Abstract
Escherichia coli strain Nissle 1917 has been widely used as a probiotic for the treatment of inflammatory bowel disorders and shown to have immunomodulatory effects. Nissle 1917 expresses a K5 capsule, the expression of which often is associated with extraintestinal and urinary tract isolates of E. coli. In this paper, we investigate the role of the K5 capsule in mediating interactions between Nissle 1917 and intestinal epithelial cells. We show that the loss of capsule significantly reduced the level of monocyte chemoattractant protein 1 (MCP-1), RANTES, macrophage inflammatory protein 2α (MIP-2α), MIP-2β, interleukin-8, and gamma interferon-inducible protein 10 induction by Nissle 1917 in both Caco-2 cells and MCP-1 induction in ex vivo mouse small intestine. The complementation of the capsule-minus mutation confirmed that the effects on chemokine induction were capsule specific. The addition of purified K5, but not K1, capsular polysaccharide to the capsule-minus Nissle 1917 at least in part restored chemokine induction to wild-type levels. The purified K5 capsular polysaccharide alone was unable to stimulate chemokine production, indicating that the K5 polysaccharide was acting to mediate interactions between Nissle 1917 and intestinal epithelial cells. The induction of chemokine by Nissle 1917 was generated predominantly by interaction with the basolateral surface of Caco-2 cells, suggesting that Nissle 1917 will be most effective in inducing chemokine expression where the epithelial barrier is disrupted.
A probiotic has been defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (20). These benefits include the balancing and restoration of the intestinal microflora, repair of intestinal barrier functions (54), expression of bacteriocins (36), immunomodulatory effects (18, 43, 47, 53), and antagonizing epithelial colonization and invasion by pathogens (2). Escherichia coli strain Nissle 1917 was isolated from the feces of a soldier who did not develop diarrhea during a severe outbreak of shigellosis (38). Despite exhibiting a serotype (O6:K5:H1) that is characteristic of E. coli strains associated with urinary tract infections, Nissle 1917 apparently is nonpathogenic (25, 53) and has been used widely in preventing infectious diarrheal diseases (7, 14, 27, 37, 52, 53), the treatment of inflammatory bowel diseases such as ulcerative colitis and Crohn's disease (7, 23, 32, 33), and to prevent the colonization of the digestive tract of neonates by pathogens (35). Recently, there has been a growing interest in investigating the immunomodulatory effect of Nissle 1917. Previous studies showed that colonization by Nissle 1917 may lead to an alteration of the hosts' cytokine repertoire (13, 49), increased immunoglobulin A secretion (14), lymphocyte or macrophage activation (13), the modulation of CD4+ clonal expansion (47), the stimulation of antimicrobial peptide production by intestinal epithelial cells (39, 52, 54), and alterations of the pro- and anti-inflammatory balance of local cytokines (49). Recently it has been shown that Nissle 1917 activates γδT cells, stimulating CXCL8 and interleukin-6 (IL-6) release but inhibiting tumor necrosis factor alpha (TNF-α) secretion (26). Following activation, Nissle 1917 induced apoptosis in activated γδT cells, indicating a key role for Nissle 1917 in interacting with the subset of T cells that operate at the interface between the adaptive and innate immune responses (26). Nissle 1917 also has been shown to express a direct anti-inflammatory activity on epithelial cells by blocking TNF-α-induced IL-8 secretion through a NF-κB-independent mechanism (28). Although the immunomodulatory effects of Nissle 1917 are well documented, the contribution of individual microbial components in mediating such effects is less well understood. So far, only a role for flagellin in mediating the induction of human β-defensin expression by Nissle 1917 has been established (44). Nissle 1917 expresses a K5 capsule on its cell surface, and a number of roles for polysaccharide capsules in the virulence of E. coli have been proposed, including resistance to phagocytosis and complement-mediated killing and the increased colonization of the host (42). In contrast, in the case of other encapsulated pathogens, it has been shown that the expression of a polysaccharide capsule can affect the induction of chemokines following attachment to host cells (6, 17, 22, 24, 40, 41, 45, 50). The aim of the present study was to investigate the role of the K5 capsule in mediating the immunomodulatory activity of Nissle 1917.
MATERIALS AND METHODS
Preparation of bacteria.
For all experiments, bacteria were grown overnight in Luria-Bertani (LB) broth at 37°C and with shaking at 200 rpm. The cultures then were diluted 1:100 in fresh LB broth and reincubated in the same conditions until mid-log phase (optical density at 600 nm, 0.5). Where appropriate, the medium was supplemented with ampicillin (100 μg/ml) or chloramphenicol (25 μg/ml). Escherichia coli strain Nissle 1917 was a generous gift of U. Dobrindt from the University of Wurzburg, Germany. The kfiC knockout mutant lacking a K5 capsule first was generated in strain MS101 (46) according to a method previously described (15) by the replacement of the kfiC gene with a chloramphenicol resistance cassette amplified from pKD3 using the primers 5′-AATGCAGTTTTATTCTCATTTTATTTATCATTAAGTGAATGTATGGTGTAGGCTGGAGCTGCTTCG-3′ and 5′-CC AAAAATTTAATATATATAGAATAACAAACTATTGTTCAAATATTCCTG ACATATGAATATCCTAATTAG-3′. The mutation then was moved into Nissle 1917 to generate EcNK5− by P1 transduction, and the loss of K5 capsule expression was confirmed by assaying sensitivity to K5-specific bacteriophage. Plasmid pBkfiCD was generated by cloning a 2.7-kb PCR fragment encoding the kfiCD genes into XhoI- and XbaI-digested pBluescript II SK(+) (Fermentas, York, United Kingdom). The kfiCD amplicon was generated using the primers KfiCDF (5′-ATATCT AGATAACTGACTGACTAGGGAGTGATATACTGAACGCAGAATATATA TATAAATTTAGTTGAACG-3′) and KfiCDR (5′-ATACTCGAGTTAGTCACATTTAACAAATCGCGAC-3′). The introduction of plasmid pBkfiCD into strain EcNK5− restored capsule expression, as measured by sensitivity to K5-specific bacteriophage.
Purification of K1 and K5 polysaccharide.
The K1 polysaccharide was prepared from strain LE392(pKT272) (8), and the K5 polysaccharide was prepared from strain MS101 as described previously (12). Lipopolysaccharide (LPS) was removed by polymyxin B treatment, and the final polysaccharide preparation was assayed for LPS contamination using a Limulus amoebocyte lysate according to the manufacturer's instructions (AMS Biotechnology Ltd., Abingdon, United Kingdom). Both the K1 and K5 polysaccharide preparations were free of detectable contaminating LPS.
Purification and analysis of LPS.
LPS samples were prepared and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by silver staining, as described previously (31).
Cell culture conditions.
The human colon adenocarcinoma cell line Caco-2 (19) was maintained in IMDM cell culture medium (Sigma Aldrich, Irvine, Ayrshire, United Kingdom) containing 10% fetal bovine serum (FBS; Invitrogen, Life Technologies, Paisley, United Kingdom) at 37°C in the presence of 5% CO2. Caco-2 cells between passages 10 and 20 were used for experiments. Cells were split twice a week at a ratio of 1:3. Nonpolarized Caco-2 cells were obtained by culture in 6-well plates (Costar Corning) for 4 days until a confluent monolayer was obtained. Polarized Caco-2 cell monolayers were obtained as described previously (11). Briefly, the cells were seeded onto Transwell polycarbonate filters (24 mm; 0.4-μm pore size; Costar Corning) at a concentration of 2 × 105 cells/cm2 in Dulbecco's modified essential medium supplemented with 10% FBS for 21 to 24 days at 37°C. Under these conditions, monolayers develop a transepithelial electrical resistance (TEER) of ∼200 Ω·cm2, indicating the formation of a polarized cell layer.
