Abstract
Aichi virus has been associated with acute gastroenteritis in adults and children. Stool samples were collected from 788 Tunisian children suffering from diarrhea. Aichi virus was found in 4.1% of the cases. The high proportion of monoinfections and the high frequency of hospitalizations support the role of Aichi virus in pediatric gastroenteritis.
Aichi virus was detected in 1989 as being responsible for oyster-associated nonbacterial gastroenteritis in a patient in Aichi, Japan (11). This virus is a new member of the Picornaviridae family and is assigned to a new genus named Kobuvirus (6, 14). Two genotypes, genotypes A and B, have been described (15). More recently, a third genotype, genotype C, has been proposed on the basis of phylogenetic analyses of both 3CD and VP1 sequences (1).
Very few studies report on the epidemiology of Aichi virus and its impact on human health. Its presence in stool samples collected from nonbacterial gastroenteritis outbreaks, especially due to oyster consumption, has been documented in Japan (12, 15, 16), Germany (4), and France (1). Moreover, the presence of Aichi virus was reported in oyster samples during a French outbreak of gastroenteritis occurring after oyster consumption (3). Aichi virus was also found as the etiologic agent of sporadic gastroenteritis in Japanese tourists from Southeast Asia (13). However, this virus was not detected in The Netherlands in 10 years of research (10). These rare reports on the presence of Aichi virus in diarrheic stool samples from adults reflect a low incidence of Aichi virus infections. Just as in adults, there has been limited knowledge about the epidemiology of Aichi virus infection in children. Its presence in fecal specimens of children suffering from diarrhea has been demonstrated in several Asian countries (5, 13), in Brazil (4), and in France (1). Nevertheless, despite the low incidence of Aichi virus encountered in sporadic and epidemic cases, several studies of seroprevalence conducted in Japan (12), Germany (4), Spain (J. Buesa, personal communication, 28 September 2007), and more recently in France (2) indicated that this virus is quite common. The high level of seroprevalence in adults indicates widespread exposure to Aichi virus during childhood.
In a previous paper describing a 2-year study, we reported on the presence of Aichi virus in Tunisian children suffering from gastroenteritis (8). To gain more insight into the epidemiology of the Aichi virus circulating in the pediatric Tunisian population and to characterize the strains at the molecular level, we expanded the survey to a period of more than 4 years.
Diarrheic stool samples were collected from 788 children that were <12 years old (74.6% were <2 years old) in Monastir, Tunisia: 408 samples were collected from January 2003 to April 2007 from hospitalized children within 24 h of admission (inpatients), and 380 were collected between January 2003 and May 2004 from outpatient children presenting in the dispensaries for gastrointestinal symptoms. The samples were all negative for bacterial pathogens and parasites (9). Aichi virus was detected by reverse transcription-PCR using the primer pair 6261 and 6779 (15) and a Qiagen OneStep reverse transcription-PCR kit (Qiagen, Hilden, Germany) to amplify a 519-bp fragment at the 3CD junction. Genotyping was performed by direct sequencing of the PCR products with the same primers. Phylogenetic and statistical analyses were performed as previously described (8).
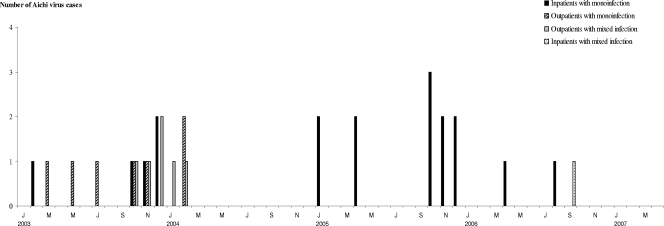
Thirty-two (4.1%) samples were positive for Aichi virus. Screening for other viruses showed that group A rotaviruses were predominant (27%), followed by noroviruses (16.2%). Astroviruses, adenoviruses 40/41, and sapoviruses were also detected in 3.6%, 2.3% and 0.8% of stool specimens, respectively. Of the 32 samples positive for Aichi virus, 25 (78.1%) were monoinfections, whereas 3 (9.4%), 1 (3.1%), and 1 (3.1%) were also positive for rotavirus, astrovirus, and norovirus, respectively. Moreover, two (6.2%) samples were infected by a combination of Aichi virus, rotavirus, and astrovirus. Among the monoinfections, Aichi virus was detected in 18 stool samples from inpatients and in 7 specimens from outpatients, which corresponded to 4.4% and 1.8%, respectively. The monthly distribution of the Aichi virus cases is presented in Fig. 1.
FIG. 1.
Monthly distribution of Aichi virus in stool samples from children with acute gastroenteritis in Monastir, Tunisia, between January 2003 and April 2007. Abbreviations: J, January; M, March; May; J, July; S, September; N, November.
