Abstract
When cultured in a low-iron medium, Legionella pneumophila secretes a siderophore (legiobactin) that is both reactive in the chrome azurol S (CAS) assay and capable of stimulating the growth of iron-starved legionellae. Using anion-exchange high-pressure liquid chromatography (HPLC), we purified legiobactin from culture supernatants of a virulent strain of L. pneumophila. In the process, we detected the ferrated form of legiobactin as well as other CAS-reactive substances. Purified legiobactin had a yellow-gold color and absorbed primarily from 220 nm and below. In accordance, nuclear magnetic resonance spectroscopy revealed that legiobactin lacks aromatic carbons, and among the 13 aliphatics present, there were 3 carbonyls. When examined by HPLC, supernatants from L. pneumophila mutants inactivated for lbtA and lbtB completely lacked legiobactin, indicating that the LbtA and LbtB proteins are absolutely required for siderophore activity. Independently derived lbtA mutants, but not a complemented derivative, displayed a reduced ability to infect the lungs of A/J mice after intratracheal inoculation, indicating that legiobactin is required for optimal intrapulmonary survival by L. pneumophila. This defect, however, was not evident when the lbtA mutant and its parental strain were coinoculated into the lung, indicating that legiobactin secreted by the wild type can promote growth of the mutant in trans. Legiobactin mutants grew normally in murine lung macrophages and alveolar epithelial cells, suggesting that legiobactin promotes something other than intracellular infection of resident lung cells. Overall, these data represent the first documentation of a role for siderophore expression in the virulence of L. pneumophila.
The gram-negative bacterium Legionella pneumophila is the principal etiologic agent of Legionnaires’ disease, a common and serious form of pneumonia that often afflicts immunocompromised individuals (33, 38). Human infection occurs after the inhalation of Legionella-contaminated water droplets that can originate from a wide variety of aerosol-generating devices. Within the lung, L. pneumophila replicates primarily within the resident macrophages that line the alveolus. Protein secretion systems are well known to contribute greatly to the organism's facility to grow within that intracellular niche (49, 88). Iron acquisition is another key requirement for L. pneumophila replication, intracellular infection, and virulence (21-23). Previously, we determined that when L. pneumophila is grown in a low-iron, chemically defined medium (CDM) it secretes a low-molecular-weight, siderophore activity that is detected by chrome azurol S (CAS), a reagent that identifies high-affinity ferric iron chelators independently of structure (55, 87). Supernatants containing this CAS-reactive material stimulate the growth of iron-starved L. pneumophila, including the wild type and a mutant lacking ferrous iron transport (feoB) function (1). We have named the secreted CAS-reactive material that stimulates bacterial growth legiobactin. Other Legionella species also appear to express legiobactin (1, 89). To determine the role of legiobactin in infection, we have sought to test L. pneumophila mutants specifically lacking the siderophore in the murine model of Legionnaires’ disease. Previously, we had identified two linked genes, lbtA and lbtB, that appeared to be required for the expression of legiobactin, i.e., supernatants from mutants inactivated for lbtA or lbtB showed both a 40 to 70% loss in CAS reactivity and a complete inability to stimulate the growth of iron-starved legionellae (1). LbtA has homology to siderophore synthetases of Bordetella spp., Escherichia coli, Erwinia chrysanthemi, Francisella tularensis, Vibrio parahaemolyticus, and others (1, 27, 30, 40, 44, 57, 58, 90, 93). LbtB is akin to inner membrane siderophore exporters of Azotobacter sp., Bordetella sp., Escherichia sp., and others (1, 10, 26, 40, 42, 62, 72, 94). Thus, we believe that cytoplasmic LbtA is involved in the synthesis of legiobactin whereas LbtB promotes transit across the inner membrane prior to final export. As a necessary prelude to assessing lbtA or lbtB mutants in disease models, we now report the purification of legiobactin and the demonstration of these mutants specifically and completely lacking this molecule. Legiobactin mutants, but not their complemented derivatives, were then found to be defective for infection of the mammalian lung, indicating, for the first time, the importance of a siderophore in L. pneumophila virulence.
MATERIALS AND METHODS
Bacterial strains, growth media, and chemicals.
L. pneumophila strain 130b (American Type Culture Collection [ATCC] strain BAA-74, also known as AA100) was our wild-type strain (1, 55, 84). Mutants of 130b used in this study were NU300, which has a kanamycin resistance cassette inserted into lbtA; NU302, which has an unmarked deletion mutation in lbtA; NU303, which has a gentamicin resistance cassette inserted into lbtB; and NU269, which has a kanamycin resistance cassette inserted into feoB (1, 78). Complemented derivatives that contain lbtA or lbtB cloned into pMMB2002 (i.e., plbtA or plbtB) have also been described previously (1). Legionellae were routinely grown at 37°C on buffered charcoal yeast extract (BCYE) agar or in buffered yeast extract broth (1, 55). In order to obtain siderophore-containing supernatants, strains were grown in a deferrated version of CDM (1). Unless otherwise noted, chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO).
Siderophore assays.
L. pneumophila supernatant samples were prepared and tested for siderophore chelating activity using the CAS assay as previously described (1, 55, 87, 89). However, to facilitate the testing of fractions obtained during purification (see below), the assay was also done in a 96-well-plate format using 50 μl of CAS solution and 100 μl of test material along with 50 μl of 50 mM K2HPO4 (pH 7) added to speed the reaction. Supernatants and fractions were also tested for siderophore biological activity by examination of their ability to promote the growth of the NU269 feoB mutant on non-iron-supplemented BCYE agar as previously described (1, 78). NU269 lacks an inner membrane ferrous iron permease and therefore is impaired for uptake of ferrous but not ferric iron (78). The feoB mutant also has a reduced ability to grow when cultured on low-iron BCYE agar or in low-iron buffered yeast extract broth (1, 78), correlating with appreciable levels of ferrous iron in the cultures as a result of l-cysteine that is standardly added to the yeast extract base (37). This mutant growth deficit can be reversed by the addition of ferric iron salts or supernatants containing legiobactin, a mediator of ferric iron uptake (1).
Purification of legiobactin.
Supernatants were prepared as described above but scaled up to yield more material for purification. In preliminary experiments, we tested the binding of the CAS reactivity in L. pneumophila supernatants to hydrophobic and charged resins. Whereas 73% of the CAS reactivity bound to hydrophobic resin XAD-16, 98% of the CAS reactivity was removed from supernatants upon incubation with the cation-exchange resin CM Sephadex C-25 or the anion-exchange resin DEAE Sephadex A-50. There was no binding to Sephadex G-25 and G-50 beads, indicating that the high level of binding to the cation and anion exchangers was specific. The A-50 resin was chosen for the next step in legiobactin purification. Five liters of CAS-reactive supernatant was harvested from strain 130b cultures, and 500-ml batches were loaded onto DEAE Sephadex A-50 columns (Amersham Pharmacia Biotech, Uppsala, Sweden). Supernatant expressing bound CAS activity was washed with 2 column volumes of MOPS (morpholinepropanesulfonic acid) buffer and then eluted after exposure to increasing concentrations of NaCl. All CAS-reactive supernatant eluted in 300 to 400 mM NaCl. CAS-reactive A-50 fractions (500 ml) were concentrated to 10 ml by rotary evaporation, filtered, and desalted by filtration through Sephadex G-10 columns (Amersham). Fractions were concentrated down to 4 ml prior to high-pressure liquid chromatography (HPLC) analysis. Legiobactin was finally purified by HPLC using a water-400 mM NaCl gradient method. An anion-exchange column (TSK-GEL DEAE-2SW, 5 μm, 4.6 mm by 250 mm; Tosoh Bioscience, Montgomeryville, PA) was connected to a Waters 1525 binary HPLC pump system equipped with a Waters 717 Plus auto sampler, a 2996 photodiode array detector, and a Fraction Collector II (Waters Corporation, Milford, MA). Wavelengths were set to include 220 nm, an absorbance for detecting compounds eluting from the column. All solvents were degassed by a Waters in-line degasser after being filtered through 0.45-μm filters (Gelman Sciences, Ann Arbor, MI). The concentrated, semipurified supernatant (500 to 900 μl) was injected during each run. No CAS reactivity was detected in the solvent front, suggesting that legiobactin bound quite well to the column and was not being lost due to overloading. The elution gradient used consisted of a combined flow rate of 0.7 ml min−1 of 100% A (double-distilled water [ddH2O], pH 5.8) for 5 min, which then decreased to 40% A while B (400 mM NaCl in ddH2O, pH 5.8) increased to 60% for the following 120 min. Over the next 2 min (min 125 to 127), the percentage of A declined to 0 and the percentage of B increased to 100, where it remained for 10 min (min 127 to 137). From min 137 to 140, the percent A increased from 0 to 100 while the percent B declined from 100 to 0. During the last 10 min (min 140 to 150), these percentages were maintained prior to injection of another sample. The CAS- and bioassay-reactive legiobactin eluted at or near 90 min, the time 45% B (45% of 400 mM NaCl) had been obtained. Over the course of our experiments, the column had to be replaced due to unexplained pressure increases; there appeared to be a component in supernatants that irreversibly bound to the resin and eventually caused clogging. This resulted in a small variation in the times that CAS-reactive material and legiobactin eluted in the different experiments. After being concentrated and desalted as described above, legiobactin-containing fractions were deferrated with 8-hydroxyquinoline (16). Briefly, siderophore in ddH20 (5 to 7 ml) was mixed with 2.5% 8-hydroxyquinoline in 20 ml dichloromethane and incubated at 4°C. After 2 h of incubation, the characteristic green color of ferriquinoline was obvious. Deferrated legiobactin in ddH2O was then mixed with 20 ml dichloromethane three times to remove the remaining 8-hydroxyquinoline. Legiobactin was lyophilized and kept at −80°C.
