Abstract
Brucella abortus is an intracellular pathogen that persists within phagocytic cells of the reticuloendothelial system. To identify in vivo interactions between B. abortus and the host that lead to persistent infection, we studied the persistence of B. abortus and an isogenic virB mutant deficient in the VirB type IV secretion system (T4SS) in knockout mice. In contrast to control mice, mice lacking B cells (Igh6−/−) were permissive for infection with the attenuated virB mutant. To determine the basis for this phenotype, we characterized immune functions of Igh6−/− mice in the context of B. abortus infection. Igh6−/− mice had greater numbers of extracellular bacteria in the spleen and increased early expression of proinflammatory cytokines during B. abortus infection. Further, a virB mutant, despite its wild-type level of survival, failed to elicit microgranuloma formation in the spleens of Igh6−/− mice, suggesting a requirement for the T4SS to elicit this pathological change. Passive transfer of immunoglobulin G from naïve mice restored the ability of Igh6−/− mice to control the persistence of the virB mutant by a complement-independent mechanism. Further, adoptive transfer of CD11b+ cells from C57BL/6 mice to Igh6−/− mice restored the ability of the knockout mice to limit the replication of the virB mutant in the spleen, suggesting that the Igh6−/− mutation affects phagocyte function and that phagocyte function can be restored by natural antibody.
Human brucellosis is a febrile disease resulting from the transmission of Brucella abortus, B. suis, B. melitensis, or B. canis from its respective zoonotic reservoir in cattle, swine, goats and sheep, or dogs (47). These pathogens are endemic in many areas of the world, including Central and South America, the Mediterranean, and Central Asia, and are responsible for an estimated 500,000 new brucellosis cases each year (1). In human brucellosis, as well as in the zoonotic reservoir species, bacteria may persist for long periods of time in the reticuloendothelial system (7). This aspect of infection can be modeled in the mouse, which has been used to identify and characterize the virulence factors involved in the systemic persistence of Brucella spp. (2, 22).
One essential virulence factor of the human pathogenic Brucella species is the type IV secretion system (T4SS) encoded by the virB locus on chromosome II (18, 29, 40). The T4SS of Brucella spp., similar to those of other bacterial pathogens, mediates the translocation of proteins into host cells; however, the functions of two newly identified B. abortus T4SS substrates, VceA and VceC, is not yet known (12, 25, 39, 45, 46). In cultured macrophages and dendritic cells (DC), the T4SS is essential for the intracellular replication and persistence of B. abortus (13, 36, 40). The T4SS mediates exclusion of late endosomal/lysosomal markers from the Brucella-containing vacuole and targeting of B. abortus to exit sites of the endoplasmic reticulum, where replication occurs (5, 6, 12, 41), suggesting that T4SS effectors are involved in this function. After intraperitoneal (i.p.) inoculation of mice, the T4SS is required not for the initial systemic dissemination of B. abortus but rather for persistence in the reticuloendothelial system (31, 34). In order to better understand the interactions between the host and B. abortus that lead to the persistence of wild-type (WT) strains and the eventual clearance of virB mutants, we examined the immune mechanisms required for clearance of the virB mutant. Unexpectedly, mice lacking B cells (Igh6−/−) were permissive for the splenic persistence of the virB mutant, while the persistence of WT B. abortus was not increased. When cultured ex vivo, macrophages from Igh6−/− mice behaved identically to macrophages from control mice in their ability to control the intracellular replication of the virB mutant while permitting the replication of WT B. abortus (34).
In this study, we attempted to pinpoint the defect in Igh6−/− mice that renders them permissive for persistent infection by the virB mutant. Our results show that nonspecific antibody can reverse the defect of these mice in controlling virB mutant replication without affecting WT B. abortus. These results suggest that the T4SS mediates the evasion of a natural antibody-dependent immune clearance function by B. abortus during persistence in vivo.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
The bacterial strains used in this study were B. abortus vaccine strain RB51, WT stain 2308, and its isogenic mutant BA41, which has an insertion of mTn5Km2 at nucleotide 1232 of the B. abortus virB locus (GenBank accession number AF226278). This insertion is located 59 bp downstream of the virB1 gene and is polar on the expression of downstream genes in the virB operon. Strains were cultured on tryptic soy agar (TSA; Difco/Becton-Dickinson, Sparks, MD) or in tryptic soy broth at 37°C on a rotary shaker. Bacterial inocula for infection of mice were cultured on TSA plus 5% blood. For cultures of strain BA41, kanamycin was added to the culture medium at 100 mg/liter. All work with live B. abortus was performed in a biosafety level 3 facility.
Infection of mice.
Female 6- to 8-week-old B6.129S2.Igh-6tm1Cgn (Igh6−/−) (21) mice carrying a targeted knockout of the gene that encodes the immunoglobulin mu chain and age- and sex-matched C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Female 6- to 8-week-old B6.129P2-FceR1gtm1Rav N12 (FcRγ−/−) (42) mice deficient in the gamma chain subunit of the FcγRI, FcγRIII, and FcɛRI receptors and age- and sex-matched C57BL/6 mice were obtained from Taconic Farms (Germantown, NY). Mice were held in microisolator cages with sterile bedding and water and irradiated feed in a biosafety level 3 facility. For infection experiments, groups of 5 to 10 knockout mice or 5 to 10 C57BL/6 mice were inoculated i.p. with 0.1 ml of phosphate-buffered saline (PBS) containing 5 × 104 CFU of a 1:1 mixture of WT and virB mutant B. abortus. For single infections, four mice per group were infected with 5 × 104 CFU of WT or virB mutant B. abortus. Twenty-one days after infection, the mice were euthanized by CO2 asphyxiation and their spleens were collected aseptically at necropsy. The spleens were homogenized in 3 ml of PBS, and serial dilutions of the homogenate were plated on TSA and TSA plus kanamycin for enumeration of CFU. All animal experiments were approved by the University of California at Davis Laboratory Animal Care and Use Committee and were conducted in accordance with institutional guidelines.
Histopathology and histomorphometry.
Spleen samples were fixed in buffered formalin, processed according to standard procedures for paraffin embedding, sectioned at 5 μm, and stained with hematoxylin and eosin. A veterinary pathologist (M.N.X.) examined the slides by using blinded sample analysis. Volumetric proportions of splenic tissue components, including red pulp, lymphoid tissue, microgranulomas, and perifollicular macrophages, were measured with a 25-point circular grid (Zeiss KPL ocular 6.3× with 25 points) over 10 randomly selected microscopic fields (20× objective) for a total of 250 points.
Immunization of mice.
C57BL/6 mice were inoculated i.p. with 0.1 ml of PBS containing 5 × 104 CFU of B. abortus 2308. Ten weeks later, serum and splenocytes were collected to perform transfer experiments. Age- and sex-matched mice were treated with 0.1 ml PBS alone, and at 10 weeks, serum and splenocytes were also collected.
Splenocyte isolation and transfer.
