
| Baseline | 3.04 ± 0.20 (3-5)b | 3.93 ± 1.24 (1-14)c | 0.008 |
| 12 mo | 2.08 ± 0.13 (1-5) | 3.31 ± 1.18 (1-11)c | 0.0001 |
| PI | |||
| Baseline | 0.26 ± 0.10 (0-1) | 0.38 ± 0.40 (0-2)c | 0.0134 |
| 12 mo | 0.03 ± 0.02 (0-0.75)b | 0.04 ± 0.04 (0-0.75)b | 0.5104 |
| GI | |||
| Baseline | 0.29 ± 0.11 (0-1)b | 0.49 ± 0.29 (0-2)b,c | 0.001 |
| 12 mo | 0.02 ± 0.02 (0-0.75) | 0.08 ± 0.10 (0-0.75)c | 0.001 |
P values for baseline versus 12-month data are as follows: PD, 0.00001 (non-JP2) and 0.0083 (JP2); CAL, 0.00001 (non-JP2) and 0.1298 (JP2); PI, 0.00001 (non-JP2) and 0.00012 (JP2); GI, 0.002 (non-JP2) and 0.0001 (JP2).
Statistically significant difference in comparison of data between baseline and 12 months (ANOVA and Student t tests or ANOVA and Wilcoxon tests) within the same A. actinomycetemcomitans strain-infected group.
Statistically significant difference in comparison of data between non-JP2-strain-infected and JP2-strain-infected groups at each time point (ANOVA and Student t tests).
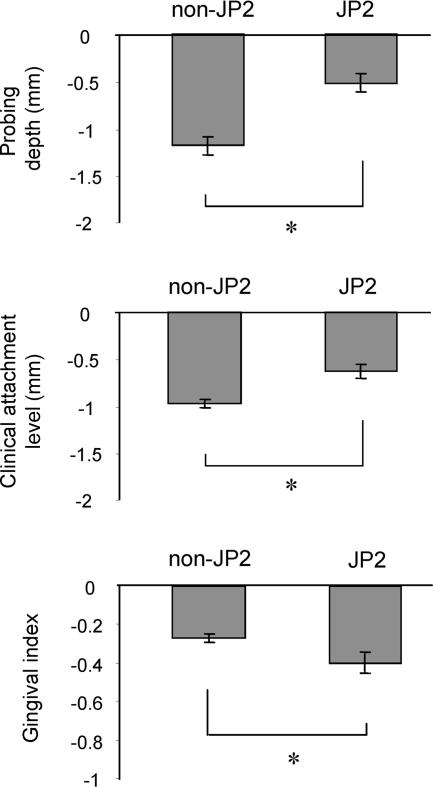
The response to treatment by the JP2-strain-infected and non-JP2-strain-infected groups was further evaluated by examining the extent of reduction of the pathogenic clinical parameters PD, CAL, and GI (Fig. 1). In comparison of the data obtained pretherapy and posttherapy, PD and CAL in the non-JP2-strain-infected group showed significantly more reduction than did these clinical parameters in the JP2-strain-infected group (Fig. 1). Interestingly, compared to the reduction rate of PD and CAL in the non-JP2-strain-infected group, an opposing trend occurred in the JP2-strain-infected group where the GI score was evidently more reduced than that parameter in the non-JP2-strain-infected group (Fig. 1). A possible reason that explains the different response patterns between the GI score and the scores of both PD and CAL found in the JP2-strain-infected group is addressed below. In any case, it is noteworthy that the raw GI score of the JP2-strain-infected group measured at 12 months was still significantly higher than that of the non-JP2-strain-infected group (Table 4). Overall, and more to the point of this paper, these analyses suggested that the treatment response of patients infected with non-JP2 strains, as monitored by clinical scores of PD and CAL, was better than the response of patients infected with JP2 strains.
FIG. 1.
Reduction of clinical parameters at posttherapy examination. The treatment response by JP2-strain-infected and non-JP2-strain-infected groups (n = 25 for both) was evaluated by examining the reduction of pathogenic clinical parameters PD, CAL, and GI. The differences between the clinical parameters measured at baseline pretreatment examination (day 0) and posttreatment examination (12 months) were calculated and expressed. The columns and bars indicate averages and standard errors, respectively. *, statistically significant difference, comparing the non-JP2-strain-infected group to the JP2-strain-infected group by ANOVA and Student's t tests.
