Abstract
Infection with Chlamydia muridarum in the mouse urogenital tract can induce both protective immunity and inflammatory pathologies, which has been used as a model for understanding the immune and pathogenic mechanisms of C. trachomatis infection. We compared the roles of CD28- and CD40 ligand (CD40L)-mediated costimulation in C. muridarum infection. Mice with CD28 or CD80/CD86 gene knockout (KO) displayed an infection course similar to that of wild-type mice during both primary and secondary infection, suggesting that CD28-mediated costimulation is not required for protection against C. muridarum infection. However, mice deficient in CD40L or CD40 displayed a prolonged infection course after primary or secondary infection, suggesting that CD40-CD40L costimulation plays an essential role in the development of anti-C. muridarum immunity. Interestingly, the CD28- or CD80/CD86-deficient mice displayed significantly lower levels of inflammatory pathologies in the upper genital tracts after primary infection, although the attenuation in inflammation was no longer significant during secondary infection. However, the CD40L or CD40 KO mice developed inflammatory pathologies as severe as those in wild-type mice following either primary or secondary infection despite the obvious deficits in adaptive immunity in these KO mice. The resistance of CD28 or CD80/CD86 KO mice to chlamydial infection correlated with production of gamma interferon, while the development of inflammatory pathologies in CD40L or CD40 KO mice correlated with the production of other proinflammatory cytokines in mouse urogenital tracts during the early stages of the infection. These observations together suggest that C. muridarum-induced protective immunity and inflammatory pathologies can be mediated by distinct costimulatory signals.
Chlamydia trachomatis consists of multiple biovars with different tissue tropism. The three human biovars include the trachoma biovar that infects human ocular epithelium, causing preventable blindness (43); the genital biovar that invades human urogenital tract epithelial tissues, potentially leading to complications such as ectopic pregnancy and infertility in the affected women (40); and the LGV biovar (lymphogranuloma venereum) that can cause systemic infection (36). The mouse biovar known as mouse pneumonitis agent (MoPn) is now classified as a new species, C. muridarum. Although C. muridarum organisms cause no known human diseases, they have been extensively used to study the mechanisms of C. trachomatis pathogenesis and immunity (10, 24, 28, 30, 32). Using the C. muridarum urogenital tract infection mouse model in combination with strategies such as in vivo depletion and gene knockout (KO), CD4+ T-cell-dependent and gamma interferon (IFN-γ)-mediated immunity has been identified as a major protective mechanism for mice to control chlamydial infection (27). However, the same Th1 response may also contribute to the chlamydia-induced inflammatory pathologies. Since costimulation systems impact both T-cell activation and phenotypes, we compared the roles of CD28- and CD40 ligand (CD40L)-mediated costimulation in C. muridarum urogenital tract infection in the present study.
T-cell activation requires both an antigen-specific signal generated by T-cell-receptor engagement with antigenic peptide-major histocompatibility complex on antigen-presenting cells, and a costimulatory signal that can be provided by CD28 or CD40L on T cells through ligation with their counterparts (CD80 [B7-1] and CD86 [B7-2] for CD28 or CD40 for CD40L) on antigen-presenting cells (18, 19, 23). The CD28-mediated costimulatory signal can result in an enhanced T-cell proliferation and cytokine production (23) and contribute to the development of various inflammatory diseases (6, 21, 31, 42, 44, 47). However, the role of CD28 costimulatory system in host defense against microbial infection has been less consistent (9, 13, 14, 26, 33, 46), and it is not known whether the CD28-mediated costimulation plays any role in C. muridarum infection. In contrast, the CD40L-CD40 costimulation system is known to play a critical role in host defense against intracellular pathogen infection by promoting Th1-dominant immunity (1, 12, 15, 16, 34, 41). Since Th1-dominant immunity is essential for controlling chlamydial infection, we hypothesize that the CD40L-CD40 costimulation is critical for developing antichlamydia immunity.
In the present study, we found that CD28-mediated costimulation was not required for protection against C. muridarum infection but contributed to the development of inflammatory pathologies, whereas CD40L costimulation was necessary for developing anti-C. muridarum immunity but not required for inducing the inflammatory damages. These observations have clearly demonstrated that C. muridarum-induced protective immunity and inflammatory pathologies can be mediated via distinct costimulatory mechanisms.
MATERIALS AND METHODS
Chlamydial organisms and infection.
The C. muridarum (also called MoPn) organisms (Nigg strain) were used to infect mice and mouse peritoneal macrophages (Mφs), as well as HeLa cells (human cervical carcinoma epithelial cells, ATCC catalog no. CCL2). The organisms were propagated, purified, divided into aliquots, and stored as described previously (7, 8). Female C57BL/6J mice—either wild type (stock number 000664) or with a gene deficiency in CD28 (B6.129S2-Cd28tm1Mak/J, stock number 002666), CD80/CD86 (B6.129S4-Cd80tm1shrCd86tm2Shr/J, stock number 003610), CD40L (B6.129S2-Cd40lgtm1Imx/J, stock number 002770), or CD40 (B6.129P2-Cd40tm1Kik/J, stock number 002928)—were purchased at the age of 5 to 6 weeks from Jackson laboratories (Bar Harbor, ME). Each mouse was inoculated intravaginally with 104 inclusion-forming units (IFU) of live C. muridarum organisms in 20 μl of SPG (sucrose-phosphate-glutamate buffer consisting of 218 mM sucrose, 3.76 mM KH2PO4, 7.1 mM K2HPO4, and 4.9 mM glutamate [pH 7.2]). At 5 days prior to infection, each mouse was injected subcutaneously with 2.5 mg of Depo-Provera (Pharmacia Upjohn, Kalamazoo, MI) to synchronize the estrus cycle and increase mouse susceptibility to chlamydial infection. For some mice, a secondary infection was similarly carried out at day 73 after the first infection. Depo-Provera was also applied 5 days prior to the secondary infection. For in vitro infection of Mφs, mouse Mφs were collected from the peritoneal cavity as described previously (52). Briefly, the mouse peritoneal cavity was injected with 4 to 5 ml of cold Hanks buffer (2.5 mM HEPES [pH 7.4], 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM Na2HPO4, 25 mM glucose, 0.05% bovine serum albumin) using a 27-gauge needle. After gentle massage, the solution was slowly withdrawn from the mouse peritoneal cavity using a 20-gauge needle. After the total number of viable cells was determined, the peritoneal cavity-derived cells were resuspended in RPMI 1640 with 10% fetal calf serum, and then 10 μg of gentamicin/ml and 2 × 105 cells were added to each well of 48-well plates. The plates were incubated at 37°C for 2 h in a CO2 incubator to allow macrophages to adhere. The adherent Mφs were cultured overnight prior to chlamydial inoculation. Chlamydial organisms diluted in cell growth medium were directly inoculated onto the cell monolayers at a multiplicity of infection of 5. The infected cultures were incubated for 24 h at 37°C in a CO2 incubator before harvesting for cytokine measurement by enzyme-linked immunosorbent assay (ELISA). To infect HeLa cells, HeLa cells grown on coverslips in 24-well plates containing Dulbecco modified Eagle medium (Gibco-BRL, Rockville, MD) with 10% fetal calf serum (Gibco-BRL) at 37°C in an incubator supplied with 5% CO2 were inoculated with C. muridarum organisms as described previously (7, 8). The infected cultures were processed for immunofluorescence assay as described below.
