Abstract
Leishmania mexicana cysteine peptidases (CPs) have been identified as important parasite virulence factors. More recently, a natural inhibitor of CPs (ICP) from L. mexicana has been characterized, and ICP mutants have been created. Infection of BALB/c mice with ICP null mutants or ICP reexpressing mutants resulted in nonhealing, progressively growing lesions albeit slightly attenuated compared with the growth of lesions produced by wild-type parasites. In contrast, BALB/c mice infected with mutants overexpressing ICP were able to significantly control lesion growth or heal. While BALB/c mice infected with wild-type parasites, ICP null mutants, or ICP reexpressing mutants produced significant antibody responses, including immunoglobulin E (IgE), no Th1 response, as indicated by antigen-induced splenocyte gamma interferon (IFN-γ) production, could be demonstrated. In contrast, BALB/c mice infected with mutants overexpressing ICP produced significantly less antibody, particularly IgE, as well as significantly reduced splenocyte interleukin-4 and enhanced IFN-γ production. BALB/c mice were able to resolve infection following infection with one ICP overexpressing clone, which was subsequently used for vaccination studies with BALB/c mice. However, no protection was afforded these mice when they were challenged with wild-type parasites. Nevertheless, two other mouse strains susceptible to L. mexicana, C3H and C57BL/6, vaccinated with overexpressing ICP mutants were able to control challenge infection associated with an enhanced Th1 response. This study confirms that L. mexicana CPs are virulence factors and that ICPs have therapeutic potential.
Leishmaniasis encompasses a large spectrum of clinical diseases which, depending upon the parasite species and the host's immune response, can have various outcomes (16). Our understanding of the factors that lead to this diversity of clinical symptoms has, in large part, come from studies using murine models and particularly Leishmania major. While the majority of mouse strains can control infection with L. major, virtually all develop nonhealing lesions full of parasites when infected cutaneously with L. mexicana (4). Studies with the BALB/c mouse have indicated that the nonhealing response of this mouse strain to infection with L. major is LACK antigen dependent (13), while the nonhealing response to L. mexicana is independent of this antigen (27). Conversely, our studies have implicated cysteine peptidases (CPs) as major virulence factors for L. mexicana (reviewed in reference 17). Information about the apparent functions and importance of CPs in the host-parasite interaction was obtained via the generation of mutants deficient in the CPA and CPB genes (Δcpa and Δcpb mutants). L. mexicana mutants deficient in the multicopy CPB gene array (Δcpb mutants) have reduced virulence, with poor lesion growth, for BALB/c mice (2, 18). Only the reexpression of multiple CPB genes from a cosmid significantly restored virulence (8), suggesting that the multiple genes have complementary functions. CPA CPB double null mutant parasites (Δcpa Δcpb mutants) were less infective for BALB/c mice than Δcpb mutants (2, 18), which not only implicates CPA as a virulence factor but also indicates that there is some redundancy in function between CPA and CPB. CPA, together with CPB1 and CPB2 (the CPB isoforms encoded by the first 2 genes of the 19-gene CPB tandem array), is expressed in metacyclic promastigotes, suggesting roles for these CPs in the virulence of this life cycle stage. Indeed, the L. mexicana Δcpa Δcpb mutants are defective in metacyclogenesis, due to an impairment of autophagy-dependent protein turnover required for differentiation (28).
Small-molecule inhibitors of CPB have some efficacy against Leishmania, both in vitro and in vivo (24). These inhibitors are thought to be active against not only CPB but also CPA and CPC, and their toxicity to Leishmania may be a consequence of the inhibition of multiple enzymes. Nevertheless, these results confirm the importance of CPs in the host-parasite interaction. Treatment with a natural CP inhibitor, cystatin, promoted a protective response against Leishmania infection and a switch from a predominately Th2 to a Th1 response (7). Leishmania lacks cystatins, but the parasite possesses an unusual inhibitor of CPs (designated ICP), which does not occur in mammals (22). Leishmania ICP has been shown to be a potent inhibitor of clan CA, family C1 CPs, such as CPB and mammalian cathepsin L (22), and the structure has recently been solved by nuclear magnetic resonance (25). Analysis of L. mexicana mutants lacking ICP implicated the inhibitor in the host-parasite interaction (3), but whether ICP plays an influential immune modulatory role awaited examination. The present study used ICP null mutants (Δicp mutants), ICP reexpressing parasites in which ICP had been expressed in the null mutant (Δicp::PrRNAICP), and null mutant parasites overexpressing ICP (Δicp[pXG ICP]) to determine the influence of ICP on the immune response and developing disease phenotype. Significantly, while ICP-deficient parasites remained comparatively virulent, ICP mutants overexpressing ICP did not promote significant lesion growth in BALB/c mice and developed a Th1 response. The vaccine potential of the mutant parasites was subsequently examined.
MATERIALS AND METHODS
Generation of L. mexicana Δicp null mutant promastigotes reexpressing and overexpressing ICP.
All ICP transgenic cell lines were generated as described by Besteiro, Coombs, and Mottram (3). They include two independent ICP null mutants (Δicp1 and Δicp3 mutants), where the two alleles coding for the gene were replaced by selection markers using homologous recombination. Clones of the mutants were subsequently used to produce reexpressing cell lines. One had an integrated single-gene copy at the rRNA locus conferring expression in both amastigote and promastigote stages of the parasite (i.e., Δicp::PrRNAICP) and providing a level of expression of ICP similar to that of wild-type parasites. The second had extrachromosomal expression of ICP, via the pXG multicopy expression plasmid, to yield ICP overexpressing cell lines (Δicp[pXG ICP]).
Culture of mutant parasites.
L. mexicana (MNYC/BZ/M379) promastigotes were cultured at 25°C in modified Eagle's medium (Invitrogen, Paisley, United Kingdom) with 10% (vol/vol) heat-inactivated fetal calf serum (Harlan Sera-Lab, United Kingdom). The appropriate antibiotics were then added to the culture to select the mutant parasites as previously described (3).
Western blotting.
Western blotting was carried out as previously described (3). Affinity-purified antiserum specific for L. mexicana ICP was prepared as previously described (22). A 1:1,000 dilution of the affinity-purified anti-ICP antibody and a 1:10,000 dilution of mouse monoclonal anti-Trypanosoma brucei elongation factor 1α (Upstate/Millipore) were applied, followed by a 1:5,000 dilution of secondary antibody conjugated to horseradish peroxidase.
Mice.
