Abstract
The process of host cell invasion by Trypanosoma cruzi depends on parasite energy. What source of energy is used for that event is not known. To address this and other questions related to T. cruzi energy requirements and cell invasion, we analyzed metacyclic trypomastigote forms of the phylogenetically distant CL and G strains. For both strains, the nutritional stress experienced by cells starved for 24, 36, or 48 h in phosphate-buffered saline reduced the ATP content and the ability of the parasite to invade HeLa cells proportionally to the starvation time. Inhibition of ATP production by treating parasites with rotenone plus antimycin A also diminished the infectivity. Nutrient depletion did not alter the expression of gp82, the surface molecule that mediates CL strain internalization, but increased the expression of gp90, the negative regulator of cell invasion, in the G strain. When l-proline was given to metacyclic forms starved for 36 h, the ATP levels were restored to those of nonstarved controls for both strains. Glucose had no such effect, although this carbohydrate and l-proline were transported in similar fashions. Recovery of infectivity promoted by l-proline treatment of starved parasites was restricted to the CL strain. The profile of restoration of ATP content and gp82-mediated invasion capacity by l-proline treatment of starved Y-strain parasites was similar to that of the CL strain, whereas the Dm28 and Dm30 strains, whose infectivity is downregulated by gp90, behaved like the G strain. l-Proline was also found to increase the ability of the CL strain to traverse a gastric mucin layer, a property important for the establishment of T. cruzi infection by the oral route. Efficient translocation of parasites through gastric mucin toward the target epithelial cells in the stomach mucosa is an essential requirement for subsequent cell invasion. By relying on these closely associated ATP-driven processes, the metacyclic trypomastigotes effectively accomplish their internalization.
Host cell invasion by Trypanosoma cruzi, which is critical for the establishment of infection in mammalian hosts, is a multistep process involving various parasite and host cell molecules that, in a concerted series of events, leads to intracellular Ca2+ mobilization in both types of cells (5, 12, 32). The first step of this process, namely, T. cruzi attachment to target cells, requires parasite energy (23). The main source of energy for this event is unknown. Also unknown is whether there are differences in energy requirements among different T. cruzi strains that use distinct mechanisms to enter target cells.
Epimastigotes, the proliferative and noninfective developmental forms of T. cruzi, use mainly glucose, l-proline, and l-glutamic acid as carbon sources and to obtain energy through respiration (27). In these parasite forms, the active transport of glucose, l-proline, or l-glutamic acid has been previously demonstrated (2, 24, 25, 29). With regard to l-proline, which is one of the prominent constituents of the hemolymph and tissue fluids of hematophagous insect vectors (1, 6), its uptake by active proline transport systems has been found in epimastigotes (24) and in tissue culture trypomastigotes that correspond to the bloodstream parasites and intracellular epimastigotes and amastigotes (30). Concerning the metacyclic trypomastigotes, which are found in the terminal portions of the digestive tract of triatomine insects and constitute the parasite forms responsible for the initial interaction with host cells, there is no information regarding whether they transport and utilize as an energy source the compounds referred to above.
The replicative epimastigotes and the infective metacyclic trypomastigotes may differentially use the resources available in the extracellular milieu for the production of energy required for diverse biological processes. Epimastigotes were shown to transform into metacyclic trypomastigotes within 48 h when l-proline or l-glutamate was added to the medium (15). Another study, using a different T. cruzi strain, showed a high rate of differentiation of epimastigotes to metacyclic forms in a medium composed of artificial triatomine urine supplemented with proline (7). l-Proline was also shown to induce transformation of the intracellular epimastigote-like stage into the trypomastigote stage (30), and it seems to be a main carbon source in the intracellular stages, since its development throughout the intracellular life cycle stages is dependent on the presence of proline and since glucose is not taken up by amastigotes (A. M. Silber, R. R. Tonelli, G. C. Lopes, N. L. Cunha e Silva, A. C. T. Torrecilhas, R. I. Schumacher, W. Colli, and M. J. M. Alves, submitted for publication). The most prominent function of metacyclic forms is to invade host cells, and different T. cruzi strains may use distinct strategies to accomplish that function, as illustrated by studies conducted with the CL and G strains, which belong to two highly divergent genetic subgroups (4). Metacyclic forms of the CL strain engage the stage-specific surface gp82 glycoprotein to bind to host cells and induce actin cytoskeleton disruption and parasite internalization (9). On the other hand, G strain metacyclic forms preferentially use the mucin-like molecules gp35 and gp50 to induce the recruitment of target cell actin to the site of parasite entry (13). In addition, G strain infectivity is influenced by gp90, a metacyclic-stage-specific molecule that downregulates host cell invasion (17). We addressed here issues related to energy requirements in T. cruzi infection by analyzing CL and G strains and, based on the findings with these strains, by extending the study to include other CL-like or G-like parasite strains.
MATERIALS AND METHODS
Parasites, mammalian cell culture, and invasion assays.
The following T. cruzi strains were used: CL, which was isolated from the insect Triatoma infestans, in the southern state of Rio Grande do Sul, Brazil (3); G, which was isolated from an opossum in the Brazilian Amazon (31); Y, which was derived from a patient sample (26); and Dm28 and Dm30, which were isolated from Didelphis marsupialis in Venezuela by Victor Contreras. The parasites were maintained cyclically in mice and in liver infusion tryptose medium containing 5% fetal calf serum. For differentiation of epimastigotes into metacyclic trypomastigotes, the parasites were grown for one passage in Grace's medium (Invitrogen) or TC100 medium (Cultilab, Brazil). Metacyclic forms from cultures at the stationary-growth phase were purified by passage through a DEAE-cellulose column, as described previously (28). HeLa cells, the human carcinoma-derived epithelial cells, were grown at 37°C in Dulbecco's minimum essential medium supplemented with 10% fetal calf serum, streptomycin (100 μg/ml), and penicillin (100 U/ml) in a humidified 5% CO2 atmosphere. Cell invasion assays (33) were performed by growing 1.5 × 105 HeLa cells in each well of 24-well plates containing 13-mm-diameter round glass coverslips. After 16 to 24 h of culture growth, the cells were washed with phosphate-buffered saline++ (PBS++) (PBS containing [per liter] 140 mg of CaCl2, 400 mg of KCl, 100 mg of MgCl2·6H2O, 100 mg of MgSO4·7H2O, 350 mg of NaHCO3). Parasites were then added to HeLa cells, at a multiplicity of infection of 10:1 or 20:1, depending on the T. cruzi strain, and the incubation proceeded for 1 h at 37°C in PBS++. The duplicate or triplicate coverslips were fixed in Bouin solution, stained with Giemsa, and sequentially dehydrated in acetone, a graded acetone-xylol series (9:1, 7:3, and 3:7), and xylol.