Monitoring mouse shedding of live chlamydial organisms.
To monitor live organism shedding, vaginal swabs were taken on different days after the intravaginal infection (once every 3 to 4 days for the first 30 days and once per week thereafter). Each swab was dissolved in 500 μl of SPG, followed by sonication on ice, and the released organisms were titrated on HeLa cell monolayers in duplicates as described previously (29). Briefly, serially diluted swab samples were inoculated onto HeLa cell monolayers grown on coverslips in 24-well plates. After incubation for 24 h in the presence of 2 μg of cycloheximide/ml, the cultures were processed for immunofluorescence assay as described below. The inclusions were counted under a fluorescence microscope. Five random fields were counted per coverslip. For coverslips with less than 1 IFU per field, the entire coverslips were counted. Coverslips showing obvious cytotoxicity of HeLa cells were excluded. The total number of IFU per swab was calculated based on the number of IFU per field, the number of fields per coverslip, dilution factors, and inoculation and total sample volumes. An average was taken from the serially diluted and duplicate samples for any given swab. The calculated total number of IFU/swab was converted into log10, and the log10 IFU was used to calculate the means and standard deviations for each group at each time point.
Evaluating mouse genital tract tissue pathologies and histological scoring.
Mice were sacrificed 80 (with primary infection only) or 115 (with a secondary infection on day 73) days after the primary infection, and the mouse urogenital tract tissues were isolated. Before the tissues were removed from the mouse body, an in situ gross examination was performed for evidence of hydrosalpinx and any other related abnormalities. The excised tissues were then fixed in 10% neutral formalin and embedded in paraffin and serially sectioned longitudinally (5 μm/section). Efforts were made to include the cervix, both uterine horns, and oviducts, as well as the luminal structures of each tissue in each section. The sections were stained with hematoxylin and eosin (H&E) as described elsewhere (37). The H&E-stained sections were assessed by a certified pathologist (I-T.Y.) blinded to mouse treatment and scored for the severity of inflammation and pathologies based on the modified schemes established previously (20, 29, 37). The uterine horns and oviducts were scored separately. Scoring for the dilatation of the uterine horn or oviduct was as follows: 0, no significant dilatation; 1, mild dilatation of a single cross-section; 2, one to three dilated cross-sections; 3, more than three dilated cross-sections; and 4, confluent pronounced dilation. Scoring for the inflammatory cell infiltrates (at the chronic stage of infection, the infiltrates mainly contain mononuclear cells) was as follows: 0, no significant infiltration; 1, infiltration at a single focus; 2, infiltration at two to four foci; 3, infiltration at more than four foci; and 4, confluent infiltration. Scores assigned to individual mice were calculated into means ± the standard errors for each group of animals (n = 5 to 11, as indicated in the corresponding data table and figures).
Immunofluorescence assay.
HeLa cells grown on coverslips were fixed with 2% paraformaldehyde (Sigma, St. Luis, MO) dissolved in phosphate-buffered saline for 30 min at room temperature, followed by permeabilization with 2% saponin (Sigma) for an additional 1 h. After washing and blocking steps, the cell samples were subjected to staining. Hoechst stain (blue; Sigma) was used to visualize nuclear DNA. For titrating IFU from mouse vaginal swab samples, a mouse anti-chlamydial lipopolysaccharide antibody (clone MB5H9, unpublished observation) plus a goat anti-mouse immunoglobulin G (IgG) conjugated with Cy3 (red; Jackson Immunoresearch Laboratories, Inc., West Grove, PA) were used to visualize chlamydial inclusions. For titrating and isotyping mouse antisera, C. muridarum-infected HeLa cells grown on coverslips were used as antigens. Binding of the serially diluted mouse antisera to antigens were detected with the fluorescence-conjugated goat antibodies against mouse μ (for IgM), γ (for total IgG), γ1 (IgG1), γ2a (IgG2a), γ2b (IgG2b), or γ3 (IgG3) chains (Jackson Immunoresearch). The detection antibody alone-labeled samples were used as controls. The coverslips were observed under an AX-70 fluorescence microscope equipped with multiple filter sets (Olympus, Melville, NY).
ELISA.
Cytokines from mouse vaginal swab samples, Mφ culture and mouse spleen cell restimulation culture supernatants were measured by using commercially available ELISA kits. The kits for mouse interleukin-1α (IL-1α; catalog no. DY400), IL-6 (catalog no. DY406), tumor necrosis factor alpha (TNF-α; catalog no. DY410), macrophage inflammatory protein 2 (MIP-2; mouse homolog of hIL-8, DY452), IL-12p70 (catalog no. DY419), IFN-γ (catalog no. DY485), IL-4 (catalog no. DY404), and IL-5 (catalog no. DY405) were all obtained from R&D Systems, Inc. (Minneapolis, MN). The ELISA was performed according to the instructions provided by the manufacturer or as described elsewhere (39, 50, 51). Briefly, 96-well ELISA microplates (Nunc, Rochester, NY) were coated with a capture antibody and, after blocking, the cytokine samples or standards were added to the coated plates, followed by a biotin-conjugated detection antibody. The antibody binding was detected with a horseradish peroxidase-conjugated avidin plus a soluble colorimetric substrate ABTS [2,2′azinobis(3-ethylbenzthiazolinesulfonic acid)]. The absorbance taken at 405 nm using a microplate reader (Molecular Devices Corp., Sunnyvale, CA) was used to calculate cytokine concentrations (in pg or ng per ml).
To prepare vaginal swab samples for cytokine measurements, each mouse vaginal swab was sonicated in 0.5 ml of SPG on ice as described above. After a portion was used for titrating live organisms, the rest vaginal swab samples were stored at −80°C as source materials for cytokine measurements. The frozen vaginal swab samples were completely thawed on ice and mixed well before dilution. Both neat and serially diluted vaginal swab samples were applied to the corresponding ELISA plate wells with 50 μl/well. The Mφ cultures were set up and infected with MoPn organisms as described above, and the supernatants were collected and applied to ELISA plates with or without dilution. To prepare mouse spleen cell in vitro restimulation cultures, splenocytes were harvested from mice infected with MoPn on day 80 or 115 after the primary infection and cultured in 96-well plates at a density of 5 × 106 cells/well with or without stimulation with UV-inactivated MoPn elementary bodies at a final concentration of 106 IFU/ml. After culture for 3 days, the culture supernatants were collected for cytokine measurements.
Statistical analysis.
