Abstract
Mycobacterium abscessus is the most common cause of rapidly growing mycobacterial chronic lung disease. Recently, two new M. abscessus-related species, M. massiliense and M. bolletii, have been described. Health care-associated outbreaks have recently been investigated by the use of molecular identification and typing tools; however, very little is known about the natural epidemiology and pathogenicity of M. massiliense or M. bolletii outside of outbreak situations. The differentiation of these two species from M. abscessus is difficult and relies on the sequencing of one or more housekeeping genes. We performed extensive molecular identification and typing of 42 clinical isolates of M. abscessus, M. massiliense, and M. bolletii from patients monitored at the NIH between 1999 and 2007. The corresponding clinical data were also examined. Partial sequencing of rpoB, hsp65, and secA led to the unambiguous identification of 26 M. abscessus isolates, 7 M. massiliense isolates, and 2 M. bolletii isolates. The identification results for seven other isolates were ambiguous and warranted further sequencing and an integrated phylogenetic analysis. Strain relatedness was assessed by repetitive-sequence-based PCR (rep-PCR) and pulsed-field gel electrophoresis (PFGE), which showed the characteristic clonal groups for each species. Five isolates with ambiguous species identities as M. abscessus-M. massiliense by rpoB, hsp65, and secA sequencing clustered as a distinct group by rep-PCR and PFGE together with the M. massiliense type strain. Overall, the clinical manifestations of disease caused by each species were similar. In summary, a multilocus sequencing approach (not just rpoB partial sequencing) is required for division of M. abscessus and closely related species. Molecular typing complements sequence-based identification and provides information on prevalent clones with possible relevant clinical aspects.
Rapidly growing mycobacteria (RGM) are ubiquitous organisms increasingly emerging as important human pathogens. Mycobacterium abscessus is commonly associated with wound infections and abscess formation and is the most frequent RGM causing chronic lung disease, often in immunocompromised patients (15, 22, 24). M. abscessus is also notable for its resistance to treatment and the poor clinical outcome of infection with the organism (22, 24). Within the past decade, two new species of mycobacteria closely related to M. abscessus, M. massiliense and M. bolletii, have been described (1, 3). Information on the pathogenic role of M. massiliense and M. bolletii is still scant. Recent reports have described the isolation of M. massiliense from two patients in the United States (29) and one patient in Italy (35) and, lately, the identification of M. massiliense and M. bolletii among South Korean isolates (18). Both M. massiliense and M. bolletii have also been linked to health care-associated outbreaks (8, 19, 37).
The species-level identification of RGM can provide the first indication of antibiotic susceptibility and can suggest the appropriate type of patient management. For example, M. abscessus is more resistant to many antibiotics both in vivo and in vitro than M. fortuitum and M. mucogenicum, but it is usually susceptible to amikacin and clarithromycin (6, 15, 24). M. massiliense was originally reported to be distinguishable from M. abscessus and related species by its susceptibility to doxycycline (3); however, resistant isolates have since been described (19, 37), suggesting that antibiotic susceptibility results may not reliably differentiate among these closely related species.
Although 16S rRNA gene sequencing has been used for the identification of nontuberculous mycobacteria (NTM), including RGM, it has limited value in distinguishing among some closely related species (9, 14). Therefore, the use of several other gene targets for the identification of mycobacteria has been proposed (2, 5, 11, 23, 25, 31, 32, 39, 41). Discrimination among M. abscessus, M. massiliense, and M. bolletii (which have identical 16S rRNA gene sequences) has proven to be difficult, with sequencing of different gene targets often providing conflicting results. Among these gene targets, partial sequencing of rpoB has increasingly been used (1, 19, 29, 37).
Genotypic analysis of NTM has proven useful not only in the investigation of outbreaks and pseudo-outbreaks (38) but also in characterizing the molecular epidemiology of strains, and in assessing clonal distribution and expansion (4, 7, 13, 17). In particular, molecular typing has recently been used for the characterization of health care-related outbreaks of M. massiliense and M. bolletii (19, 37).
We sought to perform a thorough molecular investigation, including strain identification and typing, for a series of 42 clinical isolates (CIs) of M. abscessus, M. massiliense, and M. bolleti from patients monitored in our institution between 1999 and 2007. A retrospective patient chart review assessed demographics, underlying conditions, and clinical history.
The 42 CIs and 3 type strains were subjected to multilocus sequence analysis, including sequencing of rpoB, hsp65, secA, and the internally transcribed spacer (ITS) region. The relatedness among the isolates was assessed by use of an automated repetitive-sequence-based PCR (rep-PCR) and pulsed-field gel electrophoresis (PFGE). This is the most extensive molecular characterization of non-outbreak-related isolates from patients with M. abscessus, M. massiliense, and M. bolletii infections.
MATERIALS AND METHODS
Bacterial strains.
Forty-two CIs collected from 1999 to 2007 under appropriate clinical protocols with informed consent were included in this study. They were grown from sputum (n = 31), bronchoalveolar lavage fluid (n = 3), abscesses (n = 3), blood cultures (n = 2), skin (n = 2), or a lymph node (n = 1). The methods used for the identification of the CIs included assessments of pigment production, growth rate, and colony characteristics, followed by multilocus sequencing. Species identification was considered unambiguous only when there was agreement in the species assignation by hsp65, rpoB, and secA gene sequencing analysis.
Type strains of M. abscessus (ATCC 19977), M. massiliense (CIP 108297), and M. bolletii (CIP 108541) were used. The bacterial strains were stored at −70°C in Tween albumin broth (Remel, Lenexa, KS). Prior to use, the strains were subcultured onto Middlebrook 7H11 agar (Remel).
DNA isolation, PCRs, and sequencing.
DNA was extracted from a 10-μl loopful of each mycobacterial colony by use of an UltraClean microbial DNA isolation kit (Mo Bio Laboratories, Solana Beach, CA), according to the manufacturer's instructions. PCRs were carried out with Illustra PuReTaq Ready-To-Go PCR beads (GE Healthcare, Buckinghamshire, United Kingdom). Unless stated otherwise, the amplified PCR fragments were sequenced under contract with the Laboratory of Molecular Technology in Frederick, MD.
Partial amplification of the hsp65 gene was performed with primers Tb11 (5′-ACC AAC GAT GGT GTG TCC AT-3′) and Tb12 (5′-CTT GTC GAA CCG CAT ACC CT-3′) (33) that were tailed with the M13 sequencing primer sites M13F (5′-GTA AAA CGA CGG CCA G-3′) and M13R (5′-CAG GAA ACA GCT ATG AC-3′), respectively. Sequencing of the 401-bp fragment was carried out at the NIH with tail primers M13F and M13R, as described elsewhere (39).