BALB/c mice were obtained from Harlan UK Limited (Oxon, United Kingdom) and were bred in-house under barrier conditions. C3H and C57BL/6 mice were purchased from Harlan UK Limited (Oxon, United Kingdom) and maintained in a specific-pathogen-free environment. Animal experiments were performed in accordance with United Kingdom Home Office guidelines.
L. mexicana infections.
Groups of at least five 6- to 8-week-old female mice were inoculated subcutaneously with 2 × 105 to 5 × 105 stationary-phase promastigotes grown in modified Eagle's medium into the hind footpad. The lesion size was monitored weekly using a sliding-gauge micrometer. Lesions were excised from L. mexicana-infected mice at the termination of experiments and disrupted through a metal mesh with 5 ml of RPMI 1640 (Cambrex Bio Science Verviers, Belgium). The parasites were washed twice at 350 × g in RPMI and enumerated using an improved Neubauer hemocytometer as previously described (3).
Vaccination studies.
Groups of at least five 6- to 8-week-old female BALB/c, C3H, and C57BL/6 mice were vaccinated subcutaneously with 2 × 105 to 5 × 105 Δicp1[pXG ICP] or Δicp3[pXG ICP] parasites into the left hind footpad in RPMI 1640. At 14 weeks postvaccination, mice were challenged with 2 × 105 to 5 × 105 wild-type L. mexicana metacyclic promastigotes in RPMI 1640 subcutaneously into the right hind footpad. The lesion size was monitored weekly using a sliding-gauge micrometer.
Splenocyte stimulation and cytokine detection.
Splenocytes were isolated from infected mice and cultured for 72 h in 96-well plates in the presence or absence of L. mexicana antigenic lysate or 0.5 μg/ml anti-CD3 (C363.29B; Southern Biotechnology Associates Inc., AL) as previously described (1, 23). Gamma interferon (IFN-γ) levels in the supernatants were detected by capture enzyme-linked immunosorbent assay (ELISA). Briefly, the wells of Immulon 1B flat-bottomed microtiter plates (ThermoLabsystems, MA) were coated with 50 μl of 1 μg/ml purified anti-mouse IFN-γ capture antibody R4-6A2 (PharMingen; supplied by Insight Biotechnology, Wembley, United Kingdom) in phosphate-buffered saline (PBS) (pH 9.0) or 500 ng/ml interleukin-4 (IL-4) capture antibody 11B11 (PharMingen) overnight at 4°C. Supernatants were then added to the individual wells, and either 30 μl recombinant mouse IFN-γ (R&D Systems Europe, Abingdon, United Kingdom) or recombinant mouse IL-4 (Genzyme, Cambridge, United Kingdom) was added to individual wells in duplicate in a doubling dilution with a solution of pH 7.4 PBS supplemented with 10% (vol/vol) fetal calf serum (Harlan Sera-Lab, Crawley, United Kingdom), ranging from 20 ng ml−1 to 0.01 ng ml−1 (IFN-γ) or 2 ng ml−1 to 0.977 pg ml−1 (IL-4). The plates were then incubated for 2 h at 37°C. The bound cytokines were incubated with either biotin rat anti-mouse IFN-γ monoclonal antibody XMG1.2 (PharMingen) or biotin rat anti-mouse IL-4 antibody BVD6-24G2 (PharMingen) and detected with either conjugated streptavidin-alkaline phosphatase or conjugated streptavidin-horseradish peroxidase (PharMingen), respectively. The respective substrate, p-nitrophenyl-phosphate (Sigma-Aldrich, Poole, United Kingdom) or tetramethylbenzidine in pH 5.5 sodium acetate buffer containing 0.0003% hydrogen peroxide (BDH, Poole), was then added to the wells. Finally, the plates were read at an absorbance of 405 nm for IFN-γ or at 450 nm for IL-4.
Detection of L. mexicana-specific IgG1, IgG2a, and total IgE.
L. mexicana-specific immunoglobulin G1 (IgG1) and IgG2a in the plasma of infected mice were detected by ELISA as previously described (1, 23). Briefly, Immulon 1B flat-bottomed microtiter plates were coated with 100 μl 10 μg/ml L. mexicana lysate (lysate was prepared as previously described [23]) in PBS (pH 9.0) overnight at 4°C. Plasma samples were serially diluted in duplicate, followed by a 1-h incubation at 37°C. Bound Leishmania-specific antibodies were detected with a 1-h incubation with horseradish peroxidase-conjugated goat anti-mouse IgG1 (Southern Biotechnology Associates Inc., AL) or horseradish peroxidase-conjugated goat anti-mouse IgG2a (Southern Biotechnology Associates Inc., AL). The substrate tetramethylbenzidine in pH 5.5 sodium acetate buffer containing 0.0003% hydrogen peroxide (BDH, Poole) was then added to the wells, and following color development, the reaction of the substrate with the conjugated horseradish peroxidase was stopped by the addition of 10% sulfuric acid to the wells. The absorbance was then measured at 450 nm using a plate reader with SOFTmax PRO software (Molecular Devices), and the endpoint dilution was determined. Total IgE in the plasma of infected mice was detected by capture ELISA as previously described (23), using R35-72 capture IgE monoclonal antibody (PharMingen) and biotinylated rat anti-mouse IgE (Southern Biotechnology Associates). Antibody titers were compared using the Mann-Whitney U test, and all other comparisons were made using the Student t test.
IFN-γ depletion and parasite enumeration.
In vivo depletion of IFN-γ was achieved by the intraperitoneal inoculation of 150 μg rat hybridoma R4-6A2 (PharmMingen) on day 0 and on days 4 and 8 postinfection of mice with 5 × 106 L. mexicana amastigotes into the footpad. Three animals comprised each experimental group, and four samplings were taken from each footpad. Parasite numbers were quantified by limiting dilution as previously described (6).
RESULTS
Western blot analysis of ICP mutant L. mexicana stationary-phase promastigotes.
