Abstract
Streptococcus pneumoniae is the most common pathogen associated with otitis media. To examine the role of Toll-like receptor 2 (TLR2) in host defense against Streptococcus pneumoniae infection in the middle ear, wild-type (WT; C57BL/6) and TLR2-deficient (TLR2−/−) mice were inoculated with Streptococcus pneumoniae (1 × 106 CFU) through the tympanic membrane. Nineteen of 37 TLR2−/− mice showed bacteremia and died within 3 days after the challenge, compared to only 4 of 32 WT mice that died. Of those that survived, more severe hearing loss in the TLR2−/− mice than in the WT mice was indicated by an elevation in auditory-evoked brain stem response thresholds at 3 or 7 days postinoculation. The histological pathology was characterized by effusion and tissue damage in the middle ear, and in the TLR2−/− mice, the outcome of infection became more severe at 7 days. At both 3 and 7 days postchallenge, the TLR2−/− mice had higher blood bacterial titers than the WT mice (P < 0.05), and typical bacteria were identified in the effusion from both ears of both mouse groups by acridine orange staining. Moreover, by 3 days postchallenge, the mRNA accumulation levels of NF-κB, tumor necrosis factor alpha, interleukin 1β, MIP1α, Muc5ac, and Muc5b were significantly lower in the ears of TLR2−/− mice than in WT mice. In summary, TLR2−/− mice may produce relatively low levels of proinflammatory cytokines following pneumococcal challenge, thus hindering the clearance of bacteria from the middle ear and leading to sepsis and a high mortality rate. This study provides evidence that TLR2 is important in the molecular pathogenesis and host response to otitis media.
Streptococcus pneumoniae, a gram-positive bacterium, is one of the two most common pathogens involved in acute middle ear infection, which frequently leads to acquired hearing loss and communication disorders in children (20). The first line of host defense against bacterial infection by the innate immune system is essentially initiated by Toll-like receptors (TLRs), family-pattern-recognition receptors that detect and respond to microbial ligands (3). TLR2 mediates host responses to gram-positive bacterial cell wall components such as peptidoglycan (PGN), lipoteichoic acids (LTA), and lipoproteins (1, 37). TLR2 may function as a regulator of inflammation, and abnormal immune inflammatory responses develop in the absence of TLR2. In humans, one mutation in the TLR2 gene results in an Arg753Gln polymorphism that predisposes individuals to life-threatening bacterial infections (22). TLR2-deficient (TLR2−/−) mice succumb to Mycobacterium tuberculosis infection (6) and are highly susceptible to Staphylococcus aureus infection (32). TLR2−/− mice show delayed pneumococcal phagocytosis and impaired oxidative killing by granulocytes (17). Studies have also demonstrated that TLR2 participates in the mediation of the immune response in experimental pneumococcal meningitis (16, 18) and that mice with a targeted disruption of the TLR2 gene are more susceptible to meningitis-induced intracranial complications (7, 8). However, a TLR2-mediated immune response in S. pneumoniae-induced acute otitis media (AOM) has not yet been reported.
In this study, we established an AOM mouse model by challenging TLR2−/− mice with S. pneumoniae. We investigated the immune responses of the challenged mice by observing the fate of the mice and by analyzing the histological and molecular pathology of the mouse ears. The evidence presented here indeed supports a role for TLR2 in the pathology of and host response to pneumococcal otitis media infection.
MATERIALS AND METHODS
Mice.
Tlr2tm1Kir (TLR2−/−) (36) and C57BL/6J (wild-type [WT]) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). A total of 37 TLR2−/− mice (17 male and 20 female) and 32 WT mice (17 male and 15 female) aged 4 to 5 weeks were used. These studies were conducted in accordance with the principles set forth in the Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council, revised 1996, and were approved by the Case Western Reserve University Health Sciences Institutional Animal Use and Care Committee.
ABR thresholds and tympanometry analysis.
Auditory-evoked brain stem response (ABR) is an evoked potential measure of auditory activity in the brain stem that is commonly used for predicting hearing levels in animals and young children. It is now a method of first choice for screening for hearing impairment in mice. A computer-aided evoked potential system (Intelligent Hearing Systems) was used to test the mouse ABR thresholds as previously described (38). Broadband-click (8 to 16 kHz) and 8-, 16-, and 32-kHz pure-tone bursts were, respectively, presented to mice. Tympanometry measurement, an examination used to test the condition of the middle ear and the mobility of the tympanic membrane and conduction bones by creating variations of air pressure in the ear canal, was performed following the procedures described before (39) by using an MT10 tympanometer from Interacoustics (Assens, Denmark). Prior to ABR or tympanometric measurement, mice were anesthetized with avertin (0.5 mg/g mouse mass) by intraperitoneal injection. Both assays were performed prior to bacterial challenge and at 3 and 7 days following the challenge.
Mouse model of AOM.
Encapsulated S. pneumoniae serotype 6A (N.Carolina6A-23, ATCC BAA-659) was purchased from ATCC and plated onto Trypticase blood agar plates. The plates were incubated at 37°C in 5% CO2 for 20 h. Colonies from the initial culture plates were harvested in phosphate-buffered saline (PBS), resuspended and inoculated onto five or six plates, and cultured as described above. The bacteria were then collected, suspended in PBS, and plated for titer determination (CFU/ml) by serial 10-fold dilution. Five microliters of S. pneumoniae suspension (1 × 106 CFU), immediately after preparation, was inoculated into the right middle ear of each WT and TLR2−/− mouse through the tympanic membrane under anesthetized conditions. Each mouse also received an equivalent volume of PBS through the left tympanic membrane. The viable status of the mice was examined every h thereafter.
Blood bacterial titer determination.
For mice that died after the challenge, the heart was exsanguinated immediately under sterile conditions. For the surviving mice, retro-orbital blood was collected using sterile microtubes before the mice were euthanized for further investigation at 3 and 7 days postinoculation. The blood bacterial titers of infected mice were determined by plating 50-μl volumes of 10-fold diluted blood. The recovery of S. pneumoniae isolates was confirmed by the typical colonial morphology of the isolates on Trypticase blood agar plates.
Histological analysis of middle and inner ears.