Assays of adherence to and invasion of cultured mammalian cells.
Bacterial adherence and invasion into Caco-2 cells were assessed as described previously (1) and carried out as follows. Mid-log-phase bacteria were washed with phosphate-buffered saline (PBS) and resuspended in IMDM to the required final count, corresponding to multiplicities of infection (MOIs) of 1 to 100. Mammalian cells were grown to a confluent monolayer in 6-well tissue culture plates. After the washing of the monolayer, an aliquot of 2 ml of the adjusted bacterial suspension was added to each well, and the mammalian cells then were incubated with bacteria for 2, 4, or 6 h at 37°C in 5% CO2 to allow bacterial adherence. After the removal of nonadhered bacteria by repeated washing, the infected cells were treated with 1% saponin (Sigma Aldrich Company, Irvine, Ayrshire, United Kingdom) for 5 min and serially diluted, and the number of bacteria was assessed by viable counting. This represents the total number of adherent and internalized bacteria. Invasion assays were carried out using a similar protocol, except that the infected monolayer was treated for 2 h with gentamicin (200 μg/ml) to kill extracellular bacteria prior to the addition of saponin. Adherent viable bacteria were quantified as the difference between the total number of cell-associated bacteria (adherent plus internalized) and the number of internalized bacteria. The results are expressed as the number of adhering bacteria per Caco-2 cell.
Stimulation of Caco-2 cells.
Confluent Caco-2 cells in 6-well plates (about 1 × 106 cells per well) were washed with PBS and incubated for 6 h at 37°C and 5% CO2 with a bacterial suspension made in IMDM medium at an MOI of 1. Polarized Caco-2 monolayers grown in Transwells were treated in the same way, except that the bacteria were added to either the apical or basal reservoir. Controls were carried out in a similar way, except that the bacterial suspension was replaced by IMDM medium. In the case of polarized cells, monolayer integrity was monitored throughout the experiment by measuring TEER. At the end of the incubation, culture supernatants were collected and stored at −80°C for the analysis of cytokine secretion, and the cell layer was extracted for RNA as described below.
Ex vivo challenging of mice intestinal epithelial cells.
The ex vivo challenging of mice intestinal epithelial cells was performed by following a modified protocol described by Ukena et al. (49). Briefly, small intestine was dissected, washed, and cut into 5-mm-long pieces. These pieces were opened longitudinally and washed with PBS containing dithiothreitol (50 μM) for 15 min with shaking at 37°C to remove the excess mucus. Tissue pieces then were washed with PBS, followed by two washing steps with cold IMDM supplemented with 2% FBS. Five tissue pieces per well were placed in a 6-well plate in IMDM. Bacteria then were applied at 5 × 105 CFU/ml in IMDM and incubated with tissue for 6 h prior to washing the tissue and extracting its RNA.
RNA extraction and quantification.
RNA from cells and tissue was extracted using an RNeasy minikit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Total RNA from independent triplicate experiments was isolated. The extracted RNA was exposed twice to DNA digestion using RNase-free DNase (Qiagen, Hilden, Germany) to exclude any genomic DNA contamination. The absence of DNA in isolated RNA was confirmed by performing PCR in the absence of a reverse transcriptase step. Two micrograms of each isolated RNA then was reverse transcribed for 60 min at 42°C in 25-μl assays containing 25 pM oligo(dT) primer, 12.5 mM deoxynucleoside triphosphates, 40 U avian myeloblastosis virus reverse transcriptase (Roche Diagnostics, Mannheim, Germany), and 20 U of RNase inhibitor (Roche Diagnostics, Mannheim, Germany). For quantitative real-time PCR, equivalent amounts of cDNAs (2 μl) and 300 nM each of the corresponding forward and reverse primers were used with a SYBR green PCR kit from Eurogentic. The specific primers listed in Table 1 were designed to amplify 90- to 250-bp fragments from the cDNA under investigation. The PCRs were carried out in the ABI PRISM 7000 cycler (Applied Biosystems, CA). Cycling conditions for PCR amplification were 95°C for 10 min and then 40 cycles of denaturation at 95°C for 15 s, annealing, and extension at 58°C for 1 min. The housekeeping genes used were RPS9 (ribosomal protein S9) in the case of Caco-2 cells and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) in the case of ex vivo mouse tissues. The housekeeping genes from each sample were assessed in parallel with each reaction as internal standards. A threshold was set in the linear part of the amplification curve, and the number of cycles needed to reach it was calculated for every gene. Relative mRNA levels were determined by using included standard curves for each individual gene, and further normalization to the housekeeping gene was carried out.
TABLE 1.
Primers used for real-time qRT-PCR
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| RPS-9 | CGCAGGCGCAGACGGTGGAAGC | CGAAGGGTCTCCGCGGGGTCACAT |
| MCP-1 | CGCACTCTCGCCTCCAGCATGAAG | CGCGAGCCTCTGCACTGAGATCTTC |
| IL-8 | CCTACAACAGACCCACAACAATACATG | CTGATGGAAGAGAGCTCTGTCTGGAC |
| MIP-2α | TAGGTCAAACCCAAGTTAGTTCAATC | GATTCCTCAGCCTCTATCACAG |
| MIP-2β | AAGAAGCTTATCAGCGTATCATTGAC | TTTCTTACGAGGGTTCTACTTATTTATG |
| RANTES | CTCCCAAGCTAGGACAAGAGCAAG | TCCAACCCAGCAGTCGTCTTTG |
| IP-10 | GTTTTAAGGAGATCTTTTAGACATTTC | CTCTAAGTGGGCATTCAAGGAGTAC |
| MCP-1a | CCTGCTTGCTGGTGATTCCTCTTGATG | CCATGCAGGTCCCTGTCATGC |
| GAPDHa | TGCACCACCAACTGCTTAG | GATGCAGGGATGATGTTC |
Mouse gene.
Cytokine analysis by cytometric bead array.
The levels of chemokines in the Caco-2 culture supernatants were quantified using the BD Biosciences cytometric bead array human chemokine kit I with antibodies specific for IL-8, RANTES, and monocyte chemoattractant protein 1 (MCP-1) (BD Biosciences, Heidelberg, Germany). This was performed according to the manufacturer's instructions and as previously described (10) with a FACSCalibur flow cytometer (BD Biosciences) using two-color detection. Data analysis was performed by BD Biosciences cytometric bead array software.
Statistical analysis.
The significance of differences between means were tested using two-tailed Student t tests and analysis of variance. The differences between means were considered statistically significant when P ≤ 0.01. The SPSS statistical package was used for analysis.
RESULTS
Effect of the K5 capsule on chemokine induction by Nissle 1917.
