Abstract
In recent reports it was shown that genetically modified choline-free strains of Streptococcus pneumoniae (D39Cho−licA64 and D39ChiplicB31) expressing the type II capsular polysaccharide were virtually avirulent in the murine sepsis model, in sharp contrast to the isogenic and highly virulent strains D39Cho− and D39Chip, which have retained the choline residues at their surface. We now demonstrate that this choline-associated virulence is independent of Toll-like receptor 2 recognition. Also, despite the lack of virulence, choline-free strains of S. pneumoniae were able to activate splenic dendritic cells, induce secretion of proinflammatory cytokines, and produce specific protective immunity against subsequent challenge. However, after this transient engagement of the immune system the choline-free bacteria were rapidly cleared from the blood, while the isogenic virulent strain D39Cho− continued to grow, accompanied by prolonged expression of cytokines, eventually killing the experimental animals. The critical contribution of choline residues to the virulence potential of pneumococci appears to be the role that these amino alcohol residues play in a pneumococcal immune evasion strategy, the mechanism of which is unknown at the present time.
A unique characteristic of the species Streptococcus pneumoniae is its auxotrophic requirement for choline (14). The bacterium takes up choline from the growth medium and incorporates this amino alcohol into the cell wall teichoic acid (2, 18) and the membrane-anchored glycolipid lipoteichoic acid polymers (1) that are located on the pneumococcal cell surface (7). A choline-independent strain, R6Cho−, capable of growing in choline-free medium acquired heterologous genetic elements during transformation of the S. pneumoniae strain R6 with DNA from Streptococcus oralis (10, 17), a streptococcal species that contains choline in its cell wall but does not require choline for growth (9). The choline independence of another, recently isolated mutant, R6Chip, is based on a single point mutation in the tacF gene, which encodes a teichoic acid flippase (4). However, these mutant strains were still able to utilize environmental choline. In order to prevent this, mutant derivatives of R6Cho− and R6Chip were prepared in which genes in the lic1 operon responsible for the cellular uptake and metabolism of choline were inactivated (11). Such mutants, for instance, R6Cho−licA64, were able to maintain a choline-free phenotype in the choline-containing in vivo environment. R6Cho−licA64 grew in long autolysis-defective chains both in choline-containing and choline-free medium, and D39Cho−licA64, a derivative of the strain expressing capsular polysaccharide II, could grow both in vitro and also in vivo in a murine model of pneumococcal infection (11).
In a previous study it was demonstrated that the choline-free strains D39Cho−licA64 and D39ChiplicB31, expressing the polysaccharide capsular type II, were virtually avirulent in the mouse model of sepsis (4, 11). Nevertheless, following intraperitoneal inoculation, D39Cho−licA64 was able to invade the bloodstream and replicate for a limited time, after which the bacteria were cleared from the blood. The purpose of the study described here was to determine to what extent these avirulent strains have the capacity to engage the host immune system during their transient period of growth within the host.
MATERIALS AND METHODS
Cultivation of bacteria and preparation of inocula.
For murine in vivo assays the S. pneumoniae strains D39Cho−, D39Cho−licA64, and D39ChiplicB31 (4) were grown in choline-free Cden medium at 37°C without aeration (19). By using choline-free Cden medium all strains showed a chain-forming phenotype just prior to intraperitoneal inoculation into the mice. Strains D39 and SV36 (the latter producing a capsular polysaccharide type III) (13) were grown in C+Y medium at 37°C without aeration (9). For inoculum preparation exponentially growing cultures of the strains were back-diluted in the respective growth medium and were allowed to grow to an optical density at 590 nm of 0.6. Bacterial pellets were washed twice with 0.9% NaCl. The cultures were further diluted in 0.9% NaCl and adjusted to the desired inoculum concentrations.
Murine intraperitoneal sepsis model.
For the majority of the experiments 8-week-old female CD1 mice (Charles River Laboratories, Wilmington, MA) were used. Time course studies on the in vivo growth of bacteria were repeated three times. Mice were injected in the peritoneal cavity with 0.5 ml of the prepared inocula containing 106 CFU of bacteria. In each experiment blood from two mice per time point was collected by cardiac puncture and pooled. For bacterial titer determinations serial dilutions of the blood samples were plated on 3% sheep blood agar plates supplemented with 5 μg/ml gentamicin and incubated at 37°C in a 5% CO2 atmosphere. Prior to the assay of serum cytokine levels, 10 μg/ml mitomycin C was added to the blood samples followed by incubation for 1 h at 37°C, in order to kill the bacteria. Control experiments showed that mitomycin C did not trigger cytokine production in uninfected blood samples. After centrifugation the serum was collected and stored at −80°C.