Four each of TLR2−/− mice and WT mice on days 3 and 7 postinjection were sacrificed, and the bullae (including both the middle and inner ear) were isolated. Histological analyses were performed following the methods described previously (13). Briefly, the middle and inner ears from TLR2−/− and WT mice were dissected, perfused with Bouin's fixative, immersed in the same for 48 h, decalcified with Cal-EX solution for 6 h, and embedded in paraffin. Sections (7 μm) were cut, mounted on glass slides, and counterstained with hematoxylin/eosin.
In situ staining for bacteria in the middle ear.
To identify S. pneumoniae in the effusion of both middle ears, a BD* acridine orange stain kit (catalog no. 212536; BD Diagnostic Systems, Sparks, MD) was used following the supplier's protocol. Briefly, the sections prepared as described above were deparaffinized in 3 aliquots of xylene for 5 min each; hydrated in aliquots of 100%, 95%, and 70% ethanol for 5 min each; and washed in distilled water at room temperature for 5 min sequentially. The slides were then fixed in 100% methanol for 2 min, flooded with acridine orange stain for 2 min, and rinsed thoroughly with tap water. The tissue sections were examined using a fluorescent microscope (Leica). A cultured S. pneumoniae smear stained bright orange and was used as a positive control.
Semiquantitative RT-PCR for determining mRNA accumulation of TLR2-related genes.
Total RNA (DNA-free) was prepared from the right bullae (containing multiple cell types in the middle and inner ears) of 4 mice from both mouse groups using the pure-Link TM Micro-to-Midi Total RNA Purification System (Invitrogen). First strand cDNA was synthesized using a SuperScript First-Strand Synthesis System with 2 μg of total RNA template from each sample. Reverse transcriptase PCR (RT-PCR) was carried out to reveal the profiles of mRNA accumulation of different genes (e.g., cytokines and TLR2-related molecules in the cell signal transduction pathways). The primers used for these genes are listed in Table 1. The 20-μl reaction mixture contained 50 mM KCl, 10 mM Tris-HCl (pH 9.0, 25°C), 0.01% Triton X-100, 2 mM MgCl2, 250 nM of each primer (forward and reverse), 200 μM deoxynucleoside triphosphate, 1 μl of cDNA, and 0.5 U of Taq DNA polymerase (New England BioLabs). PCR was performed in a Bio-Rad PTC-200 Peltier thermal cycler. The amplification consisted of an initial denaturation at 94°C for 2 min followed by 28 cycles of 94°C for 30 s, 60°C for 40 s, and 72°C for 50 s and then a final extension of 72°C for 2 min. A total of 10 μl of each PCR product was subjected to agarose gel electrophoresis. The gray intensity of each band on the agarose gel was digitized using ImageJ software (NIH) and corrected by the coefficient of the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene expression level of the same sample.
TABLE 1.
Sequences of primers used in the RT-PCR
| Primer | Oligonucleotide sequence (5′-3′) | Size (bp) | Reference |
|---|---|---|---|
| GapdhF | AACTTTGGCATTGTGGAAGG | 351 | 4 |
| GapdhR | GGAGACAACCTGGTCCTCAG | ||
| TLR2F | GAGCGAGCTGGGTAAAGTAGAAA | 528 | 34 |
| TLR2R | AGCCGAGGCAAGAACAAAGA | ||
| MYD88F | ACTGGCCTGAGCAACTAGGA | 356 | 34 |
| MYD88R | CTTCTTTTCTGGGGGTAGGG | ||
| Nod2F | AGCAGAACTTCTTGTCCCTGA | 515 | 27 |
| Nod2R | TCACAACAAGAGTCTGGCGT | ||
| IkkβF | GGGAGAATGACGTGAAGCAT | 163 | |
| IkkβR | GTCTCTTGGCTTCTCCCTGA | ||
| NF-κB1 F | CGGCAACTCACAGACAGAGA | 182 | |
| NF-κB1 R | ATTCGGGGCTTTGCTATCAT | ||
| IL-1βF | TCATGGGATGATGATGATAACCTGCT | 502 | 35 |
| IL-1βR | CCCATACTTTAGGAAGACACGGATT | ||
| TNF-αF | CCACCACGCTCTTCTGTCTAC | 303 | |
| TNF-αR | CCTTGAAGAGAACCTGGGAGT | ||
| MIP1αF | CTCAACATCATGAAGGTCTC | 285 | 2 |
| MIP1αR | GGCATTCAGTTCCAGGTCAG | ||
| Muc5acF | TGGAAGGATGCTATCCCAAG | 202 | |
| Muc5acR | CACCAGCATTGTGGGTACAG | ||
| Muc5bF | GACACCATCTATGGGGTTGG | 243 | |
| Muc5bR | CAGGACTGTTCACCCAGGTT |
Immunostaining for NF-κB and TNF-α.
Sections were deparaffinized as described above. The samples in the sections were then covered with 0.05% trypsin solution (containing 0.1% calcium chloride [pH 7.8]) and incubated in a humidified chamber at 37°C for 20 min. After being washed twice in 1× PBS (5 min each) and blocked in 5% bovine serum albumin for 1 h, the samples were immersed in rabbit anti-mouse polyclonal antibodies (1:200 dilution) and incubated at 4°C overnight. The NF-κB p105/p50 antibody (ab19285, 1.000 mg/ml) and tumor necrosis factor alpha (TNF-α) antibody (ab9739, 0.500 mg/ml) were purchased from Abcam, Inc. (Cambridge, MA). Then the samples were washed twice in 1× PBS for 5 min each, immersed in goat anti-rabbit secondary antibody conjugated to Alexa Fluor 488 (1:500 dilution; Invitrogen) and incubated at room temperature for 1 h. The mounted samples were observed under an immunofluorescence microscope (Leica).
Statistical methods.
Data were analyzed using the Student t test, analysis of variance, or the log-rank test. A P value of <0.05 was considered significant.
RESULTS
Elevated death rate in TLR2−/− mice follows pneumococcal challenge.