Partial amplification of the rpoB sequence was carried out with the primer pair Myco-F (5′-GGC AAG GTC ACC CCG AAG GG-3′) and Myco-R (5′-AGC GGC TGC TGG GTG ATC ATC-3′) (2). A 711-bp sequence was derived from the amplicon by using the same primer pair and also sequencing primers MycoseqF (5′ GAA GGG TGA GAC CGA GCT GAC-3′) and MycoseqR (5′-GCT GGG TGA TCA TCG AGT ACG G-3′) (2).
Partial amplification of the secA1 gene was carried out with primers Mtu.For1 (5′-GAC AGY GAG TGG ATG GGY CGS GTG CAC CG-3′) and Mtu.Rev490 (5′-GCG GAC GAT GTA RTC CTT GTC SCG-3′) tailed with the M13F and M13R sequencing primer sites, respectively (39). Sequencing of a 465-bp fragment was carried out with tail primers M13F and M13R, along with the PCR primers Mtu.For and Mtu.Rev490.
Partial amplification and sequencing of the 16S-23S rRNA gene ITS region was performed with primers Sp1 (5′-ACC TCC TTT CTA AGG AGC ACC-3′) and Sp2 (5′-GAT GCT CGC AAC CAC TAT CCA-3′), as described elsewhere (26).
Sequence and phylogenetic analysis.
The Lasergene (version 5.51) program (DNAStar, Inc., Madison, WI) was used for sequence assembly. Multiple-sequence alignment was carried out by the method with the CLUSTAL W (version 2.0) program.
Phylogenetic analyses of rpoB and multiple concatenated sequences were constructed by using the PhyML (version 2.4.5) program and PHYLIP (phylogeny inference package, version 3.5c; J. Felsenstein, University of Washington, Seattle) (the method of Fitch in Dnapars software). A superdistance matrix (SDM) was generated from each gene's distance matrix by using SDM software (10), followed by tree construction with the Fitch method. The resulting trees were depicted by using the FigTree (version 1.1.2) program (Institute of Evolutionary Biology, University of Edinburgh). The reliability of each tree was assessed by the bootstrap method with a total of 1,000 bootstrapped iterations.
Mycobacterial typing.
The DiversiLab Mycobacterium typing kit (Bacterial Barcodes, Inc., a subsidiary of bioMerieux, Inc.) was used for rep-PCR typing of the mycobacterial isolates, according to the manufacturer's instructions. Amplified fragments of various sizes and intensities were separated and detected by using a microfluidics chip with the Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA). Further analysis was performed with DiversiLab software (version 3.3), which uses the Pearson correlation coefficient to determine distance matrices and the unweighted pair group method with arithmetic means to create dendrograms (16). The reports generated included dendrograms and virtual gel images.
Analysis of large restriction fragment profiles by PFGE was carried out as described previously (40) with few modifications. In brief, organisms cast into low-melting-point agarose plugs were lysed with lysozyme, sodium dodecyl sulfate, and proteinase K. The modified protocol included the addition to the mycobacterial cultures of cycloserine (1 mg/ml) and ampicillin (0.1 mg/ml) and incubation for 24 h at 30°C just before the plugs were made. The genomic DNA contained in the plugs was digested with the restriction endonuclease AseI and separated by PFGE with a CHEF Mapper system (Bio-Rad Laboratories, Richmond, CA). The PFGE band patterns were analyzed with the BioNumerics (version 4.01) program (Applied Maths, Inc., Austin, TX).
Clinical data.
A retrospective chart review was performed for 40 patients identified by the NIH Microbiology Laboratory as having had a positive culture for M. abscessus, M. massiliense, or M. bolletii between January 1999 and December 2007. Two patients whose isolates were included in the molecular portion of this study (one M. abscessus isolate and one M. massiliense isolate) were excluded from clinical analysis due to a lack of relevant clinical data. The study was approved by the NIH Office of Human Subjects Research. Pertinent data from the date of the first positive M. abscessus, M. massiliense, or M. bolletii culture at the NIH were accrued by examination of the medical record and electronic laboratory database. The basic demographics, underlying comorbidities, and clinical histories of the patients were recorded. The source of the positive culture, other microbial pathogens isolated, and the type of mycobacterial infection (pulmonary, disseminated, or soft tissue infection and/or lymphadenitis) were determined. For those patients with pulmonary isolates, the results of computed tomography of the chest performed at or near the time of the positive culture were reviewed (20) by a single investigator (K.N.O.) blinded to the microbiology data.
Descriptive and comparative statistics were performed with Prism (version 4.0c) software (GraphPad Software, Inc., San Diego, CA). Comparison between the M. abscessus and M. massiliense groups was performed by Fisher's exact test for categorical variables and Student's t test or the Mann-Whitney test for continuous variables. Two-tailed P values were recorded, and significance was set at an alpha value of <0.05. The data for the cases of M. bolletii infection were not included in the comparative statistics, due to the small sample size.
RESULTS
Identification of CIs.
Comparison of the hsp65 sequences of the 42 CIs and 3 type strains led to the identification of 27 CIs as M. abscessus, 12 CIs as M. massiliense, and 3 CIs as M. bolletii (Table 1). A similar analysis conducted by sequencing of the rpoB gene assigned the 42 CIs as follows: 33 M. abscessus isolates, 7 M. massiliense isolates, and 2 M. bolletii isolates (Table 1).
TABLE 1.
Comparison of genes sequences from clinical isolates and reference strains of M. abscessus, M. massiliense, and M. bolletii
| Organism |
hsp65
|
rpoB
|
secA
|
|||
|---|---|---|---|---|---|---|
| No. of isolates | % Identity | No. of isolates | % Identity | No. of isolates | % Identity | |
| M. abscessus | 27 | 100 | 26 | 100 | 19 | 100 |
| 7a | 99.9 | 6 | 99.8 | |||
| 1 | 99.6 | |||||
| M. massiliense | 12 | 100 | 7 | 99.7 | 7 | 100 |
| 2 | 99.8 | |||||
| 3 | 99.6 | |||||
| M. bolletii | 1 | 100 | 1 | 99.9 | 2 | 100 |
| 2b | 99.8 | 1 | 99.7 | 2c | 99.8 | |
Includes clinical isolates 71, 73, 76, 510, and 1210 identified as M. massiliense by secA and hsp65 sequencing.
Includes clinical isolate 79 identified as M. abscessus by rpoB sequencing.