An analysis of variance (ANOVA; http://www.physics.csbsju.edu/stats/anova.html) was performed to analyze the IFU and cytokine measurement data from multiple groups and a two-tailed Student t test (Microsoft Excel) to compare two given groups. The semiquantitative pathology score data were analyzed with a Student t test or a Wilcoxon-signed rank test (an alternative nonparametric method of the Student t test [http://faculty.vassar.edu/lowry/wilcoxon.html]). The Fisher exact test (http://www.danielsoper.com/statcalc/calc29.aspx) was used to analyze the data on incidence of uterine dilation and oviduct hydrosalpinx shown in Table 1.
TABLE 1.
Incidence of gross pathologiesa
| Mouse group | No. of miceb sacrificed after:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary infection
|
Secondary infection
|
|||||||||||
| Uterine horn dilation
|
Hydrosalpinx
|
Uterine horn dilation
|
Hydrosalpinx
|
|||||||||
| None | Single | Both | None | Single | Both | None | Single | Both | None | Single | Both | |
| Wild type | 0 | 0 | 10 | 0 | 0 | 10 | 0 | 0 | 8 | 0 | 0 | 8 |
| CD28 KO | 5* | 2 | 4 | 0 | 5 | 6 | 0 | 0 | 9 | 0 | 0 | 9 |
| CD80/86 KO | 2** | 2 | 1 | 0 | 2 | 3 | 0 | 0 | 5 | 0 | 0 | 5 |
| CD40L KO | 0 | 0 | 10 | 0 | 2 | 8 | 0 | 0 | 8 | 0 | 0 | 8 |
| CD40 KO | 0 | 1 | 9 | 0 | 0 | 10 | 0 | 0 | 8 | 0 | 0 | 8 |
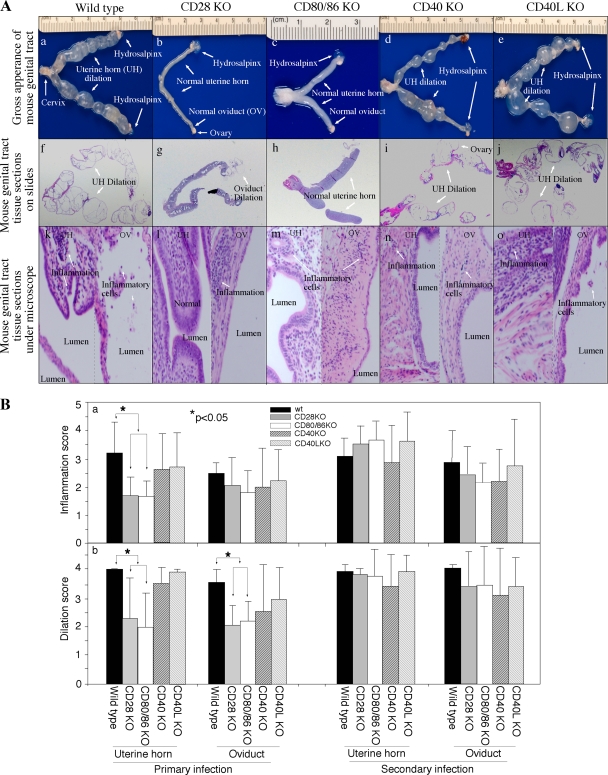
Mice were sacrificed after primary infection alone or after secondary infection, and the reproductive tissues were isolated and inspected for gross pathologies, including uterine horn dilatation and oviduct hydrosalpinx (see Fig. 2A for images).
The number of mice showing the pathologies on single or both sides of the reproductive tissues were recorded and tabulated. The total uterine horn dilation incidence (both single and bilateral) of CD28 KO mice (*, P = 0.02) or CD80/86 KO mice (**, P = 0.09) was compared to that of wil-type mice using the Fisher exact test.
RESULTS
Mice deficient in CD28- but not CD40L-mediated costimulation remain resistant to urogenital tract infection with C. muridarum organisms.
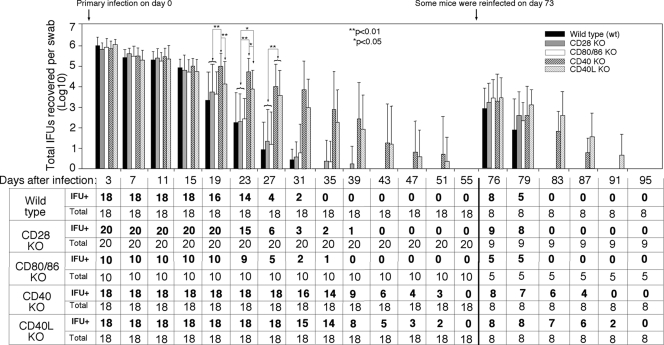
We compared mice deficient in CD28 (n = 20 mice), CD80/CD86 (n = 10), CD40L (n = 18), or CD40 (n = 18) to wild-type mice (n = 18) in their susceptibility to C. muridarum infection in the urogenital tract. The five groups of mice were intravaginally infected with C. muridarum organisms, and the vaginal shedding of live organisms was monitored over the course of infection (Fig. 1). All mice regardless of the genotypes were infected with C. muridarum organisms and shed similar levels of live organisms up to 2 weeks after infection. However, by day 19, the wild-type mice and mice deficient in CD28, CD80/CD86, or CD40L significantly reduced their live organism shedding compared to the mice deficient in CD40. By day 23, the wild-type and CD28 or CD80/CD86 KO mice continued to rapidly decrease their shedding, while the mice deficient in CD40 maintained a high level of shedding. Although the level of shedding by the CD40L KO mice was significantly lower than that of the CD40 KO mice, both groups produced significantly higher levels of shedding than either the wild-type mice or mice deficient in CD28 or CD80/CD86. Starting on day 27, the difference between the CD40 KO and CD40L KO mice disappeared, and both groups of mice maintained significantly higher levels of shedding than either the wild-type or CD28 or CD86/CD80 KO mice. By day 35, all 18 wild-type mice cleared the infection, and only 2 of the 20 CD28 KO and 1 of the 10 CD80/CD86 KO mice remained positive in shedding. However, most of the mice deficient in either CD40 (14 of 18) or CD40L (14 of 18) continued to shed significant level of live organisms, and the shedding remained detectable until day 51 after infection. Apparently, deficiency in CD40 or CD40L extended primary infection time course by more than 2 weeks. On day 73, some of the mice from each group were reinfected with the same amount of C. muridarum organisms (eight mice from the wild-type, CD40, or CD40L KO groups, respectively, nine mice from CD28 KO, and five mice from CD80/CD86 KO groups were reinfected). The mice without reinfection were sacrificed on day 80 for pathology evaluation. Upon reinfection, only about 1,000 live organisms were recovered from all mice on day 3 regardless of the mouse genotypes. This represented an ∼1,000-fold decrease in live organism shedding compared to the shedding after primary infection, indicating that all of the mice had developed protective immunity against C. muridarum reinfection. However, the CD40 or CD40L KO mice continued to shed live organisms beyond 2 weeks, while both wild-type and CD28 or CD80/CD86 KO mice completely cleared the infection within a 1-week period, demonstrating that CD40-CD40L costimulation is required for rapid clearance of the C. muridarum challenge infection. Together, these observations suggest that the CD28-mediated costimulation is dispensable, while the CD40L-CD40 costimulation system is essential for the development of protective immunity against C. muridarum infection in the mouse urogenital tract.