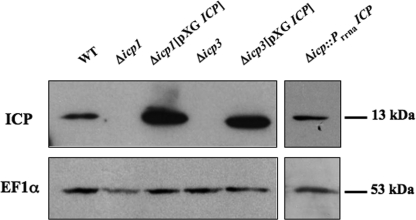
ICP null mutants were produced as described previously (3), and two independent clones (Δicp1 and Δicp3 mutants) were isolated. The two clones were used for producing ICP overexpressing cell lines (Δicp1[pXG ICP] and Δicp3[pXG ICP]), whereas clone 1 only was used to produce a cell line reexpressing the protein from a single ICP copy integrated in its genome (Δicp::PrRNAICP). The expression of ICP in stationary-phase promastigotes from wild-type and transgenic L. mexicana cell lines was assessed by Western blot analysis (Fig. 1). This confirmed that no ICP was present in Δicp1 and Δicp3 promastigotes. Expression of ICP in the Δicp::PrRNAICP mutant was similar to that in the wild type, whereas Δicp1[pXG ICP] and Δicp3[pXG ICP] had a significantly higher level of ICP expression (∼5-fold increase) (Fig. 1).
FIG. 1.
Western blot analysis of whole-cell lysates prepared from wild-type (WT), Δicp1, Δicp3, Δicp1[pXG ICP], Δicp3[pXG ICP], and Δicp::PrRNAICP stationary-phase L. mexicana promastigotes by using affinity-purified antisera raised against ICP and monoclonal antibodies raised against elongation factor 1α (used as an internal loading control). The equivalent of 5 × 106 parasites was loaded per lane for sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and molecular masses of the proteins of interest are indicated on the right.
L. mexicana mutants overexpressing ICP have significantly reduced virulence for BALB/c mice.
Rapidly growing, nonhealing lesions were induced following inoculation of BALB/c mice with 5 × 105 wild-type, Δicp1, and Δicp3 stationary-phase promastigotes into the hind footpad (Fig. 2a). However, lesion growth was significantly (P < 0.05) reduced, from week 8 postinfection, following inoculation with Δicp1 and Δicp3 parasites compared with that following inoculation with wild-type parasites. Reexpression of the ICP gene with the integrative construct restored some virulence, and 10 weeks elapsed before a significant difference in lesion growth kinetics was observed between BALB/c mice infected with Δicp::PrRNAICP and those infected with wild-type parasites. Overexpression of ICP resulted in a significant change in disease phenotype: BALB/c mice inoculated with Δicp3[pXG ICP] stationary-phase promastigotes developed small lesions which ceased to grow after week 5, while those infected with Δicp1[pXG ICP] stationary-phase promastigotes apparently resolved infections by week 13. Significantly reduced parasite burdens were also found in the lesions at 13 weeks postinfection of the BALB/c mice infected with Δicp1, Δicp3, Δicp3[pXG ICP], and Δicp::PrRNAICP parasites relative to the parasite burden for those infected with wild-type parasites (Fig. 2b). No parasites could be detected nor promastigotes derived from the site of infection with Δicp1[pXG ICP] stationary-phase promastigotes. When possible, lesion-derived Δicp3[pXG ICP] parasites were differentiated back to promastigotes in vitro without selective pressure and tested for ICP expression by Western blotting. Surprisingly, and in spite of the absence of antibiotic selection within the animal (which could lead to a loss of the plasmid expressing ICP), ∼70% of the clones still expressed ICP, although at a level greatly reduced from the initial level (data not shown). When these parasites were used to infect mice, still in the absence of selection, they were able to produce lesions (data not shown), possibly due to their lowered ICP expression levels. Indeed, as discussed in our previous work (3), differences in virulence could be due to variations in ICP expression level between the cell lines. Indeed, wild-type ICP protein is downregulated in amastigotes (22), whereas the integrated reexpressing mutant Δicp::PrRNAICP has a constitutive expression throughout the stages. Hence, both cell lines have similar ICP expression levels in promastigotes, but in differentiated amastigotes the protein is likely to be slightly overexpressed in Δicp::PrRNAICP compared to the level in the wild type.
FIG. 2.
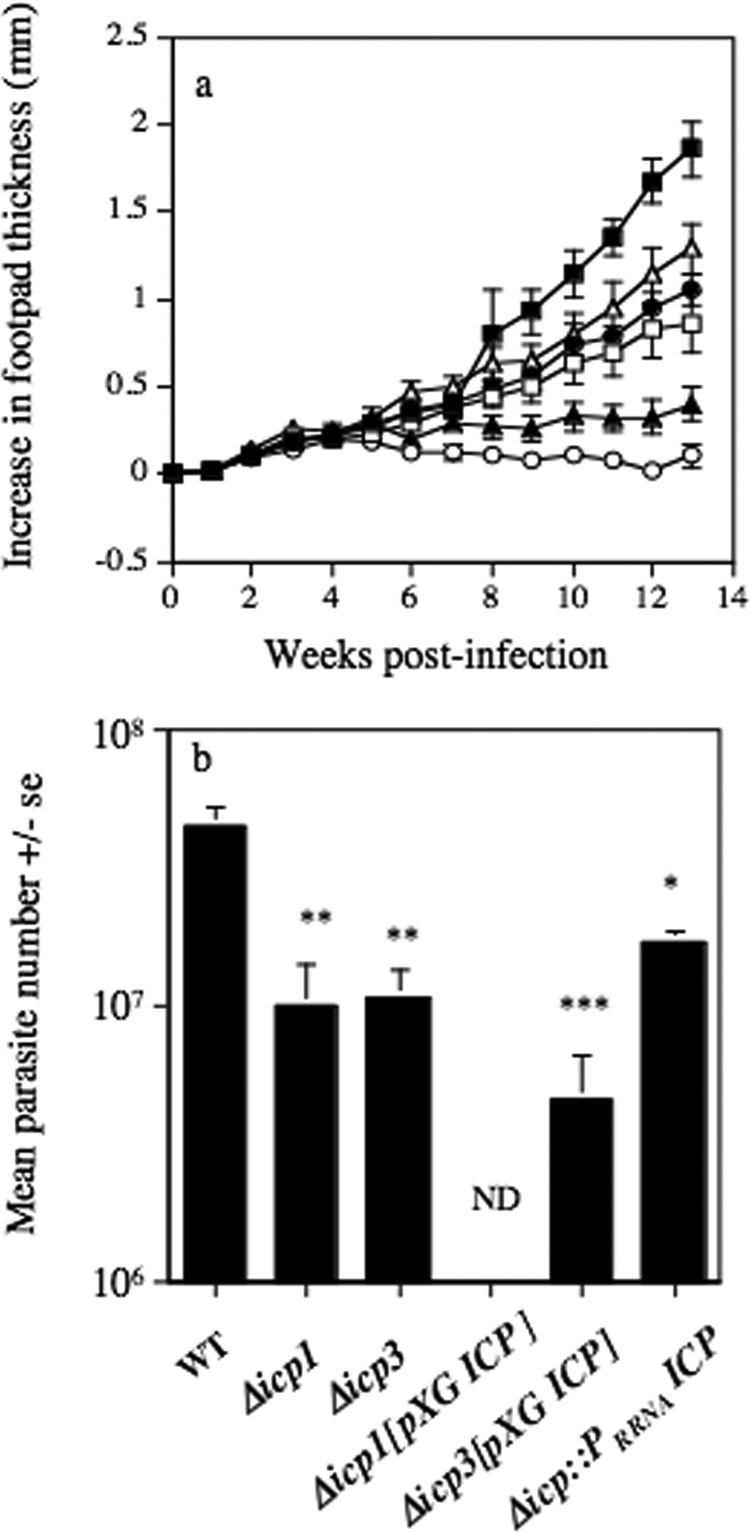
(a) Mean increases in lesion diameter for BALB/c mice following subcutaneous infection into the hind footpad with 5 × 105 wild-type (▪), Δicp1 (□), Δicp3 (•), Δicp1[pXG ICP] (○), Δicp3[pXG ICP] (▴), and Δicp::PrRNAICP (▵) L. mexicana stationary-phase promastigotes. (b) Mean numbers of parasites isolated at week 13 postinfection from BALB/c mice infected with 5 × 105 wild-type (WT), Δicp1, Δicp3, Δicp1[pXG ICP], Δicp3[pXG ICP], and Δicp::PrRNAICP L. mexicana stationary-phase promastigotes (* P < 0.05; **, P < 0.01; ***, P < 0.005). ND, not detected. Bars represent standard errors (se) of the means. Results are representative of three independent experiments.