For some patients in both JP2-strain-infected and non-JP2-strain-infected groups at the 12-month (i.e., 8-month-posttherapy) examination, the colonization by A. actinomycetemcomitans appeared to become lower than the detection limit as shown by PCR-based bacterial DNA analysis. Therefore, to relate clinical parameters to the persistence of bacteria in the patients at the 12-month examination and to determine whether patients were still positive or negative for their respective strains of A. actinomycetemcomitans, intragroup analyses of pathogenic clinical parameters (GI, PD, and CAL) (Fig. 2) were carried out among patients in each group. Among those subjects in the non-JP2-strain-infected group, no significant differences were found for PD and CAL between the patients who stayed positive for non-JP2 strains and those who became negative for non-JP2 strains. Similarly, no significant differences of PD and CAL between the JP2-strain-negative and JP2-strain-positive patients were observed among patients in the JP2-strain-infected group. However, among subjects who were originally JP2 strain positive, as measured at 12 months, the patients who remained JP2 strain positive showed significantly higher GIs than those who lost the detectable JP2 clone (Fig. 2). For subjects representing the latter case, this would account for the opposing trend which occurred in the JP2-strain-infected group, as suggested above, where the GI score was evidently more reduced than that parameter in the non-JP2-strain-infected group (Fig. 1). However, this phenomenon has further implications, particularly since there was no difference of GI between non-JP2-strain-positive and non-JP2-strain-negative patients among individuals in the non-JP2-strain-infected group who were also measured at the 12-month examination.
GI is a clinical measurement which indicates the inflammatory response triggered by currently occurring tissue damage. Therefore, among those patients still showing detectable JP2 A. actinomycetemcomitans infection, inflammation of gingival tissue, as determined by GI, is persistently induced, in contrast to patients who became JP2 strain negative and thus showed no evidence of gingival inflammation. In other words, the removal of H-LTX A. actinomycetemcomitans diminished inflammation, while the removal of non-JP2 strains did not show any significant difference in the GI scores. These results indicated that the early identification and elimination of JP2+ A. actinomycetemcomitans from the periodontal pocket is a key factor in preventing the progression of periodontitis associated with JP2+ A. actinomycetemcomitans infection by allowing optimal results from the usual three-step mechanical-chemical therapies.
DISCUSSION
In response to the therapy administered in this study, the incidence of JP2 and non-JP2 A. actinomycetemcomitans diminished in both groups of patients. At the same time, no cross-infection between JP2 and non-JP2 strains was detected at the 12-month measurement. At baseline, clinical measurements of PD, CAL, GI, and PI were statistically higher in the JP2-strain-positive group than the non-JP2-strain-positive group. In response to treatment, however, PD, CAL, GI, and PI scores for both the JP2-strain-positive and non-JP2-strain-positive groups decreased significantly, but non-JP2-strain-positive patients showed greater decreases in the clinical pathogenic parameters PD and CAL than did the JP2-strain-positive patients. These results indicated that non-JP2-strain-positive patients responded more efficiently to the periodontal therapy applied in this study than did the JP2-strain-positive patients. Although it is unclear precisely which clinical approach or specific treatment modality accounts for this result, the findings obtained in our study indicate that the identification of JP2 and non-JP2 strain infection at baseline diagnosis can, at the very least, be predictive of potentially successful clinical treatment for these A. actinomycetemcomitans strains. In addition, because elimination of A. actinomycetemcomitans JP2 strains resulted in the improvement of the GI score (Fig. 2), any treatment that might be able to remove A. actinomycetemcomitans JP2 strains from patients would be of particular interest.
A recent study which analyzed the multilocus sequence of 82 A. actinomycetemcomitans strains, including 66 JP2 clones, indicated that the JP2 clone initially emerged as a distinct genotype in the part of Africa along the Mediterranean approximately 2,400 years ago (22). Later, this specific strain appeared to spread throughout the whole continent of Africa (22). The emergence of the JP2 clone in North, Central, and South America is considered to have occurred during the transatlantic slave trade from Africa (22). The population of Brazil is a more diverse mixture of ethnic origins than those of other countries in North and South America. However, since the ethnic background of individual citizens is not officially registered in Brazil, it would be difficult to determine the ethnic background of the participants in this study. Nonetheless, based on the geometrical origin of the JP2 clone, as noted above, the JP2 clones found in this study are believed to have originated from Africa. Thus, in order to conclude the possible association between the origin of a given highly leukotoxic strain clone and ethnicity, it is necessary to correlate the clonal diversity of highly leukotoxic strains with the defined ethnic background of patients infected with, for example, the JP2 clone. This question will be addressed in a future study.
