Abstract
Helicobacter pylori is one of the most common bacterial pathogens, infecting up to 50% of the world's population. The host is not able to clear the infection, leading to life-long chronic inflammation with continuous infiltration of lymphocytes and granulocytes. The migration of leukocytes from the blood into inflamed tissue is dependent on adhesion molecules expressed on the vascular endothelium. The aim of this study was to characterize the effect of H. pylori-induced gastritis with regard to the expression of endothelial adhesion molecules in the gastric mucosa and compare this to other types of chronic mucosal inflammations. Our results demonstrate an increased level of expression of the adhesion molecule E-selectin, but not of intracellular adhesion molecule 1, vascular adhesion molecule 1, or vascular adhesion protein 1, in H. pylori-induced gastritis but not in gastritis induced by acetylsalicylic acid or pouchitis. The upregulated E-selectin expression was determined to be localized to the gastric mucosa rather than being a systemic response to the infection. Moreover, the H. pylori type IV secretion system encoded by the cag pathogenicity island (cagPAI) was found to be an important determinant for the upregulation of human endothelial E-selectin expression in vitro, and this process is probably dependent on the CagL protein, mediating binding to α5β1 integrins. Thus, endothelial E-selectin expression induced by H. pylori probably contributes to the large influx of neutrophils and macrophages seen in infected individuals, and our results suggest that this process may be more pronounced in patients infected with cagPAI-positive H. pylori strains and may thereby contribute to tissue damage in these individuals.
Helicobacter pylori is a gram-negative, microaerophilic rod and one of the most common bacterial pathogens, colonizing the stomach epithelium of up to 50% of the world's population (7, 8). The prevalence of infection varies among geographic regions and ethnicities and can be correlated with a low socioeconomic status (23). Once the bacterium has colonized the mucosa, chronic inflammation with a characteristic histological profile involving the degeneration of the surface epithelium and infiltration of acute and chronic inflammatory cells into the gastric mucosa will develop (7). Although an immune response is evoked in the host, the infection is not eradicated but usually persists throughout life. Virulent H. pylori strains harbor a type IV secretion system (T4SS) encoded by the cag pathogenicity island (cagPAI), with CagA being the only known effector protein (3). Within the CagPAI, CagL is a protein that is highly conserved among pathogenic H. pylori strains. It was recently demonstrated that CagL is targeted to the pilus surface and that it is involved in triggering the injection of CagA upon contact with host cells. CagL interacts with host cells through α5β1 integrin, most likely in an arginine-glycine-aspartate (RGD)-dependent manner (21).
The transendothelial migration of lymphocytes is essential in steady state as well as for inflammation, and interactions between leukocytes and epithelial cells play an important role in H. pylori-associated gastric inflammation (26). The recruitment of leukocytes from the bloodstream into tissues is a multistep process involving selectins, chemokines, and integrins in a coordinated fashion (37). Selectins are a family of adhesive receptors expressed on platelets, leukocytes, and endothelial cells (6). E-selectin is normally expressed on endothelial cells following exposure to inflammatory chemokines but not on the surface of resting endothelial cells (6, 27). Other adhesion molecules exposed by the activated endothelium include vascular adhesion molecule 1 (VCAM-1) and intracellular adhesion molecule 1 (ICAM-1), which are members of the immunoglobulin superfamily and are expressed on the endothelium as a response to the exposure of proinflammatory cytokines (39).
The endothelial expression of VCAM-1 and ICAM-1 is upregulated in the acute phase of H. pylori infection, and neutrophils may be recruited to the mucosa via binding to these adhesion molecules. Previous studies have shown elevated levels of VCAM-1 and ICAM-1 in H. pylori-associated gastritis in patients with dyspepsia compared to levels in H. pylori-uninfected individuals (15). Vascular adhesion protein 1 (VAP-1) is another endothelial molecule that supports the binding of leukocytes to the endothelium (33). The surface expression of VAP-1 is upregulated after prolonged chronic inflammation in the gut (2, 34). In addition, VAP-1 also seems to play a major role in determining the flux of lymphocytes in healthy vascular beds (42). Another recently described adhesion molecule, common lymphatic endothelial receptor 1 (CLEVER-1), is a glycoprotein that mediates the binding of lymphocytes to the surface of both the lymphatic endothelium and high endothelial venules (18). However, the expressions of VAP-1 and CLEVER-1 have not yet been examined with regard to gastric inflammation.
We have previously shown that only H. pylori-infected individuals mount a vaccine-specific immune response in the gastric mucosa following oral immunization. Duodenal B-cell responses are similar in H. pylori-infected and -uninfected individuals, while gastric responses have been reported only for patients with H. pylori-induced gastritis (24, 29). This effect is dependent on the migration of effector B cells from the circulation, rather than local antigen uptake and B-cell differentiation, and cannot be mimicked by chemical gastritis induced by acetylsalicylic acid (ASA) (29). Since the level of expression of the immunoglobulin superfamily member mucosal addressin cellular adhesion molecule 1 is not changed by H. pylori infection (28), we hypothesized that other adhesion molecules may contribute to lymphocyte recruitment in H. pylori-induced gastritis.
We therefore investigated the effect of chronic H. pylori-induced inflammation on the expression of endothelial adhesion molecules in the gastrointestinal mucosa and compared that to data for chemical gastritis induced by ASA and to data for patients suffering from pouchitis. We show that the expression of E-selectin but not those of ICAM-1, VCAM-1, and VAP-1 is selectively upregulated on endothelial cells in the human gastric mucosa following infection with H. pylori and that an increased level of E-selectin expression is a local effect of the H. pylori infection rather than a systemic response. Finally, a functional cagPAI with CagL is necessary for H. pylori-induced E-selectin expression on human endothelial cells in vitro.
MATERIALS AND METHODS
Volunteers and collection of specimen.
This study was performed following approval from the human ethics committee of the Medical Faculty at the University of Gothenburg, and all volunteers gave informed consent to participate in the study. Sixteen H. pylori-infected volunteers (6 females and 10 males aged 26 to 67 years) and 17 uninfected individuals (eight females and nine males aged 24 to 54 years) were recruited from among blood donors at Sahlgrenska University Hospital. Helicobacter infection was confirmed or excluded by culture on Scirrow agar plates as well as serology and pathology reports. Ten antrum biopsy specimens were taken from each volunteer by endoscopy. Three of the biopsy specimens were embedded in optimal cutting temperature, snap-frozen, and stored at −70°C for subsequent immunohistochemical analyses. Three biopsy specimens were snap-frozen and stored in −70°C for subsequent protein extraction. An additional three biopsy specimens were put in RNA Later (Ambion) overnight and then moved to −70°C until RNA isolation. One biopsy specimen from each volunteer was also formalin fixed, paraffin embedded, and analyzed by an experienced histopathologist for grade of gastritis as well as the presence of Helicobacter-like organisms (HLOs) using the updated Sydney system (9). Two weeks later, the uninfected volunteers received a 3-day treatment with ASA (1.5 g/day).
