Abstract
A new Tetratrichomonas species was identified by molecular and phylogenetic approaches in the pleural fluid from a patient with encysted empyema leading to dyspnea. This observation raised the questions of the real prevalence of pulmonary trichomonosis in humans, the zoonotic potential of trichomonads, and the existence of human-host-adapted strains.
CASE REPORT
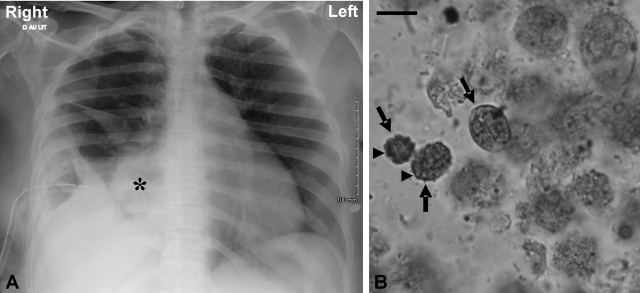
A 40-year-old woman was admitted to the emergency unit with a bacterial infection and a history of fever, fatigue, vomiting, and respiratory distress for 2 weeks before admission. On physical examination, the patient was irritable with dyspnea. Her vital signs were as follows: temperature, 39.2°C; blood pressure, 168/81 mm Hg; heart rate, 110 beats/min; respiration, 30 breaths/min. A chest X-ray followed by a computed tomography scan revealed encysted empyema of the right lung with a large pleural effusion (Fig. 1A). Foul-smelling and purulent pleural fluid was removed from the right chest cavity by thoracentesis. Examination of the pleural fluid showed numerous motile and flagellated protozoa, which were provisionally identified as trichomonads by their morphological characteristics, including form, size, and motility. These flagellates were not cultured, and their identification was confirmed by microscopic examination of May-Grünwald-Giemsa- and Merthiolate-iodine-Formalin (MIF)-stained smears (Fig. 1B). Moreover, this pleural fluid was used for subsequent molecular characterization of these protozoa. Bacteriological cultures of the fluid yielded gram-positive cocci. On day 2, a chest tube was inserted for drainage of the right pleura and fibrinolytic therapy with urokinase, and mechanical ventilation was instituted. Pleural fluid was drained and was positive for Streptococcus constellatus and Streptococcus gordonii, both anaerobic and aerobic bacteria, and the anaerobic bacterium Prevotella sp. in addition to the trichomonad species mentioned above. Antibiotherapy with amoxicillin (amoxicilline) was continued, and treatment with metronidazole (Flagyl) was started. On day 10, the patient suffered from diarrhea with the emergence of Clostridium difficile. Hence, the use of metronidazole was delayed and the patient was treated with vancomycin per os in addition to amoxicillin. On day 18, a follow-up chest computed tomography scan showed partial clearing of the pleural effusion. Consequently, the patient did not require further surgical cleaning of the pleural cavity. On day 24, the patient was successfully weaned from the ventilator, and by day 26, her general health condition had improved and she presented neither fever nor dyspnea.
FIG. 1.
Chest radiography on admission of the patient and cytological appearance of trichomonad cells in the pleural fluid (MIF staining). (A) Chest X-ray revealing encysted empyema with massive right pleural effusion represented by an asterisk. (B) MIF-stained smear of empyema fluid showing three trichomonad cells (arrows). Note the ovoid or ellipsoidal shape of the microorganisms. Internal structures such as the axostyle-pelta complex and flagella are not clearly visible. Only a well-developed undulating membrane (arrowheads), considered a typical cytoskeletal structure of trichomonads, is identifiable. Bar = 10 μm.