TLR2−/− mice and C57BL/6J (WT) mice were challenged with 106 viable S. pneumoniae bacteria injected into the right middle ear. Within 3 days after inoculation, 21 (11 male and 10 female) of the 37 TLR2−/− mice and 4 (3 male and 1 female) of the 32 WT mice had died (P < 0.01). A time course observation of the mortality rates in the two mouse groups is listed in Table 2, and a survival curve to reveal the survival rates (%) of the WT and TLR2−/− mice at different time points within 3 days postinoculation is shown in Fig. 1A, in which the survival rate of the WT mice was significantly higher than that of the TLR2−/− mice (P < 0.05). The surviving mice (16 TLR2−/− mice and 28 WT mice) were further investigated for bacteremia, hearing loss, middle ear inflammation, and proinflammatory gene expression as follows.
TABLE 2.
Mouse death rates within 3 days after S. pneumoniae middle ear challengea
| Mouse | Total no. of deaths at indicated h postinoculation
|
No. of deaths within 3 days/total no. of mice | P value | |||||
|---|---|---|---|---|---|---|---|---|
| 12 | 24 | 36 | 48 | 60 | 72 | |||
| WT | 1 | 3 | 4 | 4 | 4 | 4 | 4/32 | <0.01 |
| TLR2−/− | 7 | 15 | 18 | 19 | 21 | 21 | 21/37 | <0.01 |
Dose of S. pneumoniae, 1 × 106 CFU.
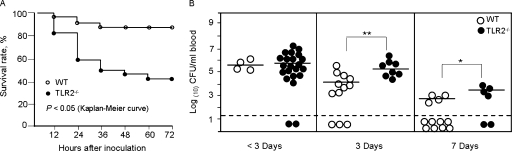
FIG. 1.
(A) Survival rate of TLR2−/− (n = 37) and WT (n = 32) mice within 3 days after tympanic inoculation of 1 × 106 CFU of S. pneumoniae. The survival rate of the WT mice was significantly higher than that of the TLR2−/− mice (P < 0.05, log-rank test). (B) Bacterial titers in the blood of WT (n = 32) and TLR2−/− mice (n = 37) after inoculation of 1 × 106 CFU of S. pneumoniae. Blood collected up to 3 days (<3) after the challenge was from mice that died. At 3 days, blood was collected from surviving mice (selected at random), and at 7 days, blood was collected from all remaining mice. Each point plotted represents the titer from one mouse, with the mean indicated by a horizontal line in each cluster. Mice with negative blood culture results are shown below the dashed horizontal line. Blood bacterial counts were significantly higher in TLR2−/− mice than those in WT mice at 3 and 7 days postinoculation. *, P < 0.05; **, P < 0.01.
High titers in the TLR2−/− mouse blood cultures for S. pneumoniae.
Cardiac blood or retro-orbital blood from each mouse was cultured to establish whether the pneumococci had spread from the right ear to the blood. The positive blood culture rates and blood bacterial titers are listed in Table 3 and Table 4, respectively. Blood culture results revealed an S. pneumoniae bacteremia in 19 of 21 TLR2−/− mice and in all 4 WT mice that died within 3 days postinoculation. Though the mean titer of S. pneumoniae in the blood of the TLR2−/− mice was higher than that of the WT mice, there was no significance. All 8 TLR2−/− mice and 9 of 12 WT mice sacrificed on day 3 were positive for S. pneumoniae in their blood cultures; 4 of 6 TLR2−/− mice and 4 of 16 WT mice were positive on day 7. The TLR2−/− and WT mice showed similar rates of positive culture at both time points; however, the blood bacterial titers were significantly higher in the TLR2−/− mice at 3 and 7 days postinoculation than the titers in the WT mice (P < 0.01 and 0.05, respectively). Additionally, the positive culture rate of S. pneumoniae in WT mice was significantly higher at 3 days (9/12) than at 7 days (4/16) postinoculation (P < 0.01), indicating the probable resolution of the infection. The death rate in TLR2−/− mice gave too small a sample for a similar statistical comparison of the day 3 and 7 culture rates within the TLR2−/− group. A summary of the blood culture results is given in Fig. 1B.
TABLE 3.
Positive culture rates post-middle ear inoculation for S. pneumoniae in mouse blooda
| Mice | No. of positive blood cultures/total no. of mice at indicated time of death (days)
|
||
|---|---|---|---|
| <3 | 3 | 7 | |
| Wild-type | 4/4 | 9/12b | 4/16b |
| TLR2−/− | 19/21 | 8/8 | 4/6 |
Dose of S. pneumoniae, 1 × 106 CFU.
P < 0.01.
TABLE 4.
Blood titers of S. pneumoniae in TLR2−/− and WT mice
| Days postinoculation | Mice | Log(10) CFU/ml blood (no. of mice) | P value |
|---|---|---|---|
| <3 | WT (died) | 5.595 ± 0.352 (n = 4) | >0.05 |
| TLR2−/− (died) | 5.818 ± 0.916 (n = 19) | ||
| 3 | WT | 4.370 ± 0.746 (n = 9) | <0.01 |
| TLR2−/− | 5.425 ± 0.594 (n = 8) | ||
| 7 | WT | 2.824 ± 0.427 (n = 4) | <0.05 |
| TLR2−/− | 3.597 ± 0.441 (n = 4) |
ABR thresholds indicate greater hearing loss during AOM in TLR2−/− mice than in WT mice.
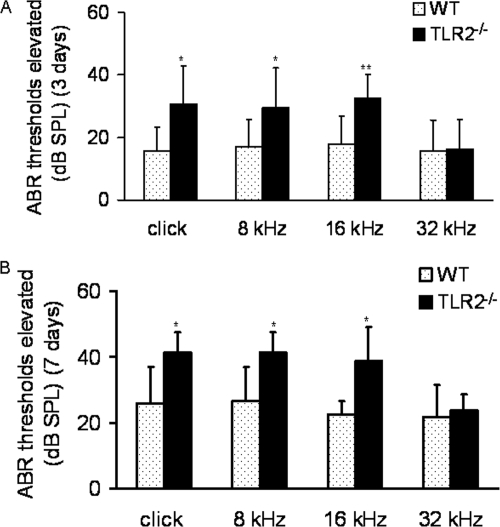
The ABR thresholds of the WT mice and TLR2−/− mice in response to broadband-click (8 to 16 kHz) and pure-tone (8 kHz, 16 kHz, and 32 kHz auditory) stimuli were determined immediately prior to (baseline) and at 3 days and 7 days after pneumococcal challenge. The postchallenge elevation in the mean ABR thresholds was calculated relative to the mean baseline measurement. The increased ABR thresholds in TLR2−/− mice were significantly higher than those in WT mice at relatively low stimulus frequencies (P < 0.05, 0.05, and 0.01 for the broadband-click stimuli and 8-kHz and 16-kHz pure-tone stimuli, respectively; P > 0.05 for the 32-kHz pure-tone stimuli) at both time points (Fig. 2).