FIG. 1.
Effect of CD28, CD80/CD86, CD40, or CD40L deficiency on live organism shedding following chlamydial infection. Wild-type mice (black bar) or mice deficient in CD28 (CD28 KO, gray bar), CD80/CD86 (open bar), CD40 (CD40 KO, heavily hatched bar), or CD40L (CD40L KO, lightly hatched bar) were infected intravaginally with C. muridarum organisms, and vaginal swabs were obtained along the infection course as indicated along the x axis for measuring the number of live organisms (in IFU). The IFU count from each swab was converted into log10, and the log10 IFU values were used to calculate the means and standard deviations for each mouse group at each time point as presented along the y axis. The CD28 KO group started with a total of 20 mice and the CD80/CD86 KO group started with 10 mice while the wild-type, CD40 KO, and CD40L KO groups each started with 18. The number of mice with detectable IFU (IFU+) at each time point is listed in the bottom table. On day 73 after the primary infection, five to nine mice from each group were reinfected with C. muridarum organisms. The log10 IFU values along the time course were first analyzed by ANOVA and reanalyzed between the wild-type and KO groups (that displayed significant differences under ANOVA) by using a two-tailed Student t test (**, P < 0.01; *, P < 0.05). The wild-type and CD28 KO mice resolved their infections within 30 days after primary infection and within 7 days after secondary infection. However, mice deficient in CD40 or CD40L maintained statistically significant higher levels of live organism shedding and displayed extended infection courses (to 50 days after primary infection and 20 days after secondary infection).
Deficiency in CD28-mediated but not CD40L-mediated costimulation protected mice from upper genital tract pathologies after C. muridarum infection.
A total of 80 (with primary infection only) or 115 (plus a secondary infection) days after the primary infection, mice were sacrificed for evaluating the pathologies in the genital tract tissues. Inflammatory infiltrates and luminal dilation under a microscope, together with hydrosalpinx and uterine horn dilation in gross appearance, are hallmarks of the urogenital tract pathologies caused by chlamydial vaginal infection in mice (37). When the gross appearance of the isolated genital tracts was compared (Fig. 2A and Table 1), we found that all or most wild-type mice and mice deficient in CD40 or CD40L developed obvious bilateral hydrosalpinx and uterine horn dilation, while only 50% of the CD28 or CD80/CD86 KO mice developed visible uterine horn dilatation, and 50% developed bilateral hydrosalpinx after primary infection. It is worth noting that C57 wild-type mice developed an extremely high level of hydrosalpinx in the current experiment, while previous studies have reported a wide range of hydrosalpinx rates in these mice, suggesting that infection conditions may affect the incidence of chlamydia-induced pathologies. Thus, the levels of pathologies can only be compared between mice infected under the identical infection condition. Nevertheless, the differences in pathologies between different groups disappeared after reinfection (Table 1). The H&E-stained histological sections were semiquantitatively scored under a microscope for inflammation and luminal dilation. A significantly reduced level of luminal dilation was observed in both the uterine horn and oviduct tissue sections from the CD28- or CD80/CD86-deficient mice compared to wild-type mice after primary infection (Fig.2Bb, P < 0.05). The uterine horn tissues of the CD28 or CD80/CD86 KO mice also displayed a significantly lower inflammation infiltration score (Fig.2Ba, P < 0.05). However, mice deficient in CD40 or CD40L developed inflammation and dilatation scores similar to those for wild-type mice. These observations together demonstrated that CD28-mediated but not CD40L-mediated costimulation contributed to inflammatory pathologies induced by C. muridarum infection.
FIG. 2.
Effect of CD28, CD80/CD86, CD40, or CD40L deficiency on the development of inflammatory pathologies in the mouse urogenital tract after chlamydial infection. (A) When urogenital tract tissues from wild-type (a, f, and k), CD28 KO (b, g, and l), CD80/CD86 KO (c, h, and m), CD40 KO (d, i, and n), and CD40L KO (e, j, and o) mice were examined for gross appearance (a to e), on slides (f to j), and under a microscope (k to o), obvious inflammatory pathologies were noted in the representative images from wild-type, CD40 KO, and CD40L KO mice but not CD28 or CD80/CD86 KO mice. The pathologies were recorded as hydrosalpinx and uterine horn (UT) dilatation observable with the naked eye (a, d, and e, white arrows, see Table 1 for quantitative data), as well as extensive infiltration of mononuclear cells and oviduct (OV) luminal dilation observed under microscope after H&E staining (k, n, and o, white arrows). The representative images were from mice sacrificed on day 80 after primary infection. (B) Inflammation and lumen dilatation of both uterine horns and oviducts were semiquantitatively scored under a microscope, and the scores were used to calculate the means and standard errors for each group as shown along the y axis. The various tissue and mouse groups were indicated along the x axis. The solid bar represents tissue samples from wild-type mice, the gray bar represents tissue samples from C28 KO mice, the open bar represents tissue samples from CD80/CD86 KO mice, the heavily hatched bar represents tissue samples from CD40 KO mice, and the lightly hatched bar represents tissue samples from CD40L KO mice. The number of mice in each group is listed Table 1. Statistically significant differences in inflammation scores (*, P < 0.05) of uterine horn tissues and dilatation scores of both uterine horn and oviduct tissues were found between wild-type and CD28 or CD80/CD86 KO mice after primary but not after secondary infection.
Effect of CD28- and CD40L-mediated costimulation signals on antigen-specific immune responses to C. muridarum urogenital infection.
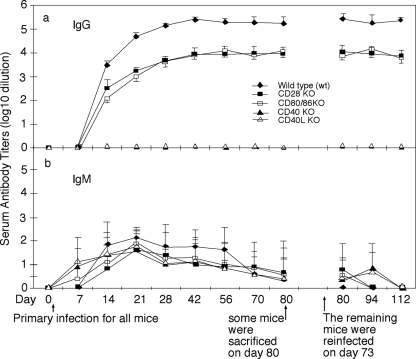
We monitored the mouse serum antibody responses to C. muridarum urogenital infection. The wild-type mice developed a robust anti-C. muridarum organism IgG antibody response over the entire infection courses (Fig. 3). However, the C. muridarum-specific IgG antibody level in the CD28 or CD80/CD86 KO mice was more than 10-fold lower than that in wild-type mice after either primary or secondary infection (Fig. 3a), a finding consistent with the fact that basal immunoglobulin concentrations in CD28-deficient mice are about one-fifth of those found in wild-type controls (38). As expected, mice deficient in CD40 or CD40L failed to produce any detectable levels of C. muridarum-specific IgG antibodies. All mice developed similar levels of IgM antibodies, and no hyper-IgM was detected in mice deficient in CD40L or CD40 (Fig. 3b). Although humans lacking functional CD40L are known to develop hyper-IgM syndrome (22), mice deficient in CD40L-CD40 costimulation do not exhibit hyper-IgM (49). We further compared the isotypes of IgG antibody responses between wild-type and CD28 or CD80/CD86 KO mice and found that CD28 or CD80/CD86 KO mice exhibited a preferential lack of IgG1 isotype during both primary and secondary infection with C. muridarum (data not shown). Although the CD28 KO mice are known to develop low titers of both IgG1 and IgG2b with an increased level of IgG2a (38), interestingly, only IgG1 isotype switching was lacking in response to C. muridarum urogenital infection.
