Abstract
Different Escherichia coli strains generally have the same metabolic capacity for growth on sugars in vitro, but they appear to use different sugars in the streptomycin-treated mouse intestine (Fabich et al., Infect. Immun. 76:1143-1152, 2008). Here, mice were precolonized with any of three human commensal strains (E. coli MG1655, E. coli HS, or E. coli Nissle 1917) and 10 days later were fed 105 CFU of the same strains. While each precolonized strain nearly eliminated its isogenic strain, confirming that colonization resistance can be modeled in mice, each allowed growth of the other commensal strains to higher numbers, consistent with different commensal E. coli strains using different nutrients in the intestine. Mice were also precolonized with any of five commensal E. coli strains for 10 days and then were fed 105 CFU of E. coli EDL933, an O157:H7 pathogen. E. coli Nissle 1917 and E. coli EFC1 limited growth of E. coli EDL933 in the intestine (103 to 104 CFU/gram of feces), whereas E. coli MG1655, E. coli HS, and E. coli EFC2 allowed growth to higher numbers (106 to 107 CFU/gram of feces). Importantly, when E. coli EDL933 was fed to mice previously co-colonized with three E. coli strains (MG1655, HS, and Nissle 1917), it was eliminated from the intestine (<10 CFU/gram of feces). These results confirm that commensal E. coli strains can provide a barrier to infection and suggest that it may be possible to construct E. coli probiotic strains that prevent growth of pathogenic E. coli strains in the intestine.
When a bacterial species indefinitely persists in stable numbers in the intestine of an animal, without repeated introduction of the bacterium to that animal, the animal's intestine is said to be colonized with that bacterium. The mammalian intestine is colonized with thousands of species (50), collectively known as the indigenous intestinal microbiota. Once established, the intestinal microbiota is quite stable, and most invading microorganisms fail to colonize. This phenomenon, referred to as colonization resistance (60), can be explained in part by a lag phase caused by both short-chain fatty acids and hydrogen sulfide, which are metabolic end products of the metabolism of the indigenous microbiota (22, 31). Thus, if the numbers of an invading bacterium are small, they may be completely eliminated from the intestine before exiting the lag phase. However, even when the numbers of an invading bacterial species are large, the complete intestinal microbiota, in most instances, still prevents its establishment, suggesting that colonization resistance cannot be completely explained by an extended lag phase (21, 23, 60).
An analogy can be drawn between the mammalian intestine and a chemostat (23, 38). Two different microorganisms cannot coexist in a chemostat when competing for a single limiting nutrient; the one that utilizes that nutrient even slightly more efficiently will eventually outcompete the other (18). However, if two microorganisms utilize different growth-limiting nutrients in a chemostat, they can coexist and maintain stable populations (18, 58). Work with continuous-flow cultures in chemostats designed to mimic the intestine (21, 23) led to the theory that being physically attached to the intestinal wall allows a bacterial species to remain in the intestine despite growing at a rate lower than the washout rate from the intestine. Moreover, the theory predicts that two bacterial strains competing for the same limiting nutrient can coexist in the intestine if the metabolically less-efficient one is attached to the intestinal wall (23). In addition, the data obtained from continuous-flow cultures show that if an established bacterium and an invading one are equally fit to compete for the same limiting nutrient, the invading bacterium will be eliminated by the established bacterium if it is attached to the intestinal wall, because large wall populations can reduce the limiting nutrient concentration to the point that an invader will not be able to multiply in the lumen of the intestine at a rate fast enough to resist washout (19, 22). Thus, according to the theory, the mammalian intestine can be thought of as a chemostat in which thousands of species of bacteria are in equilibrium, many being physically attached to the host intestinal wall in large numbers, and all competing for resources from a mixture of limiting nutrients.
In support of the theory, when healthy human volunteers are fed Escherichia coli strains isolated from their own feces, those strains do not colonize (1). However, despite colonization resistance, there appears to be a continuous succession of commensal E. coli strains in the mammalian intestine. In fact, an average of five different E. coli strains can be found at any one time in the feces of individual humans (2). Some strains are present for months to years, while others persist only transiently, i.e., for a few days. It therefore appears that, for commensal E. coli strains, while colonization resistance is a powerful mechanism, it is not completely effective. As such, it is therefore possible that pathogenic E. coli strains take advantage of incomplete colonization resistance among commensal E. coli strains to initiate infection of the human intestine.
Why is colonization resistance incomplete for commensal E. coli strains in the human intestine? One possibility is that there are different epithelial cell receptors for different E. coli strains, thereby allowing an invading commensal strain to resist washout by first binding to the epithelium and then competing with an established strain for the same limiting nutrient(s). However, when the location of the human commensal strain E. coli MG1655 was examined in the streptomycin-treated mouse large intestine using in situ hybridization with species-specific rRNA probes (51), it was found that E. coli MG1655 was not associated with the intestinal epithelium (46) but was dispersed as either single cells or dividing doublets in the mucus layer, which overlays the epithelium and turns over about every 2 h (52). The same was true of the rat commensal strain E. coli BJ4 (51). Therefore, if incomplete colonization resistance among different commensal E. coli strains also occurs in the mouse intestine, it is not likely due to adhesion of the strains to different receptors on the intestinal epithelium.
Incomplete colonization resistance could be explained nutritionally if different E. coli strains were to use different nutrients for growth in the intestine. Indeed, we recently reported that the human commensal E. coli MG1655 and the human enterohemorrhagic E. coli EDL933 do not use all the same sugars for growth when each is the only E. coli strain in the streptomycin-treated mouse intestine, despite being able to use them all for growth in vitro (17). We found that E. coli EDL933 and E. coli MG1655 both use l-arabinose, l-fucose, d-maltose, and N-acetyl-d-glucosamine in the mouse intestine, but E. coli EDL933 uses three sugars not used by E. coli MG1655 (d-galactose, d-mannose, and d-ribose), whereas E. coli MG1655 uses two sugars not used by E. coli EDL933 (sialic acid and d-gluconate) (17). Moreover, different E. coli commensals using different nutrients in the mouse intestine was shown by the fact that, although the human commensal strains E. coli MG1655 and E. coli Nissle 1917 both use l-arabinose and l-fucose in the mouse intestine, E. coli Nissle 1917 also uses d-mannose, which is not used by E. coli MG1655 (13).
Clearly, the finding that different commensal E. coli strains use different nutrients for growth in the mouse intestine is consistent with incomplete colonization resistance among different commensal E. coli strains in the human intestine. However, whether the streptomycin-treated mouse intestine displays incomplete colonization resistance among different E. coli strains has not been examined. In the present study, we show that in this regard the mouse intestine mimics the human intestine, i.e., when the streptomycin-treated mouse intestine is precolonized with a commensal E. coli strain, it displays colonization resistance against invasion by the same strain but allows the growth of different commensal E. coli strains from low to high numbers. Moreover, we show that although several different human commensal E. coli strains individually display incomplete colonization resistance against E. coli EDL933 (an O157:H7 strain) to various degrees, simultaneous precolonization of the streptomycin-treated mouse intestine with three different commensal E. coli strains results in a completely effective barrier to subsequent E. coli EDL933 invasion.
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains used in this study are listed in Table 1. The original E. coli strain K-12 was obtained from a stool sample from a convalescing diphtheria patient in Palo Alto, CA, in 1922 (4). The sequenced E. coli MG1655 strain (CGSC 7740) was derived from the original K-12 strain, having only been cured of the temperate bacteriophage lambda and the F plasmid by means of UV light and acridine orange (4). It has an IS1 element in the flhDC promoter (6). The E. coli MG1655 strain used in the present investigation is the sequenced MG1655 strain, but lacking the IS1 element in the flhDC promoter (Table 1) (26). E. coli HS is a human commensal strain isolated from a laboratory scientist at the Walter Reed Army Institute of Research (39). E. coli Nissle 1917 is a human commensal strain that has been used as a probiotic agent since the early 1920s (55), and E. coli EDL933 is an O157:H7 strain isolated from an outbreak caused by contaminated beef in 1982 (53). E. coli EFC1 and E. coli EFC2 were each isolated from the feces of a healthy human (45).
TABLE 1.