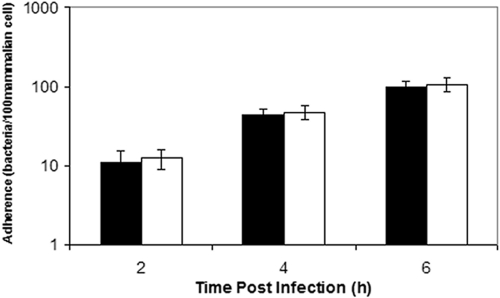
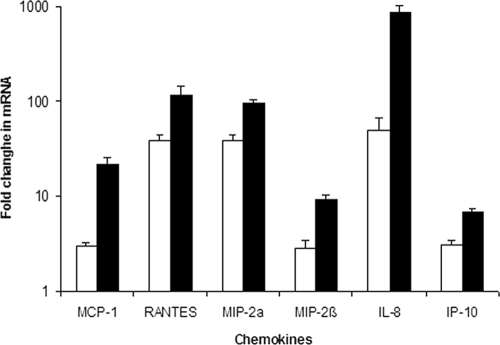
To determine a role for the K5 capsule in mediating interactions between Nissle 1917 and gut epithelial cells, we compared the induction of cytokine gene expression in Caco-2 cells by Nissle 1917 to that of a knockout mutant lacking a K5 capsule (designated EcNK5−), as described previously (49). The first stage was to establish that the loss of capsule had no effect on the attachment of Nissle 1917 to Caco-2 cells. The incubation of Caco-2 cells with either Nissle 1917 or EcNK5− at an MOI of 1 showed no significant difference in the ability of the two strains to attach to Caco-2 cells during a 6-h time period (Fig. 1). The infection of Caco-2 cells with Nissle 1917 stimulated the expression of an array of chemokine genes, including MCP-1, RANTES, macrophage inflammatory protein 2α (MIP-2α), MIP-2β, IL-8, and gamma interferon-inducible protein 10 (IP-10), as measured by quantitative reverse transcription-PCR (qRT-PCR) (Fig. 2). This was in keeping with previous findings (49). In the case of Caco-2 cells infected with EcNK5−, the same chemokine genes were induced, but the level of induction was significantly reduced compared to that of infection with Nissle 1917 (P ≤ 0.01) (Fig. 2). For example, IL-8 and MCP-1 secretion in response to EcNK5− was 10- and 6-fold lower, respectively, than that observed with Nissle 1917 (Fig. 2). However, EcNK5− still induced significant expression of these chemokine genes compared to that of uninfected cells (P ≤ 0.01) (Fig. 2).
FIG. 1.
Adherence of Nissle 1917 (EcNK5) and EcNK5− to Caco-2 cells. Mid-log-phase bacteria were added to a confluent lawn of Caco-2 cells at an MOI of 1, as described in Materials and Methods. After 2, 4, or 6 h, the cells were washed extensively before lysis, and the adherent bacteria enumerated by plating on L agar. The results are expressed as the number of adherent bacteria per 100 Caco-2 cells. The white bars are EcNK5−, while the filled bars are Nissle 1917. The figure is representative of three independent experiments, each done at least in quadruple. Data are expressed as means ± standard deviations.
FIG. 2.
Effect of Nissle 1917 and EcNK5− on chemokine expression by Caco-2 cells. Caco-2 cells were infected with bacteria, RNA was extracted, and qRT-PCR was performed as described in Materials and Methods. Relative chemokine mRNA levels were expressed as the change (n-fold) from the corresponding levels in the uninfected Caco-2 cells. The white bars are EcNK5−, while the filled bars are Nissle 1917. The figure is representative of three independent experiments, each done at least in quadruple. Data are expressed as means ± standard deviations. In all cases, there was a significant difference in chemokine expression between Nissle 1917 and EcNK5− (P ≤ 0.01).
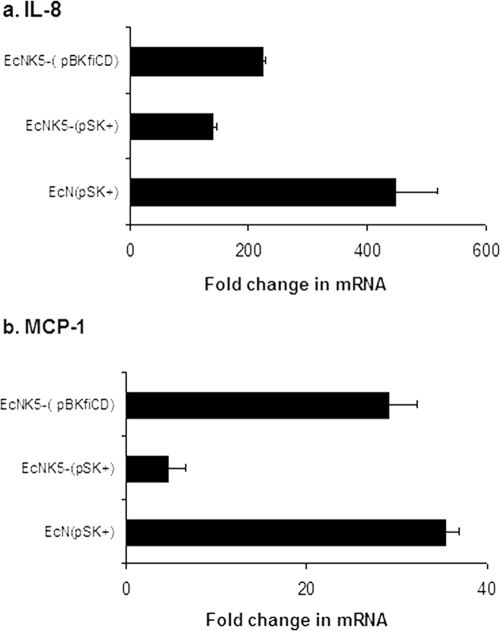
To confirm that the differences in chemokine induction were due to the lack of K5 capsule, the experiment was repeated using strain EcNK5−(pBkfiCD), in which the kfiC mutation is complemented by plasmid pBkfiCD. This restores K5 capsule expression as detected by sensitivity to K5-specific bacteriophage (data not shown). The infection of Caco-2 cells with strain EcNK5−(pBkfiCD) induced MCP-1 gene expression to levels comparable to that seen with Nissle 1917 harboring the empty vector plasmid pBluescript II SK(+) (Fig. 3). The restoration of K5 capsule expression to EcNK5− also significantly increased (P ≤ 0.01) the induction of IL-8 gene expression compared to that of EcNK5− carrying the empty vector plasmid pBluescript II SK(+), although the effect was more modest than that seen with MCP-1 (Fig. 3).
FIG. 3.
Effect of restoring K5 capsule expression to strain EcNK5− on the induction of IL-8 and MCP-1 by Caco-2 cells. Caco-2 cells were infected with bacteria, RNA was extracted, and qRT-PCR was performed as described in Materials and Methods. Relative chemokine mRNA levels were expressed as the change (n-fold) in the corresponding levels in the uninfected Caco-2 cells. The figure is representative of three independent experiments, each done at least in quadruple. Data are expressed as means ± standard deviations. EcN, strain Nissle 1917; pSK+, pBluescript II SK(+).
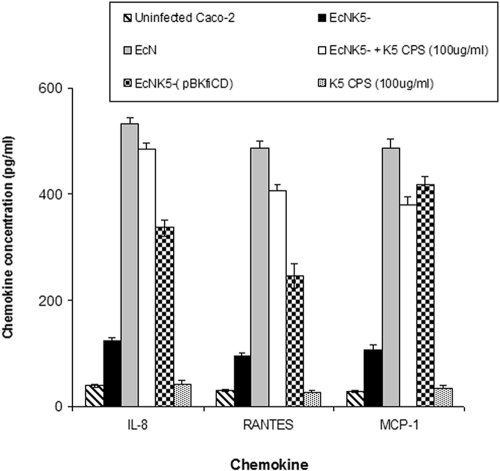
To confirm that the changes in gene expression reflect changes in chemokine production, supernatants from uninfected Caco-2 cells and those infected with Nissle 1917 or EcNK5− were collected and analyzed for IL-8, MCP-1, and RANTES (Fig. 4). Although both Nissle 1917 and EcNK5− induced chemokine secretion by Caco-2 cells, which was not seen with noninfected controls, there was a significant difference between their levels of induction (Fig. 4). Infection with Nissle 1917 led to a 14- to 17-fold increase in chemokine production, which was significantly different (P ≤ 0.01) from the 3- to 4-fold increase seen with EcNK5− (Fig. 4). The restoration of K5 capsule expression in strain EcNK5−(pBkfiCD) was accompanied by a significant rise (P ≤ 0.01) in the secretion of all three chemokines compared to that caused by EcNK5− (Fig. 3). Taken together, these data confirm that the expression of a K5 capsule is modulating the induction of chemokine production.
FIG. 4.
Levels of chemokines in culture supernatants of Caco-2 cells infected with different Nissle 1917 strains. Caco-2 cells were cocultured with Nissle 1917 (EcN), EcNK5−(pBkfiCD), EcNK5− supplemented with purified K5 polysaccharide (CPS; 100 ug·ml−1), or just purified K5 polysaccharide (100 μg·ml−1) for 6 h. Culture supernatants were analyzed for IL-8, RANTES, and MCP-1 using cytometric bead array kit I from BD Bioscience. The cytokine quantity is expressed as picograms per milliliter. Data are presented as the means from two independent experiments, each done in quadruple. Data are expressed as means ± standard deviations.