NMR spectroscopy.
Proton nuclear magnetic resonance (NMR) and carbon-13 NMR were performed with purified legiobactin dissolved in D2O at 4°C on a Varian NMR spectrophotometer (Varian, Inc., Palo Alto, CA) operating at 300-MHz 1H frequency and 75-MHz 13C frequency. Chemical shifts are reported in parts per million, using dimethyl-2-silapentane-5-sulfonate sodium salt (DSS) as a reference. DSS was placed externally to relate the chemical shifts of legiobactin and to avoid contaminating the sample.
Analysis of gene expression.
Reverse transcription-PCR was done as described before (1). L. pneumophila RNA was isolated using RNA STAT-60 (Tel-Test B, Friendswood, TX). The primers (Integrated DNA Tech., Coralville, IA) used were lbtAF1RT (5′-CATTTGATCGATGGCCTCTT) and lbtAR1RT (5′-GCGCGGAAATTAGGATGATA) to amplify a 226-bp internal fragment of lbtA and lbtAF4RT (5′-GGATCCTGCTAAAACAAATTGCA) and lbtBR2RT (5′-CACTACCGATGGTACTGGTG) to amplify a 324-bp fragment encompassing the 3′ end of lbtA and the 5′ end of lbtB. Controls in which reverse transcriptase was omitted from the PCR were done to rule out contributions of contaminating DNA in the DNase-treated RNA samples.
Pulmonary infection of A/J mice with L. pneumophila.
Female, 6- to 8-week-old A/J mice (Jackson Laboratory, Bar Harbor, ME) were anesthetized and then inoculated intratracheally with legionellae (13, 79). As we have done before (28, 83), to determine the relative abilities of strains to replicate and survive in mouse lungs, groups of mice (n = 5) were infected separately with 106 CFU of wild-type and mutant bacteria, and at various hours postinoculation, the bacterial CFU in the lungs were determined by plating on BCYE agar. Competition assays were also performed as we have described previously (28, 78, 79, 82, 83); mice were inoculated with 105 CFU of a ca. 1:1 ratio of wild-type and mutant bacteria, and then 1 and 3 days later, the ratios of wild type to mutant in lung homogenates were determined by plating. Animal experiments were approved by the Animal Care and Use Committee of Northwestern University.
Intracellular infection with L. pneumophila.
Bone marrow-derived macrophages and explanted alveolar macrophages were obtained from A/J mice and infected with L. pneumophila as previously described (29, 41, 91). Briefly, monolayers consisting of 1.0 × 105 to 2.5 × 105 macrophages were infected with bacteria at a multiplicity of infection equal to 1 (for bone marrow-derived cells) or 5 (for alveolar cells), incubated for 2 h to allow bacterial entry, and then washed three times with media to remove unincorporated bacteria. At various times postinoculation, infected monolayers were lysed and serial dilutions were plated on BCYE agar in order to determine the numbers of legionellae. The A549 alveolar epithelial cell line (ATCC CCL-185) was maintained and infected with legionellae at a multiplicity of infection equal to 10, as previously described (43).
RESULTS
Purification of legiobactin and detection of ferrilegiobactin.
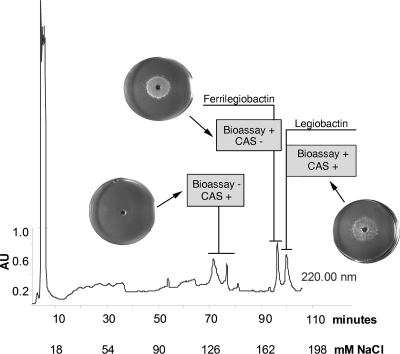
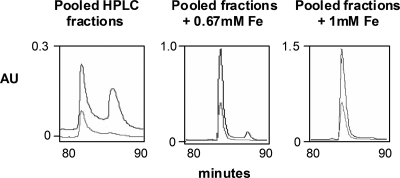
Concentrated CAS-reactive supernatants of strain 130b were subjected to anion-exchange HPLC analysis (Fig. 1). Three CAS-reactive peaks were detected, with each displaying absorbance at 220 nm. The first two CAS-reactive peaks were not readily separated from each other and, over the course of our experiments (for reasons noted above), eluted at different times within the 55- to 75-min range. The third CAS-reactive peak eluted in 180 mM NaCl. In the HPLC run depicted in Fig. 1, this corresponded to an elution time of 102 min; however, over the course of later trials (for reasons noted above), this peak appeared at or near 90 min (e.g., see Fig. 2). These data confirmed that L. pneumophila supernatants contain more than one CAS-reactive substance (1). Importantly, however, the only CAS-positive peak that supported the growth of the feoB mutant on low-iron medium was that which eluted in 180 mM NaCl and at 90 to 102 min (Fig. 1). Thus, we concluded that the last CAS-positive peak represents the siderophore legiobactin. During HPLC analysis, we consistently observed a peak that eluted just before legiobactin and was CAS negative but bioassay positive (Fig. 1). We hypothesized that this peak was iron-loaded legiobactin, i.e., ferrilegiobactin. To investigate this, we pooled legiobactin fractions obtained from five HPLC runs and reinjected that material either following no further treatment or after ferration. When the untreated, pooled legiobactin peaks were reinjected, we saw not only the elution of the CAS-positive, bioassay-positive (legiobactin) peak at nearly 90 min but also the emergence of the earlier CAS-negative peak eluting nearer to 80 min (Fig. 2, left). This suggested that legiobactin was acquiring iron upon isolation through the HPLC lines and/or during the subsequent HPLC, resulting in a downshift in elution time and an increased absorbance at 254 nm for ferrilegiobactin. More dramatically, when pooled legiobactin samples were treated with 0.67 mM or 1 mM FeCl3 and then subjected to another round of HPLC, we observed a large diminution or complete loss of the CAS-positive peak coincident with a large increase in the preceding peak (Fig. 2A, center and right). When the material in the earlier peaks was treated with the iron-binding reagent 8-hydroxyquinoline, there was a restoration of CAS reactivity. Thus, upon exposure to ferric iron, legiobactin undergoes a reversible shift in elution and a loss of CAS reactivity that are compatible with the creation of ferrilegiobactin. As expected, ferrilegiobactin preparations were very capable of rescuing the growth of the feoB mutant on low-iron media (data not shown).
FIG. 1.