Cell binding assay.
HeLa cells were seeded onto 24-well plates containing 13-mm-diameter glass coverslips, at 1.5 × 105 cells per well, and grown for 16 h. After washings in PBS, the cells were fixed with 3.5% formaldehyde in PBS for 30 min at room temperature. Following fixation, the cells were immediately washed with PBS, incubated with 50 mM NH4Cl for 30 min, and then washed with PBS. Parasites were added to fixed cells, in PBS++, and the incubation proceeded for 1 h at 37°C. Soon after being washed with PBS, the coverslips were processed for staining with Giemsa.
Determination of T. cruzi ATP content.
To measure the parasite ATP content, a bioluminescent somatic cell assay kit (Sigma) was used. Fifty microliters of PBS was added to 100 μl of cell ATP-releasing reagent, followed by 50 μl of a suspension containing 5 × 106 parasites. Controls contained standard ATP, at various dilutions, instead of parasites. Subsequently, 50 μl of the mix was transferred to each well of white UB 96-well plates (Uniscience) containing ATP assay mix working solution. Light emission levels were measured using a SpectraMaxL luminometer (Molecular Devices) at 570 nm. ATP concentrations were determined by interpolation from a standard curve obtained with diluted ATP solutions.
Transport assays.
The assays were performed according to a protocol described elsewhere (24). Briefly, purified metacyclic forms were washed three times with PBS, and the final suspension, containing 2 × 108 parasites/ml, was distributed in aliquots of 100 μl (2 × 107 cells each). Thereafter, 100 μl of the desired dilution of l-proline, l-glutamate, or glucose, in PBS, was added to the assay tubes and traced with 0.5 μCi of [14C]glucose, l-[3H]proline (at a concentration of either 0.75 mM or 3.0 mM), or 0.1 μCi of l-[3H]glutamic acid (NEN Life Science Products, Boston, MA). Final concentrations for each metabolite were those that were able to saturate each transport system: 0.75 and 3 mM for proline (24), 1 mM for glutamate (25), and 3 mM for glucose (29). Transport was stopped by adding 800 μl of cold 50 mM glucose-, l-proline-, or l-glutamic acid-PBS, followed by two fast washes in PBS. After washings, the pellets were resuspended in scintillation liquid, the incorporated radioactivity was measured with a scintillation counter, and the obtained values were used to calculate the uptake of each substrate.
Parasite migration assay.
Polycarbonate Transwell filters (Costar, Cambridge, MA) (3-μm pores, 6.5-mm diameter) were coated with 50 μl of a preparation containing 10 mg/ml gastric mucin from porcine stomach (Sigma) in water. Nutrient-deprived T. cruzi metacyclic forms, in 600 μl of PBS or PBS containing either 1 mM glucose or 3 mM l-proline, were added to the bottom of 24-well plates (2 × 107 parasites/well) and incubated for 1 h at 37°C. Thereafter, the mucin-coated Transwell filters were placed onto parasite-containing wells, and 100 μl of PBS was added to the filter chamber. At different time points of incubation at 37°C, 10-μl volumes were collected from the filter chamber for determination of parasite numbers, and the volume in this chamber was corrected by adding 10 μl of PBS.
Flow cytometry analysis.
Metacyclic trypomastigotes (1 × 107) were fixed with 4% paraformaldehyde in PBS, washed, and incubated with 50 mM NH4Cl for 30 min. After being washed in PBS, the parasites were incubated for 1 h at room temperature with one of the following monoclonal antibodies (MAb): 3F6, directed to the surface molecule gp82; 10D8, directed to mucin-like gp35 and gp50 glycoproteins; or 1G7, directed to the surface glycoprotein gp90. Thereafter, Alexa 488-conjugated anti-mouse immunoglobulin G was added and the reaction proceeded for 1 h at room temperature. Following three more washes, the parasites were analyzed in a Becton Dickinson FACScan cytometer.
Lysis assay.
For the complement-mediated lysis assay, 10 μl of a suspension of purified metacyclic forms (108/ml) was incubated with 40 μl of ascitic fluid containing the 1G7 MAb. After 10 min of incubation of the mixture at room temperature, 50 μl of normal human serum was added as a source of complement, and the parasites were further incubated at 37°C for 30 min. The numbers of live motile parasites were counted using a Neubauer chamber.
Statistical analysis.
The results were analyzed by using the program GraphPad InStat to determine significance according to Student's t test.
RESULTS
Decreased ATP content in T. cruzi metacyclic trypomastigotes results in reduced host cell invasion.