Effect of purified K5 and K1 capsular polysaccharide on chemokine expression by Caco-2 cells.
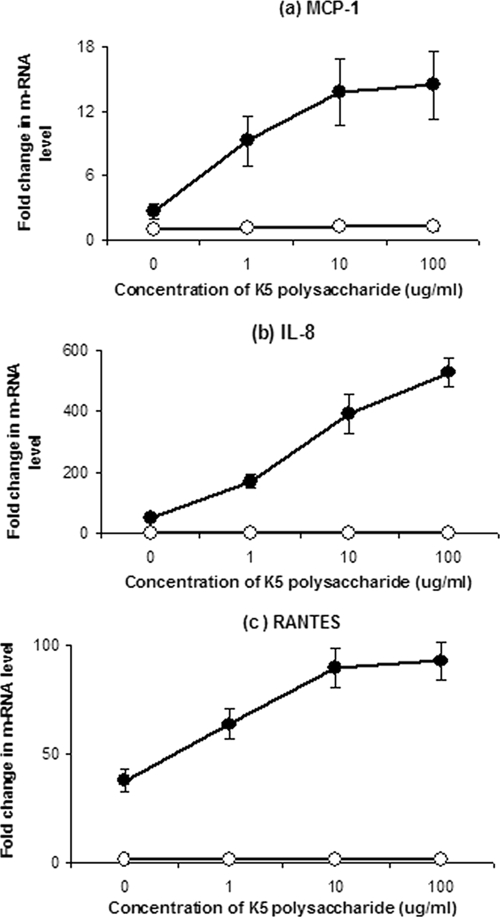
To establish whether the K5 polysaccharide alone was capable of inducing chemokine gene expression, Caco-2 cells were incubated with increasing concentrations of purified K5 polysaccharide, and the expression of chemokine genes was assessed by qRT-PCR. The addition of purified K5 polysaccharide alone did not induce the expression of IL-8, MCP-1, or RANTES (Fig. 4). However, the addition of purified K5 polysaccharide to cells infected with EcNK5− resulted in a dose-dependent induction of chemokine gene expression in Caco-2 cells (Fig. 5). To confirm that these changes in mRNA corresponded to chemokine production, the levels of IL-8, MCP-1, and RANTES were determined in the culture supernatants of EcNK5−-infected Caco-2 cells in the presence of 100 μg·ml−1 K5 polysaccharide. The presence of exogenous K5 polysaccharide together with EcNK5− resulted in a significant (P ≤ 0.01) fourfold increase in IL-8, MCP-1, and RANTES secretion compared to that seen with Caco-2 cells infected with EcNK5− alone (Fig. 4). Purified K5 polysaccharide alone had no effect on stimulating IL-8, MCP-1, or RANTES (Fig. 4). These data indicate that the presence of exogenous K5 polysaccharide can substitute for the expression of a K5 capsule and stimulate chemokine expression to levels induced by the encapsulated Nissle 1917. To determine if this effect was K5 specific, the experiments were repeated using increasing concentrations of purified K1 capsular polysaccharide. The purified K1 polysaccharide was unable to stimulate chemokine gene expression and also was unable to potentiate the induction of chemokine gene expression when coincubated with EcNK5− (data not shown). These data indicate that the ability of exogenous K5 polysaccharide to potentiate the induction of chemokine expression by EcNK5− is K5 specific.
FIG. 5.
Effect of the addition of soluble K5 capsular polysaccharide on the expression of MCP-1, IL-8, and RANTES by Caco-2 cells in the presence or absence of EcNK5−. Caco-2 cells either were infected with EcNK5− together with increasing concentrations of purified K5 polysaccharide (filled circles) or exposed to purified K5 polysaccharide alone (open circles) prior to RNA extraction and qRT-PCR, as described in Materials and Methods. Relative chemokine mRNA levels were expressed as the change (n-fold) in the corresponding levels in the uninfected Caco-2 cells. The results are expressed as the change (n-fold) in mRNA level from that of the noninfected control. The figure is representative of three independent experiments, each done at least in quadruple. Data are expressed as means ± standard deviations.
Induction of MCP-1 response in mouse small intestine after challenge with Nissle 1917 strains.
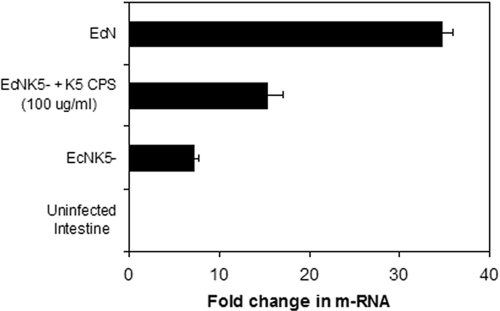
The effect of K5 capsule expression on the ability of Nissle 1917 to induce chemokine responses in mouse intestinal tissue was assayed by infecting ex vivo pieces of small intestine from BALB/c mice with either Nissle 1917 or EcNK5−. The incubation of mouse intestine for 6 h with Nissle 1917 resulted in a significant (P ≤ 0.01) upregulation in MCP-1 mRNA expression, 35-fold compared to that of the uninfected control (Fig. 6). Incubation with EcNK5− also induced MCP-1 expression, but the increase was significantly smaller (sevenfold; P ≤ 0.01) than that seen with Nissle 1917, confirming the findings for Caco-2 cells. The coincubation of 100 μg/ml K5 polysaccharide together with EcNK5− significantly increased (P ≤ 0.01) MCP-1 expression compared to that of EcNK5− alone (Fig. 6). These data confirm and extend the tissue culture data. They demonstrate that the ability of Nissle 1917 to induce MCP-1 and the role of the K5 capsule in this process was not confined to tissue culture cells. However, because of the possibility of tissue breakdown during the time course of the experiment, one cannot draw any conclusions about the site of interaction between the Nissle 1917 and epithelial cells in this experiment.
FIG. 6.
Ex vivo induction of MCP-1 gene expression in BALB/c mouse small intestine by Nissle 1917 and EcNK5−. After the removal of the mucus layer, five tissue pieces from small intestine were cocultured with either Nissle 1917 (EcN), EcNK5−, or EcNK5− supplemented with purified K5 polysaccharide (CPS; 100 μg·ml−1) for 6 h. The results are expressed as the change (n-fold) in mRNA level from that of the noninfected control. The gene coding for GAPDH was used as the housekeeping gene, and MCP-1 expression was normalized with respect to its level. Relative mRNA amounts were normalized with respect to expression levels. The figure is representative of two independent experiments, each done in quadruple. An asterisk indicates significant difference in chemokine expression compared to that caused by noncapsulated mutant EcNK5− (P ≤ 0.01).
Proinflammatory responses of polarized Caco-2 cells challenged either apically or basolaterally with Nissle 1917 or EcNK5−.