Bacterial strains
| E. coli strain | Genotype/phenotype | Referred to in text as: | Source/reference |
|---|---|---|---|
| MG1655 (−IS1) Strr | No IS1 element in the flhDC promoter; resistant to streptomycin | MG1655 and MG1655 Strr | 26 |
| MG1655 (−IS1) Strr Nalr | Spontaneous nalidixic acid-resistant mutant of MG1655 (−IS1) Strr | MG1655 and MG1655 Strr Nalr | 26 |
| HS | Human commensal strain | Not applicable | James Nataro |
| HS Strr | Spontaneous streptomycin-resistant mutant of HS | HS and HS Strr | This study |
| HS Strr Nalr | Spontaneous nalidixic acid-resistant mutant of HS Strr | HS and HS Strr Nalr | This study |
| Nissle 1917 Strr | Spontaneous streptomycin-resistant mutant of Nissle 1917 | Not applicable | 3 |
| Nissle 1917 Strr Nalr | Spontaneous nalidixic acid-resistant mutant of Nissle 1917 Strr | Nissle 1917 and Nissle 1917 Strr Nalr | 3 |
| Nissle 1917 Strr ΔlacZ::cat | Mutant of Nissle 1917 Strr, unable to make β-galactosidase, resistant to streptomycin and chloramphenicol | Nissle 1917 ΔlacZ and Nissle 1917 Strr ΔlacZ::cat | This study |
| EFC1 | Human commensal strain | Not applicable | Michael Donnenberg |
| EFC1 Strr | Spontaneous streptomycin-resistant mutant of EFC1 | EFC1 and EFC1 Strr | This study |
| EFC2 | Human commensal strain | Not applicable | Michael Donnenberg |
| EFC2 Strr | Spontaneous streptomycin-resistant mutant of EFC2 | EFC2 and EFC2 Strr | This study |
| F-18 Strr Nalr | Spontaneous streptomycin and nalidixic acid-resistant mutant of F-18 | F-18 | 11 |
Media and growth conditions.
LB broth Lennox (Difco Laboratories, Detroit, MI), LB agar Lennox (Difco), and MacConkey agar (Difco) were prepared according to the package instructions. SOC medium was prepared as described by Datsenko and Wanner (15). For colonization experiments, E. coli strains were grown overnight in LB broth Lennox from an inoculum of about 106 CFU/ml to about 109 CFU/ml. Cultures (10 ml) were incubated at 37°C with shaking in 125-ml tissue culture bottles. For testing the growth of strains in M9 minimal medium (43) containing lactose as the sole carbon and energy source, overnight cultures grown in LB broth Lennox were washed twice in M9 minimal medium (no carbon source), 100 μl of the washed cultures was transferred to 10 ml of M9 minimal medium containing lactose (0.4%, wt/wt) (Bacto-Lactose [Difco]), and cultures were incubated at 37°C with shaking overnight in 125-ml tissue culture bottles. Growth was monitored spectrophotometrically (A600) using a Pharmacia Biotech Ultrospec 2000 UV/visible spectrophotometer.
Construction and characterization of E. coli Nissle 1917 Strr ΔlacZ::cat.
E. coli Nissle 1917 Strr ΔlacZ::cat was constructed by allelic exchange mutagenesis using a chloramphenicol cassette, as described by Datsenko and Wanner (15). Primers were designed by referring to the complete genome of E. coli MG1655 (6). The primers used to construct the mutants were as follows: forward, 5′-ACATGTCTGACAATGGCAGATCCCAGCGGTCAAAACAGGCGGCAGTAAG GTGTAGGCTGGAGCTGCTTCG-3′; and reverse, 5′-TTTTACAACGTCGTGACTGGGAAAACCCTGGCGTTACCCAACTTAATCGCATATGAAT ATCCTCCTTAGT-3′. The construct was verified by PCR and sequencing. As expected, the E. coli Nissle 1917 Strr ΔlacZ::cat mutant failed to grow in M9 minimal medium containing lactose (0.4%, wt/wt) as the sole carbon source. The 2,700-bp deletion in the lacZ gene begins 76 bp downstream of the lacZ start codon and ends 341 base pairs upstream of the TAA stop codon. The primers used for confirming the size of the deletion by sequencing were as follows: forward, 5′-ATTGTAACAGTGGCCCGAAG-3′; and reverse, 5′-CGGATATCTCGGTAGTGGGA-3′. For sequencing, PCR products were purified with a Qiagen Qiaquick PCR purification kit, following the manufacturer's instructions. PCR products were submitted to the Rhode Island Genomics and Sequencing Center at the University of Rhode Island. After completion of the cycle sequencing, samples were purified with Agencourt's CleanSEQ SPRI reagent and separated on an Applied Biosystems 3130xl genetic analyzer (50-cm capillary array with POP7 polymer).
Mouse colonization experiments.
Streptomycin-treated mice have been used since 1954 to overcome the colonization resistance encountered in conventional animals (8). In large part, colonization resistance is overcome in streptomycin-treated mice by the loss of facultative anaerobes from the microbiota and by the observed decrease in the concentrations of short-chain fatty acids and hydrogen sulfide (32). The streptomycin-treated CD-1 mouse was used here to study the competition in the intestine between streptomycin-resistant, wild-type E. coli strains. Since the numbers of a strain of E. coli in mouse feces are a reflection of their numbers in the mouse large intestine (14, 36), fecal counts were used to judge the relative colonizing abilities of various E. coli strains. All the strains used in this study are spontaneous streptomycin-resistant mutants, resistant to greater than 2 mg/ml of streptomycin sulfate. E. coli MG1655 Strr, E. coli HS Strr, and E. coli EDL933 Strr all contain the same point mutation in rpsL previously reported for high-level streptomycin resistance in E. coli (24), in which amino acid 44 has been changed from lysine (5′-AAA-3′) to threonine (5′-ACA-3′) (M. P. Leatham, unpublished data), thereby eliminating the possibility that differences observed in colonizing abilities among these strains could be due to mutations in different genes that confer streptomycin resistance. E. coli Nissle 1917 Strr also has a point mutation in rpsL in amino acid 44, resulting in a change from lysine (5′-AAA-3′) to arginine (5′-AGA-3′) (Leatham, unpublished). In addition to being streptomycin resistant, some of the wild-type E. coli strains used in the colonization experiments are resistant to either chloramphenicol, nalidixic acid, or rifampin, genetic markers that have no effect on the colonization abilities of the strains used in these studies (9, 44, 46).
Mice are given streptomycin sulfate in their drinking water (5 g/liter) over the entire course of these experiments, which selectively removes facultative anaerobic E. coli, enterococci, streptococci, lactobacilli, and anaerobic lactobacilli and bifidobacteria (32). Nevertheless, the overall populations of anaerobes, including Bacteroides and Eubacterium, in the cecal contents following streptomycin treatment are unchanged (32). Therefore, the streptomycin-treated mouse model allows colonization by experimentally introduced E. coli strains and competition with large numbers of strict anaerobes, and thus it is our model of choice for studying competition among E. coli strains in the intestine (14, 36).