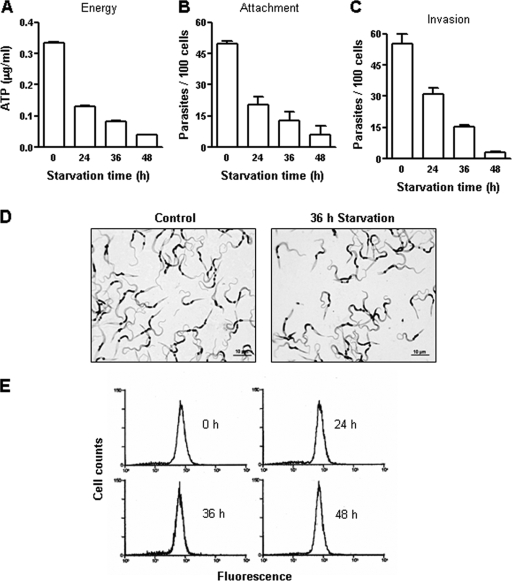
First, experiments were performed to establish the conditions under which the endogenous energy store of the parasite was reduced. Metacyclic forms of CL and G strains were maintained under conditions of nutritional stress in PBS, at 27°C, for various time periods and, along with the nonstarved controls, were then analyzed with regard to the ATP content and the ability to adhere to or to enter epithelial HeLa cells. In cells of the CL strain starved for 24, 36, and 48 h, the ATP levels were reduced by ∼60%, ∼75%, and ∼87.5%, respectively, compared to the control results (Fig. 1A), and the ability of the parasite to bind to and invade HeLa cells decreased accordingly (Fig. 1B and C). In repeated experiments, similar results were obtained. Nutritional depletion reduced the motility of metacyclic forms, while fully preserving their morphology, so that the appearances of control and starved parasites were similar under conditions of phase-contrast microscopy and indistinguishable upon Giemsa staining (Fig. 1D). That starved parasites preserved their integrity was also confirmed by the observation that the results of staining by propidium iodide of starved and control parasites were similar. Furthermore, subsequent experiments showed that parasites starved for 36 h retained their infective potential (see Fig. 3A). As CL strain metacyclic forms engage the surface gp82 glycoprotein for host cell adhesion and invasion (20), we checked whether expression of gp82 diminished during nutritional stress and whether it thus contributed to the decrease in parasite infectivity. No alterations in gp82 expression levels in starved parasites were observed, as ascertained by flow cytometry analysis (Fig. 1E).
FIG. 1.
ATP content and adhesion-invasion capacity of T. cruzi metacyclic forms (CL strain) subjected to nutritional stress. (A, B, and C) Parasites were maintained in PBS for the indicated time periods and were then analyzed with regard to the ATP content (A) and the ability to attach to (B) or to enter (C) HeLa cells. Representative results of one out of three experiments are shown. Values represent the means ± standard deviations of the results of triplicate experiments. The numbers of attached or intracellular parasites represented in panels B and C were counted in 250 cells. (D) Giemsa-stained control and starved parasites. (E) The expression levels of the metacyclic-stage-specific surface gp82 molecule in starved parasites were determined by flow cytometry, using the specific MAb 3F6.
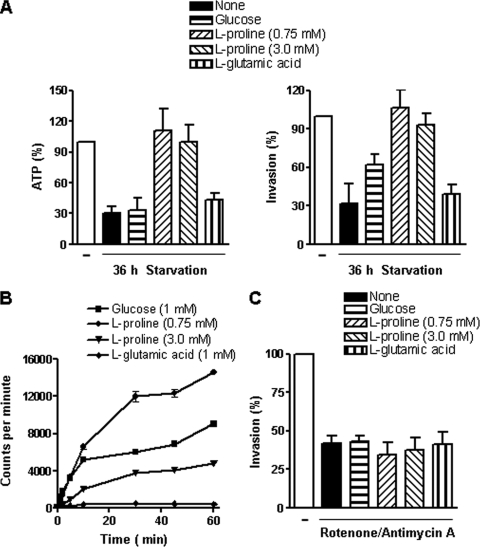
FIG. 3.
Effect of l-proline on ATP production and infectivity of starved T. cruzi metacyclic forms (CL strain). (A) Parasites previously maintained in PBS for 36 h were incubated for 1 h with glucose (1 mM), l-proline (0.75 mM or 3.0 mM), or l-glutamic acid (1 mM). Afterward, the parasite ATP content and the ability to enter HeLa cells were examined. The numbers of intracellular parasites in 250 Giemsa-stained cells were counted. A reference value of 100% was assigned to the controls. Values represent the means ± standard deviations of the results of three independent experiments performed in duplicate. The differences between the results for the 36-h-starved and the l-proline-supplemented parasites were significant (P < 0.005). (B) Transport of glucose, l-proline, or glutamate was assayed by using radioactive compounds as described in Materials and Methods. At different time points after the addition of the radioactive reagent, the uptake was stopped by adding cold glucose, l-proline, or l-glutamic acid in PBS. Values represent the means ± standard deviations of the results of triplicate experiments. (C) Parasites were treated with inhibitors of energy production (rotenone [200 μM] and antimycin A [0.5 μM]) for 30 min, washed in PBS, and then used for cell invasion assays in the absence or presence of 1 mM glucose, 0.75 mM l-proline, or 1 mM l-glutamate. Intracellular parasites were counted in 250 Giemsa-stained cells. Values represent the means ± standard deviations of the results of three independent experiments performed in duplicate.
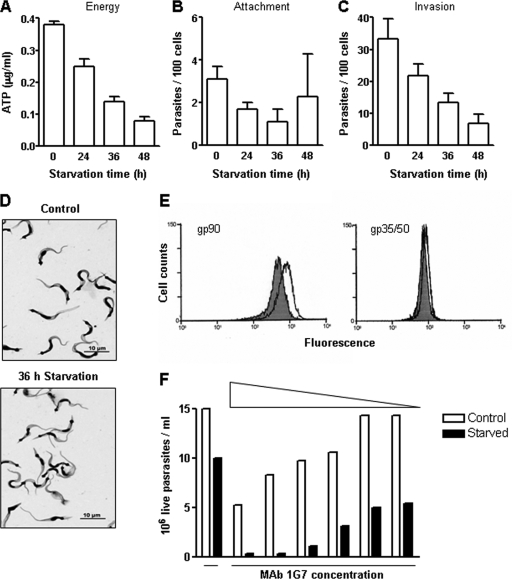
Concerning the G strain, the rates of decrease in the ATP content were on the order of 35%, 65%, and 80% in parasites starved for 24, 36, and 48 h, respectively (Fig. 2A), and the infectivity with respect to HeLa cells diminished concomitantly with the decrease in ATP levels (Fig. 2C). Unlike the results seen with the CL strain (Fig. 1B), G strain binding to HeLa cells was minimal, regardless of the ATP content (Fig. 2B). With the morphology fully preserved under conditions of nutritional stress, the control and starved parasites were visibly similar (Fig. 2D). Given that G strain invasion capacity is influenced by gp90, the surface molecule that acts as a downward modulator of invasion (17), expression of this molecule was examined in parasites starved for 36 h compared to controls. By the use of MAb 1G7 directed to gp90, increased expression of gp90 was detected in starved parasites by flow cytometry analysis and by complement-mediated lysis (Fig. 2E and F). In the latter assay, no parasite lysis was observed in the absence of antibody or in the presence of MAb 5E7 directed to a cryptic epitope on gp90, detectable in fixed but not in live parasites. The result shown in Fig. 2E suggests that the decreased infectivity of the G strain (Fig. 2C) may have been due at least in part to a higher level of gp90 expression. We also checked the expression levels of the surface glycoproteins gp35 and gp50, which promote host cell invasion of G strain metacyclic forms (32, 33), and found that levels were only slightly augmented in starved parasites (Fig. 2E).
