Abstract
After acute brucellosis infection, symptoms persist in a minority of patients for more than 1 year. Such patients are defined as having chronic brucellosis. Since no objective laboratory methods exist to confirm the presence of chronic disease, these patients suffer delays in both diagnosis and treatment. The aim of the current study was to evaluate the usefulness of quantitative real-time PCR (Q-PCR) in the diagnosis and follow-up of these patients. Thirty-five subjects with a well-documented history of brucellosis that had been diagnosed between 2 and 33 years previously were screened by Q-PCR for the presence of Brucella melitensis DNA and by serological tests and blood culture. Subjects were divided into three groups: 8 (23%) focal-disease subjects, 9 (26%) nonfocal-disease subjects with subjective complaints, such as fatigue, malaise, arthralgia, and/or myalgia, and 18 (51%) asymptomatic subjects. All (100%) focal-disease patients and symptomatic nonfocal-disease patients had at least one positive Q-PCR sample. Only six (33%) of the asymptomatic subjects had Q-PCR-positive samples (P < 0.05). Eleven patients (five focal-disease patients and six nonfocal-disease patients with subjective complaints) received therapy during the study. For those patients who completed treatment, six (60%) still had Q-PCR-positive samples at the posttreatment follow-up. The proportion of individuals with B. melitensis DNA was significantly higher for symptomatic nonfocal-disease patients than for asymptomatic subjects. Therefore, Q-PCR appears to be a useful method for identifying chronic brucellosis patients.
Human brucellosis is a multisystem disease that may present with a broad spectrum of clinical manifestations. The causative organisms, Brucella spp., are facultatively intracellular bacteria that are capable of evading a number of host defense mechanisms and can survive within phagocytic cells for long periods. These properties may account for focal complications, relapses, and chronic disease (21).
The chronic course of the disease was initially explored during the 1930s by Evans (6a) and was further explored in the 1950s by Davies (5). In 1951, Spink and associates stated, with respect to the duration of the illness, that the majority of patients with brucellosis recover within a year after the administration of an antibiotic, while a small but significant number of patients continue to have clinical manifestations despite such therapy (22). These patients can be divided into two groups: those with a focal disease, such as spondylitis, and those without a focal disease who complain, nevertheless, of poor health and have symptoms such as chronic fatigue syndrome (CFS), musculoskeletal pain, depression, or anxiety.
The diagnosis of chronic brucellosis is often based on clinical complaints together with the presence of high immunoglobulin G titers (2). However, the specificity of current serological assays is considered to be low, since titers may remain positive for years after the successful resolution of symptoms. In 1980, Buchanan and Faber described a serological test for evaluation of the effectiveness of treatment and for excluding a diagnosis of chronic brucellosis (3). The authors observed that, in assays with 2-mercapthoethanol, results remained positive for 9% of patients 1 year after the initiation of treatment. Among these patients, 50% still had signs and symptoms of brucellosis and required further treatment. Recently, PCR has been used to detect Brucella spp. in the diagnosis of primary infections, relapse, and focal complications of the disease (12, 13, 20). Initially, the persistence of Brucella sp. DNA after therapy was linked to relapse (9, 12, 17). However, our group and others have demonstrated the persistence of Brucella sp. DNA for long periods of time after the conclusion of therapy in asymptomatic patients (10, 15, 24). The practical role of quantitative real-time PCR (Q-PCR) in the laboratory diagnosis of chronic brucellosis and the assessment of clinical manifestations remains to be demonstrated. In the present study, we examined 35 subjects with a well-documented history of brucellosis and followed them to evaluate the usefulness of Q-PCR and the relationship between a positive result by the Q-PCR assay and clinical course.
MATERIALS AND METHODS
Subjects and samples.
Between February 2003 and October 2008, we screened patients with a well-documented history of brucellosis for symptoms and the persistence of Brucella melitensis DNA. A diagnosis of acute brucellosis had been made between 2 and 33 years previously according to one or both of the following criteria: isolation of Brucella spp. from blood or any sample of body fluid or tissue and/or the presence of a compatible clinical picture together with the demonstration of specific antibodies at significant titers (≥1:160 by the Wright test or ≥1:320 by the Coombs test) or seroconversion. All of the isolated strains were sent to the Centro de Investigación y Tecnología Agroalimentaria (Zaragoza, Spain) for definitive identification and biotyping. All isolates were identified as B. melitensis biotype 1 and B. melitensis biotype 3.