For experiments studying the effect of Toll-like receptor 2 (TLR-2) on the infection, 8-week-old male B6.129-Tlr2tm1Kir/J mice (The Jackson Laboratories, Bar Harbor, ME) were used. For survival curves five B6.129-Tlr2tm1Kir/J mice were compared to five C57BL/6 mice, which had the appropriate genetic background to serve as controls. Both groups (n = 5 animals) were infected with 105 CFU D39Cho−, and survival of the mice was monitored.
Cytokine determinations.
The concentrations of cytokines from mouse sera were measured by using Luminex (Millipore Corporation, St. Charles, MO), according to the manufacturer's protocol: 25-μl serum samples were incubated for 15 min with 25 μl of a serum diluent, followed by a 2-h incubation with Beadmates coated with anticytokine monoclonal antibodies. The plate was washed once, and biotin-conjugated anticytokine monoclonal antibodies were added for 1.5 h, followed by 30 min of incubation with Beadlyte streptavidin-phycoerythrin. Samples were measured in duplicate with Luminex and analyzed using Beadview software (Millipore Corporation, St. Charles, MO).
Maturation of murine splenic dendritic cells.
To analyze the activation state of dendritic cells in mice infected with the different bacterial strains, one mouse spleen per bacterial strain was collected at 6 and 9 h postinfection. The tissues were transferred to petri dishes containing Hank's medium supplemented with collagenase. After flushing with medium using a syringe, the spleen tissues were homogenized and incubated at 37°C for 25 min. Next, 100 μl of 0.5 M EDTA was added followed by incubation at 37°C for 5 min, after which the cell suspensions were strained through a mesh and centrifuged. ACK lysing buffer (1.5 ml; BioSource, Rockville, MD) was added and mixtures were incubated for 4 min at room temperature to allow erythrocyte lysis. To stop lysis 13 ml of fluorescence-activated cell sorting buffer (phosphate-buffered saline [PBS] plus 5% fetal calf serum) was added and cells were centrifuged for 10 min. CD11c+ CD8+ and CD11c+ CD8− dendritic cells were analyzed by fluorescence-activated cell sorting for the presence of cell surface maturation markers CD80 and CD86. Two independent experiments were performed.
Maturation of human monocyte-derived dendritic cells.
Buffy coats purchased from the New York Blood Center were used as a source of mononuclear cells from healthy donors. Peripheral blood mononuclear cells were isolated from the peripheral blood by density gradient centrifugation (Ficoll-Paque Plus; GE Healthcare). Monocytes were separated from the peripheral blood mononuclear cells by using CD14 microbeads (Miltenyi Biotech). Dendritic cells (DCs) were generated from purified blood monocytes as previously described (5). The CD14+ monocytes were cultured in RPMI 1640 (Biowhittaker) supplemented with 1% plasma in the presence of 800 U/ml of granulocyte-macrophage colony-stimulating factor (Immunex) and 1,000 U/ml of interleukin-4 (IL-4; R&D Systems). The cultures were supplemented with cytokines on days 1, 3, and 5 of culture. On day 5, immature DCs were allowed to mature overnight with 100 ng/ml lipopolysaccharide (LPS; Sigma-Aldrich).
Various bacteria as well as bacterial cell wall preparations were tested for their potential to induce DC maturation after incubation for 36 h. Cultures of the choline-containing strains D39Cho− and the isogenic choline-free derivative D39Cho−licA64 were grown in C+Y medium at 37°C. When the cell concentration in the cultures reached about 107 CFU per ml the cultures received 10 μg/ml mitomycin C and were incubated for 1 h at 37°C to kill the bacteria, which was confirmed by plating on blood agar. CFU equivalents of 108 of the nonviable bacterial suspensions were applied to assay dendritic cell maturation.
Choline-free and choline-containing cell walls were prepared and purified by a published procedure (17) from the nonencapsulated strain R6Cho− grown in Cden medium that was either free of choline or was supplemented with 5 μg/ml choline. Suspensions of cell walls were adjusted to the desired concentration in PBS, and approximately 107 CFU equivalents were used in the assays.
Dendritic cell maturation was monitored by flow cytometry (FACSCalibur; BD Biosciences) based on the presence of various maturation markers, CD80, CD83, and CD86 (BD Biosciences). Each experiment was repeated three times using fresh batches of dendritic cells and freshly prepared mitomycin C-killed bacteria.
Induction of protective immunity.
Mice were injected intraperitoneally (i.p.) with 103 CFU of the avirulent D39Cho−licA64 strain expressing the type II capsular polysaccharide (n = 4 animals per group). Control mice (n = 5 animals) were injected with saline. On day 10 after inoculation with the avirulent strain the animals were challenged with 104 CFU of the highly virulent strain, D39Cho−, and survival was monitored.