FIG. 2.
Comparison of the degree of elevation from baseline measurements (preinjection) in mean ABR thresholds between right ears of WT and TLR2−/− mice at 3 days (A; n = 12 and 8, respectively) and 7 days (B; n = 6 and 6, respectively) post-pneumococcal challenge. On the y axis, 0 represents the baseline, and the number of dB sound pressure levels (dB SPL) represents the increase in mean ABR threshold. The degree of increase in TLR2−/− mice was significantly higher than that in WT mice at stimulus frequencies of click, 8 kHz, and 16 kHz at both time points. Each error bar represents the standard deviation calculated for each mean. Significant differences are indicated by asterisks (*, P < 0.05; **, P < 0.01).
Tympanometry assessment reveals possible structural alteration in the ears of TLR2−/− mice.
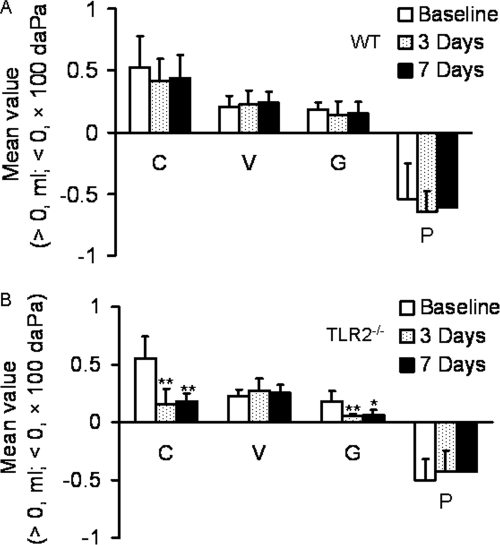
The tympanometric values of compliance (C, the peak amplitude of the tympanogram), gradient (G, the flatness of the tympanogram), volume (V) of the ear canal, and pressure (P) of the middle ears were not significantly changed in the WT mice (Fig. 3A) at either time point after the challenge (P > 0.05) relative to the baseline measurements, whereas the C and G values were significantly lower on average in the TLR2−/− mice at both 3 and 7 days postchallenge (Fig. 3B) (P < 0.01 and 0.01 for C, P < 0.01 and 0.05 for G, respectively). The values of P and V remained relatively constant throughout the course of the experiment (Fig. 3).
FIG. 3.
Time course comparison of the values of tympanometry parameters in the right ears of mice inoculated with S. pneumoniae. Baseline, preinjection (panel A, n = 29; panel B, n = 18); 3 Days, 3 days postinjection (panel A, n = 8; panel B, n = 8); 7 Days, 7 days postinjection (panel A, n = 7; panel B, n = 6). (A) For WT mice, the mean values of compliance (C), gradient (G), volume (V), and pressure (P) are shown and were not significantly changed (P > 0.05) postchallenge from the baseline values. (B) For TLR2−/− mice, the mean C, G, V and P values are shown with significant decreases from the baseline readings for C and G indicated by asterisks. Each error bar represents the standard deviation calculated for each mean. *, P < 0.05; **, P < 0.01.
A histological observation of the middle ears reveals a severe degree of tissue damage in TLR2−/− mice.
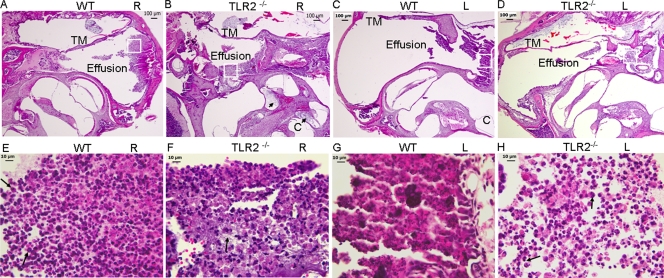
The bullae from eight TLR2−/− mice or WT mice were examined. All TLR2−/− mice and WT mice observed showed signs of inflammation in the right middle ears. Mucosal inflammation was characterized by middle ear effusion and leukocytic infiltration (mainly polymorphonuclear cells) and release of tissue debris at both time points, with fibrous proliferation being evident at 7 days postinoculation. In some mice, especially the TLR2−/− mice, the inner ear was also involved with effusion (Fig. 4B). Inflammation was also observed in the left ears in WT (4/8) and TLR2−/− mice (7/8) injected only with PBS. Therefore, the outcome of the middle ear infection was graded at the histological level, based on the amount of effusion and cellular infiltration, the degree of tissue destruction and proliferation, and whether the infection had spread to the inner ear as well as the middle ear (Table 5). There was a significant difference between the WT and TLR2−/− mice in the outcome of middle ear infection 7 days postchallenge (P < 0.05). There was no significant difference in the degree of middle ear damage between the TLR2−/− and WT mice at 3 days postchallenge (data not shown). Additionally, acridine orange staining demonstrated that morphologically typical S. pneumoniae was present in the effusion from both ears of the mice from both groups at 7 days postinoculation (Fig. 5).
FIG. 4.
Representative images of hematoxylin/eosin-stained right (R) and left (L) middle ears of the WT and TLR2−/− mice 7 days following the inoculation of S. pneumoniae in the right middle ears. Inflammatory fluid accumulation is seen in both the right (A and B) and left (C and D) middle ear space in WT and TLR2−/− mice. Middle ear tissue destruction and the release of tissue debris are evident in panel B. The tympanic membranes (TM) were thickened. The cochleae (C) were also involved with effusion, especially as indicated in panel B by arrows. (E to H) Enlarged images of each region outlined by a square in panels A to D to show the cell types present in the effusion of each middle ear. The inflammatory cells were mainly composed of polymorphonuclear cells as indicated by arrows in panels E and H. There was also fibrous proliferation in panel F as indicated by the arrow. Scale bars, 100 μm for panels A to D and 10 μm for panels E to H.