Anion-exchange HPLC analysis of L. pneumophila CDM culture supernatants. Concentrated supernatants obtained from deferrated CDM cultures of wild-type strain 130b were injected onto a TSK-GEL DEAE-2SW anion-exchange column and then subjected to NaCl elution over a 120-min period. Fractions obtained were analyzed at A220 and tested for their reactivity (positive [+] or negative [−]) in the CAS assay and the feoB bioassay. Images showing the ability or inability of a supernatant fraction to stimulate the growth of the feoB mutant on low-iron BCYE plates are inserted over the A220 scan. The results presented are representative of at least four independent experiments. AU, arbitrary units.
FIG. 2.
Anion-exchange HPLC analysis of legiobactin samples treated with iron. HPLC fractions containing legiobactin (i.e., both CAS-positive and bioassay-positive material) were pooled and reinjected into the HPLC following no addition of iron (left), addition of 0.67 mM FeCl3 (middle), or addition of 1 mM FeCl3 (right). Fractions were eluted with NaCl over a 120-min period. Shown here are those peaks eluted between 80 and 90 min, as detected at A220 (top line) and A254 (bottom line). The results presented are representative of at least two independent experiments. AU, arbitrary units.
Absorption and NMR analysis of legiobactin.
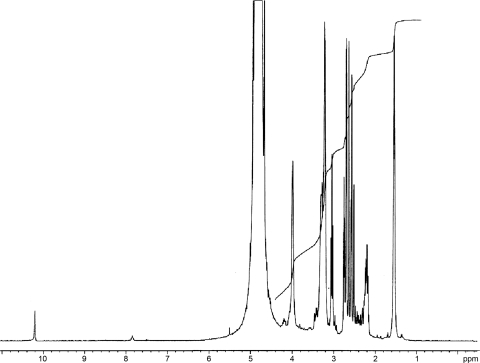
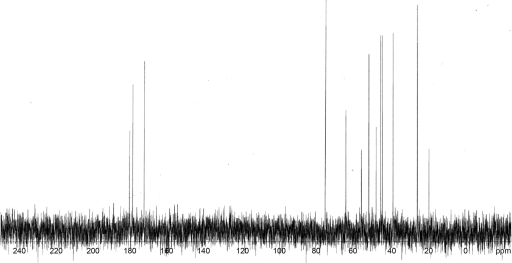
Purified legiobactin had a yellow-gold color in ddH2O, a trait common to carboxylate siderophores (102). The Legionella siderophore absorbed only in deep UV, from 220 nm and below, with a small shoulder at 325 to 350 nm, correlating with the visible yellow color it emits (data not shown). In accordance, 300-MHz proton NMR (Fig. 3) indicated that the protons in legiobactin are not associated with any aromatic carbons, which would have given absorbance in the visible range. The protons were instead associated with aliphatic carbons, as evidenced by their detection from 0 to 4.2 ppm (Fig. 3). Based upon the peaks observed from 1.1 to 2.5 ppm, there are likely methyl, methylene, and methine protons, with some of these shifted downfield to 2.5 to 4 ppm due to a carbonyl carbon in close proximity. A hydrogen atom(s) associated with a nitrogen atom(s) would also be localized to the 2.5- to 4-ppm range. Thus, legiobactin appeared composed of mainly methylene, methine, and amine hydrogens. Proton-decoupled 13C NMR was done to determine what types of carbons compose legiobactin (Fig. 4). The C-13 scan showed the presence of only aliphatic carbons, as seen from 0 to 80 ppm, confirming the proton NMR results. There appeared to be 13 carbons, with 3 of them being carbonyls, as seen from 170 to 181 ppm. These data suggest that legiobactin's iron-binding capacity comes from carbonyl oxygens, like those found in carboxylates, as in many other polycarboxylate siderophores (102).
FIG. 3.
Proton NMR spectrum of legiobactin. Purified legiobactin in deuterium oxide was subjected to 300-MHz proton NMR. The protons of legiobactin were detected from 1.1 to 4.2 ppm. A large DOH peak was present at 4.8 ppm due to the deuterium picking up a hydrogen atom, and water suppression experiments showed that no legiobactin-related peaks were hidden under the D2O peak. The peak near 10 ppm was not observed in repeat experiments. The results presented are representative of three independent experiments.
FIG. 4.
Proton-decoupled C-13 NMR spectrum of legiobactin. Purified legiobactin in deuterium oxide revealed 13 aliphatic carbons (0 to 80 ppm), with 3 of those being carbonyls (170 to 181 ppm). The results presented are representative of two independent experiments.
Absence of legiobactin and ferrilegiobactin in lbtA and lbtB mutant supernatants.
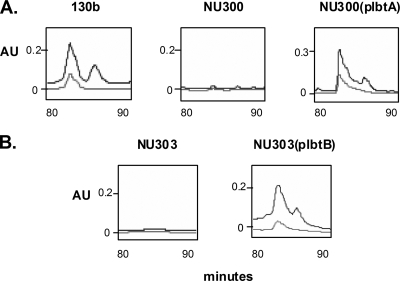
Previously, we had identified two neighboring genes, lbtA and lbtB, that appeared to be required for the expression of legiobactin (1). Using reverse transcription-PCR, we confirmed that lbtA and lbtB are cotranscribed in strain 130b (data not shown). To clarify the role of lbtA and lbtB in legiobactin expression, the supernatants of lbtA mutant NU300 and lbtB mutant NU303 were analyzed by HPLC, as described for parent 130b. The legiobactin and ferrilegiobactin peaks were completely absent from the supernatants of both mutants, and reintroduction of lbtA or lbtB into the corresponding mutant on pMMB2002 restored the siderophore peaks (Fig. 5). LbtA and LbtB mutants containing only the vector did not show restoration of siderophore activity. Whereas legiobactin peaks from wild-type and complemented mutant samples promoted feoB mutant growth, fractions from the mutants lacked bioactivity (data not shown). Together, these data confirm that lbtA and lbtB are absolutely required for the expression of legiobactin and that lbtA and lbtB mutants of strain 130b completely lack legiobactin and ferrilegiobactin, as opposed to simply having altered levels of expression. Thus, the residual CAS reactivity that is present in mutant supernatants is most likely due to a nonspecific (nonsiderophore) CAS-reactive species or another siderophore that is not detected by the bioassays.
FIG. 5.
HPLC analysis of CDM culture supernatants from lbtA and lbtB mutants. Concentrated supernatants obtained from deferrated CDM cultures of wild-type 130b, lbtA mutant NU300, and complemented mutant NU300(plbtA) (A) as well as lbtB mutant NU303 and complemented mutant NU303(plbtB) (B) were injected onto a TSK-GEL DEAE-2SW anion-exchange column and then subjected to NaCl elution over a 120-min period. Shown here are those peaks eluted between 80 and 90 min, as detected at A220 (top line) and A254 (bottom line). The results presented are representative of at least three independent experiments. AU, arbitrary units.
Importance of legiobactin in L. pneumophila lung infection.