Originally, LTX was found to destroy neutrophils (26) and macrophages (37). Since this activity helps bacteria avoid host innate immune responses, LTX was considered to play an important role in the pathogenesis elicited by A. actinomycetemcomitans. In 1997, Haubek et al. (17) originally reported that only highly leukotoxic strains showed beta-hemolysis activity on blood agar, while the bacterial molecule that is responsible for this hemolysis activity was not clear. However, Balashova et al. (3) revealed that the hemolysis activity in A. actinomycetemcomitans is derived from LTX. Therefore, the periodontal tissue damage related to JP2 infectious strains could be associated with the neutrophil-killing activity of LTX as well as its hemolytic activity. Actually, the elevated GI score in patients who remained JP2 strain positive at 12 months (i.e., 8 months posttherapy) (Fig. 2) might be explained by locally produced LTX hemolytic action, resulting in the continuing colonization by the JP2 clone and hence sustained inflammation in the periodontal pocket.
The successful treatment of the patients with LAP was originally related to the reduction of A. actinomycetemcomitans, whereas failure was simply attributed to the persistence of high levels of this microorganism in the diseased lesion (15, 31). However, when these clinical investigations were carried out in the 1980s, it was still unclear if A. actinomycetemcomitans could be subclassified into pathogenic and nonpathogenic strains based on the difference in LTX promoters. A recent study by Haubek et al. (21) has now revealed that the non-JP2 clones can be considered commensal bacteria in oral ecology because non-JP2 clones are found in successfully treated LAP patients, as well as some periodontally healthy subjects. On the other hand, the persistence of A. actinomycetemcomitans in patients with LAP may now be attributed to the strong correlation between JP2 clones and continuing A. actinomycetemcomitans pathogenesis (21). Therefore, based on the genotype determined by the presence or absence of a specific 530-bp sequence in the LTX promoter region, we followed up these clinical studies and presented data further demonstrating that identification of A. actinomycetemcomitans at baseline examination could be a reasonable predictor of treatment response outcomes because patients with JP2+ clones were observed to be more resistant to clinical treatment than were those harboring non-JP2+ clones.
A recent study examining adolescents in Morocco demonstrated that healthy carriers of the JP2 clone are at prominently higher risk for developing aggressive periodontitis than are those who carry the non-JP2 clones (21, 23). This finding clarified that aggressive periodontitis is caused by the JP2 clone of A. actinomycetemcomitans, which is a monoinfection, compared to chronic adult periodontitis, which is associated with polymicrobial infection. However, adolescent periodontitis is also associated with non-JP2 clones of A. actinomycetemcomitans (36) in China (34), Brazil (8), and North America (16). Furthermore, it is true that more than one serotype or clonal type is associated with the onset of periodontitis found in adolescents in the United States (12). Based on this evidence, the results obtained in this study could be interpreted as follows: that monoinfection with the JP2 clone develops an aggressive type of periodontitis similar to the cases found in Morocco, whereas non-JP2 clone infections may involve polymicrobial infection in which the reduction of total plaque, rather than the elimination of specific bacteria, results in the amelioration of the disease. Our data clearly demonstrated that diminishing levels of A. actinomycetemcomitans JP2 strains resulted in a significant decrease of inflammatory clinical GI score, while the presence or absence of non-JP2 strains had no effect on the GI score measured at 12 months (Fig. 2). Therefore, the early identification of JP2+ A. actinomycetemcomitans and its elimination from the oral cavity are key steps to be taken as part of successful therapy against periodontitis.
Since many different bacteria are reported to colonize the gingival crevice and since putative periodontal pathogens, such as Porphyromonas gingivalis or Tannerella forsythia, are found predominantly in patients' gingival crevice (periodontal pocket), it is conceivable that periodontal treatment applied to patients also reduced the number of other putative periodontal pathogens, which may also affect the results obtained in this study. Nonetheless, the statistically significant difference found in the change of clinical parameters between the JP2 strain and the non-JP2 strain clearly indicated that original colonization by the JP2 strain lowers the patient's responsiveness to periodontal therapy. Future studies will address the influence of multiple species of subgingival bacteria on the patients' responsiveness to periodontal therapy in relation to the presence or absence of JP2 or non-JP2 strains in the patients with periodontal disease.