TABLE 5.
Semiquantitative evaluation of the outcome of infection in the middle ears of TLR2−/−mice and control mice 7 days postchallenge
| Mice | Eara | Degree of pathological alteration inb:
|
Positive ratec | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Middle ear effusion | Inflammatory cells | Tissue proliferation | Tissue debris | Inner ear effusion | ||||
| TLR2−/− | 1R | + | + | ++ | + | + | 57/120 | <0.05 |
| 1L | ++ | ++ | + | ++ | − | |||
| 2R | ++ | ++ | ++ | ++ | − | |||
| 2L | + | + | + | ++ | + | |||
| 3R | ++ | ++ | ++ | ++ | ++ | |||
| 3L | + | + | + | ++ | + | |||
| 4R | ++ | ++ | + | ++ | ++ | |||
| 4L | + | + | + | + | + | |||
| WT | 1R | +++ | +++ | + | ++ | − | 38/120 | <0.05 |
| 1L | + | + | + | + | − | |||
| 2R | + | + | + | + | − | |||
| 2L | + | + | ++ | + | − | |||
| 3R | ++ | ++ | + | ++ | + | |||
| 3L | + | + | + | + | − | |||
| 4R | + | + | + | + | − | |||
| 4L | − | − | − | − | − | |||
Both the right (R) and left (L) ears of each of the four mice in each group were evaluated.
To show the degrees of pathological alteration in the middle ears, the symbols −, +, ++, and +++ were used, with − indicating no pathology and +, ++, or +++ indicating small, medium, and great degrees of pathology, respectively, regarding middle ear effusion, inflammatory cells, tissue proliferation, tissue debris, or inner ear effusion.
Numerical scores were assigned to allow for the semiquantitative analysis of the pathology (1 point was given for each +, for a total maximum possible score of 30 per mouse). The total positive rate (57/120) of the five indicators in the TLR2−/− mice was significantly higher than that (38/120) in the WT mice (P < 0.05).
FIG. 5.
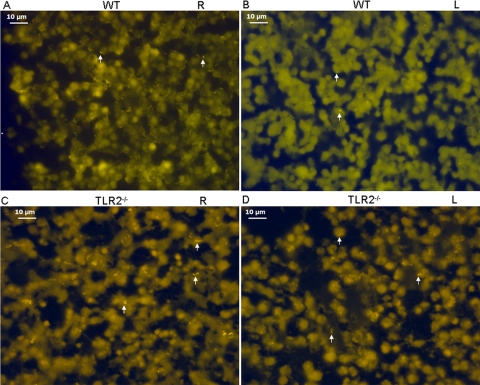
S. pneumoniae revealed by acridine orange in the effusion of the right (R) and left (L) middle ears of WT and TLR2−/− mice 7 days postchallenge. S. pneumoniae isolates were stained bright orange (indicated by arrows) and appear in all four panels. Typical S. pneumoniae isolates were evident as diplococci, indicated by arrows. Single (top arrow in panel B) and short chains (top and bottom arrows in panel C) of the bacteria can also be seen. Scale bar = 10 μm.
TLR2−/− mice show decreased levels of proinflammatory gene expression at the level of mRNA accumulation.
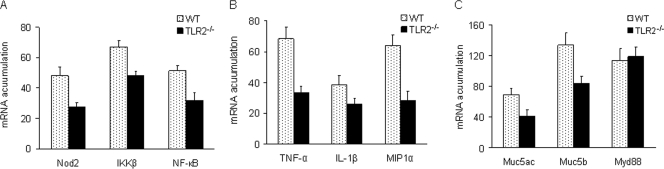
Of those exhibiting S. pneumoniae-positive blood cultures, four mice were chosen randomly from each of the TLR2−/− and WT groups to be assessed for mRNA accumulation levels of proinflammatory cytokines. GAPDH-corrected RT-PCR results showed that the mRNA accumulations of Nod2, IκB kinase β (IKKβ), NF-κB, TNF-α, interleukin-1β (IL-1β), MIP1α, Muc5ac, and Muc5b in the right ears of the TLR2−/− mice were significantly lower at 3 days postchallenge than the WT control mRNA levels (P < 0.01, 0.01, 0.01, 0.01, 0.05, 0.01, 0.01, and 0.05, respectively), whereas Myd88 was not significantly changed (P < 0.05) (Fig. 6).
FIG. 6.
mRNA accumulation levels measured 3 days after S. pneumoniae challenge of TLR2-related genes from middle and inner right ears of WT and TLR2−/− mice. After 2% agarose gel electrophoresis of the RT-PCR products, the bands were digitized for GAPDH, Nod2, IKKβ, and NF-κB (A); TNF-α, IL-1β, and MIP1α (B); and Muc5ac, Muc5b, and Myd88 (C). The GAPDH-corrected mRNA accumulation levels were averaged for each genotype and plotted, with error bars indicating standard deviations from the means. The mRNA accumulation levels of all the genes except Myd88 were significantly higher in WT mice than in TLR2−/− mice (P < 0.05).
Immunostaining for NF-κB and TNF-α reveals a decrease in the proinflammatory protein levels in TLR2−/− mice.
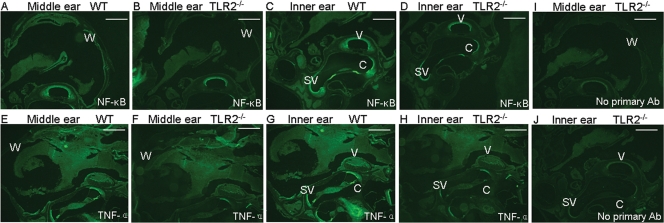
To confirm that the decrease in the mRNA accumulation of the inflammatory genes was correlated with protein expression levels, we chose two of the proteins for which well-characterized reagents are available and analyzed their expression in situ. Paraffin sections of the middle and inner right ears from WT and TLR2−/− mice were stained with fluorescein isothiocyanate (FITC)-anti-NF-κB and FITC-anti-TNF-α at 7 days post-pneumococcal challenge. The fluorescence from the NF-κB and TNF-α antibodies was diffuse, but the intensity of the staining in the wall of the middle ear (for NF-κB), the stapedial artery, the stria vascularis, and the organ of Corti was much stronger in WT mice; control sections stained with primary antibody omission showed less recordable imaging in the same anatomic sites described above (Fig. 7).