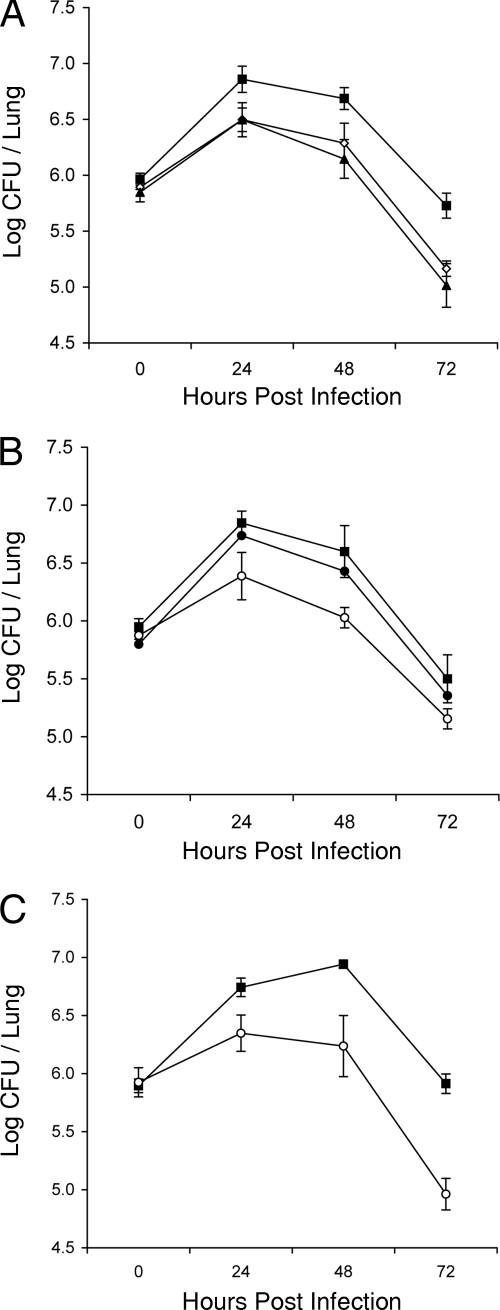
Having validated the nature of our lbtA and lbtB mutants, we next used an lbtA mutant in order to determine the importance of legiobactin in pathogenesis. Thus, we monitored the replication and persistence of wild-type 130b versus those of lbtA mutant NU300 following intratracheal inoculation into separate groups of A/J mice (Fig. 6A). As we and others have seen before (13, 28), the number of wild-type bacteria in the lungs increased ca. 10-fold during the first 24 h and then gradually declined over time. At 24, 48, and 72 h postinoculation, however, the lbtA mutant exhibited a statistically significant three- to fourfold decrease in CFU relative to the level for the wild type. In a previous study, we had found that the L. pneumophila feoB mutant has a reduced ability to grow in the murine lung (78). The magnitude of the defect exhibited by the lbtA mutant was nearly identical to that of the ferrous iron transport mutant (Fig. 6A). In a follow-up experiment, the independently derived lbtA deletion mutant NU302 behaved similarly to NU300 (Fig. 6B), indicating that the reduced CFU observed in vivo was due to the mutation in lbtA, versus a second-site mutation. As confirmation, the complemented derivative of NU302 containing an intact copy of lbtA on a plasmid behaved as the wild type did in the mouse lung (Fig. 6B). In these two experiments, the lbtA mutants’ defects were evident at 24 h and the size of the difference in recovery between the mutant and the wild type did not increase much over the next 48 h. In a third experiment, however, the magnitude of the defect of mutant NU302 did increase over time, going from ca. threefold at 24 h to ninefold at 72 h (Fig. 6C). In a final trial that compared 130b to NU300, the size of the defect went from 5-fold at 24 h to 13-fold at 48 h (data not shown). Although we do not know the reason for the modest variation in size of the mutant defect, the defect appeared greatest in those trials in which the attainment of peak growth by the wild type was delayed (e.g., compare Fig. 6A and B to C). Together, these data demonstrate that lbtA is required for optimal lung infection by L. pneumophila and that mutants lacking legiobactin display an in vivo defect that ranges from 3-fold to 13-fold. Previously, in a preliminary examination of the role of lbtA in vivo, we examined the ability of lbtA mutant NU300 to grow in the A/J mouse lung when coinoculated with parental 130b (1). In that in vivo competition, the ratio of wild type to mutant at day 1 and day 3 postinoculation did not deviate significantly from the initial ratio of 1:1. In light of the findings just described, we repeated the in vivo competition assay on the chance that NU300 might have changed phenotype. However, the mutant once again did not show a competitive disadvantage (data not shown). These data do not question the newfound importance of lbtA in L. pneumophila infection but simply indicate that legiobactin secreted by the wild type can promote in trans the growth of an lbtA mutant when the two are coinfecting the lung.
FIG. 6.
Growth and survival of wild-type and lbtA mutant L. pneumophila in the lungs of infected mice. A/J mice were intratracheally inoculated with equal numbers of wild-type and mutant bacteria, and then at various time points, the CFU in infected lungs were determined by plating. (A) Infections with wild-type strain 130b (▪), lbtA mutant NU300 (⋄), and feoB mutant NU269 (▴). (B) Infections with 130b (▪), lbtA mutant NU302 (○), and complemented mutant NU302(plbtA) (•). (C) Another infection with wild-type 130b (▪) versus lbtA mutant NU302 (○). Data are the means and standard deviations (error bars) obtained from five infected animals. Significant differences were obtained between the CFU recovered from mice infected with 130b or the complemented mutants and those infected with NU300, NU302, or NU269 at 24, 48, and 72 h postinoculation (Student's t test, P < 0.05).
Lack of a required role for legiobactin in L. pneumophila intracellular infection of lung macrophages and epithelia.
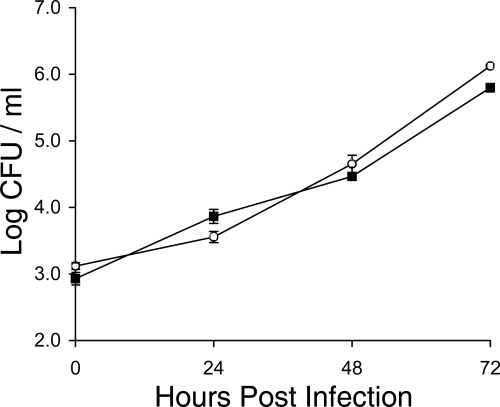
As a first step toward identifying the reason(s) for the reduced ability of legiobactin mutants to infect the lung, we assessed the capacity of an lbtA mutant to infect A/J mouse macrophages. However, mutant NU302 grew like the wild type did in both bone marrow-derived macrophages (not shown) and explanted alveolar macrophages (Fig. 7). Previously, we found that lbtA mutants also grow normally in the human macrophage-like U937 cell line, even though lbtA is expressed by the wild type when growing in those host cells (1). In addition to infecting macrophages, L. pneumophila is able to invade and grow within alveolar epithelial cells (25, 43, 64, 80). Therefore, we next determined the relative ability of an lbtA mutant to infect A549 cells, an alveolar epithelial line that is known to support L. pneumophila infection (18, 36, 43, 69, 70, 103). However, the mutant infected these cells as well as parental 130b did (not shown). These data indicate that lbtA is not needed for multiple forms of intracellular infection and that the importance of legiobactin in the mammalian lung involves something other than intracellular growth in resident alveolar macrophages or lung epithelia.
FIG. 7.
Intracellular infection of murine alveolar macrophages by wild-type and lbtA mutant L. pneumophila. Explanted alveolar macrophages from A/J mice were infected with wild-type strain 130b (▪) and lbtA mutant NU302 (○), and then at various time points, the CFU in infected monolayers were determined by plating. Data are the means and standard deviations (error bars) obtained from four infected wells. No significant differences were obtained between the CFU recovered from cells infected with the wild type and those infected with the mutants at 0, 24, 48, and 72 h postinoculation (Student's t test, P > 0.05). The results presented are representative of two independent experiments.
DISCUSSION
Based on the behavior of legiobactin null mutants in a murine model of Legionnaires’ disease, we have documented that a siderophore is required for optimal infection of the lung by L. pneumophila. That legiobactin promotes legionellosis is in keeping with our understanding of the role of siderophores in infection. The importance of siderophores in mammalian infection has been shown for many bacteria, including Bacillus anthracis, Bordetella bronchiseptica, Bordetella pertussis, Brucella abortus, Burkholderia cenocepacia, Escherichia coli, Francisella tularensis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella enterica, Shigella flexneri, Staphylococcus aureus, Vibrio vulnificus, Yersinia enterocolitica, and Yersinia pestis (4, 6, 9, 11, 12, 17, 26, 27, 31, 53, 56, 60, 63, 67, 76, 77, 92, 96, 98, 100, 101). Because L. pneumophila lbtA mutants grew normally in lung macrophages and epithelial cells, the importance of legiobactin appears to most significantly involve a process other than intracellular growth in resident lung cells. On the one hand, legiobactin could promote growth and/or survival of a subset of legionellae that reside in the extracellular milieu. That extracellular growth and survival are components of L. pneumophila infection has been suggested before, when various other mutants were found to be more defective in vivo than during intracellular-infection assays (28, 35, 65, 79, 83). On the other hand, legiobactin might be critical for promoting intracellular growth after the innate immune system has been triggered. For example, gamma interferon-activated macrophages contain reduced levels of iron for L. pneumophila growth (7, 50, 51, 66). That fewer numbers of lbtA mutant bacteria were recovered during the first 24 h postinoculation is compatible with legiobactin promoting growth in the lung in extracellular compartments and/or in an immune-activated intracellular niche (13, 14). Since the in vivo defect increased in magnitude over time in some of our experiments, legiobactin may also be needed in later stages of persistence. The infectious role of siderophores has been examined for several other pathogens that inhabit the lung or are facultative intracellular parasites. That a siderophore can promote extracellular growth and/or survival in lungs is evident from studies of B. bronchiseptica, B. pertussis, B. cenocepacia, K. pneumoniae, and P. aeruginosa (11, 53, 77, 92, 98). However, siderophores have also been shown to be necessary for optimal growth in (non-immune-activated) macrophages, in the case of B. anthracis, B. abortus, Mycobacterium tuberculosis, and S. enterica (17, 32, 45, 73). In a situation perhaps reminiscent of ours, S. flexneri siderophore mutants are not defective for intracellular infection of host cells but are nonetheless defective when examined in an in vivo model of infection (67, 74). Finally, the fungus Aspergillus fumigatus produces different siderophores that operate during the different extra- and intracellular stages of infection (86). Thus, although the importance of legiobactin for pathogenesis is clear, the most critical site of action for the siderophore is yet to be defined. But, in light of the field's major emphasis on studying L. pneumophila macrophage infection, our data implicating a role for legiobactin in extracellular survival or growth in immune-activated host cells should lead to an increased understanding of an understudied aspect of Legionella pneumonia.