In addition, plasma samples from 14 H. pylori-infected individuals (3 females and 11 males aged 21 to 69 years) and 14 uninfected individuals (3 females and 11 males aged 24 to 69 years) were collected from among blood donors at Sahlgrenska University Hospital.
In the second part of the study, patients (two women and three men aged 43 to 52 years) who had undergone colectomies due to ulcerative colitis were recruited from the follow-up program at the Department of Surgery at Sahlgrenska University Hospital. The patients had undergone continence surgery 5 to 12 years prior to this study by the construction of a pelvic pouch with an ileoanal anastomosis. The maximal extent of the small-bowel resection was 10 cm of the distal ileum. None of the participating patients had episodes of acute pouchitis or extraintestinal manifestations of ulcerative colitis during the 3 years prior to the study. Subclinical pouchitis, however, results in neutrophil-mediated inflammation with no need for medication, and all participating patients were in good general health. Five biopsy specimens were collected from the ileal pouch and 12 pinch biopsy specimens were collected from the duodenum from each patient (20).
Immunohistochemistry.
The expression of the adhesion molecules ICAM-1 (clone 6.5B5; Dako), VCAM-1 (clone 51-10C9; BD Pharmingen), E-selectin (clone 1.2B6; Dako), CLEVER-1, and VAP-1 (clones 3-266 and 2D10 SFS, respectively [kindly provided by professor S. Jalkanen, University of Turku]) as well as the endothelial marker Von Willebrand factor were detected using immunohistochemical staining. Cryo-cut tissue sections (8 μm) were fixed in ice-cold acetone and air dried. Endogenous peroxidase was blocked with glucose-oxidase (Sigma-Aldrich). The slides used for ICAM-1 and VAP-1 staining were also blocked with 1% dry milk in phosphate-buffered saline (PBS) for 30 min at room temperature, and these antibodies were also diluted in 1% dry milk in PBS. All slides were incubated with optimal dilutions of the respective primary antibody determined from pilot experiments or with mouse immunoglobulin G1 (IgG1) isotype control (Dako) in PBS at room temperature for 1 h, followed by a 30-min incubation with horseradish peroxidase-conjugated rabbit antibodies to mouse IgG1 in PBS complemented with 5% human AB+ serum. Signal was detected by use of an AEC detection system (Vector, United Kingdom), and the sections were then counterstained with Mayer's hematoxylin and mounted. The total number of vessels, as well as expressing adhesion molecules, was determined using a Zeiss Axioskop 2 apparatus (Carl Zeiss). The area of each tissue section was measured using Zeiss AxioVisoin (Carl Zeiss), and staining was expressed as the number of positive vessels/total number of vessels per μm2. There was no difference in the total numbers of vessels between H. pylori-infected and -uninfected volunteers or before and after ASA treatment.
H. pylori strains and culturing conditions.
The following isogenic mutants of cagPAI-positive strains P1 and P12 were produced by the insertion of a chloramphenicol or kanamycin resistance gene cassette: P1ΔvirD4, P1ΔvirB10, P1ΔcagA, P1ΔnapA, P1ΔvacA, P1ΔureA, and P12ΔcagL (21, 35). All H. pylori strains were cultured on Columbia ISO-A plates in a microaerophilic milieu at 37°C with the addition of 6 μg/ml chloramphenicol or 12 μg/ml kanamycin. After 4 days, bacteria were scraped off the plates and resuspended in 600 μl PBS, and the optical density (OD) of the suspension was determined at 600 nm. The final working solution was adjusted to an OD of 1.0 in PBS, which corresponds to approximately 5 × 109 H. pylori bacteria/ml.
Subculture and stimulation of human endothelial cells.
Human umbilical vein cells (HUVECs) from the first passage were grown in M200 medium supplemented with a Low Serum Growth supplement kit (all from Cascade Biologics Inc.) containing penicillin, streptomycin, amphotericin B, fetal bovine serum, hydrocortisone, human epidermal growth factor, basic fibroblast growth factor, and heparin. Cells were incubated in a humidified atmosphere of 5% CO2 and 95% air at 37°C with a change of medium every 48 h. In passage 4, cells were frozen in fetal calf serum containing 10% dimethyl sulfoxide and stored in liquid nitrogen. Cells were used within passages 4 to 7 in all experiments.
HUVECs were diluted to a concentration of 50,000 cells/ml in supplemented M200 medium. Two milliliters of this suspension was then added to each well of a 12-well plate (Nunc, Roskilde, Denmark) and incubated for 48 h at 37°C in 5% CO2. Before stimulation, old medium was removed, and 1 ml fresh medium was added to each well. Fifty microliters of live bacteria at an OD of 1.0, 50 μl of PBS, or 100 ng of tumor necrosis factor alpha (TNF-α) (Peprotech, United Kingdom) in a volume of 50 μl PBS was added. Plates were then incubated for 6 h at 37°C in 5% CO2. The incubation time had been optimized for studying the expression of E-selectin in preceding pilot experiments (data not shown).
FACS analysis of E-selectin, ICAM-1, and VCAM-1 expression in HUVECs.