FIG. 3.
Effect of CD28, CD80/CD86, CD40, or CD40L deficiency on mouse serum IgG and IgM antibody production in response to chlamydial infection. The various mouse groups were infected with chlamydial organisms as described in the Fig. 1 legend, and the mice were bled on different days after primary infection, as indicated along the x axis. Serum samples displayed on the right side of the figure were from the reinfected mice only. The Chlamydia-specific IgG (a) and IgM (b) antibodies in the mouse sera were measured using C. muridarum-infected HeLa cells as antigens in an immunofluorescence assay. The highest dilution at which a given mouse serum still positively stained the C. muridarum inclusions was determined as the titer of that serum. The serum dilutions were converted into log10 titers for calculating the means and standard deviations, as displayed along the y axis. The wild-type mice (solid diamond, n = 10) developed a robust serum IgG response, while the CD28 (n = 12) or CD80/CD86 (n = 10) KO mice (solid or open square) developed a reduced level of IgG antibodies. However, no Chlamydia-specific IgG antibodies were detected in mice deficient in either CD40 (solid triangle, n = 10) or CD40L (open triangle, n = 10), although all mice developed Chlamydia-specific serum IgM antibodies.
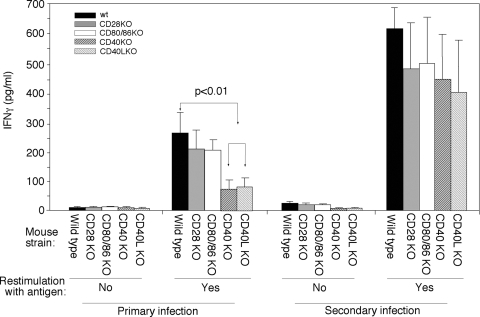
We also compared the antigen-specific T-cell responses between the five groups of mice (Fig. 4). Despite our repeated efforts in measuring both Th2 (IL-4 and IL-5) and Th1 (IFN-γ) cytokines from the in vitro restimulated splenocyte culture supernatants, we were only able to reproducibly detect significant levels of IFN-γ but not of IL-4 and IL-5. The levels of IL-4 and IL-5 were below our ELISA detection sensitivity level (data not shown). Importantly, both the wild-type mice and mice deficient in CD28 or CD80/CD86 produced significantly higher levels of IFN-γ than the CD40 or CD40L KO mice after the primary infection with Chlamydia. However, the difference in IFN-γ level between different groups of mice was no longer significant after reinfection. These observations suggest that CD40L-mediated costimulation signaling is important for IFN-γ production. However, mice lacking the CD40L costimulation system can still maintain the ability to eventually produce significant amounts of IFN-γ after reinfection with chlamydial organisms.
FIG. 4.
Effect of CD28, CD80/CD86, CD40, or CD40L deficiency on chlamydial antigen-specific T-cell responses. Splenocytes were harvested from wild-type (solid bar), CD28 KO (gray bar), CD80/CD86 KO (open bar), CD40 KO (heavily hatched bar), or CD40L KO (lightly hatched bar) mice and restimulated with chlamydial organism antigens for 72 h. The culture supernatants were measured for IFN-γ using ELISA, and the results were expressed as pg/ml as shown along the y axis. There were five mice for each group after primary infection and five for the CD80/CD86 KO mice and three for the remaining groups after secondary infection. Spleen cells from CD40 or CD40L KO mice failed to produce significantly high levels of IFN-γ (P < 0.01, Student t test).
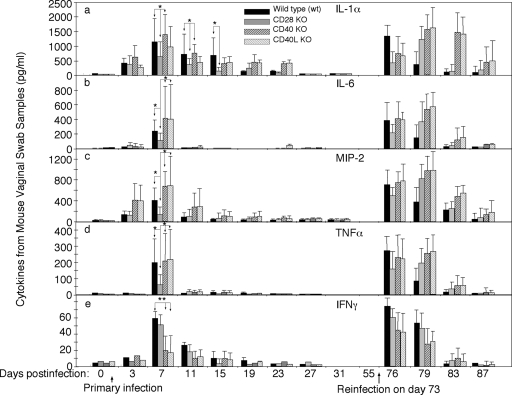
Effect of CD28- and CD40L-mediated costimulation signals on inflammatory cytokine production.
Since C. muridarum-induced pathologies in the urogenital tract are largely due to infection-activated inflammatory responses, we monitored the inflammatory cytokine production in the urogenital tracts during C. muridarum infection. All wild-type mice produced IL-1α (Fig. 5a), IL-6 (b), MIP-2 (c), and TNF-α (d) with a peak on day 7 after primary infection and on day 3 after secondary infection, while IL-12 was not detectable from any of these samples (data not shown), which is consistent with previous observations (8). Interestingly, mice deficient in CD28 or CD80/CD86 (data not shown) produced significantly lower levels of IL-1α, IL-6, MIP-2, and TNF-α after the primary infection, while mice deficient in either CD40L or CD40 produced as much of these cytokines as the wild-type mice. The reduced cytokine production in the genital tracts of CD28 or CD80/CD86 but not CD40 or CD40L KO mice was a surprise to us. We then further tested whether the gene deficiency in CD28 or CD80/CD86 can affect the ability of mouse inflammatory cells such as macrophages to produce cytokines. We found that mouse peritoneal macrophages harvested from these mice produced similar amounts of cytokines as the wild-type mice did upon C. muridarum organism stimulation in vitro (data not shown). Thus, the decreased cytokine production by CD28 or CD80/CD86 KO mice during C. muridarum infection was not due to the inability of inflammatory cells to produce cytokines but likely due to the lack of CD28-mediated costimulation. We also monitored the level of IFN-γ in the mouse urogenital tract (Fig. 5e) since IFN-γ can directly inhibit chlamydial growth. Interestingly, the CD28 or CD80/CD86 (data not shown) KO mice produced as much IFN-γ as wild-type mice, which is consistent with their ability to resist chlamydial infection. As expected, the CD40L or CD40 KO mice failed to produce significant levels of IFN-γ, which correlates with their extended infection time course. However, upon reinfection with chlamydial organisms, the difference in IFN-γ levels between the various groups of mice is no longer significant, demonstrating that mice deficient in CD40L costimulation system can still make IFN-γ after reinfection with chlamydial organisms.