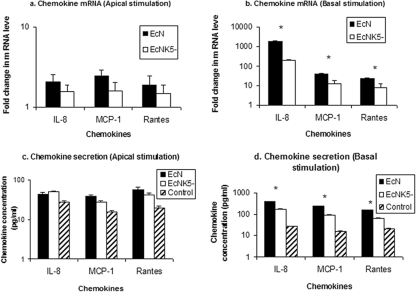
To determine if the site of interaction between Nissle 1917 and epithelial cells is important in mediating the epithelial cell response, Caco-2 monolayers grown on Transwell supports were incubated with Nissle 1917 or EcNK5− either on the apical or the basolateral surface. After 6 h, the mRNA of IL-8, MCP-1, and RANTES were quantified, and levels of each chemokine in the growth medium were determined. Compared to that of noninfected control monolayers, apical stimulation with Nissle 1917 resulted in very modest increases in chemokine mRNA expression, ranging from 1.5- to 2.4-fold, with similar changes (1.6 to 2.9-fold) in the levels of secreted chemokine (Fig. 7A and C). Apical EcNK5− produced a similar effect with no significant difference in the induction of chemokine expression compared to that of Nissle 1917, indicating that the presence of a K5 capsule was not important in stimulating Caco-2 cells on the apical surface. In contrast, Nissle 1917 exposure on the basolateral surface resulted in large increases in both mRNA and secreted protein for all three chemokines. For example, there was a 15-fold increase in secreted IL-8 and a >1,000-fold increase in IL-8 mRNA (Fig. 7B and D). Likewise, there also were significant increases in both the mRNA and chemokine levels for MCP-1 and RANTES when Nissle 1917 was applied to the basolateral surface (Fig. 7B and D). Basolateral exposure to EcNK5− also induced chemokine expression, but it did so to a significantly (P ≤ 0.01) lesser extent than that seen with Nissle 1917 (Fig. 7B and D). These data clearly demonstrate that Nissle 1917 activates chemokine induction predominantly by interacting with the basolateral surface of Caco-2 cells, and that this effect is dependent partly on the presence of a K5 capsule.
FIG. 7.
Induction of chemokine expression by apical or basal stimulation of polarized Caco-2 cells using Nissle 1917 and EcNK5−. Polarized Caco-2 cells were challenged with Nissle 1917 (EcN) or EcNK5− either from apical or basal sides. After 6 h of incubation, IL-8, MCP-1, and RANTES expression were measured by real-time quantitative PCR on RNA extracted from the apically (a) and basally (b) stimulated cells. The culture media of apically (c) and basally (d) stimulated cells were collected, and the levels of secreted chemokine were determined. Relative chemokine messenger mRNA levels were expressed as the change (n-fold) from the corresponding levels in the uninfected Caco-2 cells, while the secreted chemokine level is expressed as picograms per milliliter. Data are means ± standard deviations of two independent experiments, each done in duplicate. An asterisk indicates significant difference in chemokine expression or secretion in the case of Nissle 1917 and EcNK5−.
DISCUSSION
The data presented here show that a K5 capsule is crucially important in mediating the immunomodulatory effects of the probiotic Nissle 1917 on gut epithelial cells. Both wild-type Nissle 1917 and the K5 capsule-minus mutant, EcNK5−, were able to stimulate Caco-2 cells to express a broad array of proinflammatory chemokines, including MCP-1, RANTES, MIP-2α, MIP-2β, IL-8, and IP-10. However, the lack of a K5 capsule dramatically reduced the level of chemokine induction following attachment, indicating a role for the capsule in mediating interactions between Nissle 1917 and Caco-2 cells. The induction of MCP-1 together with MIP-2α and MIP-2β by Nissle 1917 is consistent with previous reports (49). The expression of proinflammatory chemokines by a probiotic organism at first sight appears curious. However, IL-8, MCP-1, and RANTES are all chemoattractant for cells of the innate immune system such as neutrophils and monocytes (3) and are, as such, an important signaling system for the initiation of an inflammatory response when the host epithelial surface lining is invaded by microbial pathogens. In the case of MCP-1, which also is induced when macrophages are infected with the probiotic strain Lactobacillus rhamnosus GG (51), it has been proposed that release serves to generate a strong T-cell response, thereby protecting the host from bacterial infection (51). In the case of IL-8 and RANTES, it is possible that the exposure of the host epithelia to Nissle 1917 triggers a limited local response that offers protection against the invasion of pathogenic bacteria. Indeed, it is known that Nissle 1917 is capable of inhibiting the invasion of a number of intracellular pathogens into epithelial cells (2). It is clear from the literature that there are conflicting data on the effects of probiotic bacteria on the secretion of immunomodulatory factors by gut epithelial cells (5, 12, 34), and that this probably reflects differences in the target cells, the types of probiotic bacteria, and the experimental protocol used in each case.
The impaired immunomodulatory effect of the EcNK5− strain also was apparent in ex vivo mouse small intestine. As with Caco-2 cells, both Nissle 1917 and EcNK5− induced the expression of MCP-1 in the mouse small intestine, but induction was more pronounced with Nissle 1917 than with EcNK5−. This confirms that the effects observed here are not limited to transformed cells in tissue culture, and that the effect of the K5 capsule in maximizing the induction of chemokines following the attachment of Nissle 1917 can be seen in epithelial cells derived from both human and mouse intestine.
The mechanism by which Nissle 1917 stimulates epithelial immune responses in vivo was studied in Caco-2 monolayers grown on Transwell supports that mimic the polarized epithelium. The apical application of Nissle 1917 to Caco-2 cells simulates the normal environment in the intact gut in which bacteria can access the luminal, but not the basal, surface of the gut epithelium. Under these conditions, Nissle 1917 stimulated only a modest activation of IL-8 secretion. In contrast, the application of Nissle 1917 directly to the basolateral surface, which would be expected to occur in vivo in situations where the epithelial barrier had been damaged, stimulated a dramatic increase in IL-8 gene and protein expression (Fig. 7). A similar pattern was observed for RANTES and MCP-1. In line with the data from nonpolarized Caco-2 cells, the ability of EcNK5− to stimulate chemokine secretion after apical or basolateral exposure was limited. These data suggest that Nissle 1917 is more effective in stimulating mucosal immunity in situations where the epithelial barrier has been compromised (e.g., as a result of disease) and the bacteria can gain access to the basolateral surface, and that the expression of a K5 capsule is important in potentiating these responses.
The mechanism by which Nissle 1917 stimulates chemokine secretion in Caco-2 is unclear, although the activation of Toll-like receptors (TLRs) that are involved in the innate response and the recognition of microbial pathogens (4, 16, 48) are most likely. The LPS (TLR4) and flagellin (TLR5) receptors are specific for gram-negative pathogens and are localized primarily to the basolateral surfaces in well-differentiated polarized cells (21, 29). As such, Nissle 1917 will have relatively little access to these receptors during health, when the epithelial barrier is intact. Conversely, when the epithelium is disrupted, Nissle 1917 may cross the epithelial barrier and activate basolateral TLR. It is known that flagellin produced by Nissle 1917 is important in stimulating the secretion of the antimicrobial peptide Hbd-2 by Caco-2 cells (44). Also, Nissle 1917 has been reported to ameliorate experimental colitis by the activation of TLR4 and TLR2 signaling pathways (23). It is likely, therefore, that the Nissle 1917 induction of chemokines following the basolateral stimulation of Caco-2 cells is mediated by the activation of similar processes. It has been shown that TLR signals by commensal bacteria are needed by the host to maintain defense against pathogenic bacteria. Without these signals, susceptibility to antibiotic-resistant strains of bacteria develops (9). Specifically, these TLR signals enhance the production of antimicrobial peptides such as Reg III and Hbd-2, both of which are produced following Nissle 1917 treatment (52).