Includes clinical isolates 79 and 1214 identified as M. abscessus by rpoB sequencing.
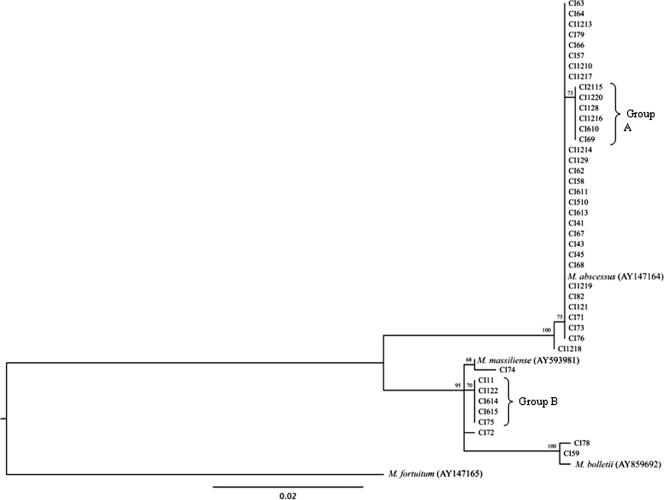
A phylogenetic tree of the partial rpoB gene sequences (Fig. 1) separated a monophyletic cluster of M. abscessus from a cluster that included M. massiliense and M. bolletii. The last two species were further separated from each other with a high bootstrap value. Among the isolates identified as M. abscessus by rpoB gene sequencing, comparison of the sequences of the CIs with the corresponding sequence of the type strain revealed 100% identity for 26 isolates. The sequences of seven other isolates showed 99.9% identity with the sequence of the type strain; the lack of complete identity was due to a single nucleotide difference: six CIs (group A in Fig. 1) exhibited a one-base substitution at position 187 (C to T), while CI1218 showed a T-to-C transition at base 169.
FIG. 1.
Phylogenetic tree derived from rpoB sequences of 42 CIs and the type strains of M. abscessus, M. massiliense, and M. bolletti. The tree was constructed by using the PhyML (version 2.4.5) program and M. fortuitum as the outgroup. The numbers on the branches represent the percentage of 1,000 bootstrap samples supporting the branch.
None of the CIs identified as M. massiliense by rpoB gene sequencing showed a perfect match with the corresponding type strain. All of them had a two-base change that led to 99.7% identity with the type strain. Five isolates differed from the type strain at base 152 (C to T) and base 343 (T to C) (group B in Fig. 1). CI74 showed substitutions at base 67 (T to G) and base 517 (G to A), while for CI72, the substitutions were at position 343 (T to C) and position 490 (G to A).
The two CIs identified as M. bolletii by rpoB gene sequencing showed one- and two-base differences with the type strain, respectively. CI78 and CI59 showed a G-to-C transversion at position 13. In addition, CI78 had an additional C-to-T substitution at position 634.
Evaluation of a novel target, secA, for identification of M. abscessus group.
To evaluate the performance of a novel gene target, the secA gene, recently reported to have been used for the identification of mycobacteria (39), we amplified a 465-bp fragment (after subtraction of the primer sequences) for all CIs and type strains. The sequences of the amplified fragment were aligned and compared with those of the type strains of M. abscessus, M. massiliense, and M. bolletii. Results obtained by secA analysis were compared with those generated by sequencing of rpoB, a gene target increasingly used for discrimination among these and other related species (1, 19, 29, 37).
By secA sequencing, 26 CIs were identified as M. abscessus (Table 1), all of which had been identified as such by hsp65 and rpoB gene sequencing. secA analysis identified 12 isolates as M. massiliense, 7 of which had been assigned to this species by rpoB and hsp65 gene sequencing (Table 1). The secA gene sequences of the other five CIs (CI71, CI73, CI76, CI510, CI1210) showed 100% similarity to the secA gene of the M. massiliense type strain. However, they were identified as M. abscessus by rpoB gene sequencing and as M. massiliense by hsp65 gene sequencing and showed a 100% match to the corresponding type strains. Four CIs were identified as M. bolletii by secA sequencing; two of them (CI78, CI59) were identified as such by rpoB and hsp65 gene sequencing.
Integrated phylogenetic analysis.
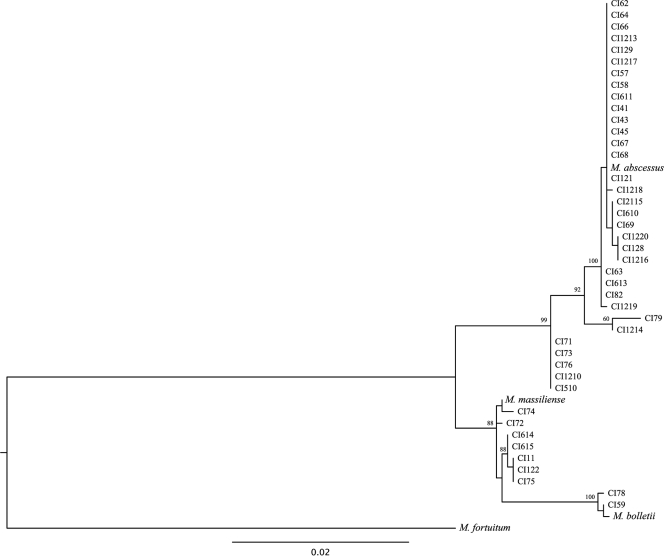
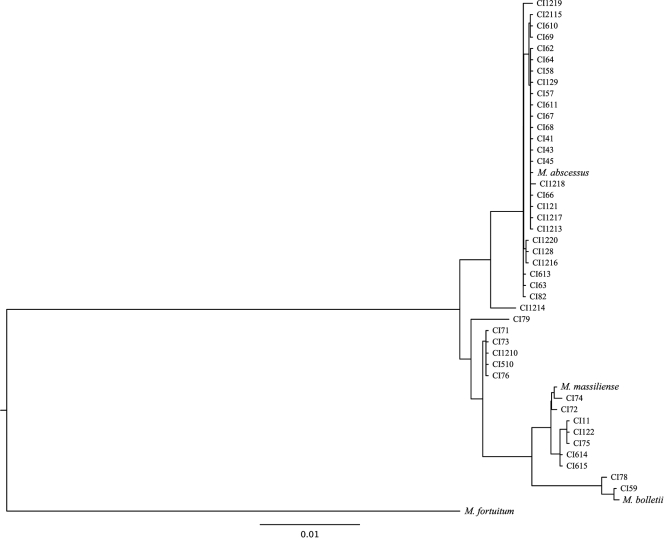
We sought to integrate the phylogenetic analysis of the three housekeeping genes using a gene concatenation approach and SDM approach (10). The maximum-likelihood method (Fig. 2) and the distance-based and maximum-parsimony methods (data not shown) were used to construct trees from the concatenated gene alignments. Five isolates (CI71, CI73, CI76, CI510, CI1210) with conflicting species assignments by use of the individual phylogenetic trees formed a separate clade between the M. abscessus and the M. massiliense groups. Isolates CI79 and CI1214 also formed a separate group closer to M. abscessus. This topology was supported by the maximum-likelihood and distance-based trees, while the maximum-parsimony method was inconclusive with regard to these seven isolates. Finally, an additional tree generated by the SDM method (10) (Fig. 3) also supported the grouping of the five isolates with ambiguous identities separate from the M. abscessus and M. massiliense groups.