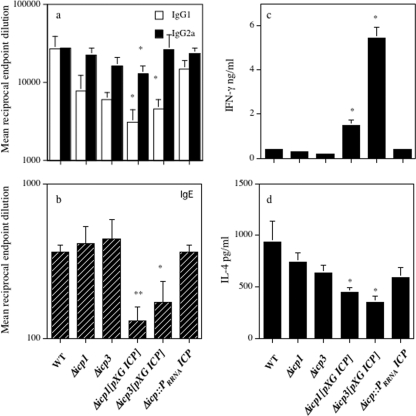
BALB/c mice infected with L. mexicana mutants overexpressing ICP develop a modified, Th1/Th2-biased response.
At the termination of the experiment at week 13, no significant differences in L. mexicana-specific IgG1 or IgG2a levels were displayed in BALB/c mice inoculated with the Δicp1 mutant, the Δicp3 mutant, or Δicp::PrRNAICP compared with the levels displayed in BALB/c mice inoculated with 5 × 105 wild-type stationary-phase promastigotes (Fig. 3a). However, infection with Δicp1[pXG ICP] and Δicp3[pXG ICP] resulted in significantly (P < 0.05) lower levels of L. mexicana-specific IgG1 than did infection with wild-type parasites. Infection with Δicp1[pXG ICP] also resulted in significantly (P < 0.05) lower levels of IgG2a than did infection with wild-type parasites (Fig. 3a). Similarly, no significant difference was noted in the total IgE levels for BALB/c mice infected with the Δicp1 mutant, the Δicp3 mutant, or Δicp::PrRNAICP compared with the levels for BALB/c mice inoculated with 5 × 105 wild-type stationary-phase promastigotes, whereas ICP overexpressing cell lines produced significantly (P < 0.05) lower levels of IgE than did wild-type parasites (Fig. 3b). In addition, IgE levels were normalized against levels for noninfected control sera, and absorbances of greater than three times the level for the control sera were deemed indicative of significant IgE production. This revealed that while five of five BALB/c mice infected with wild-type parasites or Δicp::PrRNAICP and four of five mice infected with the Δicp1 or Δicp3 mutant had elevated IgE production, only one of five of those mice infected with Δicp1[pXG ICP] or Δicp3[pXG ICP] had raised IgE levels. In vitro stimulation with L. mexicana antigenic lysate induced significantly (P < 0.05) increased levels of IFN-γ from splenocytes isolated from BALB/c mice inoculated with Δicp1[pXG ICP] or Δicp3[pXG ICP] in comparison with levels for the BALB/c mice inoculated with wild-type L. mexicana (Fig. 3c). In contrast, anti-CD3-induced IL-4 production was significantly less (P < 0.05) from splenocytes isolated from BALB/c mice inoculated with Δicp1[pXG ICP] or Δicp3[pXG ICP] than from splenocytes isolated from BALB/c mice inoculated with wild-type L. mexicana (Fig. 3d). No significant differences in IFN-γ and IL-4 production (Fig. 3c and d) were detected in the splenocytes isolated from the BALB/c mice infected with the Δicp1 mutant, the Δicp3 mutant, or Δicp::PrRNAICP compared with the levels for BALB/c mice inoculated with wild-type parasites.
FIG. 3.
L. mexicana-specific IgG1 and IgG2a levels (a) and total IgE levels (b) in BALB/c mice in response to infection with 5 × 105 wild-type (WT), Δicp1, Δicp3, Δicp1[pXG ICP], Δicp3[pXG ICP], and Δicp::PrRNAICP stationary-phase promastigotes into the hind footpad (*, P < 0.05; **, P < 0.01). In vitro production of IFN-γ (c) and IL-4 (d) by splenocytes, in the presence of 10 μg/ml L. mexicana antigen and 0.5 μg/ml anti-CD3, respectively, from BALB/c mice infected with 5 × 105 wild-type, Δicp1, Δicp3, Δicp1[pXG ICP], Δicp3[pXG ICP], and Δicp::PrRNAICP L. mexicana stationary-phase promastigotes at week 13 postinfection (*, P < 0.05). Bars represent standard errors of the means. Results are representative of three independent experiments.
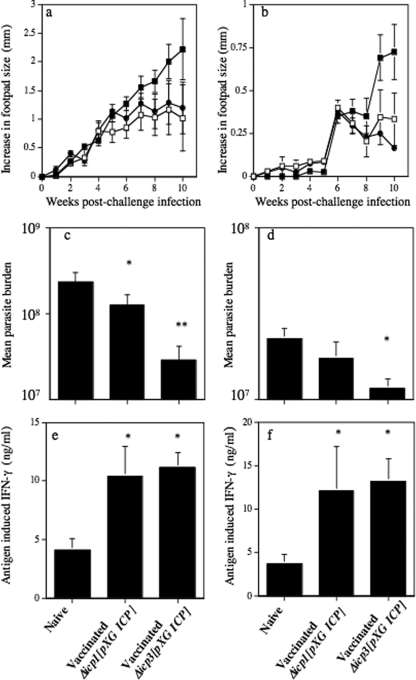
Vaccination with L. mexicana mutants overexpressing ICP fails to protect BALB/c mice against challenge with wild-type parasites.