Subjects were divided into three groups. Group A consisted of eight (23%) focal-disease subjects. Group B comprised nine (26%) nonfocal-disease subjects who complained of subjective symptoms, such as fatigue, malaise, arthralgia, and/or myalgia. Group C included 18 (51%) asymptomatic subjects.
Each time a patient visited our office for a checkup, blood and serum samples were taken for analysis by Q-PCR, blood culture, and serological and laboratory evaluations. Laboratory evaluations included complete blood counts, erythrocyte sedimentation rates, routine biochemical tests (for alanine aminotransferase, alkaline phosphatase, gamma glutamyl transpeptidase, and creatinine), and tests for C-reactive protein and rheumatoid factor. We also analyzed one sample of cerebrospinal fluid (CSF) and one sample of synovial fluid (SF) for two focal-disease patients.
Definitions.
Because we observed various patterns of disease, we used the following operational definitions for purposes of clarity and classification. Chronic brucellosis patients included all patients whose symptoms, whether nonspecific or symptoms of focal disease, had persisted for more than 1 year after the initial episode. Diagnosis of focal disease, such as spondylitis, sacroiliitis, hip arthritis, knee arthritis, spleen abscess, hepatic abscess, or multifocal motor neuropathy, was made on the basis of appropriate findings upon physical examination and radiological analysis, bone scintigraphy, magnetic resonance studies, or electroneurograms.
Treatment.
Treatment regimens were based on patients' preferences and/or focal complications. Therapeutic efficacy was evaluated as either (i) a treatment effect of no benefit or a (ii) temporary or (iii) permanent resolution of symptoms or signs of the disease at the end of antibiotic treatment.
Control group.
Control samples of whole blood and serum were obtained from 15 healthy donors at the University General Hospital of Albacete (Albacete, Spain). The study was approved by the institutional review board at the center, and informed consent was obtained from all patients.
Microbiological studies.
Tissue samples were cultured according to standard microbiological techniques (7). Blood culture, the rose bengal test, and the Wright test were performed as described elsewhere (15). The anti-Brucella Coombs test was performed by the standard method (19).
Extraction of DNA.
DNA was extracted from whole-blood samples, serum samples, CSF, and SF with the UltraClean DNA-BloodSpin kit (Mo Bio Laboratories, Carlsbad, CA) in accordance with the manufacturer's instructions but with the following three modifications: (i) in step 1, 40 μl of proteinase K (6 mg/ml) was added; (ii) in step 6, incubation at 65°C was performed for 15 min; and (iii) in the final step, DNA was eluted in 50 μl of elution buffer for samples of whole blood, CSF, and SF and in 40 μl for serum samples. All of the final concentrations of DNA, as well as purity, were determined with a Nanodrop spectrophotometer, model ND-1000 (Nanodrop Technologies, Wilmington, DE).
Q-PCR.
After DNA extraction, amplification was performed as previously described (15) with slight modifications. The amount of human DNA included in each 20 μl of Q-PCR mixture was 200 ng when DNA had been extracted from whole-blood samples and 2 μl when DNA had been extracted from serum, CSF, or SF. For construction of a standard curve, 10-fold serial dilutions of the 251-bp fragment of B. melitensis DNA were included in duplicate, at levels ranging from 106 to 100 copies per reaction mixture. The standard curve had a correlation coefficient (R2) of 1, a slope of −3.4, and an intercept of 40. Q-PCR efficiency (E = 10−1/slope) was 1.97.
To avoid potential carryover contamination of samples with previously amplified products of Q-PCR, tubes with water only were distributed among the tubes with clinical samples, and all were handled in the same way as the tubes of samples during DNA extraction and Q-PCR. The mixture for Q-PCR was prepared in a laminar-flow hood, and positive controls were manipulated in a separate room with a different set of instruments.
Each sample was analyzed in triplicate. A sample was considered positive when at least one of the three replicates gave an amplified product. Each assay included one positive control consisting of a dilute solution of B. melitensis DNA and one negative control lacking template DNA. Data (bacterial DNA loads) were expressed as copies of B. melitensis DNA per milliliter of sample ± standard deviation (SD).
Statistical analysis.
The chi-square test and Fisher's exact test were used to compare categorical variables in different groups. Student's t test and the Wilcoxon rank-sum test were used to compare continuous variables. A P value equal to or less than 0.05 was considered to indicate a statistically significant difference.