FIG. 7.
Representative sections of middle and inner right ears from WT and TLR2−/− mice 7 days after S. pneumoniae challenge. (A to D) Anti-NF-κB-FITC staining of ear tissue from WT (A and C) and TLR2−/− (B and D) mice. (E to H) Anti-TNF-α staining of ear tissue from WT (E and G) and TLR2−/− (F and H) mice. Panels A and C show the middle and inner ears from one mouse, as do panels B and D, E and G, and F and H. The staining intensity of both antibodies was much stronger in WT ear tissue than TLR2−/− tissue. (I and J) Control sections of the middle ear (I) and inner ear (J) stained with primary antibody (Ab) omission. W, wall of middle ear; V, stapedial artery; C, organ of Corti; SV, stria vascularis. Scale bar = 500 μm.
DISCUSSION
In this study, we established comparative mouse models of AOM to investigate the role of TLR2 in OM by challenging TLR2−/− mice and WT mice with the gram-positive bacterium S. pneumoniae. An earlier report (24) regarding the dose of inoculation of viable S. pneumoniae serotype 3 (105 CFU) in C57BL/6 mice was published in which the intrabullar route was used and the number of mice tested was limited. In the present study, a dose of 106 CFU for S. pneumoniae serotype 6 for each mouse through the tympanic membrane proved successful in establishing AOM. At this dosage, we have shown that 56.8% of 37 TLR2−/− mice died within 3 days postinoculation, compared to only 12.5% of 32 WT mice. Our results demonstrate that TLR2−/− mice are more vulnerable to the challenge with S. pneumoniae at the dosage used. Upon histological observation of the surviving mice, the TLR2−/− mice showed more severe degrees of tissue damage and less ability to localize the infection with regard to the intactness of the middle ear structures and the involvement of the inner ears and left ears. It was reported that there was a propensity for mice to develop bacteremia and to succumb to systemic infections after the middle ear inoculation of S. pneumoniae (24). The high positive-culture rate of S. pneumoniae in blood and the presence of bacteria revealed by acridine orange in the effusion of both middle ears supported our hypothesis that S. pneumoniae spread from the right ear to the left ear mainly via the blood. Because high blood titers of S. pneumoniae were found in mice from both groups, but especially in TLR2−/− mice, sepsis was probably the cause of the high rate of mouse mortality.
A combination of tympanometry assessment and ABR testing can provide detailed information about mouse middle ear inflammation and hearing ability. Tympanometry gives a quick assessment of middle ear status (39). However, many factors influence this measurement, especially the intactness and stiffness of the tympanic membrane and ossicle chain and the content of the effusion in the middle ear. The loss of compliance in some mouse strains could result from a combination of OM and OM-related complications in the middle ear (39). Though the values of C and G were significantly decreased in the TLR2−/− mice in the 7-day period after bacterial challenge, neither factor changed significantly in WT mice at either time point, suggesting that the structural damage caused by S. pneumoniae infection in the middle ears of WT mice was not severe enough to affect those parameters. The results were consistent with our histological observation. It is now accepted that ABR offers a valid and simple physiologic test of mouse middle ear inflammation (23). All mice with AOM in our study exhibited an elevation in ABR thresholds; however, the magnitude of elevation was significantly higher in TLR2−/− mice than in WT mice at relatively low stimulus frequencies (Fig. 2). The increase in ABR threshold was probably caused mainly by abnormal sound transduction in these TLR2−/− mice.
Pneumococcal OM may involve the altered expression of broad arrays of inflammatory genes (20). Studies by Santos-Sierra and colleagues showed that the stimulation of TLR2 by components of gram-positive cell walls (PGN and LTA) leads to the production of proinflammatory cytokines, such as TNF-α, IL-6, and IL-1β in mouse macrophages (31). These effector molecules mediate inflammatory responses and drive T helper 1 (TH1) cell development, thus causing the activation of adaptive immunity. Chemokines, such as MCP1, MIP1α, and MIP1β, which act as chemoattractants and activators of specific leukocytes at the site of inflammation, can recruit inflammatory cells to the site of the infection. In the absence of TLR2, the response to cell wall extracts from gram-positive bacteria, such as PGN, was impaired in mouse macrophages (33). In the present investigation, the mRNA accumulations of TNF-α, IL-1β, and MIP1α genes were at significantly low levels in the ears of TLR2−/− mice compared to the levels in the WT controls (though limited numbers of mice were tested because of high mortality rates), indicating impaired inflammatory responses of the TLR2−/− mice to the S. pneumoniae challenge.
It has been reported that NOD2 is required for the recognition of PGN in the cytoplasm and thus leads to strong synergistic effects on the TLR2-mediated production of proinflammatory cytokines (TNF-α and IL-1β) in mouse macrophages (26). Nod2 mRNA expression was upregulated after pneumococcal infection, and NF-κB activation by S. pneumoniae was dependent on Nod2 in HEK293 cells (10, 27). To release NF-κB from IκB in the cytoplasm, the phosphorylation of IκB by IKKs is required (29). The low-level mRNA accumulation of Nod2 and IKKβ genes may downregulate the level of NF-κB mRNA in the ears of TLR2−/− mice. Therefore, a deficiency in TLR2 expression may hinder the expression of NF-κB and its related cytokines or chemokines in response to S. pneumoniae infection. In our study, we found that NF-κB protein levels in middle ear tissue were below detectable levels, consistent with what has been published regarding the relationship between TLR2 and NF-κB expression. Furthermore, mRNA levels were low for NOD2, NF-κB, and IKKβ, indicating that the lack of TLR2 has affected the entire pathway in these mice and that the infection does not circumvent this pathway.