The specifics of the method used to compare the large intestine colonizing abilities of E. coli strains in mice have been described previously (41, 56, 57, 62). Briefly, three male CD-1 mice (5 to 8 weeks old) were given drinking water containing streptomycin sulfate (5 g/liter) for 24 h to eliminate resident facultative bacteria (42). Following 18 h of starvation for food and water, the mice were fed 1 ml of 20% (wt/vol) sucrose containing 105 CFU of LB broth Lennox-grown E. coli strains, as described in Results. After ingesting the bacterial suspension, both the food (Teklad mouse and rat diet; Harlan, Madison, WI) and streptomycin-water were returned to the mice, and 1 g of feces was collected after 5 h, 24 h, and on odd-numbered days at the indicated times. Mice were housed individually in cages without bedding and were placed in clean cages at 24-h intervals. Fecal samples (one gram) were therefore no older than 24 h. Each fecal sample was homogenized in 10 ml of 1% Bacto-Tryptone (Difco), diluted in the same medium, and plated on MacConkey agar plates with appropriate antibiotics. When appropriate, 1 ml of a fecal homogenate (sampled after the feces had settled) was centrifuged at 12,000 × g, resuspended in 100 μl of 1% Bacto-Tryptone, and plated on a MacConkey agar plate with appropriate antibiotics. This procedure increases the sensitivity of the assay from 102 CFU/gram of feces to 10 CFU/per gram of feces. To distinguish the various E. coli strains in feces, dilutions were plated on lactose MacConkey agar containing streptomycin sulfate (100 μg/ml); streptomycin sulfate (100 μg/ml) and nalidixic acid (50 μg/ml); streptomycin sulfate (100 μg/ml) and chloramphenicol (30 μg/ml); or streptomycin sulfate (100 μg/ml) and rifampin (50 μg/ml). Streptomycin sulfate, chloramphenicol, and nalidixic acid were purchased from Sigma-Aldrich (St. Louis, MO). Rifampin was purchased from Fisher Scientific (Pittsburgh, PA). All plates were incubated for 18 to 24 h at 37°C prior to counting. When necessary, i.e., to distinguish strains, 100 colonies from plates containing streptomycin were toothpicked onto MacConkey agar plates containing streptomycin and nalidixic acid or onto MacConkey agar plates containing streptomycin and chloramphenicol. Each colonization experiment was replicated at least twice, with essentially identical results. Pooled data from at least two independent experiments (a total of six mice) are presented in the figures.
Isolation and enumeration of E. coli strains from mouse intestinal mucus.
E. coli HS, E. coli MG1655, and E. coli Nissle 1917 ΔlacZ were each fed to sets of three mice. On day 22 postfeeding, the mice were sacrificed, and the ileum, the rest of the small intestine, the cecum, and the colon were removed from each mouse. Each section of the intestine was washed extensively with HEPES-Hanks buffer (pH 7.2), and the mucus from each section of the intestine was scraped into 5 ml of HEPES-Hanks buffer (pH 7.2), as described previously (10). Each sample was homogenized by vortexing and then plated on MacConkey agar with appropriate antibiotics. Plates were incubated for 18 to 24 h at 37°C prior to counting. To distinguish strains, 100 colonies were toothpicked as described above. The number of CFU per intestinal section for each strain was calculated from the CFU per milliliter by multiplying by the total volume (in milliliters) of each mucus sample.
Colicin and microcin assays.
Colicin and microcin activity was assayed as described by Patzer et al. (49). Briefly, bacteria to be tested for colicin or microcin activity were streaked on nutrient broth dipyridyl plates (nutrient broth [Difco], 8 g/liter; NaCl, 5 g/liter; Bacto agar [Difco], 15 g/liter; 2,2′ dipyridyl [Sigma-Aldrich Corp., St. Louis, MO], 0.2 mM) and incubated overnight at 37°C. Indicator strains were grown overnight in LB broth Lennox at 37°C with shaking in 125-ml tissue culture bottles. For colicin or microcin testing, 105 CFU or 106 CFU of an indicator strain was added to 3 ml of nutrient broth soft agar (nutrient broth, 8 g/liter; NaCl, 5 g/liter; Bacto agar, 15 g/liter), which was then poured onto a 20-ml nutrient broth dipyridyl plate. After the nutrient broth soft agar solidified, strains to be tested for colicin or microcin activity on the indicator strain were toothpicked onto the plate. Plates were incubated overnight at 37°C, and zones of growth inhibition were measured.
RESULTS
Mice precolonized with a human commensal E. coli strain are resistant to subsequent intestinal colonization by the same strain.
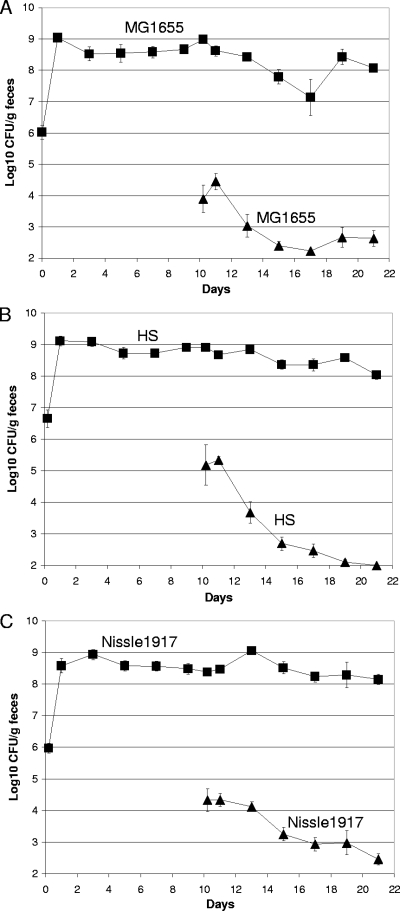
When healthy human volunteers are fed E. coli strains isolated from their own feces, those strains do not colonize (1). This is an example of colonization resistance (2). If colonization resistance occurs in the streptomycin-treated mouse for individual E. coli strains, it would be expected that if mice were precolonized with a human commensal E. coli strain for 10 days and were then fed low numbers of the same strain, the strain fed at day 10 would have a difficult time colonizing the mouse intestine. To test this hypothesis, mice were precolonized for 10 days with any of three human commensal strains: E. coli MG1655 Strr, E. coli Nissle 1917 Strr Nalr, or E. coli HS Strr. On day 10, the mice precolonized with E. coli MG1655 Strr were fed 105 CFU of E. coli MG1655 Strr Nalr, the mice precolonized with E. coli Nissle 1917 Strr Nalr were fed 105 CFU of E. coli Nissle 1917 Strr Rifr, and the mice precolonized with E. coli HS Strr were fed 105 CFU of E. coli HS Strr Nalr. Precolonized E. coli MG1655 nearly eliminated the E. coli MG1655 that was fed to the mice on day 10 (Fig. 1A), precolonized E. coli HS nearly eliminated the E. coli HS that was fed to the mice on day 10 (Fig. 1B), and precolonized E. coli Nissle 1917 nearly eliminated the E. coli Nissle 1917 that was fed to the mice on day 10 (Fig. 1C). Thus, the streptomycin-treated mouse model of intestinal colonization also exhibits colonization resistance, as observed in humans (1). When a mouse is fed an E. coli strain that is already residing in its intestine, the new strain has great difficulty in colonizing.
FIG. 1.
A precolonized E. coli strain prevents the same strain from colonizing the mouse intestine. Sets of three mice were fed 105 CFU of a human commensal strain and 10 days later were fed 105 CFU of the same strain. (A) E. coli MG1655 Strr (▪) and, 10 days later, E. coli MG1655 Strr Nalr (▴). (B) E. coli HS Strr (▪) and, 10 days later, E. coli HS Strr Nalr (▴). (C) E. coli Nissle 1917 Strr Nalr (▪) and, 10 days later, 105 CFU of E. coli Nissle 1917 Strr Rifr (▴). At the indicated times, fecal samples were homogenized, diluted, and plated as described in Materials and Methods. Data from two independent experiments (six mice) are shown. Bars represent the standard errors of the log10 means of CFU per gram of feces for six mice.
Mice precolonized with a human commensal E. coli strain cannot prevent subsequent intestinal colonization by a different human commensal E. coli strain.