As infection with Δicp1[pXG ICP] stationary-phase promastigotes induced a diminished type 2 and an enhanced type 1 response in BALB/c mice, which were also able to control infection with this mutant, the vaccine potential of Δicp1[pXG ICP] was assessed. Consequently, at 14 weeks after vaccination with Δicp1[pXG ICP], BALB/c mice were challenged, along with naïve, sex- and age-matched counterparts, with wild-type L. mexicana metacyclic promastigotes. However, subcutaneous vaccination of BALB/c mice with 2 × 105 Δicp1[pXG ICP] parasites 14 weeks prior to challenge inoculation did not induce resistance to infection with wild-type L. mexicana metacyclic promastigotes. No significant difference was noted in lesion development for vaccinated BALB/c mice infected with wild-type parasites in the contralateral footpad compared with that for similarly infected nonvaccinated BALB/c mice (Fig. 4a). Similarly, prior vaccination with Δicp1[pXG ICP] L. mexicana promastigotes did not alter the parasite burdens for the BALB/c mice at the termination of the experiment compared with those for infected, nonvaccinated BALB/c mice (Fig. 4b). There were also no differences in the immune responses between vaccinated and nonvaccinated BALB/c mice at the termination of the challenge infection (data not shown). Vaccination of BALB/c mice with Δicp3[pXG ICP] stationary-phase promastigotes also failed to induce protection against wild-type challenge (data not shown).
FIG. 4.
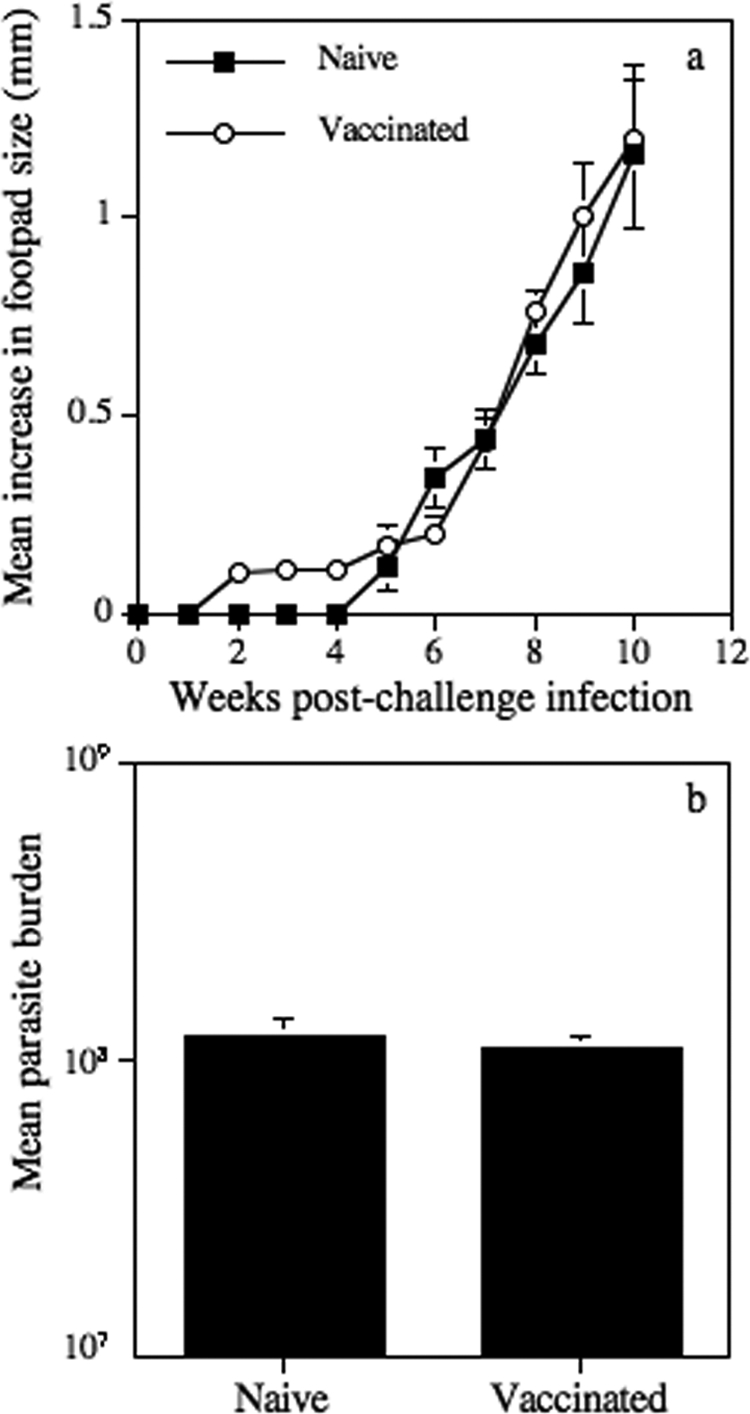
Mean increases in hind-footpad diameter (a) and parasite burdens (b) following subcutaneous infection with 5 × 105 L. mexicana stationary-phase promastigotes into naïve BALB/c mice and BALB/c mice vaccinated with Δicp1[pXG ICP]. Bars represent standard errors, and results are representative of three independent experiments.
Vaccination with L. mexicana mutants overexpressing ICP protects C3H and C57BL/6 mice against challenge with wild-type parasites.