Spleens were obtained from immunized or naïve C57BL/6 mice 10 weeks after infection. After gently teasing apart the spleens, cells were passed through a 70-μm cell strainer and treated with ACK buffer (0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM Na2EDTA, pH 7.2) to lyse red blood cells. Cells were washed with PBS (Gibco) containing 1% bovine serum albumin (PBS-BSA). Cells were counted, and 1 × 106 viable cells were transferred intravenously into Igh6−/− mice. One group received PBS-BSA alone. Two days later, the Igh6−/− mice were infected with a 1:1 mixture of B. abortus WT strain 2308 or the virB mutant, and the CFU in their spleens were enumerated 21 days later.
Ex vivo Gm protection assay.
Spleens were obtained 21 days after infection from groups of four C57BL/6 or Igh6 mice infected with B. abortus 2308 or the virB mutant and gently teased apart to obtain single-cell suspensions. The splenocyte suspension of each spleen was divided in two. One half was treated with gentamicin (Gm, 50 μg/ml) for 4 h, and the other half was left untreated. After Gm treatment, supernatants were removed and plated (extracellular fraction) while splenocytes were lysed with 0.5% Tween 20 and plated (intracellular fraction). The fraction of intracellular or extracellular bacteria in the spleens of C57BL/6 or Igh6 mice infected with either B. abortus 2308 or the virB mutant was calculated as the percentage of the CFU of 2308 or virB mutant bacteria recovered in the intracellular or extracellular fraction divided by the total number of CFU of strain 2308 or the virB mutant.
Serum transfer.
Blood was collected from sex- and age-matched immunized or naïve C57BL/6 mice, as well as naïve Igh6−/− mice, and serum was obtained. Serum was diluted 1:10 in PBS and transferred intravenously into Igh6−/− mice at days 5, 11, and 17 after infection. Spleens were collected at day 21 postinfection, and CFU were enumerated.
IgG purification and transfer.
Immunoglobulin G (IgG) from naïve or immunized C57BL/6 mice was purified by using Hi-Trap protein G HP columns (Amersham Biosciences, Piscataway, NJ) and following the manufacturer's instructions. Briefly, serum was passed through the column and washed three times with binding buffer (20 mM sodium phosphate, pH 7.0). IgG was eluted with 0.1 M glycine-HCl (pH 2.7) and dialyzed twice against PBS (pH 7.4). After checking the concentration of purified IgG, 75 μg was transferred intravenously three times into Igh6−/− mice at days 5, 11, and 17 after infection. Spleens were collected at day 21 postinfection, and CFU of B. abortus were enumerated.
For transfer of Fc and Fab fragments, purified IgG, Fc fragment, and Fab fragment were purchased from Sigma (Saint Louis, MO). Equal molar amounts of IgG, Fc fragment, or Fab fragment were transferred intravenously into Igh6−/− mice at days 5, 11, and 17 postinfection. Spleens were collected at day 21 postinfection, and CFU were enumerated.
Complement-antibody sensitivity assay.
B. abortus 2308 or RB51 or the virB mutant was incubated in the presence of naïve or Brucella-specific serum supplemented with 30% fresh or heat-inactivated (56°C for 1 h) complement for 24 h at 37°C as described by Corbeil et al. (10). The numbers of CFU in triplicate cultures were determined by plating serial dilutions.
CD11b+ cell isolation and transfer.
Macrophages (CD11b+ cells) were isolated from the spleens of naïve C57BL/6 or Igh6−/− mice with a MACS CD11b MicroBeads magnetic cell sorting kit from Miltenyi Biotech (Auburn, CA) by following the manufacturer's instructions. Briefly, after gently teasing apart the spleens, cells were passed through a 70-μm cell strainer and treated with ACK buffer (0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM Na2EDTA, pH 7.2) to lyse red blood cells. Cells were washed with PBS-BSA, counted, and incubated with CD11b MicroBeads. Cells were applied to a magnetic column, washed, eluted, and counted. Viable cells (1 × 106) were transferred intravenously into Igh6−/− mice. One group received PBS-BSA alone. Two days later, the Igh6−/− mice were infected with a 1:1 mixture of B. abortus WT strain 2308 or the virB mutant, and 21 days later, spleens were collected and CFU were enumerated.
Bone marrow-derived macrophages.
Bone marrow-derived macrophages were differentiated from bone marrow precursors of C57BL/6, Igh6−/−, or FcRγ−/− mice by following a previously published procedure (34). Igh6−/− and C57BL/6 bone marrow macrophages were also differentiated in medium containing no or very little IgG. Ultra-low-IgG fetal bovine serum (Invitrogen) containing less than 5 μg of IgG/ml was further depleted of IgG by passage through a Hi-Trap protein G HP column (Amersham Biosciences, Piscataway, NJ) three times.
Macrophage infection.
To evaluate the intracellular survival and growth of B. abortus, we used a previously described Gm protection assay (34). Briefly, 24-well microtiter plates were seeded with macrophages at a concentration of 2 ×105/well and incubated overnight at 37°C. Approximately 2 × 107 bacteria in 0.5 ml of RPMIsup or RPMIsup-IgG, containing B. abortus 2308 (wild type) or its isogenic virB mutant, were added to each well of macrophages. After the addition of Gm (50 μg/ml), wells were sampled at various time points after infection to determine intracellular CFU. Each infection was performed in triplicate, and C57BL/6 macrophages were always assayed together with Igh6−/− or FcRγ−/− macrophages.
RNA isolation.
RNA from spleens of C57BL/6 or Igh6 mice infected with B. abortus 2308 or the virB mutant for 1 or 3 days postinfection was isolated with Tri-Reagent (Molecular Research Center, Inc., Cincinnati, OH) by following the manufacturer's instructions. Briefly, spleen samples were homogenized in 1 ml of Tri-Reagent and incubated for 5 min at room temperature. RNA was extracted by adding 0.2 ml of chloroform and centrifuging the samples at 10,000 rpm for 15 min at 4°C. RNA was precipitated with 0.5 ml of isopropanol and resuspended in H2O.
cDNA.
One microgram of RNA was transcribed to cDNA with TaqMan reverse transcription reagents (Applied Biosystems, Branchburg, NJ). The RNA was mixed with 5 μl of 10× buffer, 11 μl of 20 mM MgCl2, 10 μl of deoxynucleoside triphosphates, 2.5 μl of random hexamers, 1 μl of reverse transcriptase, and H2O to a final volume of 50 μl. The PCR profile used to transcribe the samples required an incubation step performed at 25°C for 10 min, followed by the reverse transcription step done at 48°C for 30 min and an inactivation step performed at 95°C for 5 min.
Real-time PCR.
Reverse-transcribed cDNA was amplified with published primer sets for mouse interleukin-6 (IL-6), IL-10, IL-12p40, chemokine CXCL1 (KC), MIP-2, gamma interferon (IFN-γ), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (30) with SYBR green PCR master mix (Applied Biosystems) and an ABI PRISM 7900HT detection system (Applied Biosystems) by following the manufacturer's instructions. n-Fold induction of mRNA was determined from the threshold cycle (CT) values normalized for GAPDH expression and then normalized to the value derived from the mock-infected C57BL/6 control mice.
Statistical analysis.
For determination of the statistical significance of differences between experimental groups, a Student t test was performed on the data after logarithmic conversion. A P value of <0.05 was considered significant. For histomorphometry, volumetric proportion data expressed as percentages were submitted to analysis of variance after angular transformation and comparison by the Tukey test. Analysis of variance was used to analyze the significance of differences between real-time PCR data.