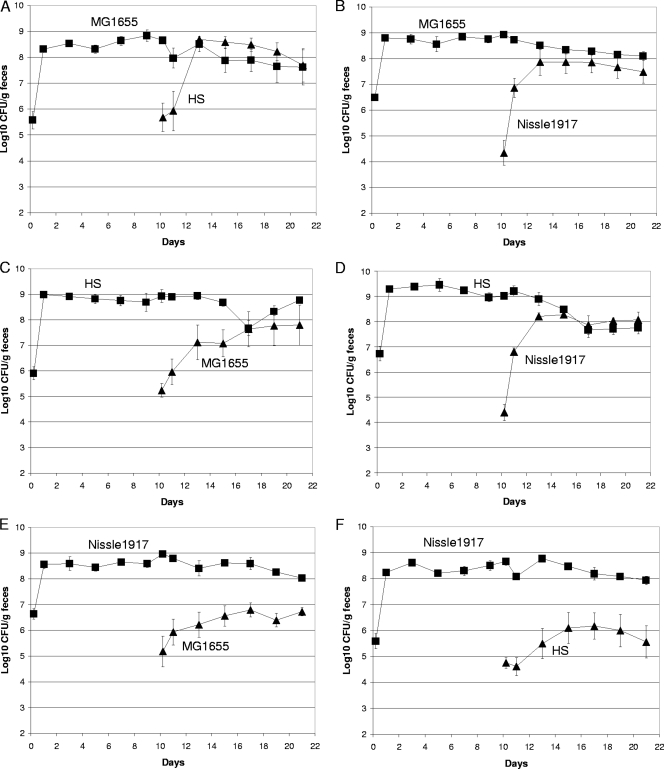
It was not surprising that mice precolonized with a human commensal E. coli strain were resistant to colonization by the same strain fed to the mice 10 days later, since both the precolonized strain and the strain fed at day 10 are isogenic and presumably utilize all nutrients equally well, and the precolonized strain had the advantage of 10 days to adapt physiologically and genetically (26, 27, 37) to the intestinal environment. However, evidence is mounting that different human E. coli strains are different with respect to nutrient utilization in the mouse intestine (13, 17). In view of this evidence, it seemed possible that mice precolonized with one human commensal E. coli strain might allow subsequent intestinal colonization by a different human commensal E. coli strain. To this end, mice precolonized with E. coli MG1655 Strr were fed 105 CFU of E. coli HS Strr Nalr, mice precolonized with E. coli MG1655 Strr Nalr were fed 105 CFU of E. coli Nissle 1917 Strr Rifr, mice precolonized with E. coli HS Strr were fed 105 CFU of either E. coli MG1655 Strr Nalr or E. coli Nissle 1917 Strr Rifr, and mice precolonized with E. coli Nissle 1917 Strr Rifr were fed 105 CFU of either E. coli MG1655 Strr Nalr or E. coli HS Strr Nalr. Indeed, E. coli HS and E. coli Nissle 1917 were able to grow in the intestine from low numbers to about the level of precolonized E. coli MG1655 (Fig. 2A and B), E. coli MG1655 and E. coli Nissle 1917 were able to grow from low numbers to about the level of E. coli HS in the intestines of mice precolonized with E. coli HS (Fig. 2C and D), and E. coli MG1655 and E. coli HS were able to grow from low to relatively high numbers in the intestines of mice precolonized with E. coli Nissle 1917, although they did not reach the level of E. coli Nissle 1917 (Fig. 2E and F). Since each of the precolonized commensal E. coli strains nearly eliminated its isogenic strain from the mouse intestine (Fig. 1), and since different E. coli strains use different nutrients for growth in the mouse intestine (13, 17), these data suggest that each different commensal E. coli strain either uses one or more nutrients not used by the other two commensal E. coli strains to grow in the mouse intestine or uses one or more nutrients better than each of the other two strains.
FIG. 2.
E. coli human commensal strains can colonize the intestines of mice precolonized with different human E. coli commensal strains. (A) Sets of three mice were fed 105 CFU of E. coli MG1655 Strr (▪) and, 10 days later, were fed 105 CFU of E. coli HS Strr Nalr (▴). (B) Sets of three mice were fed 105 CFU of E. coli MG1655 Strr Nalr (▪) and, 10 days later, were fed 105 CFU of E. coli Nissle 1917 Strr Rifr (▴). (C) Sets of three mice were fed 105 CFU of E. coli HS Strr (▪) and, 10 days later, were fed 105 CFU of E. coli MG1655 Strr Nalr (▴). (D) Sets of three mice were fed 105 CFU of E. coli HS Strr Nalr (▪) and, 10 days later, were fed 105 CFU of E. coli Nissle 1917 Strr Rifr (▴). (E) Sets of three mice were fed 105 CFU of E. coli Nissle 1917 Strr Rifr (▪) and, 10 days later, were fed 105 CFU of E. coli MG1655 Strr Nalr (▴). (F) Sets of three mice were fed 105 CFU of E. coli Nissle 1917 Strr Rifr (▪) and, 10 days later, were fed 105 CFU of E. coli HS Strr Nalr (▴). Data were collected and plotted as described in the legend to Fig. 1.
Growth of wild-type E. coli EDL933 in mice precolonized with either E. coli MG1655, E. coli HS, E. coli Nissle 1917, E. coli EFC1, or E. coli EFC2.
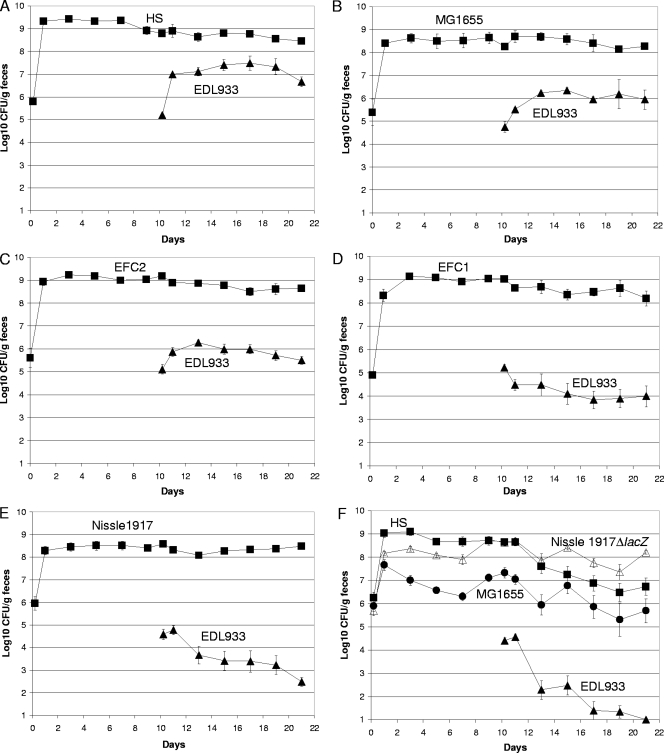
Since commensal E. coli strains are normal members of the human intestinal microbiota, and a healthy human carries an average of five different E. coli strains in the intestine (2), it seems reasonable to assume that any human that becomes infected with an enterohemorrhagic E. coli (EHEC) strain was colonized with at least one commensal E. coli strain prior to becoming infected. EHEC strains, such as E. coli EDL933, grow from very low numbers to extremely high numbers in the intestines of humans that develop disease (34). In fact, many people shed high numbers of EHEC (106 to 108 CFU/gram of feces) for several weeks after the onset of diarrhea (35). Therefore, it appears that invading EHEC strains can grow in the presence of commensal strains that inhabit the intestine. However, many humans that eat EHEC-contaminated foods do not develop disease symptoms (47). It therefore seemed possible, in view of the fact that different E. coli strains use different sugars for growth in the intestine (13, 17), that some precolonized commensal E. coli strains do not allow EHEC strains to grow to high numbers in the intestine. To address this possibility, mice were fed any of five human commensal strains (E. coli MG1655 Strr Nalr, E. coli HS Strr Nalr, E. coli Nissle 1917 Strr Nalr, E. coli EFC1 Strr, or E. coli EFC2 Strr) and 10 days later were fed 105 CFU of the EHEC strain E. coli EDL933 Strr Rifr. Indeed, three different results were obtained. In mice precolonized with E. coli HS, E. coli EDL933 grew from 105 CFU/gram of feces to about 5 × 107 CFU/gram of feces (Fig. 3A). This level of growth of an E. coli O157:H7 strain has been observed previously in mice precolonized with a commensal E. coli strain (25). In mice precolonized with either E. coli MG1655 or E. coli EFC2, E. coli EDL933 grew from 105 CFU/gram of feces to about 106 CFU/gram of feces (Fig. 3B and C). However, in mice precolonized with either E. coli EFC1 or E. coli Nissle 1917, E. coli EDL933 dropped from 105 CFU/gram of feces to between 103 and 104 CFU/gram of feces (Fig. 3D and E). Therefore, different resident commensal E. coli strains vary by as much as 10,000-fold with respect to the level of carriage of the E. coli EDL933 that they allow in the mouse intestine.
FIG. 3.