The magnitude of the in vivo defect exhibited by lbtA mutants is entirely compatible with the current understanding of bacterial iron acquisition during mammalian infection. Indeed, it is well known that pathogens have multiple pathways for iron assimilation and that the elimination of a single pathway generally does not completely abolish in vivo growth and virulence (39, 85). L. pneumophila is no exception, as we have found that the organism has, among other things, a secreted pyomelanin that has ferric reductase activity, a ferrous iron (feoB) transport system, heme-binding capability, and a possible iron peptide (iraAB) transporter (19, 24, 48, 68, 71, 75, 78, 81, 99). Based on our HPLC detection of additional CAS-reactive substances in wild-type supernatants as well as the presence of an lbtA-like gene (frgA) in the L. pneumophila genome (1, 46), it is also conceivable that the organism secretes another siderophore. In keeping with the “modest” defect observed for the lbtA mutant, L. pneumophila feoB, iraAB, and frgA mutants are only partly defective when assayed for intracellular infection or lung infection (46, 78, 99).
The preceding discussion has highlighted the role of legiobactin as a direct mediator of ferric iron acquisition, a logical supposition based upon the typical role of siderophores in many other bacteria (61); however, there are other potential ways in which a siderophore, like legiobactin, might promote virulence. For example, the P. aeruginosa siderophore pyoverdine, in addition to its role as an iron scavenger, acts as a signaling molecule, regulating the expression of other virulence factors, including exotoxin A and a protease (5, 52). Also, pyoverdine is implicated as having a role in biofilm formation and surface motility (59, 97), and another P. aeruginosa secreted factor, PQS, can function as both an iron chelator and a quorum-sensing molecule (8). Furthermore, P. aeruginosa pyochelin is implicated, by virtue of being a catalyst for generating a hydroxyl radical, as a mediator of tissue damage (15). Finally, several different siderophores are capable of directly altering, at least in vitro, the viability and function of cells of the immune system, including T cells and macrophages (2, 3, 20, 47, 54, 95). Thus, future examination of the role of legiobactin in infection needs to consider the variety of ways that siderophores can act.
Our lung infection data highlight the importance of using multiple assays when judging the role of a secreted factor in pathogenesis. Indeed, uncovering the lbtA mutant defect required that the mutant be inoculated apart from the wild type. Clearly, coinoculation, i.e., a competition assay, was not effective in discerning the importance of legiobactin. In contrast, the feoB mutant, due to its lack of a membrane transporter, showed a defect whether it was tested in separate animals, as was done here, or by coinoculation, as we had done in the past (78). However, for L. pneumophila studies, this, interestingly, has not always been the case. For example, when we tested mutants lacking in type II protein secretion, we observed the mutant defect in both in vivo assays (28, 83). Thus, in order for the wild type to assist the lbtA mutant, but not the type II mutant, it would appear that the secreted siderophore is made in a larger amount, is more stable or more diffusible, or is able to exert an effect over a larger distance.
The purification of legiobactin was a critical first step in allowing us to document the importance of legiobactin in L. pneumophila lung infection, i.e., by purifying the wild-type siderophore and then comparing the HPLC profiles of the lbtA and lbtB mutants to that of the wild type, we were able to discern the true lack of siderophore in our mutants and thereby use those strains to assess the role of legiobactin in infection. However, the purification scheme developed here can also be used in future studies aimed at determining the structure of legiobactin. Based upon the inability of CAS-reactive supernatants to give a positive reaction in the Arnow and Csaky assays (55), legiobactin appears not to be a typical catecholate or hydroxamate. The biochemical analyses of purified legiobactin presented here further indicate that the Legionella siderophore has the traits of a (noncatecholate, nonhydroxamate) carboxylate siderophore, e.g., a yellow color, a weak absorption near 335 nm, the absence of aromatic carbons, and the presence of multiple carbonyls (102). Another clue to structure is the fact that LbtA has sequence similarity to amide bond-forming siderophore enzymes that are associated with the production of carboxylates, including achromobactin of E. chrysanthemi, rhizoferrin of F. tularensis, and vibrioferrin of V. parahaemolyticus (1, 30, 40, 90, 93, 102). Some nonclassical siderophores contain diamines that serve as carriers for iron-chelating substructures (34). In F. tularensis, the LbtA homolog FslA/FigA is believed to form an amide bond between putrescine and citric acid to create a siderophore similar to rhizoferrin, whereas in V. parahaemolyticus, the LbtA homolog PvsB or PvsD links 2-oxoglutaric acid to l-alanine through an amine bond in vibrioferrin (30, 90, 93, 102). However, based upon the number of carbons detected, legiobactin appears to be unique from the other carboxylates that are synthesized via LbtA-like proteins, i.e., whereas legiobactin has 13 carbons, achromobactin has 22, rhizoferrin 16, and vibrioferrin 15 (40, 90, 93, 102). In a similar vein, legiobactin appears distinct from other carboxylates, such as rhizobactin DM4 and the staphyloferrins, which contain amide linkages (102). Ultimately, knowledge of the structure of legiobactin might help us to better understand the way(s) in which this siderophore promotes infection and lead to the generation of siderophore inhibitors that control bacterial growth (61).
Acknowledgments
We thank members of the Cianciotto lab for helpful discussions and Ruella Rouf for technical assistance.
This work was funded by NIH grant AI34937 awarded to N.P.C.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 27 April 2009.