In a second experiment, animals were immunized i.p. with 104 CFU of the avirulent D39ChiplicB31 strain (n = 5 animals per group). Five control mice were injected with saline. On day 10 postinfection the animals were challenged with a potentially lethal dose (104 and 106 CFU) of either of the highly virulent strains D39 (capsule type II) or SV36 (capsular type III), and survival was monitored. All mice that survived this first challenge were next challenged on day 25 with a lethal dose of 104 CFU of strain SV36.
Detection of capsule-specific IgM antibodies with an enzyme-linked immunosorbent assay (ELISA).
Capsular-specific antibodies were quantified using a modified version of a previously described protocol (3). Plates (96-well; Nunc) were coated with 1 μg/well of type II or type III capsular polysaccharide in carbonate buffer, pH 9.5, overnight at 4°C. Plates were washed with 0.05% Tween 20-PBS and blocked for 6 h with 1% bovine serum albumin in PBS. Next, plates were washed and sera of mice, collected at day 10 after infection with D39ChiplicB31, were analyzed. Samples were plated out in duplicate serial threefold dilutions, starting at a 1:6 dilution, and incubated overnight at 4°C. The plates were washed, and goat anti-mouse immunoglobulin M (IgM) antibodies conjugated to horseradish peroxidase (Bethyl) were plated at 0.2 μg/ml and incubated for 4 h at room temperature. The plates were washed and developed with 3,3′,5,5′-tetramethylbenzidine (KPL, Gaithersburg, MD) per the manufacturer's instructions, and the absorbance was read at 405 nm.
RESULTS
Replication of the avirulent choline-free strain in the bloodstream of infected mice.
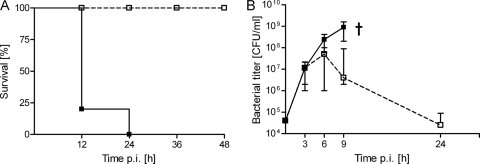
D39Cho− and D39Cho−licA64 were previously tested in a mouse sepsis model, and a drastic reduction of virulence was observed during infection with the choline-free double mutant (11). Figure 1 demonstrates a repeat of this experiment: 8-week-old female CD1 mice were infected i.p. with 106 CFU of either D39Cho− or D39Cho−licA64. Infection with the highly virulent D39Cho− was fatal, while D39Cho−licA64 was virtually avirulent (Fig. 1A). In each of the three experiments two mice at each time point were sacrificed to determine bacterial load in the blood (Fig. 1B). Samples taken by heart puncture about 5 min after the intraperitoneal inoculation showed comparable viable titers (about 104 CFU/ml blood) for both bacterial strains. By the third hour after infection the viable titer of both D39Cho− and D39Cho−licA64 had increased to about 107 CFU/ml blood, indicating that both the choline-containing (virulent) and the choline-free (avirulent) strains were able to multiply in the blood during the first 6 h after i.p. inoculation. After the 6-h time point the viable titer of the choline-containing strain D39Cho− continued to increase steadily, reaching a titer of close to 109 CFU/ml at the ninth hour postinfection. In contrast, the titer of the choline-free double mutant began to decline after the sixth hour and the bacteria were eventually cleared from the bloodstream.
FIG. 1.
Survival of mice and bacterial titers in blood. (A) Survival of animals in the course of infection with either 106 CFU of D39Cho− (solid lines and solid squares) or 106 CFU of D39Cho−licA64 (dashed lines and open squares) (11). (B) Bacterial titers in blood. Mice were infected i.p. with 106 CFU of bacteria. D39Cho− (solid lines and solid squares) was able to multiply while D39Cho−licA64 (dashed lines and open squares) was cleared from the blood. The median plus range of the bacterial growth of three independent experiments is displayed. †, death of animals.
Transient expression of cytokines in serum.