In summary, considering all traditional periodontal clinical parameters, our findings suggested that patients infected with the JP2 clone of A. actinomycetemcomitans showed poorer responses to the periodontal therapies applied in this study than did subjects infected with the non-JP2 clones. Since 56% of subjects remained positive for JP2 clones, even after the intensive treatment employed in this study, establishment of an innovative supplemental therapy that can eliminate highly leukotoxic A. actinomycetemcomitans is required.
Acknowledgments
Support for this study was provided by grants DE18310 and DE18499 from NIDCR.
Footnotes
Published ahead of print on 6 May 2009.
REFERENCES
- 1.Akincibay, H., S. O. Orsal, D. Sengün, and T. F. Tözüm. 2008. Systemic administration of doxycycline versus metronidazole plus amoxicillin in the treatment of localized aggressive periodontitis: a clinical and microbiologic study. Quintessence Int. 39e33-39. [PubMed] [Google Scholar]
- 2.Araujo, M. W. B., K. M. Hovey, J. R. Benedek, S. G. Grossi, J. Dorn, J. Wactawski-Wende, R. J. Genco, and M. Trevisan. 2003. Reproducibility of probing depth measurement using a constant-force electronic probe: analysis of inter- and intraexaminer variability. J. Periodontol. 741736-1740. [DOI] [PubMed] [Google Scholar]
- 3.Balashova, N. V., J. A. Crosby, L. Al Ghofaily, and S. C. Kachlany. 2006. Leukotoxin confers beta-hemolytic activity to Actinobacillus actinomycetemcomitans. Infect. Immun. 742015-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berglundh, T., L. Krok, B. Liljenberg, E. Westfelt, G. Serino, and J. Lindhe. 1998. The use of metronidazole and amoxicillin in the treatment of advanced periodontal disease. A prospective, controlled clinical trial. J. Clin. Periodontol. 25354-362. [DOI] [PubMed] [Google Scholar]
- 5.Brogan, J. M., E. T. Lally, K. Poulsen, M. Kilian, and D. R. Demuth. 1994. Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect. Immun. 62501-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christgau, M., T. Manner, S. Beuer, K. A. Hiller, and G. Schmalz. 2006. Periodontal healing after non-surgical therapy with a modified sonic scaler: a controlled clinical trial. J. Clin. Periodontol. 33749-758. [DOI] [PubMed] [Google Scholar]
- 7.Cortelli, J. R., S. C. Cortelli, V. Haraszthy, S. F. Jordan, and J. J. Zambon. 2005. Prevalence of periodontal pathogens in Brazilians with aggressive or chronic periodontitis. J. Clin. Periodontol. 32860-866. [DOI] [PubMed] [Google Scholar]
- 8.Cortelli, S. C., A. O. C. Jorge, J. R. Cortelli, S. F. Jordan, and V. Haraszthy. 2003. Detection of highly and minimally leukotoxic Actinobacillus actinomycetemcomitans strains in patients with periodontal disease. Braz. Oral Res. 17183-188. [DOI] [PubMed] [Google Scholar]
- 9.Cortelli, S. C., M. Feres, J. A. Shibli, L. C. Figueiredo, V. Padilha de Oliveira, and J. R. Cortelli. 2005. Presence of Actinobacillus actinomycetemcomitans on the Community Periodontal Index (CPI) teeth in periodontally healthy individuals. J. Contemp. Dent. Pract. 15:85-93. [PubMed] [Google Scholar]
- 10.Dahlén, G., M. Wikström, and S. Renvert. 1996. Treatment of periodontal disease based on microbiological diagnosis. A 5-year follow-up on individual patterns. J. Periodontol. 67879-887. [DOI] [PubMed] [Google Scholar]
- 11.DiRienzo, J. M., J. Slots, M. Sixou, M. A. Sol, R. Harmon, and T. L. McKay. 1994. Specific genetic variants of Actinobacillus actinomycetemcomitans correlate with disease and health in a regional population of families with localized juvenile periodontitis. Infect. Immun. 623058-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine, D. H., K. Markowitz, D. Furgang, K. Fairlie, J. Ferrandiz, C. Nasri, M. McKiernan, and J. Gunsolley. 2007. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: longitudinal cohort study of initially healthy adolescents. J. Clin. Microbiol. 453859-3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gafan, G. P., V. S. Lucas, G. J. Roberts, A. Petrie, M. Wilson, and D. A. Spratt. 2004. Prevalence of periodontal pathogens in dental plaque of children. J. Clin. Microbiol. 424141-4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunsolley, J. C., J. J. Zambon, C. A. Mellott, C. N. Brooks, and C. C. Kaugars. 1994. Periodontal therapy in young adults with severe generalized periodontitis. J. Periodontol. 65268-273. [DOI] [PubMed] [Google Scholar]