FIG. 2.
Phylogenetic tree derived from concatenated hsp65, rpoB, and secA sequences of 42 CIs and the type strains of M. abscessus, M. massiliense, and M. bolletti. The tree was constructed by using the PhyML (version 2.4.5) program and M. fortuitum as the outgroup. The numbers on the branches represent the percentage of 1,000 bootstrap samples supporting the branch.
FIG. 3.
Phylogenetic tree derived from concatenated hsp65, rpoB, and secA sequences of 42 CIs and the type strains of M. abscessus, M. massiliense, and M. bolletti. The tree was constructed by using the SDM generated from each gene's distance matrix with SDM software (10).
In order to complement the results obtained by the integrated phylogenetic analysis of concatenated housekeeping genes, we also sequenced the ITS region, a nonhousekeeping gene region previously used to discriminate between M. massiliense and M. abscessus (3). Sequencing of the ITS region confirmed the species assignments of all seven CIs previously identified as M. massiliense by hsp65, rpoB, and secA gene sequencing. The five CIs with ambiguous identities identified as M. massiliense by both secA and hsp65 sequencing but as M. abscessus by rpoB sequencing were identified as M. massiliense by sequencing of the ITS region. Each of them had a perfect match to the M. massiliense type strain, including a characteristic A-to-G transition and a C insertion (3).
Typing of M. abscessus, M. massiliense, and M. bolletii by rep-PCR.
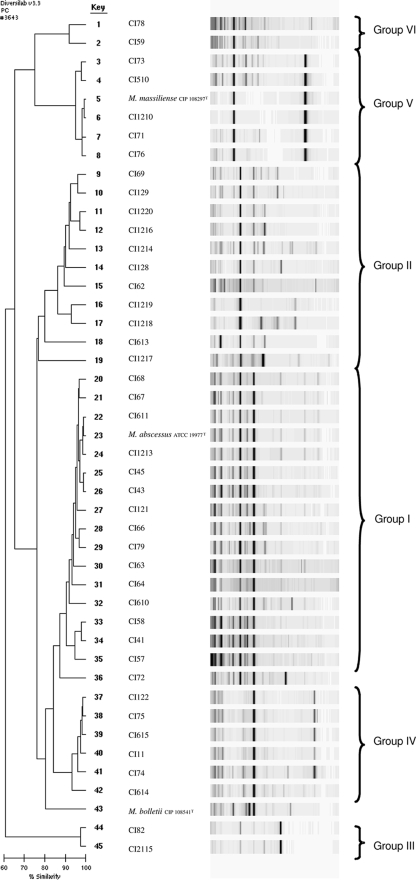
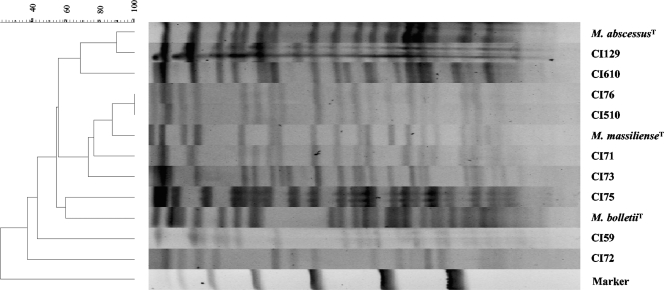
Following the species assignment of all 42 CIs, we assessed the relatedness among the clinical and reference strains for each of the species. For that purpose, we chose a commercial high-throughput rep-PCR developed for molecular typing of microorganisms (16) using a typing kit designed for Mycobacterium spp. DNA was extracted from all clinical and reference isolates and then subjected to fingerprinting by rep-PCR. The results of rep-PCR analysis of all CIs as well as the corresponding type strains are shown in Fig. 4. Most M. abscessus isolates showed similar patterns and clustered together with the M. abscessus type strain (group I in Fig. 4). A second group of M. abscessus isolates (n = 11) clustered together as a clearly separate but less homogeneous group (group II). Finally, a separate branch was observed for two distinct clinical M. abscessus isolates (group III).
FIG. 4.
Rep-PCR-based dendrogram and virtual gel image fingerprints obtained from 42 CIs of M. abscessus, M. massiliense, and M. bolletii and the corresponding type strains (ATCC 19977, CIP 108297, and CIP 108541, respectively). The DiversiLab system with the Mycobacterium sp. fingerprinting kit was used. Pearson's correlation coefficient was used to create a pairwise percent similarity matrix, and the tree was generated by the unweighted pair group method with arithmetic means (UPGMA). Bar, percent similarity among strains.
Six CIs identified as M. massiliense by rpoB sequencing clustered together (group IV) and were separated from CI72. Interestingly, five CIs ambiguously identified as M. abscessus by rpoB sequencing but as M. massiliense by sequencing of the hsp65 and secA genes and the ITS region formed a distinct cluster that appeared as a separate branch in the tree that also included the M. massiliense type strain (group V).
Finally, the two CIs of M. bolletii showed similar patterns (group VI) which were different from that of the M. bolletii type strain.
Typing of representative strains of M. abscessus, M. massiliense, and M. bolletii by PFGE.
In order to confirm the typing results obtained by rep-PCR, 12 selected strains of M. abscessus, M. massiliense, and M. bolletii were subjected to PFGE analysis. The strains were compared for their relatedness by the method of Tenover et al. (34), with minor modifications, as described previously (40). As shown in Fig. 5, distinct patterns were observed for the type strains of M. abscessus, M. massiliense, and M. bolletii. Both CIs of M. abscessus clustered together with the type strain. The type strain of M. massiliense was closely related (few band differences) to CIs 71, 76, 510, and 73, while several band differences were observed for the type strain of M. massiliense compared to those observed for CI72 and CI75, suggesting that the last two isolates are unrelated. M. bolletii CI59 showed a distinct pattern which was different from that of the M. bolletii type strain.