RESULTS
Study subjects.
There were 35 subjects in the study, each with a well-documented history of brucellosis, diagnosed between 2 and 33 years previously. Twelve subjects were women (34%), and 23 were men (66%). The mean age was 48 years (range, 24 to 83 years). Subjects were followed for 1 to 2,166 days (mean, 657 days). Patients' epidemiological features and clinical findings upon entry into the study are summarized in Table 1. Diffuse polyarthralgia and fatigue were the most frequent symptoms. None of the patients was febrile. Five patients had been diagnosed with CFS and one with fibromyalgia.
TABLE 1.
Clinical and epidemiological details and results of serological tests and Q-PCR assays of 17 chronic brucellosis patients upon entry into the study
| Patient no. | Dates of sample collection | Age (yr)/sexa | Yr of infection | Focal complication | Associated diseaseb | Clinical manifestation(s) | Result by the following testc:
|
Treatment (days)d | Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RB | STA | Coombs | Q-PCR
|
||||||||||
| Blood | Serum | ||||||||||||
| Group A (focal-disease patients) | |||||||||||||
| 1 | Apr. 2005-Feb. 2009 | 66/M | 1998 | Spleen abscess | Diabetes | Polyarthralgia | + | 1,280 | 5,120 | 6 | 0 | 1, Dox (60) + Strep (15); 2, Dox (60) + Rif (60) + TMP-SMZ (60); 3, Dox (180) | Polyarthralgia |
| 2 | Aug. 2005-Jan. 2009 | 51/M | 1978 | Pain in right hip | Hypertension, dyslipidemia, depression, glaucoma | Pain in right hip | − | − | 1,280 | 271 | 154 | Dox (90) + Rif (90) | Pain in right hip |
| 3 | June 2006-Jan. 2009 | 44/M | 1990 | Multiple multifocal motor neuropathy | Multiple multifocal motor neuropathy | Loss of right-hand strength | − | − | 40 | 461 | 0 | 1, Dox (240) + Rif (240); 2, Dox (180) + Rif (180) | Loss of right-hand strength |
| 4 | Sept. 2006-Oct. 2007 | 30/M | 1998 | Pain in right knee | Epileptic crisis | Polyarthralgia | − | − | 40 | 0e | 0e | Dox (45) + Gent (7) | Polyarthralgia |
| 5 | Nov. 2003-Feb. 2009 | 35/M | 1999 | Sacroiliitis | Hemochromatosis, obesity, seborrheic dermatitis | Osteoarticular pain | + | 20 | 1,280 | 136 | 35 | None recommended | Polyarthralgia |
| 6 | Mar. 2007-Jan. 2009 | 40/F | 1993 | Spondylitis L5 | Fibromyalgia, asthma | Arthromyalgia, lumbar pain | − | − | 80 | 125 | 94 | Dox (90) + Rif (90) | Osteomuscular pain |
| 7 | Dec. 2006-Sept. 2008 | 64/M | 2000 | Spondylitis | None | None | + | 20 | 640 | 96 | 0 | None recommended | Asymptomatic |
| 8 | July 2003-Dec. 2008 | 60/M | 2000 | Hepatic abscess | None | None | − | − | 640 | 72 | 359 | None recommended | Asymptomatic |
| Group B (nonfocal-disease patients with subjective complaints) | |||||||||||||
| 9 | Mar. 2003-Nov. 2008 | 44/M | 1990 | None | CFS, hepatitis C | Osteomuscular pain | − | − | − | 178 | 132 | None recommended | Osteomuscular pain |
| 10 | Feb. 2003-Jan. 2008 | 57/M | 1997 | None | Prostatitis | Polyarthralgia | − | − | 1,280 | 6 | 0 | 1, Dox (60); 2, Dox (60); 3, Dox (60); 4, Dox (60) | Polyarthralgia |
| 11 | Jan. 2006-Feb. 2009 | 49/F | 2006 | None | CFS, DM II | Polyarthralgia, asthenia | + | 80 | 320 | 3,755 | 0 | 1, Dox (60); 2, Dox (45) + Strep (14); 3, TMP-SMZ (90); 4, Dox (90) | Polyarthralgia |
| 12 | Dec. 2002-Feb. 2009 | 54/M | 2002 | None | Schizophrenia | Pain in right shoulder | + | 40 | 20,480 | 0 | 797 | None recommended | Asymptomatic |
| 13 | Apr. 2003-Feb. 2009 | 42/F | 1994 | None | CFS | Polyarthralgia, fatigue, asthenia | − | 40 | 40 | 112 | 0 | Dox (180) | Osteomuscular pain |
| 14 | June 2006-Jan. 2009 | 36/F | 1996 | None | None | Polyarthralgia | + | 80 | 2,560 | 0 | 182 | 1, Dox + Rif (interrupted); 2, TMP-SMZ (90); 3, Dox (90) | Polyarthralgia |
| 15 | July 2006-Oct. 2008 | 35/M | 1997 | None | Hyperlipidemia | Polyarthralgia | − | − | − | 32 | 366 | Dox (120) + Rif (120) | Polyarthralgia |
| 16 | Oct. 2007-Jan. 2009 | 37/M | 1991 | None | CFS | Polyarthralgia | − | − | − | 0 | 121 | None recommended | Polyarthralgia |
| 17 | Feb. 2008-Jan. 2009 | 56/M | 1975 | None | CFS, hypothyroidism, hypertension | Polyarthralgia | − | 40 | 2,560 | 25 | 0 | Rif (interrupted) | Polyarthralgia |
M, male; F, female.