After stimulation, culture medium was removed, and cells were washed with PBS three times. HUVECs were detached by using 300 μl trypsin-EDTA solution/well, and 1 ml of trypsin neutralizer solution (both from Cascade Biologics Inc.) was then added to neutralize the enzyme. Cell suspensions were added to 1.5-ml tubes and centrifuged at 1,500 rpm for 10 min. Supernatants were discarded, and cells were resuspended in fluorescence-activated cell sorter (FACS) wash buffer (PBS containing 1.46 g of EDTA/liter, 2.5 g of serum albumin/liter, 0.2 g of NaN3/liter, and 2% AB+ human serum), divided into six aliquots, and incubated with mouse IgG1 monoclonal antibody to human E-selectin (CD62E), phycoerythrin-conjugated anti-VCAM-1, fluorescein isothiocyanate-conjugated anti-ICAM-1, isotype control mouse IgG1, or phycoerythrin- or fluorescein isothiocyanate-conjugated mouse negative controls (both from DakoCytomation) diluted in FACS wash buffer for 30 min at 4°C. After incubation, cells were washed once in FACS wash buffer, and a rat antibody to mouse IgG1 (Becton Dickinson) diluted 1:50 in FACS wash buffer was added to wells previously incubated with E-selectin-specific or isotype control mouse IgG1. After incubation for 30 min at 4°C, cells were washed and finally fixed using 150 μl Cellfix (Becton Dickinson, Belgium) and then analyzed for adhesion molecule expression by flow cytometry (FACSCalibur; Becton Dickinson).
Protein extraction.
Three biopsy specimens from each volunteer (eight H. pylori-infected and nine uninfected volunteers) were incubated in PBS containing saponin, soybean trypsin inhibitor, Pefabloc, and bovine serum albumin overnight at 4°C as previously described (5). Each suspension was then centrifuged, and supernatants were collected for detection of E-selectin. Total protein concentrations in the extracts were measured using the BCA protein assay (Thermo Scientific) following desalting on Zeba Micro Desalt spin columns (Pierce).
Detection of soluble E-selectin in plasma and protein extracts.
The concentrations of soluble E-selectin in the plasma and protein extracts of H. pylori-infected and -uninfected individuals (eight and nine samples, respectively, for saponin and 14 samples each, respectively, for plasma) were determined by using a Duoset enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Abingdon, United Kingdom) according to the manufacturer's instructions. Serum samples were diluted 10-fold prior to analysis, and protein extracts were diluted 3-fold. The detection limit of the assay was 42 pg/ml. E-selectin concentrations in protein extracts were adjusted to total protein concentrations.
RNA isolation.
Biopsy specimens collected from H. pylori-infected and -uninfected individuals (eight and nine individuals, respectively) were homogenized using a tissue lyser (TissueLyserII; Retsch, Qiagen) in the presence of lysis buffer (RNeasy minikit; Qiagen, Germany), and total RNA was extracted according to the manufacturer's descriptions. Extracted RNA was run on a 1% agarose gel to check RNA integrity, and the concentration was measured using NanoDrop ND-1000 spectrophotometer software, version 2.5.1. Extracted RNA was stored at −70°C until further use.
DNA isolation.
H. pylori strains were available from five of the infected volunteers and were grown for 3 days on Columbia ISO-A plates. If the strains were not available, frozen biopsy specimens of antrum tissue were used. DNA was extracted from bacterial centrifugal pellets or from frozen biopsy specimens. A Qiagen DNeasy tissue kit was used according to the manufacturer's instructions, with pretreatment for gram-negative bacteria, and eluted in 100 μl elution buffer. DNA was kept at −20°C until further use.
Quantitative real-time reverse transcription (RT)-PCR.
To detect E-selectin expression in gastric tissue, preparation of cDNA was performed using the Omniscript kit (Qiagen, Germany). For each 20-μl reaction mixture, 500 ng total RNA was used. The gene expression assay for E-selectin was obtained from Applied Biosystems. The relative-quantification PCR mixtures, with a total volume of 20 μl, were run in 96-well plates using standard amplification conditions for the 7500 real-time PCR system (Applied Biosystems). Each reaction mixture contained 1 μl cDNA (25 ng), 2× universal PCR master mix (Roche), RNase-free water, and 1 μl primer. All reactions were run in duplicate, and the hypoxanthine phosphoribosyltransferase (HPRT) housekeeping gene was used as a reference gene throughout the entire experiment. For calculating the difference in expression levels, we used the comparative threshold cycle method, also called the 2−ΔΔCT method. Data are presented as the change in mRNA expression normalized to the sample expressing the smallest amounts of E-selectin.
To determine the presence of the cagPAI in H. pylori strains, absolute-quantification real-time PCRs were run in 96-well plates with a total volume of 20 μl in each reaction mixture. The PCR mix contained 10 μl SYBR green real-time PCR master mix (Applied Biosystems), 10 pmol of each primer, 2 μl of sample, and 6 of μl DNase-free water. Primers for cagA and hpaA were obtained from Applied Biosystems. A negative control as well as a standard curve made from RNase-treated genomic DNA extracted from H. pylori strain J99 were included in each run. The standard curve ranged from 101 to 105 copies per μl. Analysis was performed by use of a 7500 real-time PCR system (Applied Biosystems) using the default settings, with the modification that 45 cycles were run. The program ended with a dissociation step for melting-temperature analysis of each product to confirm amplification specificity. Analysis and quantification were performed using the instrument's software.
Statistics.
All statistical analyses were carried out with SPSS 14.0 using a Wilcoxon or a two-tailed Mann-Whitney test. P values of less than 0.05 were considered to be significant.
RESULTS
Inflammation and bacterial load.
Biopsy samples from uninfected subjects were histologically normal without inflammation or HLOs. In contrast, active chronic inflammation and HLOs were observed in biopsy specimen samples from all H. pylori-infected subjects. The chronic inflammation score according to the updated Sydney system was 1.88 ± 0.46 (mean ± standard deviation), and the active inflammation score was 1.0 ± 0.93. The HLO score was 1.75 ± 0.46. No athrophy or intestinal metaplasia was seen in any of the subjects (data not shown).
Adhesion molecule expression by vessel endothelium in H. pylori-induced gastritis.
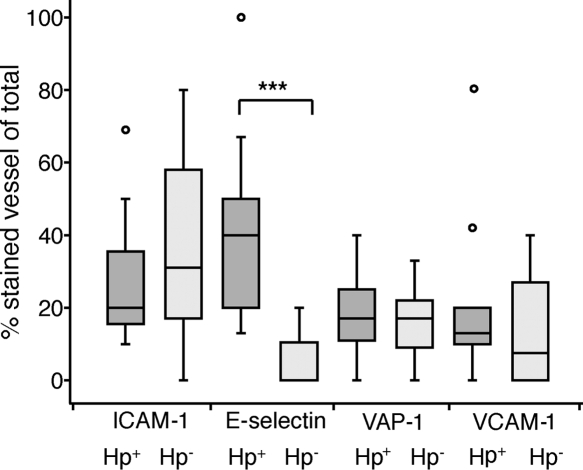
In a first set of experiments, we aimed to examine differences in expression levels of inflammation-related adhesion molecules on endothelial cells in H. pylori-infected individuals and healthy volunteers. For this purpose, cryo-cut sections of gastric biopsy specimens collected from individuals with H. pylori-induced gastritis as well as healthy volunteers were analyzed using immunohistochemistry. The level of expression of E-selectin was low in most uninfected volunteers, while H. pylori-infected individuals had a significantly higher level of expression of E-selectin (Fig. 1 and 2). In contrast, the levels of expression of ICAM-1 varied considerably in the group of uninfected volunteers, as did VCAM-1 and VAP-1 expression levels although to a somewhat lesser extent, and H. pylori infection did not result in any major changes in this pattern (Fig. 2).