- 15.Haffajee, A. D., S. S. Socransky, J. L. Ebersole, and D. J. Smith. 1984. Clinical, microbiological and immunological features associated with the treatment of active periodontosis lesions. J. Clin. Periodontol. 11600-618. [DOI] [PubMed] [Google Scholar]
- 16.Haraszthy, V. I., G. Hariharan, E. M. Tinoco, J. R. Cortelli, E. T. Lally, E. Davis, and J. J. Zambon. 2000. Evidence for the role of highly leuotoxic Actinobacillus actinomycetemcomitans in the pathogenesis of localized juvenile and other forms of early-onset periodontitis. J. Periodontol. 71912-922. [DOI] [PubMed] [Google Scholar]
- 17.Haubek, D., J. M. Dirienzo, E. M. Tinoco, J. Westergaard, N. J. Lopez, C. P. Chung, K. Poulsen, and M. Kilian. 1997. Racial tropism of a highly toxic clone of Actinobacillus actinomycetemcomitans associated with juvenile periodontitis. J. Clin. Microbiol. 353037-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haubek, D., O. K. Ennibi, K. Poulsen, S. Poulsen, N. Benzarti, and M. Kilian. 2001. Early-onset periodontitis in Morocco is associated with the highly leukotoxic clone of Actinobacillus actinomycetemcomitans. J. Dent. Res. 801580-1583. [DOI] [PubMed] [Google Scholar]
- 19.Haubek, D., O. K. Ennibi, K. Poulsen, N. Benzarti, and V. Baelum. 2004. The highly leukotoxic JP2 clone of Actinobacillus actinomycetemcomitans and progression of periodontal attachment loss. J. Dent. Res. 83767-770. [DOI] [PubMed] [Google Scholar]
- 20.Haubek, D., and J. Westergaard. 2004. Detection of a highly toxic clone of Actinobacillus actinomycetemcomitans (JP2) in a Moroccan immigrant family with multiple cases of localized aggressive periodontitis. Int. J. Paediatr. Dent. 1441-48. [DOI] [PubMed] [Google Scholar]
- 21.Haubek, D., A. Havemose-Poulsen, and J. Westergaard. 2006. Aggressive periodontitis in a 16-year-old Ghanaian adolescent, the original source of Actinobacillus actinomycetemcomitans strain HK1651—a 10-year follow up. Int. J. Paediatr. Dent. 16370-375. [DOI] [PubMed] [Google Scholar]
- 22.Haubek, D., K. Poulsen, and M. Kilian. 2007. Microevolution and patterns of dissemination of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans. Infect. Immun. 753080-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haubek, D., O. K. Ennibi, K. Poulsen, M. Vaeth, S. Poulsen, and M. Kilian. 2008. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. Lancet 371237-242. [DOI] [PubMed] [Google Scholar]
- 24.He, T., T. Nishihara, D. R. Demuth, and I. Ishikawa. 1999. A novel insertion sequence increases the expression of leukotoxicity in Actinobacillus actinomycetemcomitans clinical isolates. J. Periodontol. 701261-1268. [DOI] [PubMed] [Google Scholar]
- 25.Henderson, B., S. P. Nair, J. M. Ward, and M. Wilson. 2003. Molecular pathogenicity of the oral opportunistic pathogen Actinobacillus actinomycetemcomitans. Annu. Rev. Microbiol. 5729-55. [DOI] [PubMed] [Google Scholar]
- 26.Iwase, M., H. M. Korchak, E. T. Lally, P. Berthold, and N. S. Taichman. 1992. Lytic effects of Actinobacillus actinomycetemcomitans leukotoxin on human neutrophil cytoplasts. J. Leukoc. Biol. 52224-227. [DOI] [PubMed] [Google Scholar]
- 27.Leung, N. M., R. Chen, and J. D. Rudney. 2006. Oral bacteria in plaque and invading buccal cells of young orthodontic patients. Am. J. Orthod. Dentofacial Orthop. 130:698.e11-18. [DOI] [PubMed] [Google Scholar]
- 28.Löe, H., and J. Silness. 1963. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol. Scand. 21533-551. [DOI] [PubMed] [Google Scholar]