FIG. 5.
Effect of CD28, CD80/CD86, CD40, or CD40L deficiency on cytokine production in the mouse genital tract after chlamydial infection. On different days after intravaginal infection, vaginal swabs were collected from various groups of mice including wild-type (solid bar), CD28 KO (gray bar), CD40 KO (heavily hatched bar), or CD40L KO (lightly hatched bar) mice, as described in Fig. 1 legend and as displayed along the x axis. A portion of each vaginal swab sample was used to measure IL-1α (a), IL-6 (b), MIP-2 (a mouse homologue of IL-8 [c]), TNF-α (d), and IFN-γ (e) using ELISA, and the results were expressed as pg/ml along the y axis. The data came from 8 to 12 mice per group after primary infection and 3 to 6 mice per group after secondary infection. The varied number of mouse samples in each group was largely caused by the limited amount of each vaginal swab sample. No single sample had sufficient amount for measuring all five cytokines. The cytokine levels from CD80/CD86 KO mice were similar to those from CD28 KO mice and were not included into the figure. Please note that most cytokines peaked on day 7 after primary infection. The CD28 KO mice produced significantly lower levels of IL-1α, IL-6, MIP-2, and TNF-α (*, P < 0.05 [Student t test]), but not IFN-γ, whereas CD40 or CD40L KO mice failed to produce a significant level of IFN-γ (**, P < 0.01). Upon secondary infection, the cytokine peaks in most mice moved to day 3 and extended to day 6 or beyond.
DISCUSSION
Previous studies using gene KO mice have established a critical role of the Th1-dominant response in both Chlamydia-induced protective immunity and pathogenic responses (27). In the present study, we have demonstrated that the CD40L-CD40 costimulation system is required for the development of protective immunity, while the CD28-CD80/CD86 costimulation signal significantly contributes to the development of inflammatory pathologies. The differential effects of CD40L and CD28 costimulation systems correlate with the different levels of IFN-γ and other proinflammatory cytokines produced by these mice during early stages of the chlamydial infection.
The CD40L-CD40 costimulation system is known to play important roles in host defense against microbial infection, especially by intracellular pathogens (34, 41, 48). This is because CD40L-CD40 costimulation system is required for producing a robust Th1 dominant response. We indeed found that the CD40L or CD40 KO mice failed to produce significant levels of IFN-γ following a primary infection with chlamydial organisms. Thus, these KO mice experienced a prolonged infection time course. Interestingly, these KO mice eventually resolved the chlamydial infection while IFN-γ, IFN-γ receptor, or CD4 KO mice often fail to resolve infection (11, 27), suggesting that CD40L-independent costimulation may be able to compensate for the loss of CD40L costimulation function and allow the CD40L or CD40 KO mice to eventually develop protective immunity for clearing chlamydial infection. This hypothesis is supported by our findings that the CD40L or CD40 KO mice produced significant amounts of IFN-γ after reinfection with chlamydial organisms, and the reinfection time course in the CD40L or CD40 KO mice was shortened by ∼5 weeks compared to the primary infection time course.
Although CD28 may play important roles in many different aspects of immune responses, we found that the development of protective immunity against C. muridarum organism infection did not require CD28 or CD80/CD86. However, a recent study showed that CD28 appeared to be required for mouse resistance to infection with the human C. trachomatis serovar D organisms (25). The role of the CD28-CD80/CD86 costimulatory system in host defense against microbial infection varies depending on the type of infection. During viral infection, CD28 is required for host resistance to infection by ectromelia virus (14) but not lymphocytic choriomeningitis virus (38). During parasitic infection, CD28 is necessary for controlling infection by Trypanosoma cruzi (26) but not Heligmosomoides polygyrus (17) or Leishmania major (4). After infection with Toxoplasma gondii, CD28 is required for generating the memory responses but not for resolving the primary infection (45). These varied roles of CD28 costimulation in host defense may be due to the fact that a strong T-cell-receptor signal can activate T cells in the absence of CD28 and/or alternative (CD28-independent) costimulation systems may be activated upon severe infection (2). It has been hypothesized that productive and/or severe infections can induce immunity without the need of CD28, while weak or abortive infections may fail to do so (14). The C. muridarum organisms are obviously more invasive than serovar D organisms in infecting the mouse urogenital tract. Thus, the development of protective immune responses during serovar D infection may depend on CD28, while C. muridarum may be able to induce immunity without the requirement of CD28 costimulation. However, it is not clear whether C. muridarum infection bypasses the CD28 costimulation by providing high-affinity major histocompatibility complex/peptide complexes to T cells or by activating CD28-independent costimulation systems or both. Regardless of how C. muridarum infection bypasses CD28 costimulation in CD28 KO mice, these analyses strongly suggest that different infection conditions can activate different immune mechanisms.
Despite the fact that CD28 KO mice mounted normal protective immunity against C. muridarum infection, these mice developed significantly lower levels of inflammatory pathologies, suggesting that CD28 may significantly contribute to the pathology development in wild-type mice after C. muridarum infection. This observation is consistent with the well-established role of CD28 costimulation in enhancing T-cell proliferation and cytokine production and promoting inflammatory pathologies (6, 21, 23, 31, 33, 35, 42, 44, 46, 47). We found that the decreased pathologies correlated well with the reduced proinflammatory cytokine production in the urogenital tracts of the CD28 KO mice, suggesting that CD28-mediated costimulation may promote inflammatory cytokine production.
To our surprise, the CD40 and CD40L KO mice developed severe pathologies after C. muridarum infection, although these KO mice displayed severe deficits in protective immunity. We hypothesize that C. muridarum can activate CD40L/CD40-independent costimulation systems such as the CD28-CD80/CD86 pathway for inducing chlamydial pathologies in the CD40L or CD40 KO mice. The extended infection time courses in the CD40L or CD40 KO mice may provide the CD40L/CD40 costimulation-independent host responses extra time to exacerbate inflammatory pathologies. Obviously, more studies are required to further define the CD40L/CD40 costimulation-independent immune components involved in the C. muridarum induction of pathologies. Nevertheless, by comparing the roles of CD28-CD80/CD86 and CD40L/CD40 costimulation systems in chlamydial infection, the present study has demonstrated that protective immunity and inflammatory pathologies induced during chlamydial infection can be mediated by distinct costimulation pathways with CD28-mediated costimulation dispensable for protective immunity but required for inflammatory pathologies and the opposite for the CD40L-CD40 costimulation system. Although we have focused our discussions on the costimulatory roles of CD28, CD80/CD86, CD40L, and CD40 in chlamydial infection and pathology, it is worth noting that these costimulatory molecules may also affect chlamydial infection and pathology using mechanisms that are independent of costimulation.