Most studies in the literature reported a low incidence of Aichi virus in the pediatric population: 2.3% in Pakistani children (13), 0.9% in French children hospitalized for acute gastroenteritis (1), and 3.1% in children from Japan, Bangladesh, Thailand, and Vietnam (5). In the Tunisian pediatric population, the frequency of detection of Aichi virus was then relatively higher than usually observed. Aichi virus has been proposed as an etiologic agent of oyster-related gastroenteritis outbreaks (11, 12, 15, 16), but the almost systematic presence of mixed infections with other viruses led us to consider Aichi virus an indicator of infection rather than the only causative agent of gastroenteritis (1). However, the high incidence of monoinfections observed in our study supported the idea that Aichi virus is a pathogenic agent responsible for gastroenteritis, although at levels far below norovirus and rotavirus, which are the main agents. Moreover, the presence of Aichi virus in pediatric stool samples suggests that it can be transmitted by ways other than oysters, since young children usually are not consumers of these products. In addition, we observed that monoinfections due to Aichi virus were significantly more frequent among the hospitalized pediatric population than among outpatients (P = 0.04). There is no data in the literature about the implication of Aichi virus in the hospitalization of children with acute gastroenteritis, but our results suggested that this virus could be virulent enough to require hospitalization. However, we cannot exclude the possibility that other pathogens that are unknown, undetected, or not tested for could be responsible for gastroenteritis.
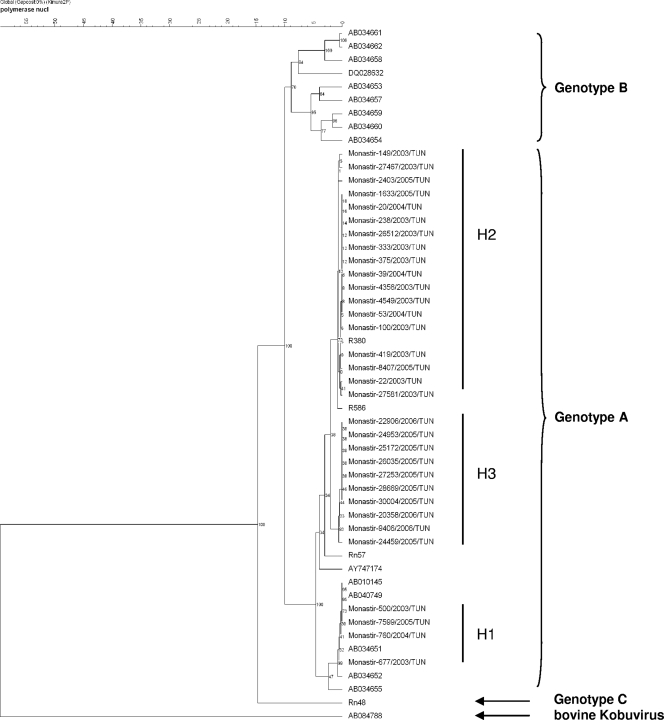
The phylogenetic analysis of Aichi virus strains showed that all samples were classified as genotype A and divided into three groups, H1, H2, and H3, according to their nucleotide identities (Fig. 2). Despite the limited data in the literature, different studies suggested some geographical distribution of the genotypes. Only genotype A has been found in Germany and France (1, 4), and genotype A is also predominant in Japan (5, 15). Only genotype B has been isolated from Pakistani children (15), and genotype B was predominant in Bangladeshi children (5). Genotype B was also found in Brazil (4) and Malaysia (15). The epidemiology of Aichi virus in Africa is not yet known. One child hospitalized for gastroenteritis just after returning from a trip to Mali was found positive for a strain of genotype C (1). Additional studies are required to learn about the epidemiology of Aichi virus in Africa, but according to our results, genotype A seems to be predominant in the Tunisian child population.
FIG. 2.
Phylogenetic analysis based on the partial nucleotide sequence (468 bp) of the 3CD-encoding gene of the Aichi virus strains associated with pediatric gastroenteritis in Monastir, Tunisia, between January 2003 and April 2007. The tree was constructed by clustering using the unweighted-pair group method using average linkages. The reference strains are identified in this tree by their GenBank accession numbers. nucl, nucleotides. Number on the scale bar at the top of the figure represent percent similarity of nucleotides.
In conclusion, the high incidence of monoinfections observed and the relatively high frequency of hospitalizations due to Aichi virus infections support the role of Aichi virus as a causative agent of pediatric diarrhea. However, further studies must be conducted notably on controls to check that healthy children are free of Aichi virus.
Nucleotide sequence accession numbers.
The nucleotide sequences reported here have been deposited in the GenBank sequence database under accession numbers FJ872477 to FJ872508.
Acknowledgments
The study was partly supported by the CMCU project (code 04/S0813) and the National Reference Center (NRC) for Enteric Viruses (Dijon, France).