E. coli EDL933 colonization of the mouse intestine precolonized with different commensal strains. (A) Sets of three mice were fed 105 CFU of E. coli HS Strr Nalr (▪) and, 10 days later, were fed 105 CFU of E. coli EDL933 Strr Rifr (▴). (B) Sets of three mice were fed 105 CFU of E. coli MG1655 Strr Nalr (▪) and, 10 days later, were fed 105 CFU of E. coli EDL933 Strr Rifr (▴). (C) Sets of three mice were fed 105 CFU of E. coli EFC2 Strr (▪) and, 10 days later, were fed 105 CFU of E. coli EDL933 Strr Rifr (▴). (D) Sets of three mice were fed 105 CFU of E. coli EFC1 Strr (▪) and, 10 days later, were fed 105 CFU of E. coli EDL933 Strr Rifr (▴). (E) Sets of three mice were fed 105 CFU of E. coli Nissle Strr Nalr (▪) and, 10 days later, were fed 105 CFU of E. coli EDL933 Strr Rifr (▴). (F) Sets of three mice were fed 105 CFU of E. coli HS Strr (▪), E. coli MG1655 Strr Nalr (•), and E. coli Nissle 1917 Strr ΔlacZ::cat (▵) and, 10 days later, were fed 105 CFU of E. coli EDL933 Strr Rifr (▴). Data were collected and plotted as described in the legend to Fig. 1.
Growth of wild-type E. coli EDL933 in mice precolonized with E. coli MG1655, E. coli HS, and E. coli Nissle 1917.
If different commensal E. coli strains use different nutrients for growth in the intestine, it would be expected that nutrients would be more limited for E. coli EDL933 growth in mice precolonized with three different commensal E. coli strains than in mice precolonized with only one commensal E. coli strain. To address this possibility, mice were precolonized with E. coli MG1655 Strr Nalr, E. coli HS Strr, and E. coli Nissle 1917 Strr ΔlacZ::cat. In control experiments, E. coli Nissle 1917 ΔlacZ had the same colonizing ability as wild-type E. coli Nissle 1917 (data not shown) and was used because it is chloramphenicol resistant (Table 1) and can be selected on plates containing chloramphenicol. Ten days after precolonizing with the three commensal E. coli strains, the mice were fed 105 CFU of E. coli EDL933 Strr Rifr (Fig. 3F). In these experiments, the limit of detection of E. coli EDL933 was 10 CFU/gram of feces (see Materials and Methods). After E. coli EDL933 was fed to the mice, E. coli Nissle 1917 ΔlacZ colonized at a level of about 108 CFU/gram of feces, E. coli HS at a level of about 107 CFU/gram of feces, and E. coli MG1655 at a level between 105 and 106 CFU/gram of feces (Fig. 3F). In contrast, E. coli EDL933 dropped from about 105 CFU/gram of feces at 1 day postfeeding to <10 CFU/gram of feces 10 days later, a 10,000-fold decrease to an undetectable level. This difference represents a 50-fold reduction in E. coli EDL933 in mice precolonized with E. coli MG1655, E. coli HS, and E. coli Nissle 1917 compared to the level in mice precolonized with E. coli Nissle 1917 alone (compare Fig. 3E and F). Clearly, the three commensal strains were far more effective in protecting against E. coli EDL933 growth in the mouse intestine than any of the commensal strains alone.
Colicin and microcin production.
E. coli Nissle 1917 is known to produce microcins M and H47 (49), and many E. coli strains produce colicins. Therefore, the ability of the different commensal E. coli strains to limit E. coli EDL933 colonization to various degrees could be due in part to colicin or microcin production. However, when E. coli Nissle 1917, E. coli MG1655, and E. coli HS were tested for the ability to inhibit E. coli EDL933 growth on nutrient broth dipyridyl plates (see Materials and Methods), none were able to do so. If E. coli Nissle 1917 microcins were active against E. coli EDL933, growth inhibition would have been seen, since E. coli Nissle 1917 inhibited the growth of E. coli MG1655 (∼1.5-mm zone of inhibition). E. coli Nissle 1917 did not inhibit the growth of E. coli HS. Furthermore, E. coli MG1655 did not inhibit the growth of E. coli HS or E. coli Nissle 1917, and E. coli HS did not inhibit the growth of E. coli MG1655 or E. coli Nissle 1917. In further support of the validity of the assay, E. coli F-18, which produces ColV (30), inhibited the growth of E. coli MG1655 (∼4.0-mm zone of inhibition), E. coli HS (∼9.0-mm zone of inhibition), and to a very limited extent, the growth of E. coli EDL933 (∼1.0-mm zone of inhibition). These results suggest that none of the commensal strains used in the colonization experiments produce an antimicrobial that limits the growth of E. coli EDL933.
Location of E. coli HS, E. coli MG1655, and E. coli Nissle 1917 ΔlacZ along the length of the mouse gastrointestinal tract.
The ability of several E. coli strains, including E. coli EDL933, to grow in mouse intestinal mucus has been correlated with their ability to colonize the mouse large intestine, i.e., they grow rapidly in cecal mucus in vitro but far more slowly or not at all in cecal luminal contents (40, 48, 56, 57, 61). However, it was still possible that the ability of either E. coli HS, E. coli MG1655, or E. coli Nissle 1917 to grow from low to higher numbers in the intestines of mice precolonized with a different E. coli strain was due to the preference of each strain for a different location in the intestine, e.g., one strain might grow preferentially in the cecum and another in the ileum. To test this hypothesis, 22 days after feeding the mice 105 CFU of E. coli HS, E. coli MG1655, and E. coli Nissle 1917 ΔlacZ, the numbers of each strain in the ileal mucus, the mucus isolated from the rest of the small intestine, the cecal mucus, and the colonic mucus were determined. This experiment was conducted at the conclusion of the experiment shown in Fig. 3F. As shown in Table 2, although the numbers of each strain were highest in cecal and colonic mucus, there were considerable numbers of each strain in small intestine mucus and in ileal mucus. Moreover, the relative numbers of the strains in each mucus preparation reflected the relative numbers of the strains in the other mucus preparations and in feces (Table 2). It therefore appears that neither E. coli HS, E. coli MG1655, nor E. coli Nissle 1917 ΔlacZ has a preference for a specific site when competing with the others in the mouse intestine. It should also be mentioned that although E. coli EDL933 was not detectable in the mucus preparations in these experiments, it is also found in the mucus in each of the intestinal sections when it is the only E. coli strain fed to mice (44).
TABLE 2.
E. coli HS, E. coli MG1655, and E. coli Nissle 1917 lacZ::cat in small intestine mucus, ileal mucus, cecal mucus, colonic mucus, and feces
| Location | Log10 CFUa
|
||
|---|---|---|---|
| E. coli HS | E. coli MG1655 | E. coli Nissle 1917 ΔlacZ | |
| Small intestine mucusb | 4.4 ± 0.3 | 2.3 ± 0.5 | 4.7 ± 0.3 |
| Ileal mucus | 3.2 ± 0.6 | 1.9 ± 0.4 | 4.1 ± 0.4 |
| Cecal mucus | 4.8 ± 0.4 | 3.8 ± 0.6 | 6.4 ± 0.2 |
| Colonic mucus | 4.6 ± 0.4 | 3.4 ± 0.6 | 5.9 ± 0.4 |
| Feces | 6.9 ± 0.3 | 5.7 ± 0.5 | 8.2 ± 0.1 |
The values are log10 means ± standard errors of the means for six mice. Mucus preparations were isolated on day 22 after feeding. The CFU value for each mucus preparation is corrected for the entire volume of the preparation. The fecal values are the CFU/gram of feces at 21 days after feeding.
Mucus from immediately below the stomach to the proximal ileum.
DISCUSSION
In the present study, we demonstrate colonization resistance in mice precolonized with a specific human commensal E. coli strain and subsequently fed the same strain 10 days later, i.e., the strain fed at day 10 is nearly eliminated (Fig. 1). However, despite the fact that different human commensal strains compete with each other in all sections of the intestine (Table 2), it appears that colonization resistance is not effective when mice precolonized with one human commensal E. coli strain are fed 105 CFU of a different human commensal E. coli strain 10 days later. That is, the strain fed at day 10 grows from low to higher numbers in the mouse intestine and persists in high numbers along with the precolonized strain (Fig. 2).