DM II, diabetes mellitus type II.
RB, rose bengal; STA, standard tube agglutination; +, positive; −, negative. Standard tube agglutination and Coombs test results are expressed as reciprocal serology titers. Q-PCR results are expressed as copies of B. melitensis DNA per milliliter of sample.
Dox, doxycycline (100 mg/12 h); Strep, streptomycin (1 g/24 h); Rif, rifampin (900 mg/24 h); Gent, gentamicin (240 mg/24 h); TMP-SMZ, cotrimoxazole (trimethoprim [TMP], 160 mg/12 h; sulfamethoxazole [SMZ], 800 mg/12 h). Numbers followed by commas indicate the first, second, third, or fourth course of treatment.
Upon entry into the study, the SF sample was positive by the Q-PCR assay, with 175 copies/ml.
Serological analysis and blood culture.
We performed serological analysis on a total of 246 serum samples, including 90 from focal-disease patients, 122 from symptomatic nonfocal-disease patients, and 34 from asymptomatic subjects. The number of serologically positive samples tested for each group of patients is summarized in Table 2.
TABLE 2.
Serological results for the three groups of patients
| Groupa (n) | Wright test
|
Coombs test
|
No. positive by the rose bengal test/total (%) | ||
|---|---|---|---|---|---|
| No. with a score of ≥1:160/total (%) | Rangeb | No. with a score of ≥1:320/total (%) | Range | ||
| A (8) | 6/90 (7) | Neg-1:1,280 | 45/90 (50) | Neg-1:5,120 | 19/90 (21) |
| B (9) | 17/122 (14) | Neg-1:160 | 62/122 (51) | Neg-1:40,960 | 34/122 (28) |
| C (18) | 0/34 (0) | Neg-1:40 | 5/34 (15) | Neg-1:640 | 0/34 (0) |
Group A, focal-disease patients; group B, nonfocal-disease patients; group C, asymptomatic subjects.
Neg, negative.
None of the 60 blood samples collected yielded positive cultures. The sample of CSF collected from the patient with neuropathy and the sample of SF from the patient with chronic right-knee pain also gave negative results in culture.
Quantification of B. melitensis DNA loads in clinical samples.
The numbers of positive Q-PCR samples and the quantification of B. melitensis DNA loads over time for the three groups of subjects are summarized in Table 3.
TABLE 3.
Results of Q-PCR positivity and B. melitensis DNA loads in blood and serum samples over time for the three groups of patients
| Patient groupa (n) | No. of positive Q-PCR samples/total samples analyzed (B. melitensis DNA loadb) at the following point:
|
|||||
|---|---|---|---|---|---|---|
| Baseline
|
Treatment period
|
Posttreatment follow-up
|
||||
| Blood | Serum | Blood | Serum | Blood | Serum | |
| A (8) | 12/39 (244 ± 253) | 7/38 (432 ± 505) | 9/27 (316 ± 479) | 4/26 (650 ± 767) | 1/29 (15) | 2/29 (2,516 ± 2,109) |
| B (9) | 14/67 (515 ± 1,524) | 12/65 (243 ± 222) | 1/18 (599) | 2/18 (142 ± 180) | 7/33 (128 ± 199) | 6/33 (384 ± 218) |
| C (18) | 5/36 (171 ± 136) | 2/36 (53 ± 11) | ||||
Group A, focal-disease patients; group B, nonfocal-disease patients; group C, asymptomatic subjects.