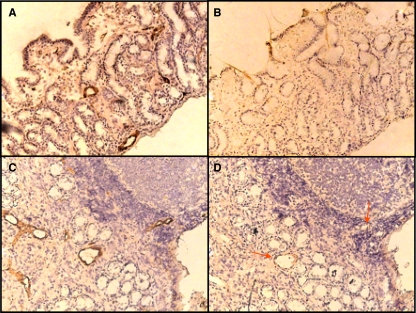
FIG. 1.
Expression of E-selectin in gastric mucosa. Von Willebrand factor and E-selectin expression levels were determined by immunohistochemistry, and sections were counterstained with hematoxylin. (A) Total number of vessels in a noninfected individual as determined by Von Willebrand factor. (B) Expression of E-selectin in a noninfected individual. (C) Total number of vessels in an H. pylori-infected individual as determined by Von Willebrand factor. (D) Expression of E-selectin in an H. pylori-infected individual. Arrows indicate examples of E-selectin-positive blood vessels. Magnification, ×20.
FIG. 2.
Endothelial expression of adhesion molecules in individuals with H. pylori-induced gastritis (H. pylori positive [Hp+])and healthy noninfected individuals (H. pylori negative [Hp−]). ICAM-1, E-selectin, VAP-1, and VCAM-1 expression levels were determined by immunohistochemistry and are presented as the proportions of positive vessels compared to all vessels (stained with Von Willebrand factor). The box represents the middle 50% of the data, and the line represents the median. The difference of the end of the whiskers is the range, and the circles are outliers. ***, P < 0.001.
Adhesion molecule expression by vessel endothelium in jejunal pouchitis.
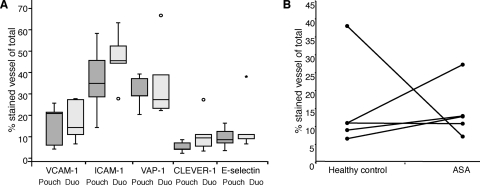
To examine the levels of expression of adhesion molecules on the surface of the vascular endothelium during another chronic mucosal inflammation, tissue specimens from colectomized patients with jejunal pouchitis were compared to healthy duodenum samples from the same individuals. We found that there was no change in levels of expression of any of the adhesion molecules ICAM-1, VCAM-1, E-selectin, VAP-1, or CLEVER-1 as a result of jejunal pouchitis (Fig. 3A).
FIG. 3.
(A) VCAM-1, ICAM-1, E-selectin, VAP-1, and CLEVER-1 expression in jejunal pouchitis (Pouch) and healthy duodenum (Duo). Adhesion molecule expression was determined by immunohistochemistry and is presented as the proportion of positive vessels out of all vessels (stained with Von Willebrand factor). The box represents the middle 50% of the data, and the line represents the median. The difference of the end of the whiskers is the range, and the circles are outliers. (B) Expression of E-selectin in gastric mucosa of healthy individuals before and after treatment with ASA. E-selectin expression levels were determined by immunohistochemistry and are presented as the percentage of E-selectin-positive vessels out of all vessels (stained with Von Willebrand factor). Circles show individual values before and after ASA treatment.
E-selectin expression by vessel endothelium in ASA-induced gastritis.
Next, we wanted to investigate if upregulated E-selectin expression was specific to H. pylori-induced gastritis or if it could be mimicked by chemically induced gastritis. We used ASA to induce gastritis in the same healthy volunteers 2 weeks after the initial endoscopy with biopsy specimen sampling. ASA treatment leads to a relatively mild gastritis associated with a high level of production of proinflammatory cytokines and recruitment of neutrophils (13). This is not a replicate of H. pylori-induced gastritis: the cytokines produced differ partly but represent a possibility for the investigation of another type of gastric inflammation. Immunohistochemistry was performed on cryo-cut sections from healthy volunteers before and after ASA-induced gastritis. Our results revealed that the level of expression of E-selectin was not significantly changed during chemical gastritis (Fig. 3B).
Gastric and serum levels of E-selectin in H. pylori-infected and -uninfected volunteers.
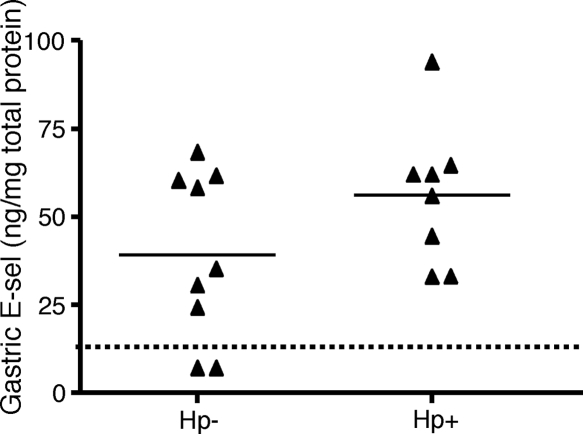
Since immunohistochemistry data showed an increased level of expression of E-selectin in H. pylori-infected individuals, we wished to verify and better quantitate the level of gastric E-selectin expression. We first used saponin-extracted biopsy specimens from the antrum of H. pylori-infected and -uninfected individuals to determine gastric E-selectin protein levels using ELISA. The amount of E-selectin in the gastric samples was generally low, and in two samples, it was below the detection limit (Fig. 4). We could not detect any significant difference in the amounts of E-selectin between the two groups, but the data were nevertheless skewed toward an increased concentration of E-selectin in the H. pylori-infected individuals compared to that in uninfected individuals (means, 39.7 and 56.3 ng/mg total protein, respectively) (Fig. 4).
FIG. 4.