RESULTS
Adoptive transfer of splenocytes from WT mice restores the ability of Igh6−/− mice to control the virB mutant.
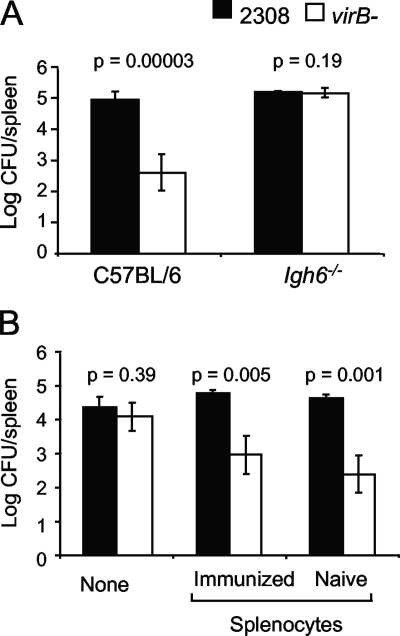
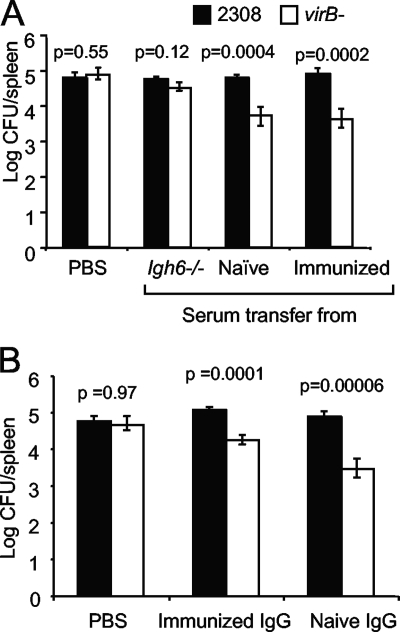
B. abortus 2308 is able to disseminate from the injection site and establish persistent infection of the spleen after i.p. inoculation of C57BL/6 mice. In contrast, a virB mutant can disseminate to the spleen and persist only briefly (5 days) before its numbers begin to decline (34). We reported previously that Igh6−/− mice, which do not produce mature B cells due to a targeted inactivation of the IgM heavy chain (21), permit the persistence of a virB mutant at the same level as WT B. abortus for at least 21 days, suggesting that the T4SS encoded by the virB locus contributes to the evasion of an immune mechanism that is defective in Igh6−/− mice (Fig. 1A) (34). Based on a time course experiment showing the greatest difference in the virB mutant colonization of Igh6−/− mice and control mice at 21 days postinfection, we chose this time point for subsequent experiments designed to reconstitute the immune defect of the Igh6−/− mice (34). To determine whether this defect could be restored by adoptive transfer, we transferred 1 × 106 splenocytes from either naïve mice or mice that had been infected 10 weeks previously with B. abortus 2308. Two days after the adoptive transfer of splenocytes, mice were infected with a 1:1 mixture of B. abortus 2308 and the virB mutant. Both naïve and immune splenocytes transferred the ability to control the persistence of the virB mutant but did not affect the number of WT B. abortus CFU recovered from the spleen at 21 days after i.p. infection (Fig. 1B).
FIG. 1.
(A) Bacterial loads in the spleen 21 days after infection of C57BL/6 or Igh6−/− mice with a mixed inoculum containing B. abortus 2308 (WT) and the virB mutant. Mice (n = 5) were inoculated i.p. with a 1:1 mixture of WT and virB mutant bacteria. The number of CFU of each bacterial strain was determined by differential plating. The significance of differences (P < 0.05) between the virB mutant and the WT was determined with the Student t test. (B) Bacterial loads in the spleens of Igh6−/− mice after splenocyte transfer. Splenocytes from immunized or naïve C57BL/6 mice were transferred intravenously into 10 Igh6−/− mice per group 2 days before they were inoculated i.p. with a 1:1 mixture of WT and virB mutant bacteria. Numbers of CFU from the spleens were determined 21 days after infection. The significance of differences (P < 0.05) between the virB mutant and the WT was determined by Student's t test.
B. abortus in the spleens of Igh6−/− mice are more susceptible to killing by Gm.
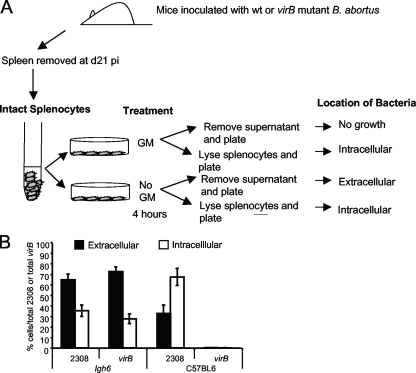
Our previous work showed that primary macrophages from Igh6−/− mice infected ex vivo were as bactericidal as macrophages from control mice against both WT B. abortus and the virB mutant, suggesting that increased survival of the virB mutant in these mice was not the result of defective macrophage function (34). However, a previous report showed that after uptake by epithelial cells in vitro, a nonpolar virB10 mutant was recycled to the surface (9). This raised the possibility that in Igh6−/− mice the virB mutant might persist in an extracellular location, since antibody, which is absent in the Igh6−/− mice, might contribute to uptake and killing of extracellular bacteria in vivo. To test this possibility, C57BL/6 or Igh6−/− mice were inoculated with either B. abortus 2308 or the virB mutant BA41. At day 21, when equal numbers of both strains were present in the spleens of Igh6−/− mice, the spleens were excised and single-cell suspensions were prepared by gently teasing apart the spleens. This suspension was either treated for 4 h with Gm to kill extracellular bacteria or left untreated to determine the total number of bacteria (Fig. 2A). Data in Fig. 2B show that in C57BL/6 mice, approximately one-third of the B. abortus WT bacteria were in a Gm-sensitive or likely extracellular location, while two-thirds were resistant to Gm killing, which is indicative of intracellular residence. As expected, no virB mutant bacteria were recovered from C57BL/6 mouse spleens. In Igh6−/− mice, the ratio of Gm-susceptible to Gm-resistant bacteria was reversed. Approximately two-thirds of the bacteria were killed by Gm, suggesting an extracellular location, and one-third were Gm resistant or likely to be intracellular. This relationship of intracellular to extracellular bacteria was the same for both the WT and the virB mutant, suggesting that the increase in extracellular bacteria is a characteristic of Igh6−/− mice and not of the virB mutant strain.
FIG. 2.
Ex vivo Gm protection assay of intracellular B. abortus 2308 (WT) or the virB mutant in the spleens of C57BL/6 mice or Igh6−/− mice. (A) Schematic representation of the experiment. Groups of four C57BL/6 or Igh6−/− mice were inoculated with either B. abortus 2308 or the virB mutant. At day 21 postinfection (d21 pi), spleens were removed and teased apart to generate a cell suspension. Each splenocyte suspension was divided into two parts. One half was treated with Gm for 4 h, and the other half was left untreated. After Gm treatment, supernatants of treated and untreated cells were removed and plated to enumerate extracellular (Gm-sensitive) bacteria, while splenocytes were lysed with 0.5% Tween 20 and plated to count intracellular (Gm-protected) bacteria. (B) Percentages of intracellular (white bars) or extracellular (black bars) bacteria in the spleens of C57BL/6 or Igh6−/− mice infected with either B. abortus 2308 or the virB mutant were calculated as the percentage of the total CFU (intracellular or extracellular)/total CFU for both strain 2308 and the virB mutant.