A recent study (25) supports our results in showing that macrophages in TLR2−/− mice downregulated proinflammatory cytokine gene transcription (such as TNF-α) in response to a complex macromolecule of S. pneumoniae cell wall fragments (PnCM); however, the results also implied that inflammatory cytokine production was TLR2 dependent and NOD2 independent as tested in WT and TLR2−/− and Nod2−/− mouse macrophages. Because the mammalian immune system may need multiple, redundant means to recognize and respond to complex antigens, there may be compensatory pathways that function in the absence of NOD2.
Molecules that generate TLR2-dependent signals are dependent upon MyD88 and subsequently activate NF-κB. In this investigation, the TLR2−/− mice showed the same Myd88 mRNA levels as the WT mice. This result can be explained by the fact that MyD88 is one of the general adaptor molecules for several TLRs (1, 12). MyD88 may be participating in TLR2-independent pathways in response to S. pneumoniae challenge in these mice.
Mucins are important structural components of the mucociliary transport system that protects the epithelium against invading microorganisms. S. pneumoniae is a potent inducer of mucin or mucin-like glycoprotein in the middle ear (21). The cellular production of mucin is controlled by at least eight different mucin genes in the respiratory epithelium of humans, and MUC5AC and MUC5B are the major constituents of airway mucus (14, 15). Human studies showed that the MUC5B mucin gene was upregulated 4.2-fold in middle ears with chronic OM (19), and MUC5AC/GAPDH and MUC5B/GAPDH mRNA ratios in the middle ear effusions from a test group were significantly increased compared to the ratios of the control groups (9). A recent report revealed that IL-1β and TNF-α upregulated mucin secretion from human middle ear epithelial cells and that the upregulation was inhibited by IL-1 receptor antagonist and anti-TNF antibody (30). The activation of NF-κB in murine middle ear epithelial cells also induced Muc5b promoter activation and gene expression (28). Therefore, low levels of mRNA accumulation of Muc5ac and Muc5b in TLR2−/− mice may result from decreased mRNA levels of NF-κB, TNF-α, and IL-1β, which then impairs the physical defense barrier in the middle and inner ear.
The low-level expression of cytokines, chemokines, and mucins may limit the ability of TLR2−/− mice to clear bacteria from the middle ear in the initial days following S. pneumoniae challenge, leading to sepsis and a high death rate in TLR2−/− mice. However, WT mice mounted a strong immune response to the challenge, had a lower mortality rate, and showed a much better outcome following infection.
Gene expression profiles from the TLR2−/− mice in response to S. pneumoniae challenge reveal that the TLR2 pathway plays an important role in the molecular mechanism of OM. The development of therapeutic approaches for OM and its complications may require regulation of this pathway or targeting components of the pathway.
Acknowledgments
The work is supported by NIH grants R01DC007392, R01DC009246, and R01DC008165 from the National Institute on Deafness and Other Communication Disorders (NIDCD).
The TLR2−/− mice were generously provided by the Jackson Laboratory.
Editor: A. Camilli
Footnotes
Published ahead of print on 4 May 2009.
REFERENCES
- 1.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124783-801. [DOI] [PubMed] [Google Scholar]
- 2.Behera, A. K., E. Hildebrand, R. T. Bronson, G. Perides, S. Uematsu, S. Akira, and L. T. Hu. 2006. Myd88 deficiency results in tissue-specific changes in cytokine induction and inflammation in interleukin-18-independent mice infected with Borrelia burgdorferi. Infect. Immun. 741462-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bornstein, S. R., P. Zacharowski, R. R. Schumann, A. Barthel, N. Tran, C. Papewalis, V. Rettori, S. M. McCann, K. Schulze-Osthoff, W. A. Scherbaum, J. Tarnow, and K. Zacharowski. 2004. Impaired adrenal stress response in Toll-like receptor 2-deficient mice. Proc. Natl. Acad. Sci. USA 10116695-16700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, M., V. O. Ona, M. Li, R. J. Ferrante, K. B. Fink, S. Zhu, J. Bian, L. Guo, L. A. Farrell, S. M. Hersch, W. Hobbs, J. P. Vonsattel, J. H. Cha, and R. M. Friedlander. 2000. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med. 6797-801. [DOI] [PubMed] [Google Scholar]
- 5.Reference deleted.
- 6.Drennan, M. B., D. Nicolle, V. J. Quesniaux, M. Jacobs, N. Allie, J. Mpagi, C. Fremond, H. Wagner, C. Kirschning, and B. Ryffel. 2004. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am. J. Pathol. 16449-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Echchannaoui, H., K. Frei, C. Schnell, S. L. Leib, W. Zimmerli, and R. Landmann. 2002. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J. Infect. Dis. 186798-806. [DOI] [PubMed] [Google Scholar]
- 8.Echchannaoui, H., P. Bachmann, M. Letiembre, M. Espinosa, and R. Landmann. 2005. Regulation of Streptococcus pneumoniae distribution by Toll-like receptor 2 in vivo. Immunobiology 210229-236. [DOI] [PubMed] [Google Scholar]
- 9.Elsheikh, M. N., and M. E. Mahfouz. 2006. Up-regulation of MUC5AC and MUC5B mucin genes in nasopharyngeal respiratory mucosa and selective up-regulation of MUC5B in middle ear in pediatric otitis media with effusion. Laryngoscope 116365-369. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez, O., C. Pipaon, N. Inohara, A. Fontalba, Y. Ogura, F. Prosper, G. Nunez, and J. L. Fernandez-Luna. 2002. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J. Biol. Chem. 27741701-41705. [DOI] [PubMed] [Google Scholar]
- 11.Reference deleted.