Expressed as mean copies per milliliter ± SD.
All (100%) symptomatic focal-disease patients and nonfocal-disease patients with subjective complaints had at least one Q-PCR-positive sample (whole blood and/or serum). This percentage was significantly higher than the 33% (6 out of 18 patients) of asymptomatic subjects who tested positive by Q-PCR. Concerning bacterial load, statistically significant differences were found between groups A and B, B and C, and C and A in both whole-blood and serum samples collected at baseline.
Group A: focal-disease patients.
Three to 28 serial whole-blood and serum samples were obtained from each focal-disease patient. In total, 190 samples, including 95 whole-blood samples, 93 serum samples, 1 CSF sample, and 1 SF sample, were analyzed by Q-PCR. At baseline, the mean bacterial loads were 244 ± 253 copies/ml (12/39 samples positive) and 432 ± 505 copies/ml (7/38 samples positive) for the whole-blood and serum samples, respectively (P < 0.05). The SF sample yielded a positive result, with 175 copies of B. melitensis DNA per ml. The CSF sample was negative.
Group B: nonfocal-disease subjects.
Nine to 21 serial whole-blood and serum samples from each symptomatic nonfocal-disease patient were analyzed by Q-PCR. In total, 234 samples, including 118 whole-blood and 116 serum samples, were included. At baseline, the mean bacterial DNA loads were 515 ± 1,524 copies/ml (14/67 samples positive) and 243 ± 222 copies/ml (12/65 samples positive) for the whole-blood and serum samples, respectively (P > 0.05).
Group C: asymptomatic subjects.
One to 12 serial whole-blood and serum samples were obtained from each asymptomatic subject, with 36 whole-blood and 36 serum samples in total. The bacterial DNA loads for these six subjects were 171 ± 136 copies/ml (5/36 samples positive) and 53 ± 11 copies/ml (2/36 samples positive) for whole-blood and serum samples, respectively (P < 0.05).
Control subjects.
All of the 15 whole-blood and 15 serum samples from the 15 healthy donors that were analyzed by Q-PCR produced negative results.
B. melitensis DNA changes in treated chronic brucellosis patients.
Eleven patients (five focal-disease patients and six symptomatic nonfocal-disease patients) received one to four courses of antibiotics, consisting of doxycycline (100 mg/12 h) either alone or in combination with rifampin (rifampicin) (900 mg/24 h), gentamicin (240 mg/24 h), streptomycin (1 g/24 h), or cotrimoxazole (trimethoprim, 160 mg/12 h; sulfamethoxazole, 800 mg/12 h). The duration of therapy ranged from 45 to 495 days (mean, 191 days). Five patients experienced a transitory resolution of symptoms, and two showed long-term improvement. The remaining four patients experienced no beneficial effects of treatment, including one patient who interrupted treatment because of adverse effects. After the completion of antibiotic treatment, follow-up for these patients ranged from 87 to 1,050 days (mean, 472 days).
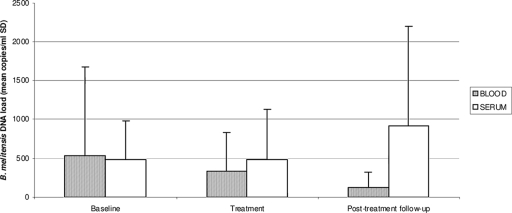
We analyzed the bacterial DNA loads from diagnosis to beyond the termination of therapy for the 10 chronic brucellosis patients who completed treatment. The changes in bacterial DNA loads for these patients are shown in Fig. 1.
FIG. 1.
Changes in mean levels of Brucella melitensis DNA (mean copies per milliliter ± SD) over time in treated patients (five focal-disease patients and five nonfocal-disease patients).