REFERENCES
- 1.Allard, K. A., V. K. Viswanathan, and N. P. Cianciotto. 2006. lbtA and lbtB are required for production of the Legionella pneumophila siderophore legiobactin. J. Bacteriol. 1881351-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autenrieth, I., K. Hantke, and J. Heesemann. 1991. Immunosuppression of the host and delivery of iron to the pathogen: a possible dual role of siderophores in the pathogenesis of microbial infections? Med. Microbiol. Immunol. 180135-141. [DOI] [PubMed] [Google Scholar]
- 3.Autenrieth, I. B., E. Bohn, J. H. Ewald, and J. Heesemann. 1995. Deferoxamine B but not deferoxamine G1 inhibits cytokine production in murine bone marrow macrophages. J. Infect. Dis. 172490-496. [DOI] [PubMed] [Google Scholar]
- 4.Bearden, S. W., J. D. Fetherston, and R. D. Perry. 1997. Genetic organization of the yersiniabactin biosynthetic region and construction of avirulent mutants in Yersinia pestis. Infect. Immun. 651659-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beare, P. A., R. J. For, L. W. Martin, and I. L. Lamont. 2003. Siderophore-mediated cell signalling in Pseudomonas aeruginosa: divergent pathways regulate virulence factor production and siderophore receptor synthesis. Mol. Microbiol. 47195-207. [DOI] [PubMed] [Google Scholar]
- 6.Bellaire, B. H., P. H. Elzer, S. Hagius, J. Walker, C. L. Baldwin, and R. M. Roop II. 2003. Genetic organization and iron-responsive regulation of the Brucella abortus 2,3-dihydroxybenzoic acid biosynthesis operon, a cluster of genes required for wild-type virulence in pregnant cattle. Infect. Immun. 711794-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhardwaj, N., T. W. Nash, and M. A. Horwitz. 1986. Interferon-gamma-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J. Immunol. 1372662-2669. [PubMed] [Google Scholar]
- 8.Bredenbruch, F., R. Geffers, M. Nimtz, J. Buer, and S. Haussler. 2006. The Pseudomonas aeruginosa quinolone signal (PQS) has an iron-chelating activity. Environ. Microbiol. 81318-1329. [DOI] [PubMed] [Google Scholar]
- 9.Brem, D., C. Pelludat, A. Rakin, C. A. Jacobi, and J. Heesemann. 2001. Functional analysis of yersiniabactin transport genes of Yersinia enterocolitica. Microbiology 1471115-1127. [DOI] [PubMed] [Google Scholar]
- 10.Brickman, T. J., and S. K. Armstrong. 2005. Bordetella AlcS transporter functions in alcaligin siderophore export and is central to inducer sensing in positive regulation of alcaligin system gene expression. J. Bacteriol. 1873650-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brickman, T. J., and S. K. Armstrong. 2007. Impact of alcaligin siderophore utilization on in vivo growth of Bordetella pertussis. Infect. Immun. 755305-5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brickman, T. J., T. Hanawa, M. T. Anderson, R. J. Suhadolc, and S. K. Armstrong. 2008. Differential expression of Bordetella pertussis iron transport system genes during infection. Mol. Microbiol. 703-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brieland, J., P. Freeman, R. Kunkel, C. Chrisp, M. Hurley, J. Fantone, and C. Engleberg. 1994. Replicative Legionella pneumophila lung infection in intratracheally inoculated A/J mice. A murine model of human Legionnaires’ disease. Am. J. Pathol. 1451537-1546. [PMC free article] [PubMed] [Google Scholar]
- 14.Brieland, J. K., D. G. Remick, M. L. LeGendre, N. C. Engleberg, and J. C. Fantone. 1998. In vivo regulation of replicative Legionella pneumophila lung infection by endogenous interleukin-12. Infect. Immun. 6665-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Britigan, B. E., G. T. Rasmussen, and C. D. Cox. 1997. Augmentation of oxidant injury to human pulmonary epithelial cells by the Pseudomonas aeruginosa siderophore pyochelin. Infect. Immun. 651071-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buyer, J. S., J. M. Wright, and J. Leong. 1986. Structure of pseudobactin A214, a siderophore from a bean-deleterious Pseudomonas. Biochemistry 255492-5499. [DOI] [PubMed] [Google Scholar]
- 17.Cendrowski, S., W. MacArthur, and P. Hanna. 2004. Bacillus anthracis requires siderophore biosynthesis for growth in macrophages and mouse virulence. Mol. Microbiol. 51407-417. [DOI] [PubMed] [Google Scholar]
- 18.Chang, B., F. Kura, J. Amemura-Maekawa, N. Koizumi, and H. Watanabe. 2005. Identification of a novel adhesion molecule involved in the virulence of Legionella pneumophila. Infect. Immun. 734272-4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatfield, C. H., and N. P. Cianciotto. 2007. The secreted pyomelanin pigment of Legionella pneumophila confers ferric reductase activity. Infect. Immun. 754062-4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi, E. Y., E. C. Kim, H. M. Oh, S. Kim, H. J. Lee, E. Y. Cho, K. H. Yoon, E. A. Kim, W. C. Han, S. C. Choi, J. Y. Hwang, C. Park, B. S. Oh, Y. Kim, K. C. Kimm, K. I. Park, H. T. Chung, and C. D. Jun. 2004. Iron chelator triggers inflammatory signals in human intestinal epithelial cells: involvement of p38 and extracellular signal-regulated kinase signaling pathways. J. Immunol. 1727069-7077. [DOI] [PubMed] [Google Scholar]
- 21.Cianciotto, N. P. 2007. Iron acquisition by Legionella pneumophila. Biometals 20323-331. [DOI] [PubMed] [Google Scholar]
- 22.Cianciotto, N. P. 2008. Iron assimilation and type II protein secretion, p. 33-48. In P. S. Hoffman, H. Friedman, and M. Bendinelli (ed.), Legionella pneumophila: pathogenesis and immunity. Springer, New York, NY.
- 23.Cianciotto, N. P. 2008. Secretion and export in Legionella, p. 153-166. In K. Huener and M. S. Swanson (ed.), Legionella molecular microbiology. Horizon Biosciences, Norwick, United Kingdom.
- 24.Cianciotto, N. P., P. Cornelis, and C. Baysse. 2005. Impact of the bacterial type I cytochrome c maturation system on different biological processes. Mol. Microbiol. 561408-1415. [DOI] [PubMed] [Google Scholar]
- 25.Cianciotto, N. P., J. Kim Stamos, and D. W. Kamp. 1995. Infectivity of Legionella pneumophila mip mutant for alveolar epithelial cells. Curr. Microbiol. 30247-250. [DOI] [PubMed] [Google Scholar]
- 26.Crouch, M. L., M. Castor, J. E. Karlinsey, T. Kalhorn, and F. C. Fang. 2008. Biosynthesis and IroC-dependent export of the siderophore salmochelin are essential for virulence of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 67971-983. [DOI] [PubMed] [Google Scholar]
- 27.Dale, S. E., A. Doherty-Kirby, G. Lajoie, and D. E. Heinrichs. 2004. Role of siderophore biosynthesis in virulence of Staphylococcus aureus: identification and characterization of genes involved in production of a siderophore. Infect. Immun. 7229-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DebRoy, S., J. Dao, M. Soderberg, O. Rossier, and N. P. Cianciotto. 2006. Legionella pneumophila type II secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung. Proc. Natl. Acad. Sci. USA 10319146-19151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng, J. C., K. Tateda, X. Zeng, and T. J. Standiford. 2001. Transient transgenic expression of gamma interferon promotes Legionella pneumophila clearance in immunocompetent hosts. Infect. Immun. 696382-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng, K., R. J. Blick, W. Liu, and E. J. Hansen. 2006. Identification of Francisella tularensis genes affected by iron limitation. Infect. Immun. 744224-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Der Vartanian, M., B. Jaffeux, M. Contrepois, M. Chavarot, J. P. Girardeau, Y. Bertin, and C. Martin. 1992. Role of aerobactin in systemic spread of an opportunistic strain of Escherichia coli from the intestinal tract of gnotobiotic lambs. Infect. Immun. 602800-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Voss, J. J., K. Rutter, B. G. Schroeder, H. Su, Y. Zhu, and C. E. Barry III. 2000. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc. Natl. Acad. Sci. USA 971252-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diederen, B. M. 2008. Legionella spp. and Legionnaires’ disease. J. Infect. 561-12. [DOI] [PubMed] [Google Scholar]
- 34.Drechsel, H., M. Tschierske, A. Thieken, G. Jung, H. Zahner, and G. Winkelmann. 1995. The carboxylate type siderophore rhizoferrin and its analogs produced by directed fermentation. J. Ind. Microbiol. 14105-112. [Google Scholar]