FIG. 5.
Dendrogram of PFGE patterns of M. abscessus, M. massiliense, and M. bolletii isolates digested with AseI. The comparison was performed with the BioNumerics (version 4.01) program (Applied Maths, Inc.).
Clinical characteristics.
Among the 40 patients evaluated, 27 were classified as having M. abscessus infections, 11 were classified as having M. massiliense infections, and 2 were classified as having M. bolletii infections by our intensive molecular approach. The demographic and clinical characteristics of the patients are summarized in Table 2.
TABLE 2.
Patient demographics and clinical characteristics classified by organism
| Clinical characteristic | Result for the following organisma:
|
||
|---|---|---|---|
| M. abscessus (n = 27) | M. massiliense (n = 11) | M. bolletii (n = 2) | |
| Gender (no. [%] of patients) | |||
| Male | 8 (29.6) | 5 (45.5) | 1 (50) |
| Female | 19 (70.4) | 6 (54.5) | 1 (50) |
| Median (range) age (yr) | 54 (10-79) | 27 (14-55) | 31 (14-48) |
| Median (range) BMI (kg/m2) | 22.5 (14.5-30.6)b | 24.4 (15.4-34.2) | 17.7 (15.0-20.4) |
| Type of mycobacterial infection (no. [%] of patients) | |||
| Pulmonary | 23 (85.2) | 7 (63.6) | 1 (50.0) |
| Disseminated | 3 (11.1) | 2 (18.2) | 1 (50.0) |
| Skin, soft tissue | 1 (3.7) | 1 (9.1) | 0 |
| Lymphadenitis | 0 | 1 (9.1) | 0 |
| Comorbidities (no. [%] of patients) | |||
| Pulmonary | |||
| Prior pulmonary MAC infection | 8 (29.6) | 0 | 0 |
| Bronchiectasis (preceding NTM infection)c | 8 (29.6) | 6 (54.5) | 0 |
| Asthma | 3 (11.1) | 2 (18.2) | 0 |
| COPDd | 1 (3.7) | 1 (9.1) | 0 |
| Cystic fibrosis | 3 (11.1) | 3 (27.3) | 0 |
| Primary ciliary dyskinesia | 0 | 1 (9.1) | 0 |
| Immunodeficiency | |||
| IFN-γe receptor deficiency | 0 | 0 | 1 (50) |
| IFN-γ autoantibody | 1 (3.7) | 1 (9.1) | 0 |
| Monocytopenia | 1 (3.7) | 0 | 0 |
| Severe combined immunodeficiency | 0 | 1 (9.1) | 0 |
| Job syndrome | 1 (3.7) | 1 (9.1) | 0 |
| HIVf infection | 0 | 0 | 0 |
| Unclassified | 3 (11.1) | 0 | 0 |
| Active systemic immunosuppression | 3 (11.1) | 0 | 1 (50) |
| History of transplant | 0 | 1 (9.1) | 1 (50) |
| Malignancy | 3 (11.1) | 2 (18.2) | 1 (50) |
| History of chemotherapy | 2 (7.4) | 2 (18.2) | 1 (50) |
| Diabetes | 1 (5.9) | 3 (27.3) | 0 |
| Autoimmune disorder | 2 (7.4) | 2 (18.2) | 0 |
| Symptoms (no. [%] of patients) | |||
| Fever | 5 (18.5) | 2 (18.2) | 2 (100) |
| Chills and/or night sweats | 6 (22.2) | 2 (18.2) | 0 |
| Weight loss | 7 (25.9) | 3 (27.3) | 1 (50) |
| Fatigue | 13 (48.2) | 3 (27.3) | 1 (50) |
| Dyspnea | 13 (48.2) | 2 (18.2) | 1 (50) |
| Cough | 22 (81.5) | 6 (54.6) | 1 (50) |
| Hemoptysis | 4 (14.8) | 0 | 0 |
| Lymphadenopathy | 2 (7.4) | 3 (27.3) | 2 (100) |
| Wound or skin lesion | 4 (14.8) | 1 (9.1) | 0 |
Species classification is based on consensus agreement among all identification methods used.
BMI, body mass index. Body mass index data were missing for one subject infected with M. abscessus.
Specifically refers to documented bronchiectasis preceding diagnosis with an NTM infection.
COPD, chronic obstructive pulmonary disease.
IFN-γ, gamma interferon.
HIV, human immunodeficiency virus.
The majority (89%) of patients infected with M. abscessus identified their race as white, whereas 55% of the patients (odds ratio, 6.6; 95% confidence interval, 1.2 to 36.1; P < 0.05) infected with M. massiliense and 50% of the patients infected with M. bolletii identified their race as white. The median age at the time of M. abscessus isolation was 54 years (range, 10 to 79 years), whereas the median age at the time of M. massiliense isolation was 27 years (range, 14 to 55 years) (P < 0.05). The latter group had a bimodal age distribution, with six patients being between the ages of 14 and 27 years and five patients being between the ages of 47 and 55 years.
In our cohort, pulmonary infection was more common than disseminated disease, soft tissue infection, or lymphadenitis, accounting for over 77% of total infections. All patients with pulmonary infection had abnormalities on chest imaging: 97% had nodules, 43% had cavities and nodules, and over 93% had bronchiectasis. Bilateral disease was present in 90% and involved an average of five lobes (the lingula was counted as a distinct lobe). Among the patients with pulmonary infections, 15 (48%) had a history of chronic pulmonary disease (i.e., cystic fibrosis, primary ciliary dyskinesia, asthma, idiopathic bronchiectasis, etc.) that predated a diagnosis of pulmonary NTM infection. An additional eight patients (all infected with M. abscessus) had a history of M. avium complex (MAC) pulmonary infection without having known lung disease preceding the diagnosis of MAC infection (one patient had a prior MAC infection and preexisting lung disease). Interestingly, all 7 of the patients with M. massiliense pulmonary infection had known lung disease prior to their diagnosis of NTM infection, whereas only 8 (35%) of the 23 patients with M. abscessus infection carried a diagnosis of chronic pulmonary disease before they were diagnosed with either MAC or M. abscessus infection (odds ratio, 27.4; 95% confidence interval, 1.4 to 540.2; P < 0.01).