Regardless of the precise mechanisms how these molecules exert their effects during chlamydial infection, the information obtained from the current study can be utilized to facilitate chlamydial vaccine development. Immunogens and/or immunization regimes that can selectively activate CD40L-CD40 but not CD28-CD80/CD86 costimulatory signaling pathways may be chosen to induce better protection against chlamydial infection and Chlamydia-induced diseases. In fact, activation of CD40 has been evaluated as an adjuvant strategy to enhance vaccine immunogenicity (3, 5).
Acknowledgments
This study was supported in part by grants (to G.Z.) from the U.S. National Institutes of Health.
Editor: S. R. Blanke
Footnotes
Published ahead of print on 27 April 2009.
REFERENCES
- 1.Armitage, R. J., C. R. Maliszewski, M. R. Alderson, K. H. Grabstein, M. K. Spriggs, and W. C. Fanslow. 1993. CD40L: a multi-functional ligand. Semin. Immunol. 5401-412. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann, M. F., E. Sebzda, T. M. Kundig, A. Shahinian, D. E. Speiser, T. W. Mak, and P. S. Ohashi. 1996. T-cell responses are governed by avidity and co-stimulatory thresholds. Eur. J. Immunol. 262017-2022. [DOI] [PubMed] [Google Scholar]
- 3.Barr, T. A., J. Carlring, and A. W. Heath. 2005. CD40 antibody as a potent immunological adjuvant: CD40 antibody provides the CD40 signal to B cells, but does not substitute for T-cell help in responses to TD antigens. Vaccine 233477-3482. [DOI] [PubMed] [Google Scholar]
- 4.Brown, D. R., J. M. Green, N. H. Moskowitz, M. Davis, C. B. Thompson, and S. L. Reiner. 1996. Limited role of CD28-mediated signals in T helper subset differentiation. J. Exp. Med. 184803-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairing, J., T. Barr, and A. W. Heath. 2005. Adjuvanticity of anti-cD40 in vaccine development. Curr. Opin. Mol. Ther. 773-77. [PubMed] [Google Scholar]
- 6.Chang, T. T., C. Jabs, R. A. Sobel, V. K. Kuchroo, and A. H. Sharpe. 1999. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J. Exp. Med. 190733-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, C., D. Chen, J. Sharma, W. Cheng, Y. Zhong, K. Liu, J. Jensen, R. Shain, B. Arulanandam, and G. Zhong. 2006. The hypothetical protein CT813 is localized in the Chlamydia trachomatis inclusion membrane and is immunogenic in women urogenitally infected with C. trachomatis. Infect. Immun. 744826-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, W., P. Shivshankar, Z. Li, L. Chen, I. T. Yeh, and G. Zhong. 2008. Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection. Infect. Immun. 76515-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Compton, H. L., and J. P. Farrell. 2002. CD28 costimulation and parasite dose combine to influence the susceptibility of BALB/c mice to infection with Leishmania major. J. Immunol. 1681302-1308. [DOI] [PubMed] [Google Scholar]
- 10.Cotter, T. W., Q. Meng, Z. L. Shen, Y. X. Zhang, H. Su, and H. D. Caldwell. 1995. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect. Immun. 634704-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotter, T. W., K. H. Ramsey, G. S. Miranpuri, C. E. Poulsen, and G. I. Byrne. 1997. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect. Immun. 652145-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durie, F. H., T. M. Foy, S. R. Masters, J. D. Laman, and R. J. Noelle. 1994. The role of CD40 in the regulation of humoral and cell-mediated immunity. Immunol. Today 15406-411. [DOI] [PubMed] [Google Scholar]
- 13.Elias, R. M., L. R. Sardinha, K. R. Bastos, C. A. Zago, A. P. da Silva, J. M. Alvarez, and M. R. Lima. 2005. Role of CD28 in polyclonal and specific T- and B-cell responses required for protection against blood stage malaria. J. Immunol. 174790-799. [DOI] [PubMed] [Google Scholar]
- 14.Fang, M., and L. J. Sigal. 2006. Direct CD28 costimulation is required for CD8+ T-cell-mediated resistance to an acute viral disease in a natural host. J. Immunol. 1778027-8036. [DOI] [PubMed] [Google Scholar]
- 15.Foy, T. M., J. D. Laman, J. A. Ledbetter, A. Aruffo, E. Claassen, and R. J. Noelle. 1994. gp39-CD40 interactions are essential for germinal center formation and the development of B-cell memory. J. Exp. Med. 180157-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foy, T. M., S. R. Masters, and R. J. Noelle. 1994. Hyper IgM syndrome: two mutations distinguish HIM. J. Clin. Investig. 941349-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gause, W. C., P. Lu, X. D. Zhou, S. J. Chen, K. B. Madden, S. C. Morris, P. S. Linsley, F. D. Finkelman, and J. F. Urban. 1996. Heligmosomoides polygyrus: B7-independence of the secondary type 2 response. Exp. Parasitol. 84264-273. [DOI] [PubMed] [Google Scholar]
- 18.Greenwald, R. J., G. J. Freeman, and A. H. Sharpe. 2005. The B7 family revisited. Annu. Rev. Immunol. 23515-548. [DOI] [PubMed] [Google Scholar]
- 19.Grewal, I. S., and R. A. Flavell. 1996. The role of CD40 ligand in costimulation and T-cell activation. Immunol. Rev. 15385-106. [DOI] [PubMed] [Google Scholar]
- 20.Imtiaz, M. T., J. H. Schripsema, I. M. Sigar, J. N. Kasimos, and K. H. Ramsey. 2006. Inhibition of matrix metalloproteinases protects mice from ascending infection and chronic disease manifestations resulting from urogenital Chlamydia muridarum infection. Infect. Immun. 745513-5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimzey, S. L., P. Liu, and J. M. Green. 2004. Requirement for CD28 in the effector phase of allergic airway inflammation. J. Immunol. 173632-640. [DOI] [PubMed] [Google Scholar]
- 22.Kroczek, R. A., D. Graf, D. Brugnoni, S. Giliani, U. Korthuer, A. Ugazio, G. Senger, H. W. Mages, A. Villa, and L. D. Notarangelo. 1994. Defective expression of CD40 ligand on T cells causes “X-linked immunodeficiency with hyper-IgM (HIGM1)”. Immunol. Rev. 13839-59. [DOI] [PubMed] [Google Scholar]
- 23.Lenschow, D. J., T. L. Walunas, and J. A. Bluestone. 1996. CD28/B7 system of T-cell costimulation. Annu. Rev. Immunol. 14233-258. [DOI] [PubMed] [Google Scholar]