Igh6−/− mice respond to B. abortus infection with expression of proinflammatory cytokines and chemokines.
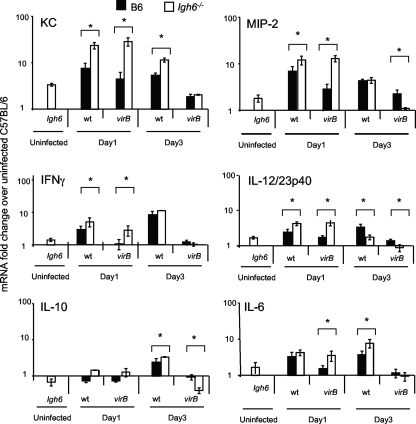
A possible mechanism underlying the permissive phenotype of Igh6−/− mice for the virB mutant is a deficient innate immune response. We therefore tested the ability of these mice to respond to B. abortus infection with early (1 to 3 days) transcriptional activation of the proinflammatory chemokines KC and MIP-2 and the cytokines IFN-γ, IL-6, tumor necrosis factor alpha, and IL-12/23p40, which are produced early in Brucella infection of mice (33, 35, 38, 48). Igh6−/− mice expressed higher baseline levels of KC, MIP-2, IFN-γ, IL-12/23p40, and IL-6 than did control (C57BL/6) mice (Fig. 3). Similarly, at day 1 postinfection, Igh6−/− mice had higher levels of KC, MIP-2, IFN-γ, and IL-12/23p40 than control mice did. This result suggested that the cytokine milieu in the spleen very early in infection differs between Igh6−/− and control mice in that slightly higher levels of proinflammatory cytokines and chemokines are expressed in Igh6−/− mice. By day 3 postinfection, both Igh6−/− and control mice had similar responses to both WT B. abortus and the virB mutant (Fig. 3).
FIG. 3.
Quantification of proinflammatory gene transcripts in the spleens of Igh6−/− and C57BL/6 mice infected with B. abortus 2308 or the virB mutant. Transcript levels in spleens from groups of four mice infected with either B. abortus 2308 (black bars) or the virB mutant (white bars) were assayed by real-time reverse transcriptase PCR at different time points postinoculation. Increases in transcript levels were calculated by normalizing CT values for each of the gene levels to CT values for GAPDH levels and to CT values for each of the gene levels measured in a group of mock-infected C57BL/6 mice. Asterisks indicate significant differences (α = 0.05) between Igh6−/− and control mice as calculated by analysis of variance.
The VirB T4SS is required for microgranuloma formation in the spleens of Igh6−/− mice.
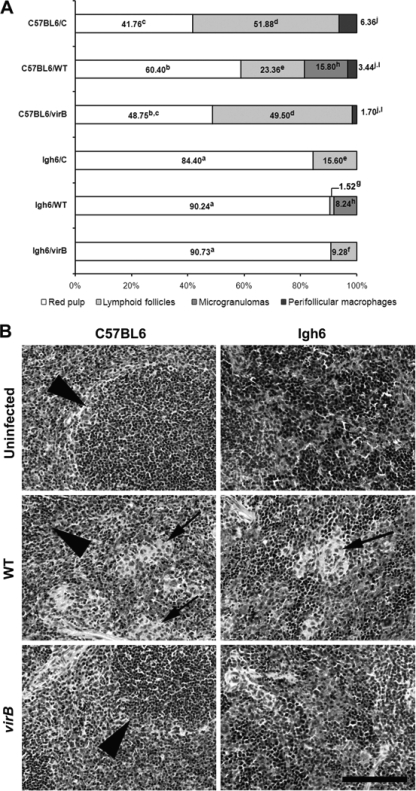
B. abortus infection is associated with the formation of focal granulomatous lesions in the spleen, liver, and lymphoid tissues of both humans and rodents, starting at 2 to 3 weeks postinfection (14, 15). To determine whether these later immune responses to B. abortus infection may be altered in Igh6−/− mice, we performed histopathologic and morphometric analyses of spleen tissue from mice infected for 21 days with either WT or virB mutant B. abortus. These data are summarized in Fig. 4. As expected, Igh6−/− mice did not have well-organized lymphoid follicles, unlike parental strain C57BL/6 mice, as B cells are required for this process (16, 17). Infection with WT B. abortus resulted in the formation of microgranulomas in the spleens of both C57BL/6 and Igh6−/− mice, which was associated with a decrease in the volumetric proportion of lymphoid follicles. Conversely, the virB mutant did not cause marked morphological changes in the spleens of either C57BL6 or Igh6−/− mice, with absence of microgranulomas. Interestingly, in spite of its ability to persist at WT levels in Igh6−/− mice, the virB mutant does not induce microgranuloma formation in these mice. Importantly, WT B. abortus is capable of inducing microgranulomas in Igh6−/− mice, indicating that the absence of microgranulomas in Igh6−/− mice infected with the virB mutant is likely to be due to a nonfunctional T4SS rather than the absence of mature B cells.
FIG. 4.
(A) Morphometric analysis of spleens from C57BL/6 and Igh6−/− mice. Columns indicate volumetric proportions of tissue components (n = 5 per group). Different superscript letters in the same component indicate statistically significant differences (P < 0.05). (B) Representative micrographs of hematoxylin-and-eosin-stained sections of spleen tissue from C57BL/6 and Igh6−/− mice infected with WT B. abortus or the virB mutant or from uninfected controls, as indicated. Arrows indicate microgranulomas, and arrowheads indicate lymphoid tissue. Bar = 100 μm.
Passive transfer of natural antibody restores the ability of Igh6−/− mice to limit the persistence of the virB mutant.
Igh6−/− mice lack mature B cells and are therefore unable to produce antibodies (21). To test the idea that antibody deficiency may underlie the increased susceptibility of these mice to persistent infection with the B. abortus virB mutant, we transferred serum from C57BL/6 mice that were either naïve or had been infected 10 weeks previously with B. abortus 2308. To mimic the development of an antibody response during infection, we transferred the serum to Igh6−/− mice on days 5, 11, and 17 after inoculation with B. abortus. Figure 5A shows that transfer of either naïve or immune serum restored the ability of Igh6−/− mice to control the persistence of the virB mutant but did not affect the persistence of WT B. abortus 2308. Serum from Igh6−/− mice had no effect on the persistence of the virB mutant (Fig. 5A), suggesting that antibody may be the serum component responsible for this activity. Following up on this observation, we purified serum IgG from either naïve or Brucella-immune mice with protein G columns and administered it to Igh6−/− mice 5, 11, and 17 days after i.p. inoculation with a 1:1 mixture of B. abortus 2308 and the virB mutant (Fig. 5B). A similar trend was observed as in the previous experiment, with IgG from both naïve and B. abortus-infected mice conferring the ability to reduce the colonization levels of the virB mutant. These results were surprising and suggested that a lack of immunoglobulin per se, and not the ability to mount an adaptive antibody response, contributed to the reduced ability of Igh6−/− mice to limit the persistence of virB mutant B. abortus.