FIG. 2.
ATP content and adhesion-invasion capacity of T. cruzi metacyclic forms (G strain) subjected to nutritional stress. (A, B, and C) Parasites were maintained in PBS for the indicated time periods and were then analyzed with regard to the ATP content (A) and the ability to attach to (B) or to enter (C) HeLa cells. Representative results of one out of three experiments are shown. Values represent the means ± standard deviations of the results of triplicate experiments. In panels B and C, after 1 h of incubation with parasites, HeLa cells were stained with Giemsa and the numbers of attached or intracellular parasites in 250 cells were counted. (D) Giemsa-stained control and starved parasites. (E) The expression levels of the surface molecules gp90 and gp35-gp50 were determined in control (shaded) and starved (unshaded) parasites by flow cytometry, using MAb 1G7 and MAb 10D8, respectively. (F) The susceptibility of control and starved parasites to complement-mediated lysis by anti-gp90 MAb 1G7 at various concentrations was determined.
ATP levels and infectivity are restored by l-proline treatment of starved CL strain metacyclic forms.
To identify the source of parasite energy required for target cell invasion, assays were performed by incubating metacyclic forms starved for 36 h with 1 mM glucose, 0.75 mM or 3.0 mM l-proline, or 1 mM l-glutamic acid at room temperature for 1 h. l-Proline concentrations of 0.75 mM and 3.0 mM are the saturating substrate concentrations chosen for high-affinity transporter system A and low-affinity system B, respectively, as described previously in studies of epimastigotes (24). After washings in PBS, the ATP levels, as well as the parasite infectivity, were examined. l-Proline treatment fully restored both the ATP content and the ability of starved parasites to enter HeLa cells; glucose treatment exhibited some effect, whereas l-glutamic acid treatment had no effect (Fig. 3A). Nutritional depletion for 48 h, which resulted in less than 15% of the control ATP levels and complete loss of motility, affected the parasites in a manner that was not reversible by l-proline treatment. To ascertain the presence of transporters for glucose, l-proline, or l-glutamic acid, we measured the uptake of these compounds by metacyclic forms. Incorporation of l-proline through high-affinity binding system A and low-affinity binding system B as well as the transport of glucose was confirmed, but glutamate uptake levels were negligible (Fig. 3B). Similar results were obtained in repeated experiments. In addition to l-proline, the effect of d-proline was tested. When added to 36-h-starved CL metacyclic forms, d-proline was capable of restoring the parasite ATP content, and the ability of the parasite to enter HeLa cells, as effectively as its enantiomer (data not shown). This effect of d-proline may be due in part to the presence of l-proline, provided that proline racemase is present in T. cruzi (21). We found that, upon incubation with l-proline, the starved parasites with restored ATP content expressed gp82 levels similar to those of controls. When incubated for 1 h with ATP (200 μg/ml), the starved parasites recovered their cell adhesion and invasion capabilities to achieve levels corresponding to ∼72% and 86.3% of control levels, respectively.
The requirement of ATP for cell invasion was further ascertained by treating parasites with a combination of rotenone and antimycin A, drugs that affect ATP production. Antimycin A prevents the formation of ATP by inhibiting the oxidation of ubiquinol in the electron transport chain, and rotenone acts by interfering with the electron transport chain in mitochondria, thus preventing NADH from being converted into usable cellular energy. The parasites were treated with rotenone (200 μM) plus antimycin A (0.5 μM) for 30 min, washed in PBS, and then incubated for 1 h with 1 mM glucose, 0.75 mM l-proline, or 1 mM l-glutamate. After washings in PBS, the parasites were used for cell invasion assays. Treatment with rotenone plus antimycin A, which inhibited parasite locomotion but preserved the flagellar movement, significantly inhibited entry of the CL strain into HeLa cells in a manner not reversible by treatment with glucose or l-proline (Fig. 3C). Experiments with lower concentrations of rotenone were also performed. Parasites were treated with antimycin A (0.5 μM) plus rotenone at 1, 10, or 50 μM for 30 min, washed, and then incubated with l-proline before measurement of the ATP content or seeding onto HeLa cells. Decreases in ATP levels and infectivity, observed at all rotenone concentrations, could be partially reversed by treatment with l-proline (Table 1).
TABLE 1.
Effect of rotenone plus antimycin A on T. cruzi ATP content and infectivitya
| T. cruzi strain | Concn (μM)
|
ATPb (%) | HeLa cell invasionb (%) | ||
|---|---|---|---|---|---|
| Antimycin A | Rotenone | l-proline | |||
| CL | 0 | 0 | 0 | 100.0 | 100.00 |
| 0.5 | 1 | 0 | 35.3 + 3.9 | 41.3 + 0.8 | |
| 0.5 | 1 | 3.0 | 57.8 + 2.4 | 68.2 + 5.9 | |
| 0.5 | 10 | 0 | 30.8 + 2.3 | 34.0 + 5.6 | |
| 0.5 | 10 | 3.0 | 49.2 + 6.4 | 67.7 + 7.3 | |
| 0.5 | 50 | 0 | 32.2 + 3.7 | 28.8 + 2.5 | |
| 0.5 | 50 | 3.0 | 59.0 + 2.5 | 66.5 + 8.4 | |
| G | 0 | 0 | 0 | 100.0 | 100.00 |
| 0.5 | 1 | 0 | 135.7 + 11.7 | 120.94 + 5.6 | |
| 0.5 | 1 | 3.0 | 225.8 + 4.4 | 89.8 + 4.9 | |
| 0.5 | 10 | 0 | 44.9 + 2.1 | 45.9 + 2.8 | |
| 0.5 | 10 | 3.0 | 79.0 + 2.0 | 50.8 + 3.7 | |
| 0.5 | 50 | 0 | 23.3 + 1.6 | 32.3 + 2.1 | |
| 0.5 | 50 | 0.75 | 45.1 + 0.6 | 36.5 + 3.2 | |
Parasites were incubated with antimycin A plus rotenone at the indicated concentrations for 30 min. After washings in PBS, l-proline was added and incubation proceeded for 1 h before ATP measurement and determination of infectivity.