- 24.Lu, H., and G. Zhong. 1999. Interleukin-12 production is required for chlamydial antigen-pulsed dendritic cells to induce protection against live Chlamydia trachomatis infection. Infect. Immun. 671763-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marks, E., M. Verolin, A. Stensson, and N. Lycke. 2007. Differential CD28 and inducible costimulatory molecule signaling requirements for protective CD4+ T-cell-mediated immunity against genital tract Chlamydia trachomatis infection. Infect. Immun. 754638-4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martins, G. A., A. P. Campanelli, R. B. Silva, C. E. Tadokoro, M. Russo, F. Q. Cunha, L. V. Rizzo, and J. S. Silva. 2004. CD28 is required for T-cell activation and IFN-gamma production by CD4+ and CD8+ T cells in response to Trypanosoma cruzi infection. Microbes Infect. 61133-1144. [DOI] [PubMed] [Google Scholar]
- 27.Morrison, R. P., and H. D. Caldwell. 2002. Immunity to murine chlamydial genital infection. Infect. Immun. 702741-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison, S. G., and R. P. Morrison. 2005. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J. Immunol. 1757536-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murthy, A. K., J. P. Chambers, P. A. Meier, G. Zhong, and B. P. Arulanandam. 2007. Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production. Infect. Immun. 75666-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murthy, A. K., J. Sharma, J. J. Coalson, G. Zhong, and B. P. Arulanandam. 2004. Chlamydia trachomatis pulmonary infection induces greater inflammatory pathology in immunoglobulin A deficient mice. Cell. Immunol. 23056-64. [DOI] [PubMed] [Google Scholar]
- 31.Nickoloff, B. J., F. O. Nestle, X. G. Zheng, and L. A. Turka. 1994. T lymphocytes in skin lesions of psoriasis and mycosis fungoides express B7-1: a ligand for CD28. Blood 832580-2586. [PubMed] [Google Scholar]
- 32.Pal, S., E. M. Peterson, and L. M. de la Maza. 2005. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect. Immun. 738153-8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reichmann, G., E. N. Villegas, L. Craig, R. Peach, and C. A. Hunter. 1999. The CD28/B7 interaction is not required for resistance to Toxoplasma gondii in the brain but contributes to the development of immunopathology. J. Immunol. 1633354-3362. [PubMed] [Google Scholar]
- 34.Reichmann, G., W. Walker, E. N. Villegas, L. Craig, G. Cai, J. Alexander, and C. A. Hunter. 2000. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect. Immun. 681312-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saha, B., D. M. Harlan, K. P. Lee, C. H. June, and R. Abe. 1996. Protection against lethal toxic shock by targeted disruption of the CD28 gene. J. Exp. Med. 1832675-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schachter, J., and J. Moncada. 2005. Lymphogranuloma venereum: how to turn an endemic disease into an outbreak of a new disease? Start looking. Sex. Transm. Dis. 32331-332. [DOI] [PubMed] [Google Scholar]
- 37.Shah, A. A., J. H. Schripsema, M. T. Imtiaz, I. M. Sigar, J. Kasimos, P. G. Matos, S. Inouye, and K. H. Ramsey. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex. Transm. Dis. 3249-56. [DOI] [PubMed] [Google Scholar]
- 38.Shahinian, A., K. Pfeffer, K. P. Lee, T. M. Kundig, K. Kishihara, A. Wakeham, K. Kawai, P. S. Ohashi, C. B. Thompson, and T. W. Mak. 1993. Differential T-cell costimulatory requirements in CD28-deficient mice. Science 261609-612. [DOI] [PubMed] [Google Scholar]
- 39.Sharma, J., A. M. Bosnic, J. M. Piper, and G. Zhong. 2004. Human antibody responses to a Chlamydia-secreted protease factor. Infect. Immun. 727164-7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherman, K. J., J. R. Daling, A. Stergachis, N. S. Weiss, H. M. Foy, S. P. Wang, and J. T. Grayston. 1990. Sexually transmitted diseases and tubal pregnancy. Sex. Transm. Dis. 17115-121. [DOI] [PubMed] [Google Scholar]
- 41.Soong, L., J. C. Xu, I. S. Grewal, P. Kima, J. Sun, B. J. Longley, Jr., N. H. Ruddle, D. McMahon-Pratt, and R. A. Flavell. 1996. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity 4263-273. [DOI] [PubMed] [Google Scholar]
- 42.Tada, Y., K. Nagasawa, A. Ho, F. Morito, O. Ushiyama, N. Suzuki, H. Ohta, and T. W. Mak. 1999. CD28-deficient mice are highly resistant to collagen-induced arthritis. J. Immunol. 162203-208. [PubMed] [Google Scholar]
- 43.Taylor, H. R., S. L. Johnson, J. Schachter, H. D. Caldwell, and R. A. Prendergast. 1987. Pathogenesis of trachoma: the stimulus for inflammation. J. Immunol. 1383023-3027. [PubMed] [Google Scholar]
- 44.Verwilghen, J., R. Lovis, M. De Boer, P. S. Linsley, G. K. Haines, A. E. Koch, and R. M. Pope. 1994. Expression of functional B7 and CTLA4 on rheumatoid synovial T cells. J. Immunol. 1531378-1385. [PubMed] [Google Scholar]
- 45.Villegas, E. N., M. M. Elloso, G. Reichmann, R. Peach, and C. A. Hunter. 1999. Role of CD28 in the generation of effector and memory responses required for resistance to Toxoplasma gondii. J. Immunol. 1633344-3353. [PubMed] [Google Scholar]
- 46.Villegas, E. N., U. Wille, L. Craig, P. S. Linsley, D. M. Rennick, R. Peach, and C. A. Hunter. 2000. Blockade of costimulation prevents infection-induced immunopathology in interleukin-10-deficient mice. Infect. Immun. 682837-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Windhagen, A., J. Newcombe, F. Dangond, C. Strand, M. N. Woodroofe, M. L. Cuzner, and D. A. Hafler. 1995. Expression of costimulatory molecules B7-1 (CD80), B7-2 (CD86), and interleukin 12 cytokine in multiple sclerosis lesions. J. Exp. Med. 1821985-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winkelstein, J. A., M. C. Marino, H. Ochs, R. Fuleihan, P. R. Scholl, R. Geha, E. R. Stiehm, and M. E. Conley. 2003. The X-linked hyper-IgM syndrome: clinical and immunologic features of 79 patients. Medicine 82373-384. [DOI] [PubMed] [Google Scholar]
- 49.Xu, J., T. M. Foy, J. D. Laman, E. A. Elliott, J. J. Dunn, T. J. Waldschmidt, J. Elsemore, R. J. Noelle, and R. A. Flavell. 1994. Mice deficient for the CD40 ligand. Immunity 1423-431. [DOI] [PubMed] [Google Scholar]
- 50.Zhong, G., I. Toth, R. Reid, and R. C. Brunham. 1993. Immunogenicity evaluation of a lipidic amino acid-based synthetic peptide vaccine for Chlamydia trachomatis. J. Immunol. 1513728-3736. [PubMed] [Google Scholar]
- 51.Zhong, G. M., and R. C. Brunham. 1990. Immunoaccessible peptide sequences of the major outer membrane protein from Chlamydia trachomatis serovar C. Infect. Immun. 583438-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhong, G. M., and L. M. de la Maza. 1988. Activation of mouse peritoneal macrophages in vitro or in vivo by recombinant murine gamma interferon inhibits the growth of Chlamydia trachomatis serovar L1. Infect. Immun. 563322-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]