- 12.Jo, E. K., C. S. Yang, C. H. Choi, and C. V. Harding. 2007. Intracellular signaling cascades regulating innate immune responses to Mycobacteria: branching out from Toll-like receptors. Cell. Microbiol. 91087-1098. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, K. R., L. H. Gagnon, L. S. Webb, L. L. Peters, N. L. Hawes, B. Chang, and Q. Y. Zheng. 2003. Mouse models of USH1C and DFNB18: phenotypic and molecular analyses of two new spontaneous mutations of the Ush1c gene. Hum. Mol. Genet. 233075-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawano, H., M. M. Paparella, S. B. Ho, P. A. Schachern, N. Morizono, C. T. Le, and J. Lin. 2000. Identification of MUC5B mucin gene in human middle ear with chronic otitis media. Laryngoscope 110668-673. [DOI] [PubMed] [Google Scholar]
- 15.Kim, D. H., S. C. Chu, J. Y. Lee, S. J. Hwang, S. H. Lee, and H. M. Lee. 2004. Up-regulation of MUC5AC and MUC5B mucin genes in chronic rhinosinusitis. Arch. Otolaryngol. Head Neck Surg. 130747-752. [DOI] [PubMed] [Google Scholar]
- 16.Koedel, U., B. Angele, T. Rupprecht, H. Wagner, A. Roggenkamp, H. W. Pfister, and C. J. Kirschning. 2003. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J. Immunol. 170438-444. [DOI] [PubMed] [Google Scholar]
- 17.Letiembre, M., H. Echchannaoui, P. Bachmann, F. Ferracin, C. Nieto, M. Espinosa, and R. Landmann. 2005. Toll-like receptor 2 deficiency delays pneumococcal phagocytosis and impairs oxidative killing by granulocytes. Infect. Immun. 738397-8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Letiembre, M., H. Echchannaoui, F. Ferracin, S. Rivest, and R. Landmann. 2005. Toll-like receptor-2 deficiency is associated with enhanced brain TNF gene expression during pneumococcal meningitis. J. Neuroimmunol. 16821-33. [DOI] [PubMed] [Google Scholar]
- 19.Lin, J., V. Tsuprun, H. Kawano, M. M. Paparella, Z. Zhang, R. Anway, and S. B. Ho. 2001. Characterization of mucins in human middle ear and Eustachian tube. Am. J. Physiol. Lung Cell. Mol. Physiol. 280L1157-L1167. [DOI] [PubMed] [Google Scholar]
- 20.Lin, J., Y. Tsuboi, W. Pan, G. S. Giebink, G. L. Adams, and Y. Kim. 2002. Analysis by cDNA microarrays of altered gene expression in middle ears of rats following pneumococcal infection. Int. J. Pediatr. Otorhinolaryngol. 65203-211. [DOI] [PubMed] [Google Scholar]
- 21.Lin, J., A. Vambutas, A. Haruta, M. M. Paparella, G. S. Giebink, and Y. Kim. 1999. Pneumococcus activation of the 5-lipoxygenase pathway and production of glycoproteins in the middle ear of rats. J. Infect. Dis. 1791145-1151. [DOI] [PubMed] [Google Scholar]
- 22.Lorenz, E., J. P. Mira, K. L. Cornish, N. C. Arbour, and D. A. Schwartz. 2000. A novel polymorphism in the Toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect. Immun. 686398-6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacArthur, C. J., S. H. Hefeneider, J. B. Kempton, S. K. Parrish, S. L. McCoy, and D. R. Trune. 2006. Evaluation of the mouse model for acute otitis media. Hear. Res. 21912-23. [DOI] [PubMed] [Google Scholar]
- 24.Melhus, A., and A. F. Ryan. 2003. A mouse model for acute otitis media. APMIS 111989-994. [DOI] [PubMed] [Google Scholar]
- 25.Moreira, L. O., K. C. El Kasmi, A. M. Smith, D. Finkelstein, S. Fillon, Y. G. Kim, G. Núñez, E. Tuomanen, and P. J. Murray. 2008. The TLR2-MyD88-NOD2-RIPK2 signalling axis regulates a balanced pro-inflammatory and IL-10-mediated anti-inflammatory cytokine response to Gram-positive cell walls. Cell. Microbiol. 102067-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Netea, M. G., G. Ferwerda, D. J. de Jong, T. Jansen, L. Jacobs, M. Kramer, T. H. Naber, J. P. Drenth, S. E. Girardin, B. J. Kullberg, G. J. Adema, and J. W. Van der Meer. 2005. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J. Immunol. 1746518-6523. [DOI] [PubMed] [Google Scholar]
- 27.Opitz, B., A. Püschel, B. Schmeck, A. C. Hocke, S. Rosseau, S. Hammerschmidt, R. R. Schumann, N. Suttorp, and S. Hippenstiel. 2004. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J. Biol. Chem. 27936426-36432. [DOI] [PubMed] [Google Scholar]
- 28.Preciado, D., J. Lin, B. Wuertz, and M. Rose. 2008. Cigarette smoke activates NF kappa B and induces Muc5b expression in mouse middle ear cells. Laryngoscope 118464-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryo, A., F. Suizu, Y. Yoshida, K. Perrem, Y. C. Liou, G. Wulf, R. Rottapel, S. Yamaoka, and K. P. Lu. 2003. Regulation of NF-kappa B signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol. Cell 121413-1426. [DOI] [PubMed] [Google Scholar]
- 30.Samuel, E. A., A. Burrows, and J. E. Kerschner. 2008. Cytokine regulation of mucin secretion in a human middle ear epithelial model. Cytokine 4138-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos-Sierra, S., D. T. Golenbock, and P. Henneke. 2006. Toll-like receptor-dependent discrimination of streptococci. J. Endotoxin Res. 12307-311. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and Myd88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 1655392-5396. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11443-451. [DOI] [PubMed] [Google Scholar]
- 34.Tang, S. C., T. V. Arumugam, X. Xu, A. Cheng, M. R. Mughal, D. G. Jo, J. D. Lathia, D. A. Siler, S. Chigurupati, X. Ouyang, T. Magnus, S. Camandola, and M. P. Mattson. 2007. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc. Natl. Acad. Sci. USA 10413798-13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulett, G. C., N. Ketheesan, and R. G. Hirst. 2000. Cytokine gene expression in innately susceptible BALB/c mice and relatively resistant C57BL/6 mice during infection with virulent Burkholderia pseudomallei. Infect. Immun. 682034-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168348-355. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarski, and D. Golenbock. 1999. Cutting edge: recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 1631-5. [PubMed] [Google Scholar]
- 38.Zheng, Q. Y., K. R. Johnson, and L. C. Erway. 1999. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear. Res. 13094-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng, Q. Y., Y. C. Tong, K. N. Alagramam, and H. Yu. 2007. Tympanometry assessment of 61 inbred strains of mice. Hear. Res. 23123-31. [DOI] [PMC free article] [PubMed] [Google Scholar]