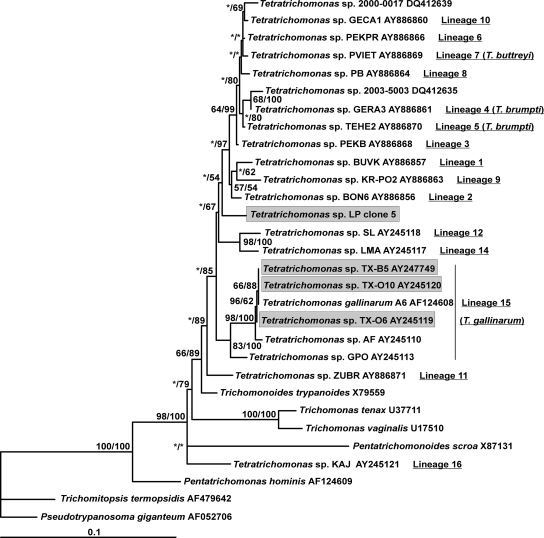
In order to identify the trichomonad species involved in this case report, DNA was extracted from pleural fluid using the High Pure PCR template preparation kit (Roche Applied Science, Mannheim, Germany) according to the manufacturer's protocol. In a first step, the internal transcribed spacer 1 (ITS1)-5.8S rRNA-ITS2 region was amplified as described previously (6, 12). PCR was carried out with a 50-μl volume according to the standard conditions for Platinum Taq high-fidelity DNA polymerase (Invitrogen, Groningen, The Netherlands). The PCR product was separated by agarose gel electrophoresis, and a band of the expected size (around 400 bp) was purified using the Wizard SV Gel and PCR clean-up system (Promega, Madison, WI). The purified PCR product was cloned in the T vector pCR 2.1-TOPO (Invitrogen) and amplified in Escherichia coli One Shot TOP10 competent cells. Minipreparations of plasmid DNA were done using the QIAprep Spin miniprep kit (Qiagen). Four clones containing inserts of approximately the expected size were arbitrarily selected and sequenced on both strands. These sequences were aligned with the use of the BioEdit v7.0.1 package (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). The four sequenced clones exhibited 98.6 to 99.7% identity. The differences observed between the clones were likely due to normal variation within the multiple copies of the RNA genes in any given genome. These sequences were compared with all of the trichomonad ITS1-5.8S rRNA-ITS2 sequences available from the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov) using the BLAST program and showed a high degree of similarity (85 to 90%) to sequences of Tetratrichomonas sp. strains isolated from different hosts. These results showed that the trichomonad isolate found in the lungs of this patient (strain LP) belonged to the Tetratrichomonas genus. Although ITS1-5.8S rRNA-ITS2 could be a valuable marker for genotyping approaches, the limited number of positions used in phylogenetic tree construction could represent a restrictive factor for taxonomic considerations. Therefore, in order to formally identify this strain, a small-subunit (SSU) rRNA gene sequence was subsequently obtained by PCR. DNA amplification was carried out using the sense primer Euk1900 and the antisense primer Euk3 as described previously (17, 19). The PCR product of the expected size (around 1,600 bp) was purified and cloned as described above. Four clones were arbitrarily isolated and sequenced. These clones exhibited 99.4 to 99.8% identity, indicating that they all derived from the same species. Therefore, only one of these sequences was aligned with a set of trichomonad sequences, including 22 other Tetratrichomonas sequences retrieved from databases using the ARB package (http://www.arb-home.de/). This set of Tetratrichomonas sequences was representative of the full genetic diversity of this genus. We restricted the phylogenetic inferences to 1,290 sites that could be unambiguously aligned. Phylogenetic analyses were carried out using MrBAYES 3_0b4 and PHYML with parameters identical to those described previously (9). Only the burn-in values in the Bayesian analysis (1,000 in place of 100,000) and the number of bootstrap values in the maximum likelihood analysis (500 in place of 100 replicates) were modified. The unrooted trees constructed in this study (Fig. 2) confirmed the unexpected diversity in the genus Tetratrichomonas, as previously suggested (2, 3, 9), and the identification of at least 16 lineages that could probably represent separate, mostly new, species. Interestingly, the SSU rRNA sequence of strain LP did not show any close relationship with any of the previously identified Tetratrichomonas lineages. Moreover, the evolutionary distances observed between Tetratrichomonas strain LP and the other Tetratrichomonas strains included in our phylogenetic analysis were comparable to those observed among the 16 different Tetratrichomonas lineages. In addition, strain LP did not cluster with representatives of lineage 15 (Tetratrichomonas gallinarum), which included human strains previously isolated from the oral cavities (TX-O6 and TX-O10) and bronchi (TX-B5) of patients suffering from different chronic pulmonary diseases (2). These data unequivocally identified strain LP as an as-yet-undescribed Tetratrichomonas species and as a potential novel parasite in humans.