When the precolonized E. coli strain and the strain fed at 10 days are isogenic and utilize all nutrients equally well, the precolonized strain has the advantage of having had 10 days to adapt to the intestinal environment. The mechanisms involved in adaptation that result in colonization resistance are largely unknown. Freter argued that adhesion to the intestinal epithelium would impart a major advantage to the precolonized strain, resulting in the elimination of the invading strain (19, 20, 21). However, this explanation is unlikely in the present case, since the human commensal strain E. coli MG1655 does not associate with the intestinal epithelium (46) but still displays colonization resistance against itself (Fig. 1A). It has been shown that precolonized commensal E. coli strains can adapt genetically to the mouse intestine such that they become better colonizers of the mouse intestine than their parents by using nutrients more efficiently (26, 27, 37). Whatever the mechanism of adaptation of a precolonized strain to the mouse intestine, whether it be genetic, physiological, or both, it is clear that colonization resistance is effective when mice precolonized with a commensal E. coli strain are fed the same E. coli strain (Fig. 1) but ineffective when precolonized mice are fed a different E. coli strain (Fig. 2). Furthermore, since it appears that different E. coli strains have different nutritional programs for growth in the intestine (13, 17), it seems likely that nonisogenic strains fail to display colonization resistance for nutritional reasons. That is, when the precolonized E. coli strain and the E. coli strain fed at day 10 are strains isolated from different humans, and the strain fed at day 10 grows from low to higher numbers, without eliminating the precolonized strain, we hypothesize that it does so either by using one or more nutrients not being used by the precolonized strain or by outcompeting it for one or more nutrients; however, we fully recognize that E. coli colonization may be impacted by several other factors, including interaction with the indigenous microbiota (21, 50), innate immunity (12), and competition for iron (59).
As stated above, the results presented here are consistent with our previous finding that different E. coli strains have different nutritional programs in the mouse intestine (13, 14). In this vein, it will be of great interest to determine whether a specific commensal E. coli strain uses the same nutrients for growth when it is the only E. coli strain in the mouse intestine as it does when it grows from low to high numbers in mice precolonized with a different commensal E. coli strain. Of equal interest will be to determine whether a specific commensal E. coli strain uses the same or different nutrients for growth in the intestines of mice precolonized with different commensal E. coli strains, e.g., does E. coli Nissle 1917 use the same nutrients to grow from low to high numbers in mice precolonized with E. coli MG1655 as it does in mice precolonized with E. coli HS?
Precolonized E. coli Nissle 1917 allowed growth of both E. coli MG1655 and E. coli HS to between 106 and 107 CFU/gram of feces (Fig. 2E and F) but limited E. coli EDL933 to levels between 103 and 104 CFU/gram of feces (Fig. 3E). Therefore, it appears that in the mouse intestine E. coli Nissle 1917 allows commensal E. coli strains to grow to levels up to 1,000-fold greater than the levels of EHEC strain E. coli EDL933 that it allows. E. coli Nissle 1917 is a commensal strain that has been used as a probiotic agent to treat gastrointestinal infections in humans since the early 1920s (54). Several features of E. coli Nissle 1917 have been proposed to be responsible for its probiotic nature, including its ability to express two microcins (35), the absence of known protein toxins, its semirough lipopolysaccharide, and hence its serum sensitivity (7, 30), and the presence of six iron uptake systems (29). At the present time, we cannot rule out the possibility that E. coli Nissle 1917 inhibits the growth of E. coli EDL933 in the mouse intestine via a secreted inhibitory substance; however, as reported here, E. coli Nissle 1917 produces no inhibitory substance against E. coli EDL933 in microcin assays. Thus, we favor the hypothesis that the nutrients available to E. coli EDL933 and their concentrations in the intestine are far less in mice precolonized with E. coli Nissle 1917 than are available to either E. coli MG1655 or E. coli HS, e.g., for reasons presently unknown, it may be that E. coli Nissle 1917 is able to outcompete E. coli EDL933, but not E. coli MG1655 or E. coli HS, for one or more major nutrients in the intestine.
It is interesting to note that E. coli EDL933 was able to grow from 105 CFU/gram of feces to 5 × 107 CFU per gram of feces in mice precolonized with E. coli HS (Fig. 3A) but the level dropped to 5 × 103 CFU/gram of feces in mice precolonized with E. coli Nissle 1917 (Fig. 3E). How can these data be explained on a nutritional basis? It should be noted that E. coli Nissle 1917 was able to grow to the level of E. coli HS in mice precolonized with E. coli HS (Fig. 2D); although E. coli HS was able to grow from 105 CFU/gram of feces to 106 CFU/gram of feces in mice precolonized with E. coli Nissle 1917, that level was still 100-fold lower than that of E. coli Nissle 1917 (Fig. 2F). Thus, it would appear that although E. coli HS can either use a nutrient(s) that E. coli Nissle 1917 does not use or uses it better, overall E. coli 1917 is the nutritionally superior strain in the intestine, most likely filling more nutritional niches than E. coli HS. If so, it is not surprising that E. coli EDL933 was able to grow much better in mice precolonized with E. coli HS than in mice precolonized with E. coli Nissle 1917.
The fact that E. coli EDL933 growth in the intestine is severely limited, such that it persists in the intestine at a level of almost 5 orders of magnitude lower than E. coli Nissle 1917 in mice precolonized with E. coli Nissle 1917, says nothing as to whether the same scenario in the human intestine would or would not lead to disease. It must be remembered that colonization is only the first step in infection and that the mouse intestine is strictly a model for E. coli EDL933 colonization, not pathogenesis. Hemorrhagic colitis in humans is characterized by hemorrhage and edema in the lamina propria and bloody diarrhea (28, 34). It is possible that although an EHEC strain in the human might also be initially limited to a low level of growth in the intestine due to limiting nutrient levels caused by the resident E. coli strain, as long as the relatively few EHEC cells are healthy and able to persist at that low level, they might be able to initiate the pathogenic process by damaging the mucosa. If so, blood would enter the intestine and expose the EHEC cells to a new rich source of nutrients, leading to increased EHEC growth and subsequent disease. If this scenario is true, one approach to preventing disease would be to precolonize humans with one or more E. coli strains that would not allow any growth of ingested EHEC cells, i.e., that would occupy all E. coli nutritional niches, thereby leading to complete EHEC elimination from the intestine prior to the onset of disease. That this approach to preventing EHEC colonization may have merit is shown by the fact that E. coli EDL933 fed at day 10 was eliminated (<10 CFU/gram of feces) from the intestines of mice precolonized with E. coli Nissle 1917, E. coli HS, and E. coli MG1655, rather than colonizing at a level of about 5 × 103 CFU/gram of feces for several days as in mice precolonized with just E. coli Nissle 1917 (compare Fig. 3E and F).
The usual commentary in E. coli comparative genomics papers is that the genomic core genomes and hence the metabolomes of various E. coli strains are nearly identical (33, 55). If so, why is it that commensal E. coli strains fed to mice at day 10 can grow from low to high numbers in mice precolonized with different commensal E. coli strains, i.e., why do they use different nutrients? With rare exceptions, it is certainly not because those strains that do not use a particular nutrient in the intestine do not have the ability to use it, e.g., E. coli MG1655 uses sialic acid for growth in the intestine whereas E. coli EDL933 does not, but both strains can use sialic acid for growth in vitro (17). We think it is possible that the observed colonization differences in competition between different E. coli commensals, as well as between commensal E. coli strains and E. coli EDL933, may be manifestations of the differing efficiencies with which each strain can occupy specific nutritional niches. That is, both strains might compete for a specific nutrient, but not equally well, e.g., one strain might have more kinetically efficient pathways for uptake and catabolism of that nutrient or each strain might have the same pathways for uptake and catabolism of the nutrient, but those pathways might be more highly induced in one strain than in the other. Alternatively, it may be that non-core genes (5, 16) play a major role in choosing the nutrients that are used by different E. coli strains in the intestine. Further research designed to understand the mechanisms by which different E. coli strains choose specific nutrients for growth in the intestine should provide a nutritional framework for the rational design of E. coli commensal strains (i.e., probiotics) that can serve as the first line of defense in protecting humans against colonization by E. coli intestinal pathogens.