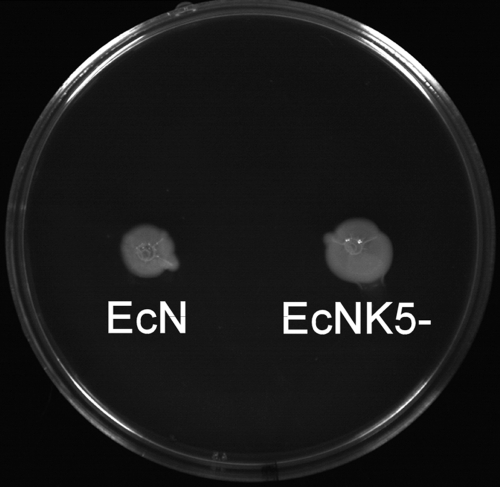
The observation that strain EcNK5− had a reduced ability to induce chemokines shows, for the first time, that a polysaccharide capsule of E. coli has an important role in mediating the host response following attachment to gut epithelial cells. Since the complementation and restoration of K5 capsule expression leads to an increase in chemokine induction, it is clear that the loss of the K5 capsule is responsible for the observed reduction in chemokine induction with strain EcNK5−. Similarly, the failure of purified E. coli K1 polysaccharide to enhance chemokine induction when coincubated with EcNK5− indicates that the effect on amplifying chemokine expression is K5 polysaccharide specific and not merely due to the presence of a negatively charged E. coli capsular polysaccharide molecule. The observations that there were no detectable differences between the protein profiles of the outer membranes of strains Nissle 1917 and EcNK5− (data not shown) and that both strains had the same pattern of adhesion to Caco-2 cells supports the notion that the effects on chemokine induction are a consequence of a lack of K5 capsule. The observation that both Nissle 1917 and EcNK5− were equally motile (Fig. 8) would indicate that the loss of capsule is not affecting flagellin expression. This is important, considering the known role of flagellin in activating TLR5 (21). In addition, there was no detectable change in the migration of purified LPS on SDS-PAGE when purified from either Nissle 1917 or EcNK5− (Fig. 9), indicating no change in LPS structure as a consequence of the loss of the K5 capsule. Overall, these results suggest that the phenotype of the EcNK5− strain is a consequence of a loss of capsule. There was no evidence that the K5 capsule per se has immunomodulatory effects, since the addition of purified K5 polysaccharide to Caco-2 cells in the absence of EcNK5− produced no significant increase in chemokine secretion. This is in contrast to the effects described when using the purified capsular polysaccharide A of the human gut symbiont Bacteroides fragilis. In this case, polysaccharide A has been shown to have anti-inflammatory properties and prevent inflammatory disease in mice infected with Helicobacter hepaticus (36). In the case of the K5 polysaccharide, it appears that it is acting to mediate interactions between Nissle 1917 and gut epithelial cells to elicit the maximum and appropriate chemokine response. A number of functions have been assigned for polysaccharide capsules in the virulence of extraintestinal pathogenic E. coli and uropathogenic E. coli, including resistance to phagocytosis and complement-mediated killing (42) and survival during the transcytosis of endothelial cells in crossing the blood-brain barrier (30). The data here describing a role for the K5 capsule in mediating chemokine secretion following the attachment of Nissle 1917 to epithelial cells is the first example of an E. coli capsule acting in such a fashion to affect the immunomodulatory properties of a nonpathogenic E. coli strain recognized primarily for its probiotic properties.
FIG. 8.
Motility of strains Nissle 1917 (EcN) and EcNK5−. Equal numbers (5 × 108) of both strains were inoculated into an L agar plate containing 0.5% (wt/vol) agar and incubated overnight at 37°C. There was no detectable loss of motility in strain EcNK5− as a consequence of the loss of capsule.
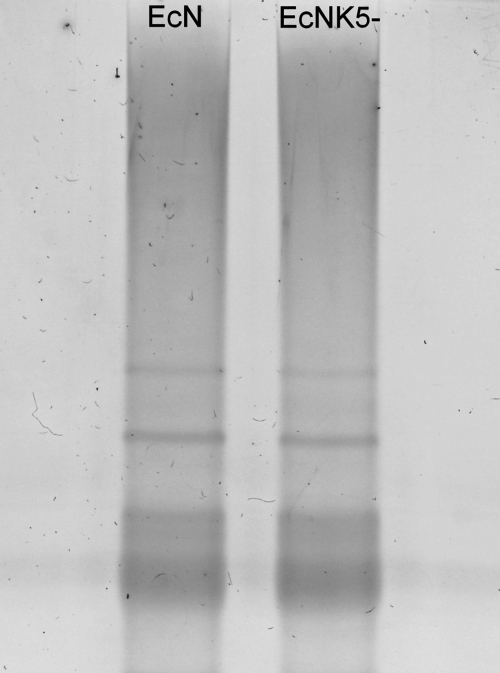
FIG. 9.
Silver-stained SDS-PAGE of LPS preparations from strains Nissle 1917 (EcN) and EcNK5−. LPS was purified from strains Nissle 1917 and EcNK5− and analyzed by SDS-PAGE, followed by silver staining. The LPS profiles of strains Nissle 1917 and EcNK5− are identical, indicating that the kfiC mutation in strain EcNK5− has no detectable effect on the structure of the LPS.
With other encapsulated bacterial pathogens, capsular polysaccharides have been demonstrated to have immunomodulatory effects. In the case of Staphylococcus aureus, capsular polysaccharide types 5 and 8 were able to bind to epithelial cells and induce IL-8 expression, as well as inducing IL-8, IL-6, IL-1β, and TNF-α from monocytes (43). It was proposed that these capsular polysaccharides were acting as adhesins to promote attachment to epithelial cells while at the same expressing immunomodulatory effects (45). Likewise, the serotype K1 polysaccharide from the oral pathogen Porphyromonas gingivalis elicits MIP-2, RANTES, and MCP-1 secretion from murine macrophages (17). In the case of the type 2 capsule of Streptococcus suis, the presence of a capsule disrupts interactions between cell wall components and TLRs, probably through steric effects, thereby reducing cytokine responses to adherent S. suis (24). However, a capsule was required for maximal MCP-1 production by monocytes through a TLR2/MyD88-independent pathway, and purified type 2 polysaccharide was able to stimulate MCP-1 release (24). The purified polysaccharide also induced the late activation of CD14 and TLR2, but this probably was a consequence of MCP-1 activation (24). The type II polysaccharide of Streptococcus pneumoniae is able to stimulate cytokine release from macrophages in part through TLR2-CD14 pathways (5). In contrast, in Salmonella enterica serotype Typhi, the Vi capsular antigen reduces TLR-dependent IL-8 production from intestinal mucosa (40) and IL-17 secretion (40). This indicates that in this case, the Vi antigen is acting to reduce intestinal inflammation, possibly playing a role in reducing the influx of neutrophils to the site of infection (40, 41). Therefore, it is clear that capsular polysaccharides have a number of immunomodulatory effects, and these effects can be dependent on whether the polysaccharide is cell associated as a capsule or free as a polysaccharide. The fact that capsular polysaccharides are shed from the surface of pathogens during infections (42) means that the immunomodulatory effects of capsular polysaccharides may not be localized merely to the immediate site of infection.
In summary, this paper demonstrates that the K5 capsule plays a vital role in mediating the immunomodulatory effects of Nissle 1917. While Nissle 1917 is a probiotic strain, E. coli K5 strains usually are associated with extraintestinal infections, and it will be interesting to determine what role the K5 capsule plays in modulating local inflammatory responses during the course of an infection. Currently, experiments are in progress to understand the mechanism by which the K5 polysaccharide interacts with epithelial cells.
Acknowledgments
The work in the laboratory of I.S.R. is supported by the BBSRC and MRC of the United Kingdom. The laboratory of R.G. is supported by the Wellcome Trust. M.H. gratefully acknowledges the award of a postdoctoral scholarship from the Ministry of Higher Education Egypt.