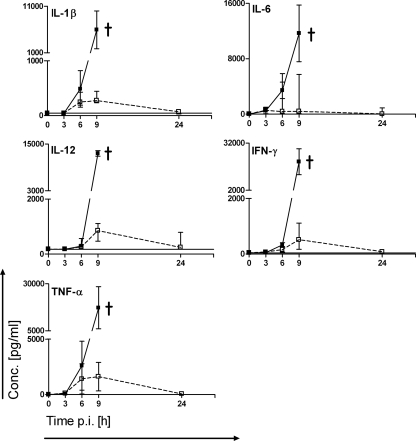
There was a potent and quantitatively comparable production of the proinflammatory cytokines IL-1β, IL-6, IL-12(p70), tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ) stimulated by both choline-free and choline-containing strains during the initial 6-h time period after inoculation (Fig. 2). Although the high range in the expression of some cytokines (e.g., IL-6) suggests that some mice might be more affected by the infection with D39Cho−licA64 than others, none of the animals showed obvious phenotypical symptoms of pneumococcal disease. However, after the 6-h time point, cytokine levels induced by the two strains began to differ: there was a continued production of proinflammatory cytokines in parallel with the increase in the titer of the choline-containing D39Cho− cells in the blood. In contrast and consistent with their clearance from the blood, the choline-free double mutant D39Cho−licA64 did not induce further expression of cytokines between 6 and 24 h after infection. Figure 2 shows the time course of cytokine production from three independent experiments. The cytokines granulocyte-macrophage colony-stimulating factor, IL-2, IL-4, IL-5, and IL-10 were also tested in the assay, but their titers were below detection limits and are not shown in the figure.
FIG. 2.
Time course of cytokine expression in serum. In each experiment mice (two animals for each time point) were infected i.p. with 106 CFU of D39Cho− (solid lines and solid squares) or D39Cho−licA64 (dashed lines and open squares). The production of IL-1β, IL-6, IL-12(p70), TNF-α, and IFN-γ was monitored over time. The median plus range from three independent experiments is shown. †, death of animals. The solid line indicates cytokine expression in naive serum. p.i., postinfection.
Role of TLR-2 in survival of infected mice.
Several reports have considered the involvement of TLR-2 in the host immune response to invasion by gram-positive pathogens, specifically through the recognition of structural features of the lipoteichoic acid component of the bacteria (15, 16, 20). Since choline is a structural component of the pneumococcal cell wall and cell membrane teichoic acids, we tested the possible role of the TLR-2 receptor in the pathophysiology of infection by the choline-containing strain.
Two types of experiments were performed. The genetic background of the TLR-2 mouse mutant is C57BL/6, which is different from the genetic background of the CD1 mouse in which all the previous virulence studies had been performed. Therefore, prior to using the TLR-2 mouse mutant we first tested the virulence properties of the two S. pneumoniae strains—the choline-containing strain D39Cho− and the choline-free D39Cho−licA64 strain—in the background of C57BL/6, used as the control mice.
Infection of the C57BL/6 mice with various concentrations (n = 5 animals per bacterial concentration) of either the choline-containing or the choline-free strain reproduced the same findings previously documented with the CD1 mouse: D39Cho− was highly virulent while D39Cho−licA64 was virtually avirulent in the C57BL/6 mice.
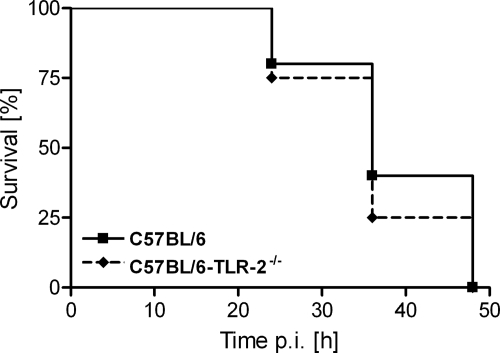
In the second experiment we proceeded to test the possible contributions of TLR-2 to the lethality of infection by the choline-containing strain D39Cho−. Groups of the control mice C57BL/6 and the Toll-like receptor mutant C57BL/6-TLR-2−/− were infected i.p. with the choline-containing D39Cho− (five mice per group), and mouse survival was monitored over time. There was no significant difference in the susceptibilities of the two mouse strains: both showed similar high susceptibility to infection, resulting in the death of all animals within 48 h. Figure 3 shows survival curves of the tested mouse strains after i.p. infection with 105 CFU D39Cho−.
FIG. 3.
Effect of TLR-2 on survival of mice. C57BL/6 and C57BL/6 TLR-2−/− mice (five animals per group) were infected with 105 CFU of D39Cho− and survival was monitored over time.
Maturation of murine splenic dendritic cells.