Acknowledgments
This research was supported by Public Health Service grant AI48945 to T.C. and P.S.C.; R.M.-L. was supported by a USDA Strengthening Research Grant, entitled “Environmental Biotechnology at URI,” to P.S.C.
We thank James P. Nataro, University of Maryland School of Medicine, for kindly providing E. coli HS and Michael S. Donnenberg, University of Maryland Medical Center, for kindly providing E. coli EFC1 and E. coli EFC2.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 13 April 2009.
REFERENCES
- 1.Anderson, J. D., W. A. Gillespie, and M. H. Richmond. 1973. Chemotherapy and antibiotic-resistance transfer between Enterobacteria in the human gastro-intestinal tract. J. Med. Microbiol. 6461-473. [DOI] [PubMed] [Google Scholar]
- 2.Apperloo-Renkema, H. Z., B. D. van der Waaij, and D. van der Waaij. 1990. Determination of colonization resistance of the digestive tract by biotyping of Enterobacteriaceae. Epidemiol. Infect. 105355-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autieri, S. M., J. J. Lins, M. P. Leatham, D. C. Laux, T. Conway, and P. S. Cohen. 2007. l-Fucose stimulates utilization of d-ribose by Escherichia coli MG1655 ΔfucAO and E. coli Nissle 1917 ΔfucAO mutants in the mouse intestine and in M9 minimal medium. Infect. Immun. 755465-5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann, B. J. 1996. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12, p. 2460-2488. In F. C. Neidhardt, J. I. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed., vol. 2. American Society for Microbiology, Washington, DC. [Google Scholar]
- 5.Bergthorsson, U., and H. Ochman. 1998. Distribution of chromosome length variation in natural isolates of Escherichia coli. Mol. Biol. Evol. 156-16. [DOI] [PubMed] [Google Scholar]
- 6.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 2771453-1474. [DOI] [PubMed] [Google Scholar]
- 7.Blum, G., R. Marre, and J. Hacker. 1995. Properties of Escherichia coli strains of serotype O6. Infection 23234-236. [DOI] [PubMed] [Google Scholar]
- 8.Bohnhoff, M., B. L. Drake, and C. P. Miller. 1954. Effect of streptomycin on susceptibility of intestinal tract to experimental Salmonella infection. Proc. Soc. Exp. Biol. Med. 86132-137. [DOI] [PubMed] [Google Scholar]
- 9.Chang, D. E., D. J. Smalley, D. L. Tucker, M. P. Leatham, W. E. Norris, S. J. Stevenson, A. B. Anderson, J. E. Grissom, D. C. Laux, P. S. Cohen, and T. Conway. 2004. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl. Acad. Sci. USA 1017427-7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen, P. S., and D. C. Laux. 1995. Bacterial adhesion to and penetration of intestinal mucus in vitro. Methods Enzymol. 253309-314. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, P. S., R. Rossoll, V. J. Cabelli, S. L. Yang, and D. C. Laux. 1983. Relationship between the mouse colonizing ability of a human fecal Escherichia coli strain and its ability to bind a specific mouse colonic mucous gel protein. Infect. Immun. 4062-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole, A. M., and T. Ganz. 2005. Defensins and other antimicrobial peptides: innate defense of mucosal surfaces, p. 17-34. In J. P. Nataro, P. S. Cohen, H. L. T. Mobley, and J. N. Weiser (ed.), Colonization of mucosal surfaces. ASM Press, Washington, DC.
- 13.Conway, T., and P. S. Cohen. 2007. Escherichia coli at the intestinal mucosal surface, p. 175-196. In K. A. Brogden, F. C. Minion, N. Cornick, T. B. Stanton, Q. Zhang, L. K. Nolan, and M. J. Wannemuehler (ed.), Virulence mechanisms of bacterial pathogens, 4th ed. ASM Press, Washington, DC.
- 14.Conway, T., K. A. Krogfelt, and P. S. Cohen. 29 December 2004. The life of commensal Escherichia coli in the mammalian intestine. In R. Curtiss III et al. (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. http://www.ecosal.org. [DOI] [PubMed]
- 15.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobrindt, U., F. Agerer, K. Michaelis, A. Janka, C. Buchrieser, M. Samuelson, C. Svanborg, G. Gottschalk, H. Karch, and J. Hacker. 2003. Analysis of genome plasticity in pathogenic and commensal Escherichia coli isolates by use of DNA arrays. J. Bacteriol. 1851831-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabich, A. J., S. A. Jones, F. Z. Chowdhury, A. Cernosek, A. Anderson, D. Smalley, J. W. McHargue, G. A. Hightower, J. T. Smith, S. M. Autieri, M. P. Leatham, J. J. Lins, R. L. Allen, D. C. Laux, P. S. Cohen, and T. Conway. 2008. Comparative carbon nutrition of pathogenic and commensal Escherichia coli in the mouse intestine. Infect. Immun. 761143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredrickson, A. G. 1977. Behavior of mixed cultures of microorganisms. Annu. Rev. Microbiol. 3163-87. [DOI] [PubMed] [Google Scholar]
- 19.Freter, R. 1983. Mechanisms that control the microflora in the large intestine, p. 33-54. In D. J. Hentges (ed.), Human intestinal microflora in health and disease. Academic Press, Inc., New York, NY.
- 20.Freter, R. 1988. Mechanisms of bacterial colonization of the mucosal surfaces of the gut, p. 45-60. In Virulence mechanisms of bacterial pathogens. American Society for Microbiology, Washington, DC.
- 21.Freter, R. 1992. Factors affecting the microecology of the gut, p. 111-144. In R. Fuller (ed.), Probiotics: the scientific basis. Chapman & Hall, London, United Kingdom.
- 22.Freter, R., H. Brickner, M. Botney, D. Cleven, and A. Aranki. 1983. Mechanisms that control bacterial populations in continuous-flow culture models or mouse large intestinal flora. Infect. Immun. 39676-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freter, R., H. Brickner, J. Fekete, M. M. Vickerman, and K. E. Carey. 1983. Survival and implantation of Escherichia coli in the intestinal tract. Infect. Immun. 39686-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funatsu, G., and H. G. Wittmann. 1972. Ribosomal proteins. 33. Location of amino-acid replacements in protein S12 isolated from Escherichia coli mutants resistant to streptomycin. J. Mol. Biol. 68547-550. [DOI] [PubMed] [Google Scholar]
- 25.Gamage, S. D., A. K. Patton, J. E. Strasser, C. L. Chalk, and A. A. Weiss. 2006. Commensal bacteria influence Escherichia coli O157:H7 persistence and Shiga toxin production in the mouse intestine. Infect. Immun. 741977-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauger, E. J., M. P. Leatham, R. Mercado-Lubo, D. C. Laux, T. Conway, and P. S. Cohen. 2007. Role of motility and the flhDC operon in Escherichia coli MG1655 colonization of the mouse intestine. Infect. Immun. 753315-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giraud, A., S. Arous, M. D. Paepe, V. Gaboriau-Routhiau, J.-C. Bambou, S. Rakotobe, A. B. Lindner, F. Taddei, and N. Cerf-Bensussanet. 2008. Dissecting the genetic components of adaptation of Escherichia coli to the mouse gut. PLoS Genet. 4e2. doi: 10.1371/journal.pgen.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffin, P. M., L. C. Olmstead, and R. E. Petras. 1990. Escherichia coli O157:H7-associated colitis. A clinical and histological study of 11 cases. Gastroenterology 99142-149. [DOI] [PubMed] [Google Scholar]
- 29.Grozdanov, L., C. Raasch, J. Schulze, U. Sonnenborn, G. Gottschalk, J. Hacker, and U. Dobrindt. 2004. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J. Bacteriol. 1865432-5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grozdanov, L., U. Zähringer, G. Blum-Oehler, L. Brade, A. Henne, Y. A. Knirel, U. Schombel, J. Schulze, U. Sonnenborn, G. Gottschalk, J. Hacker, E. T. Rietschel, and U. Dobrindt. 2002. A single nucleotide exchange in the wzy gene is responsible for the semirough O6 lipopolysaccharide phenotype and serum sensitivity of Escherichia coli strain Nissle 1917. J. Bacteriol. 1845912-5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hentges, D. J. 1983. Role of the intestinal microflora in host defense against infection, p. 311-331. In D. J. Hentges (ed.), Human intestinal microflora in health and disease. Academic Press, Inc., New York, NY.