We thank U. Dobrindt for the generous gift of E. coli Nissle 1917.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 20 April 2009.
REFERENCES
- 1.Albiger, B., L. Johansson, and A. B. Jonsson. 2003. Lipooligosaccharide-deficient Neisseria meningitidis shows altered pilus-associated characteristics. Infect. Immun. 71155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altenhoefer, A., S. Oswald, U. Sonnenborn, C. Enders, J. Schulze, J. Hacker, and T. A. Oelschlaeger. 2004. The probiotic Escherichia coli strain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol. Med. Microbiol. 40223-229. [DOI] [PubMed] [Google Scholar]
- 3.Baggiolini, M., P. Loetscher, and B. Moser. 1995. Interleukin-8 and the chemokine family. Int. J. Immunopharmacol. 17103-108. [DOI] [PubMed] [Google Scholar]
- 4.Beutler, B., and E. M. Moresco. 2008. The forward genetic dissection of afferent innate immunity. Curr. Top. Microbiol. Immunol. 3213-26. [DOI] [PubMed] [Google Scholar]
- 5.Boirivant, M., and W. Strober. 2007. The mechanism of action of probiotics. Curr. Opin. Gastroenterol. 23679-692. [DOI] [PubMed] [Google Scholar]
- 6.Bootsma, H. J., M. Egmont-Petersen, and P. W. Hermans. 2007. Analysis of the in vitro transcriptional response of human pharyngeal epithelial cells to adherent Streptococcus pneumoniae: evidence for a distinct response to encapsulated strains. Infect. Immun. 755489-5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boudeau, J., A. L. Glasser, S. Julien, J. F. Colombel, and A. Darfeuille-Michaud. 2003. Inhibitory effect of probiotic Escherichia coli strain Nissle 1917 on adhesion to and invasion of intestinal epithelial cells by adherent-invasive E. coli strains isolated from patients with Crohn's disease. Aliment. Pharmacol. Ther. 1845-56. [DOI] [PubMed] [Google Scholar]
- 8.Boulnois, G. J., I. S. Roberts, R. Hodge, K. R. Hardy, K. Jann, and K. N. Timmis. 1987. Analysis of the K1 capsule biosynthesis genes of Escherichia coli: definition of three functional gene blocks for capsule production. Mol. Gen. Genet. 208242-246. [DOI] [PubMed] [Google Scholar]
- 9.Brandl, K., G. Plitas, C. N. Mihu, C., Ubeda, T. Jia, M. Fleisher, B. Schnabl, R. P. DeMatteo, and E. G. Pamer. 2008. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 455804-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, R., L. Lowe, J. D. Wilson, E. Crowther, K. Tzeggai, J. E. Bishop, and R. Varro. 1999. Simultaneous quantification of six human cytokines in a single sample using microparticle-based flow cytometric technology. Clin. Chem. 451693-1694. [PubMed] [Google Scholar]
- 11.Clark, E. C., C. Hoare, J. Tanianis-Hughes, C. J. Watson, G. L. Carlson, and G. Warhurst. 2005. Interferon-gamma induces translocation of commensal. E. coli across gut epithelial cells via a lipid raft-mediated process. Gastroenterology 1281258-1267. [DOI] [PubMed] [Google Scholar]
- 12.Clarke, B. R., F. Esumeh, and I. S. Roberts. 2000. Cloning, expression, and purification of the K5 capsular polysaccharide lyase (KflA) from coliphage K5A: evidence for two distinct K5 lyase enzymes. J. Bacteriol. 1823761-3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross, M. L., A. Ganner, D. Teilab, and L. M. Fray. 2004. Patterns of cytokine induction by gram-positive and gram-negative probiotic bacteria. FEMS Immunol. Med. Microbiol. 42173-180. [DOI] [PubMed] [Google Scholar]
- 14.Cukrowska, B., R. Lodinova-Zadnikova, C. Enders, U. Sonnenborn, J. Schulze, and H. Tlaskalova-Hogenova. 2002. Specific proliferative and antibodyresponses of premature infants to intestinal colonization with non-pathogenic probiotic E. coli strain Nissle 1917. Scand. J. Immunol. 55204-209. [DOI] [PubMed] [Google Scholar]
- 15.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de la Barrera, S., M. Aleman, and C. Sasiain-Medel. 2006. Toll-like receptors in human infectious diseases. Curr. Pharm. Des. 124173-4184. [DOI] [PubMed] [Google Scholar]
- 17.d'Empaire, G., M. T. Baer, and F. C. Gibson III. 2006. The K1 serotype capsular polysaccharide of Porphyromonas gingivalis elicits chemokine production from murine macrophages that facilitates cell migration. Infect. Immun. 746236-6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncker, S. C., A. Lorentz, B. Schroeder, G. Breves, and S. C. Bischoff. 2006. Effect of orally administered probiotic E. coli strain Nissle 1917 on intestinal mucosal immune cells of healthy young pigs. Vet. Immunol. Immunopathol. 111239-250. [DOI] [PubMed] [Google Scholar]
- 19.Fogh, J., W. C. Wright, and J. D. Loveless. 1977. Absence of HeLa cell contamination in 169 cell lines derived from human tumors. J. Natl. Cancer Inst. 58209-214. [DOI] [PubMed] [Google Scholar]
- 20.Food and Agricultural Organization of the United Nations and World Health Organization. 2002. Guidelines for the evaluation of probiotics in food. Working group report. Food and Agricultural Organization of the United Nations and World Health Organization, Geneva, Switzerland.