The mean bacterial loads prior to antibiotic therapy were 534 ± 1,149 copies/ml (10/37 samples positive) and 479 ± 506 copies/ml (6/37 samples positive) for the whole-blood and serum samples, respectively (P > 0.05). During treatment, the mean bacterial loads decreased to 343 ± 486 copies/ml (10/42 samples positive) in whole-blood samples and slightly increased to 480 ± 654 copies/ml (2/42 samples positive) in serum samples, without reaching statistical significance (P > 0.05). Upon the completion of treatment, the bacterial DNA loads decreased slightly in whole-blood samples, to 147 ± 211 copies/ml (6/58 samples positive), and increased considerably in serum samples, to 917 ± 1,282 copies/ml (8/58 samples positive), in six (60%) patients (two patients with focal complaints and four nonfocal-disease patients).
DISCUSSION
In the current study, we evaluated the usefulness of Q-PCR in the diagnosis and follow-up of chronic brucellosis patients. We studied a cohort of 35 subjects with a history of brucellosis diagnosed between 2 and 33 years previously. The most significant characteristic of infection was the long-term persistence of B. melitensis DNA in nonfocal-disease patients complaining of nonspecific symptoms undiagnosable by classical methods. This observation is very important insofar as it offers hope to a not insignificant group of patients who wander from physician to physician, remaining undiagnosed even though their symptoms (weakness, easy fatigability, anxiety, nervousness, and vague aches) persist. In such situations, Q-PCR could help physicians to diagnose chronic infection. Since other intracellular bacteria, such as Mycoplasma spp., have been linked to a number of syndromes of as yet unknown etiology, which include fibromyalgia, CFS, and sarcoidosis (4, 6, 14), it will be of interest to investigate the potential role of Brucella sp. infection in such syndromes.
With respect to the specificity of Q-PCR, we did not detect bacterial DNA in either whole-blood or serum samples from healthy donors who had no history of brucellosis. However, we were able to detect and measure levels of B. melitensis DNA in asymptomatic subjects with a history of brucellosis, albeit in a smaller proportion of patients than that in the group of symptomatic chronic patients. The clinical significance of the persistence of bacterial DNA in asymptomatic patients for long periods after antibiotic treatment is still unclear. We do not know whether this indicates that these patients could develop symptoms of brucellosis in the future. The persistence of bacterial DNA after the completion of antimicrobial treatment has also been reported for patients with other infectious diseases, such as tuberculosis, leptospirosis, and Lyme disease (8, 11, 18, 23, 25). Asymptomatic former brucellosis patients with bacterial DNA have not been adequately described in the literature, and it seems appropriate to perform follow-up studies of these patients, who probably require special vigilance in the posttreatment phase.
The detection of bacterial DNA despite negative blood cultures in symptomatic patients without focal complications suggests that they might remain infected with nondividing but nonetheless live, infectious bacteria that persist in a latent form under the control of the host's immune system. Some authors (8, 11, 18) have suggested that DNA from dead microbes is rapidly cleared from the blood, presumably by nucleolytic activity present in tissues and body fluids. In an experimental model, DNA from heat-killed Borrelia burgdorferi was injected into the skin of an uninfected dog. PCR analysis detected B. burgdorferi DNA for a maximum of 3 weeks after injection, implying that during natural, acute infection, the DNA of killed microorganisms is removed quickly and completely within a few days (23). These findings could corroborate our theory that the B. melitensis DNA detected at least 2 years following initial brucellosis infection is derived from live bacteria with infectious, symptom-causing potential.
Since we were able to detect B. melitensis DNA after therapy in 80% of patients, it seems that the duration, dose, and combination of current antibiotic treatments are not effective in eradicating the bacteria. It is not clear whether therapy of longer duration or new treatments with different combinations of antibiotics would result in better outcomes. Prolonged evaluation of patients after antibiotic treatment is needed in order to determine whether the decrease in the bacterial DNA load after treatment is transient or definitive, resulting in total eradication of the bacteria.
With respect to the intermittent detection of bacterial DNA, it seems possible that in chronic brucellosis patients, the concentrations of B. melitensis DNA in body fluids, such as blood or serum, may be transient or too low to allow consistent detection. Furthermore, it has been reported that nucleic acids are probably released from bacteria into the circulation as breakdown products (26), which might hinder amplification by PCR. Thus, a negative result in a Q-PCR assay may not exclude the disease. Some authors have suggested testing several replicates of the purified DNA from clinical specimens in parallel to increase the probability of a positive result (1). However, in tissue samples where bacteria are concentrated, such as the bone marrow or SF from focal-disease patients, it was easier to detect B. melitensis DNA, as seen in the sample of SF from the young man with right-knee pain and in the bone marrow of a woman with spondylitis (16).