FIG. 2.
Unrooted maximum likelihood tree of trichomonads based on the SSU rRNA gene sequences. Tetratrichomonas strains isolated from humans (including strain LP obtained in this study) are boxed. Tetratrichomonas lineages described by Cepicka et al. (3) are represented. Species identifications of some of these lineages are indicated in parentheses after the corresponding lineage. Note that Tetratrichomonas sp. strain LP formed an independent lineage within the Tetratrichomonas genus and did not group with other strains isolated from humans. Numbers near the individual nodes indicate bootstrap values (left of the slash) and Bayesian posterior probabilities (right of the slash) given as percentages by the two different reconstruction methods (maximum likelihood and MrBAYES). Asterisks designate nodes with values below 50%. Scale bar indicates 0.1 substitution (corrected) per base pair.
Trichomonads are flagellated protists frequently found in the urogenital tracts (Trichomonas vaginalis), oral cavities (Trichomonas tenax), and intestinal tracts (Pentatrichomonas hominis and Dientamoeba fragilis) of humans (11). Only D. fragilis and T. vaginalis are considered pathogenic and are the causative agents of a common form of chronic diarrhea and of vaginitis, respectively. It was thought that each human species had a specific tropism for its site of infection. However, it has been recently shown that these microorganisms could be found outside their natural habitats, such as in the lungs (pulmonary trichomonosis). For instance, T. tenax has been identified in numerous cases in the upper or lower respiratory tracts of humans (1, 14-16). This organism is usually regarded as a harmless commensal of the human mouth associated with poor dentition and oral hygiene and is thought to enter the respiratory tract by aspiration of oropharyngeal secretions. Interestingly, other trichomonad species have been identified in human lungs using immunological and molecular tools. These species include T. vaginalis (4); P. hominis (12); Tritrichomonas foetus (6), a genital trichomonad found in bovids; and a Tetratrichomonas sp. (13). Several Tetratrichomonas strains were isolated from the oral cavities and bronchi of patients with chronic pulmonary diseases. SSU rRNA sequences obtained from these different strains were highly similar and clustered together in the same lineage 15 that includes the avian species T. gallinarum (2, 3). In the present study, we have identified a Tetratrichomonas species that differs from the other Tetratrichomonas strains previously characterized in humans and likely represents a new lineage and species. The identification of strains belonging to the genera Tritrichomonas and Tetratrichomonas in humans clearly raised the question of the as yet poorly understood zoonotic potential of trichomonads, since these taxa were thought to be of animal origin. Alternatively, these isolates could represent human-host-adapted species, since Kutisova et al. (13) failed to transmit Tetratrichomonas strains of human origin to birds.
Regarding the recent literature, the presence of trichomonads in the human respiratory tract cannot be hereafter considered unusual. Indeed, these microorganisms are found frequently in the course of human Pneumocystis pneumonia (PCP) (5, 7) and of acute respiratory distress syndrome (ARDS) (8). More precisely, trichomonads were detected as coinfecting agents with Pneumocystis in 60% of PCP patients and were found in 30% of ARDS patients. Because these protozoa are microaerophilic, they are likely not able to cause pulmonary disease by themselves and require favorable conditions for their development. With regard to PCP and ARDS, it was hypothesized that the development of these protozoa in the alveolar lumens was linked to local hypoxic conditions rather than immunodepression. Indeed, in these two clinical contexts, the alveolar lumens are obliterated by fungi in the case of PCP or by fibrin and cellular debris in the case of ARDS. The proliferation of trichomonads in the pleural cavity has been reported in 16 cases of empyema, including that reported by Wang et al. (20), and appeared to depend on the presence of bacterial species in addition to anaerobic conditions (10, 14, 20). This is due to the fact that these parasites are known to feed on bacteria. Besides, antibiotics and metronidazole against coinfection with empyema, as well as drugs active against PCP, have consistently cured patients of pulmonary trichomonosis (1, 4, 6). The question of whether trichomonads actually occur in the respiratory tracts of patients with underlying pulmonary pathologies is raised, and the frequency of this occurrence is probably broadly underestimated for three main reasons. First, most trichomonad cells detected in different clinical contexts undergo deep morphological modifications that involve a change from a flagellate to an amoeboid form (4, 5, 7, 8). Such change renders the identification of these parasites extremely difficult for observers not aware of the pleomorphism of these parasites. Second, and as previously suggested (12), trichomonads might be more frequently detected if a preliminary microscopic examination of wet mount smears was systematically performed with high-risk patients with lung diseases. And third, inappropriate storage conditions of the samples and too-long delays between collection and analysis could also lead to undiagnosed pulmonary trichomonosis (1).