- 35.Edelstein, P. H., M. A. Edelstein, F. Higa, and S. Falkow. 1999. Discovery of virulence genes of Legionella pneumophila by using signature tagged mutagenesis in a guinea pig pneumonia model. Proc. Natl. Acad. Sci. USA 968190-8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edelstein, P. H., B. Hu, F. Higa, and M. A. Edelstein. 2003. lvgA, a novel Legionella pneumophila virulence factor. Infect. Immun. 712394-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ewann, F., and P. S. Hoffman. 2006. Cysteine metabolism in Legionella pneumophila: characterization of an l-cystine-utilizing mutant. Appl. Environ. Microbiol. 723993-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fields, B. S., R. F. Benson, and R. E. Besser. 2002. Legionella and Legionnaires’ disease: 25 years of investigation. Clin. Microbiol. Rev. 15506-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischbach, M. A., H. Lin, D. R. Liu, and C. T. Walsh. 2006. How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat. Chem. Biol. 2132-138. [DOI] [PubMed] [Google Scholar]
- 40.Franza, T., B. Mahe, and D. Expert. 2005. Erwinia chrysanthemi requires a second iron transport route dependent of the siderophore achromobactin for extracellular growth and plant infection. Mol. Microbiol. 55261-275. [DOI] [PubMed] [Google Scholar]
- 41.Fujio, H., S. Yoshida, H. Miyamoto, M. Mitsuyama, and Y. Mizuguchi. 1992. Investigation of the role of macrophages and endogenous interferon-gamma in natural resistance of mice against Legionella pneumophila infection. FEMS Microbiol. Immunol. 4183-191. [DOI] [PubMed] [Google Scholar]
- 42.Furrer, J. L., D. N. Sanders, I. G. Hook-Barnard, and M. A. McIntosh. 2002. Export of the siderophore enterobactin in Escherichia coli: involvement of a 43 kDa membrane exporter. Mol. Microbiol. 441225-1234. [DOI] [PubMed] [Google Scholar]
- 43.Gao, L. Y., B. J. Stone, J. K. Brieland, and Y. Abu Kwaik. 1998. Different fates of Legionella pneumophila pmi and mil mutants within macrophages and alveolar epithelial cells. Microb. Pathog. 25291-306. [DOI] [PubMed] [Google Scholar]
- 44.Giardina, P. C., L. A. Foster, S. I. Toth, B. A. Roe, and D. W. Dyer. 1997. Analysis of the alcABC operon encoding alcaligin biosynthesis enzymes in Bordetella bronchiseptica. Gene 19419-24. [DOI] [PubMed] [Google Scholar]
- 45.Gorbacheva, V. Y., G. Faundez, H. P. Godfrey, and F. C. Cabello. 2001. Restricted growth of ent and tonB mutants of Salmonella enterica serovar Typhi in human Mono Mac 6 monocytic cells. FEMS Microbiol. Lett. 1967-11. [DOI] [PubMed] [Google Scholar]
- 46.Hickey, E. K., and N. P. Cianciotto. 1997. An iron- and fur-repressed Legionella pneumophila gene that promotes intracellular infection and encodes a protein with similarity to the Escherichia coli aerobactin synthetases. Infect. Immun. 65133-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hileti, D., P. Panayiotidis, and A. V. Hoffbrand. 1995. Iron chelators induce apoptosis in proliferating cells. Br. J. Haematol. 89181-187. [DOI] [PubMed] [Google Scholar]
- 48.Huston, W. M., J. Naylor, N. P. Cianciotto, M. P. Jennings, and A. G. McEwan. 2008. Functional analysis of the multi-copper oxidase from Legionella pneumophila. Microbes Infect. 10497-503. [DOI] [PubMed] [Google Scholar]
- 49.Isberg, R. R., T. J. O'Connor, and M. Heidtman. 2009. The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat. Rev. Microbiol. 713-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jensen, W. A., R. M. Rose, A. S. Wasserman, T. H. Kalb, K. Anton, and H. G. Remold. 1987. In vitro activation of the antibacterial activity of human pulmonary macrophages by recombinant gamma interferon. J. Infect. Dis. 155574-577. [DOI] [PubMed] [Google Scholar]
- 51.Klein, T. W., Y. Yamamoto, H. K. Brown, and H. Friedman. 1991. Interferon-gamma induced resistance to Legionella pneumophila in susceptible A/J mouse macrophages. J. Leukoc. Biol. 4998-103. [DOI] [PubMed] [Google Scholar]
- 52.Lamont, I. L., P. A. Beare, U. Ochsner, A. I. Vasil, and M. L. Vasil. 2002. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 997072-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawlor, M. S., C. O'Connor, and V. L. Miller. 2007. Yersiniabactin is a virulence factor for Klebsiella pneumoniae during pulmonary infection. Infect. Immun. 751463-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee, H. J., S. C. Choi, E. Y. Choi, M. H. Lee, G. S. Seo, E. C. Kim, B. J. Yang, M. S. Lee, Y. I. Shin, K. I. Park, and C. D. Jun. 2005. Iron chelator induces MIP-alpha/CCL20 in human intestinal epithelial cells: implication for triggering mucosal adaptive immunity. Exp. Mol. Med. 37297-310. [DOI] [PubMed] [Google Scholar]
- 55.Liles, M. R., T. Aber Scheel, and N. P. Cianciotto. 2000. Discovery of a nonclassical siderophore, legiobactin, produced by strains of Legionella pneumophila. J. Bacteriol. 182749-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Litwin, C. M., T. W. Rayback, and J. Skinner. 1996. Role of catechol siderophore synthesis in Vibrio vulnificus virulence. Infect. Immun. 642834-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lynch, D., J. O'Brien, T. Welch, P. Clarke, P. O. Cuiv, J. H. Crosa, and M. O'Connell. 2001. Genetic organization of the region encoding regulation, biosynthesis, and transport of rhizobactin 1021, a siderophore produced by Sinorhizobium meliloti. J. Bacteriol. 1832576-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez, J. L., M. Herrero, and V. de Lorenzo. 1994. The organization of intercistronic regions of the aerobactin operon of pColV-K30 may account for the differential expression of the iucABCD iutA genes. J. Mol. Biol. 238288-293. [DOI] [PubMed] [Google Scholar]
- 59.Matilla, M. A., J. L. Ramos, E. Duque, J. de Dios Alche, M. Espinosa-Urgel, and M. I. Ramos-Gonzalez. 2007. Temperature and pyoverdine-mediated iron acquisition control surface motility of Pseudomonas putida. Environ. Microbiol. 91842-1850. [DOI] [PubMed] [Google Scholar]
- 60.Meyer, J. M., A. Neely, A. Stintzi, C. Georges, and I. A. Holder. 1996. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect. Immun. 64518-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miethke, M., and M. A. Marahiel. 2007. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 71413-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miethke, M., S. Schmidt, and M. A. Marahiel. 2008. The major facilitator superfamily-type transporter YmfE and the multidrug-efflux activator Mta mediate bacillibactin secretion in Bacillus subtilis. J. Bacteriol. 1905143-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milne, T. S., S. L. Michell, H. Diaper, P. Wikstrom, K. Svensson, P. C. Oyston, and R. W. Titball. 2007. A 55 kDa hypothetical membrane protein is an iron-regulated virulence factor of Francisella tularensis subsp. novicida U112. J. Med. Microbiol. 561268-1276. [DOI] [PubMed] [Google Scholar]
- 64.Mody, C. H., R. Paine III, M. S. Shahrabadi, R. H. Simon, E. Pearlman, B. I. Eisenstein, and G. B. Toews. 1993. Legionella pneumophila replicates within rat alveolar epithelial cells. J. Infect. Dis. 1671138-1145. [DOI] [PubMed] [Google Scholar]
- 65.Moffat, J. F., P. H. Edelstein, D. P. Regula, Jr., J. D. Cirillo, and L. S. Tompkins. 1994. Effects of an isogenic Zn-metalloprotease-deficient mutant of Legionella pneumophila in a guinea-pig pneumonia model. Mol. Microbiol. 12693-705. [DOI] [PubMed] [Google Scholar]
- 66.Nash, T. W., D. M. Libby, and M. A. Horwitz. 1988. IFN-gamma-activated human alveolar macrophages inhibit the intracellular multiplication of Legionella pneumophila. J. Immunol. 1403978-3981. [PubMed] [Google Scholar]
- 67.Nassif, X., M. C. Mazert, J. Mounier, and P. J. Sansonetti. 1987. Evaluation with an iuc::Tn10 mutant of the role of aerobactin production in the virulence of Shigella flexneri. Infect. Immun. 551963-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naylor, J., and N. P. Cianciotto. 2004. Cytochrome c maturation proteins are critical for in vivo growth of Legionella pneumophila. FEMS Microbiol. Lett. 241249-256. [DOI] [PubMed] [Google Scholar]