Values represent means + standard deviations of the results of triplicate experiments.
The ability of CL strain metacyclic forms to traverse gastric mucin is enhanced by l-proline.
Studies performed with the CL strain have indicated that the establishment of infection in mice by the oral route is associated with the ability of parasites to bind to gastric mucin through gp82, this being the first step for translocation toward underlying epithelial cells (8, 19). Here we examined the effect of treatment with glucose or l-proline on the ability of starved metacyclic forms to traverse the gastric mucin layer. Parasites maintained for 36 h in PBS were added to the bottom of 24-well plates, in the absence or presence of glucose or l-proline. After 1 h of incubation at 37°C, polycarbonate Transwell filters coated with gastric mucin were placed onto parasite-containing wells. At different time points, 10-μl samples from the filter chamber were collected to determine parasite numbers. Compared to nonstarved controls, the number of starved parasites that translocated through the gastric mucin layer was much lower. Upon incubation with l-proline, but not with glucose, that number increased to levels even higher than the control numbers (Fig. 4).
FIG. 4.
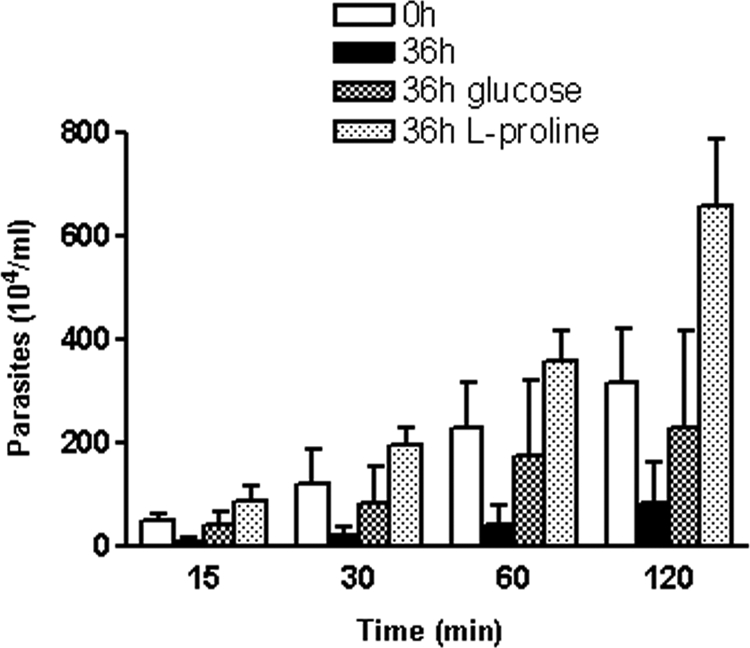
Effect of l-proline on migration of T. cruzi metacyclic forms through gastric mucin. Parasites previously maintained for 36 h in PBS were added to the bottom of 24-well plates, in the absence or presence of 1 mM glucose or 3 mM l-proline. After 1 h of incubation at 37°C, polycarbonate Transwell filters coated with gastric mucin were placed onto parasite-containing wells. At different time points, samples from the filter chamber were collected and the numbers of parasites counted. Values represent the means ± standard deviations of the results of three independent experiments performed in duplicate.
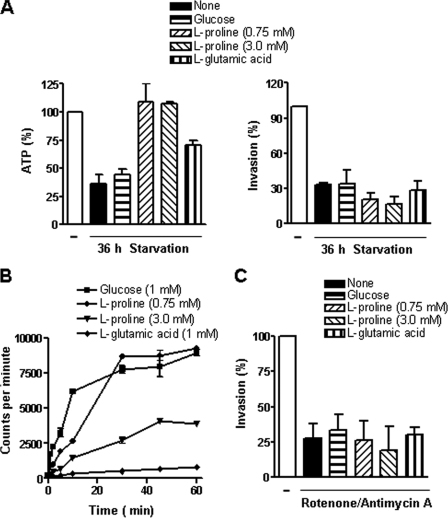
l-Proline restores ATP levels but not infectivity in starved G strain metacyclic forms.
Although G strain metacyclic forms starved for 36 h recovered the normal ATP content after treatment with l-proline, the infectivity toward HeLa cells could not be restored (Fig. 5A), and this was not due to inefficient uptake of l-proline. As shown in Fig. 5B, there was effective l-proline transport, particularly through high-affinity binding system A. The lack of recovery of the infectivity of starved parasites was presumably due to increased regulatory activity of overexpressed gp90 that was capable of neutralizing the effect of l-proline. The energy requirements of the G strain for host cell invasion were also confirmed by treatment with 200 μM rotenone plus 0.5 μM antimycin A, which significantly inhibited HeLa cell entry in a manner not reversible by glucose or l-proline treatment (Fig. 5C). The G strain behaved differently from the CL strain upon incubation with lower concentrations of rotenone. When treated with antimycin A (0.5 μM) plus rotenone at 1 μM, unexpectedly, the ATP levels and the invasion capacity were augmented (Table 1). At rotenone concentrations of 10 and 50 μM, there was a proportional decrease in ATP content and infectivity, with the ATP levels but not infectivity being partially restored by l-proline (Table 1).
FIG. 5.