Expression of E-selectin in gastric tissues. The levels of E-selectin (E-sel) in saponin-extracted gastric biopsy specimens were measured by ELISA and adjusted to total protein levels in the extract. Symbols represent individual values, lines represent the means, and the dotted line represents the detection limit. Hp, H. pylori.
We also investigated whether the increased level of expression of E-selectin seen on the endothelial cells of H. pylori-infected individuals was a local response or if there was a general upregulation of E-selectin in a systemic response to the infection. H. pylori-infected and healthy volunteers were recruited among blood donors, and the serum levels of E-selectin were determined by ELISA. The mean serum concentration of E-selectin for the H. pylori-infected group was 18.8 ng/ml (range, 11.5 to 31.5 ng/ml), and that for the uninfected group was 18.9 ng/ml (range, 9.4 to 49.3 ng/ml), and there was no statistically significant difference in the concentrations of E-selectin between the groups (data not shown). These results clearly indicate that the increased level of expression of E-selectin is restricted to the endothelium in the gastric mucosa upon H. pylori infection rather than a systemic response to the infection.
Expression of E-selectin mRNA in H. pylori-infected and -uninfected mucosa.
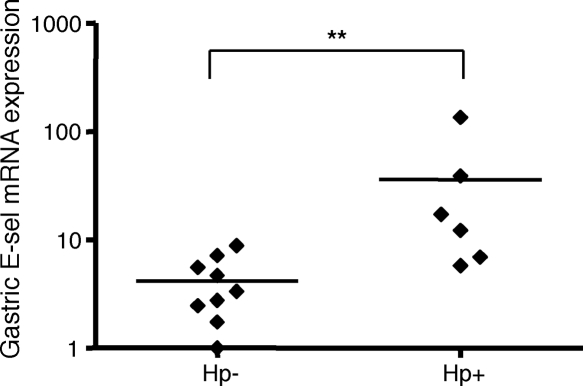
Given that E-selectin expression was selectively upregulated on the gastric blood vessel walls of H. pylori-infected individuals, we also examined whether this increase was caused by a parallel increase in E-selectin mRNA levels. The levels of E-selectin mRNA in H. pylori-infected and uninfected mucosa were analyzed using relative-quantification real-time RT-PCR. The mean change was standardized to an endogenous reference gene (HPRT) and then normalized to the lowest value of E-selectin expression. HPRT expression as well as blood vessel density were not influenced by H. pylori infection (data not shown), while there was a statistically significant difference in the levels of expression of E-selectin between the groups (P = 0.0048), indicating that the increase in levels of E-selectin was indeed due to an increased level of transcription of the E-selectin gene (Fig. 5).
FIG. 5.
E-selectin mRNA levels in gastric tissues. The mRNA levels of E-selectin in the mucosa of H. pylori-infected and -uninfected individuals were measured by relative-quantification real-time RT-PCR. The E-selectin mRNA expression level is presented as the change standardized to an endogenous reference gene (HPRT) and then normalized to the sample expressing the smallest amount of E-selectin mRNA. Symbols represent individual values, and lines represent the means. **, P < 0.005. Hp, H. pylori.
Adhesion molecule expression on human endothelial cells after H. pylori stimulation.
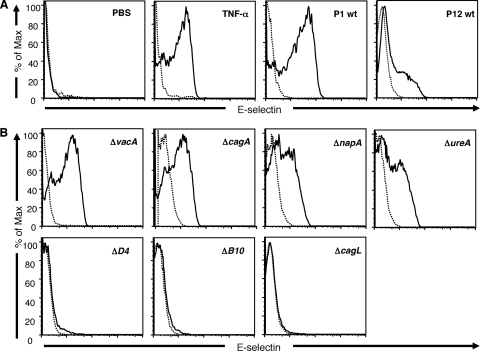
With the knowledge that H. pylori is able to induce endothelial E-selectin expression in vivo, we wanted to investigate if the bacteria are able to directly induce E-selectin expression on cultured human endothelial cells and to identify the bacterial factor(s) mediating the effect. For this purpose, HUVECs were incubated with cagPAI-positive H. pylori strains P1 and P12 for 6 h, and the levels of expression of E-selectin, ICAM-1, and VCAM-1 were analyzed by flow cytometry. H. pylori strains P1 and P12, as well as the positive control TNF-α (100 ng/ml), always induced a significantly increased level of expression of E-selectin compared to that for the unstimulated control (PBS), even though the levels of expression of E-selectin varied between experiments and strains (Fig. 6A). We then used the isogenic mutants P1ΔvacA, P1ΔcagA, P1ΔnapA, P1ΔureA, P1ΔvirD4, and P1ΔvirB10 of strain P1 and P12ΔcagL of strain P12 to stimulate human endothelial cells in parallel with wild-type (wt) strains P1 and P12. Stimulation with the P1ΔvacA mutant induced E-selectin expression to the same extent as did stimulation with wt P1, demonstrating that the VacA cytotoxin is not involved in the induction of E-selectin expression on human endothelial cells (Fig. 6B). Furthermore, the isogenic mutants P1ΔcagA, P1ΔnapA, and P1ΔureA were all able to induce a significant level of expression of E-selectin but to a somewhat lesser extent than did wt P1 (Fig. 6B). In contrast, the P1ΔvirD4 and P1ΔvirB10 mutants, which carry mutations in two of the structural proteins of the T4SS in the cagPAI, totally abolished E-selectin expression on endothelial cells (Fig. 6B). Also, the P12ΔcagL mutant was unable to induce E-selectin expression on HUVECs, while a significant upregulation was detected by parental wt strain P12 (Fig. 6A and B). Taken together, these results imply that a functional cagPAI expressing CagL is important for the H. pylori-induced endothelial upregulation of E-selectin.
FIG. 6.
Expression of E-selectin in HUVECs after stimulation with H. pylori bacteria. The expression of E-selectin was evaluated on HUVECs by flow cytometry after 6 h of stimulation with a 50-μl H. pylori suspension (OD of 1.0), 100 ng/ml of TNF-α, or PBS (unstimulated control). (A) E-selectin (solid lines) and isotype control (dashed lines) expression levels on HUVECs incubated with PBS (negative control), TNF-α (positive control), and H. pylori wt strains P1 and P12. (B) E-selectin (solid line) and isotype control (dashed line) expression levels on HUVECs after stimulation with H. pylori isogenic mutants P1ΔvacA, P1ΔcagA, P1ΔnapA, P1ΔureA, P1ΔvirD4, and P1ΔvirB10. The results shown are from one representative experiment out of three to six experiments.