FIG. 5.
(A) Bacterial loads in the spleens of Igh6−/− mice after serum transfer. Igh6−/− mice were inoculated i.p. with a 1:1 mixture of WT and virB mutant bacteria. Serum from naive Igh6−/− mice, naïve C57BL/6, or immunized C57BL/6 mice was transferred intravenously into 10 Igh6−/− mice per group at days 5, 11, and 17 after infection. CFU were recovered from the spleens 21 days after infection. The significance of differences (P < 0.05) between the virB mutant and the WT was determined by Student's t test. (B) Bacterial loads in the spleens of Igh6−/− mice after IgG transfer. Igh6−/− mice were inoculated i.p. with a 1:1 mixture of WT and virB mutant bacteria. IgG purified from naïve or immunized C57BL/6 mice was transferred intravenously into five Igh6−/− mice per group at days 5, 11, and 17 after infection. CFU from the spleens were enumerated 21 days after infection. The significance of differences (P < 0.05) between the virB mutant and the WT was determined by Student's t test.
Natural IgG does not affect complement-mediated killing of the virB mutant.
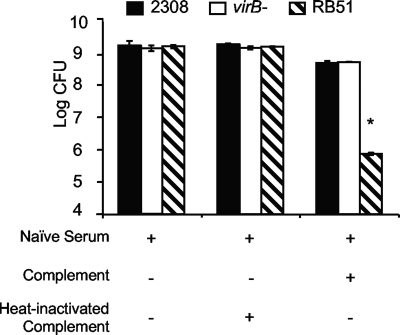
The previous results showed that passive transfer of IgG mediates increased control of virB mutant replication in the spleens of Igh6−/− mice. One function mediated by immunoglobulin is fixation of complement, with the Fab portion binding to the bacterium and complement fixation occurring at the Fc end of the molecule. Since Igh6−/− mice are deficient in antibody but not in complement, it was possible that passive transfer of natural antibodies could lead to increased complement fixation and/or killing of the virB mutant, compared to WT B. abortus. To test this idea, we compared the susceptibilities of B. abortus 2308, its isogenic virB mutant BA41, and rough vaccine strain RB51 to complement-mediated killing. As expected, incubation of bacteria with serum from naïve C57BL/6 mice and fresh rabbit complement reduced the number of CFU of RB51 by >2 orders of magnitude but only led to a fivefold reduction in the number of viable WT B. abortus CFU (10). No difference was observed between the killing of B. abortus 2308 and that of the virB mutant, showing that evasion of complement-mediated killing does not contribute to the increased survival of the virB mutant in Igh6−/− mice (Fig. 6).
FIG. 6.
Susceptibility of B. abortus 2308 or RB51 or the virB mutant to complement-antibody-mediated killing. Bacteria were treated with fresh or heat-inactivated complement and naïve serum, and CFU were subsequently determined. Bars represent the mean ± standard deviation of a single experiment that was representative of duplicate experiments performed. The asterisk denotes a statistically significant difference (P < 0.05) determined with the Student t test.
Transfer of CD11b+ cells into Igh6−/− mice restores their ability to limit the persistence of the virB mutant.
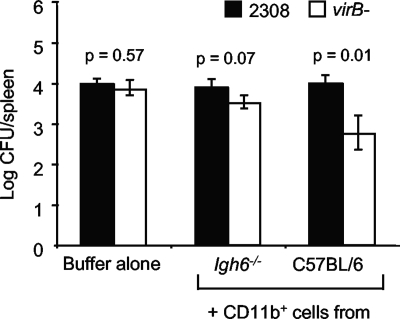
B. abortus has been shown to localize to macrophages of the reticuloendothelial system during infection (19, 23, 28). Therefore, we considered defective phagocyte function as a possible reason for the inability of Igh6−/− mice to eliminate the virB mutant from the spleen. Previously, we showed that macrophages isolated from the spleen or peritoneal cavity or differentiated from the bone marrow of Igh6−/− mice behaved like those isolated from WT mice in that they permitted intracellular replication of WT B. abortus but killed the virB mutant (34). Therefore, we considered that our ex vivo culture conditions for macrophages may not completely reflect in vivo conditions. One possible difference was that the macrophages of Igh6−/− mice are not exposed to antibody in vivo, but ex vivo they were cultured in the presence of bovine serum, which contains IgG. To more closely mimic the in vivo conditions in Igh6−/− mice, we cultured macrophages in serum depleted of IgG; however, IgG-depleted serum did not support the growth of either primary cells or J774 macrophages (data not shown). To avoid culturing macrophages ex vivo, we tested their function by enriching macrophages from spleens of C57BL/6 or Igh6−/− mice with anti-CD11b magnetic beads and IgG-free buffers and adoptively transferring them to Igh6−/− mice. These mice were subsequently inoculated i.p. with a 1:1 mixture of WT and virB mutant B. abortus, and the number of CFU of each strain in the spleen was determined at 21 days after infection (Fig. 7). Adoptive transfer of CD11b+ cells from Igh6−/− mice led to a slight reduction in the recovery of the virB mutant from the spleens of the mice, but this was not significant. However, mice that received CD11b+ cells from C57BL/6 mice reduced the load of the virB mutant by more than 10-fold relative to that of WT B. abortus, suggesting that macrophages from Igh6−/− mice have a reduced function in vivo.
FIG. 7.
Bacterial loads in the spleens of Igh6−/− mice after adoptive transfer. CD11b+ cells from Igh6−/− or C57BL/6 mice were transferred intravenously into five Igh6−/− mice per group 2 days before i.p. infection with a 1:1 mixture of WT and virB mutant bacteria. CFU of WT and mutant bacteria in the spleens were enumerated 21 days after infection. The statistical significance of differences (P < 0.05) between the virB mutant and the WT was determined with the Student t test.
Role of Fc receptors in limiting virB mutant persistence in the spleen.
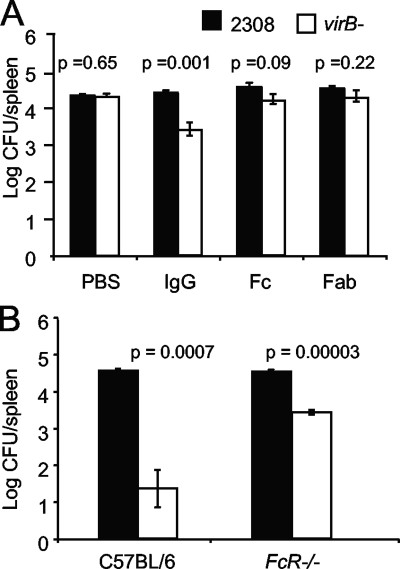
Nonspecific IgG is known to have immunomodulatory properties and is used to treat human autoimmune diseases (8, 20, 43). Evidence has been presented for mechanisms of action mediated by both IgG binding to autoantigens via the Fab domain (3) and binding of the Fc portion to a receptor for the Fc portion of IgG, FcγRII (37). To determine whether one of these mechanisms could contribute to the effect of IgG on Igh6−/− mice, we treated mice with purified fragments of IgG (Fig. 8A). As shown above (Fig. 5), treatment with intact IgG led to a significant reduction in the number of virB mutant CFU in the spleens of Igh6−/− mice at 21 days postinfection. Treatment with equimolar amounts of the Fc or Fab portion of IgG did not significantly reduce the persistence of the virB mutant.