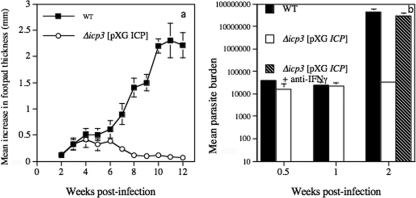
Previously, we have shown that other L. mexicana-susceptible mouse strains are more responsive to vaccination with CP mutants than BALB/c mice (2, 5). In addition, other mouse strains, such as C3H and C57BL/6, were able to control lesion growth with mutants that continued to cause nonhealing disease, albeit limited, in BALB/c mice (2, 6). Consequently, infection of these mouse strains with Δicp3[pXG ICP] and, to a lesser extent, Δicp1[pXG ICP] resulted in control of lesion growth. In a typical experiment, in contrast to the nonhealing response of C3H mice infected with wild-type parasites, mice infected with Δicp3[pXG ICP] developed small lesions that peaked at 4 to 6 weeks and consequently proceeded to heal (Fig. 5a). Parasite burdens were similar for wild-type- and Δicp3[pXG ICP]-infected mice up to 1 week postinfection (Fig. 5b). However, by week 2 postinfection parasite burdens were approaching 3 orders of magnitude greater than those for mice infected with Δicp3[pXG ICP] (P < 0.01). However, treatment of Δicp3[pXG ICP]-infected mice with neutralizing IFN-γ resulted in a significant increase in mutant parasite growth, also approaching 3 orders of magnitude greater (Fig. 5b). This not only confirmed the ability of Δicp3[pXG ICP] to multiply within the host but also confirmed that growth was limited due to the generation of IFN-γ. Neutralizing IFN-γ also increased Δicp3[pXG ICP] growth in BALB/c mice, indicating that the role of IFN-γ in protection was independent of the mouse strain used (data not shown). Consequently, we studied the ability of ICP overexpressing mutants to protect C3H and C57BL/6 mice against challenge with wild-type parasites. Lesions were smaller in both Δicp1[pXG ICP]- and Δicp3[pXG ICP]-vaccinated C3H and C57BL/6 mice at the termination of the experiment, at week 10, than in nonvaccinated mice following challenge with 5 × 105 wild-type stationary-phase promastigotes (Fig. 6a and b). However, only in C57BL/6 mice vaccinated with Δicp3[pXG ICP] was this difference significant (P < 0.05). No increase in lesion growth was detected from 5 weeks after challenge infection in vaccinated C3H mice, whereas continuous significant lesion growth was noted for the nonvaccinated challenged group. Control of the challenge infection due to prior vaccination of C3H mice with Δicp1[pXG ICP] or Δicp3[pXG ICP] and C57BL/6 mice with Δicp3[pXG ICP], though not Δicp1[pXG ICP], stationary-phase promastigotes was also demonstrated by the significantly (P < 0.05) reduced parasite burdens compared with those for nonvaccinated mice infected with 5 × 105 wild-type promastigotes (Fig. 6c and d). Subcutaneous vaccination of C3H and C57BL/6 mice with 2 × 105 Δicp3[pXG ICP] or Δicp1[pXG ICP] parasites 14 weeks prior to inoculation with wild-type parasites resulted, at the termination of this experiment, in a significantly (P < 0.05) higher increase in antigen-induced IFN-γ production from splenocytes than in antigen-induced IFN-γ production from splenocytes of naïve C3H or C57BL/6 mice infected with 5 × 105 wild-type L. mexicana stationary-phase promastigotes (Fig. 6e and f).
FIG. 5.
(a) Mean increases in hind-footpad thickness for C3H mice infected with wild-type (WT) and Δicp3[pXG ICP] promastigotes. (b) The parasites in the footpad were quantified by limiting dilution for up to 2 weeks postinfection, and the effects of intraperitoneal anti-IFN-γ treatment (150 μg rat hybridoma R4-6A2 on day 0, day 4, and day 8) on the growth of Δicp3[pXG ICP] were assessed. Bars represent standard errors of the means. Results are representative of two independent experiments.
FIG. 6.
Mean increases in hind-footpad diameter (a and b) and parasite burdens (c and d) following subcutaneous infection with 5 × 105 wild-type L. mexicana stationary-phase promastigotes into naïve (▪) C3H mice (a and c) and C57BL/6 mice (b and d) and mice vaccinated with 2 × 105 stationary-phase Δicp1[pXG ICP] (□) or Δicp3[pXG ICP] (•) promastigotes (a, b, c, and d). Mean levels of IFN-γ production from antigen-stimulated splenocytes isolated from wild-type-L. mexicana-infected naïve C3H (e) and C57BL/6 (f) mice and mice vaccinated with Δicp1[pXG ICP] or Δicp3[pXG ICP] stationary-phase promastigotes. *, P < 0.05; **, P < 0.01. Bars represent standard errors of the means. Results are representative of two independent experiments.
DISCUSSION
Previous studies of the characterization and function of L. mexicana ICP have suggested that it may play a role in protecting the parasite from the degradative activity of not only parasite CPB and CPA in particular but also host CPs which are found colocalized with ICP in the parasite endocytic vesicles (3). Consequently, ICP-deficient L. mexicana mutants lose some virulence for BALB/c mice, as measured by slightly reduced lesion growth, which was somewhat restored by reexpressing normal levels of ICP in the mutants (3). In spite of the reduced virulence of the Δicp mutant, there are no discernible immunological consequences for the host or parasite, as demonstrated in the present study by antibody levels as well as IFN-γ and IL-4 production. In contrast, we demonstrate not only that parasites overexpressing ICP have greatly reduced virulence for mice but also that this is associated with a switch from a Th2- to a Th1-biased response. Consequently, when ICP is overexpressed by mutant parasites, it influences the infectivity of the parasite in ways other than those merely associated with efficient housekeeping and fitness activities.
Our previous studies utilizing mutants of L. mexicana deficient in the CPs have revealed the importance of both clan CA, family C1 peptidases CPB and CPA for virulence. Δcpb mutant amastigotes, although equally as infective for macrophages in vitro as wild-type parasites (10), have a reduced ability to induce lesion growth in BALB/c mice (2, 8). Δcpa Δcpb mutants were less infective for BALB/c mice than Δcpb mutants (2, 18), which also implicates CPA as a virulence factor. As ICP has been shown to preferentially inhibit clan CA, family C1, cathepsin L-like peptidases, such as CPB (22), and to bind specifically to CPA and CPB (3), this could be the likely mechanism by which ICP overexpressing mutants lose virulence. Although expressed primarily in wild-type promastigotes rather than amastigotes (22), ICP is released in significant quantities by the amastigotes of overexpressing clones Δicp1[pXG ICP] and Δicp3[pXG ICP] (3). The virulence of L. mexicana for BALB/c mice has been associated with the ability of CPB/CPA to induce a Th2 response in BALB/c mice. In infected mice, Δcpb and Δcpa Δcpb mutants induce a Th1 response characterized by increased IFN-γ production, and consequently, lesion growth is inhibited (2, 5). Similar immunological and disease phenotypes were found in mice infected by ICP overexpressing mutants in the present study, suggesting that ICP acts to inhibit CPB/CPA immunomodulatory activity.