Unstimulated HUVECs did not express VCAM-1, and the stimulation of HUVECs with any of the H. pylori strains used in the study did not upregulate the expression of VCAM-1, whereas TNF-α induced a high level of expression of VCAM-1 (data not shown). On the other hand, when examining ICAM-1 expression levels, we saw that this more or less correlated with the level of expression of E-selectin for the isogenic mutants tested (data not shown), with the exception that untreated HUVECs already expressed some ICAM-1.
Detection of CagA DNA in mucosa and clinical isolates from H. pylori-infected and -uninfected individuals.
Since a functional cagPAI was important for H. pylori to be able to induce E-selectin expression in vitro, the next step was to see if the H. pylori strains from our clinical specimens express CagA, which is used as a marker for the cagPAI. The presence or absence of the CagA gene was analyzed using absolute-quantification real-time RT-PCR on DNA from five clinical isolates and four antrum biopsy specimens collected from volunteers when no H. pylori isolates were available. We were able to retrieve bacterial DNA from all clinical isolates and three of the biopsy samples as indicated by our positive control, hpaA (data not shown). Out of these eight specimens, all were positive for the cagA gene, indicating that the E-selectin expression which we demonstrated in vivo may indeed be a result of a functional cagPAI.
DISCUSSION
During infections, endothelial cells have a key function in promoting leukocyte entry into inflamed areas. This is dependent largely upon the expression of several adhesion molecules on endothelial cells in the vascular system. In this study, we show that H. pylori-induced gastritis results in a selective upregulation of the adhesion molecule E-selectin, while other adhesion molecules remained at the same levels as those seen in healthy gastric mucosa. This was further confirmed at the mRNA level, showing that H. pylori-induced inflammation induces substantially increased levels of transcription of E-selectin mRNA. The increased E-selectin expression level was specific for H. pylori-induced inflammation and could not be seen for chemical gastritis or jejunal pouchitis. When we examined the sera of H. pylori-infected and -uninfected volunteers for soluble E-selectin, we could not record any difference between the two groups, suggesting that the upregulation of E-selectin is a local effect rather than part of a systemic response to H. pylori infection.
E-selectin is present on endothelial cells and plays a central role in the interaction with carbohydrates expressed on the leukocyte surface during the recruitment of leukocytes to inflamed tissue. It is not present on unstimulated endothelial cells, but levels of gene transcription and protein expression are rapidly increased upon activation by proinflammatory molecules such as TNF-α and lipopolysaccharides (19, 37). In addition, our results from this study also show that endothelial E-selectin expression can be directly induced by live H. pylori bacteria. In vivo, however, other factors like cytokines may also influence the expression of E-selectin. Sialyl− Lewisx and sialyl− Lewisa are ligands for E-selectin and are expressed on both neutrophils and monocytes (22). Previous studies have also shown that the lipopolysaccharides of H. pylori strains contain sequences that are related to the sialyl− Lewisx and sialyl− Lewisa antigens and that the bacteria are in fact able to bind E-selectin (1, 12). Increased levels of endothelial expression of E-selectin would probably lead to an increased recruitment of neutrophils and monocyte-derived macrophages to the tissue. In fact, this correlates well with the histological changes seen in H. pylori-induced gastritis, which are characterized by a large influx of neutrophils and mononuclear cells and an increase in the macrophage population (3, 11). These cells are likely to contribute to tissue damage by their production of reactive oxygen species and matrix-degrading enzymes (4, 30). It is less likely, though, that E-selectin is involved in the recruitment of effector B cells to the gastric mucosa, since lymphocytes lack the expression of sialylated Lewisx or Lewisa unless they are transformed by Epstein-Barr virus infection (25). Instead, other factors such as chemokine production may contribute to B-cell accumulation (14).
In contrast to our findings, a study reported previously by Hatz et al. (15) showed increased levels of expression of the adhesion molecules VCAM-1 and ICAM-1 but no significant increase in the level of expression of E-selectin in H. pylori-induced gastritis. Also, Higuchi et al. previously found an increase in levels of ICAM-1 expression but almost no expression of E-selectin in the lamina propria of H. pylori-infected individuals (16). The difference between the studies may be caused by the recruitment of volunteers donating specimens or possibly by the use of different antibody clones to detect endothelial adhesion molecules. In our study, all participants were asymptomatic, even those with H. pylori-induced gastritis, whereas in the study described previously Hatz and coworkers, the participants were recruited among patients suffering from dyspepsia. Furthermore, our previous in vitro studies showed that different clinical isolates of H. pylori influence the expression of adhesion molecules unequally even if they all express the major virulence factors cagPAI and VacA similarly (17). Differences in H. pylori strains may also affect the results in different studies.
In a previous in vitro study, we demonstrated that live H. pylori cells stimulate HUVECs to upregulate the expression of the adhesion molecules VCAM-1, ICAM-1, and E-selectin (17). There was a large variation in the levels of expression of E-selectin induced by the different clinical isolates used in the previous study, but we were unable to identify any specific H. pylori factors regulating E-selectin expression. In the current study, however, the use of isogenic H. pylori strains deficient in selected bacterial virulence factors enabled the identification of the T4SS encoded by the cagPAI and, in particular, the CagL protein as being the most important E-selectin inducing H. pylori components. These findings are in agreement with our previous findings that the ΔvirD4 and ΔvirB7 mutants also had a reduced ability to activate endothelial cells to support T-cell transendothelial migration (10). Interestingly, the CagA protein was not necessary for increased levels of E-selectin expression, suggesting a mechanism other than CagA phosphorylation for signal transduction. A strong candidate is the CagL protein, which is the only product encoded by the cagPAI that contains an arginine-glycine-aspartate (RGD) motif (21), enabling it to serve as a site of recognition for integrins (32). CagL has recently been shown to be targeted to the pilus surface, where it interacts with integrin β1, leading to the activation of Src and FAK, which in turn activates JUN N-terminal kinase (JNK). Recent studies also prove that CagL indeed activates JNK and downstream signaling via integrin β1 in gastric epithelial cells (36). This signal pathway is most likely to participate in H. pylori-induced E-selectin expression since JNK is necessary for the endothelial expression of E-selectin (31, 36, 41). In addition, part of the cagPAI-dependent signaling may be mediated by Nod1, reacting with peptidoglycan introduced by the T4SS, as previously described by Viala et al. (40). Nod1 activation will result in the activation of NF-κB as well as mitogen-activated protein kinase and JNK (38), which are all involved in the endothelial expression of E-selectin (31). Our hypothesis that the cagPAI is important for inducing E-selectin expression on human endothelial cells is further supported by the demonstration that all H. pylori strains isolated from volunteers with increased E-selectin expression levels in vivo were positive for cagA, which was used as a marker for the presence of the cagPAI.