Effect of l-proline on ATP production and infectivity of T. cruzi metacyclic forms (G strain) subjected to nutritional stress. (A) Parasites previously maintained in PBS for 36 h were incubated for 1 h with glucose (1 mM), l-proline (0.75 mM or 3.0 mM), or l-glutamic acid (1 mM). After washings, the parasite ATP content and the ability to enter HeLa cells were examined. Intracellular parasite numbers were counted in 250 cells stained with Giemsa. A reference value of 100% was assigned to controls. Values represent the means ± standard deviations of the results of three independent experiments performed in duplicate. The differences in the levels of ATP content between 36-h-starved and l-proline-supplemented parasites were significant (P < 0.005). (B) Transport of glucose, l-proline, or glutamate was assayed by using radioactive compounds as described in Materials and Methods. At different time points after the addition of the radioactive reagent, the uptake was stopped by adding cold glucose, l-proline, or l-glutamic acid in PBS. Values represent the means ± standard deviations of the results of triplicate experiments. (C) Parasites were treated with inhibitors of energy production (rotenone [200 μM] and antimycin A [0.5 μM]) for 30 min, washed in PBS, and then used for cell invasion assays in the absence or presence of 1 mM glucose, 0.75 mM l-proline, or 1 mM l-glutamate. Intracellular parasite numbers were counted in 200 Giemsa-stained cells. Values represent the means ± standard deviations of the results of three independent experiments performed in duplicate.
Effect of l-proline on the ATP production and cell invasion capacity of CL-like and G-like T. cruzi strains.
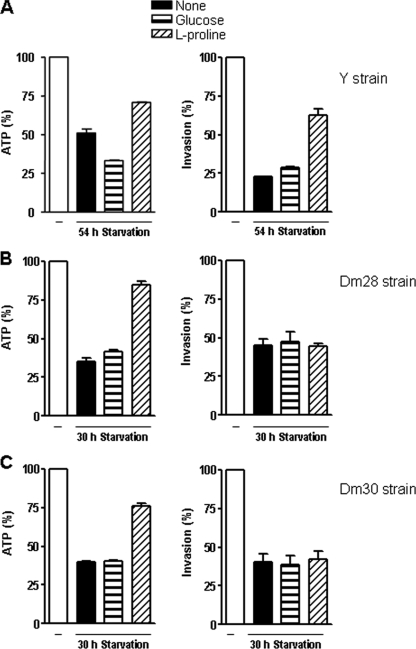
As there was a marked difference between CL and G strains concerning the effect of l-proline treatment in promoting the recovery of infectivity in starved parasites, we asked whether other CL-like or G-like strains behaved accordingly. Y strain metacyclic forms, which have a surface profile similar to that of CL strain and also rely on gp82 molecules to invade target cells, required 54 h of maintenance in PBS for a reduction in ATP content of ∼50%. Upon incubation with l-proline, starved Y parasites partially recovered their ATP content and infectivity with respect to HeLa cells (Fig. 6A). In Dm28 and Dm30 strains, which exhibit a surface profile similar to that of the G strain, the ATP levels dropped by 60 to 65% after 30 h of starvation, and l-proline restored the ATP content to ∼80% of the control levels, but the parasite remained poorly infective (Fig. 6B and C). Expression of gp90 was augmented in starved G-like strains, which, as a consequence, were more susceptible to complement-dependent MAb 1G7-mediated lysis (data not shown).
FIG. 6.
Effect of l-proline on ATP production and infectivity of different T. cruzi strains. Metacyclic forms of the indicated strains were subjected to nutritional stress for 54 h (Y strain) or 30 h (Dm28 and Dm30 strains) and were thereafter incubated for 1 h with glucose (1 mM), l-proline (0.75 mM or 3.0 mM), or l-glutamic acid (1 mM). After washings, the parasite ATP content and the ability to enter HeLa cells were examined. Intracellular parasite numbers were counted in 250 cells stained with Giemsa. A reference value of 100% was assigned to controls. Values represent the means ± standard deviations of the results of one representative experiment out of three experiments performed. The differences in ATP content and infectivity of 54-h-starved and l-proline-supplemented Y strain parasites were significant (P < 0.0005). With regard to the Dm28 and Dm30 strains, the differences in the levels of ATP content of 30-h-starved and l-proline-supplemented parasites were significant (P < 0.0001).
DISCUSSION
We show in this report that metacyclic trypomastigotes of the genetically divergent CL and G strains, which invade host cells through distinct mechanisms by engaging different surface molecules, require energy to complete that process. For both strains, depletion of ATP content by maintaining the parasites under conditions of nutritional stress for different periods of time resulted in proportionally decreased infectivity for epithelial HeLa cells (Fig. 1 and 2).
The CL and G strains differed, however, in several aspects. Metacyclic forms of the CL strain, which had their ATP levels and the ability to invade HeLa cells decreased by ∼70% after 36 h of nutritional stress, recovered the normal ATP content and full infectivity when l-proline was provided (Fig. 3A and B). In G strain metacyclic forms starved for 36 h, the ATP levels were fully restored by l-proline treatment, but recovery of infectivity toward HeLa cells was not seen (Fig. 5A and B). This could have been due, at least in part, to the enhanced expression in starved parasites of gp90 (Fig. 2E), the major determinant of metacyclic trypomastigote infectivity. Gp90-mediated interaction of G strain metacyclic forms with target cells relays inhibitory signals to both cells, blocking the intracellular Ca2+ mobilization essential for parasite internalization (22). In starved parasites, the increased gp90-mediated association with host cells may exert such a negative effect on cell invasion as to counteract the effect of l-proline. However, the experiments with antimycin A plus rotenone (Table 1), showing that l-proline treatment partially restores G strain ATP content but not infectivity, indicate the involvement of other, as-yet-unknown mechanism(s), with gp90 playing a minor role. We do not know the mechanism of upregulation of gp90. One possibility is that it is connected with the differentiation process. Transformation of epimastigotes into metacyclic trypomastigotes, which implies the expression of the stage-specific gp90 molecule, is triggered by nutritional stress. Metacyclic forms collected from culture medium at the stationary phase, which were purified and placed in PBS for starvation, may not have reached the full expression of gp90, which would be completed under conditions of further nutrient deprivation. With regard to the surface glycoproteins gp35 and gp50, also expressed in epimastigotes and engaged by the G strain to enter target cells (32), they were expressed similarly in control and starved parasites (Fig. 2E) and were therefore unrelated to the starvation-associated variation in infectivity. Two additional G-like strains, Dm28 and Dm30, which enter host cells in a mode similar to that of the G strain and which exhibited increased gp90 expression when starved, failed to recover their infectivity after treatment with l-proline, despite the recovery of ATP content (Fig. 6B and C). Unlike that of G-like strains, the internalization of the CL and Y strains, which express gp90 at low levels, is not influenced by this surface molecule (32) and is ensured by the ATP-driven binding of parasites to host cells, concomitant with the recognition of the surface molecule gp82, whose expression level remains constant throughout starvation.