FIG. 8.
(A) Bacterial loads in the spleens of Igh6−/− mice after passive transfer of IgG, IgG Fc fragment, or IgG Fab fragment. Igh6−/− mice were inoculated i.p. with a 1:1 mixture of WT and virB mutant bacteria. Equimolar amounts of purified IgG, Fc fragment, or Fab fragment were transferred intravenously into five Igh6−/− mice per group at days 5, 11, and 17 after infection. CFU were recovered from the spleens 21 days after infection. (B) Bacterial loads in the spleens of C57BL/6 or FcR−/− mice 21 days after infection with a mixed inoculum containing B. abortus 2308 (WT) and the virB mutant. Mice (four per group) were inoculated i.p. with a 1:1 mixture of WT and virB mutant bacteria. The number of CFU of each bacterial strain was determined by differential plating. The significance of differences (P < 0.05) between the virB mutant and the WT was evaluated with the Student t test.
These results suggested that reduced colonization of the virB mutant mediated by passive transfer of IgG requires both Fc and Fab portions of the molecule; however, since Fab fragments are cleared from the circulation more rapidly than intact IgG (11), we could not exclude the possibility that reduced stability of Fc and Fab fragments led to an underestimation of their activity in our experiments. Since receptors for the Fc portion of IgG are present on DC and macrophages, two cell types in which Brucella survive intracellularly (4, 36), we reasoned that in normal mice, Fc receptors may have low-affinity interactions with IgG that contribute to cellular functions involved in killing the virB mutant. To test this idea, we obtained FcRγ−/− mice lacking the Fc receptor gamma chain required for FcγRI, the high-affinity receptor for IgG; FcγRIII, the low-affinity IgG Fc receptor; and FcγRIV. These mice were used to determine whether IgG-Fc receptor interactions are required for controlling the systemic persistence of the virB mutant (Fig. 8B). For these experiments, FcRγ−/− mice and C57BL/6 controls obtained from the same supplier were inoculated with a 1:1 mixture of B. abortus 2308 and the virB mutant as described above. At 21 days postinfection, absence of FcγR did not affect the ability of WT B. abortus to persist in vivo, since both C57BL/6 and FcRγ−/− mice had equal splenic loads of WT bacteria. However, there were 100-fold more of the virB mutant bacteria in the spleens of FcRγ−/− mice, suggesting that these mice are deficient in limiting the splenic persistence of the virB mutant. This defect was not as severe as that of Igh6−/− mice (Fig. 5A), which permitted the persistence of the virB mutant at the same level as that of WT B. abortus, but was significantly different from C57BL/6 mice. Taken together, these results suggested that both natural IgG and Fc receptors are required for limiting the persistence of the virB mutant.
DISCUSSION
This study was undertaken to define interactions between B. abortus and the host that result in persistent infection. Previously, we characterized several knockout mice lacking components of adaptive immunity to identify a mouse in which the persistence of WT B. abortus was unaffected but in which the attenuated phenotype of a virB mutant was rescued. We hypothesized that this strategy would enable us to identify immune functions that are evaded by B. abortus during infection by using the T4SS. Igh6−/− mice, which lack functional B cells and therefore do not make antibody (21), allowed the virB mutant to survive in the spleen at WT levels after inoculation with either individual strains or a mixed inoculum of the WT and the virB mutant (34), suggesting that this phenotype was not due to rescue of the virB mutant by WT B. abortus, as proposed based on in vitro studies (26). The finding that virB mutants were rescued in Igh6−/− mice but the survival of WT B. abortus was unaffected suggested that the T4SS contributes to the evasion of a response that depends either directly or indirectly on the function of B cells.
Since B. abortus has been localized to phagocytic cells in tissues, one possible explanation for the increased survival of the virB mutant in Igh6−/− mice was that their phagocytes were defective in killing the virB mutant. However, ex vivo, splenic, peritoneal, and bone marrow-derived macrophages exhibited no defects in killing either WT or virB mutant B. abortus (34). We therefore asked whether B. abortus may be extracellular in these mice. Since evidence of intracellular localization in vivo is based on microscopy, it is not known to what extent individual bacteria may be in an intracellular or extracellular location during infection. Ex vivo Gm protection assays showed that in control mice, the majority (65%) of bacteria were in a Gm-resistant, or likely intracellular, location (Fig. 2). However, in Igh6−/− mice, the majority of both WT and mutant bacteria (65 to 70%) were killed by Gm. The finding of any extracellular bacteria in either mouse strain was unexpected, since B. abortus is considered to be an intracellular pathogen. However, it is possible that a portion of the bacteria within the host is extracellular or that we observed a transient extracellular phase of infection. Although we cannot exclude with our experimental design the possibility that, in Igh6−/− mice, B. abortus was in a cell type that is more easily lysed during the preparation of splenocyte suspensions, this result suggested that one possible reason for virB mutant survival in these mice is that it is predominantly in an extracellular location, where WT B. abortus would have no survival advantage over the virB mutant.
Study of early host responses to B. abortus revealed that very early (1 day) postinfection, Igh6−/− mice expressed higher levels of proinflammatory chemokines and cytokines than did control mice. Although this difference disappeared by day 3, it is possible that these early differences in the cytokine milieu also affect the survival of the virB mutant in Igh6−/− mice, either by affecting polarization of the immune response to infection or by affecting the ability of phagocytic cells to take up and/or restrict the replication of the B. abortus virB mutant (Fig. 3). Interestingly, at a later stage of infection (21 days), the VirB T4SS was required to elicit microgranuloma formation in the spleens of Igh6−/− mice, a phenotype that could not be observed previously because of the rapid clearance of the mutant from tissues of control mice. Together with our previous observation that the T4SS is required to elicit proinflammatory responses in the spleen during B. abortus infection (35), this observation suggests that the T4SS is required to elicit responses required for microgranuloma formation. This response is independent of bacterial numbers, since both WT and virB mutant B. abortus bacteria were present in equal numbers in the spleens of these mice.
To examine the mechanistic basis for the rescue of the virB mutant phenotype in Igh6−/− mice, we used several approaches to reconstitute B-cell-dependent functions in Igh6−/− mice. Surprisingly, we found that the ability to limit the persistence of the virB mutant could be rescued by passive transfer of IgG purified from B. abortus-naïve mice (Fig. 5). Further, the effect of B. abortus-immune IgG was the same as or less than that of naïve IgG. This result suggested that the defect of Igh6−/− mice that rescues the virB mutant is not in adaptive antibody responses to B. abortus per se but rather in an innate mechanism (natural antibody) that contributes either directly or indirectly to the control of bacterial infection. An analysis of the effector functions of natural antibody showed that this effect is not related to opsonization of bacteria or to complement-mediated killing of B. abortus (Fig. 6 and reference 34). However, it did involve the activity of a population of CD11b+ cells, since transfer of these cells to Igh6−/− mice restored their ability to eliminate the virB mutant from the spleen (Fig. 7). Therefore, our results suggest that, as a result of antibody deficiency, Igh6−/− mice have a defect in a population of CD11b+ cells.