We could not detect any significant differences in the levels of expression of E-selectin on endothelial cells in tissues with chemically induced gastritis compared to those in normal mucosa or for jejunal pouchitis. These are both conditions characterized by a preferential recruitment of neutrophils and the production of proinflammatory cytokines. They were, however, not used as models for H. pylori gastritis but rather for two other types of mucosal inflammation in order to determine specific components of H. pylori-induced inflammation. The large influx of neutrophils in chemical gastritis and pouchitis may lead one to anticipate a high level of expression of E-selectin. Instead, H. pylori-induced gastritis, which also involves a large accumulation of mononuclear cells in the gastric mucosa, shows a higher level of E-selectin expression. These findings indicate that E-selectin and its ligands are preferentially mediating mononuclear cell extravasation and migration into inflamed tissues in the setting of mucosal inflammation. The specific upregulation of E-selectin and the relative inability of the host to respond with significantly increased VCAM-1, ICAM-1, and VAP-1 levels in H. pylori-induced gastritis may contribute to the persistence of the bacterium at the epithelial cell surface.
In conclusion, we show that endothelial E-selectin is significantly and exclusively upregulated in H. pylori-induced gastritis, most likely in a cagPAI-dependent manner. Our findings implicate E-selectin as being an important mediator of the continuous recruitment of inflammatory cells during H. pylori-induced gastritis and emphasize the importance of the cagPAI in the maintenance of the inflammatory response.
Acknowledgments
This work was supported by the Swedish Science Council (06X-13428), Signhild Engkvist's foundation, the Kungliga och Hvitfeldtska Foundation, and Wilhelm & Martina Lundgren's foundation.
We are grateful for the volunteers who participated in this study as well as to My Engström, Eva Peras, and Mikael Hermansson for their valuable help with tissue collection. We are also thankful to Anders Janzon for help with the absolute-quantification real-time PCR.
We declare no financial or commercial conflicts of interest.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 4 May 2009.
REFERENCES
- 1.Appelmelk, B. J., M. A. Monteiro, S. L. Martin, A. P. Moran, and C. M. Vandenbroucke-Grauls. 2000. Why Helicobacter pylori has Lewis antigens. Trends Microbiol. 8565-570. [DOI] [PubMed] [Google Scholar]
- 2.Arvilommi, A. M., M. Salmi, K. Kalimo, and S. Jalkanen. 1996. Lymphocyte binding to vascular endothelium in inflamed skin revisited: a central role for vascular adhesion protein-1 (VAP-1). Eur. J. Immunol. 26825-833. [DOI] [PubMed] [Google Scholar]
- 3.Backert, S., and M. Selbach. 2008. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell. Microbiol. 101573-1581. [DOI] [PubMed] [Google Scholar]
- 4.Bergin, P. J., E. Anders, W. Sicheng, J. Erik, A. Jennie, L. Hans, M. Pierre, P. H. Qiang, and Q. J. Marianne. 2004. Increased production of matrix metalloproteinases in Helicobacter pylori-associated human gastritis. Helicobacter 9201-210. [DOI] [PubMed] [Google Scholar]
- 5.Bergquist, C., A. Mattsson-Rydberg, H. Lonroth, and A. Svennerholm. 2000. Development of a new method for the determination of immune responses in the human stomach. J. Immunol. Methods 23451-59. [DOI] [PubMed] [Google Scholar]
- 6.Bevilacqua, M. P., and R. M. Nelson. 1993. Selectins. J. Clin. Investig. 91379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodger, K., and J. E. Crabtree. 1998. Helicobacter pylori and gastric inflammation. Br. Med. Bull. 5411. [DOI] [PubMed] [Google Scholar]
- 8.Covacci, A., et al. 1999. Helicobacter pylori virulence and genetic geography. Science 2846. [DOI] [PubMed] [Google Scholar]
- 9.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1997. Histological classification of gastritis and Helicobacter pylori infection: an agreement at last? The International Workshop on the Histopathology of Gastritis. Helicobacter 2(Suppl. 1)S17-S24. [DOI] [PubMed] [Google Scholar]
- 10.Enarsson, K., M. Brisslert, S. Backert, and M. Quiding-Jarbrink. 2005. Helicobacter pylori induces transendothelial migration of activated memory T cells. Infect. Immun. 73761-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst, P. B., and B. D. Gold. 2000. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 5426. [DOI] [PubMed] [Google Scholar]
- 12.Galustian, C., N. Elviss, H. Chart, R. Owen, and T. Feizi. 2003. Interactions of the gastrotropic bacterium Helicobacter pylori with the leukocyte-endothelium adhesion molecules, the selectins—a preliminary report. FEMS Immunol. Med. Microbiol. 36127-134. [DOI] [PubMed] [Google Scholar]
- 13.Hamlet, A., C. Lindholm, O. Nilsson, and L. Olbe. 1998. Aspirin-induced gastritis, like Helicobacter pylori-induced gastritis disinhibits acid secretion in humans: relation to cytokine expression. Scand. J. Gastroenterol. 33346-356. [DOI] [PubMed] [Google Scholar]
- 14.Hansson, M., M. Hermansson, H. Svensson, A. Elfvin, L. E. Hansson, E. Johnsson, A. Sjoling, and M. Quiding-Jarbrink. 2008. CCL28 is increased in human Helicobacter pylori-induced gastritis and mediates recruitment of gastric immunoglobulin A-secreting cells. Infect. Immun. 763304-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatz, R. A., G. Rieder, M. Stolte, E. Bayerdorffer, G. Meimarakis, F. W. Schildberg, and G. Enders. 1997. Pattern of adhesion molecule expression on vascular endothelium in Helicobacter pylori-associated antral gastritis. Gastroenterology 1121908-1919. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi, K., T. Arakawa, T. Uchida, K. Nakagawa, S. Nakamura, T. Matsumoto, T. Fukuda, K. Kobayashi, and T. Kuroki. 1997. In situ expression of cell adhesion molecules in chronic gastritis with Helicobacter pylori infection. J. Clin. Gastroenterol. 25(Suppl. 1)S215-S221. [DOI] [PubMed] [Google Scholar]