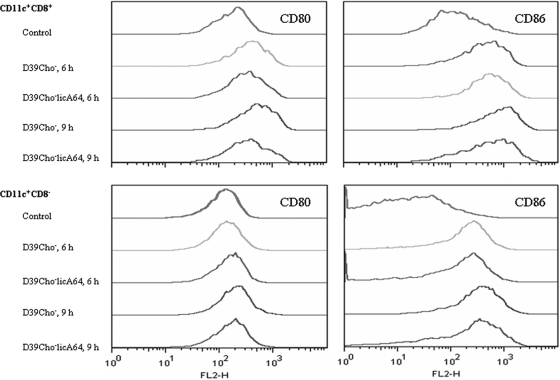
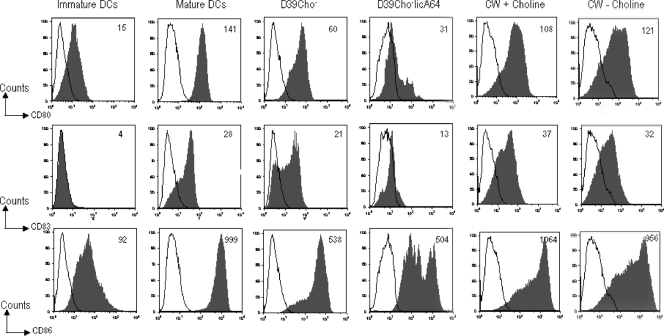
Spleen-derived dendritic cells from mice infected with either D39Cho− or D39Cho−licA64 were compared to dendritic cells isolated from healthy control mice. Both bacterial strains caused comparable expression patterns of costimulatory molecules on dendritic cells (Fig. 4). CD11c+ CD8+ dendritic cells showed upregulation of both of the tested costimulatory molecules at 6 h and 9 h after infection. On CD11c+ CD8− dendritic cells only CD86 was induced at these time points. The results indicate that both the choline-containing and choline-free bacterial strains were able to stimulate dendritic cell maturation to a comparable degree.
FIG. 4.
Maturation of murine splenic dendritic cells in vivo. CD11c+ CD8+ and CD11c+ CD8− dendritic cells, isolated from the spleen, were analyzed for the presence of CD80 and CD86. Dendritic cells from a control mouse and from mice infected i.p. with 106 CFU of either D39Cho− or D39Cho−licA64 were compared at 6 h and 9 h after infection.
In vitro maturation of human monocyte-derived dendritic cells.
When human monocyte-derived dendritic cells (106) were incubated with 108 cell equivalents of mitomycin C-killed cells of either the choline-containing or the choline-free strain, upregulation of the costimulatory molecules was detected when compared to controls of immature and/or fully matured LPS-treated dendritic cells. Evidence for the maturation of human dendritic cells was shown by the expression of CD80, CD83, and CD86. However, the choline-containing bacterium was a slightly stronger inducer (Fig. 5).
FIG. 5.
Maturation of human monocyte-derived dendritic cells in vitro. Immature DCs were cultured for 36 h with either 108 CFU equivalents of mitomycin C-killed D39Cho− or D39Cho−licA64 or 107 CFU equivalents of purified cell walls with or without choline (CW + or − choline) or left untreated. LPS-matured (LPS-treated) DCs were used as a positive control. Cells were harvested and stained for cell surface expression of CD80, CD83, and CD86. Data shown are gated for the live population. The numbers represent the mean fluorescence intensities. Open histograms represent isotype controls.
Most interestingly, cell walls prepared from either the choline-containing or the choline-free bacteria used at concentrations representing 107 cell equivalents were able to induce comparable expression of the same dendritic cell markers (Fig. 5).
Induction of protective immunity in mice surviving infection with avirulent choline-free pneumococci.
Two groups of mice (five animals per group) were inoculated i.p. either with 103 CFU of the choline-free (avirulent) strain D39Cho−licA64 or with 0.5 ml saline in the control group. On day 10 postinoculation all the surviving mice were challenged i.p. with 104 CFU of the choline-containing (highly virulent) strain D39Cho−. All five animals from the saline control group died within 48 h after challenge, whereas four of the five animals inoculated with the choline-free (avirulent) strain survived for at least 25 days of the follow-up period without showing any signs of disease.
In a follow-up experiment another choline-free and avirulent S. pneumoniae strain, D39ChiplicB31 (10), was used instead of D39Cho−licA64 to test the protective effect. A group of 16 mice, each inoculated with 104 CFU of D39ChiplicB31, was distributed into four groups (four animals per group) and challenged on day 10 after inoculation by either one of two highly virulent S. pneumoniae strains: strain D39 (capsular type II) or strain SV36 (capsular type III). The two challenge strains were introduced at either 104 or 106 CFU per mouse, using four animals for each inoculum concentration. All mice challenged with the capsular type III strain SV36 died within 48 h after inoculation. In contrast, only one of the four mice challenged with the capsular type II strain D39 at 104 CFU per animal died, and all four animals challenged with strain D39 at 106 CFU survived.
The seven mice surviving the D39 challenge were next inoculated, on day 25 post-“immunization,” with the capsular type III strain SV36 at 104 CFU per animal. All seven mice died within 2 days after the challenge (Table 1).