DISCUSSION
M. abscessus is a commonly isolated RGM. Recently, two new related species, M. massiliense and M. bolletii, have been described (1, 3). To investigate these new species, we performed a multilocus sequencing approach for species-level identification. We also explored an alternative typing method other than the lengthy and technically demanding PFGE by assessing strain relatedness with a rapid PCR-based commercial system using a protocol developed for Mycobacterium spp. Multilocus identification including a novel gene target (secA), and molecular typing gave us the opportunity to evaluate both the clinical relevance of these distinctions and the performance of different molecular identification and typing approaches.
Initial molecular identification by partial sequencing of the hsp65 gene classified these strains as M. abscessus (n = 27), M. massiliense (n = 12), and M. bolletii (n = 3) (Table 1). All 27 strains identified as M. abscessus and all 12 isolates identified as M. massiliense showed perfect matches to the corresponding type strain, as reported previously (29). Two hsp65 sequevars for M. abscessus corresponding to type I and type II hsp65 by PCR-restriction fragment length polymorphism analysis have been described (12, 21, 25). Our hsp65 sequencing results indicate that all the M. abscessus CIs were the type I sequevar and all the M. massiliense CIs were the type II sequevar.
On the basis of previous reports suggesting that rpoB gene sequencing may yield better discrimination among these closely related species, we partially sequenced the rpoB gene of our CIs. The rpoB sequencing results matched those obtained by hsp65 sequencing for most isolates; however, rpoB sequencing identified more isolates (n = 33) as belonging to M. abscessus than did hsp65 sequencing (n = 27). While most isolates identified as M. abscessus showed 100% identity to the type strain, none of the isolates identified as M. massiliense or M. bolletti showed perfect matches to the corresponding type strains. It is interesting to note that the sequences from neither of two CIs of M. massiliense from the United States (29) and Brazil (37) reported recently matched the rpoB sequence of the type strain with 100% identity. However, a perfect match to the sequence of the M. massiliense type strain was observed among isolates in a recent outbreak of M. massiliense in South Korea (19).
In general, the RGM interspecies sequence divergence determined by partial sequencing of rpoB is >3%, and it has been proposed that isolates with less than 1.7% sequence divergence are considered to belong to the same species (2). All seven CIs identified as M. massiliense by rpoB sequencing showed 99.7% similarity (0.3% divergence) to the type strain. The five CIs identified as M. abscessus by rpoB sequencing and as M. massiliense by sequencing of the three different targets (see below) also exhibited less than 1.7% sequence divergence from the type strain of M. abscessus (99.9% identity) and >3% divergence from the type strains of M. massiliense and M. bolletii (96.5% and 95.5% identities, respectively) by rpoB sequencing.
In order to resolve the discrepancies in the identification of seven isolates, we performed an integrated phylogenetic analysis of hsp65, rpoB, and secA using different methods. By such analyses, isolates CI79 and CI1214 were most closely related to M. abscessus. On the other hand, a group of five isolates with a perfect match to the M. abscessus type strain by rpoB sequencing and the M. massiliense type strain by hsp65 and secA sequencing repeatedly emerged as an independent branch between M. abscessus and M. massiliense in different phylogenetic analyses. Sequencing of the nonhousekeeping ITS region showed a perfect match to the ITS region sequence of the M. massiliense type strain, with the characteristic A-to-G transition and a C insertion being detected (3).
Multilocus sequencing combined with molecular typing provides valuable information on the identities of and the clonal relationship among bacterial isolates (30, 36). We used a rep-PCR method optimized for Mycobacterium spp. (16) which was shown to be useful for the typing of M. chelonae, M. abscessus, and M. fortuitum (27, 28). Dendrograms and virtual gel images were generated for all isolates belonging to each of the three species (Fig. 4). Rep-PCR analysis grouped the large majority of M. abscessus CIs into two major clusters (groups I and II; Fig. 4). Only two M. abscessus isolates (group III) showed a distinct pattern unrelated to these two major clusters. Most isolates of M. massiliense clustered into a major group (group IV) separated from one clinical isolate (CI72) and the type strain. Interestingly, all five isolates (CI71, CI73, CI76, CI510, CI1210) that formed a separate clade between M. abscessus and M. massiliense (Fig. 2) clustered together with the M. massiliense type strain by rep-PCR.
PFGE analysis of representative strains confirmed the results obtained by rep-PCR. Distinct PFGE patterns could be observed for isolates of each of the species. Within each species, a comparable degree of relatedness was observed by both techniques. For example, the rep-PCR patterns of M. massiliense showed three easily distinguishable groups (group V, CI72, and group VI). Similarly, PFGE clustered together members of group IV, which exhibited few or no band differences. On the other hand, very distinct patterns were observed with CIs 72 and 75, with the latter isolate belonging to rep-PCR group V.
Combining the results of multilocus sequencing and molecular typing was very informative. CI79 and CI1214, which were identified as M. abscessus by rpoB sequencing but as M. bolletii (CI79) and M. abscessus (CI1214) by hsp65 sequencing and as M. bolletii (both CI79 and CI1214) by secA sequencing, showed the rep-PCR pattern of M. abscessus group II and group I, respectively (Fig. 4). This supports the fact that both isolates are in fact M. abscessus.
The five CIs that showed discordant results, M. abscessus by rpoB sequencing and M. massiliense by hsp65, secA, and ITS region sequencing, clustered together with the type strain of M. massiliense (Fig. 4). PFGE analysis confirmed that these five isolates were highly related to the M. massiliense type strain (Fig. 5), supporting their identification as M. massiliense.
Our clinical data suggest possible differences between patients who develop infections with M. abscessus and those who become infected with M. massiliense. However, the strength and generalizability of this observation are limited by the small cohort size and the retrospective nature of the study. A prospective investigation is needed to determine whether any differences in host factors, treatment response, and clinical outcome exist among patients infected with these species.
Our multilocus sequencing approach is useful for the molecular identification of the newly described species M. massiliense and M. bolletii, as some strains show equivocal results when only one or a few gene targets are used. Partial sequencing of rpoB alone did not reliably differentiate all the CIs. Interestingly, in a recent study Kim et al. reported on two isolates which showed discordant results, M. massiliense by rpoB sequence analysis and M. abscessus by hsp65 sequence analysis, and identified them as M. massiliense on the basis of the additional analysis of sodA and the ITS region (18). Unfortunately, that study did not include an integrated phylogenetic analysis or molecular typing of the strains to assess the relatedness of the strains to each another or other clinical or reference strains.