- 69.Newton, H. J., F. M. Sansom, V. Bennett-Wood, and E. L. Hartland. 2006. Identification of Legionella pneumophila-specific genes by genomic subtractive hybridization with Legionella micdadei and identification of lpnE, a gene required for efficient host cell entry. Infect. Immun. 741683-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.N′Guessan, P. D., M. O. Etouem, B. Schmeck, A. C. Hocke, S. Scharf, K. Vardarova, B. Opitz, A. Flieger, N. Suttorp, and S. Hippenstiel. 2007. Legionella pneumophila-induced PKCalpha-, MAPK-, and NF-kappaB-dependent COX-2 expression in human lung epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 292L267-L277. [DOI] [PubMed] [Google Scholar]
- 71.O'Connell, W. A., E. K. Hickey, and N. P. Cianciotto. 1996. A Legionella pneumophila gene that promotes hemin binding. Infect. Immun. 64842-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Page, W. J., E. Kwon, A. S. Cornish, and A. E. Tindale. 2003. The csbX gene of Azotobacter vinelandii encodes an MFS efflux pump required for catecholate siderophore export. FEMS Microbiol. Lett. 228211-216. [DOI] [PubMed] [Google Scholar]
- 73.Parent, M. A., B. H. Bellaire, E. A. Murphy, R. M. Roop II, P. H. Elzer, and C. L. Baldwin. 2002. Brucella abortus siderophore 2,3-dihydroxybenzoic acid (DHBA) facilitates intracellular survival of the bacteria. Microb. Pathog. 32239-248. [DOI] [PubMed] [Google Scholar]
- 74.Payne, S. M., E. E. Wyckoff, E. R. Murphy, A. G. Oglesby, M. L. Boulette, and N. M. Davies. 2006. Iron and pathogenesis of Shigella: iron acquisition in the intracellular environment. Biometals 19173-180. [DOI] [PubMed] [Google Scholar]
- 75.Pope, C. D., W. O'Connell, and N. P. Cianciotto. 1996. Legionella pneumophila mutants that are defective for iron acquisition and assimilation and intracellular infection. Infect. Immun. 64629-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramakrishnan, G., A. Meeker, and B. Dragulev. 2008. fslE is necessary for siderophore-mediated iron acquisition in Francisella tularensis Schu S4. J. Bacteriol. 1905353-5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Register, K. B., T. F. Ducey, S. L. Brockmeier, and D. W. Dyer. 2001. Reduced virulence of a Bordetella bronchiseptica siderophore mutant in neonatal swine. Infect. Immun. 692137-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robey, M., and N. P. Cianciotto. 2002. Legionella pneumophila feoAB promotes ferrous iron uptake and intracellular infection. Infect. Immun. 705659-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robey, M., W. O'Connell, and N. P. Cianciotto. 2001. Identification of Legionella pneumophila rcp, a pagP-like gene that confers resistance to cationic antimicrobial peptides and promotes intracellular infection. Infect. Immun. 694276-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodgers, F. G. 1979. Ultrastructure of Legionella pneumophila. J. Clin. Pathol. 321195-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rossier, O., and N. P. Cianciotto. 2005. The Legionella pneumophila tatB gene facilitates secretion of phospholipase C, growth under iron-limiting conditions, and intracellular infection. Infect. Immun. 732020-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rossier, O., J. Dao, and N. P. Cianciotto. 2008. The type II secretion system of Legionella pneumophila elaborates two aminopeptidases as well as a metalloprotease that contributes to differential infection among protozoan hosts. Appl. Environ. Microbiol. 74753-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rossier, O., S. Starkenburg, and N. P. Cianciotto. 2004. Legionella pneumophila type II protein secretion promotes virulence in the A/J mouse model of Legionnaires’ disease pneumonia. Infect. Immun. 72310-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saito, A., R. D. Rolfe, P. H. Edelstein, and S. M. Finegold. 1981. Comparison of liquid growth media for Legionella pneumophila. J. Clin. Microbiol. 14623-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schaible, U. E., and S. H. Kaufmann. 2004. Iron and microbial infection. Nat. Rev. Microbiol. 2946-953. [DOI] [PubMed] [Google Scholar]
- 86.Schrettl, M., E. Bignell, C. Kragl, Y. Sabiha, O. Loss, M. Eisendle, A. Wallner, H. N. Arst, Jr., K. Haynes, and H. Haas. 2007. Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 31195-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 16047-56. [DOI] [PubMed] [Google Scholar]
- 88.Shin, S., and C. R. Roy. 2008. Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell. Microbiol. 101209-1220. [DOI] [PubMed] [Google Scholar]
- 89.Starkenburg, S. R., J. M. Casey, and N. P. Cianciotto. 2004. Siderophore activity among members of the Legionella genus. Curr. Microbiol. 49203-207. [DOI] [PubMed] [Google Scholar]
- 90.Sullivan, J. T., E. F. Jeffery, J. D. Shannon, and G. Ramakrishnan. 2006. Characterization of the siderophore of Francisella tularensis and role of fslA in siderophore production. J. Bacteriol. 1883785-8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Swanson, M. S., and R. R. Isberg. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 633609-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takase, H., H. Nitanai, K. Hoshino, and T. Otani. 2000. Impact of siderophore production on Pseudomonas aeruginosa infections in immunosuppressed mice. Infect. Immun. 681834-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tanabe, T., T. Funahashi, H. Nakao, S. Miyoshi, S. Shinoda, and S. Yamamoto. 2003. Identification and characterization of genes required for biosynthesis and transport of the siderophore vibrioferrin in Vibrio parahaemolyticus. J. Bacteriol. 1856938-6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tanabe, T., H. Nakao, T. Kuroda, T. Tsuchiya, and S. Yamamoto. 2006. Involvement of the Vibrio parahaemolyticus pvsC gene in export of the siderophore vibrioferrin. Microbiol. Immunol. 50871-876. [PubMed] [Google Scholar]
- 95.Tanji, K., T. Imaizumi, T. Matsumiya, H. Itaya, K. Fujimoto, X. Cui, T. Toki, E. Ito, H. Yoshida, K. Wakabayashi, and K. Satoh. 2001. Desferrioxamine, an iron chelator, upregulates cyclooxygenase-2 expression and prostaglandin production in a human macrophage cell line. Biochim. Biophys. Acta 1530227-235. [DOI] [PubMed] [Google Scholar]
- 96.Torres, A. G., P. Redford, R. A. Welch, and S. M. Payne. 2001. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect. Immun. 696179-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Visca, P., F. Imperi, and I. L. Lamont. 2007. Pyoverdine siderophores: from biogenesis to biosignificance. Trends Microbiol. 1522-30. [DOI] [PubMed] [Google Scholar]
- 98.Visser, M. B., S. Majumdar, E. Hani, and P. A. Sokol. 2004. Importance of the ornibactin and pyochelin siderophore transport systems in Burkholderia cenocepacia lung infections. Infect. Immun. 722850-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Viswanathan, V. K., P. H. Edelstein, C. D. Pope, and N. P. Cianciotto. 2000. The Legionella pneumophila iraAB locus is required for iron assimilation, intracellular infection, and virulence. Infect. Immun. 681069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Williams, P. H., W. Rabsch, U. Methner, W. Voigt, H. Tschape, and R. Reissbrodt. 2006. Catecholate receptor proteins in Salmonella enterica: role in virulence and implications for vaccine development. Vaccine 243840-3844. [DOI] [PubMed] [Google Scholar]
- 101.Williams, P. H., and P. J. Warner. 1980. ColV plasmid-mediated, colicin V-independent iron uptake system of invasive strains of Escherichia coli. Infect. Immun. 29411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Winkelmann, G., and H. Drechsel. 1997. Microbial siderophores, p. 199-246. In H.-J. Rehm and G. Reed (ed.), Biotechnology: products of secondary metabolism, vol. 7. VCH, Weinheim, Germany. [Google Scholar]
- 103.Yaradou, D. F., D. Raze, C. Ginevra, F. Ader, A. Doleans-Jordheim, F. Vandenesch, F. D. Menozzi, J. Etienne, and S. Jarraud. 2007. Zinc-dependent cytoadherence of Legionella pneumophila to human alveolar epithelial cells in vitro. Microb. Pathog. 43234-242. [DOI] [PubMed] [Google Scholar]