We thank the members of the NRC for their technical support.
Footnotes
Published ahead of print on 27 May 2009.
REFERENCES
- 1.Ambert-Balay, K., M. Lorrot, F. Bon, H. Giraudon, J. Kaplon, M. Wolfer, P. Lebon, D. Gendrel, and P. Pothier. 2008. Prevalence and genetic diversity of Aichi virus strains in stool samples from community and hospitalized patients. J. Clin. Microbiol. 461252-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyer, M., L. S. Aho, J. B. Bour, K. Ambert-Balay, and P. Pothier. 2008. Seroprevalence distribution of Aichi virus among a French population in 2006-2007. Arch. Virol. 1531171-1174. [DOI] [PubMed] [Google Scholar]
- 3.Le Guyader, F. S., J. C. Le Saux, K. Ambert-Balay, J. Krol, O. Serais, S. Parnaudeau, H. Giraudon, G. Delmas, M. Pommepuy, P. Pothier, and R. L. Atmar. 2008. A French oyster-related gastroenteritis outbreak: Aichi virus, norovirus, astrovirus, enterovirus, and rotavirus all involved in clinical cases. J. Clin. Microbiol. 464011-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh, D. Y., P. A. Silva, B. Hauroeder, S. Diedrich, D. D. Cardoso, and E. Schreier. 2006. Molecular characterization of the first Aichi viruses isolated in Europe and in South America. Arch. Virol. 1511199-1206. [DOI] [PubMed] [Google Scholar]
- 5.Pham, N. T., P. Khamrin, T. A. Nguyen, D. S. Kanti, T. G. Phan, S. Okitsu, and H. Ushijima. 2007. Isolation and molecular characterization of Aichi viruses from fecal specimens collected in Japan, Bangladesh, Thailand, and Vietnam. J. Clin. Microbiol. 452287-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pringle, C. R. 1999. Virus taxonomy at the XIth International Congress of Virology, Sydney, Australia, 1999. Arch. Virol. 1442065-2070. [DOI] [PubMed] [Google Scholar]
- 7.Reference deleted.
- 8.Sdiri-Loulizi, K., H. Gharbi-Khelifi, A. de Rougemont, S. Chouchane, N. Sakly, K. Ambert-Balay, M. Hassine, M. N. Guediche, M. Aouni, and P. Pothier. 2008. Acute infantile gastroenteritis associated with human enteric viruses in Tunisia. J. Clin. Microbiol. 461349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sdiri-Loulizi, K., K. Ambert-Balay, H. Gharbi-Khelifi, N. Sakly, M. Hassine, S. Chouchane, M. N. Guediche, P. Pothier, and M. Aouni. 2009. Molecular epidemiology of norovirus gastroenteritis investigated using samples collected from children in Tunisia during a four-year period: detection of the norovirus variant GGII.4 Hunter as early as January 2003. J. Clin. Microbiol. 47421-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svraka, S., E. Duizer, H. Vennema, E. de Bruin, B. van der Veer, B. Dorresteijn, and M. Koopmans. 2007. Etiological role of viruses in outbreaks of acute gastroenteritis in The Netherlands from 1994 through 2005. J. Clin. Microbiol. 451389-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita, T., S. Kobayashi, K. Sakae, S. Nakata, S. Chiba, Y. Ishihara, and S. Isomura. 1991. Isolation of cytopathic small round viruses with BS-C-1 cells from patients with gastroenteritis. J. Infect. Dis. 164954-957. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita, T., K. Sakae, Y. Ishihara, S. Isomura, and E. Utagawa. 1993. Prevalence of newly isolated, cytopathic small round virus (Aichi strain) in Japan. J. Clin. Microbiol. 312938-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita, T., K. Sakae, S. Kobayashi, Y. Ishihara, T. Miyake, A. Mubina, and S. Isomura. 1995. Isolation of cytopathic small round virus (Aichi virus) from Pakistani children and Japanese travelers from Southeast Asia. Microbiol. Immunol. 39433-435. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita, T., K. Sakae, H. Tsuzuki, Y. Suzuki, N. Ishikawa, N. Takeda, T. Miyamura, and S. Yamazaki. 1998. Complete nucleotide sequence and genetic organization of Aichi virus, a distinct member of the Picornaviridae associated with acute gastroenteritis in humans. J. Virol. 728408-8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamashita, T., M. Sugiyama, H. Tsuzuki, K. Sakae, Y. Suzuki, and Y. Miyazaki. 2000. Application of a reverse transcription-PCR for identification and differentiation of Aichi virus, a new member of the picornavirus family associated with gastroenteritis in humans. J. Clin. Microbiol. 382955-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamashita, T., M. Ito, H. Tsuzuki, and K. Sakae. 2001. Identification of Aichi virus infection by measurement of immunoglobulin responses in an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 394178-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]