We hypothesize that our five related CIs, CI71, CI73, CI76, CI510, and CI1210, that formed a separate clade between M. abscessus and M. massiliense (Fig. 2) but that were identified as M. massiliense by sequencing of the ITS region and that appeared to be related to the M. massiliense type strain by two independent typing methods are M. massiliense. On the basis of the molecular diversity, we cannot rule out the possibility of distinct taxa (i.e., at the subspecies level) within M. massiliense. Comparative genomic hybridizations with a mycobacterial chip will be performed to address this issue. Beyond taxonomic considerations, we believe that it is important to the clinical microbiologist to use a multilocus sequencing approach combined with typing techniques in order to identify such groups within the bacterial population. These groups likely share defined microbiologic, epidemiologic, and possibly virulence features.
In summary, a multilocus sequencing approach is required for discrimination among the closely related species M. abscessus, M. massiliense, and M. bolletii. Partial sequencing of rpoB (711 bp) alone does not reliably differentiate these three species. We suggest that these organisms first be identified by secA sequencing, a target routinely used in our laboratory for direct detection as well as the identification of all Mycobacterium spp. The identities of isolates identified as M. abscessus, M. massiliense, or M. bolletii by secA sequencing are confirmed by sequencing of additional gene targets. Sequence-based identification combined with molecular typing supports the identification of isolates with ambiguous identities and provides information on prevalent clones with potentially relevant clinical features. The molecular, biological, and clinical characteristics of these strains will help us to better understand and treat severe infections due to RGM.
Acknowledgments
We thank Frank G. Witebsky and Patrick R. Murray for critically reviewing the manuscript and Patricia Conville for the bionumeric analysis.
This research was supported by the Intramural Research Program of the NIH, the NIH Clinical Center, and the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 6 May 2009.
REFERENCES
- 1.Adekambi, T., P. Berger, D. Raoult, and M. Drancourt. 2006. rpoB gene sequence-based characterization of emerging non-tuberculous mycobacteria with descriptions of Mycobacterium bolletii sp. nov., Mycobacterium phocaicum sp. nov. and Mycobacterium aubagnense sp. nov. Int. J. Syst. Evol. Microbiol. 56133-143. [DOI] [PubMed] [Google Scholar]
- 2.Adekambi, T., P. Colson, and M. Drancourt. 2003. rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. J. Clin. Microbiol. 415699-5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adekambi, T., M. Reynaud-Gaubert, G. Greub, M. J. Gevaudan, B. La Scola, D. Raoult, and M. Drancourt. 2004. Amoebal coculture of “Mycobacterium massiliense” sp. nov. from the sputum of a patient with hemoptoic pneumonia. J. Clin. Microbiol. 425493-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbeit, R. D., A. Slutsky, T. W. Barber, J. N. Maslow, S. Niemczyk, J. O. Falkinham III, G. T. O'Connor, and C. F. von Reyn. 1993. Genetic diversity among strains of Mycobacterium avium causing monoclonal and polyclonal bacteremia in patients with AIDS. J. Infect. Dis. 1671384-1390. [DOI] [PubMed] [Google Scholar]
- 5.Blackwood, K. S., C. He, J. Gunton, C. Y. Turenne, J. Wolfe, and A. M. Kabani. 2000. Evaluation of recA sequences for identification of Mycobacterium species. J. Clin. Microbiol. 382846-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown-Elliott, B. A., and R. J. Wallace, Jr. 2002. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin. Microbiol. Rev. 15716-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cangelosi, G. A., R. J. Freeman, K. N. Lewis, D. Livingston-Rosanoff, K. S. Shah, S. J. Milan, and S. V. Goldberg. 2004. Evaluation of a high-throughput repetitive-sequence-based PCR system for DNA fingerprinting of Mycobacterium tuberculosis and Mycobacterium avium complex strains. J. Clin. Microbiol. 422685-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardoso, A. M., E. Martins de Sousa, C. Viana-Niero, F. Bonfim de Bortoli, Z. C. Pereira das Neves, S. C. Leao, A. P. Junqueira-Kipnis, and A. Kipnis. 2008. Emergence of nosocomial Mycobacterium massiliense infection in Goias, Brazil. Microbes Infect. 101552-1557. [DOI] [PubMed] [Google Scholar]
- 9.Cloud, J. L., H. Neal, R. Rosenberry, C. Y. Turenne, M. Jama, D. R. Hillyard, and K. C. Carroll. 2002. Identification of Mycobacterium spp. by using a commercial 16S ribosomal DNA sequencing kit and additional sequencing libraries. J. Clin. Microbiol. 40400-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Criscuolo, A., V. Berry, E. J. Douzery, and O. Gascuel. 2006. SDM: a fast distance-based approach for (super) tree building in phylogenomics. Syst. Biol. 55740-755. [DOI] [PubMed] [Google Scholar]
- 11.Dauendorffer, J. N., I. Guillemin, A. Aubry, C. Truffot-Pernot, W. Sougakoff, V. Jarlier, and E. Cambau. 2003. Identification of mycobacterial species by PCR sequencing of quinolone resistance-determining regions of DNA gyrase genes. J. Clin. Microbiol. 411311-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devallois, A., K. S. Goh, and N. Rastogi. 1997. Rapid identification of mycobacteria to species level by PCR-restriction fragment length polymorphism analysis of the hsp65 gene and proposition of an algorithm to differentiate 34 mycobacterial species. J. Clin. Microbiol. 352969-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falkinham, J. O., III. 1996. Epidemiology of infection by nontuberculous mycobacteria. Clin. Microbiol. Rev. 9177-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall, L., K. A. Doerr, S. L. Wohlfiel, and G. D. Roberts. 2003. Evaluation of the MicroSeq system for identification of mycobacteria by 16S ribosomal DNA sequencing and its integration into a routine clinical mycobacteriology laboratory. J. Clin. Microbiol. 411447-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han, X. Y., I. De, and K. L. Jacobson. 2007. Rapidly growing mycobacteria: clinical and microbiologic studies of 115 cases. Am. J. Clin. Pathol. 128612-621. [DOI] [PubMed] [Google Scholar]