Small chemical inhibitors of clan CA CPs have previously been shown to inhibit the growth of several species of Leishmania, including L. donovani (7), L. chagasi (11, 15, 26), L. tropica (15), and L. major (14, 21), and a number of mechanisms have been implicated. However, ICPs may not necessarily operate by targeting parasite CPs directly but may act by inhibiting the crucial role of host CPs in the processing of antigen via major histocompatibility complex (MHC) class II. For example, studies have shown that chemically inhibiting cathepsins B, which are involved in antigen processing, switches L. major-infected BALB/c mice from producing a nonhealing Th2 to a healing Th1 response (14), while inhibiting cathepsins L promotes a Th2 response and increased susceptibility to the parasite (19, 29). However, ICP preferentially binds and inhibits mammalian cathepsin L and has 1/1,000 less affinity for cathepsin B (22). Consequently, this specificity probably negates a role for ICP from overexpressing mutants in directly modulating host cell antigen-presenting pathways. However, L. mexicana and L. amazonensis CPs have been implicated in the inhibition of antigen presentation by degrading MHC class II molecules in the parasitophorous vacuole (9). ICP in overexpressing mutants in this study could therefore function to promote MHC class II antigen presentation indirectly by inhibiting the degradative activity of parasite CPs. Inhibitors of cathepsin L-like CPs have also been associated with modulating host cell production of nitric oxide upon infection with L. donovani (7) or L. major (21), production of tumor necrosis factor alpha upon infection with L. major (21), and production of IL-12 upon infection with L. major (21) or L. mexicana (6). All of these products are associated with, or would promote, a Th1 response and protection. There is strong evidence that L. mexicana effects on host cell IL-12 production are, in part, a consequence of CPB degrading host cell NF-κB signaling pathways and that this degradation does not occur upon infection with Δcpb mutant parasites or in the presence of cell-permeable CP inhibitors (6). Overexpressed ICP could inhibit this previously characterized CPB activity, and in addition, it could be released into the extracellular milieu (3), as previously demonstrated for CPB (12). Hence, ICP could inhibit the ability of the CP to generate IL-4 and a Th2 response (20).
As infection of BALB/c mice with L. mexicana metacyclic promastigotes of overexpressing clones Δicp1[pXG ICP] and Δicp3[pXG ICP] induced immune responses that favor resistance to infection, these genetically modified parasites were considered as vaccine candidates. Δicp1[pXG ICP] was considered the most suitable candidate for the development of a vaccine, as this mutant induced no detectable lesion progression or parasite replication. However, subcutaneous vaccination with Δicp1[pXG ICP] did not confer protection to subsequent challenge with wild-type L. mexicana promastigotes. No significant differences in lesion development or parasite burdens or subsequent immune responses were detected (data not shown) following infection of vaccinated mice with wild-type parasites or infection of naïve mice. Significantly, we have demonstrated previously that only limited resistance was conferred on BALB/c mice vaccinated with Δcpa Δcpb mutant parasites and that this required extremely large experimental groups of 20 mice to demonstrate (2). However, while the nonhealing responses of BALB/c mice for L. mexicana have been related to CP-induced IL-4 (8, 20), by contrast, the virulence of L. mexicana for the L. major-resistant C3H and C57BL/6 strains has been associated with the ability of CPB to inhibit Th1 responses rather than to induce Th2 responses (5). In mice infected with the L. mexicana Δcpb mutant, lesion resolution was associated with elevated Th1 responses, and healing was demonstrated to be Th1 dependent. Prior vaccination of these mice as well as CBA mice (2, 5) with Δcpb mutant parasites afforded significant protection against challenge with wild-type parasites. Similarly, vaccination of C57BL/6 and C3H mice with Δicp3[pXG ICP] and vaccination of C3H mice with Δicp1[pXG ICP] or Δicp3[pXG ICP] did offer some protection against wild-type challenge, as demonstrated by elevated antigen-induced spleen cell IFN-γ production, delayed lesion growth, and reduced parasite numbers.
Overall, this study not only confirms the role of L. mexicana cathepsin L-like CPs as virulence factors but also provides further evidence of the therapeutic potential of specific CP inhibitors.
Acknowledgments
This work was supported by the Wellcome Trust.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 11 May 2009.
REFERENCES
- 1.Alexander, J., F. Brombacher, H. A. McGachy, A. N. McKenzie, W. Walker, and K. C. Carter. 2002. An essential role for IL-13 in maintaining a non-healing response following Leishmania mexicana infection. Eur. J. Immunol. 322923-2933. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, J., G. H. Coombs, and J. C. Mottram. 1998. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a Th1 response. J. Immunol. 1616794-6801. [PubMed] [Google Scholar]
- 3.Besteiro, S., G. H. Coombs, and J. C. Mottram. 2004. A potential role for ICP, a leishmanial inhibitor of cysteine peptidases, in the interaction between host and parasite. Mol. Microbiol. 541224-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackwell, J. M., B. Roberts, and J. Alexander. 1985. Response of BALB/c mice to leishmanial infection. Curr. Top. Microbiol. Immunol. 12297-106. [DOI] [PubMed] [Google Scholar]
- 5.Buxbaum, L. U., H. Denise, G. H. Coombs, J. Alexander, J. C. Mottram, and P. Scott. 2003. Cysteine protease B of Leishmania mexicana inhibits host Th1 responses and protective immunity. J. Immunol. 1713711-3717. [DOI] [PubMed] [Google Scholar]
- 6.Cameron, P., A. McGachy, M. Anderson, A. Paul, G. H. Coombs, J. C. Mottram, J. Alexander, and R. Plevin. 2004. Inhibition of lipopolysaccharide-induced macrophage IL-12 production by Leishmania mexicana amastigotes: the role of cysteine peptidases and the NF-kappaB signaling pathway. J. Immunol. 1733297-3304. [DOI] [PubMed] [Google Scholar]