- 32.Hentges, D. J., J. U. Que, S. W. Casey, and A. J. Stein. 1984. The influence of streptomycin on colonization in mice. Microecol. Theor. 1453-62. [Google Scholar]
- 33.Ihssen, J., E. Grasselli, C. Bassin, P. Francois, J. C. Piffaretti, W. Koster, J. Schrenzel, and T. Egli. 2007. Comparative genomic hybridization and physiological characterization of environmental isolates indicate that significant (eco-)physiological properties are highly conserved in the species Escherichia coli. Microbiology 1532052-2066. [DOI] [PubMed] [Google Scholar]
- 34.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2123-140. [DOI] [PubMed] [Google Scholar]
- 35.Karch, H., H. Russmann, H. Schmidt, A. Schwarzkopf, and J. Heesemann. 1995. Long-term shedding and clonal turnover of enterohemorrhagic Escherichia coli O157 in diarrheal diseases. J. Clin. Microbiol. 331602-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laux, D. C., P. S. Cohen, and T. Conway. 2005. Role of the mucus layer in bacterial colonization of the intestine, p. 199-212. In J. P. Nataro, P. S. Cohen, H. L. T. Mobley, and J. N. Weiser (ed.), Colonization of mucosal surfaces. ASM Press, Washington, DC.
- 37.Leatham, M. P., S. J. Stevenson, E. J. Gauger, K. A. Krogfelt, J. J. Lins, T. L. Haddock, S. M. Autieri, T. Conway, and P. S. Cohen. 2005. Mouse intestine selects nonmotile flhDC mutants of Escherichia coli MG1655 with increased colonizing ability and better utilization of carbon sources. Infect. Immun. 738039-8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee, A. 1985. Neglected niches, the microbial ecology of the gastrointestinal tract. Adv. Microb. Ecol. 8115-162. [Google Scholar]
- 39.Levine, M. M., E. J. Bergquist, D. R. Nalin, D. H. Waterman, R. B. Hornick, C. R. Young, and S. Sotman. 1978. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet 11119-1122. [DOI] [PubMed] [Google Scholar]
- 40.Licht, T. R., T. Tolker-Nielsen, K. Holmstrøm, K. A. Krogfelt, and S. Molin. 1999. Inhibition of Escherichia coli precursor 16S rRNA processing by mouse intestinal contents. Environ. Microbiol. 123-32. [DOI] [PubMed] [Google Scholar]
- 41.McCormick, B. A., D. P. Franklin, D. C. Laux, and P. S. Cohen. 1989. Type 1 pili are not necessary for colonization of the streptomycin-treated mouse large intestine by type 1-piliated Escherichia coli F-18 and E. coli K-12. Infect. Immun. 573022-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller, C. P., and M. Bohnhoff. 1963. Changes in the mouse's enteric microbiota associated with enhanced susceptibility to Salmonella infection following streptomycin-treatment. J. Infect. Dis. 11359-66. [DOI] [PubMed] [Google Scholar]
- 43.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 44.Miranda, R. L., T. Conway, M. P. Leatham, D. E. Chang, W. E. Norris, J. H. Allen, S. J. Stevenson, D. C. Laux, and P. S. Cohen. 2004. Glycolytic and gluconeogenic growth of Escherichia coli O157:H7 (EDL933) and E. coli K-12 (MG1655) in the mouse intestine. Infect. Immun. 721666-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mobley, H. L. T., D. M. Green, A. L. Trifillis, D. E. Johnson, G. R. Chippendale, C. V. Lockatell, B. D. Jones, and J. W. Warren. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 581281-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Møller, A. K., M. P. Leatham, T. Conway, P. J. M. Nuijten, L. A. M. de Haan, K. A. Krogfelt, and P. S. Cohen. 2003. An Escherichia coli MG1655 lipopolysaccharide deep-rough core mutant grows and survives in mouse cecal mucus but fails to colonize the mouse large intestine. Infect. Immun. 712142-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neill, M. A. 1998. Treatment of disease due to Shiga toxin-producing Escherichia coli: infectious disease management, p. 357-363. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press, Washington, DC.
- 48.Newman, J. V., R. Kolter, D. C. Laux, and P. S. Cohen. 1994. The role of leuX in Escherichia coli colonization of the streptomycin-treated mouse large intestine. Microb. Pathog. 17301-311. [DOI] [PubMed] [Google Scholar]
- 49.Patzer, S. I., M. R. Baquero, D. Bravo, F. Moreno, and K. Hantke. 2003. The colicin G, H and X determinants encode microcins M and H47, which might utilize the catecholate siderophore receptors FepA, Cir, Fiu and IroN. Microbiology 1492557-2570. [DOI] [PubMed] [Google Scholar]
- 50.Peterson, D. A., D. N. Frank, N. R. Pace, and J. I. Gordon. 2008. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe 6417-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poulsen, L. K., F. Lan, C. S. Kristensen, P. Hobolth, S. Molin, and K. A. Krogfelt. 1994. Spatial distribution of Escherichia coli in the mouse large intestine inferred from rRNA in situ hybridization. Infect. Immun. 625191-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rang, C. U., T. R. Licht, T. Midtvedt, P. L. Conway, L. Chao, K. A. Krogfelt, P. S. Cohen, and S. Molin. 1999. Estimation of growth rates of Escherichia coli BJ4 in streptomycin-treated and previously germfree mice by in situ rRNA hybridization. Clin. Diagn. Lab. Immunol. 6434-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308681-685. [DOI] [PubMed] [Google Scholar]
- 54.Sartor, R. B. 2005. Probiotic therapy of intestinal inflammation and infections. Curr. Opin. Gastroenterol. 2144-50. [PubMed] [Google Scholar]
- 55.Sun, J., F. Gunzer, A. M. Westendorf, J. Buer, M. Scharfe, M. Jarek, F. Gossling, H. Blocker, and A. P. Zeng. 2005. Genomic peculiarity of coding sequences and metabolic potential of probiotic Escherichia coli strain Nissle 1917 inferred from raw genome data. J. Biotechnol. 117147-161. [DOI] [PubMed] [Google Scholar]
- 56.Sweeney, N. J., P. Klemm, B. A. McCormick, E. Moller-Nielsen, M. Utley, M. A. Schembri, D. C. Laux, and P. S. Cohen. 1996. The Escherichia coli K-12 gntP gene allows E. coli F-18 to occupy a distinct nutritional niche in the streptomycin-treated mouse large intestine. Infect. Immun. 643497-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sweeney, N. J., D. C. Laux, and P. S. Cohen. 1996. Escherichia coli F-18 and K-12 eda mutants do not colonize the streptomycin-treated mouse large intestine. Infect. Immun. 643504-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor, P. A., and P. J. Williams. 1975. Theoretical studies on the coexistence of competing species under continuous-flow conditions. Can. J. Microbiol. 2190-98. [DOI] [PubMed] [Google Scholar]
- 59.Tompkins, G. R., N. L. O'Dell, I. T. Bryson, and C. B. Pennington. 2001. The effects of dietary ferric iron and iron deprivation on the bacterial composition of the mouse intestine. Curr. Microbiol. 4338-42. [DOI] [PubMed] [Google Scholar]
- 60.van der Waaij, D., J. M. Berghuis-de Vries, and L.-V. Lekkerkerk. 1971. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J. Hyg. 69405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wadolkowski, E. A., D. C. Laux, and P. S. Cohen. 1988. Colonization of the streptomycin-treated mouse large intestine by a human fecal Escherichia coli strain: role of growth in mucus. Infect. Immun. 561030-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wadolkowski, E. A., L. M. Sung, J. A. Burris, J. E. Samuel, and A. D. O'Brien. 1990. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect. Immun. 583959-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]