The ability to bind to fixed host cells is another difference between CL and G strain metacyclic forms. Unlike the binding of the CL strain, which bound to host cells in an ATP-dependent manner (Fig. 1A and B), the level of binding of G strain parasites was negligible, regardless of the ATP content (Fig. 2A and B). As binding experiments were performed with fixed cells, one possible explanation is that G strain cytoadhesion requires active participation of target cells, an idea that is supported by the observation that parasites adhere to live cells. This difference in the cell binding capacity may be an additional factor contributing to the differential levels of infectivity shown by the two strains, which rely on distinct mechanisms to enter host cells. Gp82-mediated binding of CL strain metacyclic forms to target cells induces the disruption of the actin cytoskeleton (9), an event that facilitates invasion. By contrast, G strain metacyclic forms, which predominantly engage the mucin-like gp35-gp50 glycoproteins for target cell entry (22) and induce actin recruitment to the site of parasite invasion (13), may depend predominantly on the action of host cells for their internalization. This actin-dependent mode of entry, which is similar to the uptake of enteroinvasive Escherichia coli, is consistent with the observation that G strain parasites stimulate enteroinvasive E. coli invasion in mixed infections of HeLa cells (13).
A recent work investigated the involvement of l-proline in resistance to thermal, nutritional, and oxidative stress, showing that this amino acid participates in a myriad of biochemical and cellular processes (16). Our results with CL strain metacyclic forms further reinforce understanding of the role played by l-proline in biological processes essential for T. cruzi development. We have found that these parasites, when maintained under conditions of nutritional stress, gradually lose motility concomitantly with the decrease in the ATP levels and that the parasites starved for 36 h had a greatly reduced capacity to migrate through polycarbonate Transwell filters that were left uncoated or coated with gastric mucin. The ability of starved parasites to traverse the gastric mucin layer increased to levels even higher than those seen with the nonstarved controls when l-proline was added (Fig. 4). This property is possibly an important factor for metacyclic trypomastigotes that can infect mammalian hosts by the oral route (8, 11, 14, 19), which in some regions is the principal mode of transmission, after the main domiciliary insect vector Triatoma infestans and transmission by blood transfusion were controlled, as attested by microepidemics or outbreaks of acute Chagas' disease (10). Gp82-mediated binding to gastric mucin is a prelude for migration toward the subjacent epithelial cells, which are invaded in a gp82-dependent manner (8, 19). The increased capacity to traverse the gastric mucin layer induced by l-proline suggests that the availability of this amino acid in the gastric milieu may influence parasite infectivity, allowing a faster escape from the harsh conditions in the stomach lumen. It is of interest that Helicobacter pylori, a gram-negative bacterium whose natural habitat is the mucous layer of the human gastric epithelium, preferentially uses amino acids that predominate in samples of human gastric juice, such as l- and d-proline, in addition to l-serine and alanine (18), as a source of energy to reach the location within the mucous layer of the gastric epithelium. From the data herein presented, plus our previous studies showing that T. cruzi strains deficient in gp82 expression have a reduced capacity to bind to gastric mucin and are poorly infective by the oral route, we conclude that by using two closely associated events, namely, binding and translocation through gastric mucin and cell invasion, both of which are dependent on the presence of gp82 and driven by ATP, trypomastigotes exploit the most effective means to establish infection by the oral route.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (grants 2006/61450-0 and 2008/57596-4), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grants 473906/2008-2, 470726/2007-5, and 301409/2007-2), and Instituto Nacional de Biologia Estrutural e Química Medicinal em Doenças Infecciosas (INBEQMeDI).
Technical help by Silene Macedo and Andréa Miyake is acknowledged.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 11 May 2009.
REFERENCES
- 1.Barret, F. M. 1974. Changes in the concentration of free amino acids in the haemolymph of Rhodnius prolixus during the fifth instar. Comp. Biochem. Physiol. B 48241-250. [DOI] [PubMed] [Google Scholar]
- 2.Barret, M. P., E. Tetaud, A. Seyfang, F. Bringaud, and T. Baltz. 1998. Trypanosome glucose transporters. Mol. Biochem. Parasitol. 91195-205. [DOI] [PubMed] [Google Scholar]
- 3.Brener, Z., and E. Chiari. 1963. Variações morfológicas observadas em diferentes amostras de Trypanosoma cruzi. Rev. Inst. Med. Trop. São Paulo 5220-224. [PubMed] [Google Scholar]
- 4.Briones, M. R. S., R. P. Souto, B. S. Stolf, and B. Zingales. 1999. The evolution of two Trypanosoma cruzi subgroups inferred from rRNA genes can be correlated with the interchange of American mammalian faunas in the Cenozoic and has implications to pathogenicity and host specificity. Mol. Biochem. Parasitol. 104219-232. [DOI] [PubMed] [Google Scholar]
- 5.Burleigh, B. A., and N. W. Andrews. 1998. Signaling and host cell invasion by Trypanosoma cruzi. Curr. Opin. Microbiol. 1451-465. [DOI] [PubMed] [Google Scholar]
- 6.Bursell, E. 1981. The role of proline in energy metabolism, p. 135-154. In R. G. H. Downer (ed.), Energy metabolism in insects. Plenum Press, New York, NY.