In summary, we found that the proportion of individuals with B. melitensis DNA was significantly higher for symptomatic nonfocal-disease patients than for asymptomatic subjects. Therefore, Q-PCR appears to be a useful method for identifying chronic brucellosis patients, especially those symptomatic nonfocal-disease patients for whom the classical methods of diagnosis fail.
Acknowledgments
We thank Granada Picazo for technical support and Hernán Sandoval and Alexandra Salewski for assistance in translating the original manuscript.
The centers and investigators participating in the study of chronic brucellosis patients were as follows: the University General Hospital of Albacete, Albacete, Spain (J. Solera, M. J. Castaño, E. Navarro, J. C. Segura, E. Martínez-Alfaro, A. Navarro-Martínez, F. Medrano, I. Hermida, L. L. Garijo, M. D. Muñoz, and A. I. Martínez), the University Hospital of Pamplona, Pamplona, Spain (R. Díaz), and the University Hospital of Valladolid, Valladolid, Spain (A. Orduña).
This research was supported by the “Fondo de Investigación Sanitaria” (grant PI051858) of the Spanish Ministry of Health and by the Consejería de Sanidad (grants 06028-00 and PI-2006/43) of the Junta de Castilla-La Mancha of Spain. It was also supported by a Balagué Center S.A. grant and by a Consejería de Sanidad grant (MOV2007-JI/05) from the Junta de Castilla-La Mancha of Spain, awarded to M. J. Castaño.
Neither of the authors has any actual or potential conflicts of interest with respect to the work described here.
Footnotes
Published ahead of print on 6 May 2009.
REFERENCES
- 1.Al Dahouk, S. 2007. Evaluation of genus-specific and species-specific real-time PCR assays for the identification of Brucella spp. Clin. Chem. Lab. Med. 451464-1470. [DOI] [PubMed] [Google Scholar]
- 2.Ariza, J., T. Pellicer, R. Pallarés, A. Foz, and F. Gudiol. 1992. Specific antibody profile in human brucellosis. Clin. Infect. Dis. 14131-140. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan, T. M., and L. C. Faber. 1980. 2-Mercaptoethanol Brucella agglutination test: usefulness for predicting recovery from brucellosis. J. Clin. Microbiol. 11691-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choppa, P. C., A. Vojdani, C. Tagle, R. Andrin, and L. Magtoto. 1998. Multiplex PCR for the detection of Mycoplasma fermentans, M. hominis and M. penetrans in cell cultures and blood samples of patients with chronic fatigue syndrome. Mol. Cell. Probes 12301-308. [DOI] [PubMed] [Google Scholar]
- 5.Davies, J. E. 1957. Chronic brucellosis in general practice. Br. Med. J. 21082-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endresen, G. K. 2003. Mycoplasma blood infection in chronic fatigue and fibromyalgia syndromes. Rheumatol. Int. 23211-215. [DOI] [PubMed] [Google Scholar]
- 6a.Evans, A. C. 1939. Difficulties in the diagnosis of chronic brucellosis. Am. J. Trop. Med. 4319-325. [Google Scholar]
- 7.Hausler, W. J., N. P. Moyer, and L. A. Holcomb. 1985. Brucella, p. 328-386. In E. H. Lennette, A. Balows, W. J. Hausler, Jr., and H. J. Shadomy (ed.), Manual of clinical microbiology, 4th ed. American Society for Microbiology, Washington, DC.