- 21.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 1671882-1885. [DOI] [PubMed] [Google Scholar]
- 22.Gibson, F. C., III, A. O. Tzianabos, and A. B. Onderdonk. 1996. The capsular polysaccharide complex of Bacteroides fragilis induces cytokine production from human and murine phagocytic cells. Infect. Immun. 641065-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grabig, A., D. Paclik, C. Guzy, A. Dankof, D. C. Baumgart, J. Erckenbrecht, B. Raupach, U. Sonnenborn, J. Eckert, R. R. Schumann, B. Wiedenmann, A. U. Dignass, and A. Sturm. 2006. Escherichia coli strain Nissle 1917 ameliorates experimental colitis via Toll-like receptor 2- and Toll-like receptor 4-dependent pathways. Infect. Immun. 744075-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graveline, R., M. Segura, D. Radzioch, and M. Gottschalk. 2007. TLR2-dependent recognition of Streptococcus suis is modulated by the presence of capsular polysaccharide which modifies macrophage responsiveness. Int. Immunol. 19375-389. [DOI] [PubMed] [Google Scholar]
- 25.Grozdanov, L., C. Raasch, J. Schulze, U. Sonnenborn, G. Gottschalk, H. Hacker, and U. Dobrindt. 2004. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J. Bacteriol. 1865432-5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzy, C., D. Paclik, A. Shirbel, U. Sonnenborn, B. Wiedenmann, and A. Sturm. 2008. The probiotic Escherichia coli strain Nissle 1917 induces T cell apoptosis via caspase and FasL dependent pathways. Internat. Immunol. 20829-840. [DOI] [PubMed] [Google Scholar]
- 27.Henker J., M. Laass, B. M. Blokhin, Y. K. Bolbot, V. G. Maydannik, M. Elze, C. Wolff, and J. Schulze. 2007. The probiotic Escherichia coli strain Nissle 1917 (EcN) stops acute diarrhoea in infants and toddlers. Eur. J. Pediatr. 166311-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamada, N., K. Maeda, N. Inoue, T. Hisamatsu, S. Okamoto, K. Y. Honf, T. Yamada, N. Watanabe, K. Tsuchimoto, H. Ogata, and T. Hibi. 2008. Nonpathogenic Escherichia coli strain Nissle 1917 inhibits signal transduction in intestinal epithelial cells. Infect. Immun. 76214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly, D., S. Conway, and R. Aminov. 2005. Commensal gut bacteria: mechanisms of immune modulation. Trends Immunol. 26326-333. [DOI] [PubMed] [Google Scholar]
- 30.Kim, K. J., S. J. Elliot, F. Di Cello, M. F. Stins, and K. S. Kim. 2003. The K1 capsule modulates trafficking of E. coli-containing vacuoles and enhances intracellular bacterial survival in human brain microvascular cells. Cell Microbiol. 5245-252. [DOI] [PubMed] [Google Scholar]
- 31.Kimura, A., and E. J. Hansen. 1986. Antigenic and phenotypic variations of Haemophilus influenzae type b lipopolysaccharide and their relationship to virulence. Infect. Immun. 5169-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kruis, W., E. Schutz, P. Fric, B. Fixa, G. Judmaier, and M. Stolte. 1997. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 11853-858. [DOI] [PubMed] [Google Scholar]
- 33.Kruis, W., P. Fric, J. Pokrotnieks, M. Lukas, B. Fixa, M. Kascak, M. A. Kamm, J. Weismueller, C. Beglinger, M. Stolte, C. Wolff, and J. Schulze. 2004. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 531617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lan, J. G., S. M. Cruickshank, J. C. Singh, M. Farrar, J. P. Lodge, P. J. Felsburg, and S. R. Carding. 2005. Different cytokine response of primary colonic epithelial cells to commensal bacteria. World J. Gastroenterol. 113375-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lodinová-Zádniková, R., and U. Sonnenborn. 1997. Effect of preventive administration of a nonpathogenic Escherichia coli strain on the colonization of the intestine with microbial pathogens in newborn infants. Biol. Neonate 71224-232. [DOI] [PubMed] [Google Scholar]
- 36.Mazmanian, S. K., J. L. Round, and D. Kasper. 2008. A microbial symbiosis factor prevents inflammatory disease. Nature 453620-625. [DOI] [PubMed] [Google Scholar]
- 37.Mollenbrink, M., and E. Bruckschen. 1994. Treatment of chronic constipation with physiologic Escherichia coli bacteria. Results of a clinical study of the effectiveness and tolerance of microbiological therapy with the. E. coli Nissle 1917 strain (Mutaflor). Med. Klin. 89587-593. [PubMed] [Google Scholar]
- 38.Nissle, A. 1918. Die antagonistische Behandlung chronischer Darmstörungen mit Colibakterien. Med. Klin. 229-30. [Google Scholar]
- 39.Patzer, S. I., M. R. Baquero, D. Bravo, F. Moreno, and K. Hantke. 2003. The colicin G, H and X determinants encode microcins M and H47, which might utilize the catecholate siderophore receptors. Microbiology 1492557-2570. [DOI] [PubMed] [Google Scholar]
- 40.Raffatellu, M., D. Chessa, R. P. Wilson, R. Dusold, S. Rubino, and A. J. Baumler. 2005. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor dependent interleukin-8 expression in the intestinal mucosa. Infect. Immun. 733367-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raffatellu, M., R. L. Santos, D. Chessa, R. P. Wilson, S. E. Winter, C. A. Rossetti, S. D. Lawhon, H. Chu, T. Lau, C. L. Bevins, L. G. Bevins, and A. J. Baumler. 2007. The capsule encoding viaB locus reduces interleukin-17 expression and mucosal innate responses in the bovine intestinal mucosa during infection with Salmonella enterica serotype Typhi. Infect. Immun. 754342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts, I. S. 1996. Biochemistry and genetics of capsular polysaccharide production in bacteria. Annu. Rev. Microbiol. 50285-315. [DOI] [PubMed] [Google Scholar]
- 43.Salzman, N. H., D. Ghosh, K. M. Huttner, Y. Paterson, and C. L. Bevins. 2003. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422522-526. [DOI] [PubMed] [Google Scholar]
- 44.Schlee, M., J. Wehkamp, A. Altenhoefer, T. A. Oelschlaeger, E. F. Stange, and K. Fellermann. 2007. Induction of human B-defensin 2 by probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect. Immun. 752399-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soell, M., M. Diab, G. Haan-Archipoff, A. Beretz, C. Herbelin, B. Poutrel, and J. P. Klein. 1995. Capsular polysaccharide types 5 and 8 of Staphylococcus aureus bind specifically to human epithelial (KB) cells, endothelial cells, and monocytes and induce release of cytokines. Infect. Immun. 631380-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevens, M. P., B. R. Clarke, and I. S. Roberts. 1997. Regulation of the Escherichia coli K5 capsule gene cluster by transcription antitermination. Mol. Microbiol. 241001-1012. [DOI] [PubMed] [Google Scholar]
- 47.Sturm, A., K. Rilling, D. C. Baumgart, K. Gargas, T. Abou-Ghazale, B. Raupach, J. Eckert, R. R. Schumann, C. Enders, U. Sonnenborn, B. Wiedenmann, and A. U. Dignass. 2005. Escherichia coli Nissle 1917 distinctively modulates T-cell cycling and expansion via Toll-like receptor 2 signaling. Infect. Immun. 731452-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uematsu, S., and S. Akira. 2006. Toll-like receptors and innate immunity. J. Mol. Med. 84712-725. [DOI] [PubMed] [Google Scholar]
- 49.Ukena, S. N., A. M. Westendorf, W. Hansen, M. Rohde, R. Geffers, S. Coldewey, S. Suerbaum, J. Buer, and F. Gunzer. 2005. The host response to the probiotic Escherichia coli strain Nissle 1917: specific up-regulation of the proinflammatory chemokine MCP-1. BMC Med. Genet. 643-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Um, S. H., E. W. Son, B. O. Kim, E. Y. Moon, D. K. Rhee, and S. Pyo. 2000. Activation of murine peritoneal macrophages by Streptococcus pneumoniae type II capsular polysaccharide: involvement of CD14-dependent pathway. Scand. J. Immunol. 5239-45. [DOI] [PubMed] [Google Scholar]
- 51.Veckman, V., M. Miettinen, S. Matikainen, R. Lande, E. Giacomini, E. M. Coccia, and I. Julkunen. 2003. Lactobacilli and streptococci induce inflammatory chemokine production in human macrophages that stimulates Th1 cell chemotaxis. J. Leukoc. Biol. 74395-402. [DOI] [PubMed] [Google Scholar]
- 52.Wehkamp, B. J., J. Harder, K. Wehkamp, B. W. von Meissner, M Schlee, C Enders, U. Sonnenborn, S. Nuding, S. Bengmark, K. Fellermann, J. M. Schroder, and E. F. Stang. 2004. NFB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic. Infect. Immun. 725750-5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westendorf, A. M., F. Gunzer, S. Deppenmeier, J. K. Hunger, M. A. Schmidt, and J. Buer. 2005. Intestinal immunity of E. coli Nissle 1917: a safe carrier for therapeutic molecules. FEMS Immunol. Med. Microbiol. 43373-384. [DOI] [PubMed] [Google Scholar]
- 54.Zyrek, A. A., C. Cichon, S. Helms, C. Enders, U. Sonnenborn, and M. A. Schmidt. 2007. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCz redistribution resulting in tight junction and epithelial barrier repair. Cell. Microbiol. 9804-816. [DOI] [PubMed] [Google Scholar]