Including those described in this study, six trichomonad species have already been identified as causative agents of pulmonary trichomonosis, and the number of trichomonad taxa identified as potentially involved as coinfecting parasites of lung diseases will likely increase in the future because of the use of molecular diagnostic tools. Therefore, the significance of the pulmonary location of these parasites has to be clarified. Several observations suggest the potentially pathogenic effect of trichomonads on the alveolar epithelia of patients (7). However, physiopathological studies of these parasites have to be performed by developing in situ hybridization methods for lung tissues of patients and/or animal models of pulmonary coinfection by trichomonads and other microorganisms in order to confirm this hypothesis. Trichomonads could thus play an active role in the extension of pulmonary lesions and more generally aggravate a patient's poor physical condition and delay recovery. Hence, the impact and prevalence of pulmonary trichomonosis have to be seriously taken into consideration by the medical and public health communities, since they could be correlated with the global burden of lung diseases. Indeed, lung infections, including influenza, pneumonia, and other acute lower respiratory tract infections, cause more disease than cancer, heart attack, AIDS, tuberculosis, or malaria worldwide (18). Therefore, lung infections represent a public health priority and a large potential reservoir for trichomonad coinfection.
Nucleotide sequence accession numbers.
The nucleotide sequences obtained in this study have been deposited in GenBank under accession numbers FJ711076 to FJ711082.
Acknowledgments
This work was developed in the framework of scientific program EA3609 (French Ministry of Research) and supported by the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Institut Pasteur de Lille, and the Université Lille Nord de France.
Footnotes
Published ahead of print on 6 May 2009.
REFERENCES
- 1.Bellanger, A. P., O. Cabaret, J. M. Costa, F. Foulet, S. Bretagne, and F. Botterel. 2008. Two unusual occurrences of trichomoniasis: rapid species identification by PCR. J. Clin. Microbiol. 463159-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cepicka, I., K. Kutisova, J. Tachezy, J. Kulda, and J. Flegr. 2005. Cryptic species within the Tetratrichomonas gallinarum species complex revealed by molecular polymorphism. Vet. Parasitol. 12811-21. [DOI] [PubMed] [Google Scholar]
- 3.Cepicka, I., V. Hampl, J. Kulda, and J. Flegr. 2006. New evolutionary lineages, unexpected diversity, and host specificity in the parabasalid genus Tetratrichomonas. Mol. Phylogenet. Evol. 39542-551. [DOI] [PubMed] [Google Scholar]
- 4.Duboucher, C., C. Noël, I. Durand-Joly, D. Gerbod, P. Delgado-Viscogliosi, S. Jouveshomme, C. Leclerc, G.-L. Cartolano, E. Dei-Cas, M. Capron, and E. Viscogliosi. 2003. Pulmonary coinfection by Trichomonas vaginalis and Pneumocystis sp. as a novel manifestation of AIDS. Hum. Pathol. 34508-511. [DOI] [PubMed] [Google Scholar]
- 5.Duboucher, C., D. Gerbod, C. Noël, I. Durand-Joly, P. Delgado-Viscogliosi, C. Leclerc, S. Pham, M. Capron, E. Dei-Cas, and E. Viscogliosi. 2004. Frequency of trichomonads as coinfecting agents in Pneumocystis pneumonia. Acta Cytol. 49273-277. [DOI] [PubMed] [Google Scholar]