- 8.Kain, K. C., A. E. Brown, D. E. Lanar, W. R. Ballow, and H. K. Webster. 1993. Response of Plasmodium vivax variants to chloroquine, as determined by microscopy and quantitative polymerase chain reaction. Am. J. Trop. Med. Hyg. 49478-484. [DOI] [PubMed] [Google Scholar]
- 9.Kattar, M. M., P. A. Zalloua, G. F. Araj, J. Samaha-Kfoury, H. Shbaklo, S. S Kanj, S. Khalife, and M. Deeb. 2007. Development and evaluation of real-time polymerase chain reaction assays on whole blood and paraffin-embedded tissues for rapid diagnosis of human brucellosis. Diagn. Microbiol. Infect. Dis. 5923-32. [DOI] [PubMed] [Google Scholar]
- 10.Maas, K. S., M. Méndez, M. Zavaleta, J. Manrique, M. P. Franco, M. Mulder, N. Bonifacio, M. L. Castañeda, J. Cachaltana, E. Yaqui, R. H. Gilman, A. Guillen, D. L. Blazes, B. Espinosa, E. Hall, T. H. Abdoel, and H. L. Smits. 2007. Evaluation of brucellosis by PCR and persistence after treatment in patients returning to the hospital for follow-up. Am. J. Trop. Med. Hyg. 76698-702. [PubMed] [Google Scholar]
- 11.Malawista, S. E., S. W. Barthold, and D. H. Persing. 1994. Fate of Borrelia burgdorferi DNA in tissues of infected mice after antibiotic treatment. J. Infect. Dis. 1701312-1316. [DOI] [PubMed] [Google Scholar]
- 12.Morata, P., M. I. Queipo-Ortuño, J. M. Reguera, M. A. García-Orduñez, C. Pichardo, and J. D. Colmenero. 1999. Posttreatment follow-up of brucellosis by PCR assay. J. Clin. Microbiol. 374163-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morata, P., M. I. Queipo-Ortuño, J. M. Reguera, F. Miralles, J. J. López-González, and J. D. Colmenero. 2001. Diagnostic yield of a PCR assay in focal complications of brucellosis. J. Clin. Microbiol. 393743-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasralla, M., J. Haier, and G. L. Nicolson. 1999. Multiple mycoplasmal infections detected in blood of patients with chronic fatigue syndrome and/or fibromyalgia syndrome. Eur. J. Clin. Microbiol. Infect. Dis. 18859-865. [DOI] [PubMed] [Google Scholar]
- 15.Navarro, E., J. C. Segura, M. J. Castaño, and J. Solera. 2006. Use of real-time quantitative polymerase chain reaction to monitor the evolution of Brucella melitensis DNA load during therapy and post-therapy follow-up in patients with brucellosis. Clin. Infect. Dis. 421266-1273. [DOI] [PubMed] [Google Scholar]
- 16.Navarro-Martínez, A., E. Navarro, M. J. Castaño, and J. Solera. 2008. Rapid diagnosis of human brucellosis by quantitative real-time PCR: a case report of brucellar spondylitis. J. Clin. Microbiol. 46385-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nimri, L. F. 2003. Diagnosis of recent and relapsed cases of human brucellosis by PCR. BMC Infect. Dis. 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nocton, J. J., F. Dressler, B. J. Rutledge, R. N. Rys, D. H. Persing, and A. C. Steere. 1994. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N. Engl. J. Med. 330229-234. [DOI] [PubMed] [Google Scholar]
- 19.Otero, J. A., E. Fuertes, E. Palenque, and A. R. Noriega. 1982. Microtiter-adapted method that facilitates the Coombs test for brucellosis. J. Clin. Microbiol. 16737-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Queipo-Ortuño, M. I., P. Morata, P. Ocón, P. Manchado, and J. D. Colmenero. 1997. Rapid diagnosis of human brucellosis by peripheral-blood PCR assay. J. Clin. Microbiol. 352927-2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solera, J., E. Martínez-Alfaro, and A. Espinosa. 1997. Recognition and optimum treatment of brucellosis. Drugs 53245-256. [DOI] [PubMed] [Google Scholar]
- 22.Spink, W. W., W. H. Hall, and R. Magoffin. 1951. Follow-up study of therapy in forty-eight culturally proved cases of brucellosis; streptomycin and sulfadiazine, aureomycin, and chloramphenicol (chloromycetin). AMA Arch. Int. Med. 88419-432. [DOI] [PubMed] [Google Scholar]
- 23.Straubinger, R. K. 2000. PCR-based quantification of Borrelia burgdorferi organisms in canine tissues over a 500-day postinfection period. J. Clin. Microbiol. 382191-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vrioni, G., G. Pappas, C. Gartzonika, and S. Levidiotou. 2008. An eternal microbe: Brucella DNA load persists for years after clinical cure. Clin. Infect. Dis. 46e131-e136. [DOI] [PubMed] [Google Scholar]
- 25.Young, D., T. Hussell, and D. Dougan. 2002. Chronic bacterial infections: living with unwanted guests. Nat. Immunol. 31026-1032. [DOI] [PubMed] [Google Scholar]
- 26.Zerva, L., K. Bourantas, S. Mitka, A. Kansouzidou, and N. J. Legakis. 2001. Serum is the preferred clinical specimen for diagnosis of human brucellosis by PCR. J. Clin. Microbiol. 391661-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]