TABLE 1.
Vaccination with the choline-free strain D39ChiplicB31 induces serotype-specific protection
| No. of mice vaccinated with 104 CFU of D39ChiplicB31 (day 1) | Day 10
|
Day 25
|
||
|---|---|---|---|---|
| Challenge with: | Survival | Challenge with: | Survival | |
| 4 | D39, 104 CFU | 3/4 | SV36, 104 CFU | 0/3 |
| 4 | D39, 106 CFU | 4/4 | SV36, 104 CFU | 0/4 |
| 4 | SV36, 104 CFU | 0/4 | ||
| 4 | SV36, 106 CFU | 0/4 | ||
Production of capsule-specific antibodies.
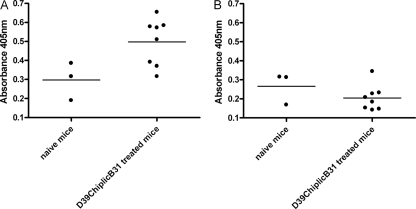
Eight mice were infected with 104 CFU of D39ChiplicB31. This strain expresses the capsular polysaccharide II on its surface. On day 10 postvaccination sera were collected and the presence of capsule-specific antibodies was determined by ELISA. Also, control sera from three naive mice were taken. Compared to the control sera, we found a significant increase (P < 0.05, Student's t test) in IgM antibody production specific to purified capsular polysaccharide II in mice that were previously immunized with strain D39ChiplicB31. In the same sera we did not detect any antibodies specific to capsular polysaccharide III (Fig. 6).
FIG. 6.
Detection of capsule-specific IgM antibodies with ELISA. Mice were vaccinated with capsule II strain D39ChiplicB31 and sera were collected at day 10 postinfection. Sera of eight mice that received the vaccination were compared to control sera of three naive mice for the production of IgM antibodies specific to pneumococcal capsular polysaccharide II (A) or pneumococcal capsular polysaccharide III (B). The scatter plots show results for individual animals and the means of the whole test group as well. We found significant (P < 0.05) production of antibodies that specifically recognized polysaccharide capsule II.
DISCUSSION
In a previous report we showed that the high degree of invasiveness of S. pneumoniae strain D39Cho− expressing the capsular polysaccharide type II could be radically reduced by genetic manipulations that resulted in the removal of choline residues from the pneumococcal surface (11). The product of such a genetic manipulation, strain D39Cho−licA64, continued to express the capsular polysaccharide II on its surface but is virtually avirulent in the murine model of pneumococcal infection.
In the studies presented here we explored several alternative mechanisms to explain the profound impact of teichoic acid choline residues on pneumococcal virulence.
During the first 6 hours after infection of the mice, the viable titers of both the choline-containing strain D39Cho− and the choline-free double mutant D39Cho−licA64 increased substantially, accompanied by expression of the proinflammatory cytokines IL-1β, IL-6, IL-12(p70), TNF-α, and IFN-γ. In contrast to the infection with choline-free bacteria, in which, past the 6-h time point, declining bacterial blood titers were paralleled by decreasing cytokine levels, the choline-containing pneumococci continued to multiply, accompanied by steadily increasing levels of proinflammatory cytokines.
These observations suggest that pneumococci have to reach a certain blood titer threshold (∼105 to 106 CFU/ml) to induce inflammation in the host. Once bacterial titers drop below this threshold due to the onset of a successful immune clearance mechanism, the inflammation ends. This hypothesis is also in line with several previous studies that claimed threshold bioactivities of various pneumococcal cell wall preparations in animal models (12).
We considered the possibility that the choline residues are directly recognized by TLR-2. A recent publication suggested that pneumococcal lipoteichoic acid is detected by Toll-like receptor 2 in vitro, resulting in the expression of the cytokines IL-1, IL-8, IL-10, TNF-α, and IFN-γ (6). This pathway could contribute to the observed overwhelming immune response induced by choline-containing bacteria as well as to their lethality. In turn, the lack of covalently attached phosphorylcholine residues may weaken the overall binding of lipoteichoic acid to TLR-2 and impair the virulence of the bacteria. If these assumptions were true, TLR-2 knockout mice would be less susceptible to choline-containing bacteria. We tested this hypothesis by infecting TLR-2 knockout mice with D39Cho− and found that the mortality was identical to what we observed in the isogenic wild-type mice, indicating that the pathway via TLR-2 has no influence on the recognition of the choline moieties and the choline-dependent virulence of the strain tested.