- 6.Duboucher, C., S. Caby, F. Dufernez, M. Chabé, N. Gantois, P. Delgado-Viscogliosi, C. Billy, E. Barré, E. Torabi, M. Capron, R. J. Pierce, E. Dei-Cas, and E. Viscogliosi. 2006. Molecular identification of Tritrichomonas foetus-like organisms as coinfecting agents of human Pneumocystis pneumonia. J. Clin. Microbiol. 441165-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duboucher, C., R. Boggia, G. Morel, M. Capron, R. J. Pierce, E. Dei-Cas, and E. Viscogliosi. 2007. Pneumocystis pneumonia: immunodepression, Pneumocystis jirovecii … and the third man. Nat. Rev. Microbiol. doi: 10.1038/nrmicro1621-c1. [DOI] [PubMed]
- 8.Duboucher, C., C. Barbier, A. Beltramini, M. Rona, J.-L. Ricome, G. Morel, M. Capron, R. J. Pierce, E. Dei-Cas, and E. Viscogliosi. 2007. Pulmonary superinfection by trichomonads in the course of acute respiratory distress syndrome. Lung 185295-301. [DOI] [PubMed] [Google Scholar]
- 9.Dufernez, F., R. L. Walker, C. Noël, S. Caby, C. Mantini, P. Delgado-Viscogliosi, M. Ohkuma, T. Kudo, M. Capron, R. J. Pierce, M. R. Villanueva, and E. Viscogliosi. 2007. Morphological and molecular identification of non-Tritrichomonas foetus trichomonad protozoa from the bovine preputial cavity. J. Eukaryot. Microbiol. 54161-168. [DOI] [PubMed] [Google Scholar]
- 10.Gilroy, S. A., E. Simcuski, R. A. Rawling, and P. A. Granato. 2007. Trichomonas species empyema coinfection in an alcoholic female. Clin. Microbiol. Newsl. 2969-71. [Google Scholar]
- 11.Honigberg, B. M. 1990. Trichomonads found outside the urogenital tract of humans, p. 342-393. In B. M. Honigberg (ed.), Trichomonads parasitic in humans. Springer-Verlag, New York, NY.
- 12.Jongwutiwes, S., U. Silachamroon, and C. Putaporntip. 2000. Pentatrichomonas hominis in empyema thoracis. Trans. R. Soc. Trop. Med. Hyg. 94185-186. [DOI] [PubMed] [Google Scholar]
- 13.Kutisova, K., J. Kulda, I. Cepicka, J. Flegr, B. Koudela, J. Teras, and J. Tachezy. 2005. Tetratrichomonads from the oral cavity and respiratory tract of humans. Parasitology 131309-319. [DOI] [PubMed] [Google Scholar]
- 14.Lewis, K. L., D. E. Doherty, J. Ribes, J. P. Seabolt, and E. S. Bensadoun. 2003. Empyema caused by Trichomonas. Chest 123291-292. [DOI] [PubMed] [Google Scholar]
- 15.Mahmoud, M. S. E., and G. A. Rahman. 2004. Pulmonary trichomoniasis: improved diagnosis by using polymerase chain reaction targeting Trichomonas tenax 18S rRNA gene in sputum specimens. J. Egypt. Soc. Parasitol. 34197-211. [PubMed] [Google Scholar]
- 16.Mallat, H., I. Podglajen, V. Lavarde, J.-L. Mainardi, J. Frappier, and M. Cornet. 2004. Molecular characterization of Trichomonas tenax causing pulmonary infection. J. Clin. Microbiol. 423886-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medlin, L., H. Elwood, S. Stickel, and M. L. Sogin. 1988. The characterization of enzymatically amplified eukaryotic 16S-like rRNA coding regions. Gene 71491-499. [DOI] [PubMed] [Google Scholar]
- 18.Mizgerd, J. P. 2006. Lung infection—a public health priority. PLoS Med. 3155-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohkuma, M., K. Ohtoko, C. Grunau, S. Moriya, and T. Kudo. 1998. Phylogenetic identification of the symbiotic hypermastigote Trichonympha agilis in the hindgut of the termite Reticulitermes speratus based on small-subunit rRNA sequence. J. Eukaryot. Microbiol. 45439-444. [DOI] [PubMed] [Google Scholar]
- 20.Wang, H.-K., J. S. Jerng, K.-E. Su, S.-C. Chang, and P.-C. Yang. 2006. Case report: Trichomonas empyema with respiratory failure. Am. J. Trop. Med. Hyg. 751234-1236. [PubMed] [Google Scholar]