- 16.Healy, M., J. Huong, T. Bittner, M. Lising, S. Frye, S. Raza, R. Schrock, J. Manry, A. Renwick, R. Nieto, C. Woods, J. Versalovic, and J. R. Lupski. 2005. Microbial DNA typing by automated repetitive-sequence-based PCR. J. Clin. Microbiol. 43199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonsson, B. E., M. Gilljam, A. Lindblad, M. Ridell, A. E. Wold, and C. Welinder-Olsson. 2007. Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis. J. Clin. Microbiol. 451497-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, H. Y., Y. Kook, Y. J. Yun, C. G. Park, N. Y. Lee, T. S. Shim, B. J. Kim, and Y. H. Kook. 2008. Proportions of Mycobacterium massiliense and Mycobacterium bolletii strains among Korean Mycobacterium chelonae-Mycobacterium abscessus group isolates. J. Clin. Microbiol. 463384-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, H. Y., Y. J. Yun, C. G. Park, D. H. Lee, Y. K. Cho, B. J. Park, S. I. Joo, E. C. Kim, Y. J. Hur, B. J. Kim, and Y. H. Kook. 2007. Outbreak of Mycobacterium massiliense infection associated with intramuscular injections. J. Clin. Microbiol. 453127-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koh, W. J., K. S. Lee, O. J. Kwon, Y. J. Jeong, S. H. Kwak, and T. S. Kim. 2005. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology 235282-288. [DOI] [PubMed] [Google Scholar]
- 21.Konig, B., I. Tammer, V. Sollich, and W. Konig. 2005. Intra- and interpatient variability of the hsp65 and 16S-23S intergenic gene region in Mycobacterium abscessus strains from patients with cystic fibrosis. J. Clin. Microbiol. 433500-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olivier, K. N., D. J. Weber, R. J. Wallace, Jr., A. R. Faiz, J. H. Lee, Y. Zhang, B. A. Brown-Elliott, A. Handler, R. W. Wilson, M. S. Schechter, L. J. Edwards, S. Chakraborti, and M. R. Knowles. 2003. Nontuberculous mycobacteria. I. Multicenter prevalence study in cystic fibrosis. Am. J. Respir. Crit. Care Med. 167828-834. [DOI] [PubMed] [Google Scholar]
- 23.Park, H., H. Jang, C. Kim, B. Chung, C. L. Chang, S. K. Park, and S. Song. 2000. Detection and identification of mycobacteria by amplification of the internal transcribed spacer regions with genus- and species-specific PCR primers. J. Clin. Microbiol. 384080-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrini, B. 2006. Mycobacterium abscessus: an emerging rapid-growing potential pathogen. APMIS 114319-328. [DOI] [PubMed] [Google Scholar]
- 25.Ringuet, H., C. Akoua-Koffi, S. Honore, A. Varnerot, V. Vincent, P. Berche, J. L. Gaillard, and C. Pierre-Audigier. 1999. hsp65 sequencing for identification of rapidly growing mycobacteria. J. Clin. Microbiol. 37852-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth, A., U. Reischl, A. Streubel, L. Naumann, R. M. Kroppenstedt, M. Habicht, M. Fischer, and H. Mauch. 2000. Novel diagnostic algorithm for identification of mycobacteria using genus-specific amplification of the 16S-23S rRNA gene spacer and restriction endonucleases. J. Clin. Microbiol. 381094-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampaio, J. L., E. Chimara, L. Ferrazoli, M. A. da Silva Telles, V. M. Del Guercio, Z. V. Jerico, K. Miyashiro, C. M. Fortaleza, M. C. Padoveze, and S. C. Leao. 2006. Application of four molecular typing methods for analysis of Mycobacterium fortuitum group strains causing mammaplasty infections. Clin. Microbiol. Infect. 12142-149. [DOI] [PubMed] [Google Scholar]
- 28.Sampaio, J. L., C. Viana-Niero, D. de Freitas, A. L. Hofling-Lima, and S. C. Leao. 2006. Enterobacterial repetitive intergenic consensus PCR is a useful tool for typing Mycobacterium chelonae and Mycobacterium abscessus isolates. Diagn. Microbiol. Infect. Dis. 55107-118. [DOI] [PubMed] [Google Scholar]
- 29.Simmon, K. E., J. I. Pounder, J. N. Greene, F. Walsh, C. M. Anderson, S. Cohen, and C. A. Petti. 2007. Identification of an emerging pathogen, Mycobacterium massiliense, by rpoB sequencing of clinical isolates collected in the United States. J. Clin. Microbiol. 451978-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh, A., R. V. Goering, S. Simjee, S. L. Foley, and M. J. Zervos. 2006. Application of molecular techniques to the study of hospital infection. Clin. Microbiol. Rev. 19512-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soini, H., and M. K. Viljanen. 1997. Diversity of the 32-kilodalton protein gene may form a basis for species determination of potentially pathogenic mycobacterial species. J. Clin. Microbiol. 35769-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takewaki, S., K. Okuzumi, I. Manabe, M. Tanimura, K. Miyamura, K. Nakahara, Y. Yazaki, A. Ohkubo, and R. Nagai. 1994. Nucleotide sequence comparison of the mycobacterial dnaJ gene and PCR-restriction fragment length polymorphism analysis for identification of mycobacterial species. Int. J. Syst. Bacteriol. 44159-166. [DOI] [PubMed] [Google Scholar]
- 33.Telenti, A., F. Marchesi, M. Balz, F. Bally, E. C. Bottger, and T. Bodmer. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 332233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tortoli, E., R. Gabini, I. Galanti, and A. Mariottini. 2008. Lethal Mycobacterium massiliense sepsis, Italy. Emerg. Infect. Dis. 14984-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Versalovic, J., and J. R. Lupski. 2002. Molecular detection and genotyping of pathogens: more accurate and rapid answers. Trends Microbiol. 10S15-S21. [DOI] [PubMed] [Google Scholar]
- 37.Viana-Niero, C., K. V. Lima, M. L. Lopes, M. C. Rabello, L. R. Marsola, V. C. Brilhante, A. M. Durham, and S. C. Leao. 2008. Molecular characterization of Mycobacterium massiliense and Mycobacterium bolletii in isolates collected from outbreaks of infections after laparoscopic surgeries and cosmetic procedures. J. Clin. Microbiol. 46850-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace, R. J., Jr., B. A. Brown, and D. E. Griffith. 1998. Nosocomial outbreaks/pseudo-outbreaks caused by nontuberculous mycobacteria. Annu. Rev. Microbiol. 52453-490. [DOI] [PubMed] [Google Scholar]
- 39.Zelazny, A. M., L. B. Calhoun, L. Li, Y. R. Shea, and S. H. Fischer. 2005. Identification of Mycobacterium species by secA1 sequences. J. Clin. Microbiol. 431051-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, Y., M. A. Yakrus, E. A. Graviss, N. Williams-Bouyer, C. Turenne, A. Kabani, and R. J. Wallace, Jr. 2004. Pulsed-field gel electrophoresis study of Mycobacterium abscessus isolates previously affected by DNA degradation. J. Clin. Microbiol. 425582-5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zolg, J. W., and S. Philippi-Schulz. 1994. The superoxide dismutase gene, a target for detection and identification of mycobacteria by PCR. J. Clin. Microbiol. 322801-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]