- 7.Das, L., N. Datta, S. Bandyopadhyay, and P. K. Das. 2001. Successful therapy of lethal murine visceral leishmaniasis with cystatin involves up-regulation of nitric oxide and a favorable T cell response. J. Immunol. 1664020-4028. [DOI] [PubMed] [Google Scholar]
- 8.Denise, H., K. McNeil, D. R. Brooks, J. Alexander, G. H. Coombs, and J. C. Mottram. 2003. Expression of multiple CPB genes encoding cysteine proteases is required for Leishmania mexicana virulence in vivo. Infect. Immun. 713190-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Souza Leao, S., T. Lang, E. Prina, R. Hellio, and J. C. Antoine. 1995. Intracellular Leishmania amazonensis amastigotes internalize and degrade MHC class II molecules of their host cells. J. Cell Sci. 1083219-3231. [DOI] [PubMed] [Google Scholar]
- 10.Frame, M. J., J. C. Mottram, and G. H. Coombs. 2000. Analysis of the roles of cysteine proteinases of Leishmania mexicana in the host-parasite interaction. Parasitology 121367-377. [DOI] [PubMed] [Google Scholar]
- 11.Gantt, K. R., S. Schultz-Cherry, N. Rodriguez, S. M. Jeronimo, E. T. Nascimento, T. L. Goldman, T. J. Recker, M. A. Miller, and M. E. Wilson. 2003. Activation of TGF-beta by Leishmania chagasi: importance for parasite survival in macrophages. J. Immunol. 1702613-2620. [DOI] [PubMed] [Google Scholar]
- 12.Ilg, T., M. Fuchs, V. Gnau, M. Wolfram, D. Harbecke, and P. Overath. 1994. Distribution of parasite cysteine proteinases in lesions of mice infected with Leishmania mexicana amastigotes. Mol. Biochem. Parasitol. 67193-203. [DOI] [PubMed] [Google Scholar]
- 13.Julia, V., M. Rassoulzadegan, and N. Glaichenhaus. 1996. Resistance to Leishmania major induced by tolerance to a single antigen. Science 274421-423. [DOI] [PubMed] [Google Scholar]
- 14.Maekawa, Y., K. Himeno, H. Ishikawa, H. Hisaeda, T. Sakai, T. Dainichi, T. Asao, R. A. Good, and N. Katunuma. 1998. Switch of CD4+ T cell differentiation from Th2 to Th1 by treatment with cathepsin B inhibitor in experimental leishmaniasis. J. Immunol. 1612120-2127. [PubMed] [Google Scholar]
- 15.Mahmoudzadeh-Niknam, H., and J. H. McKerrow. 2004. Leishmania tropica: cysteine proteases are essential for growth and pathogenicity. Exp. Parasitol. 106158-163. [DOI] [PubMed] [Google Scholar]
- 16.McMahon-Pratt, D., and J. Alexander. 2004. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol. Rev. 201206-224. [DOI] [PubMed] [Google Scholar]
- 17.Mottram, J. C., G. H. Coombs, and J. Alexander. 2004. Cysteine peptidases as virulence factors of Leishmania. Curr. Opin. Microbiol. 7375-381. [DOI] [PubMed] [Google Scholar]
- 18.Mottram, J. C., A. E. Souza, J. E. Hutchison, R. Carter, M. J. Frame, and G. H. Coombs. 1996. Evidence from disruption of the lmcpb gene array of Leishmania mexicana that cysteine proteinases are virulence factors. Proc. Natl. Acad. Sci. USA 936008-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onishi, K., Y. Li, K. Ishii, H. Hisaeda, L. Tang, X. Duan, T. Dainichi, Y. Maekawa, N. Katunuma, and K. Himeno. 2004. Cathepsin L is crucial for a Th1-type immune response during Leishmania major infection. Microbes Infect. 6468-474. [DOI] [PubMed] [Google Scholar]
- 20.Pollock, K. G., K. S. McNeil, J. C. Mottram, R. E. Lyons, J. M. Brewer, P. Scott, G. H. Coombs, and J. Alexander. 2003. The Leishmania mexicana cysteine protease, CPB2.8, induces potent Th2 responses. J. Immunol. 1701746-1753. [DOI] [PubMed] [Google Scholar]
- 21.Ponte-Sucre, A., R. Vicik, M. Schultheis, T. Schirmeister, and H. Moll. 2006. Aziridine-2,3-dicarboxylates, peptidomimetic cysteine protease inhibitors with antileishmanial activity. Antimicrob. Agents Chemother. 502439-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanderson, S. J., G. D. Westrop, J. Scharfstein, J. C. Mottram, and G. H. Coombs. 2003. Functional conservation of a natural cysteine peptidase inhibitor in protozoan and bacterial pathogens. FEBS Lett. 54212-16. [DOI] [PubMed] [Google Scholar]
- 23.Satoskar, A., H. Bluethmann, and J. Alexander. 1995. Disruption of the murine interleukin-4 gene inhibits disease progression during Leishmania mexicana infection but does not increase control of Leishmania donovani infection. Infect. Immun. 634894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selzer, P. M., S. Pingel, I. Hsieh, B. Ugele, V. J. Chan, J. C. Engel, M. Bogyo, D. G. Russell, J. A. Sakanari, and J. H. McKerrow. 1999. Cysteine protease inhibitors as chemotherapy: lessons from a parasite target. Proc. Natl. Acad. Sci. USA 9611015-11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith, B. O., N. C. Picken, G. D. Westrop, K. Bromek, J. C. Mottram, and G. H. Coombs. 2006. The structure of Leishmania mexicana ICP provides evidence for convergent evolution of cysteine peptidase inhibitors. J. Biol. Chem. 2815821-5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Somanna, A., V. Mundodi, and L. Gedamu. 2002. Functional analysis of cathepsin B-like cysteine proteases from Leishmania donovani complex. Evidence for the activation of latent transforming growth factor beta. J. Biol. Chem. 27725305-25312. [DOI] [PubMed] [Google Scholar]
- 27.Torrentera, F. A., N. Glaichenhaus, J. D. Laman, and Y. Carlier. 2001. T-cell responses to immunodominant LACK antigen do not play a critical role in determining susceptibility of BALB/c mice to Leishmania mexicana. Infect. Immun. 69617-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams, R. A., L. Tetley, J. C. Mottram, and G. H. Coombs. 2006. Cysteine peptidases CPA and CPB are vital for autophagy and differentiation in Leishmania mexicana. Mol. Microbiol. 61655-674. [DOI] [PubMed] [Google Scholar]
- 29.Zhang, T., Y. Maekawa, T. Sakai, Y. Nakano, K. Ishii, H. Hisaeda, T. Dainichi, T. Asao, N. Katunuma, and K. Himeno. 2001. Treatment with cathepsin L inhibitor potentiates Th2-type immune response in Leishmania major-infected BALB/c mice. Int. Immunol. 13975-982. [DOI] [PubMed] [Google Scholar]