How could natural antibody affect phagocytic cell function? In human medicine, intravenous IgG, a preparation of pooled IgG, is used therapeutically to treat autoimmune disease (8). Two mechanisms for the function of intravenous IgG have been proposed: one is binding of monomeric IgG to Fc receptors on phagocytic cells, resulting in activation of signal transduction cascades in the cell, and the second is activation of phagocytic cell function by binding via the Fab portion to autoantigens (27, 37). Fc receptors for IgG differ in their distribution on different cell types, in their activity, and in their affinity for different IgG isotypes (32). FcγRI on macrophages binds monomeric IgG2a at high affinity, and under physiologic conditions in vivo, most of the Fc receptors are occupied by monomeric IgG2a (44). Therefore, it is likely that passive transfer of IgG into Igh6−/− mice resulted in the binding of monomeric IgG2a to FcγRI, since we observed an effect of naïve serum that was equal to or greater than that of immune serum. This binding could trigger signaling events that either directly activate antibacterial functions of the phagocyte or, alternatively, act indirectly by stimulating the production of cytokines.
To test the first possibility, we determined whether mice defective in Fc receptors for IgG could rescue the virB mutant. This rescue was only partial, suggesting that activation of phagocyte function to kill virB mutants via IgG-Fc interaction may contribute to the effect of IgG on Igh6−/− mice (Fig. 8). An example of the second case is the genetic disease X-linked agammaglobulinemia, in which a lack of DC maturation has been reported. Addition of natural IgG and of autoreactive antibodies to DC from these patients results in their maturation, as assessed by upregulation of major histocompatibility complex class II and costimulatory molecules (3). B. abortus resides in DC in vivo, and mouse DC are able to kill virB mutants (4, 36). Since DC do not develop normally in Igh6−/− mice (24), it is possible that defective DC function is an additional factor that contributes to the persistence of the virB mutant. Our results do not allow us to define the relative contributions of Fc receptor signaling and binding of autoantigens to the reconstitution of Igh6−/− mice by natural IgG.
Taken together, our results suggest that multiple defects in Igh6−/− mice contribute either independently or in concert to the increased persistence of an attenuated B. abortus virB mutant. First, residence of increased numbers of bacteria in an extracellular location allows them to avoid phagocyte killing. Second, natural IgG is able to abolish rescue of the virB mutant, suggesting that IgG either activates phagocyte functions or contributes to intracellular localization of the bacteria. However, our previous results showing no increase in bacterial uptake by macrophages after opsonization with naïve serum (34), as well as results shown here that resistance to virB mutant infection can be adoptively transferred by CD11b+ cells, favor the former hypothesis. Together, our findings confirm the importance of phagocytic cells in vivo in restricting the growth of attenuated B. abortus strains and point to a novel role for antibody in maintaining the function of these cells.
Acknowledgments
We thank members of the Tsolis lab for critical reading of the manuscript, as well as A. Bäumler, N. Baumgarth, and C. Baldwin for helpful discussions of these results.
This work was supported by PHS award AI050553 to R.M.T. R.L.S. and M.N.X. are recipients of fellowships from CNPq (Brazil).
Editor: J. B. Bliska
Footnotes
Published ahead of print on 13 April 2009.
REFERENCES
- 1.Araj, G. F. 1999. Human brucellosis: a classical infectious disease with persistent diagnostic challenges. Clin. Lab. Sci. 12207-212. [PubMed] [Google Scholar]
- 2.Baldwin, C. L., and M. Parent. 2002. Fundamentals of host immune response against Brucella abortus: what the mouse model has revealed about control of infection. Vet. Microbiol. 90367-382. [DOI] [PubMed] [Google Scholar]
- 3.Bayry, J., S. Lacroix-Desmazes, V. Donkova-Petrini, C. Carbonneil, N. Misra, Y. Lepelletier, S. Delignat, S. Varambally, E. Oksenhendler, Y. Levy, M. Debre, M. D. Kazatchkine, O. Hermine, and S. V. Kaveri. 2004. Natural antibodies sustain differentiation and maturation of human dendritic cells. Proc. Natl. Acad. Sci. USA 10114210-14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billard, E., C. Cazevieille, J. Dornand, and A. Gross. 2005. High susceptibility of human dendritic cells to invasion by the intracellular pathogens Brucella suis, B. abortus, and B. melitensis. Infect. Immun. 738418-8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celli, J., C. de Chastellier, D. M. Franchini, J. Pizarro-Cerda, E. Moreno, and J. P. Gorvel. 2003. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 198545-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celli, J., S. P. Salcedo, and J. P. Gorvel. 2005. Brucella coopts the small GTPase Sar1 for intracellular replication. Proc. Natl. Acad. Sci. USA 1021673-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheers, C., and F. Pagram. 1979. Macrophage activation during experimental murine brucellosis: a basis for chronic infection. Infect. Immun. 23197-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clynes, R. 2007. Protective mechanisms of IVIG. Curr. Opin. Immunol. 19646-651. [DOI] [PubMed] [Google Scholar]
- 9.Comerci, D. J., M. J. Martinez-Lorenzo, R. Sieira, J. P. Gorvel, and R. A. Ugalde. 2001. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell. Microbiol. 3159-168. [DOI] [PubMed] [Google Scholar]
- 10.Corbeil, L. B., K. Blau, T. J. Inzana, K. H. Nielsen, R. H. Jacobson, R. R. Corbeil, and A. J. Winter. 1988. Killing of Brucella abortus by bovine serum. Infect. Immun. 563251-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covell, D. G., J. Barbet, O. D. Holton, C. D. Black, R. J. Parker, and J. N. Weinstein. 1986. Pharmacokinetics of monoclonal immunoglobulin G1, F(ab′)2, and Fab′ in mice. Cancer Res. 463969-3978. [PubMed] [Google Scholar]
- 12.de Jong, M. F., Y. H. Sun, A. B. den Hartigh, J. M. van Dijl, and R. M. Tsolis. 2008. Identification of VceA and VceC, two members of the VjbR regulon that are translocated into macrophages by the Brucella type IV secretion system. Mol. Microbiol. 70:1378-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.den Hartigh, A. B., Y. H. Sun, D. Sondervan, N. Heuvelmans, M. O. Reinders, T. A. Ficht, and R. M. Tsolis. 2004. Differential requirements for VirB1 and VirB2 during Brucella abortus infection. Infect. Immun. 725143-5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enright, F. M., L. N. Araya, P. H. Elzer, G. E. Rowe, and A. J. Winter. 1990. Comparative histopathology in BALB/c mice infected with virulent and attenuated strains of Brucella abortus. Vet. Immunol. Immunopathol. 26171-182. [DOI] [PubMed] [Google Scholar]
- 15.Fabyan, M. 1912. A contribution to the pathogenesis of B. abortus, Bang-II. J. Med. Res. 26441-488. [PMC free article] [PubMed] [Google Scholar]