- 7.Contreras, V. T., J. M. Salles, N. Thomas, C. M. Morel, and S. Goldenberg. 1985. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol. Biochem. Parasitol. 16315-327. [DOI] [PubMed] [Google Scholar]
- 8.Cortez, M., I. Neira, D. Ferreira, A. O. Luquetti, A. Rassi, V. D. Atayde, and N. Yoshida. 2003. Infection by Trypanosoma cruzi metacyclic forms deficient in gp82 but expressing a related surface molecule gp30. Infect. Immun. 716184-6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortez, M., V. Atayde, and N. Yoshida. 2006. Host cell invasion mediated by Trypanosoma cruzi surface molecule gp82 is associated with F-actin disassembly and is inhibited by enteroinvasive Escherichia coli. Microbes Infect. 81502-1512. [DOI] [PubMed] [Google Scholar]
- 10.Coura, J. R. 2006. Transmission of chagasic infection by oral route in the natural history of Chagas' disease. Rev. Soc. Bras. Med. Trop. 39(Suppl. 3)113-117. (In Portuguese.) [PubMed] [Google Scholar]
- 11.Covarrubias, C., M. Cortez, D. Ferreira, and N. Yoshida. 2007. Interaction with host factors exacerbate Trypanosoma cruzi cell invasion capacity upon oral infection. Int. J. Parasitol. 371609-1616. [DOI] [PubMed] [Google Scholar]
- 12.Docampo, R., and S. J. Moreno. 1996. The role of Ca2+ in the process of cell invasion by intracellular parasites. Parasitol. Today 1261-65. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira, D., M. Cortez, V. D. Atayde, and N. Yoshida. 2006. Actin cytoskeleton-dependent and -independent host cell invasion by Trypanosoma cruzi is mediated by distinct parasite surface molecules. Infect. Immun. 745522-5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoft, D. F., P. L. Farrar, K. Kratz-Owens, and D. Shaffer. 1996. Gastric invasion by Trypanosoma cruzi and induction of protective mucosal immune responses. Infect. Immun. 643800-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Homsy, J. J., B. Granger, and S. M. Krassner. 1989. Some factors inducing formation of metacyclic stages of Trypanosoma cruzi. J. Protozool. 36150-153. [DOI] [PubMed] [Google Scholar]
- 16.Magdaleno, A., I. Y. Ahn, L. S. Paes, and A. Silber. 2009. Action of a proline analogue, l-thiazolidine-4-carboxylic acid (THC), on Trypanosoma cruzi. PLoS One 4(2):e4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Málaga, S., and N. Yoshida. 2001. Targeted reduction in expression of Trypanosoma cruz surface glycoprotein gp90 increases parasite infectivity. Infect. Immun. 69353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagata, K., Y. Nagata, T. Sato, M. A. Fujino, K. Nakajima, and T. Tamura. 2003. l-Serine, d- and l-proline and alanine as respiratory substrates of Helicobacter pylori: correlation between in vitro and in vivo amino acid levels. Microbiology 1492023-2030. [DOI] [PubMed] [Google Scholar]
- 19.Neira, I., F. A. Silva, M. Cortez, and N. Yoshida. 2003. Involvement of Trypanosoma cruzi metacyclic trypomastigote surface molecule gp82 in adhesion to gastric mucin and invasion of epithelial cells. Infect. Immun. 71557-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramirez, M. I., R. C. Ruiz, J. E. Araya, J. Franco da Silveira, and N. Yoshida. 1993. Involvement of the stage-specific 82-kilodalton adhesion molecule of Trypanosoma cruzi metacyclic trypomastigotes in host cell invasion. Infect. Immun. 613636-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reina-San-Martín, B., W. Degrave, C. Rougeot, A. Cosson, N. Chamond, A. Cordeiro-da-Silva, M. Arala-Chaves, A. Coutinho, and P. Minoprio. 2000. A B-cell mitogen from a pathogenic trypanosome is a eukaryotic proline racemase. Nat. Med. 6890-897. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz, R. C., S. Favoreto, M. L. Dorta, M. E. M. Oshiro, A. T. Ferreira, P. M. Manque, and N. Yoshida. 1998. Infectivity of Trypanosoma cruzi strains is associated with differential expression of surface glycoproteins with differential Ca2+ signaling activity. Biochem. J. 330505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schenkman, S., E. Robins, and V. Nussenzweig. 1991. Attachment of Trypanosoma cruzi to mammalian cells requires parasite energy, and invasion can be independent of the target cell cytoskeleton. Infect. Immun. 59645-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silber, A. M., R. R. Tonelli, M. Martinelli, W. Colli, and M. J. M. Alves. 2002. Active transport of l-proline in Trypanosoma cruzi. J. Eukaryot. Microbiol. 49441-446. [DOI] [PubMed] [Google Scholar]
- 25.Silber, A. M., R. L. G. Rojas, U. Urias, W. Colli, and M. J. M. Alves. 2006. Biochemical characterization of the glutamate transport in Trypanosoma cruzi. Int. J. Parasitol. 36157-161. [DOI] [PubMed] [Google Scholar]
- 26.Silva, L. H. P., and V. Nussenzweig. 1953. Sobre uma cepa de Trypanosoma cruzi altamente virulenta para o camundongo branco. Folia Clin. Biol. 20191-203. [Google Scholar]
- 27.Sylvester, D., and S. M. Krassner. 1976. Proline metabolism in Trypanosoma cruzi epimastigotes. Comp. Biochem. Physiol. B 55443-447. [DOI] [PubMed] [Google Scholar]
- 28.Teixeira, M. M. G., and N. Yoshida. 1986. Stage-specific surface antigens of metacyclic trypomastigotes of Trypanosoma cruzi identified by monoclonal antibodies. Mol. Biochem. Parasitol. 18271-282. [DOI] [PubMed] [Google Scholar]
- 29.Tetaud, E., F. Bringaud, S. Chabas, M. P. Barrett, and T. Baltz. 1994. Characterization of glucose transport and cloning of a hexose transporter gene in Trypanosoma cruzi. Proc. Natl. Acad. Sci. USA 918278-8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tonelli, R., A. M. Silber, M. Almeida-de-Faria, I. Y. Hirata, W. Colli, and J. M. Alves. 2004. l-Proline is essential for the intracellular differentiation of Trypanosoma cruzi. Cell. Microbiol. 6733-741. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida, N. 1983. Surface antigens of metacyclic trypomastigotes of Trypanosoma cruzi. Infect. Immun. 40836-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida, N. 2006. Molecular basis of mammalian cell invasion of Trypanosoma cruzi. An. Acad. Bras. Ciênc. 7887-111. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida, N., R. A. Mortara, M. F. Araguth, J. C. Gonzalez, and M. Russo. 1989. Metacyclic neutralizing effect of monoclonal antibody 10D8 directed to the 35- and 50-kilodalton surface glycoconjugates of Trypanosoma cruzi. Infect. Immun. 571663-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]