We compared the maturation status of dendritic cells in CD1 mice that received the choline-free versus choline-containing bacteria. Spleens were removed from mice and costimulatory molecules on the surface of isolated splenic dendritic cells were determined as indicators of maturation. Remarkably, both the choline-free as well as choline-containing strains equally engaged dendritic cells, despite the different intensities of the induced cytokine patterns. Lymphoid CD11c+ CD8+ as well as myeloid CD11c+ CD8− DC subsets showed equal upregulation of costimulatory molecules CD80 and CD86. Although we cannot predict yet whether the initiated immune response shows a cellular or humoral profile, these results demonstrate that choline-free S. pneumoniae bacteria possess potent adjuvant properties.
We also tested the ability of the bacterial preparations to stimulate maturation of human monocyte-derived dendritic cells in vitro. Human dendritic cells were incubated with mitomycin C-killed suspensions of strains D39Cho−licA64 and D39Cho−. Both strains were able to trigger upregulation of costimulatory molecules, although the choline-containing strain D39Cho− led to a stronger activation. To narrow down the immunogenic epitope on the bacterial surface, purified cell walls differing in the presence or absence of choline were also tested in the same assay and were found to have equal potencies to induce maturation of dendritic cells. Similarly, the same pair of cell wall preparations showed identical inflammatory potentials when applied intracisternally into the cerebrospinal fluid space of infant rats (8).
These experiments suggest that the choline residues may not be directly recognized by the immune system, nor do they represent the immediate trigger of the immune response, since choline-free bacteria were still able to stimulate cytokine production and DC maturation in vivo and the cell wall preparations from both choline-containing and choline-free bacteria could activate DCs in vitro.
To understand whether the observed immunogenicity of choline-free bacteria was also sufficient to induce protective immunity, mice were immunized with a single dose of D39Cho−licA64. After 10 days the same animals were reinfected with the isogenic virulent strain D39Cho− and they showed a surprisingly strong protection. However, it was not clear from this experiment whether the induced protection was serotype specific or was possibly related to the large heterologous S. oralis elements that have been shown to be present in strain D39Cho− (10).
To address these questions we repeated the “protection” experiment using the recently isolated and genetically well-defined choline-independent mutant D39ChiplicB31, which only differs from the wild-type D39 (besides the mutation in the licB gene) by a single point mutation (4) and is therefore free of any possibly interfering, heterologous genetic elements. Table 1 shows that animals surviving infection by the avirulent double mutant D39ChiplicB31 were protected against challenge by the highly virulent, isogenic wild-type strain D39, but the same animals were still susceptible to challenge by the capsular type III strain SV36. These findings make it unlikely that the protection effect originally observed was related to the strain or to the large heterologous DNA sequences carried in D39Cho−licA64, the strain used in the original protection experiment. Instead, the results suggest that infection by the avirulent pneumococci was able to generate a capsule-specific protective immunity in the mice. Consistently, mice vaccinated with D39ChiplicB31 developed capsule II-specific IgM antibodies within 10 days. No capsule III-specific antibodies were detected in the very same sera.
These observations allow two major conclusions. (i) Despite its drastically reduced virulence, the choline-free double mutant expressing the capsular polysaccharide II was still able to engage the host immune system, as evidenced by the expression of proinflammatory cytokines, maturation of dendritic cells, and the capacity to induce a substantial degree of serotype specific protective immunity in the surviving animals. The chemical entity responsible for triggering the observed immune response appears to be a component common to both choline-containing and choline-free cell walls, most likely a component of the peptidoglycan or teichoic acid backbone.
(i) The mechanism of the strikingly different virulence of choline-containing and choline-free bacteria is not well understood. The choline-containing bacteria have a superior capacity to continue proliferation and reach higher titers in the bloodstream, thus providing a continuous stimulus for cytokine production and keeping the immune system in a highly activated state for a prolonged time. In sharp contrast, the clearance of the choline-free bacteria from the bloodstream and their decreasing titers, beyond the 6-h time point after infection, limit the pathological inflammation.
Our observations strongly suggest that the critical contribution of choline residues to the virulence potential of pneumococci may be the role that these amino alcohol residues play in a pneumococcal immune evasion strategy. Whether this mechanism is directly linked to the choline moieties or is mediated through the choline-binding proteins attached to the choline residues on the pneumococcal surface is unknown at the present time.
Acknowledgments
Partial support for these studies was provided by grants from the American Austrian Foundation and the Irene Diamond Foundation to the laboratory of Alexander Tomasz.
Editor: A. Camilli
Footnotes
Published ahead of print on 11 May 2009.