- 16.Fu, Y. X., and D. D. Chaplin. 1999. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17399-433. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez, M., F. Mackay, J. L. Browning, M. H. Kosco-Vilbois, and R. J. Noelle. 1998. The sequential role of lymphotoxin and B cells in the development of splenic follicles. J. Exp. Med. 187997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong, P. C., R. M. Tsolis, and T. A. Ficht. 2000. Identification of genes required for chronic persistence of Brucella abortus in mice. Infect. Immun. 684102-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izadjoo, M. J., Y. Polotsky, M. G. Mense, A. K. Bhattacharjee, C. M. Paranavitana, T. L. Hadfield, and D. L. Hoover. 2000. Impaired control of Brucella melitensis infection in Rag1-deficient mice. Infect. Immun. 685314-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jolles, S., W. A. Sewell, and S. A. Misbah. 2005. Clinical uses of intravenous immunoglobulin. Clin. Exp. Immunol. 1421-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350423-426. [DOI] [PubMed] [Google Scholar]
- 22.Ko, J., and G. A. Splitter. 2003. Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans. Clin. Microbiol. Rev. 1665-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meador, V. P., L. B. Tabatabai, W. A. Hagemoser, and B. L. Deyoe. 1986. Identification of Brucella abortus in formalin-fixed, paraffin-embedded tissues of cows, goats, and mice with an avidin-biotin-peroxidase complex immunoenzymatic staining technique. Am. J. Vet. Res. 472147-2150. [PubMed] [Google Scholar]
- 24.Moulin, V., F. Andris, K. Thielemans, C. Maliszewski, J. Urbain, and M. Moser. 2000. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J. Exp. Med. 192475-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagai, H., J. C. Kagan, X. Zhu, R. A. Kahn, and C. R. Roy. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295679-682. [DOI] [PubMed] [Google Scholar]
- 26.Nijskens, C., R. Copin, X. De Bolle, and J. J. Letesson. 2008. Intracellular rescuing of a B. melitensis 16M virB mutant by co-infection with a wild type strain. Microb. Pathog. 45134-141. [DOI] [PubMed] [Google Scholar]
- 27.Nimmerjahn, F., and J. V. Ravetch. 2007. The antiinflammatory activity of IgG: the intravenous IgG paradox. J. Exp. Med. 20411-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oberti, J., S. Sanchez, R. Caravano, and A. Loubatieres. 1975. Mise en evidence des antigenes brucelliens in situ dens les cellules du ganglion et de la rate de souris par la microscopie electronique. C. R. Acad. Sci. Paris 280503-506. [PubMed] [Google Scholar]
- 29.O'Callaghan, D., C. Cazevieille, A. Allardet-Servent, M. L. Boschiroli, G. Bourg, V. Foulongne, P. Frutos, Y. Kulakov, and M. Ramuz. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 331210-1220. [DOI] [PubMed] [Google Scholar]
- 30.Overbergh, L., A. Giulietti, D. Valckx, R. Decallonne, R. Bouillon, and C. Mathieu. 2003. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J. Biomol. Tech. 1433-43. [PMC free article] [PubMed] [Google Scholar]
- 31.Rajashekara, G., D. A. Glover, M. Krepps, and G. A. Splitter. 2005. Temporal analysis of pathogenic events in virulent and avirulent Brucella melitensis infections. Cell. Microbiol. 71459-1473. [DOI] [PubMed] [Google Scholar]
- 32.Ravetch, J. V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19275-290. [DOI] [PubMed] [Google Scholar]
- 33.Rolán, H. G., and R. M. Tsolis. 2008. Inactivation of the type IV secretion system reduces the Th1 polarization of the immune response to Brucella abortus infection. Infect. Immun. 763207-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rolán, H. G., and R. M. Tsolis. 2007. Mice lacking components of adaptive immunity show increased Brucella abortus virB mutant colonization. Infect. Immun. 752965-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roux, C. M., H. G. Rolán, R. L. Santos, P. D. Beremand, T. L. Thomas, L. G. Adams, and R. M. Tsolis. 2007. Brucella requires a functional type IV secretion system to elicit innate immune responses in mice. Cell. Microbiol. 91851-1869. [DOI] [PubMed] [Google Scholar]
- 36.Salcedo, S. P., M. I. Marchesini, H. Lelouard, E. Fugier, G. Jolly, S. Balor, A. Muller, N. Lapaque, O. Demaria, L. Alexopoulou, D. J. Comerci, R. A. Ugalde, P. Pierre, and J. P. Gorvel. 2008. Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp1. PLoS Pathog. 4e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samuelsson, A., T. L. Towers, and J. V. Ravetch. 2001. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 291484-486. [DOI] [PubMed] [Google Scholar]
- 38.Saunders, B. M., Z. Liu, Y. Zhan, and C. Cheers. 1993. Interleukin-6 production during chronic experimental infection. Immunol. Cell Biol. 71(Pt. 4)275-280. [DOI] [PubMed] [Google Scholar]
- 39.Schulein, R., P. Guye, T. A. Rhomberg, M. C. Schmid, G. Schroder, A. C. Vergunst, I. Carena, and C. Dehio. 2005. A bipartite signal mediates the transfer of type IV secretion substrates of Bartonella henselae into human cells. Proc. Natl. Acad. Sci. USA 102856-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sieira, R., D. J. Comerci, D. O. Sanchez, and R. A. Ugalde. 2000. A homologue of an operon required for DNA transfer in Agrobacterium is required in Brucella abortus for virulence and intracellular multiplication. J. Bacteriol. 1824849-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Starr, T., T. W. Ng, T. D. Wehrly, L. A. Knodler, and J. Celli. 2008. Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic 9678-694. [DOI] [PubMed] [Google Scholar]
- 42.Takai, T., M. Li, D. Sylvestre, R. Clynes, and J. V. Ravetch. 1994. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell 76519-529. [DOI] [PubMed] [Google Scholar]
- 43.Toubi, E., and A. Etzioni. 2005. Intravenous immunoglobulin in immunodeficiency states: state of the art. Clin. Rev. Allergy Immunol. 29167-172. [DOI] [PubMed] [Google Scholar]
- 44.Unkeless, J. C., and H. N. Eisen. 1975. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J. Exp. Med. 1421520-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vergunst, A. C., B. Schrammeijer, A. den Dulk-Ras, C. M. de Vlaam, T. J. Regensburg-Tuink, and P. J. Hooykaas. 2000. VirB/D4-dependent protein translocation from Agrobacterium into plant cells. Science 290979-982. [DOI] [PubMed] [Google Scholar]
- 46.Weiss, A. A., F. D. Johnson, and D. L. Burns. 1993. Molecular characterization of an operon required for pertussis toxin secretion. Proc. Natl. Acad. Sci. USA 902970-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young, E. 2000. Mandell, Douglas, and Bennett's principles and practice of infectious disease, vol. 2. Churchill Livingstone, Philadelphia, PA.
- 48.Zhan, Y., Z. Liu, and C. Cheers. 1996. Tumor necrosis factor alpha and interleukin-12 contribute to resistance to the intracellular bacterium Brucella abortus by different mechanisms. Infect. Immun. 642782-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]