- 17.Innocenti, M., A. C. Thoreson, R. L. Ferrero, E. Stromberg, I. Bolin, L. Eriksson, A. M. Svennerholm, and M. Quiding-Jarbrink. 2002. Helicobacter pylori-induced activation of human endothelial cells. Infect. Immun. 704581-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irjala, H., K. Elima, E. L. Johansson, M. Merinen, K. Kontula, K. Alanen, R. Grenman, M. Salmi, and S. Jalkanen. 2003. The same endothelial receptor controls lymphocyte traffic both in vascular and lymphatic vessels. Eur. J. Immunol. 33815-824. [DOI] [PubMed] [Google Scholar]
- 19.Kansas, G. S. 1996. Selectins and their ligands: current concepts and controversies. Blood 883259-3287. [PubMed] [Google Scholar]
- 20.Kilhamn, J., H. Brevinge, M. Quiding-Jarbrink, A. M. Svennerholm, and M. Jertborn. 2001. Induction and distribution of intestinal immune responses after administration of recombinant cholera toxin B subunit in the ileal pouches of colectomized patients. Infect. Immun. 693466-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwok, T., D. Zabler, S. Urman, M. Rohde, R. Hartig, S. Wessler, R. Misselwitz, J. Berger, N. Sewald, W. Konig, and S. Backert. 2007. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature 449862-866. [DOI] [PubMed] [Google Scholar]
- 22.Lasky, L. A. 1992. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science 258964-969. [DOI] [PubMed] [Google Scholar]
- 23.Malaty, H. M. 1996. Prevalence of Helicobacter pylori infection in Korean children: inverse relation to socioeconomic status despite a uniformly high prevalence in adults. Am. J. Epidemiol. 1435. [DOI] [PubMed] [Google Scholar]
- 24.Mattsson, A., H. Lonroth, M. Quiding-Jarbrink, and A. M. Svennerholm. 1998. Induction of B cell responses in the stomach of Helicobacter pylori-infected subjects after oral cholera vaccination. J. Clin. Investig. 10251-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mollicone, R., T. Caillard, J. Le Pendu, A. Francois, N. Sansonetti, H. Villarroya, and R. Oriol. 1988. Expression of ABH and X (Lex) antigens on platelets and lymphocytes. Blood 711113-1119. [PubMed] [Google Scholar]
- 26.Mori, N., A. Wada, T. Hirayama, T. P. Parks, C. Stratowa, and N. Yamamoto. 2000. Activation of intercellular adhesion molecule 1 expression by Helicobacter pylori is regulated by NF-κB in gastric epithelial cancer cells. Infect. Immun. 681806-1814. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Osborn, L., C. Hession, R. Tizard, C. Vassallo, S. Luhowskyj, G. Chi-Rosso, and R. Lobb. 1989. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 591203-1211. [DOI] [PubMed] [Google Scholar]
- 28.Quiding-Jarbrink, M., I. Ahlstedt, C. Lindholm, E. L. Johansson, and H. Lonroth. 2001. Homing commitment of lymphocytes activated in the human gastric and intestinal mucosa. Gut 49519-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quiding-Jarbrink, M., H. Lonroth, I. Ahlstedt, J. Holmgren, and A. M. Svennerholm. 2001. Human gastric B cell responses can be induced by intestinal immunisation. Gut 49512-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramarao, N., S. D. Gray-Owen, and T. F. Meyer. 2000. Helicobacter pylori induces but survives the extracellular release of oxygen radicals from professional phagocytes using its catalase activity. Mol. Microbiol. 38103-113. [DOI] [PubMed] [Google Scholar]
- 31.Read, M. A., M. Z. Whitley, S. Gupta, J. W. Pierce, J. Best, R. J. Davis, and T. Collins. 1997. Tumor necrosis factor alpha-induced E-selectin expression is activated by the nuclear factor-kappaB and c-JUN N-terminal kinase/p38 mitogen-activated protein kinase pathways. J. Biol. Chem. 2722753-2761. [DOI] [PubMed] [Google Scholar]
- 32.Ruoslahti, E. 1996. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12697-715. [DOI] [PubMed] [Google Scholar]
- 33.Salmi, M., and S. Jalkanen. 1992. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science 2571407-1409. [DOI] [PubMed] [Google Scholar]
- 34.Salmi, M., K. Kalimo, and S. Jalkanen. 1993. Induction and function of vascular adhesion protein-1 at sites of inflammation. J. Exp. Med. 1782255-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selbach, M., S. Moese, T. F. Meyer, and S. Backert. 2002. Functional analysis of the Helicobacter pylori cag pathogenicity island reveals both VirD4-CagA-dependent and VirD4-CagA-independent mechanisms. Infect. Immun. 70665-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snider, J. L., C. Allison, B. H. Bellaire, R. L. Ferrero, and J. A. Cardelli. 2008. The beta1 integrin activates JNK independent of CagA, and JNK activation is required for Helicobacter pylori CagA+-induced motility of gastric cancer cells. J. Biol. Chem. 28313952-13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Springer, T. A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76301-314. [DOI] [PubMed] [Google Scholar]
- 38.Strober, W., P. J. Murray, A. Kitani, and T. Watanabe. 2006. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 69-20. [DOI] [PubMed] [Google Scholar]
- 39.van de Stolpe, A., and P. T. van der Saag. 1996. Intercellular adhesion molecule-1. J. Mol. Med. (Berlin) 7413-33. [DOI] [PubMed] [Google Scholar]
- 40.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Girardin, A. P. Moran, R. Athman, S. Memet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 51166-1174. [DOI] [PubMed] [Google Scholar]
- 41.Yamauchi, J., T. Kawano, M. Nagao, Y. Kaziro, and H. Itoh. 2000. G(i)-dependent activation of c-Jun N-terminal kinase in human embryonal kidney 293 cells. J. Biol. Chem. 2757633-7640. [DOI] [PubMed] [Google Scholar]
- 42.Yoong, K. F., G. McNab, S. G. Hubscher, and D. H. Adams. 1998. Vascular adhesion protein-1 and ICAM-1 support the adhesion of tumor-infiltrating lymphocytes to tumor endothelium in human hepatocellular carcinoma. J. Immunol. 1603978-3988. [PubMed] [Google Scholar]