REFERENCES
- 1.Briles, E. B., and A. Tomasz. 1973. Pneumococcal Forssman antigen. A choline-containing lipoteichoic acid. J. Biol. Chem. 2486394-6397. [PubMed] [Google Scholar]
- 2.Brundish, D. E., and J. Baddiley. 1968. Pneumococcal C-substance, a ribitol teichoic acid containing choline phosphate. Biochem. J. 110573-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colino, J., Y. Shen, and C. M. Snapper. 2002. Dendritic cells pulsed with intact Streptococcus pneumoniae elicit both protein- and polysaccharide-specific immunoglobulin isotype responses in vivo through distinct mechanisms. J. Exp. Med. 1951-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damjanovic, M., A. S. Kharat, A. Eberhardt, A. Tomasz, and W. Vollmer. 2007. The essential tacF gene is responsible for the choline-dependent growth phenotype of Streptococcus pneumoniae. J. Bacteriol. 1897105-7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhodapkar, K. M., J. L. Kaufman, M. Ehlers, D. K. Banerjee, E. Bonvini, S. Koenig, R. M. Steinman, J. V. Ravetch, and M. V. Dhodapkar. 2005. Selective blockade of inhibitory Fcγ receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc. Natl. Acad. Sci. USA 1022910-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Draing, C., M. Pfitzenmaier, S. Zummo, G. Mancuso, A. Geyer, T. Hartung, and S. von Aulock. 2006. Comparison of lipoteichoic acid from different serotypes of Streptococcus pneumoniae. J. Biol. Chem. 28133849-33859. [DOI] [PubMed] [Google Scholar]
- 7.Fischer, W., T. Behr, R. Hartmann, J. Peter-Katalinic, and H. Egge. 1993. Teichoic acid and lipoteichoic acid of Streptococcus pneumoniae possess identical chain structures. A reinvestigation of teichoid acid (C polysaccharide). Eur. J. Biochem. 215851-857. [DOI] [PubMed] [Google Scholar]
- 8.Gehre, F., S. L. Leib, D. Grandgirard, J. Kummer, A. Buhlmann, F. Simon, R. Gaumann, A. S. Kharat, M. G. Tauber, and A. Tomasz. 2007. Essential role of choline for pneumococcal virlence in an experimental model of meningitis. J. Intern. Med. 264143-154. [DOI] [PubMed] [Google Scholar]
- 9.Horne, D. S., and A. Tomasz. 1993. Possible role of a choline-containing teichoic acid in the maintenance of normal cell shape and physiology in Streptococcus oralis. J. Bacteriol. 1751717-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kharat, A. S., D. Denapaite, F. Gehre, R. Bruckner, W. Vollmer, R. Hakenbeck, and A. Tomasz. 2008. Different pathways of choline metabolism in two choline-independent strains of Streptococcus pneumoniae and their impact on virulence. J. Bacteriol. 1905907-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kharat, A. S., and A. Tomasz. 2006. Drastic reduction in the virulence of Streptococcus pneumoniae expressing type 2 capsular polysaccharide but lacking choline residues in the cell wall. Mol. Microbiol. 6093-107. [DOI] [PubMed] [Google Scholar]
- 12.Majcherczyk, P. A., and P. Moreillon. 2004. Inflammation and host defense, p. 183-200. In E. Tuomanen (ed.), The pneumococcus. ASM Press, Washington DC.
- 13.Nesin, M., M. Ramirez, and A. Tomasz. 1998. Capsular transformation of a multidrug-resistant Streptococcus pneumoniae in vivo. J. Infect. Dis. 177707-713. [DOI] [PubMed] [Google Scholar]
- 14.Rane, L., and Y. Subbarow. 1940. Nutritional requirements of the pneumococcus. I. Growth factors for types I, II, V, VII, VIII. J. Bacteriol. 40695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 27815587-15594. [DOI] [PubMed] [Google Scholar]
- 16.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 27417406-17409. [DOI] [PubMed] [Google Scholar]
- 17.Severin, A., D. Horne, and A. Tomasz. 1997. Autolysis and cell wall degradation in a choline-independent strain of Streptococcus pneumoniae. Microb. Drug Resist. 3391-400. [DOI] [PubMed] [Google Scholar]
- 18.Tomasz, A. 1967. Choline in the cell wall of a bacterium: novel type of polymer-linked choline in Pneumococcus. Science 157694-697. [DOI] [PubMed] [Google Scholar]
- 19.Tomasz, A. 1964. Progr. 64th Gen. Meet. Am. Soc. Microbiol., abstr. G87. American Society for Microbiology, Washington, DC.
- 20.Weber, J. R., P. Moreillon, and E. I. Tuomanen. 2003. Innate sensors for gram-positive bacteria. Curr. Opin. Immunol. 15408-415. [DOI] [PubMed] [Google Scholar]