- 29.López, N. J., P. C. Smith, and J. Gutierres. 2002. Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: a randomized controlled trial. J. Periodontol. 73911-924. [DOI] [PubMed] [Google Scholar]
- 30.Macheleidt, A., H. P. Muller, T. Eger, M. Putzker, and L. Zoller. 1999. Clonal diversity of Actinobacillus actinomycetemcomitans isolates from young adults with minimal periodontal disease. J. Periodontal Res. 34179-187. [DOI] [PubMed] [Google Scholar]
- 31.Mandell, R. L., L. S. Tripodi, E. Savitt, J. M. Goodson, and S. S. Socransky. 1986. The effect of treatment on Actinobacillus actinomycetemcomitans in localized juvenile periodontitis. J. Periodontol. 5794-99. [DOI] [PubMed] [Google Scholar]
- 32.Meyer, D. H., J. E. Lippmann, and P. M. Fives-Taylor. 1996. Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic, multistep process. Infect. Immun. 642988-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mombelli, A., R. Gmür, C. Gobbi, and N. P. Lang. 1994. Actinobacillus actinomycetemcomitans in adult periodontitis. II. Characterization of isolated strains and effect of mechanical periodontal treatment. J. Periodontol. 65827-834. [DOI] [PubMed] [Google Scholar]
- 34.Mombelli, A., R. Gmür, N. P. Lang, E. Corbert, and J. Frey. 1999. Actinobacillus actinomycetemcomitans in Chinese adults. Serotype distribution and analysis of the leucotoxin gene promoter locus. J. Clin. Periodontol. 26505-510. [DOI] [PubMed] [Google Scholar]
- 35.Orrù, G., M. F. Marini, M. L. Ciusa, D. Isola, M. Cotti, M. Baldoni, V. Piras, E. Pisano, and C. Montaldo. 2006. Usefulness of real time PCR for the differentiation and quantification of 652 and JP2 Actinobacillus actinomycetemcomitans genotypes in dental plaque and saliva. BMC Infect. Dis. 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pihlstrom, B. L., and D. H. Fine. 2008. Aggressive periodontitis in adolescents in Morocco. Lancet 371188-189. [DOI] [PubMed] [Google Scholar]
- 37.Rabie, G., E. T. Lally, and B. J. Shenker. 1988. Immunosuppressive properties of Actinobacillus actinomycetemcomitans leukotoxin. Infect. Immun. 56122-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramfjord, S. P., and R. R. Nissle. 1974. The modified widman flap. J. Periodontol. 45601-607. [DOI] [PubMed] [Google Scholar]
- 39.Shiloah, J., M. R. Patters, J. W. Dean III, P. Bland, and G. Toledo. 1998. The prevalence of Actinonacillus actinomycetemcomitans, Porphyromonas gingivalis and Bacteroides forsythus in humans 1 year after 4 randomized treatment modalities. J. Periodontol. 691364-1372. [DOI] [PubMed] [Google Scholar]
- 40.Silness, J., and H. Löe. 1964. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 22121-135. [DOI] [PubMed] [Google Scholar]
- 41.Slots, J., H. S. Reynolds, and R. J. Genco. 1980. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect. Immun. 291013-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Winkelhoff, A. J., J. P. Rodenburg, R. J. Goene, F. Abbas, E. G. Winkell, and J. de Graaff. 1989. Metronidazole plus amoxicillin in the treatment of Actinobacillus actinomycetemcomitans associated periodontitis. J. Clin. Periodontol. 16128-131. [DOI] [PubMed] [Google Scholar]
- 43.Wu, Y. M., J. Yan, L. L. Chen, and Z. Y. Gu. 2007. Association between infection of different strains of Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans in subgingival plaque and clinical parameters in chronic periodontitis. J. Zhejiang Univ. Sci. B 8121-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, H. W., Y. F. Huang, Y. Chan, and M. Y. Chou. 2005. Relationship of Actinobacillus actinomycetemcomitans serotypes to periodontal condition: prevalence and proportions in subgingival plaque. Eur. J. Oral Sci. 11328-33. [DOI] [PubMed] [Google Scholar]
- 45.Zambon, J. J., L. A. Christersson, and J. Slots. 1983. Actinobacillus actinomycetemcomitans in human periodontal disease. Prevalence in patient groups and distribution of biotypes and serotypes within families. J. Periodontol. 54707-711. [DOI] [PubMed] [Google Scholar]